Abstract
Objective: Data on associations between facial aging and smoking or alcohol consumption are generally derived from small studies, and therefore, vary. The aim of this large multinational study was to determine more accurately which clinical signs of skin- and volume-related facial aging are associated with tobacco and alcohol use in women. Design: This was a subanalysis of a global, cross-sectional, Internet-based survey of self-reported facial aging. Participants: Women aged 18 to 75 years old (n=3,267) from the United States, Australia, Canada, and the United Kingdom who described themselves as white, Asian, black, or Hispanic were included. Measurements: Using a mirror, participants determined their own aging severity on photonumeric rating scales for 11 facial characteristics. Linear regressions were used to assess associations between each feature’s severity and smoking status (never vs. current and former smoker); smoking pack years (0 versus 1–10, 11–20, and >20 years); alcohol use (none vs. moderate and heavy); and alcoholic beverage type, after controlling for body mass index, country, age, and race. Results: Smoking was associated with an increased severity of forehead, crow’s feet, and glabellar lines; under-eye puffiness; tear-trough hollowing; nasolabial folds; oral commissures; perioral lines; and reduced lip fullness (p≤0.025) but not midface volume loss or visible blood vessels. Heavy alcohol use (≥8 drinks/week) was associated with increased upper facial lines, under-eye puffiness, oral commissures, midface volume loss, and blood vessels (p≤0.042). Conclusion: Smoking and alcohol consumption significantly but differentially impact skin and volume-related facial aging.
Keywords: Facial lines, facial skin aging, impact of tobacco use, impact of alcohol consumption, volume-related aging
Age-related skin changes are caused by a combination of intrinsic chronological processes and extrinsic factors. Ultraviolet (UV) exposure is the main extrinsic cause;1–3 other reported factors include tobacco use,4–7 high alcohol intake,4 air pollution,3 low body mass index (BMI),5,6 and lower socioeconomic status.5
Free radicals generated by smoking damage repair mechanisms and reduce extracellular matrix turnover (e.g., collagen and elastin synthesis), leading to premature signs of skin aging.3,8 Smoking also causes cutaneous microvascular constriction that increases in relation to the duration and amount of exposure.9 Alcohol consumption impairs the skin’s antioxidant defense system by decreasing dermal carotenoid concentrations.10 Alcohol also causes peripheral vasodilation,11,12 which can lead to dilated facial capillaries. Social perceptions and value judgments are often based on appearance. Wrinkles; under-eye puffiness; uneven skin tone; and volume loss around the eyes, midface, and lips add to the perception of increased age.5,13
Previous studies have shown varying associations exist between tobacco or alcohol use and skin photoaging,5,14 wrinkling,6,15–18 or facial aging in general.13,19 However, these studies were generally conducted in single countries and involved relatively small study populations (i.e., a few hundred participants or fewer). Few data are available on the patterns and specific characteristics of facial skin- and volume-related aging from large, globally dispersed populations of women who smoke and drink alcohol compared to those who abstain. Knowledge of premature facial aging patterns in women with a history of smoking and/or high alcohol consumption helps practitioners provide evidence-based advice on lifestyle modifications to help patients preserve their facial appearance. This analysis of a cross-sectional, Internet-based survey of more than 3,000 women from the United States (US), Australia, Canada, and the United Kingdom (UK) was conducted to determine the patterns of clinical signs of facial aging associated with tobacco and alcohol use.
METHODS
Study design and participants. Before the study’s initiation, ethics approval was obtained from a central institutional review board; the research protocol was approved for federal-wide assurance by the US Department of Health and Human Services.
Adults in the US, Australia, Canada, and the UK who had preconsented to complete health-related Internet surveys were recruited from the YouGov® (YouGov plc, London, United Kingdom) PollingPoint Panel, a proprietary opt-in study panel, by email invitation.20 The prespecified sampling structure of this cross-sectional survey on self-reported facial aging, the questions, and data quality assurance protocols have been previously described;20 eligibility criteria are listed in Table 1. Respondents had to consent to participate before being directed to the survey. Sampling was designed to acquire approximately equal numbers of respondents by race/ethnicity within each age band listed in Appendix 1.
TABLE 1.
Survey eligibility criteria
| INCLUSION CRITERIAa |
| Aged 18–75 years |
| E-mail address provided to YouGov was still active at the time the study invitation was issued |
| Able to read and understand English |
| Willing and able to provide informed consent by “opting-in” via a web link in the e-mail invitation |
| Qualified for one of the subgroups (based on target age, sex, race/ethnicity and country of residence distributions) for which the enrolment target had not yet been fulfilled |
| EXCLUSION CRITERIAb |
| Unwilling or unable to provide informed consent via the web link in the e-mail invitation |
| Had experienced any significant trauma or burns that significantly altered the appearance of the facial skin |
| Had undergone facial plastic surgery (e.g., eyelid surgery, brow lift, surgical implants) at any time prior to study enrollment |
| Had received treatment with injectable fillers or botulinum toxin at any time prior to study enrollment |
| Had undergone facial skin resurfacing procedures (e.g., laser, surgery) or chemical or mechanical facial peels (e.g., microdermabrasion, glycolic peels) at any time prior to study enrollment |
| Had used a prescription oral or topical retinoid or products containing ≥4% hydroquinone at any time prior to study enrollment |
| Had used prescription or over-the-counter facial products containing growth factors or hormones at any time prior to study enrollment |
Survey respondents had to meet all of these criteria
Respondents reporting any of these criteria were ineligible for enrollment
APPENDIX 1.
Baseline demographics and smoking and alcohol consumption habits of women by age
| VARIABLE | TOTAL (N=3,267) | AGE (YEARS) | P-VALUEa | |||||
|---|---|---|---|---|---|---|---|---|
| 18–29 (N=577) | 30–39 N=582) | 40–49 (N=579) | 50–59 (N=589) | 60–69 (N=611) | 70–75 (N=329) | |||
| BMI, mean (SD) | 27.0 (7.1) | 24.5 (6.9) | 25.7 (7.1) | 27.3 (6.9) | 28.0 (7.2) | 28.3 (6.7) | 28.5 (6.7) | <0. 0001 |
| Missing, n (%) | 461 (14.1) | 92 (15.9) | 94 (16.2) | 87 (15.0) | 84 (14.3) | 70 (11.5) | 34 (10.3) | |
| Race, n (%) | <0.0001 | |||||||
| White | 1317 (40.3) | 180 (13.7) | 195 (14.8) | 238 (18.1) | 263 (20.0) | 266 (20.2) | 175 (13.3) | |
| Asian | 710 (21.7) | 181 (25.5) | 178 (25.1) | 128 (18.3) | 107 (15.1) | 88 (12.4) | 28 (3.9) | |
| Black | 714 (21.9) | 124 (17.4) | 118 (16.5) | 128 (17.3) | 124 (17.4) | 123 (17.2) | 97 (13.6) | |
| Latino or Hispanic | 526 (16.1) | 92 (17.5) | 91 (17.3) | 85 (16.2) | 95 (18.1) | 134 (25.5) | 29 (5.5) | |
| Fitzpatrick Skin Type, n (%)b | 0.0642 | |||||||
| Type I + II | 924 (28.3) | 181 (31.4) | 148 (25.5) | 155 (26.7) | 187 (31.8) | 159 (26.1) | 94 (28.7) | |
| Type III + IV | 1627 (49.9) | 276 (47.9) | 290 (49.9) | 298 (51.5) | 279 (47.5) | 331 (54.3) | 153 (46.7) | |
| Type V + VI | 711 (21.8) | 119 (20.7) | 423 (24.6) | 126 (21.8) | 122 (20.8) | 120 (19.7) | 81 (24.7) | |
| Missing | 5 (0.2) | 1 (0.2) | 1 (0.2) | 1 (0.2) | 1 (0.2) | 1 (0.3) | ||
| Smoking status, n (%) | <0.0001 | |||||||
| Current smoker | 494 (15.1) | 55 (9.5) | 75 (12.9) | 131 (22.6) | 126 (21.4) | 71 (11.6) | 36 (10.9) | |
| Former smoker | 672 (20.6) | 55 (9.5) | 70 (12.0) | 88 (15.2) | 131 (22.2) | 199 (32.6) | 129 (39.2) | |
| Never smoked | 2101 (64.3) | 467 (80.9) | 437 (75.1) | 360 (62.2) | 332 (56.4) | 341 (55.8) | 164 (49.8) | |
| Months smoked‡ | <0.0001 | |||||||
| Median (min–max) | 216.0 (0–720) | 56.0 (0–216) | 120.0 (0–300) | 240.0 (0–445) | 304.0 (0–564) | 242.0 (0–672) | 300.0 (0–720) | |
| Pack years for smokingc,d | <0.0001 | |||||||
| Median (range) | 9.1 (0–100) | 1.3 (0–15) | 3.5 (0–46) | 10.0 (0–60) | 13.8 (0–88) | 12.5 (0–100) | 15.0 (0–100) | |
| Number of months since having stopped smoking | <0.0001 | |||||||
| Median (range) | 146.0 (0–684) | 30.0 (0–156) | 67.0 (1–240) | 88.0 (1–366) | 144.0 (3–540) | 212.0 (2–684) | 300.0 (1–600) | |
| Type of smoking product, n (%)e | ||||||||
| Cigarettes | 1144 (35.0) | 103 (17.9) | 142 (24.4) | 214 (37.0) | 256 (43.5) | 266 (43.5) | 163 (49.5) | <0.0001 |
| Cigars | 40 (1.2) | 16 (2.8) | 8 (1.4) | 6 (1.0) | 2 (0.3) | 6 (1.0) | 2 (0.6) | <0.0001 |
| Pipes | 7 (0.2) | 2 (0.3) | 2 (0.3) | 2 (0.3) | 1 (0.2) | <0.0001 | ||
| Number of cigarettes per day | <0.0001 | |||||||
| Mean (SD) | 12.7 (9.7) | 7.8 (6.7) | 9.2 (7.2) | 12.5 (8.3) | 14.0 (10.8) | 14.3 (9.9) | 14.6 (10.7) | |
| Number of cigars per day | 0.0779 | |||||||
| Mean (SD) | 2.1 (2.3) | 2.1 (1.6) | 3.1 (3.1) | 1.5 (1.4) | 5.5 (6.4) | 0.7 (0.5) | 0.5 (0.7) | |
| Number of pipes per day | 0.8844 | |||||||
| Mean (SD) | 3.4 (4.0) | 3.5 (3.5) | 2.5 (2.1) | 5.5 (7.8) | 1.0 (-) | |||
| Drinks alcohol, n (% yes) | 1727 (52.9) | 338 (58.6) | 288 (49.5) | 310 (53.5) | 323 (54.8) | 311 (50.9) | 157 (47.7) | 0.0078 |
| Number of drinks per week | <0.0001 | |||||||
| Mean (SD) | 3.7 (4.5) | 2.8 (3.8) | 3.2 (3.5) | 3.7 (4.6) | 4.0 (5.0) | 4.8 (5.4) | 4.0 (4.2) | |
| Median (min–max) | 2.0 (0–30) | 1.0 (0–25) | 2.0 (0–30) | 2.0 (0–30) | 2.0 (0–30) | 3.0 (0–30) | 2.0 (0–20) | |
For continuous variables, means (standard deviations) are shown—unless the data were skewed, in which case, medians and ranges are shown.
T-test of mean scores (for continuous variables) or chi-squared/Fisher’s exact (for categorical responses) by age, with p-values based on comparisons between age groups
Type I: very fair skin; Type II: fair skin; Type III: medium skin; Type IV: olive/light brown skin; Type V: brown skin; Type VI: dark brown skin
For current and former smokers
Smoking one pack of 20 cigarettes per day for one year=one pack-year
Participants were permitted to select all that applied, therefore, percentages might add up to more than 100 percent. Min: minimum; Max: maximum
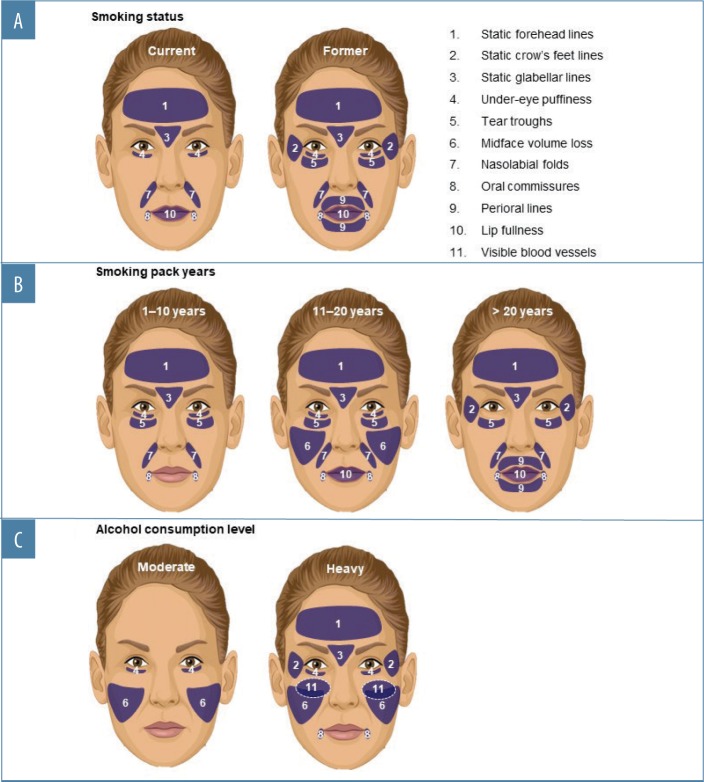
In addition to questions about demographics and lifestyle, survey respondents used a mirror and photonumeric rating scales20 to determine their severity of aging (none to severe) for 11 facial characteristics in the absence of facial expression, inlcluding forehead, crow’s feet, and glabellar lines; under-eye puffiness; tear-trough hollowing; visible blood vessels on the cheeks; midface volume loss; nasolabial folds; oral commissures; perioral lines; and lip fullness. These features were selected because they are of interest to aesthetic practitioners and validated photonumeric scales are available.21
Data from women aged 18 to 75 years who described themselves as white, Asian, black, or Latino/Hispanic were included in this subanalysis. The objectives were to compare the severity of self-reported facial aging 1) between never-smokers and current or former smokers, by status and pack year history; and 2) among women who did and did not use alcohol by frequency and beverage type.
Statistical analyses. The target sample size was originally 2,500 women and 500 men from four countries (US, Canada, UK, and Australia) to achieve a total sample of at least 3,000 panelists aged 18 to 75 years. The sampling framework was designed to achieve a study cohort composed of an approximately equal number of men and women within each country by the age and racial/ethnic subgroups of interest.
Linear regression based on a general linear model was used to assess the associations in women between smoking status (never smoked [reference] vs. current and former smoker), pack-years (0 [reference] versus 1–10, 11–20, and >20 years), alcohol use (none [reference] versus moderate and high use), and alcohol type (none [reference] versus wine only, spirit/beer only, and combination drinker), and aging severity for each facial feature. Pack-year cutoffs were generated based on decades of use, with higher pack-years indicating heavier smoking and more exposure. Alcohol beverage types were chosen to determine whether any type might be associated with less facial aging than other beverages, given that moderate consumption of wine, beer, and spirits have different impacts on cardiovascular mortality and life expectancy.22,23 Covariates included age and BMI as continuous variables; race (white [reference] vs. Asian, black, and Hispanic); and country (US [reference] versus Australia, Canada, and UK). Country was used as a proxy for sun exposure in all models because no significant associations between sun exposure during the six months preceding survey completion and aging severity of most facial features was observed in this dataset.20 Race was controlled for because the skin of black people ages differently and more slowly than the skin of white, Hispanic, and Asian people.24
Analysis of variance was used to assess the overall model. As this research was exploratory, we included the main variables of interest (i.e., pack-years, smoking status, alcohol use) in the linear regression model, and evaluated pairwise comparisons even if the overall main effect for the factor was nonsignificant. Adjusted R2 described the overall model fit, and unstandardized beta coefficients of predictor variables indicated the direction and strength of the association. To provide a visual indication of each feature’s aging trends, severity scores, stratified by age group, were plotted against these extrinsic factors. Pairwise comparisons between current versus former smoking status and pack-year history (1–10, 11–20, and >20 years) were also conducted. Interactions between smoking pack-years and alcohol use were conducted using the same covariates. Statistical analyses were carried out using SAS version 9.4 (SAS Institute, Cary, North Carolina). Any missing data were considered as missing; no data imputations were performed.
RESULTS
Study population. A total of 10,823 people responded to the survey invitation between December 2013 and February 2014; their disposition is summarized in Figure 1. This subanalysis, conducted in 2016 and 2017, included data from 3,267 women: 1,569 (48.0%) from the US, 591 (18.1%) from Canada, 588 (18.0%) from Australia, and 519 (15.9%) from the UK. The relative proportions of survey respondents were similar in each age band. Participant demographics and smoking and alcohol consumption habits of the participants are summarized by age group in Appendix 1 and Figure 2. The proportions of women with different Fitzpatrick Skin Types were similar across all age groups (p=0.0642; Appendix 1).
FIGURE 1.
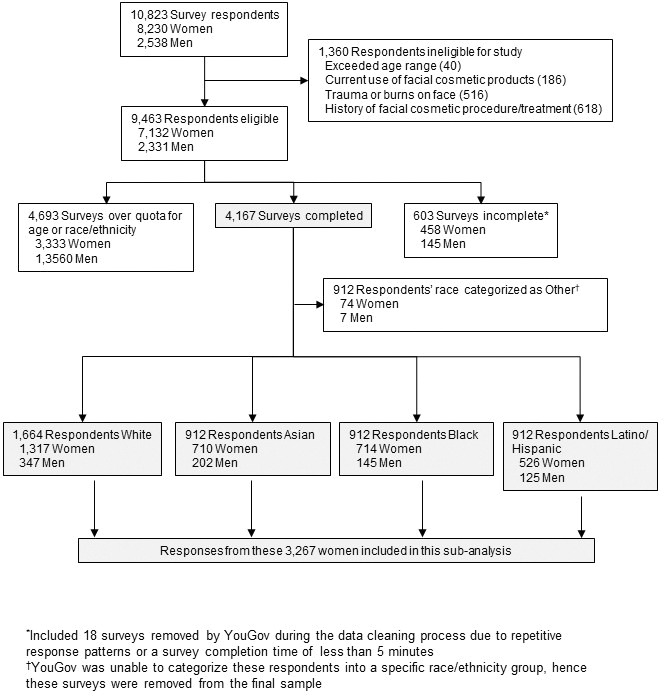
Facial skin aging; disposition of survey respondents
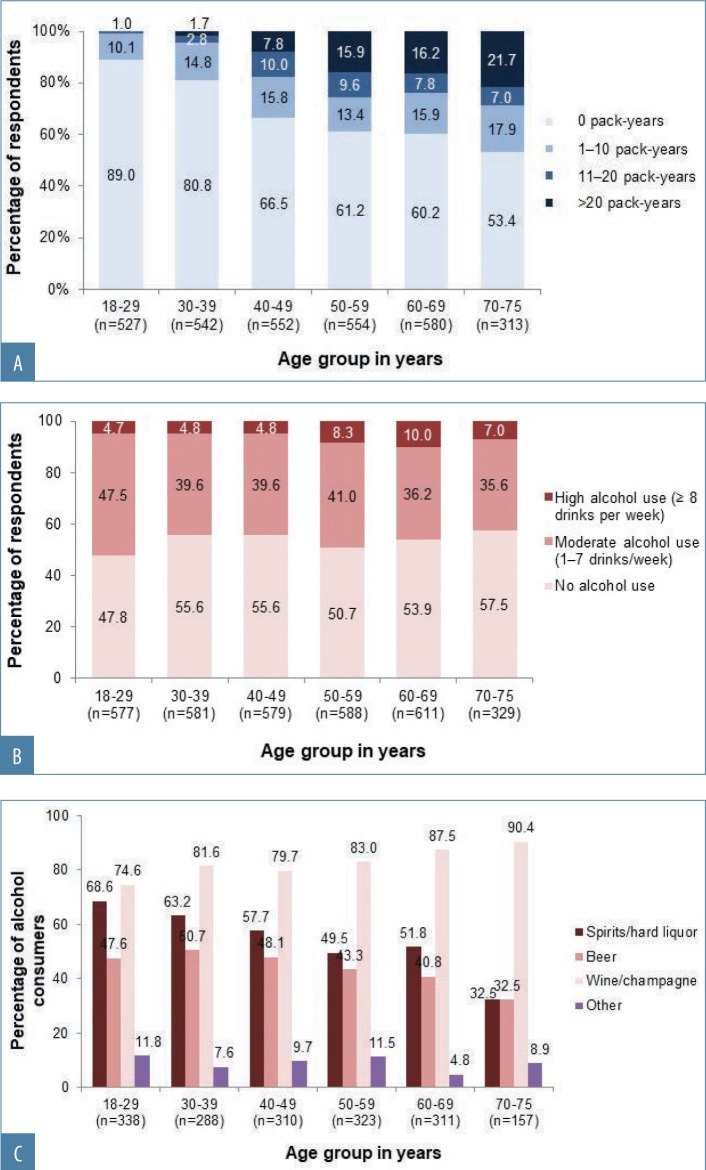
FIGURE 2A–C.
Facial skin aging. Smoking and alcohol consumption habits among survey respondents; A) Smoking history in pack-years by age;a B) alcohol use by age group, as defined in the 2015 to 2020 Dietary Guidelines for Americans;b C) types of alcoholic beverage consumed by age groupc
aData are missing from 199 respondents who stated that they were current or former smokers but did not specify pack years; bdata on alcohol use were missing for two respondents; crespondents who answered “Yes” to the question, “Do you drink alcohol?” [respondents were permitted to select all alcoholic beverage options that applied, therefore, percentages might add up to more than 100%])
Overall, 1,166 women (35.7%) were current or former smokers (Appendix 1), mostly of cigarettes (1,144/1,166; 98.1%). Women aged 50 to 75 years had the longest smoking histories (Appendix 1; Figure 2A), but the largest proportion of current smokers (131/494; 26.5%) was aged 40 to 49 years. Just over half of respondents (1,727; 52.9%) reported that they drank alcohol (Appendix 1). Of these, 1,499 (86.8%) reported consuming one drink or fewer per day (i.e., 0–7 drinks per week, defined as “consuming alcohol in moderation” in the 2015–2020 Dietary Guidelines for Americans25), and 226 women (13.1% of the drinkers) consumed eight or more drinks per week (defined as “heavy drinking”25). Women aged 18 to 29 years composed the highest proportion of alcohol drinkers, but the greatest proportion of heavy drinkers was aged 50 to 69 years (Figure 2B). Wine or champagne were the most commonly consumed alcoholic beverages by women of all age groups (Figure 2C).
Effect of smoking. Linear regression showed that current smokers had more severe signs of aging than did nonsmokers, and the associations with increased forehead and glabellar lines, under-eye puffiness, nasolabial folds, oral commissures, and reduced lip fullness were statistically significant (Appendix 2). Being a former smoker was significantly associated with more severe aging of all facial features than in nonsmokers, except for midface volume loss and visible blood vessels on the cheeks (p≤0.025; Appendix 2). Figure 3A summarizes the facial features for which statistically significant associations between smoking status and increased aging severity were identified. Comparisons between current and former smokers revealed no statistically significant differences in facial aging severity for any feature (data not shown).
APPENDIX 2.
Impact of smoking status on facial aging characteristics using linear regression controlling for age, BMI, race, and countrya
| MODEL/VARIABLEb | ANOVA MODEL SUMMARY | MAIN EFFECT FOR SMOKING STATUS | UNSTANDARDIZED COEFFICIENTS | P-VALUE | |
|---|---|---|---|---|---|
| R2 | PR>F | B | STD ERROR | ||
| Static forehead linesc | 0.24 | <0.001* | |||
| Current smoker | 0.123 | 0.041 | 0.003* | ||
| Former smoker | 0.115 | 0.038 | 0.002* | ||
| Static crow’s feet lines | 0.42 | 0.013* | |||
| Current smoker | 0.055 | 0.039 | 0.164 | ||
| Former smoker | 0.103 | 0.036 | 0.004* | ||
| Static glabellar linesc | 0.39 | 0.0001* | |||
| Current smoker | 0.185 | 0.043 | <0.0001* | ||
| Former smoker | 0.158 | 0.039 | <0.0001* | ||
| Pu_ness under eyesc | 0.21 | 0.003* | |||
| Current smoker | 0.139 | 0.045 | 0.002* | ||
| Former smoker | 0.093 | 0.042 | 0.025* | ||
| Tear troughs | 0.30 | <0.001* | |||
| Current smoker | 0.061 | 0.046 | 0.185 | ||
| Former smoker | 0.161 | 0.042 | 0.0001* | ||
| Midface volume loss | 0.25 | 0.279 | |||
| Current smoker | 0.042 | 0.056 | 0.450 | ||
| Former smoker | 0.079 | 0.051 | 0.120 | ||
| Nasolabial folds | 0.36 | <0.0001* | |||
| Current smoker | 0.186 | 0.051 | 0.0003* | ||
| Former smoker | 0.273 | 0.047 | <0.0001* | ||
| Oral commissures | 0.40 | <0.0001* | |||
| Current smoker | 0.136 | 0.041 | 0.001* | ||
| Former smoker | 0.170 | 0.038 | <0.0001* | ||
| Perioral lines | 0.31 | 0.003* | |||
| Current smoker | 0.042 | 0.038 | 0.279 | ||
| Former smoker | 0.122 | 0.035 | 0.001* | ||
| Lip fullnessc | 0.30 | <0.001* | |||
| Current smoker | 0.139 | 0.044 | 0.002* | ||
| Former smoker | 0.114 | 0.040 | 0.005* | ||
| Visible blood vessels on cheeksc | 0.04 | 0.69 | |||
| Current smoker | −0.0026 | 0.037 | 0.94 | ||
| Former smoker | 0.0276 | 0.034 | 0.42 | ||
Data for covariates of age, body mass index, race, and country not included in this table to increase clarity
Using “never smoked” as the reference variable and listed facial features as dependent variables N=2,805 for all variables except
N=2,804
Statistically significant at p<0.05
FIGURE 3.
Facial features for which the aging severity are significantly associated (p<0.05) with smoking or alcohol consumption by linear regression analysis (Appendices 2–4), after controlling for age, race, body mass index, and country, according to smoking status (A), smoking pack-year history (B), and alcohol consumption level, (C) respectively
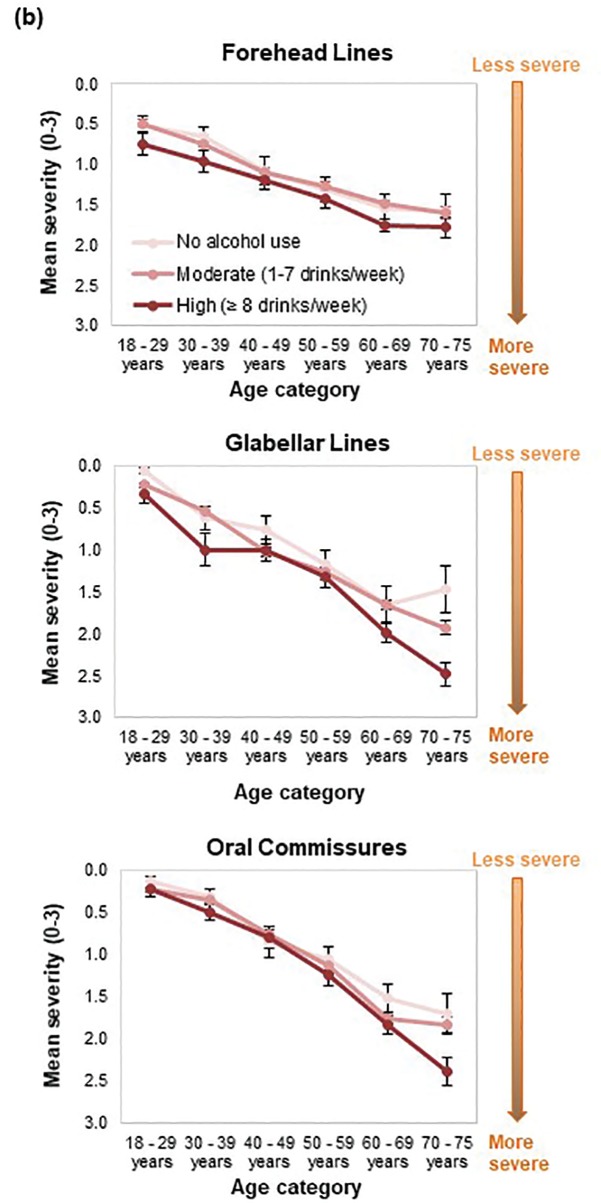
When smoking history was analyzed by pack-years, forehead and glabellar lines, under-eye puffiness, tear troughs, nasolabial folds, and deep oral commissures were significantly more likely to be present than in nonsmokers, even in women with the shortest smoking history (1– 10 pack-years; p≤0.018; Appendix 3 and Figure 3B). Significantly greater loss of midface volume and lip fullness were associated with an 11- to 20-pack-year history (p≤0.032), while crow’s feet and perioral lines were only significantly associated with a smoking history of more than 20 pack-years (p≤0.0002). In general, the mean severity over time of the analyzed features increased with smoking history, although observed differences were generally rated with one point or less on the photonumeric severity scales (Figure 4A). When comparing smoking pack-year history categories, only glabellar lines showed significant differences in severity between categories in smokers: women with 11-to-20 and 1-to-10 pack-year smoking histories had progressively less glabellar line severity than did those with greater-than-20 pack-year histories (p≤0.0089).
APPENDIX 3.
Impact of smoking in pack-yearsa on facial aging characteristics using linear regression controlling for age, body mass index, race, and countryb
| MODEL / VARIABLEb | ANOVA MODEL SUMMARY | MAIN EFFECT FOR SMOKING STATUS | UNSTANDARDIZED COEFFICIENTS | P-VALUE | |
|---|---|---|---|---|---|
| R2 | PR > F | B | STD ERROR | ||
| Static forehead lines | 0.24 | 0.002* | |||
| 1–10 pack-years | 0.156 | 0.053 | 0.003* | ||
| 11–20 pack-years | 0.157 | 0.061 | 0.010* | ||
| >20 pack-years | 0.092 | 0.043 | 0.033* | ||
| Static crow’s feet lines | 0.42 | 0.001* | |||
| 1–10 pack-years | 0.071 | 0.041 | 0.083 | ||
| 11–20 pack-years | 0.105 | 0.058 | 0.074 | ||
| >20 pack-years | 0.186 | 0.050 | 0.0002* | ||
| Static glabellar linesd | 0.39 | <0.0001* | |||
| 1–10 pack-years | 0.126 | 0.044 | 0.005* | ||
| 11–20 pack-years | 0.149 | 0.063 | 0.018* | ||
| >20 pack-years | 0.348 | 0.054 | <0.0001* | ||
| Puffiness under eyesd | 0.2 | <0.001* | |||
| 1–10 pack-years | 0.139 | 0.048 | 0.004* | ||
| 11–20 pack-years | 0.224 | 0.068 | 0.001* | ||
| >20 pack-years | 0.093 | 0.058 | 0.110 | ||
| Tear troughs | 0.29 | <0.001* | |||
| 1–10 pack-years | 0.113 | 0.048 | 0.018* | ||
| 11–20 pack-years | 0.152 | 0.068 | 0.025* | ||
| >20 pack-years | 0.199 | 0.058 | 0.001* | ||
| Midface volume loss | 0.25 | 0.177 | |||
| 1–10 pack-years | 0.049 | 0.058 | 0.397 | ||
| 11–20 pack-years | 0.177 | 0.083 | 0.032* | ||
| >20 pack-years | 0.049 | 0.071 | 0.493 | ||
| Nasolabial folds | 0.36 | <0.0001* | |||
| 1–10 pack-years | 0.2673 | 0.054 | <0.0001* | ||
| 11–20 pack-years | 0.1505 | 0.077 | 0.049* | ||
| >20 pack-years | 0.3168 | 0.066 | <0.0001* | ||
| Oral commissures | 0.40 | <0.0001* | |||
| 1–10 pack-years | 0.136 | 0.043 | 0.002* | ||
| 11–20 pack-years | 0.152 | 0.062 | 0.014* | ||
| >20 pack-years | 0.257 | 0.053 | <0.0001* | ||
| Perioral lines | 0.31 | <0.001* | |||
| 1–10 pack-years | 0.074 | 0.040 | 0.067 | ||
| 11–20 pack-years | 0.090 | 0.057 | 0.115 | ||
| >20 pack-years | 0.209 | 0.049 | <0.0001* | ||
| Lip fullnessd | 0.30 | <0.0001* | |||
| 1–10 pack-years | 0.070 | 0.046 | 0.124 | ||
| 11–20 pack-years | 0.222 | 0.065 | 0.001* | ||
| >20 pack-years | 0.212 | 0.056 | 0.0001* | ||
| Visible blood vessels on cheeksd | 0.04 | 0.303 | |||
| 1–10 pack-years | -0.035 | 0.047 | 0.457 | ||
| 11–20 pack-years | 0.036 | 0.057 | 0.514 | ||
| >20 pack-years | 0.057 | 0.038 | 0.137 |
Data for covariates of age, body mass index, race, and country not included in this table to increase clarity
Using “never smoked” as the reference variable and listed facial features as dependent variables N=2,805 for all variables except
N=2,804
Statistically significant at p<0.05
FIGURE 4A.
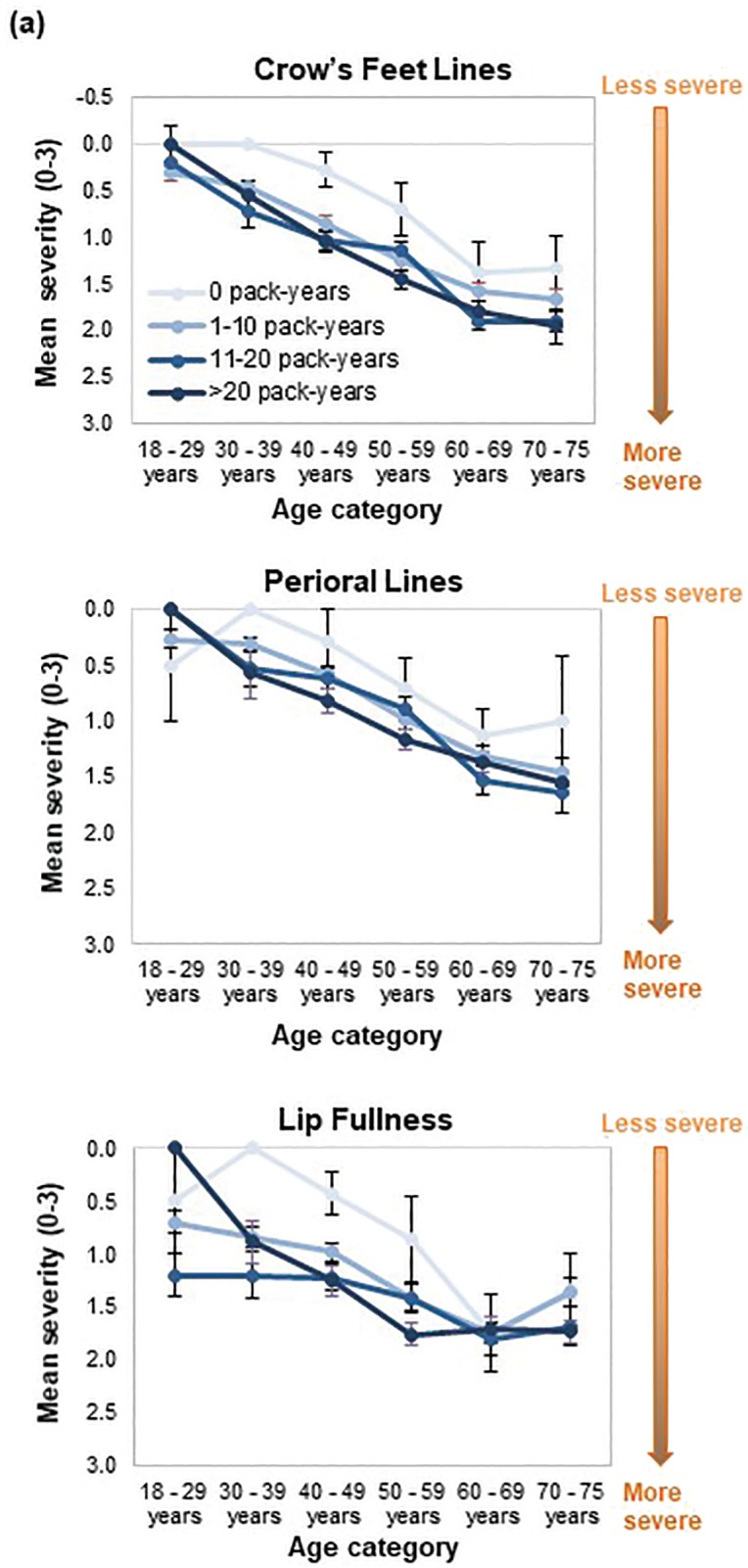
Facial skin aging; mean severity scores indicating aging trends of selected skin and volume-related features according to smoking pack years
Effect of alcohol use. Regression analyses suggested a relationship between alcohol use and aging severity that was statistically significant for under-eye puffiness, midface volume loss, and blood vessels on the cheeks (Pr > F < 0.05 in Appendix 4). In heavy drinkers (i.e., those who consumed eight or more drinks per week25), seven facial features were significantly associated with an appearance of more severe aging than in women who did not consume alcohol (p≤0.042; Appendix 4 and Figure 3C). However, drinking in moderation was only significantly associated with increased under-eye puffiness (p=0.026) and midface volume loss (p=0.041). In general, mean severity over time of the features analyzed increased to a greater extent in heavy drinkers (Figure 4B). However, comparisons between moderate and heavy drinkers revealed no significant differences in aging severity for any facial feature except visible blood vessels, which were more severe in heavy drinkers (p=0.007).
APPENDIX 4.
Impact of alcohol consumption on facial ageing characteristics using linear regression controlling for age, BMI, race, and countrya
| MODEL / VARIABLEb | ANOVA MODEL SUMMARY | MAIN EFFECT FOR SMOKING STATUS | UNSTANDARDIZED COEFFICIENTS | P-VALUE | |
|---|---|---|---|---|---|
| R2 | PR > F | B | STD ERROR | ||
| Static forehead linesc | 0.24 | 0.060 | |||
| Consumption in moderationd | -0.012 | 0.031 | 0.689 | ||
| Heavye alcohol use | 0.125 | 0.058 | 0.033* | ||
| Static crow’s feet lines | 0.42 | 0.071 | |||
| Consumption in moderationd | 0.022 | 0.029 | 0.445 | ||
| Heavye alcohol use | 0.128 | 0.056 | 0.022* | ||
| Static glabellar linesc | 0.38 | 0.107 | |||
| Consumption in moderationd | 0.017 | 0.032 | 0.589 | ||
| Heavye alcohol use | 0.128 | 0.061 | 0.035* | ||
| Puffiness under eyesc | 0.21 | 0.021* | |||
| Consumption in moderationd | 0.075 | 0.034 | 0.026* | ||
| Heavye alcohol use | 0.143 | 0.064 | 0.026* | ||
| Tear troughs | 0.29 | 0.057 | |||
| Consumption in moderationd | 0.065 | 0.034 | 0.056 | ||
| Heavye alcohol use | 0.124 | 0.065 | 0.056 | ||
| Midface volume loss | 0.25 | 0.016* | |||
| Consumption in moderationd | 0.085 | 0.042 | 0.041* | ||
| Heavye alcohol use | 0.199 | 0.079 | 0.012* | ||
| Nasolabial folds | 0.35 | 0.272 | |||
| Consumption in moderationd | 0.045 | 0.039 | 0.244 | ||
| Heavye alcohol use | 0.102 | 0.073 | 0.164 | ||
| Oral commissures | 0.40 | 0.075 | |||
| Consumption in moderationd | 0.048 | 0.031 | 0.123 | ||
| Heavye alcohol use | 0.120 | 0.059 | 0.042* | ||
| Perioral lines | 0.31 | 0.135 | |||
| Consumption in moderationd | −0.008 | 0.029 | 0.784 | ||
| Heavye alcohol use | 0.100 | 0.055 | 0.067 | ||
| Lip fullnessc | 0.30 | 0.079 | |||
| Consumption in moderationd | 0.012 | 0.033 | 0.706 | ||
| Heavye alcohol use | 0.041 | 0.062 | 0.514 | ||
| Visible blood vessels on cheeksc | 0.05 | 0.007* | |||
| Consumption in moderationd | 0.024 | 0.027 | 0.377 | ||
| Heavye alcohol use | 0.164 | 0.052 | 0.002* |
Data for covariates of age, body mass index, race, and country were not included in this table to increase clarity. Some participants responded “Yes” to the question, “Do you drink alcohol?”, but then responded “0” to the question “What is the average number of drinks that you consume per week?.” For this statistical model, weekly alcohol consumption was based only on drinks reported, i.e., n=1,329 women reported consuming 1 to 7 drinks per week, and 226 women reported consuming more than drinks per week; two responses were missing.
Using “no alcohol” as the reference variable and listed facial feature as dependent variables N=2,804 for all variables except
n=2,803
Defined as consuming up to one alcoholic drink (containing 14g/0.6 fl oz pure alcohol) per day, i.e. 1 to 7 drinks per week25
Defined as consuming more than one alcoholic drink per day, i.e., ≥8 drinks per week25
Statistically significant at p<0.05
FIGURE 4B.
Facial skin aging; mean severity scores indicating aging trends of selected skin and volume-related features according to alcohol consumption level
Linear regression analyses were also conducted to investigate whether alcoholic beverage type would impact facial aging differently. No specific beverage type was identified as being associated with an increased severity of any facial feature, although under-eye puffiness was associated with drinking a combination of beverages (p=0.001) and increased cheek blood vessels were seen among wine drinkers (p=0.0005).
When interactions between smoking history and alcohol use and their impact on aging were investigated using linear regressions, the impact of pack-years on aging severity was not dependent on alcohol use and vice versa.
DISCUSSION
This subanalysis of data from 3,267 white, Asian, black, and Hispanic women on three continents revealed associations between smoking and alcohol consumption habits and the aging severity of 11 specific skin- and volume-related facial features. A dose-dependent association was observed between pack-year smoking history and aging severity for most facial features. Greater alcohol consumption also increased the severity of some facial aging signs.
Whereas being a former smoker was associated with an increased severity of all the facial features evaluated except midface volume loss and visible blood vessels, current smoking was associated with increased aging of fewer features (Appendix 2 and Figure 3A), probably because former smokers had smoked for longer than current smokers (Appendix 1). More severe aging in the former (even though they had “given up smoking” for longer) suggests that cessation does not “reverse” the aging. Crow’s feet, tear troughs, and perioral lines were more likely to increase in severity as a longer-term consequence of smoking or depending on the amount of tobacco smoked. Analyses by smoking pack-years (Appendix 3 and Figures 3B and 4A) confirmed that the severity of glabellar lines, tear troughs, crow’s feet, perioral lines, and reduced lip fullness increased in relation to smoking duration. These observations are in agreement with previous findings in a small US twin study13 and suggest that free radicals and reactive oxygen species generated by smoking might induce aging changes not only in the epidermis and dermis (e.g., by impairing collagen biosynthesis and causing collagen degradation via the induction of matrix metalloproteases26) but also in the fat (i.e., volume-related features).27,28 As facial volume decreases, features like tear troughs and infraorbital fat pads (puffiness) become more prominent.29
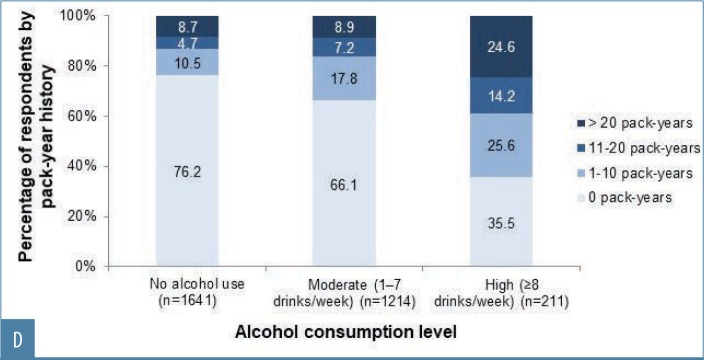
Heavy alcohol use (eight or more drinks per week)25 was associated with increased severity of nearly all features analyzed (Appendix 4 and Figures 3C and 4B). Although more heavy drinkers had also smoked (Figure 2D), linear regression analyses did not support a relationship between smoking and drinking. However, the relatively small number of heavy drinkers in this survey might have precluded identifying a relationship due to insufficient sample size. Among moderate drinkers, only midface volume loss and under-eye puffiness were associated with drinking. Additionally, only visible blood vessels were significantly increased in heavy versus moderate drinkers and in wine-only versus nondrinkers, in agreement with findings that alcohol intake is associated with rosacea.30 Significant associations between facial aging and the type of alcohol consumed were only seen for under-eye puffiness, which was significantly associated with drinking a combination of beer, wine, and spirits—possibly because those who reported drinking a combination of beverage types might have consumed more alcohol overall. In addition, the association between aging and beverage type might have been confounded by the fact that many respondents reported consuming several types (Figure 2A).
FIGURE 2D.
Facial skin aging. Smoking and alcohol consumption habits among survey respondents; smoking history by alcohol consumption levelsa,b
aData are missing from 199 respondents who stated that they were current or former smokers but did not specify pack years; bdata on alcohol use were missing for two respondents
These observations are in agreement with a US twin study in which alcohol avoidance was significantly associated with a younger appearance.19 However, other, larger studies have found no association between alcohol use and perceived age5 or wrinkling score.6 Differences between how alcohol consumption was assessed in this and other studies preclude direct comparisons of findings. Excessive alcohol consumption impacts the body in many ways (e.g., vitamin deficiency, tissue damage, disruption of inflammatory responses, and diminished ability of skin fibroblasts to produce type I collagen).10,31–33 Alcohol abuse has been reported to reduce fat mass,34 which might underlie the midface volume loss reported by heavy drinkers. The increased under-eye puffiness might have been due to the unveiling of the suborbital fat pad as the midface volume receded. Alcohol impairs the skin’s antioxidant defense system, leaving it more prone to sunburn and the aging effects of ultraviolet light.10,35 Cigarette smoking is also positively correlated with photodamage,14 most likely because smoking downregulates the aryl hydrocarbon receptor, a transcription factor that mediates the toxicity of ultraviolet B–generated photoproducts in the body.26
This study’s strength is that the data were collected from a large multiracial sample of women of all ages living in geographically dispersed countries and reported a wide range of smoking and alcohol consumption habits. This allowed for a detailed analysis of the effects of these extrinsic factors on 11 aspects of skin-and volume-related facial aging changes.
Limitations. We acknowledge several limitations. Tobacco and alcohol use were self-reported and might have been underestimated, also because the defined unit volumes of alcohol25 might have been smaller than are typically poured.36–39 However, statistically significant associations between aging progression and reported consumption levels were still detected. Aging severity was self-reported rather than assessed by a clinician, but any response bias might have been mitigated by the relatively large sample size and its geographic diversity. Although a longitudinal study design might have enabled a more effective evaluation of facial aging over time, a cross-sectional design was selected for practical purposes. Alcohol analyses were conducted using the US Department of Health and Human Services’ definitions of moderate and heavy alcohol consumption,25 which did not provide sufficient granularity. It would have been useful to determine at which level of consumption alcohol starts to increase facial aging, and the impact of excessive drinking and its relationship with the duration of tobacco exposure in a larger sample (only 7% of these women reported consuming more than eight drinks per week). Future studies could also evaluate in more detail whether giving up alcohol could halt or slow down the associated progression of facial aging as well as whether drinking predominantly red wine is associated with less progression of facial aging in comparison with other beverages due to its antioxidant content.14,40
CONCLUSION
Clear associations were found between skin-and volume-related facial aging and smoking and heavy alcohol consumption. We herein provide dermatologists with empirical data to support recommendations to their patients on lifestyle modification—namely, that never smoking and not exceeding moderate alcohol consumption can help to delay the onset of the appearance of facial aging. If they already smoke, patients (especially the young) should be advised to stop, because aging severity increases with the amount and duration of tobacco exposure. Patients with a history of smoking and high alcohol consumption who request nonsurgical treatment for facial aging might require earlier and more extensive treatment to counteract the more severe skin-and volume-related aging caused by these extrinsic factors.
REFERENCES
- 1.Fisher GJ, Kang S, Varani J et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138(11):1462–1470. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- 2.Flament F, Bazin R, Laquieze S et al. Effect of the sun on visible clinical signs of aging in Caucasian skin. Clin Cosmet Investig Dermatol. 2013;(6):221–232. doi: 10.2147/CCID.S44686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vierkotter A, Krutmann J. Environmental influences on skin aging and ethnic-specific manifestations. Dermatoendocrinol. 2012;4(3):227–231. doi: 10.4161/derm.19858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherertz EF, Hess SP. Stated age. N Engl J Med. 1993;329(4):281–282. doi: 10.1056/NEJM199307223290419. [DOI] [PubMed] [Google Scholar]
- 5.Rexbye H, Petersen I, Johansens M et al. Influence of environmental factors on facial ageing. Age Ageing. 2006;35(2):110–115. doi: 10.1093/ageing/afj031. [DOI] [PubMed] [Google Scholar]
- 6.Ekiz O, Yuce G, Ulasli SS et al. Factors influencing skin ageing in a Mediterranean population from Turkey. Clin Exp Dermatol. 2012;37(5):492–496. doi: 10.1111/j.1365-2230.2012.04386.x. [DOI] [PubMed] [Google Scholar]
- 7.Leung WC, Harvey I. Is skin ageing in the elderly caused by sun exposure or smoking? Br J Dermatol. 2002;147(6):1187–1191. doi: 10.1046/j.1365-2133.2002.04991.x. [DOI] [PubMed] [Google Scholar]
- 8.Yin L, Morita A, Tsuji T. Tobacco smoke extract induces age-related changes due to modulation of TGF-beta. Exp Dermatol. 2003;12(Suppl 2):51–56. doi: 10.1034/j.1600-0625.12.s2.8.x. [DOI] [PubMed] [Google Scholar]
- 9.Rossi M, Pistelli F, Pesce M et al. Impact of long-term exposure to cigarette smoking on skin microvascular function. Microvasc Res. 2014;93:46–51. doi: 10.1016/j.mvr.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Darvin ME, Sterry W, Lademann J et al. Alcohol consumption decreases the protection efficiency of the antioxidant network and increases the risk of sunburn in human skin. Skin Pharmacol Physiol. 2013;26(1):45–51. doi: 10.1159/000343908. [DOI] [PubMed] [Google Scholar]
- 11.Higgins E, du Vivier A. Alcohol intake and other skin disorders. Clin Dermatol. 1999;17(4):437–441. doi: 10.1016/s0738-081x(99)00038-3. [DOI] [PubMed] [Google Scholar]
- 12.Kazakevich N, Moody MN, Landau JM et al. Alcohol and skin disorders: with a focus on psoriasis. Skin Therapy Lett. 2011;16(4):5–6. [PubMed] [Google Scholar]
- 13.Okada HC, Alleyne B, Varghai K et al. Facial changes caused by smoking: a comparison between smoking and nonsmoking identical twins. Plast Reconstr Surg. 2013;132(5):1085–1092. doi: 10.1097/PRS.0b013e3182a4c20a. [DOI] [PubMed] [Google Scholar]
- 14.Martires KJ, Fu P, Polster AM et al. Factors that affect skin aging: a cohort-based survey on twins. Arch Dermatol. 2009;145(12):1375–1379. doi: 10.1001/archdermatol.2009.303. [DOI] [PubMed] [Google Scholar]
- 15.Ernster VL, Grady D, Miike R et al. Facial wrinkling in men and women, by smoking status. Am J Public Health. 1995;85(1):78–82. doi: 10.2105/ajph.85.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koh JS, Kang H, Choi SW et al. Cigarette smoking associated with premature facial wrinkling: image analysis of facial skin replicas. Int J Dermatol. 2002;41(1):21–27. doi: 10.1046/j.1365-4362.2002.01352.x. [DOI] [PubMed] [Google Scholar]
- 17.Kadunce DP, Burr R, Gress R et al. Cigarette smoking: risk factor for premature facial wrinkling. Ann Intern Med. 1991;114(10):840–844. doi: 10.7326/0003-4819-114-10-840. [DOI] [PubMed] [Google Scholar]
- 18.Gunn DA, Dick JL, van Heemst D et al. Lifestyle and youthful looks. Br J Dermatol. 2015;172(5):1338–1345. doi: 10.1111/bjd.13646. [DOI] [PubMed] [Google Scholar]
- 19.Guyuron B, Rowe DJ, Weinfeld AB et al. Factors contributing to the facial aging of identical twins. Plast Reconstr Surg. 2009;123(4):1321–1331. doi: 10.1097/PRS.0b013e31819c4d42. [DOI] [PubMed] [Google Scholar]
- 20.Goodman GJ, Armour KS, Kolodziejczyk JK et al. A comparison of self-reported signs of facial ageing among Caucasian women in Australia versus those in the United States, the United Kingdom, and Canada. Australas J Dermatol. 2017;59(2):108–117. doi: 10.1111/ajd.12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carruthers A, Carruthers J. A validated facial grading scale: the future of facial ageing measurement tools?. J Cosmet Laser Ther. 2010;12(5):235–241. doi: 10.3109/14764172.2010.514920. [DOI] [PubMed] [Google Scholar]
- 22.Streppel MT, Ocke MC, Boshuizen HC et al. Long-term wine consumption is related to cardiovascular mortality and life expectancy independently of moderate alcohol intake: the Zutphen Study. J Epidemiol Community Health. 2009;63(7):534–540. doi: 10.1136/jech.2008.082198. [DOI] [PubMed] [Google Scholar]
- 23.Strandberg TE, Strandberg AY, Salomaa VV et al. Alcoholic beverage preference, 29-year mortality, and quality of life in men in old age. J Gerontol A Biol Sci Med Sci. 2007;62(2):213–218. doi: 10.1093/gerona/62.2.213. [DOI] [PubMed] [Google Scholar]
- 24.Grimes P, Boyd C, Alexis A . Presented at the American Academy of Dermatology 74th Annual Meeting; March 4–8. Washington DC: 2016. Self-reported characteristics associated with the signs of facial aging by race/ethnic group and Fitzpatrick skin type in a diverse, multinational sample. [Google Scholar]
- 25. US Department of Health and Human Services, US Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th edition https://health.gov/dietaryguidelines/2015/resources/2015-2020_Dietary_Guidelines.pdf 1 Aug 2019.
- 26.Morita A, Torii K, Maeda A et al. Molecular basis of tobacco smoke-induced premature skin aging. J Investig Dermatol Symp Proc. 2009;14(1):53–55. doi: 10.1038/jidsymp.2009.13. [DOI] [PubMed] [Google Scholar]
- 27.Guillaumet-Adkins A, Yanez Y, Peris-Diaz MD et al. Epigenetics and oxidative stress in aging. Oxid Med Cell Longev. 2017;2017:9175806. doi: 10.1155/2017/9175806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim EJ, Kim YK, Kim JE et al. UV modulation of subcutaneous fat metabolism. J Invest Dermatol. 2011;131(8):1720–1726. doi: 10.1038/jid.2011.106. [DOI] [PubMed] [Google Scholar]
- 29.Glaser DA, Lambros V, Kolodziejczyk JK et al. Relationship between midface volume deficit and the appearance of tear troughs and nasolabial folds. Dermatol Surg. 2018;44(12):1547–1554. doi: 10.1097/DSS.0000000000001684. [DOI] [PubMed] [Google Scholar]
- 30.Li S, Cho E, Drucker AM et al. Alcohol intake and risk of rosacea in US women. J Am Acad Dermatol. 2017;76(6):1061–1067e2. doi: 10.1016/j.jaad.2017.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoyumpa AM. Mechanisms of vitamin deficiencies in alcoholism. Alcohol Clin Exp Res. 1986;10(6):573–581. doi: 10.1111/j.1530-0277.1986.tb05147.x. [DOI] [PubMed] [Google Scholar]
- 32.Jung MK, Callaci JJ, Lauing KL et al. Alcohol exposure and mechanisms of tissue injury and repair. Alcohol Clin Exp Res. 2011;35(3):392–399. doi: 10.1111/j.1530-0277.2010.01356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tipple C, Benson SM, Scholey A. A review of the physiological factors associated with alcohol hangover. Curr Drug Abuse Rev. 2016;9(2):93–98. doi: 10.2174/1874473710666170207152933. [DOI] [PubMed] [Google Scholar]
- 34.Addolorato G, Capristo E, Marini M et al. Body composition changes induced by chronic ethanol abuse: evaluation by dual energy X-ray absorptiometry. Am J Gastroenterol. 2000;95(9):2323–2327. doi: 10.1111/j.1572-0241.2000.02320.x. [DOI] [PubMed] [Google Scholar]
- 35.Li WH, Pappas A, Zhang L et al. IL-11, IL-1alpha, IL-6, and TNF-α are induced by solar radiation in vitro and may be involved in facial subcutaneous fat loss in vivo. J Dermatol Sci. 2013;71(1):58–66. doi: 10.1016/j.jdermsci.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Stockwell T, Donath S, Cooper-Stanbury M et al. Under-reporting of alcohol consumption in household surveys: a comparison of quantity-frequency, graduated-frequency and recent recall. Addiction. 2004;99(8):1024–1033. doi: 10.1111/j.1360-0443.2004.00815.x. [DOI] [PubMed] [Google Scholar]
- 37.Boniface S, Kneale J, Shelton N. Drinking pattern is more strongly associated with under-reporting of alcohol consumption than socio-demographic factors: evidence from a mixed-methods study. BMC Public Health. 2014;14:1297. doi: 10.1186/1471-2458-14-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bjerregaard P, Becker U. Validation of survey information on smoking and alcohol consumption against import statistics, Greenland 1993–2010. Int J Circumpolar Health. 2013:72. doi: 10.3402/ijch.v72i0.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilligan C, Sanson-Fisher R, Eades S et al. Assessing the accuracy of self-reported smoking status and impact of passive smoke exposure among pregnant Aboriginal and Torres Strait Islander women using cotinine biochemical validation. Drug Alcohol Rev. 2010;29(1):35–40. doi: 10.1111/j.1465-3362.2009.00078.x. [DOI] [PubMed] [Google Scholar]
- 40.Baxter RA. Anti-aging properties of resveratrol: review and report of a potent new antioxidant skin care formulation. J Cosmet Dermatol. 2008;7(1):2–7. doi: 10.1111/j.1473-2165.2008.00354.x. [DOI] [PubMed] [Google Scholar]