Abstract
Increased coronary thrombus burden is known to be a strong predictor of adverse cardiovascular (CV) outcomes. C-reactive protein to albumin ratio (CAR) can be used as a surrogate marker of pro-inflammation which is closely related to prothrombotic state. We aimed to evaluate the association between CAR and coronary thrombus burden in patients who presented with acute coronary syndrome (ACS). Patients who presented with ACS and treated with primary percutaneous coronary intervention were included in the study. Patients were divided into 2 groups as high thrombus burden and low thrombus burden. The study population included 347 patients with non-ST-segment elevation myocardial infarction (169 [48.7%]) and ST-segment elevation myocardial infarction (178 [51.3%]). The CAR was significantly higher in patients with higher thrombus burden (24.4 [1.2-30.2] vs 31.9 [2.2-31.3], P < .001). Independent predictors for increased thrombus burden were higher CRP level (odds ratio [OR]: 0.047; 95% confidence interval [CI]: 0.004-0.486; P = .010), lower serum albumin level (OR: 0.057; 95% CI: 0.033-0.990; P = .049), higher CAR (OR: 1.13; 95% CI: 1.03-1.23; P = .008), higher neutrophil–lymphocyte ratio (OR: 1.18; 95% CI: 1.05-1.31; P = .004), and baseline troponin I level (OR: 1.06; 95% CI: 1.01-1.13; P = .017). Novel CAR can be used as a reliable marker for increased coronary thrombus burden that is associated with adverse CV outcomes.
Keywords: CRP to albumin ratio, thrombus burden, inflammation, acute coronary syndrome
Introduction
Acute coronary syndrome (ACS) is the leading cardiovascular (CV) cause of mortality and necessitates prompt diagnosis and treatment. Luminal thrombus secondary to coronary plaque rupture is considered as the underlying mechanism for ACS. Preprocedural thrombus was found to be present in 15% of moderate- and high-risk patients with ACS, and intracoronary thrombus burden was a strong predictor for adverse outcomes including stent thrombosis, myocardial infarction, and mortality.1 Restoration of coronary flow in infarct-related artery by percutaneous coronary intervention (PCI) reduces the myocardial injury and improves ventricular function and long-term outcomes. On the other hand, in case of higher thrombotic burden in a coronary artery, myocardial salvage can be reduced despite the restoration of coronary flow. Impaired microvascular perfusion due to distal embolization of thrombotic material is the responsible mechanism for this “no-reflow” phenomenon.
In recent years, studies demonstrated the strong relationship between inflammation and atherosclerosis.2 Accordingly, increased C-reactive protein (CRP) and decreased albumin levels, as biomarkers for systemic inflammation, were found to be predictors of adverse CV events. In addition, it was suggested that novel CRP to albumin ratio (CAR) was more sensitive and specific for evaluating systemic inflammatory state in noncardiac diseases when compared to predictive value of these 2 markers separately.3,4 Determination of intracoronary thrombus burden and related angiographic milieu is important to manage the complications during the procedure and to prevent adverse outcomes. Thus, our aim in the present study was to analyze the association between intracoronary thrombus burden and CAR in patients with ACS.
Methods
Study Population
Single-center and retrospective analysis of patients who were presented with non-ST-segment elevation myocardial infarction (NSTEMI) and ST-segment elevation myocardial infarction (STEMI) and treated with primary PCI. Patients who were diagnosed with unstable angina pectoris were not included in the study. Patients with a history of coronary artery bypass grafting operation and/or PCI, active malignancy, infection, or connective tissue disorder were excluded from the study. Basal characteristics and clinical histories of all study patients were recorded. Medications during hospitalization period were left at the discretion of responsible physician. Stent implantation techniques and medical management during PPCI were also left at the discretion of the operator. The decision of whether to use glycoprotein IIb/IIIa antagonist (tirofiban) at the time of PCI was at the discretion of treating interventional operators. The study protocol was reviewed and approved by the institutional ethics committee and in accordance with the principles of the Helsinki Declaration.
Laboratory Measurements
Patients’ routine blood samples were obtained at admission in the emergency department. At sixth hour after the admission to the hospital, another blood sample was obtained for lipid panel and troponin-I level evaluation. Serum albumin level was analyzed using automatic photometry commercial kits using Abbott C8000i (Abbott Park, Illinois). Serum CRP levels were measured with nephelometric method (UniCel DxC 800 System; Beckman Coulter Inc, Brea, California). The CAR was calculated as the ratio of CRP to the albumin level multiplied by 100.
Angiographic Analyses
Comprehensive quantitative coronary angiography through femoral arterial access was performed in all patients. Two experienced investigators who were blinded to all clinical parameters of the patients carefully reviewed coronary angiograms. Angiographic thrombus burden was classified as follows: grade 0: no thrombus, grade 1: possible thrombus, grade 2: the thrombus’ greatest dimension is <1/2 vessel diameter, grade 3: greatest dimension >1/2 to <2 vessel diameters, grade 4: greatest dimension >2 vessel diameters, and grade 5: total vessel occlusion due to thrombus.5 Thrombus grade was evaluated just after the restoration of antegrade flow using guidewire or small balloon dilatation. The patients were stratified into low-thrombus burden (grades 1-3) and high-thrombus burden groups (grades 4 and 5) according to final thrombus score.
Statistical Analyses
All statistical analyses were performed with SPSS 18 (SPSS Inc, Chicago, Illinois). A P value of <.05 was considered significant in all statistical analyses. Continuous variables with a normal distribution were given as mean (standard deviation), while those without a normal distribution are presented as median and interquartile range values. Categorical variables were given as percentage. Normality of the data was determined using the Kolmogorov-Smirnov test. The study population was divided into 2 groups according to high thrombus and low thrombus burden. Student t test or Mann-Whitney U test was used for the distribution of data for continuous variables, and χ2 test was used for categorical variables. Thrombus burden was graded between 1 and 5 to perform correlation analysis. Pearson correlation coefficient was used for the analysis of variables that are distributed normally. Spearman rank coefficient was used for the analysis of variables that are not normally distributed. Multivariable logistic regression analyses were performed to identify the independent predictors of thrombus burden. The receiver operating characteristic (ROC) curve analysis was performed to determine cutoff value of CRP, albumin, and CAR. The predictive validities were quantified as the area under the ROC curve (c statistics), and the comparisons of c-statistics were performed by MedCalc statistic software.
Results
The study population included 347 patients with NSTEMI (169 [48.7%]) and STEMI (178 [51.3%]). Among those, 140 (40.4%) and 207 (59.6%) patients had low and high thrombus burden, respectively, according to Thrombolysis in Myocardial Infarction (TIMI) thrombus grade. Fifty-two percent of patients with low thrombus grade had NSTEMI, whereas 72% of patients with high thrombus grade had STEMI. Patients’ baseline demographic and clinical characteristics regarding TIMI thrombus grade are shown in Table 1. Patients with high thrombus burden were more likely to have diabetes (23.6% vs 33.8%, P = .043) and family history of atherosclerotic heart disease (11.4% vs 21.7%, P = .014). There was no significant difference in angiographic characteristics, except that patients with higher thrombus burden were more likely to have multivessel coronary artery disease (9.0% vs 17%, P = .025) and no-reflow (5.7% vs 13.0%, P = .029; Table 1). Laboratory measurements of study patients are shown in Table 1. Baseline troponin I, 5.5 (4.0) vs 6.8 (4.7), P = .009, and white blood cell count (WBC), 9.2 (2.5) vs 9.7 (2.5), P = .019, were significantly higher in patients with higher thrombus burden. In addition, CRP level was higher (0.90 [0.04-1.22] vs 1.15 [0.09-1.25], P < .001) and serum albumin level was lower (4.1 [3.7-4.5] vs 3.5 [3.1-4.5], P < .001) in patients with higher thrombus burden. Accordingly, CAR was significantly higher in patients with higher thrombus burden (24.4 [1.2-30.2] vs 31.9 [2.2-31.3], P < .001). We found no significant relationship between CAR and no-reflow (22.7 [15.1-39.2] vs 24.2 [15.4-46.4], P = .337).
Table 1.
Baseline Clinical and Laboratory Characteristics According to Thrombus Burden.
| Variable | Low Thrombus Burden, n= 140 | High Thrombus Burden, n=207 | P Value |
|---|---|---|---|
| Age, years | 55.7 (10) | 57.2 (13) | .267 |
| Sex, male % | 54 | 58 | .508 |
| Diabetes, % | 23.6 | 33.8 | .043 |
| Hypertension, % | 42.5 | 43.2 | .882 |
| Smoking, % | 42.1 | 44.9 | .459 |
| Dyslipidemia, % | 43.5 | 48 | .237 |
| Previous history of CAD, % | 21 | 20 | .893 |
| Family history of CAD | 11.4 | 21.7 | .014 |
| Systolic blood pressure, mm Hg | 128 (13) | 131 (20) | .600 |
| Heart rate, /min | 76.5 (16) | 74.7 (16) | .145 |
| C-reactive protein, mg/dL | 0.90 (0.04 -1.22) | 1.15 (0.09 -1.25) | <.001 |
| Albumin, g/dL | 4.1 (3.7-4.5) | 3.5 (3.1-4.5) | <.001 |
| CAR, ×100 | 24.4 (1.2-30.2) | 31.9 (2.2-31.3) | <.001 |
| Neutrophil–lymphocyte ratio | 5.8 (3.5-7.1) | 6.4 (4.4-8) | .004 |
| Hemoglobin, g/dL | 13 (11.5-14) | 12.5 (11.4-13.5) | .125 |
| White blood cell count, ×103/µL | 9.2 (2.5) | 9.7 (2.5) | .019 |
| Baseline troponin, mg/L | 5.5 (4) | 6.8 (4.7) | .009 |
| Platelet count, ×103/µL | 216 (76) | 218 (78) | .734 |
| LDL cholesterol, mg/dL | 138 (30) | 141 (32) | .328 |
| HDL cholesterol, mg/dL | 34.7 (80) | 33.3 (13) | .294 |
| Triglyceride, mg/dL | 193 (103) | 181 (97) | .262 |
| GFR, mL/min | 75 (18) | 77 (16) | .205 |
| EF,% | 45 (6) | 44 (9) | .090 |
| Previous medications, % | |||
| Aspirin | 30 | 20 | .057 |
| Statin | 16 | 15 | .881 |
| ACE inhibitors/ARB | 28 | 35 | .161 |
| β-Blocker | 12 | 11 | .867 |
| Infarct-related artery | |||
| LAD, n (%) | 35 | 27 | .233 |
| Cx, n (%) | 44 | 45 | |
| RCA, n (%) | 20 | 27 | |
| Multivessel disease, % | |||
| 1-Vessel | 60 | 46 | .025 |
| 2-Vessel | 30 | 35 | |
| 3-Vessel | 9 | 17 | |
| No reflow, % | 5.7 | 13 | .029 |
Abbreviations: CAD, coronary artery disease; CAR, C-reactive protein to albumin ratio; NLR, neutrophil to lymphocyte ratio; LDL, low-density lipoprotein; HDL, high-density lipoprotein; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; OAD, oral antidiabetic drug.
a The normal cutoff value of troponin in our laboratory <0.04 mg/L.
We performed correlation analysis between thrombus burden and CRP, serum albumin level, CAR, neutrophil to lymphocyte ratio (NLR), and age. We found that CRP (r = .378, P < .001) and CAR (r = .427, P < .001) were significantly positively correlated with thrombus burden. In addition, there was a weakly positive (r = .121, P = .013), moderately negative (r = −.419, P < .001), and weakly negative (r = −.117, P = .029) correlation between thrombus burden and NLR, serum albumin level, and age, respectively (Table 2).
Table 2.
Correlation Between Thrombus Burden and CRP, Albumin, CAR, NLR, and Age.
| Thrombus Burden | ||
|---|---|---|
| r | P | |
| CRP | .378 | <.001 |
| Albumin | −.419 | <.001 |
| CAR | .427 | <.001 |
| NLR | .121 | .013 |
| Age | −.117 | .029 |
Abbreviations: CRP, C- reactive protein; CAR, C-Reactive protein to albumin ratio; NLR, neutrophil to lymphocyte ratio.
We performed multivariate logistic regression analysis to demonstrate independent predictors for high thrombus burden. We excluded angiographic parameters in this analysis. It was shown that higher CRP level (odds ratio [OR]: 0.047; 95% confidence interval [CI]: 0.004-0.486; P = .010), lower serum albumin level (OR: 0.057; 95% CI: 0.033-0.990; P = .049), higher CAR (OR: 1.13; 95% CI: 1.03-1.23; P = 0.008), higher NLR (OR: 1.18; 95% CI: 1.05-1.31; P = .004), and baseline troponin I level (OR: 1.06; 95% CI: 1.01-1.13; P = .017) were independent predictors for increased thrombus burden (Table 3).
Table 3.
Independent Predictors of Thrombus Burden With Multivariate P Value and OR With 95% CI.
| Parameters | P Value | Odd Ratio | 95% CI |
|---|---|---|---|
| CRP | .010 | 0.047 | 0.004-0.486 |
| Albumin | .049 | 0.057 | 0.033-0.99 |
| CAR | .008 | 1.13 | 1.03-1.23 |
| NLR | .004 | 1.18 | 1.05-1.31 |
| Diabetes | .136 | 0.66 | 0.38-1.13 |
| Baseline troponin | .017 | 1.06 | 1.01-1.13 |
| Family history | .069 | 0.54 | 0.27-1.05 |
Abbreviations: CAR, C-reactive protein to albumin ratio; CI, confidence interval; CRP, C-reactive protein; NLR, neutrophil to lymphocyte ratio.
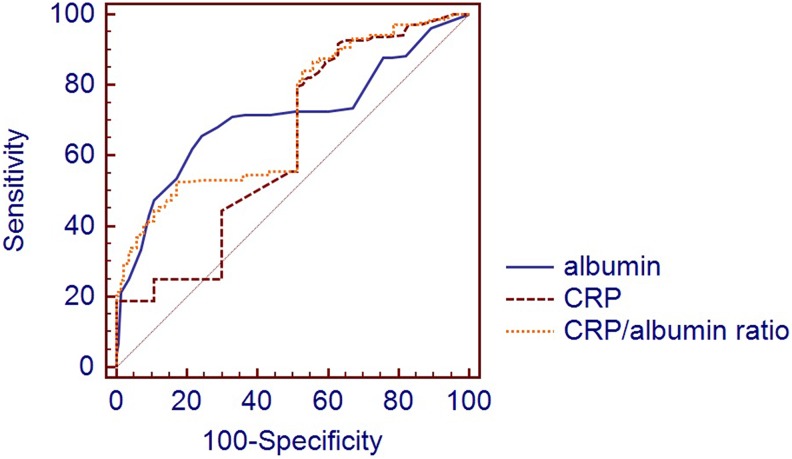
The cutoff values of CAR, albumin, and CRP for high thrombus burden were 0.15 with a sensitivity of 88.4% and a specificity of 43.6% (area under the receiver operating curve [AUC], 0.697; 95% CI: 0.646-0.745; P < .001), 3.8 with a sensitivity of 89.9% and a specificity of 37.1% (AUC: 0.661; 95% CI: 0.609-0.711; P < .001), and 0.86 with a sensitivity of 55.1% and a specificity of 72.1% (AUC: 0.653; 95% CI: 0.600-0.703; P < .001), respectively, in the ROC curve analysis
The AUC comparison of CRP-albumin and albumin-CAR did not reach statistical significance (P > .05). However, a statistically significant difference was found between CRP and CAR (P < .001; Figure 1).
Figure 1.
The receiver operating characteristic (ROC) curve comparison of C-reactive protein (CRP), albumin, and C-reactive protein to albumin ratio (CAR) in the prediction of low–high thrombus burden.
Discussion
The main findings of the present study were that coronary thrombus burden was associated with higher CRP and CAR and lower albumin level. In addition, independent predictors of thrombus burden in patients with STEMI and NSTEMI were higher CRP, CAR, NLR, and lower albumin level.
The rupture of atherosclerotic plaque with subsequent coronary artery occlusion and thrombus is the major underlying cause for ACS. It is known that in patients with ACS, increased thrombus burden is associated with distal embolization and no-reflow phenomenon which cause impaired postprocedural coronary perfusion and depressed left ventricular ejection fraction.6 Despite some contradictory results in the literature,7 most of the studies found that angiographical evidence of intracoronary thrombus was associated with worse prognosis in patients with STEMI. Increased thrombus burden (≥2× vessel diameter) was consistently found to be a strong predictor of major adverse cardiac events.8,9 In addition, damaged ischemic and necrotic myocardial cells release pro-inflammatory substances in tissue and plasma, leading to a systemic inflammatory response. Prognosis of the disease can be strongly altered through prompt and correct management of inflammatory process.10 Serum CRP level is a well-known acute-phase reactant. In several studies, it was found that there was a strong relationship between increased CRP level and atherosclerosis.11 Furthermore, CRP was demonstrated to be independently associated with endothelium-dependent and endothelium-independent coronary microvascular dysfunction in patients with coronary artery disease.12 The other evidence reported that elevated CRP level predicted microvascular dysfunction in patients with STEMI strengthened the predictive value of elevated CRP for no-reflow phenomenon.13 Hypoalbuminemia was shown to be not only a risk factor but also associated with worse prognosis in patients with STEMI.14,15 Our results were consistent with previous findings that inflammatory biomarkers including higher CRP and lower albumin levels were associated with increased thrombus burden. There was a positive moderate correlation between a novel marker CAR and thrombus burden. Also, CAR was found to be an independent predictor for increased thrombus burden.
The CAR was investigated recently as a potential biomarker for predicting major CV outcomes. In a recent study,16 Çağdaş et al demonstrated a strong correlation between CAR and coronary artery disease severity and burden. In this study, there was no correlation found between coronary artery disease severity and increased thrombus burden. It can be suggested that the exclusion of patients with STEMI can cause this discrepancy. Our results were in parallel with the previous studies demonstrating the presence of increased thrombus burden in patients with higher coronary artery disease burden and no-reflow phenomenon.17–19 In these studies, there was no significant association between family history of CAD and increased thrombus burden. On the contrary, we found that patients with a family history of CAD were more likely to have increased thrombus burden. Our results were in parallel to those of Tanboga et al.6
In patients with ACS, initial troponin level was found to be associated with larger thrombus burden within a coronary artery. Pawłowski et al showed that troponin level predicts large thrombus burden by optical coherence tomography.20 Similarly, we found that higher troponin level at admission predicted increased thrombus burden.
Pro-inflammatory and prothrombotic states are 2 closely related processes; thus, increased coronary thrombus burden can be implied as increased pro-inflammatory state.19,21 The NLR was suggested as a pro-inflammatory marker that was associated with long- and short-term mortality in patients with coronary artery disease.22,23 We found that NLR independently predicted thrombus burden in our cohort, but there was no strong positive correlation. Our results supported the potential role of systemic inflammation on the thrombus burden.
The CAR includes both CRP and albumin parameters and thus have the superiority to reflect not only pro-inflammatory state but also nutritional status. It was previously shown that in patients with malignant tumors, CAR not only predicted prognosis but also value of CAR increased as the disease progressed. Therefore, unlike the analysis of CRP and albumin separately, CAR can be used as a more reliable biomarker to predict severity and prognosis of different diseases. In addition, CAR was associated with all-cause mortality in patients with malignant tumors.24,25 Recent studies that evaluated CAR in patients with CVD had encouraging results. In a study that included patients with STEMI, researchers found that WBC, NLR, and CAR were associated with no-reflow phenomenon. They also found that after performing ROC curve analysis, CAR had higher AUC values compared to CRP and albumin level separately. Accordingly, we demonstrated that despite the fact that CAR had lower sensitivity for predicting coronary thrombus burden compared to both CRP and albumin, it had significantly higher specificity. The CAR has the potential to predict high-thrombus burden in patients with ACS, but its clinic applicability is yet to be proved. Moreover, by using CAR, it is possible to detect high-risk patients for a high intracoronary thrombus burden and modify our treatment to deal with possible adverse events related to the high-thrombus burden in patients with ACS. It can be speculated that higher specificity of CAR is attributed to its advantage to include 2 important inflammatory biomarkers.
Study Limitation
The prognostic value of CAR could not be assessed in the present study which had a limited number of patients and event rate. Our study was cross-sectional, and thus, it was not possible to obtain data for adverse events and mortality. Reperfusion success was assessed only by visual evaluation, and sensitive and specific methods such as coronary flow reserve, contrast echocardiography, and cardiac MR were not used. Although we utilized the TIMI thrombus grade, a well-known and widely used classification, this grading may not be the perfect and ideal. We could not evaluate myocardial blush grade because the duration of angiographic sine images was short. Finally, we did not routinely perform thrombus aspiration in our study.
Conclusion
C-reactive protein to albumin ratio can be easily calculated and reliably used as a predictor of increased coronary thrombus burden in patients who presented with ACS.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Goto K, Lansky AJ, Nikolsky E, et al. Prognostic significance of coronary thrombus in patients undergoing percutaneous coronary intervention for acute coronary syndromes: a subanalysis of the ACUITY (Acute Catheterization and Urgent Intervention Triage strategY) trial. JACC Cardiovasc Interv. 2011;4(7):769–777. [DOI] [PubMed] [Google Scholar]
- 2. Raggi P, Genest J, Giles JT, et al. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis. 2018;276:98–108. [DOI] [PubMed] [Google Scholar]
- 3. Kim MH, Ahn JY, Song JE, et al. The C-reactive protein/albumin ratio as an independent predictor of mortality in patients with severe sepsis or septic shock treated with early goal-directed therapy. PLoS One. 2015;10(7):e0132109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ranzani OT, Zampieri FG, Forte DN, Azevedo LCP, Park M. C-reactive protein/albumin ratio predicts 90-day mortality of septic patients. PLoS One. 2013;8(3):e59321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gibson CM, de Lemos JA, Murphy SA, et al. Combination therapy with abciximab reduces angiographically evident thrombus in acute myocardial infarction: a TIMI 14 substudy. Circulation. 2001;103(21):2550–2554. [DOI] [PubMed] [Google Scholar]
- 6. Tanboga IH, Topcu S, Aksakal E, Kalkan K, Sevimli S, Acikel M. Determinants of angiographic thrombus burden in patients with ST-segment elevation myocardial infarction. Clin Appl Thromb Hemost. 2014;20(7):716–722. [DOI] [PubMed] [Google Scholar]
- 7. Tungsubutra W, Towashiraporn K, Tresukosol D, et al. One-year clinical outcomes of ST segment elevation myocardial infarction patients treated with emergent percutaneous coronary intervention: the impact of thrombus burden. J Med Assoc Thai. 2014;97(suppl 3):S139–S146. [PubMed] [Google Scholar]
- 8. Sianos G, Papafaklis MI, Serruys PW. Angiographic thrombus burden classification in patients with ST-segment elevation myocardial infarction treated with percutaneous coronary intervention. J Invasive Cardiol. 2010;22(10):6B–14B. [PubMed] [Google Scholar]
- 9. Singh M, Berger PB, Ting HH, et al. Influence of coronary thrombus on outcome of percutaneous coronary angioplasty in the current era (the Mayo Clinic experience). Am J Cardiol. 2001;88(10):1091–1096. [DOI] [PubMed] [Google Scholar]
- 10. Kottoor SJ, Arora RR. The utility of anti-inflammatory agents in cardiovascular disease: a novel perspective on the treatment of atherosclerosis. J Cardiovasc Pharmacol Ther. 2018:1074248418778548. [DOI] [PubMed] [Google Scholar]
- 11. Yousuf O, Mohanty BD, Martin SS, et al. High-sensitivity C-reactive protein and cardiovascular disease: a resolute belief or an elusive link? J Am Coll Cardiol. 2013;62(5):397–408. [DOI] [PubMed] [Google Scholar]
- 12. Tomai F, Ribichini F, Ghini AS, et al. Elevated C-reactive protein levels and coronary microvascular dysfunction in patients with coronary artery disease. Eur Heart J. 2005;26(20):2099–2105. [DOI] [PubMed] [Google Scholar]
- 13. Huet F, Akodad M, Kuster N, et al. An hs-TNT second peak associated with high CRP at day 2 appears as potential biomarkers of micro-vascular occlusion on magnetic resonance imaging after reperfused ST-segment elevation myocardial infarction. Cardiology. 2018;140(4):227–236. [DOI] [PubMed] [Google Scholar]
- 14. Oduncu V, Erkol A, Karabay CY, et al. The prognostic value of serum albumin levels on admission in patients with acute ST-segment elevation myocardial infarction undergoing a primary percutaneous coronary intervention. Coron Artery Dis. 2013;24(2):88–94. [DOI] [PubMed] [Google Scholar]
- 15. Nelson J, Liao D, Sharrett AR, et al. Serum albumin level as a predictor of incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol. 2000;151(5):468–477. [DOI] [PubMed] [Google Scholar]
- 16. Çağdaş M, Rencüzoğullari I, Karakoyun S, et al. Assessment of relationship between C-reactive protein to albumin ratio and coronary artery disease severity in patients with acute coronary syndrome. Angiology. 2017:0003319717743325. [DOI] [PubMed] [Google Scholar]
- 17. Arısoy A, Altunkaş F, Karaman K, et al. Association of the monocyte to HDL cholesterol ratio with thrombus burden in patients with ST-segment elevation myocardial infarction. Clin Appl Thromb Hemost. 2017;23(8):992–997. [DOI] [PubMed] [Google Scholar]
- 18. Lai HM, Xu R, Yang YN, et al. Association of mean platelet volume with angiographic thrombus burden and short-term mortality in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Catheter Cardiovasc Interv. 2015;85(S1):724–733. [DOI] [PubMed] [Google Scholar]
- 19. Hamur H, Duman H, Bakirci EM, et al. Bilirubin levels and thrombus burden in patients with ST-segment elevation myocardial infarction. Angiology. 2016;67(6):565–570. [DOI] [PubMed] [Google Scholar]
- 20. Pawłowski T, Prati F, Capodanno D, Tamburino C, Gil RJ. Initial troponin level may predict thrombus burden in patients with acute coronary syndrome. Optical coherence tomography study. Kardiol Pol. 2012;70(5):457–462. [PubMed] [Google Scholar]
- 21. Andrié RP, Bauriedel G, Tuleta I, Braun P, Nickenig G, Skowasch D. Impact of intimal pathogen burden in acute coronary syndromes—correlation with inflammation, thrombosis, and autoimmunity. Cardiovasc Pathol. 2010;19(6):e205–e210. [DOI] [PubMed] [Google Scholar]
- 22. Liang Y, Chen H, Wang P. Correlation of leukocyte and coronary lesion severity of acute myocardial infarction. Angiology. 2018;69(7):591–599. [DOI] [PubMed] [Google Scholar]
- 23. Zhang S, Diao J, Qi C, et al. Predictive value of neutrophil to lymphocyte ratio in patients with acute ST segment elevation myocardial infarction after percutaneous coronary intervention: a meta-analysis. BMC Cardiovasc Disord. 2018;18(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yoshida N, Baba H. The C-reactive protein/albumin ratio may predict the long-term outcome in patients with malignant pleural mesothelioma. Ann Surg Oncol. 2018;25(6):1471–1472. [DOI] [PubMed] [Google Scholar]
- 25. Chen Z, Shao Y, Fan M, et al. Prognostic significance of preoperative C-reactive protein: albumin ratio in patients with clear cell renal cell carcinoma. Int J Clin Exp Pathol. 2015;8(11):14893. [PMC free article] [PubMed] [Google Scholar]