Abstract
Although portal vein thrombosis (PVT) commonly occurs in patients with hepatocellular carcinoma (HCC), the hypercoagulability mechanism in patients with HCC is not entirely clear. Recently, tumor-induced formation of neutrophil extracellular traps (NET) has been shown to trigger contact system activation, and contact system activation has been shown to be a new mechanism of thrombosis. Therefore, we investigated whether contact system activation and NET formation occurred in relation to PVT in HCC patients. The circulating levels of NET formation markers (DNA–histone complex, double-stranded DNA, neutrophil elastase) and contact system activation markers (factor XIIa and high-molecular-weight kininogen) were measured in 177 patients who had been diagnosed with HCC and 48 healthy controls. Presence of PVT was confirmed in 77 HCC patients. The levels of NET formation and contact system activation markers were significantly higher in patients than in healthy controls and they increased significantly with the increase in the model for end-stage liver disease (MELD) scores. Of note, these markers were significantly higher in HCC patients with PVT than in those without PVT. These NET formation markers and the contact system activation markers were significant thrombotic risk factors in HCC patients. The well-known liver injury markers (alanine transaminase, prothrombin time) significantly contributed to factor XIIa level. Contact system activation and NET formation are well correlated with liver disease severity and the markers of these can be used as thrombotic risk factors in HCC patients. In addition, therapeutics inhibiting the contact system can be potentially used to manage PVT in HCC patients.
Keywords: contact system activation, extracellular traps, liver neoplasms, venous thrombosis
Introduction
Liver cirrhosis is characterized by hemostatic imbalance with decreased production of coagulant and anticoagulant factors, which induces bleeding and thrombotic tendencies.1 Among thrombosis, portal vein thrombosis (PVT) is common in patients with liver cirrhosis. When liver cirrhosis is combined with hepatocellular carcinoma (HCC), the frequency of PVT markedly increases from <10% to 35%.2 This is because not only HCC by itself can induce a hypercoagulable state but also tumor cell invasion into the portal vein can increase the PVT frequency.3
Recently, contact system activation has emerged as a new mechanism of thrombosis.4 Neutrophils are activated by inflammatory or other stimuli and release intranuclear contents (DNA–histone complex, double-stranded DNA [dsDNA], and neutrophil elastase), called neutrophil extracellular traps (NET), into the extracellular space.5 The negatively charged histone–DNA complex can activate coagulation factor XII, which initiates contact system activation. The contact system consists of 4 plasma proteins (factor XII, factor XI, prekallikrein, high-molecular-weight kininogen [HMWK]) that assemble when factor XII comes in contact with negatively charged surfaces.6 Activated factor XII (XIIa) then activates factor XI and the downstream intrinsic coagulation pathway.7 In addition, factor XIIa converts prekallikrein to α-kallikrein, which cleaves HMWK to yield bradykinin. Factor XII activation plays an essential role in thrombosis and the inhibition of factor XII activation can block thrombosis in animals and humans.5,7–9 Although contact system activation has been suggested as a new mechanism of thrombosis,4 there have been no reports on whether the contact system is activated in patients with liver cirrhosis with HCC.
In this study, we measured the circulating levels of NET formation markers (DNA–histone complex, dsDNA, neutrophil elastase) and contact system activation markers (factor XIIa and HMWK) in patients with liver cirrhosis with HCC to investigate whether NET formation and contact system activation occurs in relation to PVT.
Methods
Patient Population
A total of 177 patients who had been diagnosed with HCC at Seoul National University Hospital were enrolled. This study was approved by the institutional review board of Seoul National University College of Medicine. The diagnosis of HCC was confirmed by clinical correlation using histologic features taken from liver biopsy as determined by a pathologist, and findings from image studies including ultrasound, computed tomography, and MRI, verified by a radiologist. Patients who had active variceal bleeding, anticoagulant or antineoplastic treatments, and hematological diseases were excluded. The cancer stage was scored by using the AJCC Cancer Staging Manual,10 based on the time point when the sample was taken. The severity of liver disease was scored in accordance with the model for end-stage liver disease (MELD) score11 on the basis of the results of clinical chemistry test and functional coagulation test. The severity was divided into 3 subgroups (MELD score <10, 10-20, and >20). The presence of PVT was confirmed by medical record review and all the recorded events were confirmed by a radiologist using imaging studies, ultrasound, contrast-enhanced, or MR. A total of 48 healthy controls who showed normal liver function test results and no evidence of liver disease were also recruited.
Blood Sampling and Routine Laboratory Testing
Peripheral venous blood samples were collected in 0.109 mol/L sodium citrate (Becton Dickinson, San Jose, California), and plasma was separated by centrifugation of whole blood at 1550g for 15 minutes. Aliquots were stored at −80°C. Fibrinogen was measured on an automated coagulation analyzer (ACL TOP; Beckman Coulter, Fullerton, California) using the HemosIL Fibrinogen-C XL reagent (Instrumentation Laboratory SpA, Milan, Italy).
Markers of NET Formation (Histone–DNA Complex, dsDNA, and Neutrophil Elastase)
The plasma level of the histone–DNA complex was quantified with a Cell Death Detection ELISA kit (Roche Diagnostics, Indianapolis, Indiana). The dsDNA level was measured using the Quant-iT PicoGreen dsDNA reagent (Molecular Probes, Eugene, Oregon) and a microplate fluorometer (Fluoroskan Ascent, Thermo Fisher Scientific Inc., Waltham, Massachusetts). The level of neutrophil elastase was measured using a Human Neutrophil Elastase Platinum ELISA kit (eBioscience, Vienna, Austria).
Markers of Contact System Activation (Factor XIIa and HMWK)
Factor XIIa activity was measured by using a chromogenic method with a CoaChrom Factor XIIa test kit (CoaChrom Diagnostica, Maria Enzersdorf, Austria). High-molecular-weight kininogen was measured with ELISA kits from Cloud-Clone Corp (Houston, Texas).
Statistical Analysis
Data were compared using χ2 test for categorical variables, and Mann-Whitney U test and Kruskal-Wallis test for continuous variables. To assess the prediction power of test variables for thrombosis, logistic regression analyses were performed by using the cutoff points of test variables that produced the best discriminative power on receiver operating characteristic (ROC) curves. The ROC curves were drawn for every variable to determine the cutoff for the best separation of thrombosis risk, and the patients were divided into 2 subgroups for each single factor (value above or below the established cutoff). To assess the variables contributing to factor XIIa level, multivariable linear regression analyses were performed. All analyses were carried out using the SPSS version 23.0 (SPSS Inc, Chicago, Illinois) and MedCalc version 14.8.1 (MedCalc Software, Ostend, Belgium).
Results
Elevation of NET Formation and Contact System Activation in Patients With HCC
Clinical and demographic characteristics of the study population are shown in Table 1. Compared to healthy controls, patients with HCC were older and the male proportion was higher. The plasma levels of fibrinogen were not different between healthy controls and patients with HCC. However, the plasma levels of NET formation markers (DNA–histone complex, dsDNA, neutrophil elastase) and a contact system marker (factor XIIa) were significantly higher in patients with HCC than in healthy controls, whereas the HMWK level was tended to be lower in patients than in healthy controls though statistically not significant.
Table 1.
Clinical and Laboratory Characteristics of the Study Population.a
| Healthy controls | Patients With Hepatocellular Carcinoma | |||||
|---|---|---|---|---|---|---|
| Total (n = 48) | Total (n = 177) | MELD < 10 (n = 65) | MELD 10-20 (n = 85) | MELD > 20 (n = 27) | P Valueb | |
| Age (years) | 37 (32-51) | 61 (36-89)c | 64 (36-89) | 61 (37-87) | 59 (47-80) | .290 |
| Male/female (n) | 25/23 | 140/37c | 47/18 | 70/15 | 23/4 | .229 |
| Child-Pugh (n) | ||||||
| Child-Pugh A | NA | 67 (37.8) | 51 (78.5) | 16 (18.8) | 0 (0) | <.001 |
| Child-Pugh B | NA | 64 (36.2) | 14 (21.5) | 45 (53.0) | 5 (18.5) | |
| Child-Pugh C | NA | 46 (26.0) | 0 (0) | 24 (28.2) | 22 (81.5) | |
| AJCC stage (n) | ||||||
| Stage I, II | NA | 69 (39.0) | 36 (52.2) | 16 (23.2) | 17 (24.6) | .016 |
| Stage III | NA | 45 (25.4) | 14 (31.1) | 18 (40.0) | 13 (28.9) | |
| Stage IV | NA | 63 (35.6) | 17 (27.0) | 30 (47.6) | 16 (25.4) | |
| PVT (n) | NA | 77 (43.5) | 15 (23.1) | 46 (54.1) | 16 (59.3) | <.001 |
| WBC (×109/L) | NA | 6.08 (1.42-20.06) | 5.85 (1.42-20.06) | 5.94 (1.58-19.28) | 7.63 (1.81-14.18) | .181 |
| Platelets (×109/L) | NA | 112 (16-578) | 141 (36-418) | 100 (16-578) | 97 (30-264) | .001 |
| AST (IU/L) | NA | 55 (15-1164) | 36 (15-351) | 76 (22-1164) | 88 (23-695) | <0.001 |
| ALT (IU/L) | NA | 38 (5-1694) | 29 (8-327) | 47 (5-1694) | 38 (9-632) | 0.010 |
| ALP (IU/L) | NA | 127 (37-811) | 107 (62-811) | 154 (37-677) | 112 (55-293) | <.001 |
| Bilirubin (mg/dL) | NA | 1.7 (0.3-43.5) | 0.8 (0.3-2.2) | 2.3 (0.4-17.6) | 10.2 (1.3-43.5) | <.001 |
| Albumin (g/L) | NA | 3.0 (0.2-4.8) | 3.6 (0.4-4.7) | 2.8 (0.3-4.8) | 2.5 (0.2-3.7) | <.001 |
| PT (seconds) | NA | 13.3 (9.7-37.7) | 12.1 (9.8-14.3) | 14.0 (9.7-23.3) | 18.3 (13.0-37.7) | <.001 |
| aPTT (seconds) | NA | 33.9 (24.2-88.1) | 31.7 (24.2-40.9) | 33.9 (27.7-44.9) | 43.7 (27.5-88.1) | <.001 |
| Fibrinogen (mg/dL) | 257 (167-450) | 277 (64-626) | 288 (119-558) | 298 (96-626) | 139 (64-431) | .003 |
| DNA–histone complex (AU) | 30 (7-252) | 115c (0-1650) | 54 (3-1547) | 139 (0-1650) | 177 (45-929) | <.0001 |
| dsDNA (ng/mL) | 75.7 (57.7-102.6) | 133.4c (73.2-456.9) | 109.4 (73.2-364.4) | 143.1 (78.5-456.9) | 144.3 (115.7-384.2) | <.001 |
| Neutrophil elastase (ng/mL) | 22.0 (9.0-301.0) | 62.7c (12.0-1190.6) | 41.9 (13.0-360.8) | 68.8 (12.0-1190.6) | 81.1 (22.5-600.8) | .014 |
| Factor XIIa (U/I) | 25.3 (14.7-39.7) | 30.4c (22.8-228.2) | 27.2 (22.8-62.7) | 33.0 (23.3-115.9) | 72.6 (27.6-228.2) | .014 |
| HMWK (ng/mL) | 3.93 (0.01-9.61) | 3.43 (0.86-7.84) | 3.74 (0.86-6.15) | 3.15 (0.95-7.84) | 2.23 (1.34-3.60) | <.001 |
Abbreviations: AJCC, American Joint Committee on Cancer; ALP, alkaline phosphatase; ALT, alanine transaminase; aPTT, activated partial thromboplastin time; AST, aspartate transaminase; dsDNA, double-stranded DNA; Factor XIIa, activated factor XII; HMWK, high-molecular-weight kininogen; MELD, model for end-stage liver disease; NA, not assessed; PT, prothrombin time; PVT, portal vein thrombosis; WBC, white blood cell.
a Data are shown as the median (range) for continuous variables or number (percentage) for categorical variables unless otherwise indicated.
b P values were calculated by χ2 test for categorical variables and Kruskal-Wallis test for continuous variables among 3 groups of hepatocellular carcinomas.
c P < .05 versus healthy controls, calculated by Mann-Whitney U test.
Correlation of NET Formation and Contact System Activation With Liver Disease Severity
The numbers of patients with MELD scores of <10, 10 to 20, and >20 were 65, 85, and 27, respectively (Table 1). There were no statistically significant differences in age, sex, or white blood cell count among the 3 MELD score subgroups. As the MELD score increased, the proportion of Child-Pugh class C patients gradually increased and PVT frequency also increased. The levels of aspartate transaminase, alanine aminotransferase (ALT), alkaline phosphatase, and bilirubin were significantly increased in high-MELD-score subgroups as well. The prothrombin time (PT) and activated partial thromboplastin time values gradually increased with increasing MELD score. Of note, the levels of all 3 NET formation markers and factor XIIa significantly increased, whereas the HMWK level gradually decreased as MELD scores increased.
Association of NET Formation and Contact System Activation With PVT
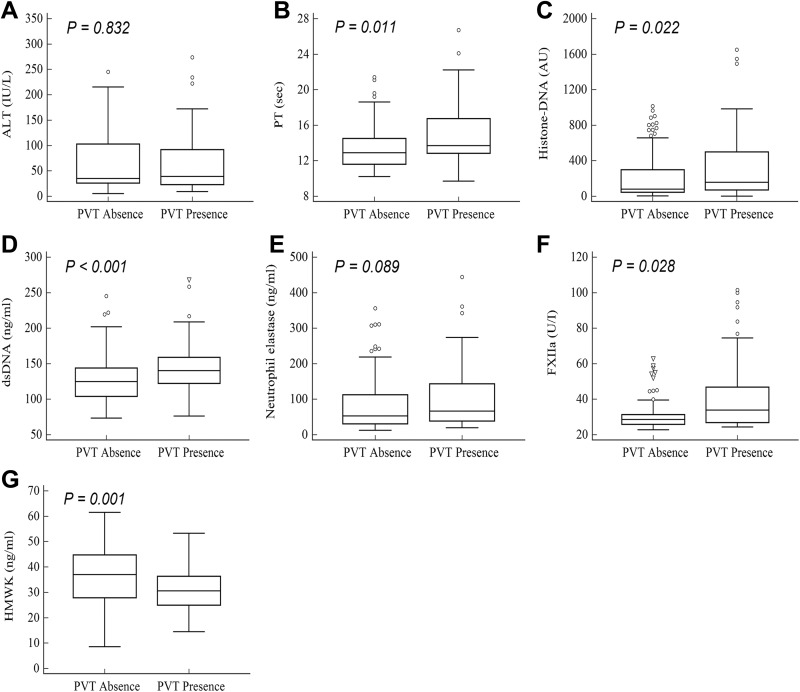
Among all patients with HCC, 77 (43.5%) patients had previous or present history of PVT, either bland thrombus or tumor thrombus. There was no significant difference in ALT (median; 43 vs 36 IU/L, P = .832) between patients with and without PVT (Figure 1). Interestingly, the levels of PT (13.7 vs 13.0 seconds, P = .011), histone–DNA complex (159 vs 83 arbitrary units [AU], P = .022), dsDNA (142.1 vs 127.0 ng/mL, P < .001), and factor XIIa (34.4 vs 29.1 U/I, P = .028) were significantly higher in patients with PVT than in those without PVT. Conversely, the HMWK level was lower in patients with PVT than in those without PVT (3.08 vs 3.70 ng/mL, P = .001).
Figure 1.
Median values of (A) alanine transaminase (ALT), (B) prothrombin time (PT), (C) histone–DNA complex, (D) double-stranded DNA (dsDNA), (E) neutrophil elastase, (F) activated factor XII (FXIIa), and (G) high-molecular-weight kininogen (HMWK). P values were calculated by Mann-Whitney U test between patients with and without portal vein thrombosis. Outliers were excluded by applying Tukey method once. ^ Outliers within the outer boundary of Tukey method after applying Tukey method once. ▽ Outliers not included in the outer boundary of the Tukey’s method after applying Tukey’s method once.
To investigate the thrombotic risks of NET formation and contact system markers, we performed logistic binary regression analysis (Table 2). In univariable logistic analysis, patients with higher PT values (>13.0 seconds) showed a significant odds ratio compared to the patients with lower PT values (≤13.0 seconds), indicating that PT was significant thrombotic risk factors. Interestingly, the NET formation and contact system markers showed significant odds ratios for thrombosis prediction. In multivariable logistic analysis, the contact system markers, factor XIIa, and HMWK remained as independent significant thrombotic risk factors.
Table 2.
Univariable and Multivariable Logistic Regression Analysis for the Assessment of Thrombotic Risk Factors.
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| ALT (>31 vs ≤31 IU/L) | 1.61 | 0.87-2.97 | .132 | 0.87 | 0.40-1.88 | .717 |
| PT (>13.0 vs ≤13.0 seconds) | 2.30 | 1.24-4.28 | .009 | 0.88 | 0.40-1.98 | .765 |
| Histone–DNA complex (>62 vs ≤62 AU) | 3.03 | 1.50-6.14 | .002 | 2.07 | 0.84-5.13 | .116 |
| dsDNA (>129.44 vs ≤129.44 ng/mL) | 2.45 | 1.31-4.57 | .005 | 1.25 | 0.56-2.81 | .588 |
| Neutrophil elastase (>46.67 vs ≤46.67 ng/mL) | 1.97 | 1.04-3.72 | .037 | 1.03 | 0.45-2.37 | .952 |
| Factor XIIa (>31.12 vs ≤31.12 U/I) | 3.14 | 1.68-5.88 | <.001 | 2.19 | 1.02-4.67 | .043 |
| HMWK (≤34.7 vs >34.7 ng/mL) | 3.27 | 1.73-6.17 | <.001 | 2.55 | 1.23-5.27 | .012 |
Abbreviations: ALT, alanine transaminase; dsDNA, double-stranded DNA; Factor XIIa, activated factor XII; HMWK, high-molecular-weight kininogen; PT, prothrombin time.
Variables Contributing to Factor XIIa Level
To identify variables contributing to factor XIIa level, we performed linear regression analysis of the factor XIIa level with other variables (Table 3). In univariable regression analysis, ALT and PT the liver injury markers, and HMWK the contact system markers were significant variables contributing to factor XIIa level. In multivariable regression analysis, ALT and PT were significant contributing variables.
Table 3.
Univariable and Multivariable Linear Regression Analysis for the Assessment of Variables Contributing to Factor XIIa Level.
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| B | 95% CI | P value | B | 95% CI | P value | |
| ALT | 0.024 | 0.000 to 0.048 | .054 | 0.028 | 0.004 to 0.053 | .022 |
| PT | 2.640 | 1.678 to 3.602 | <.001 | 2.396 | 1.281 to 3.511 | <.001 |
| Histone–DNA complex | 0.001 | −0.012 to 0.014 | .912 | −0.003 | −0.016 to 0.011 | .691 |
| dsDNA | 0.050 | −0.025 to 0.125 | .191 | 0.025 | −0.051 to 0.101 | .101 |
| Neutrophil elastase | 0.005 | −0.027 to 0.037 | .744 | −0.010 | −0.044 to 0.024 | .547 |
| HMWK | −0.533 | −0.890 to −0.205 | .004 | −0.183 | −0.580 to 0.213 | .362 |
Abbreviations: ALT, alanine transaminase; B, unstandardized regression coefficient; dsDNA, double-stranded DNA; HMWK, high-molecular-weight kininogen; PT, prothrombin time.
Discussion
This study demonstrated that the contact system was significantly more activated in patients with HCC than in healthy controls and that its activation was well correlated with liver disease severity, by comparing the concentration of NET formation markers (DNA–histone complex, dsDNA, and neutrophil elastase) and contact system activation markers (XIIa and HMWK), between healthy control and patients with HCC with diverse MELD score. Moreover, patients with PVT showed significantly higher contact system activation than those without PVT, and the markers of contact system activation, elevated factor XIIa and decreased HMWK which have the odds ratio larger than 1.00 in multivariable analysis for PVT, were revealed as independent risk factors of thrombosis. To the best of our knowledge, this is the first demonstration of contact system activation in patients with HCC with PVT. Since factor XII-driven contact activation contributes to thrombosis,4,7 our data suggest that it plays a role in thrombotic events in patients with HCC.
The pathophysiology of PVT in liver cirrhosis generally includes reduced portal blood flow, hypercoagulability, and vascular endothelial injury.2 Since PVT in liver cirrhosis commonly occurs in association with HCC, HCC as such is considered to promote PVT through direct tumor cell invasion and induction of a hypercoagulable state.2,12,13 The hypercoagulable state in HCC has been suggested to be caused by breaking of the precarious balance of procoagulant and anticoagulant protein levels.13 However, which factor initiates the coagulation cascade is not yet entirely clear. Our demonstration of a strong association of contact system activation with PVT suggests that factor XII-initiated coagulation activation initiates the coagulation cascade in PVT.
In the contact system, factor XII is activated by negatively charged surfaces. Although it can be activated in vitro by a negatively charged artificial surface such as kaolin,6 the surfaces that activate factor XII in vivo have not been identified. Recent studies have suggested that NET components released from neutrophils and polyphosphates released from activated platelets could provide negatively charged surfaces for contact system activation.4,5,14 Our results that NET formation markers were elevated in patients with HCC with PVT suggest that the NET components could indeed provide negatively charged surfaces for factor XII activation.
Cancer cells15 and cancer-derived exosomes16 can induce NET formation, which could accelerate thrombosis. Therefore, it is likely that HCC spreads within the liver and the spreading tumor cells induce NET formation and accelerate PVT.
Since factor XIIa converts prekallikrein to α-kallikrein, which cleaves HMWK to produce bradykinin,4 the circulating factor XIIa level increases and the HMWK level decreases upon contact system activation. As expected, the level of factor XIIa gradually increased and that of HMWK decreased as the MELD score increased in our study, suggesting that the contact system is activated as the severity of liver disease increases. Furthermore, ALT and PT, well-known markers of liver disease severity, were revealed as variables contributing to factor XIIa level, by showing statistically significant regression coefficient in multivariable linear regression analysis. Though these markers could be affected by many other clinical factors, therefore, it does not mean that these can be readily used as direct predictor for thrombosis risk assessment; however, these findings provide evidence of contact system activation in liver disease.
Portal vein thrombosis is considered as a poor prognostic factor in HCC.2 Prediction of PVT can be useful for management and prevention of HCC. Interestingly, our data showed that factor XIIa and HMWK were independent thrombotic risk factors. Considering that the hypercoagulable state should be actively investigated in liver cirrhosis,2 these markers of contact system activation can be suggested as predictors of thrombotic risk in patients with HCC.
Several therapeutics that inhibit contact system activation have been suggested as thrombosis inhibitors.17 Inhibiting antibodies and antisense oligonucleotides targeting factor XII or factor XI prevent thrombosis in animal and human studies.9,17 These therapeutics are not only effective for thrombosis management but also minimize bleeding risk because the contact system is involved only in thrombosis but not in hemostasis. Our results showing an association of contact system activation with PVT suggest that therapeutics inhibiting contact system activation can be potentially used to manage PVT in patients with HCC.
Our study has however several limitations. First, it was designed retrospectively. Therefore, the risk factors of PVT could be assumed only by logistic regression analysis. In the future, a prospectively designed study will be necessary to validate our results. Second, contact system components are enzymes that act for a short time and are then rapidly downregulated by plasma inhibitors such as C1 esterase inhibitor.18 Measurement of factor XIIa-C1 esterase inhibitor may increase the sensitivity of contact system activation detection. However, measuring the levels of factor XIIa and HMWK was enough to reflect contact system activation in our study.
In summary, this study demonstrated that, in patients with HCC, the contact system was gradually activated along with NET formation as the MELD score increased. Alanine aminotransferase and PT, well-known markers of liver disease, were significant variable contributing to factor XIIa level, suggesting a positive association between contact system activation and liver disease severity. Interestingly, the contact system activation markers were revealed as significant thrombotic risk factors in patients with HCC.
In conclusion, contact system activation and NET formation are well correlated with liver disease severity, and a marker of contact system activation and markers of NET formation can be used as thrombotic risk factors in patients with HCC. Our findings also suggest that therapeutics inhibiting the contact system can be potentially used to manage PVT in patients with HCC.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI17C1134) and partly by the SNUH Research Fund (grant number: 0420180330).
ORCID iD: Jong Do Seo  https://orcid.org/0000-0001-7449-7978
https://orcid.org/0000-0001-7449-7978
References
- 1. Caldwell SH, Hoffman M, Lisman T, et al. Coagulation disorders and hemostasis in liver disease: pathophysiology and critical assessment of current management. Hepatology. 2006;44(4):1039–1046. [DOI] [PubMed] [Google Scholar]
- 2. Chawla YK, Bodh V. Portal vein thrombosis. J Clin Exp Hepatol. 2015;5(1):22–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parikh S, Shah R, Kapoor P. Portal vein thrombosis. Am J Med. 2010;123(2):111–119. [DOI] [PubMed] [Google Scholar]
- 4. Long AT, Kenne E, Jung R, Fuchs TA, Renne T. Contact system revisited: an interface between inflammation, coagulation, and innate immunity. J Thromb Haemost: JTH. 2016;14(3):427–437. [DOI] [PubMed] [Google Scholar]
- 5. Gould TJ, Vu TT, Swystun LL, et al. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler Thromb Vasc Biol. 2014;34(9):1977–1984. [DOI] [PubMed] [Google Scholar]
- 6. Maas C, Oschatz C, Renne T. The plasma contact system 2.0. Semin Thromb Hemost. 2011;37(4):375–381. [DOI] [PubMed] [Google Scholar]
- 7. Muller F, Renne T. Novel roles for factor XII-driven plasma contact activation system. Curr Opin Hematol. 2008;15(5):516–521. [DOI] [PubMed] [Google Scholar]
- 8. Kleinschnitz C, Stoll G, Bendszus M, et al. Targeting coagulation factor XII provides protection from pathological thrombosis in cerebral ischemia without interfering with hemostasis. J Exp Med. 2006;203(3):513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buller HR, Bethune C, Bhanot S, et al. Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med. 2015;372(3):232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Edge SB, Compton CC. The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. [DOI] [PubMed] [Google Scholar]
- 11. Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124(1):91–96. [DOI] [PubMed] [Google Scholar]
- 12. Kim SY, Kim JE, Kim YJ, Han KS, Kim HK. Prominent protein Z-induced thrombin inhibition in cirrhosis: a new functional assay for hypercoagulability assessment. J Gastroenterol Hepatol. 2015;30(4):784–793. [DOI] [PubMed] [Google Scholar]
- 13. Kujovich JL. Coagulopathy in liver disease: a balancing act. Hematology Am Soc Hematol Educ Program. 2015;2015:243–249. [DOI] [PubMed] [Google Scholar]
- 14. Smith SA, Morrissey JH. Polyphosphate as a general procoagulant agent. J Thromb Haemost: JTH. 2008;6(10):1750–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abdol Razak N, Elaskalani O, Metharom P. Pancreatic cancer-induced neutrophil extracellular traps: a potential contributor to cancer-associated thrombosis. Int J Mol Sci. 2017;18(3):E487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leal AC, Mizurini DM, Gomes T, et al. Tumor-derived exosomes induce the formation of neutrophil extracellular traps: implications for the establishment of cancer-associated thrombosis. Sci Rep. 2017;7(1):6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Montfoort ML, Meijers JC. Recent insights into the role of the contact pathway in thrombo-inflammatory disorders. Hematology Am Soc Hematol Educ Program. 2014;2014(1):60–65. [DOI] [PubMed] [Google Scholar]
- 18. de Maat S, Tersteeg C, Herczenik E, Maas C. Tracking down contact activation—from coagulation in vitro to inflammation in vivo. Int J Lab Hematol. 2014;36(3):374–381. [DOI] [PubMed] [Google Scholar]