Abstract
Introduction
Even though the survival benefit of neoadjuvant chemotherapy (NAC) in the treatment of muscle invasive bladder cancer (MIBC) is well established, NAC has not been widely used in Poland until recently. The aim of our study was to evaluate the utilization of NAC and its association with survival in MIBC.
Material and methods
Patients who underwent radical cystectomy (RC) for MIBC between December 2012 and December 2017 were included in the study. Data were collected in the perioperative period and long-term observation was continued up to August 2018. Kaplan-Meier curves were used to estimate the probability of survival.
Results
A sample of 155 patients with a median age of 65 (IQR: 60–69) years was analyzed. In this group, 79 patients (51%) were treated with NAC prior to RC. Patients in the NAC+RC group were younger, more often had a positive smoking history, and had lower preoperative levels of hemoglobin, white blood cells and C-reactive protein. A 90-day complication rate and mortality were similar in both groups and in the entire cohort were equal to 64.5% and 5.2%, respectively. The overall survival (OS) was on average 150 days longer in the RC+NAC group compared to the RC-only group when patients were followed-up for 3 years (95%CI:3 4 – 267; p = 0.011).
Conclusions
We demonstrated a high utilization of NAC at our institution. The use of NAC was associated with a better prognosis than RC alone and was not associated with an increased morbidity or mortality. Our results support the use NAC as a safe and effective treatment modality in MIBC.
Keywords: muscle invasive bladder cancer, neoadjuvant chemotherapy
INTRODUCTION
Muscle invasive bladder cancer (MIBC) is the second cause of death among urological malignancies. The incidence of MIBC diagnosed among primary bladder tumors is up to 25% [1]. The prognosis for patients with MIBC is poor, although data concerning mortality rates after treatment of MIBC in Poland are sparse [2]. Radical cystectomy (RC) with pelvic lymphadenectomy is a surgical treatment of choice for patients with MIBC, with the 5-year survival rate around 60% [3, 4]. Despite RC, a substantial portion of patients experience cancer recurrence during follow-up. Prevention of systemic spread and early treatment of micrometastatic disease was the main rationale for introducing neoadjuvant chemotherapy (NAC) for MIBC treatment. Even though the survival benefit of cisplatin-based NAC followed by RC compared with RC alone was supported by level 1 evidence [5] and European Association of Urology guidelines recommend this treatment for MIBC, NAC was not widely used in Poland until recently. In our institution NAC was introduced in 2013. This is the first report concerning the utilization of NAC in MIBC treatment in a tertiary referral center in Poland.
MATERIAL AND METHODS
All cystectomies performed between December 2012 and December 2017 in a single academic tertiary center were reviewed (n = 233). Patients who underwent planned RC with bilateral pelvic lymphadenectomy in curative intent for muscle invasive urothelial carcinoma were included in the study. Exclusion criteria were defined as: palliative surgery (n = 21), RC for non-muscle invasive bladder cancer (NMIBC) (n = 18), urgent surgery (n = 3), tumor's histology other than transitional cell carcinoma (n = 11), concomitant malignancy (n = 19) except for low grade localized prostate cancer, benign indications for surgery (n = 6).
Medical data were collected from hospital records and medical history was obtained from patients and/or their relatives. Last follow-up telephone call was conducted in all patients or their caregivers at the end of the study period in August 2018 and in case of death, outcome documentation was obtained. The study protocol was approved by the local Ethics Committee.
Clinical stage was determined by transurethral resection of the bladder tumor (TURBt), bimanual examination, chest computed tomography (CT) or chest X-ray and abdominal and pelvic CT or magnetic resonance imaging. Pathological staging was performed according to the 2009 TNM classification [6] by pathological examination of the surgical specimens.
Statistical analysis
No formal sample size calculation was performed. We used Kaplan-Meier curves with the associated 95% confidence intervals to estimate the overall survival (OS) and recurrence-free survival (RFS) probabilities. We noted non-proportional hazards in log-log survival curves, therefore we tested the null hypothesis of no difference in restricted mean survival times between the groups instead of using the log-rank test. The average event-free survival up to 3 years was specified as a clinically important point. Deaths unrelated to bladder cancer were censored in the RFS analysis. Univariate analyses of between-group differences were performed with the Wilcoxon-Mann-Whitney, chi-squared and Fisher's exact tests. Continuous variables were described with medians and interquartile ranges. An alpha level of 0.05 was used as a cut-off for declaring statistical significance. If the cause of death could not be ascertained, patients were treated as having a recurrence.
RESULTS
Patient characteristics
One hundred and fifty-five patients that underwent RC were recruited to the study, of which 79 patients (51%) were treated with NAC prior to RC and 76 (49%) underwent RC alone. Sample characteristics are presented overall and separately for individuals treated with RC and NAC+RC (Table 1). The median age at the time of surgery was 65 (IQR: 60–69) years and patients were predominantly men (80%). Those receiving NAC and RC were younger and more often had a positive smoking history (current or former), had lower preoperative hemoglobin (Hb), white blood cells (WBC) and C-reactive protein (CRP) levels (Table 1). Otherwise the groups were similar (Table 1).
Table 1.
Patients’ characteristics
| Variable | Statistics | All (n = 155) | RC only (n = 76) | NAC+RC (n = 79) | p (RC only vs. NAC+RC) |
|---|---|---|---|---|---|
| Age | Median IQR |
65.00 60.00–69.00 |
66.50 62.00–73.00 |
64.00 58.00–67.00 |
0.004 |
| Gender (male) | n (%) | 124 (80.0) | 60 (78.9) | 64 (81) | 0.904 |
| BMI | Median IQR |
27.17 24.50–29.70 |
26.95 24.49–29.32 |
27.40 24.50–29.90 |
0.583 |
| Smokers (current/former) | n (%) | 108 (71.6) | 42 (58.3) | 66 (83.5) | 0.001 |
| Occupational exposure | n (%) | 26 (18.2) | 14 (21.9) | 12 (15.2) | 0.416 |
| ASA score (%) 1 2 3 4 |
n (%) n (%) n (%) n (%) |
1 (0.6) 104 (67.1) 49 (31.6) 1 (0.6) |
0 (0.0) 48 (63.2) 27 (35.5) 1 (1.3) |
1 (1.3) 56 (70.9) 22 (27.8) 0 (0.0) |
0.381 |
| CAD | n (%) | 37 (23.9) | 23 (30.3) | 14 (17.7) | 0.100 |
| CHF | n (%) | 15 (9.7) | 10 (13.2) | 5 (6.4) | 0.254 |
| Aortal Stenosis | n (%) | 2 (1.3) | 2 (2.6) | 0 (0) | 0.460 |
| PVD | n (%) | 9 (5.8) | 5 (6.6) | 4 (5.1) | 0.952 |
| DVT/PE | n (%) | 2 (1.3) | 1 (1.3) | 1(1.3) | 1.000 |
| HT | n (%) | 104 (67.1) | 53 (69.7) | 51 (64.6) | 0.606 |
| DM | n (%) | 32 (20.6) | 16 (21.1) | 16 (20.3) | 1.000 |
| COPD | n (%) | 12 (7.9) | 9 (11.8) | 3 (3.9) | 0.133 |
| CVE | n (%) | 5 (3.2) | 3 (4.0) | 2 (2.5) | 0.953 |
| Baseline hemoglobin (g/dL) | Median IQR |
12.10 11.00–13.20 |
12.85 11.20–14.10 |
11.80 11.00–12.60 |
0.002 |
| Baseline WBC (x10*3/uL) | Median IQR |
6.95 5.74–8.50 |
7.44 6.24–9.01 |
6.50 5.36–7.81 |
0.005 |
| Baseline PLT (x10*3/uL) | Median IQR |
226.00 177.00–300.00 |
224.00 180.25– 292.75 |
226.00 177.00– 313.50 |
0.731 |
| Baseline creatinine (umol/L) | Median IQR |
92.00 76.25–114.25 |
87.00 72.50– 108.50 |
97.00 82.50– 118.00 |
0.052 |
| Baseline albumin (g/l) | Median IQR |
42.40 39.00–45.00 |
42.50 39.00–44.00 |
42.35 40.00–45.00 |
0.369 |
| Baseline protein (g/l) | Median IQR |
68.70 65.00–72.50 |
67.50 64.75–72.00 |
69.00 65.20–72.90 |
0.324 |
| Baseline CRP (mg/ml) | Median IQR |
2.95 1.27–9.46 |
4.40 2.14–13.45 |
2.14 1.00–3.99 |
0.004 |
RC – radical cystectomy; NAC – neoadjuvant chemotherapy; IQR – Interquartile range; n (%) – number (percentage); BMI – body mass index; ASA score – American Society of Anesthesiologists score; CAD – coronary artery disease; CHF – chronic heart failure; PVD – peripheral vascular disease; DVT/PE – deep venous thrombosis/pulmonary embolism; HT – hypertension; DM – diabetes mellitus; COPD – chronic obstructive pulmonary disease; CVE – cerebral vascular event; WBC – white blood cell count; PLT – platelets; CRP – C-reactive protein
Neoadjuvant chemotherapy
The decision regarding application and choice of NAC was at the discretion of the treating oncologist. Sixty-eight patients received cisplatin-based NAC and 11 were treated with carboplatin and gemcitabine combination. Nine patients did not complete planned chemotherapy schedule due to the side effects.
Tumor characteristics
Pre-treatment clinical staging was similar in both groups, and over 40% of patients had extravesical tumor invasion. Suspicious lymph nodes were found in 21 patients (Table 2). On the other hand, the final pathology of surgical specimen differed between the groups and patients who received NAC before RC had more complete responses, more non-invasive and more localized disease. Also, fewer lymph node metastases and positive surgical margins were found in NAC+RC group (Table 2).
Table 2.
Tumor characteristics
| Variable | Statistics | All (n = 155) | RC only (n = 76) | NAC+RC (n = 79) | p (RC only vs. NAC+RC) |
|---|---|---|---|---|---|
| Clinical T-stage 2–3a 3b–4 |
n (%) n (%) |
87 (56.1) 68 (43.9) |
43 (56.6) 33 (43.4) |
44 (55.7) 35 (44.3) |
0.539 |
| Clinical N-stage 0 + |
n (%) n (%) |
134 (86.5) 21 (13.5) |
69 (90.8) 7 (9.2) |
65 (82.3) 14 (17.7) |
0.060 |
| Pathologic T-stage 0 a-1-Cis 2 3–4a |
n (%) n (%) n (%) n (%) |
22 (14.2) 25 (16.1) 39 (25.2) 69 (44.5) |
4 (5.3) 3 (3.9) 19 (25) 50 (65.8) |
18 (22.8) 22 (27.9) 20 (25.3) 19 (24.1) |
<0.001 |
| Pathologic N-stage 0 + |
n (%) n (%) |
106 (68.4) 49 (31.6) |
44 (57.9) 32 (42.1) |
62 (78.5) 17 (21.5) |
0.035 |
| Positive margin | n (%) | 19 (12.3) | 14 (18.4) | 5 (6.4) | 0.043 |
RC – radical cystectomy; NAC – neoadjuvant chemotherapy; IQR – Interquartile range; n (%) – number (percentage)
Surgery and perioperative period
Most patients underwent laparoscopic surgery and the predominant urinary diversion was an ileal conduit in both groups. A 90-days complication rate and mortality were 64.5% and 5.2%, respectively, with no statistically significant differences between groups (Table 3).
Table 3.
Perioperative characteristics
| Variable | Statistics | All (n = 155) | RC only (n = 76) | NAC+RC (n = 79) | p (RC only vs. NAC+RC) |
|---|---|---|---|---|---|
| Laparoscopic approach | n (%) | 108 (69.7) | 48 (63.2) | 60 (75.9) | 0.119 |
| Urinary diversion Bricker conduit Ureterocutaneostomy Neobladder |
n (%) n (%) n (%) |
119 (76.7) 25 (16.1) 11 (7.1) |
52 (68.4) 18 (23.7) 6 (7.9) |
67 (84.8) 7 (8.9) 5 (6.3) |
0.034 |
| CDC 90-days 0 1 2 3 4 5 |
n (%) n (%) n (%) n (%) n (%) n (%) |
55 (35.5) 6 (3.9) 62 (40) 18 (11.6) 6 (3.9) 8 (5.2) |
22 (28.9) 4 (5.3) 32 (42.1) 9 (11.8) 4 (5.3) 5 (6.6) |
33 (41.8) 2 (2.5) 30 (38) 9 (11.4) 2 (2.5) 3 (3.8) |
0.730 |
RC – radical cystectomy; NAC – neoadjuvant chemotherapy; IQR – Interquartile range; n (%) – number (percentage); CDC – Clavien-Dindo classification
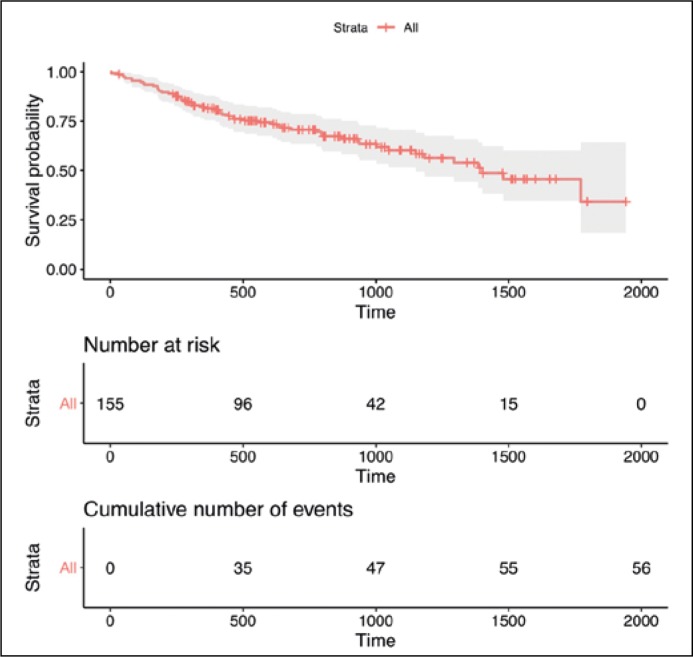
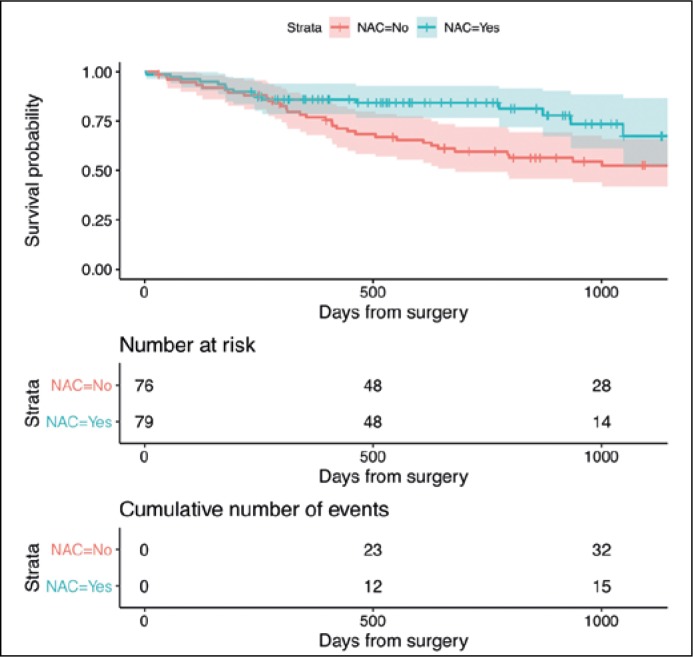
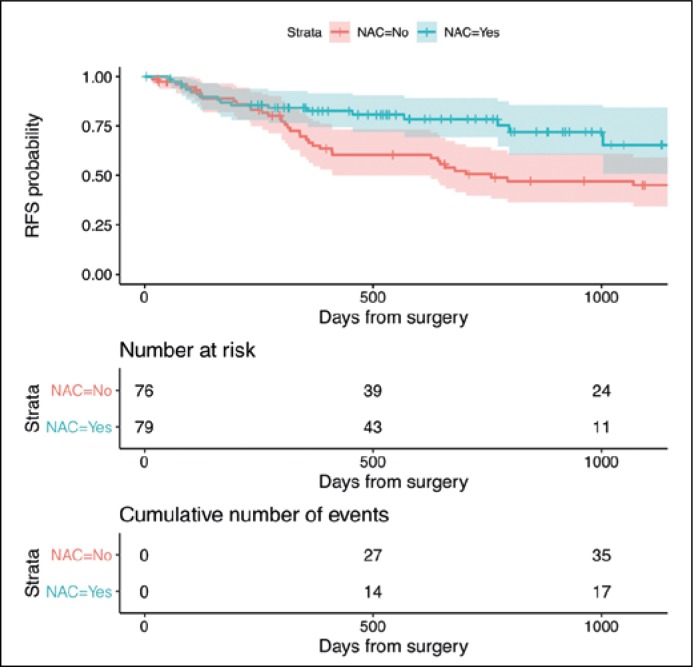
The estimated OS in the entire sample is shown in Figure 1. Patients in the RC+NAC group lived, on average, an additional 150 days in 3 years than those in the RC only group (95%CI: 34–267; P = 0.011) (Figure 2). The RFS was on average 174 days longer in the RC+NAC group than in the RC only group when patients were followed-up for 3 years (95%CI: 45–304; P = 0.008) (Figure 3). As a sensitivity analysis, we extended the restricted mean survival time to 4 years after surgery which showed an average benefit of 189 days in OS (95%CI: 8–370; P = 0.040) and 257 days in RFS (95CI: 65–449; P = 0.009).
Figure 1.
A Kaplan-Meier curve for overall survival (OS) estimated using the entire sample Footnote: Survival probability is calculated as the number of subjects surviving up to a given time interval divided by the number of patients at risk. Censored subjects (e.g. lost to follow-up) are no longer counted as at risk.
Figure 2.
Kaplan-Meier curves for OS stratified by NAC Footnote: Restricted mean survival time was calculated up to day 1095 (3-year follow-up respectively).
Figure 3.
Kaplan-Meier curves for recurrence-free survival (RFS) stratified by NAC Footnote: Restricted mean recurrence-free survival time was calculated up to day 1095 (3-year year follow-up respectively).
DISCUSSION
To the best of our knowledge, this is the first report concerning the utilization of NAC in the treatment of MIBC in an academic center in Poland. More than half of the patients received NAC preoperatively. Data from large cohort studies show that approximately 20% of patients with MIBC are treated with NAC before surgery [7, 8]. Except for one Japanese study in which 80% of subjects received carboplatin-based NAC [9], data concerning the utilization of NAC between 2013 and 2017 are lacking. The effect of NAC was studied in an experimental design and it is encouraging that our results obtained in a pragmatic setting are in line with those reported in randomized controlled trials [5]. This provides another piece of the evidence in favor of a wider usage of NAC among MIBC patients.
NAC+RC receivers in our study were younger, which is consistent with previously published data [9, 10] and might be explained by the fact that chemotherapy contradictions are less common among younger patients. Lower preoperative WBC and Hb levels in NAC+RC group most likely reflect the hematologic toxicity of NAC, and was also previously reported [10]. Interestingly, we found lower baseline CRP levels in the NAC+RC group, which are associated with a better prognosis in this population.
There were no differences in the pre-treatment clinical stage between the RC only and NAC+RC group. However, the final pathology results for NAC+RC patients was more favorable, with more patients with complete responses, more NMIBC patients, and more patients with localized disease. There were also fewer patients with metastatic lymph nodes and positive margins. Similar data were previously reported [9, 10] and this downstaging is most likely the result of introducing NAC.
The 90-days complication and mortality rates in our study were similar to those previously published [10, 11]. We found no significant differences concerning surgical outcomes between patients who received NAC and those who did not. Our results add to the current body of evidence showing that NAC in MIBC does not put patients in greater jeopardy of worse surgical outcomes.
An ileal neobladder was created only in a few patients in our study, while available data suggest that it should be the most frequently formed urinary diversion [12, 13]. However, in the recent population study concerning trends in urinary diversion in the USA, the rate of continent diversion between 2010 and 2013 was 12.1% [14]. What is interesting, fewer continent diversions were offered in centers which perform predominantly minimally invasive RC [14]. The study shows hesitancy in neobladder construction, hopefully in the next report from our center the rate of continent diversions will increase.
Our study has several limitations. First, we present limited data from a single academic center that might not reflect current practice in Poland. Second, the retrospective study design leads to incomplete data and possible selection bias. We were not able to evaluate the reasons why patients form RC only group did not receive NAC. Our sample size did not allow us to adjust the mortality prediction analysis with the known risk factors. The relatively short-term follow-up also restricted our results to some extent. Moreover, we used only overall survival as the endpoint of our study, as the cause of death could not have been determined reliably in all patients, and hence cancer-specific survival would have been likely affected. However, it should be noted that OS is the gold standard for cancer clinical research and is considered the most reliable and preferred cancer endpoint [15].
CONCLUSIONS
We successfully implemented NAC in the treatment of MIBC at our institution. NAC was associated with a better prognosis than RC alone and was not associated with an increase in the number of postoperative complications and mortality. Our study supports a wider use of NAC as safe and effective adjunct to the treatment of MIBC.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Burger M, Catto J, Dalbagni G, et al. Epidemiology and Risk Factors of Urothelial Bladder Cancer. Eur Urol. 2013;63:234–241. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 2.Dybowski B, Ossoliński K, Ossolińska A, Peller M, Bres-Niewada E, Radziszewski P. Impact of stage and comorbidities on five-year survival after radical cystectomy in Poland: single centre experience. Cent European J Urol. 2015;68:278–283. doi: 10.5173/ceju.2015.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nuhn P, May M, Sun M, et al. External Validation of Postoperative Nomograms for Prediction of All-Cause Mortality, Cancer-Specific Mortality, and Recurrence in Patients With Urothelial Carcinoma of the Bladder. Eur Urol. 2012;61:58–64. doi: 10.1016/j.eururo.2011.07.066. [DOI] [PubMed] [Google Scholar]
- 4.Stein JP, Lieskovsky G, Cote R, et al. Radical Cystectomy in the Treatment of Invasive Bladder Cancer: Long-Term Results in 1,054 Patients. J Clin Oncol. 2001;19:666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 5.Vale C. Neoadjuvant Chemotherapy in Invasive Bladder Cancer: Update of a Systematic Review and Meta-Analysis of Individual Patient Data. Eur Urol. 2005;48:202–206. doi: 10.1016/j.eururo.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Sobin L, Gospodarowicz M, Wittekind C. TNM Classification of Malignant Tumours, 7th Edition. New York, NY: John Wiley & Sons; 2011. pp. 262–265. [Google Scholar]
- 7.Reardon Z, Patel S, Zaid H, et al. Trends in the Use of Perioperative Chemotherapy for Localized and Locally Advanced Muscle-invasive Bladder Cancer: A Sign of Changing Tides. Eur Urol. 2015;67:165–170. doi: 10.1016/j.eururo.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keegan K, Zaid H, Patel S, Chang S. Increasing Utilization of Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer in the United States. Curr Urol Rep. 2014;15:394. doi: 10.1007/s11934-014-0394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anan G, Hatakeyama S, Fujita N, et al. Trends in neoadjuvant chemotherapy use and oncological outcomes for muscle-invasive bladder cancer in Japan: a multicenter study. Oncotarget. 2017;8:86130–86142. doi: 10.18632/oncotarget.20991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milenkovic U, Akand M, Moris L, et al. Impact of neoadjuvant chemotherapy on short-term complications and survival following radical cystectomy. World J Urol. 2018 doi: 10.1007/s00345-018-2584-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Gandaglia G, Popa I, Abdollah F, et al. The Effect of Neoadjuvant Chemotherapy on Perioperative Outcomes in Patients Who Have Bladder Cancer Treated with Radical Cystectomy: A Population-based Study. Eur Urol. 2014;66:561–568. doi: 10.1016/j.eururo.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Hautmann RE, Volkmer BG, Schumacher MC, Gschwend JE, Studer UE. Long-term results of standard procedures in urology: the ileal neobladder. World J Urol. 2006;24:305–314. doi: 10.1007/s00345-006-0105-z. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization (WHO) Consensus Conference on Bladder Cancer. Hautmann RE, Abol-Enein H, Hafez K, et al. Urinary diversion. Urology. 2007;69:17–49. [Google Scholar]
- 14.Lin-Brande M, Nazemi A, Pearce SM, et al. Assessing trends in urinary diversion after radical cystectomy for bladder cancer in the United States. Urol Oncol. 2019;37:180.e1–180. doi: 10.1016/j.urolonc.2018.11.003. e9. [DOI] [PubMed] [Google Scholar]
- 15.Food and Drug Administration. Guidance for Industry Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics [Internet] 2018. [cited 14 January 2019] Avaliable from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM071590.pdf.