Abstract
Heterotopic ossification (HO) is a common occurrence after multiple forms of extensive trauma. These include arthroplasties, traumatic brain and spinal cord injuries, extensive burns in the civilian setting, and combat-related extremity injuries in the battlefield. Irrespective of the form of trauma, heterotopic bone is typically endochondral in structure and is laid down via a cartilaginous matrix. Once formed, the heterotopic bone typically needs to be excised surgically, which may result in wound healing complications, in addition to a risk of recurrence. Refinements of existing diagnostic modalities, like micro- and nano-CT are being adapted toward early intervention. Trauma-induced HO is a consequence of aberrant wound healing, systemic and local immune system activation, infections, extensive vascularization, and innervation. This intricate molecular crosstalk culminates in activation of stem cells that initiate heterotopic endochondral ossification. Development of animal models recapitulating the unique traumatic injuries has greatly facilitated the mechanistic understanding of trauma-induced HO. These same models also serve as powerful tools to test the efficacy of small molecules which specifically target the molecular pathways underlying ectopic ossification. This review summarizes the recent advances in the molecular understanding, diagnostic and treatment modalities in the field of trauma-induced HO.
TRAUMA-RELATED HO: DEFINITION AND EPIDEMIOLOGY
‘Heterotopic ossification’ (HO) is a term used to refer to formation of benign mature bony elements in extra-skeletal sites, including soft tissues and joints. Heterotopic ossification is a frequent complication associated with post-traumatic healing in clinical conditions such as fractures, spinal cord injury, traumatic brain injury, blast injuries, severe burns, and extensive surgeries, such as hip arthroplasty, acetabular, and elbow surgeries.1–4 In addition to trauma-induced localized HO as described above, this pathological phenomenon is also seen systemically in a rare genetic disorder, ‘Fibrodysplasia Ossificans Progressiva’ (FOP), where heterotopic bone forms in soft tissues and joints, either sporadically or in response to an external trauma.5 Interestingly, in both nongenetic and genetic cases, this bone forms at sites with a high concentration of connective tissue cells (perimysium, periosteum, peritenon) and entheses.
Epidemiologically, trauma-related HO can be classified into 2 broad categories: civilian patients and combat casualties. While cases encompassing the former category have been listed above, the second category includes a large majority of returning service members sustaining combat-related lower extremity amputations, where trauma-induced HO is a common occurrence in the residual and salvaged limbs.6
There are distinct differences between the HO seen in traumatic civilian settings vs those seen in combat casualties, both in terms of prevalence and treatment. Under civilian settings, axial and appendicular HO is seen in around 11–20% of the patients with traumatic brain or spinal cord injuries,3 in 20% of the patients with forearm fractures, with the highest prevalence of HO among femoral shaft fractures (52%)7 and in severe burn patients (60%).8 On the other hand, the frequency of HO seen in combat casualties increases to as high as 65% in post blast, extremity-injured amputees.9 Second, the prophylactic management of HO between civilian and combat cases has significant differences. Nonsteroidal anti-inflammatory drugs (NSAIDs) and radiotherapy are the standard prophylactic means to inhibit postsurgical HO in civilian settings. Despite wide use, their efficacy is limited and the deleterious effects on local tissues caused by these modalities can preclude secondary reconstruction. Given the complexity of combat wounds, involving the salvage and reconstructive challenges (multiple organ trauma involving extensive soft tissue, osseous, vascular and neural damage, large zones of injury heavily contaminated with foreign debris that require serial debridements, trauma-related infection, and so forth), the use of NSAIDs in this setting is restrictive. Use of radiotherapy as a prophylactic agent is time-sensitive, which makes it unfeasible for use in the combat casualty care setting.
DIFFERENCES BETWEEN GENETIC HO AND TRAUMA-INDUCED HO
HO is a hallmark pathology seen in an extremely rare genetic disorder, FOP, which is characterized by a mutation in the GS regulatory domain of BMP type I receptor, ACVR1. This mutation results in a single amino acid substitution (R206H), rendering the receptor hypersensitive to activating ligands, and refractory to inhibitory signals.10 Multiple mouse models of this mutation have been developed, which have proven invaluable in dissecting the cellular and molecular mechanisms of HO, in addition to developing promising drug candidates targeting HO.11–14
Similar to trauma-induced HO, genetic HO also occurs via endochondral ossification. Hence, many findings from studies on FOP have been extended to unravel mechanisms and preventative strategies in trauma-induced HO. However, there are critical differences that set apart these two forms of HO. One of the differences is the presence of congenital malformation of the great toe, a classic diagnostic feature of FOP patients.10 Unlike trauma-induced HO, which is solely triggered by injury, genetic HO has both injury-mediated (involving skeletal muscle), and noninjury mediated (involving tendons and ligaments) components,15 though nontraumatic inflammation may still play a role. Such inflammation, usually referred to as a ‘flare-up’, has been recently reported to have propensities for specific regions in the axial and appendicular skeleton,16 which is not seen in trauma-induced HO. These regions involve the back, neck, and jaw in the axial skeleton. The worst flare-ups are in the appendicular skeleton, in particular the shoulder region and the pelvic girdle area. Another interesting observation in this study was the predominance of axial flare-ups before 8 years of age, after which appendicular flare-ups were more common. Unlike trauma-induced HO, genetic HO is progressive, resulting in the formation of an ectopic skeleton, and fusion with the existing skeleton over time. Trauma-induced HO most often results after extensive soft tissue injury and inflammation, whereas in FOP, a minor local inflammatory flare, like that induced by vaccination, is sufficient to trigger HO. Owing to this, surgical excision, which is the first-line treatment for trauma-induced HO cannot be carried out in FOP patients.10 Glucocorticoids are commonly used among FOP patients to control inflammation (flare-ups).16 Hence the combinatorial requirement of receptor mutation and injury sets apart the underlying mechanism of HO in FOP from its nongenetic, trauma-induced counterpart.
ECTOPIC BONE DEVELOPMENT POST-TRAUMA: ENDOCHONDRAL OSSIFICATION, NOT TISSUE CALCIFICATION
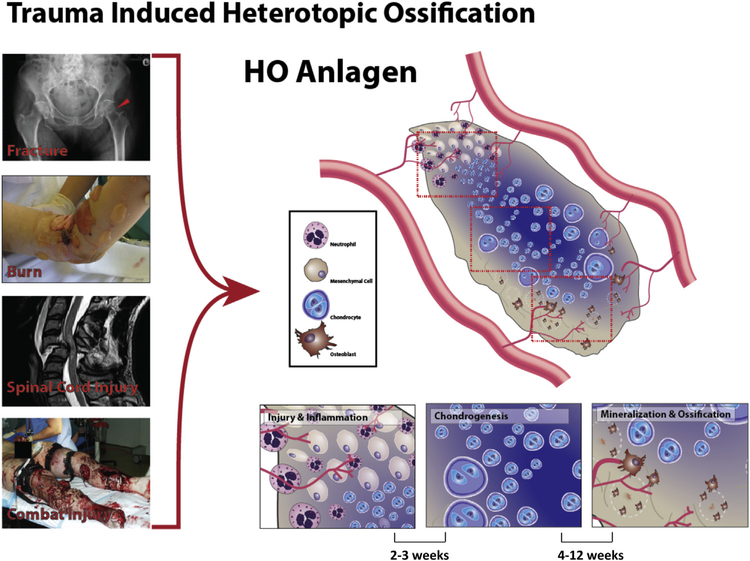
Despite the epidemiological and causative differences between the different forms of trauma-induced HO, there are striking similarities in the structure and characteristics of the ectopic bone formed in all the cases (Fig 1).
Fig 1.
A schematic representation of trauma-induced heterotopic ossification (HO). Multiple forms of trauma, like fractures, burn, spinal cord, and brain injuries and combat injury (left) result in heterotopic ossification. Ensuing the inflammatory phase post-trauma, HO occurs via the same underlying mechanism of endochondral ossification in all these cases.
Unlike tissue calcification, which occurs due to aberrant mineralization, resulting in calcium deposition, ectopic bone development after trauma follows the highly orchestrated mechanism of ‘endochondral ossification’, the phenomenon underlying embryonic development of long bones.17–20 Endochondral ossification refers to the process of formation of bone wherein a cartilage intermediate is formed and replaced by bone cells, typically seen in long bones with a central marrow element. This is in contrast to intramembranous ossification, where bone is produced from the direct conversion of mesenchymal tissue, without a cartilage intermediate as seen in craniofacial development.21
Endochondral ossification is initiated via deposition of a hyaline cartilage template by chondrocytes. Ossification of this cartilage template proceeds via 2 centers of ossification; the primary center being the middle of the cartilage shaft (diaphysis), and the second being the ends of the shaft (epiphysis). At these 2 sites, osteoclasts absorb the cartilage, endothelial cells invade, forming new blood vessels, bringing in osteoblasts which lay down a bony matrix, which forms primary trabecular bone. The outer layer of the cartilaginous matrix is replaced by bone, to form the periosteum, whereas osteoclasts breakdown the trabecular bone to form the medullary space or bone marrow cavity. This describes the progression of both embryonic long bone formation, as well as heterotopic ossification developed post-trauma.
In general, the cell fate decisions of mesenchymal stromal cells (MSCs) during bone regeneration are primarily driven by mechanical transduction mechanisms and environmental signals.22 Endochondral bone formation most often occurs external to the periosteum in areas that are mechanically less stable and adjacent to the fracture site, whereas intramembranous ossification occurs internal to the periosteum at the proximal and distal edges of the callus and forms hard callus.22,23 The mechanical stimulus/loading/strain and physical microenvironment (motion at the site of injury) associated with nonstabilized fractures promotes endochondral ossification whereas stabilized fracture healing primarily occurs via intramembranous ossification.24 Chondrocytes and osteoblasts initially secrete collagen matrices that calcify and bridge the fracture site resulting in callus formation, a process which drives the recruitment of cells from surrounding tissues for angiogenesis and remodeling,25 key components of bone repair. In addition to synthesis of random disorganized woven bone, osteoblasts synthesize and secrete into the bone matrix space a variety of bone matrix proteins, including collagen type 1 alpha 1, osteocalcin, osteopontin, alkaline phosphatase (ALP), fibronectin, and bone sialoprotein II.26 Overtime, the initial bony callus is revascularized and remodeled/reshaped, entailing multiple coupling cycles of bone deposition or production by osteoblasts and bone resorption by osteoclasts.27
DIAGNOSTICS: CT, RAMAN, NIR, ULTRASOUND
Early reliable and accurate diagnosis of HO is key to its management, prophylaxis, and treatment. Multiple radiographic and imaging modalities are currently used to diagnose HO post-trauma. Often, the initial symptomatic indicators of heterotopic ossification are those linked with aberrant wound healing, such as tissue ulceration, decreased range of motion, joint contractures, and pain. The choice of imaging modality used depends on multiple factors, such as the specific trauma in question, anatomic location of HO, swelling, extent of soft tissue involvement, and the HO burden.
Conventional radiography (X-ray) is typically the first choice to diagnose heterotopic ossification,28,29 followed by more sensitive nuclear bone scans for early detection. Ultrasonography is often used in spinal cord injuries, and post hip surgeries to detect heterotopic bone formation.30,31 Ultrasound can also indicate abnormalities in soft tissues before radiographic detection of bone,30 which serves to be a useful early diagnostic tool. However, distinguishing between nascent mineralization and mature mineralized bone in an ultrasound scan can be challenging.
Magnetic resonance imaging and computed tomography (CT) scans are other sensitive, high-resolution imaging modalities used to detect heterotopic bone formation.32–34 Magnetic resonance imaging can be confidently used for the diagnosis of exclusively mature HO, since the signal associated with early HO lesions is heterogeneous.35 Both contrast-enhanced and noncontrast CT scans are used to visualize HO, depending on the location of the ectopic bone and associated soft tissue inflammation.36,37 Although the sensitivity of micro-CT, rather than conventional CT scans, has been shown to be significantly higher in detecting early HO lesions, this may have questionable clinical utility.38,39 An emerging, high-resolution imaging technique, nano-computed tomography, is an advancement of micro-CT. Using special target transmission tubes and specific detectors, a very high spatial resolution (400 nm) can be achieved, thereby increasing its sensitivity several-fold as compared with micro-CT. Though currently being used primarily for preclinical laboratory-based studies,40 nano-CT has tremendous potential to transition to the clinic. Recent studies have demonstrated that combining MR and CT imaging greatly increases the sensitivity to distinguish early vs mature HO, infections and soft tissue inflammation.35,41 In addition, SPECT imaging modalities can be added to CT imaging to improve the assessment of sites of inflammation.42
Early diagnosis of post-traumatic heterotopic ossification is the key to timely intervention and prophylactic management of HO. Missing the early window of diagnosis results in progression of HO, which may lead to complications such as nerve impingement, joint contractures, pain, and limited range of motion. At later stages, surgical excision is the only option to remove heterotopic bone, which risks wound healing complications, delayed therapy, rehabilitation, as well as the risk of recurrence.43 Recent efforts in management of trauma-associated HO have therefore focused on diagnostics with high sensitivity and specificity for early detection of HO.
Currently, the most commonly used modality to visualize early HO is a 3-phase radionuclide bone imaging, originally developed more than 3 decades back.28,44 Using this scan, soft tissue swelling is detected after 3 weeks, and calcification is often seen a week later.44 Presence of excessive soft tissue inflammation, as seen in case of burns and combat-associated HO has been suggested to complicate diagnosis using this modality, resulting in false positives.45 A recent study reported the successful use of Raman spectroscopy ex vivo to detect very early HO lesions (,16 days post-trauma) in combat-wounded patients.46 The high sensitivity and specificity of noninvasive in vivo transcutaneous Raman spectroscopy was also demonstrated recently in a murine model of burn-induced HO.45 Using the same model, Perosky et al43 demonstrated the superior sensitivity of yet another novel imaging modality, the noninvasive ‘near infrared’ (NIR) fluorescent imaging to detect HO as early as 5 days post-trauma. Reflectance, which is a marker for vascularity and inflammation, has also recently been shown to be an option for further development.47
Hence diagnostic strategies to detect early heterotopic ossification are steadily progressing, either in terms of adding new imaging modalities or fine tuning and modifying existing techniques from other applications for early detection of HO.
Beyond imaging, serum, blood, or urine biomarkers would also allow for earlier detection and possibly prophylactic intervention into traumatic HO. Previous studies have demonstrated an increase in matrix metalloproteinase 9 (MMP9) and IL-3.48,49 However, given the amount of inflammation in high-energy wounds with or without HO, it is difficult to obtain a useful signal-to-noise ratio. Ongoing proteomic and genomic studies are attempting to further improve current technologies.
MODELS TO STUDY TRAUMA-INDUCED HO
Rat model of blast injury: blast injury, femur fracture, soft-tissue crush injury, and trans-femoral amputation through the zone of injury
Blast- and combat-related orthopedic injuries have the highest prevalence of HO among all types of trauma-induced heterotopic ossification, as stated earlier. In addition to the high prevalence, multiple aspects of blast injuries set it apart from civilian trauma. The zone of blast injury is often characterized by large open wounds, with dirt, debris, and possible infection. Each of these aspects and their combination contributes to the development of heterotopic ossification in combat-injured amputees. This unique nature of combat-related HO necessitated the development of animal models that closely resemble blast-injury–specific HO, since the existing models of HO either use bone morphogenetic proteins or genetic mutations to induce HO50,51; models that are far removed from the combat injury scenario.
Different groups have developed different methods to recreate a blast injury. One of the models utilized a manually assembled aluminum platform with a small orifice at the center, placed above a water-filled chamber. In this setup, a 0.75-g charge of pentaerythritol tetranitrate is placed at the bottom of the chamber and detonated using a commercially available detonation box, typically used for large-scale detonations. Tannous et al52 utilized this method in a rat model, to demonstrate that hindlimb amputation leads to significantly higher rates of HO than forelimb amputation. Utilizing the same procedure, Jaffe et al53 reported that the type of blast media (sand vs water) has no difference on the development or the severity of HO.
Other models utilize commercially available pneumatically driven shock tubes, where blast pressures can be regulated, timed, and quantified.54 Blast overpressure (BOP) is a critical parameter, typically encountered by combatants in the battlefield who are exposed to improvised explosive device explosions. Blast overpressure refers to the shock wave generated by pressures over and above normal atmospheric pressures and can range from a low of 30 KPa (4 psi) to a high of over 120 KPa (>15 psi).54
Such a pneumatically controlled BOP platform was utilized by Polfer et al55 to develop a rat model of trauma-induced HO. This model combines all the critical aspects of combat-mediated HO, namely blast injury, femoral fracture, crush injury, followed by trans-femoral amputation through the zone of injury. A BOP of 120 KPa (17 psi) is used, followed by creation of a midshaft femoral fracture using a commercially available drop weight apparatus, dropped from a specific height. This is immediately followed by rotating the fracture site between the 2 anvils of the support stage, in conjunction with application of a pressure of 20 psi for one minute, to simulate crush injury. Following this, a trans-femoral amputation is performed through the zone of injury.
Using this model, the authors demonstrated consistent HO in all animals subjected to BOP with amputation, HO in 66% animals with amputation alone, whereas none of the animals subjected to BOP alone developed HO. This is currently the only model which closely simulates all the aspects of a combat injury. Utilizing this model, Qureshi et al56 demonstrated the upregulation of chondrogenic and osteogenic genes in the injured soft tissue, during early phases post injury, thereby mapping the kinetics of ectopic endochondral bone development post combat injury. The same model was utilized by Pavey et al57 to explore the effect of early bacterial infection of traumatized muscle on HO development post amputation. Inoculation of injured rats with a strain of methicillin-resistant Staphylococcus aureus (MRSA) commonly found in combat wound infections, resulted in persistent colonization of the wound and bone marrow, with a significant increase in the volume of ectopic bone. This is consistent with the idea that increasing inflammation at the site of a wound increases the risk of HO. After incorporating MRSA infection post amputation in this model, the same group recently demonstrated the efficacy of a retinoic acid receptor-ɣ (RAR-ɣ) agonist, palovarotene, in inhibiting HO formation.58 Further the same group demonstrated that early vancomycin targeted antimicrobial therapy with intrawound application of vancomycin powder achieved complete eradication of infecting organisms and significantly attenuated ectopic formation59
This small animal model is the closest replication of blast-injury–induced heterotopic ossification so far. However, the model has its own limitations, in that the animals are subjected to sequential injury patterns, whereas all these injuries occur simultaneously in the battlefield. Second, limb amputation and definitive wound closure occurs shortly after injury in this model, whereas this happens at a much later stage in clinical setting.
Burn/tenotomy model of HO
Heterotopic ossification is an uncommon complication (1–3%) of burn injuries, when compared with its prevalence in the combat-injured group.60 However, this frequency goes above 50% in extensive third-degree burns.45 In the ‘burned mouse model’ typically used to simulate a burn injury, a mouse is placed in a well-insulated custom mold, such that ~30% of depilated dorsal skin is exposed to a hot water bath at 60°C for 15–20 seconds. This results in a partial thickness scald burn injury.45,61 Sterile dry gauze is used for wound debridement, and animals are resuscitated by intraperitoneal and subcutaneous administration of Ringer’s Lactate solution. After wound drying, a sterile dressing is applied to prevent wound infection. Utilizing this model, Peterson et al18 demonstrated that mesenchymal stem cells, embedded within a subcutaneously implanted collagen scaffold, resulted in significant increase in bone formation when subjected to burn injury.
Heterotopic ossification associated with burns is commonly seen to occur around joints and at sites with a large number of scleraxis-expressing cells.60,62,63 Simulation of musculoskeletal trauma in the burned mouse model is created by Achilles tendon transection (Achilles tenotomy) concomitant to a burn injury.45 In the tenotomy model, an incision is made lateral to the Achilles tendon, followed by creating a sharp transection at the midpoint, between the origin of the Achilles at the distal end of gastrocnemius and its insertion on the calcaneus. Achilles tendinopathies and tendon trauma alone often result in endochondral HO.64 However, the extent of HO in the tenotomy model, post burn was significantly higher, indicating to the role of burn injury–mediated systemic inflammation on HO.61 This bone develops with similar stages to human HO with the early inflammation and hypoxia followed by mesenchymal condensation, chondrogenesis, and subsequent osteogenesis.65,66 In addition, as in human patients, excision of HO leads to recurrence within the wound bed.67
Both combat and burn injury models simulate injury of the skeletal muscle and/or connective tissue (tendons), in addition to generation of systemic inflammation. However, considering the multiple, simultaneous impact injuries, amputation and infection involved in the combat injury model, the extent and dynamics of local and systemic inflammation in this model can be speculated to be starkly different from the burn-tenotomy injury model. It would be interesting to identify the differential acute and chronic inflammatory players in these 2 models, to understand the trauma-specific correlate of HO.
Spinal cord injury model
Patients with severe traumatic brain or spinal cord injury, with or without limb involvement, have been shown to develop HO,68–70 often referred to as ‘neurological HO’. Interestingly, the majority of neurological HO was periarticular, frequently at the hip, knee, elbow, and shoulder.71–73 Multiple risk factors for neurological HO were reported in studies of independent patient cohorts; namely injury severity, time lapsed from the injury, extent of autonomic dysregulation, and spasticity.68,72
Despite patient data, understanding of the molecular mechanisms of neurological HO is sparse, due to absence of relevant HO-specific animal models of spinal cord injury (SCI) or traumatic brain injury, till very recently. Kang et al reported the first mouse model of SCI-induced HO, which also required a subthreshold dose of bone morphogenetic protein 2 (BMP-2) in the muscle of mice, in addition to SCI.74 In this model, SCI is simulated by generating a spinal cord contusion after subjecting the mice through a dorsal midthoracic laminectomy. A 35 gm stainless steel rod is dropped on the exposed spinal cord at the thoracic (T)-10 level, from a height of 50 mm, with a penetrating depth of 1.8 mm, resulting in complete paraplegia. In this study, SCI was followed by injection of BMP-2 mixed in a heparin-chitosan hydrogel, into the quadriceps.
Recently, a more physiological SCI mouse model was reported by Genêt et al73 by combining SCI and intramuscular injury. SCI was created by transection of the spinal cord between T7-T8 vertebrae, following which the right hamstring muscle was injected with the snake venom cardiotoxin to generate acute inflammation, whereas the contralateral muscle was left uninjured. Micro-CT imaging clearly demonstrated the formation of mature HO with marrow elements, exclusively in the injured limb. Detailed histological analysis revealed homing and infiltration of macrophages around the HO.
BMP scaffold implantation model
The ligands of the BMP signaling pathway, BMPs-2 and 4 have been known to induce bone formation for several decades.75,76 The majority of studies have traditionally utilized this fact to recreate HO in vivo77–79 or dissect mechanistic basis of HO in vitro.18,80 Bone morphogenetic protein 2 is the most commonly used ligand to induce ectopic bone formation and is typically mixed with a ‘scaffold’, which can be either a resorbable synthetic polysaccharide-based hydrogels,81 or a biological matrix, like collagen or the commercially available extracellular matrix complex matrigel.77
Since the presence of BMP-2 invariably gives rise to bone formation at its site of action, use of such BMP scaffolds is common to test ectopic osteogenesis. However, the physiological relevance of this model is questionable, since most cases of genetic and trauma-induced HO is not known to be caused by local induction of a high concentration of BMP-2. Hence, in trauma-induced HO, BMP scaffolds might serve as ideal ‘positive controls’ to induce HO, in conjunction with specific trauma models to test HO.
POTENTIAL CAUSES AND MECHANISM
The development of normal healthy skeletal bone, a mineralized and vascularized tissue, is a complex and highly orchestrated physiological process. Bone healing and regeneration, akin to wound healing of any other tissue, involves hematoma formation, tissue inflammation, MSC recruitment, skeletal tissue regeneration, extracellular bone matrix accumulation, angiogenesis, and bone remodeling.82 Bone marrow and local tissue resident inflammatory macrophages likely contribute to the early inflammatory response. Bone marrow MSCs and tissue-derived MSCs contribute to tissue repair and regeneration through tightly controlled interactions involving paracrine effects and direct differentiation of osteoblasts, chondrocytes, fibroblasts, myoblasts, or adipocytes, depending on stimuli present in their microenvironment.83
Correlation between wound healing complications and HO
Bone fractures heal by 2 distinct reparative pathways, endochondral and intramembraneous ossification. In both the processes, MSCs, nonhematopoietic cells, home and migrate to the site of injured tissue in response to a gradient of complex signaling cascades involving the local release of growth factors and inflammatory cytokines/mediators.84 Once mobilized and homed to the site of injury, a variety of molecules (chemokines, adhesion molecules, and matrix degrading enzymes/inhibitors) regulate and facilitate extracellular movement of undifferentiated MSCs within the injured tissue. These recruited MSCs give rise to either mesenchymal osteoblasts, which directly form bone (intramembranous ossification) or to osteochondroprogenitors, which form an intermediate cartilaginous template, which is subsequently replaced by bone forming cells (endochondral ossification).22,27 These early dynamic developmental pathways of MSC proliferation and differentiation are governed by the master transcriptional regulators Runx2 (Cbfa1) and Sox9, respectively.
A common link among all trauma-induced heterotopic ossification is the presence of tissue injury and inflammation at the onset of trauma. Intimate involvement of the immune system has been reported, at least in the early stages of trauma-induced HO,85–88 indicating a common etiology of ectopic ossification. In fact a strong acute inflammatory response (flare-ups), mediated by minor injuries and vaccinations is also associated with the genetic form of HO, FOP.85,89
Cytokine profiling of wound effluents and serum collected from high-energy combat injury patients, before definitive wound closure found a strong association between impaired wound healing and development of HO.87 Interleukins (IL-6, IL-10) and MCP-1 in serum, and IP-10 and MIP1α in wound effluent were individually found to strongly correlate with the development of HO. A follow-up study by the same group with a larger patient population reported cytokine profiles which independently predicted HO (serum IL-3, IL-12p70 and effluent IL-3 and IL-13) or failure of wound healing (serum procalcitonin and effluent IL-6).48
An earlier study of myositis ossificans demonstrated that muscle injury, followed by necrosis results in a steady infiltration of macrophages within the necrotic muscle, before the initiation of calcification.90 This suggested that muscle injury and necrosis triggers the release of pro-osteogenic factors by homing inflammatory cells. Using a recently developed mouse model of neurological HO, Genêt et al73 demonstrated that muscle-derived mesenchymal stem cells could undergo osteogenic differentiation in the presence of serum from mice subjected to spinal cord coupled with muscle injury, and that ablation of phagocytic macrophages in the injured mice resulted in a 90% reduction of HO. These observations strongly suggests that muscle injury and necrosis trigger the release of pro-osteogenic factors by tissue-resident, and homing macrophages, thereby triggering HO. Interestingly, the same cytokines and immune cells which promote HO in trauma-induced acute inflammation, trigger bone loss during chronic inflammatory conditions,91,92 indicating to the pleiotropic effects of immune factors over time.
Innate vs adaptive immune system in trauma-induced HO
While the role of innate immunity in promoting HO via macrophages, post-trauma has been extensively demonstrated, as described in the previous section, changes in lymphocyte signaling have also been documented, especially in FOP. One study reported dysregulation of the noncanonical BMP-p38 MAPK pathway in FOP lymphocytes,5 and the same group also demonstrated the upregulation of the BMP-4 ligand in lymphoid cells isolated from FOP patients.93 Recent studies have also analyzed the role of the adaptive immune system in a trauma model of HO.94 Burn, followed by Achilles tenotomy injury, was used to induce HO in Rag1 knockout mice, deficient in both T and B lymphocytes. The authors observed 60% less HO formation in the Rag1 KO mice compared with wild-type controls. In addition, there was decrease in the osteogenic potential of MSCs isolated from these immunocompromised mice, the HO formed was immature, with foci of cartilage and disorganized trabecular bone. Interestingly, osteoclast number and activation remained unchanged in these animals. In addition to cellular adaptive immunity, lymphatic drainage has also been shown to play a central role in trauma-induced HO.95 Using the burn-tenotomy model, the authors demonstrated that removing the inguinal and popliteal lymph nodes, ipsilateral to the tenotomy site resulted in significant decrease in the volume of HO at the injury site, in addition to a reduction in frequency of CD105+ αV-Integrin+Tie2−CD45−CD90−BP1− osteogenic progenitor cells.
Most of the reports on role of immune cells in HO are in the context of the genetic disorder, FOP. With respect to innate immune cells, one such study demonstrates the involvement of mast cells from early stages of FOP, resulting in more than a 40-fold increase in their numbers in the fibroproliferative zone of FOP patients than in normal, uninjured muscle.96 Another strong link of mast cells with HO comes from the study of Salisbury et al,88 who demonstrated the role of neurogenic inflammatory mediators (Substance-P and calcitonin gene–related peptide) in mediating mast cell degranulation, such that inhibiting degranulation with cromolyn significantly reduced HO. It would be worthwhile to study the role and dynamics of mast cells in other forms of trauma-induced HO, as well as explore the involvement of other innate and adaptive immune cells in traumainduced, nongenetic forms of HO. A recent study testing the effect of a BMP Type I receptor inhibitor in the burn/tenotomy model reported reduction in neutrophil and macrophage frequency both in the soft tissue at the tenotomy site, as well as significant reduction in the frequency of Ly6G+CD11b+ neutrophils in the bone marrow.
Infection and HO
Infection is a common risk factor associated with distinct trauma–induced HO.57,97,98 Using the well-established rat model of high-energy combat injury, Pavey et al57 recently demonstrated a significantly higher volume of ectopic bone formation at the amputation site with the early colonization of MRSA. Interestingly, MRSA is also known to be the predominant pathogen in severe burn wound infections,99 where HO is a common occurrence. In a prospective study of patients with neurogenic HO, urinary tract infection was found to be a major risk factor for HO development.98
Acute or chronic infection, either systemic or locally results in a heightened innate and adaptive immune response, which in turn is a driver of HO. In fact a recent study elegantly demonstrated that the extent of the traumatic injury often dictates the downstream pathogenesis and the extent of HO. While MRSA infection of a 5% sub-acute total body surface area burn resulted in the enrichment of M1 macrophages (induce proinflammatory responses) and no sepsis in the animals, a severe (>15%) total body surface area burn resulted in isolation of predominantly M2b macrophages (CCL1+IL-10+IL-12−; induce anti-inflammatory responses), with 100% mortality following the same MRSA infection. Polarizing the M2b macrophages with CCL-1 antisense oligodeoxynucleotide resulted in enrichment of M1 macrophages, and attenuation of infection and subsequent inflammation,100 indicating the potential role of macrophages in infection-mediated HO.
Vascularization and HO
Development of long bones via endochondral ossification is intricately linked to neovascularization within primary and secondary ossification centers. In physiological bone development, vascularization is a critical link between chondrocyte maturation and osteoblast recruitment to ossification centers. Hypoxia-driven HIF1α and VEGF signaling pathways within avascular growth plates are critical for survival and growth of chondrocytes, as well as stimulation of neo-angiogenesis in the surrounding region, which seeds the circulating cells and factors for osteogenesis.101 In addition, factors from the bone marrow, like TGF-β, PDGF, IL1-β and thyroid hormone (T3) have been shown to interact with osteoblast-specific transcription factor, Cbfa1/Runx2 in nascent cartilage to induce vascularization, followed by endochondral ossification of the growth plate.102
Mice lacking various isoforms of VEGF display impaired angiogenesis and endochondral ossification.103 Ortega et al104 demonstrated intricate orchestration between VEGF-mediated vascularization of hypertrophic cartilage, MMP-9–mediated extracellular matrix remodeling and osteoclast recruitment during the physiological process of long bone development. Earlier studies have demonstrated aberrant vascularization and hypertrophic chondrocyte (HC) ossification at the growth plate in MMP9−/− mice.105 Using this mouse model, the authors showed an increase in both transcription and translation of VEGFA in late hypertrophic chondrocytes during endochondral ossification in these mice, though the release of the VEGFA protein from the HC matrix, and its subsequent activity was comparable to wild-type mice. Analysis of the extracellular matrix revealed that MMP9 deficiency affected the HC ECM structure, and thereby its degradation, resulting in low bioavailability of the VEGA protein for endothelial cell homing. In addition to impairing vascularization, VEGF deficiency also resulted in the accumulation of TRAP+ osteoclasts at the chondroosseous junction. Exogenous treatment with VEGFA resulted in improved vascularization, normalization of osteoblast number, and restored normal ossification. A similar study demonstrated the requirement of MMP-13 in the conversion of growth plate cartilage to endochondral bone,106 possibly via similar interactions with hypoxia-induced growth factors. The same hypoxia-driven pathways are essential during the initial phase of wound healing after soft tissue trauma.107,108 The link between soft tissue trauma, hypoxia and ectopic bone formation was unraveled by Agarwal et al66 in a study of trauma-induced HO in a burn-tenotomy model and in genetic models of HO, where they demonstrated a marked decrease in heterotopic ossification by pharmacological or lineage-specific genetic inhibition of HIF1α.
Neurological component of HO
The periosteal bone surface is known to be covered by primary sensory and sympathetic axons. These are present in highest density in mineralized matrix, in regions of highest turnover, and innervate the marrow cavity.109 Chemical destruction of sensory nerves in a mouse model resulted in reduced bone volume,110 and sciatic nerve resection in rats was shown to impair longitudinal bone growth and fracture healing.111 Similar findings were noted in human subjects, where there was abnormal or delayed skeletal repair in patients with poor nerve function.112 These studies clearly demonstrate an intricate relation between skeletal nerve function and bone growth.
As mentioned earlier, using a BMP-2-mediated HO model, Salisbury et al88 demonstrated sustained neurogenic inflammation, mediated by sensory nerve-associated mast cells, in response to the pain mediators, Substance-P and calcitonin gene–related peptide during the progression of HO. Furthermore, in TRPV1−/− animals lacking functional sensory neurons, or by administering inhibitors that block binding of pain mediators to their receptors, HO was significantly diminished, in the presence of BMP-2. The same group recently demonstrated that a subpopulation of endoneurium-resident claudin + cells, which also express PDGFRα, mushashi 1, p75 and Tie2, mark early osteoprogenitors in the BMP-2–induced HO model.113 It was shown that these cells migrate into circulation as early as 24 hours post BMP injection via the endoneurial vessels, and extravasate to the site of HO. This study indicates a direct contribution of neurons in the pathogenesis of HO. It would be interesting to explore if this mechanism holds true in the different forms of trauma-induced HO as well.
Most developing tissues regulate their extent of innervation by secreting neurotrophins, growth factors which activate tyrosine kinase receptors, thereby promoting the survival of neurons.114 Interestingly, a large majority of the sensory neurons in mature bone express the tyrosine kinase receptor type 1 (TrkA receptor), which has high affinity for the neurotrophin, nerve growth factor (NGF).115 Using the TrkA-LacZ mice, in which one Trka allele is replaced with LacZ, the authors observed LacZ+ axons innervating the hindlimbs via the lumbar plexus, and terminating in the perichondrial region of the femur, around embryonic day (ED) 14.5, when endochondral ossification ensues from the cartilaginous matrix.116 Using the LacZ and Thy1-YFP mice models, time-course analysis showed progressive increase in density of these axons, and by postnatal day 0, labeled nerve projections had covered the entire mineralizing bone collar, ending at the growth plate. Using a NGF-EGFP reporter mice, this group observed simultaneous expression of NGF in small groups of perichondrial cells, around ED14.5, along with the appearance of osterix-expressing chondrocytes adjacent to the NGF expressing cells. These steps preceded the vascularization of the primary ossification center. In addition expression of NGF was the highest in immature chondrocytes and osteoblasts, its levels decreasing along endochondral differentiation pathway. Inhibition of TrkA signaling was shown to attenuate innervation, vascularization, and primary ossification, indicating the involvement and importance of nerve growth factor signaling in trauma-induced HO. Considering the intricate crosstalk between nerve function and inflammation, it would be worthwhile exploring the status of nerve growth factors and their receptors in HO occurring post injury.
Stem cells responsible for HO
Much work has been done to elucidate the cells responsible for traumatic HO. Many different models have been used and the recent emergence of Cre lineage tracing systems has allowed for lineage tracing experiments. The Cre lineage systems can be difficult to interpret given that trauma can activate gene expression rendering it impossible to differentiate active expression from the original lineage. In addition, when new Cre drivers are described, their full tissue distribution may not have been defined. Inducible Cre systems (CreER) have gained increasing use for lineage marking as they allow for marking of a specific lineage in the adult animal which can be done both before and after the injury. Early work proposed vascular cells as the progenitor cells for HO117; however, recent reports indicate that endothelial to mesenchymal transition, though present, does not cause the majority of HO in trauma.65 These earlier studies used a Tie2 lineage marker which was thought to only define endothelial cells but have since also been shown to exist in inflammatory cells. Similarly Ve-Cadherin-Cre which marks endothelial cells can also mark chondrocytes which makes Ve-CadCreER a better system to track these cells. In addition, bone-chondro-stromal progenitor cells which are the cell lineage responsible for bone development do not compose the majority of developing HO tissues.118 Recent studies have revealed that the predominant cell types underlying the initiation and progression of traumatic and genetic HO comprise of connective tissue-resident progenitor cells, marked by either Prx,66 Scleraxis14,62 or Mx1.14 Prx is more widely distributed than Scleraxis, however, both mark connective tissue cells including perimysium and peritenon. Both also do not mark endothelial cells, myocytes, or cells of the hematopoietic lineage. This is consistent with what is seen clinically, as HO forms along fascial planes, in perimysium and at sites of entheses. These reports also demonstrate the differential tissue-specific responsiveness to injury and predisposition to HO. Tendons, which are highly enriched in scleraxis+ cells, are prone to HO even in the absence of injury, whereas intramuscular HO appears to be highly injury dependent.14 Whether the different lineages mark the same mesenchymal cell type in different tissues, or represent distinct progenitors, needs to be explored.
Role of BMP receptors and ligands in trauma-induced HO
Bone morphogenetic proteins (BMPs), members of the TGF-β superfamily, are the classical osteoinductive growth factors, which bind to transmembrane serine/threonine kinase receptors, and mediate signaling through Smad 1/5/8-dependent or Smad-independent mechanisms.119 The ligands BMP 2 and 4 have been routinely injected in animal models to create an osteo-permissive tissue environment, to study the mechanisms of ossification. Over the last few years, presence of BMPs and its downstream targets have been demonstrated in multiple trauma-induced HO lesions.120–124 Levels of BMP-1 and 4, TGF-β1, cartilage oligomeric matrix protein, GDF-10, and integrin-β2 were found upregulated in early fibroproliferative lesions in traumatized muscle.121,122 High levels of BMP-9 within mature HO lesion were reported in a patient with traumatic lower extremity HO.124 A study of combat injuries demonstrated significant upregulation of multiple osteogenic transcripts (BMP-2, SMAD1, ALPL, among others) in wounds with HO than those without HO.123 Put together, these studies demonstrate the presence of an osteoinductive microenvironment post trauma, which triggers onset of HO.
TREATMENT AND PROPHYLAXIS
Treatment
Surgical excision of the heterotopic bone has been the primary treatment modality for trauma-induced HO.125 Surgery must address not only the ectopic bone but also the joint and surrounding ligaments that are often contracted in the affected limb. However, the timing and efficacy of surgical excision have been controversial due to the issue of recurrence post HO excision.67,126 The extent of recurrence appears to be trauma-specific. For example, there are more reports of recurrence of HO associated with TBI67,127 than with blast injury.126,128 Traditionally, surgical intervention would be postponed for almost a year, with the belief that this delay prevents HO recurrence.129 Analysis of HO recurrence cases among traumatic brain injury patients revealed that early excision of HO did not necessarily result in later recurrence.130,131 In case of blast injury–induced HO, detailed retrospective analysis of patient data demonstrated that recurrence requiring re-excision was common when there was partial excision of the initial HO,128 and had no correlation with postoperative prophylaxis, grade of HO, or the experience of surgeons performing the excision. Also, they reported no correlation between the timing of primary HO excision and the risk of recurrence. Another retrospective study of patients with trauma-induced axial HO reported that early excision of HO is effective, resulting in low recurrence.129 Hence, despite being the mainstay approach in treatment of trauma-induced HO, excision of the heterotopic bone may or may not result in complete abrogation of HO, with a finite chance of recurrence over long term. The mechanism(s) or underlying reason(s) for recurrence following excision is not known, but multiple prophylactic approaches have been recently discovered to minimize or eliminate recurrence of HO.
Prophylactic approaches
Localized radiotherapy (RT) has been a prevalent mode of prophylaxis against HO. Radiotherapy targets osteoprogenitors underlying ectopic bone formation in the soft tissue. Radiation is typically administered 1–5 days postoperatively,132 owing to postoperative stabilization of the patient. Traditionally, multiple cycles of low-dose (2 Gy) radiation was administered over an extended period, to a total of 10–20 Gy. However, recent studies have shown the efficacy of a single dose of 6–8 Gy, administered at energies between 6 and 15 MV.133,134 Prophylactic radiation also reduces the recurrence of HO after resection of heterotopic bone, as has been extensively demonstrated in case of knee replacement surgeries.135 Despite the predominance of RT use toward HO prophylaxis, there are side-effects associated with its use, such as, increase in bony nonunions, and radiation-induced sarcoma.136
Anti-inflammatory drugs, specifically corticosteroids (prednisone, dexamethasone) and NSAIDs (Eg: aspirin, indomethacin, COX-2 specific inhibitor, celecoxib) are popular prophylactic agents against HO.137 While corticosteroids inhibit production of proinflammatory factors involved in chronic inflammation by blocking their transcription,138 NSAIDs target proinflammatory prostaglandins, which are known to potently stimulate bone formation, bone mass, and bone strength as well as heterotopic bone formation,139 and thereby limit osteogenic differentiation of progenitors.125 Clinically, most of these drugs have comparable inhibitory effect on HO, though they are limited by their side effects, such as gastric bleeding.140 However, head-to-head comparison of the broad COX inhibitor, indomethacin, and the COX-2inhibitor, celecoxib revealed significantly higher side effects of indomethacin compared with celecoxib.141 The timing of treatment also appears to be crucial in mediating specific inhibition of HO. NSAIDs and corticosteroids typically need to be administered immediately after the trauma to inhibit the development of HO. However, once formation of ectopic bone has initiated, these drugs are not effective in blocking HO.142 Despite their strong potential to block the first step of trauma-induced HO (inflammation), their use is limited due to severe adverse side effects of these drugs125,143 and in cases of fractures, the potential risk of fracture non-union.
Bisphosphonates are often the drug of choice for later stages of HO, when heterotopic bone formation has already begun.142 Bisphosphonates inhibit HO by inducing apoptosis of osteoclasts, thereby reducing calcification.144 The most commonly used bisphosphonate is disodium editronate and the effectiveness of this drug has been extensively observed in neurological HO,142,145 in addition to burn injury-induced HO.146 In this case as well, the timing of drug administration appears to be crucial, where it is most effective when administered in the early stages of HO development.142
BMP pathway inhibitors comprise another group of small molecules, targeting the cartilage and bone forming stages of HO.51,147,148 These inhibitors include the BMP inhibitor Noggin and BMP type I receptor inhibitors, which inhibit receptor dimerization and thereby Smad-dependent downstream signaling.149 Though these inhibitors show tremendous potential in blocking both early- and late-stage HO, adverse effects arising from nonspecific targeting of BMP signaling might be a concern, since BMP receptors are ubiquitous, and utilized by every organ system.
RARγ agonist (palovarotene), an activator of nuclear retinoic acid receptor γ was recently reported as a very promising small molecule inhibitor of both genetic and trauma-induced HO.12,150 Since chondrogenesis requires decrease in the levels of RAR, use of an RAR agonist inhibits chondrogenesis, and therefore heterotopic endochondral ossification.151 On the contrary, inhibition of RAR was shown to promote endochondral ossification.79 Utilizing the blast injury model in rats, Pavey et al58 reported the mitigation of blast injury-induced heterotopic ossification on treatment with the RARγ agonist, palovarotene. However, use of this therapeutic also led to substantial wound healing complications in a contaminated wound model leaving concern of its use postoperatively or in burn patients. Using the subcutaneous BMP-2 implantation model, a very recent study demonstrated that a combination of palovarotene and corticosteroids effectively inhibited HO by both distinct and common mechanisms. Both drugs led to the inhibition of NFκB and reduction in the number of mast cells and macrophages at the affected site.50
Other prophylactic means have been recently reported to inhibit trauma-induced HO. One of them is systemic administration of hydroxyethyl starch, which results in the disruption of the hypoxic microenvironment of the affected area by enhanced microcirculation,152 thereby inhibiting HO. Pulse low-intensity electromagnetic field therapy is based on the same principle as hydroxyethyl starch, whereby magnetic field is used to increase blood flow to the injured area, thereby decreasing hypoxia and toxic by-products post injury.142 The efficacy of pulse low-intensity electromagnetic field therapy was shown in preventing HO post spinal cord injury.
HIF1α and mTOR-signaling pathway inhibitors
Given the importance of early hypoxic signaling in chondrogenesis and eliciting inflammation, which precedes traumatic HO, HIF1α targeted therapies offer potential for early HO prevention. HIF1α is an appealing target as it is central to inflammatory cell migration, mesenchymal condensation, and chondrogenesis. In fact a recent study demonstrated that inhibition of the HIF1α pathway by PX-478 or rapamycin successfully abrogated both genetic and burn/tenotomy-induced heterotopic ossification.66
The current in-clinic or investigational therapies reported for HO have been listed in Table I. All current prophylactic modalities, though effective, have several important off-target effects. These off-target effects are especially important in the setting of a wound or post-surgical field as therapies that decrease inflammation may delay wound healing. Thus, the timing of treatment is as important as the mechanism of action. Ideally, a treatment would be able to be administered for a brief time course thus minimizing off target effects and delayed wound healing.
Table I.
Summary of current in-clinic or investigational therapies for trauma-induced heterotopic ossification
| S.No. | Therapy | In-clinic/ investigational | Treatment (T)/ prophylactic (P) | Timing of administration | Side effects/risks | Reference |
|---|---|---|---|---|---|---|
| 1 | Surgical excision | In-clinic | T | Early and late HO | Recurrence | 125–127 |
| 2 | Localized radiotherapy (RT) | In-clinic | T, P | Early (POD 1–5) | Bony nonunions, radiation-induced sarcomas | 125–135 |
| 3 | Corticosteroids | In-clinic (prednisone, dexamethasone) | P | Early (immediately after trauma) | Bony nonunions | 138,139 |
| 4 | NSAIDs | In-clinic (indomethacin, celecoxib) | P | Early (immediately after trauma) | Bony nonunions, gastric bleeding | 140–143 |
| 5 | Bisphosphonate | In-clinic | T | Early HO | Upper GI effects, hypocalcemia | 143,145 |
| 6 | BMP pathway inhibitors | Investigational | P | Early (immediately after trauma) | Some inhibitors myelosuppressive in trauma-induced animal models | 148–150 |
| 7 | RARg agonist (palovarotene) | Investigational | P | Early (immediately after trauma) | Wound healing complications in trauma-induced animal models | 151,152 |
| 8 | mTOR pathway inhibitors | Investigational | P | Early (immediately after trauma) | 66 | |
| 9 | HES (hydroxyethyl starch) | Investigational | P | Early | 152 | |
| 10 | Pulsed low-intensity electromagnetic field (PLIMF) | Investigational | P | Early | 143 |
Abbreviations: BMP, bone morphogenetic protein; NSAIDs, nonsteroidal anti-inflammatory drugs; POD, postoperative day.
CONCLUSION
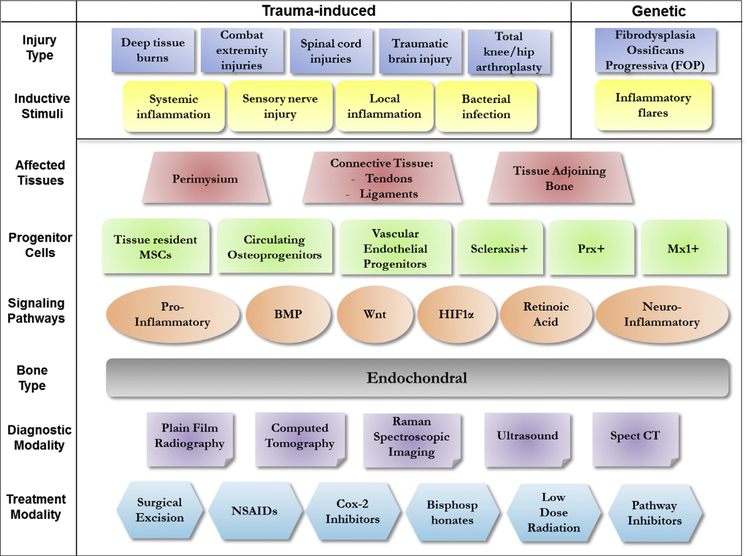
In summary, formation of extraskeletal bone is a common occurrence after extensive soft tissue injuries, like those seen in knee and hip replacement surgeries, third-degree burns, spinal cord and brain injuries, and in severely wounded service members with extremity amputations. Over the last few years, multiple animal models have been developed to study each form of trauma-induced HO in its unique settings. Despite vast differences in the initiating trauma, the fundamental mechanism of ectopic bone formation is similar for all injuries, involving inflammation at the site of injury, followed by vascularization, infection in some cases, nerve innervation, chondrogenesis, and finally culminating in osteogenic differentiation of tissue-resident progenitors (Fig 2). This common mechanistic basis of trauma-induced HO, also closely matches the genetic form of HO, FOP. This has helped develop at the preclinical level, multiple prophylactic and treatment modalities. With extensive crosstalk and ongoing collaborations among research groups working on distinct trauma-driven complications, there is tremendous hope for effective treatment(s) for patients suffering from HO.
Fig 2.
A summary of the 2 different forms of heterotopic ossification, trauma-induced and genetic HO. Multiple injury types (trauma) result in local or systemic stimuli that induce HO. The tissues predominantly susceptible to HO development include tendons, ligaments, and skeletal muscle, which is in close association with long bones. Despite the multiple progenitor cells and signaling pathways underlying initiation/development of HO, the ectopic bone is universally ‘endochondral’ in nature. In recent years, several diagnostic and treatment modalities have been developed for the management of HO.
Despite this significant progress, several knowledge gaps remain in this field. Trauma or injury is the primary trigger of HO. However, very little is known about the immune cell kinetics of HO, systemically and at the site of injury. A detailed characterization of the local and systemic signaling inflammatory-chondro-osteo-angiogenic response and immune cell changes occurring immediately after a trauma, and their impact on the development of mature ectopic bone is critical to develop effective interventions at early stages of HO initiation. These kinetics might also be different for every trauma, which in turn will dictate fine tuning of targeting strategies. Second, the origin and identity of progenitor cells underlying ectopic endochondral ossification is still a controversy. Considering the involvement of simultaneous processes during ectopic ossification (tissue remodeling, matrix formation, angiogenesis, innervation, mineral deposition), all being carried out by distinct cell types, it is possible that HO formation requires the participation of multiple progenitors, instead of a master progenitor. Here again, understanding the timing of activation of individual progenitor populations would give important insights into timeline of targeting each critical process leading to HO.
As Table I summarizes, multiple treatment and prophylactic measures targeting HO are currently in clinic. However, each of them suffers from serious limitations, reducing their effective/extensive use. There is need of exploring the clinical efficacy of the multiple investigational therapies, which have shown very promising results in animal studies, or for a few like RARγ agonists, as far as in phase I clinical trials. Another important aspect is the occurrence of bone fractures in these complex trauma cases, which often complicate specific targeting of HO, since mechanisms of fracture healing and HO development closely overlap. Determining the fine molecular differences between these two processes would greatly enable development of HO-specific therapies, without interfering with fracture healing. Put together, significant research on the cellular and molecular kinetics of trauma-induced HO is needed to develop a standard, effective, and sustainable prophylactic or treatment regimen. On the clinical front, much work needs to be done at determining the effective timing, dose, and combination of available therapeutic/prophylactic regimens to be administered to manage HO, without aberrantly altering normal wound healing.
ACKNOWLEDGMENTS
Conflicts of Interest: B.L. collaborates with Boehringer Ingleheim on a project not discussed here. We have filed a patent application for the use of Rapamycin in heterotopic ossification. IP has not yet been licensed. This work was supported in part by CDMRP grant W81XWH-14-DMRDP-CRMRP-NMSIRA 76. S.A. funded by NIH F32 AR066499, NIH Loan Repayment Program; S.J.L. and J.D. funded by Howard Hughes Medical Institute (HHMI) Medical Fellows Program; B.L. funded by DOD: W81XWH-14-DMRDPCRMRP-NMSIRA, NIH, NIGMS K08GM109105, Plastic Surgery Foundation National Endowment Award, American Association of Plastic Surgery Research Fellowship, Plastic Surgery Foundation/AAPS Pilot Research Award, ACS Clowes Award, International Fibrodysplasia Ossificans Progressiva Association Research Award, AAS Roslyn Award.
Some of the authors are the employees of the U.S. government. This work was prepared as part of their official duties. Title 17 USC. §105 provides that “Copyright protection under this title is not available for any work of the United States government.” Title 17 USC. §101 defined a U.S. government work as a work prepared by a military service member or employees of the U.S. government as part of that person’s official duties. The opinions or assertions contained herein are the private ones of the author/speaker and are not to be construed as official or reflecting the views of the Department of Defense, the Uniformed Services University of the Health Sciences or any other agency of the U.S. Government.
Abbreviations
- ACVR
activin A receptor
- ALK
activin receptor-like kinase
- BMP
bone morphogenetic protein
- BOP
blast over pressure
- COX
cyclo-oxygenase
- CT
computed tomography
- ECM
extracellular matrix
- FOP
fibrodysplasia ossificans progressive
- HES
hydroxy-ethyl starch
- HIF
hypoxia inducible factor
- HO
heterotopic ossification
- IED
improvised explosive device
- KO
knock-out
- MRSA
methicillin-resistant Staphylococcus aureus
- MSC
mesenchymal stem cells
- mTOR
mammalian target of rapamycin
- NSAID
non-steroidal anti-inflammatory drug
- PLIMF
pulsed low intensity electromagnetic field
- RAR
retinoic acid receptor
- RT
radiotherapy
- SCI
spinal cord injury
- SPECT
single photon emission computed tomography
- TBSA
total body surface area
- VEGF
vascular endothelial growth factor
Footnotes
All authors have read the journal’s authorship statement. This manuscript has been reviewed and approved by all named authors.
REFERENCES
- 1.Ritter MA, Vaughan RB. Ectopic ossification after total hip arthroplasty. Predisposing factors, frequency, and effect on results. J Bone Joint Surg Am 1977;59:345–51. [PubMed] [Google Scholar]
- 2.Gear AJ, Buckley C, Kaplan F, Vanbeek A. Multifactorial refractory heterotopic ossification. Ann Plast Surg 2004;52:319–24. [DOI] [PubMed] [Google Scholar]
- 3.Potter BK, Forsberg JA, Davis TA, et al. Heterotopic ossification following combat-related trauma. J Bone Joint Surg Am 2010;92 Suppl 2:74–89. [DOI] [PubMed] [Google Scholar]
- 4.Bowman SH, Barfield WR, Slone HS, Shealy GJ, Walton ZJ. The clinical implications of heterotopic ossification in patients treated with radial head replacement for trauma: a case series and review of the literature. J Orthop 2016;13:272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiori JL, Billings PC, de la Pena LS, Kaplan FS, Shore EM. Dysregulation of the BMP-p38 MAPK signaling pathway in cells from patients with fibrodysplasia ossificans progressiva (FOP). J Bone Miner Res 2006;21:902–9. [DOI] [PubMed] [Google Scholar]
- 6.Edwards DS, Kuhn KM, Potter BK, Forsberg JA. Heterotopic ossification: a review of current understanding, treatment, and Future. J Orthop Trauma 2016;30 Suppl 3:S27–30. [DOI] [PubMed] [Google Scholar]
- 7.Forsberg JA, Potter BK. Heterotopic ossification in wartime wounds. J Surg Orthop Adv 2010;19:54–61. [PubMed] [Google Scholar]
- 8.Vanden Bossche L, Vanderstraeten G. Heterotopic ossification: a review. J Rehabil Med 2005;37:129–36. [DOI] [PubMed] [Google Scholar]
- 9.Forsberg JA, Pepek JM, Wagner S, et al. Heterotopic ossification in high-energy wartime extremity injuries: prevalence and risk factors. J Bone Joint Surg Am 2009;91:1084–91. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan FS, Pignolo RJ, Shore EM. From mysteries to medicines: drug development for fibrodysplasia ossificans progressive. Expert Opin Orphan Drugs 2013;1:637–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal S, Loder SJ, Brownley C, et al. BMP signaling mediated by constitutively active Activin type 1 receptor (ACVR1) results in ectopic bone formation localized to distal extremity joints. Dev Biol 2015;400:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakkalakal SA, Uchibe K, Convente MR, et al. Palovarotene inhibits heterotopic ossification and Maintains limb mobility and growth in mice with the human ACVR1(R206H) fibrodysplasia ossificans progressiva (FOP) mutation. J Bone Miner Res 2016;31:1666–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agarwal S, Cholok D, Loder S, et al. mTOR inhibition and BMP signaling act synergistically to reduce muscle fibrosis and improve myofiber regeneration. JCI Insight 2016;1:e89805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dey D, Bagarova J, Hatsell SJ, et al. Two tissue-resident progenitor lineages drive distinct phenotypes of heterotopic ossification. Sci Transl Med 2016;8:366ra163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramirez DM, Ramirez MR, Reginato AM, Medici D. Molecular and cellular mechanisms of heterotopic ossification. Histol Histopathol 2014;29:1281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pignolo RJ, Bedford-Gay C, Liljesthrom M, et al. The natural history of flare-ups in fibrodysplasia ossificans progressiva (FOP): a Comprehensive Global assessment. J Bone Miner Res 2016;31:650–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson JR, Agarwal S, Brownley RC, et al. Direct mouse trauma/burn model of heterotopic ossification. J Vis Exp 2015;102:e52880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson JR, De La Rosa S, Sun H, et al. Burn injury enhances bone formation in heterotopic ossification model. Ann Surg 2014;259:993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tannous O, Stall AC, Griffith C, Donaldson CT, Castellani RJ Jr, Pellegrini VD Jr. Heterotopic bone formation about the hip undergoes endochondral ossification: a rabbit model. Clin Orthop Relat Res 2013;471:1584–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin L, Shen Q, Leng H, Duan X, Fu X, Yu C. Synergistic inhibition of endochondral bone formation by silencing Hif1alpha and Runx2 in trauma-induced heterotopic ossification. Mol Ther 2011;19:1426–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berendsen AD, Olsen BR. Bone development. Bone 2015;80: 14–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colnot C Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res 2009;24:274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury 2005;36:1392–404. [DOI] [PubMed] [Google Scholar]
- 24.Thompson Z, Miclau T, Hu D, Helms JA. A model for intramembranous ossification during fracture healing. J Orthop Res 2002; 20:1091–8. [DOI] [PubMed] [Google Scholar]
- 25.Hankenson KD, Dishowitz M, Gray C, Schenker M. Angiogenesis in bone regeneration. Injury 2011;42:556–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Florencio-Silva R, Sasso GR, Sasso-Cerri E, Simoes MJ, Cerri PS. Biology of bone tissue: structure, function, and factors that Influence bone cells. Biomed Res Int 2015;2015:421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol 2015;11:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freed JH, Hahn H, Menter R, Dillon T. The use of the three-phase bone scan in the early diagnosis of heterotopic ossification (HO) and in the evaluation of Didronel therapy. Paraplegia 1982; 20:208–16. [DOI] [PubMed] [Google Scholar]
- 29.Argyropoulou MI, Kostandi E, Kosta P, et al. Heterotopic ossification of the knee joint in intensive care unit patients: early diagnosis with magnetic resonance imaging. Crit Care 2006;10: R152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cassar-Pullicino VN, McClelland M, Badwan DA, McCall IW, Pringle RG, el Masry W. Sonographic diagnosis of heterotopic bone formation in spinal injury patients. Paraplegia 1993;31: 40–50. [DOI] [PubMed] [Google Scholar]
- 31.Rosteius T, Suero EM, Grasmucke D, et al. The sensitivity of ultrasound screening examination in detecting heterotopic ossification following spinal cord injury. Spinal Cord 2017;55:71–3. [DOI] [PubMed] [Google Scholar]
- 32.Ledermann HP, Schweitzer ME, Morrison WB. Pelvic heterotopic ossification: MR imaging characteristics. Radiology 2002;222:189–95. [DOI] [PubMed] [Google Scholar]
- 33.Wick L, Berger M, Knecht H, Glucker T, Ledermann HP. Magnetic resonance signal alterations in the acute onset of heterotopic ossification in patients with spinal cord injury. Eur Radiol 2005;15:1867–75. [DOI] [PubMed] [Google Scholar]
- 34.Nitek Z, Czwojdzinski A, Wolf-Kus A, Walecki J. Computed tomography in the diagnosis of myositis ossificans–case report. Pol J Radiol 2014;79:296–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zagarella A, Impellizzeri E, Maiolino R, Attolini R, Castoldi MC. Pelvic heterotopic ossification: when CT comes to the aid of MR imaging. Insights Imaging 2013;4:595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukutake K, Ishiwatari T, Takahashi H, et al. Investigation of ossification in the posterior longitudinal ligament using microfocus X-ray CT scanning and histological examination. Diagn Pathol 2015;10:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arduini M, Mancini F, Farsetti P, Piperno A, Ippolito E. A new classification of peri-articular heterotopic ossification of the hip associated with neurological injury: 3D CT scan assessment and intra-operative findings. Bone Joint J 2015;97-B:899–904. [DOI] [PubMed] [Google Scholar]
- 38.Brownley RC, Agarwal S, Loder S, et al. Characterization of heterotopic ossification using radiographic imaging: Evidence for a Paradigm Shift. PLoS One 2015;10:e0141432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anthonissen J, Ossendorf C, Hock JL, et al. The role of muscular trauma in the development of heterotopic ossification after hip surgery: an animal-model study in rats. Injury 2016;47:613–6. [DOI] [PubMed] [Google Scholar]
- 40.Kampschulte M, Langheinirch AC, Sender J, et al. Nanocomputed tomography: technique and applications. Rofo 2016; 188:146–54. [DOI] [PubMed] [Google Scholar]
- 41.Yabe Y, Hatori M, Kumagai J, Koizumi N, Sakuma T, Kawamura M. Heterotopic ossification of the distal portion of biceps femoris: case report and review of the literature. Ups J Med Sci 2006;111:321–7. [DOI] [PubMed] [Google Scholar]
- 42.Lima MC, Passarelli MC, Dario V, Lebani BR, Monteiro PH, Ramos CD. The use of spect/ct in the evaluation of heterotopic ossification in para/tetraplegics. Acta Ortop Bras 2014;22:12–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perosky JE, Peterson JR, Eboda ON, et al. Early detection of heterotopic ossification using near-infrared optical imaging reveals dynamic turnover and progression of mineralization following Achillestenotomyandburninjury.JOrthopRes2014;32:1416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orzel JA, Rudd TG. Heterotopic bone formation: clinical, laboratory, and imaging correlation. J Nucl Med 1985;26:125–32. [PubMed] [Google Scholar]
- 45.Peterson JR, Okagbare PI, De La Rosa S, et al. Early detection of burn induced heterotopic ossification using transcutaneous Raman spectroscopy. Bone 2013;54:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harris M, Cilwa K, Elster EA, Potter BK, Forsberg JA, Crane NJ. Pilot study for detection of early changes in tissue associated with heterotopic ossification: moving toward clinical use of Raman spectroscopy. Connect Tissue Res 2015;56:144–52. [DOI] [PubMed] [Google Scholar]
- 47.Agarwal S, Lloyd WR, Loder SJ, et al. Combined reflectance and Raman spectroscopy to assess degree of in vivo angiogenesis after tissue injury. J Surg Res 2016;209:174–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forsberg JA, Potter BK, Polfer EM, Safford SD, Elster EA. Do inflammatory markers portend heterotopic ossification and wound failure in combat wounds? Clin Orthop Relat Res 2014;472:2845–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodenberg E, Azhdarinia A, Lazard ZW, et al. Matrix metalloproteinase-9 is a diagnostic marker of heterotopic ossification in a murine model. Tissue Eng Part A 2011;17:2487–96. [DOI] [PubMed] [Google Scholar]
- 50.Sinha S, Uchibe K, Usami Y, Pacifici M, Iwamoto M. Effectiveness and mode of action of a combination therapy for heterotopic ossification with a retinoid agonist and an anti-inflammatory agent. Bone 2016;90:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu PB, Deng DY, Lai CS, et al. BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat Med 2008;14: 1363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tannous O, Griffith C, O’Toole RV, Pellegrini VD Jr. Heterotopic ossification after extremity blast amputation in a SpragueDawley rat animal model. J Orthop Trauma 2011;25:506–10. [DOI] [PubMed] [Google Scholar]
- 53.Jaffe DE, Yoo D, Blevins J, et al. Does blast Medium Affect heterotopic ossification in a blast-amputation model? Clin Orthop Relat Res 2015;473:2680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahlers ST, Vasserman-Stokes E, Shaughness MC, et al. Assessment of the effects of acute and repeated exposure to blast overpressure in rodents: toward a greater understanding of blast and the potential ramifications for injury in humans exposed to blast. Front Neurol 2012;3:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Polfer EM, Hope DN, Elster EA, et al. The development of a rat model to investigate the formation of blast-related post-traumatic heterotopic ossification. Bone Joint J 2015;97-B:572–6. [DOI] [PubMed] [Google Scholar]
- 56.Qureshi AT, Crump EK, Pavey GJ, Hope DN, Forsberg JA, Davis TA. Early characterization of blast-related heterotopic ossification in a rat model. Clin Orthop Relat Res 2015;473: 2831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pavey GJ, Qureshi AT, Hope DN, et al. Bioburden increases heterotopic ossification formation in an established rat model. Clin Orthop Relat Res 2015;473:2840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pavey GJ, Qureshi AT, Tomasino AM, et al. Targeted stimulation of retinoic acid receptor-gamma mitigates the formation of heterotopic ossification in an established blast-related traumatic injury model. Bone 2016;90:159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seavey JG, Wheatley BM, Pavey GJ, et al. Early local delivery of vancomycin suppresses ectopic bone formation in a rat model of trauma-induced heterotopic ossification. J Orthop Res 2017. [DOI] [PubMed] [Google Scholar]
- 60.Chen HC, Yang JY, Chuang SS, Huang CY, Yang SY. Heterotopic ossification in burns: our experience and literature reviews. Burns 2009;35:857–62. [DOI] [PubMed] [Google Scholar]
- 61.Rinkinen J, Hwang CD, Agarwal S, et al. The systemic effect of burn injury and trauma on muscle and bone mass and Composition. Plast Reconstr Surg 2015;136:612e–23e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Agarwal S, Loder SJ, Cholok D, et al. Scleraxis-lineage cells contribute to ectopic bone formation in muscle and tendon. Stem Cells 2017;35:705–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Richards AM,Klaassen MF. Heterotopic ossification after severe burns: a report of three cases and review of the literature. Burns 1997;23:64–8. [DOI] [PubMed] [Google Scholar]
- 64.O’Brien EJ, Frank CB, Shrive NG, Hallgrimsson B, Hart DA. Heterotopic mineralization (ossification or calcification) in tendinopathy or following surgical tendon trauma. Int J Exp Pathol 2012;93:319–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Agarwal S, Loder S, Cholok D, et al. Local and circulating endothelial cells undergo endothelial to mesenchymal transition (EndMT) in response to musculoskeletal injury. Sci Rep 2016; 6:32514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Agarwal S, Loder S, Brownley C, et al. Inhibition of Hif1alpha prevents both trauma-induced and genetic heterotopic ossification. Proc Natl Acad Sci U S A 2016;113:E338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Agarwal S, Loder S, Cholok D, et al. Surgical excision of heterotopic ossification leads to Re-Emergence of mesenchymal stem cell populations responsible for recurrence. Stem Cells Transl Med 2017;6:799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hendricks HT, Geurts AC, van Ginneken BC, Heeren AJ, Vos PE. Brain injury severity and autonomic dysregulation accurately predict heterotopic ossification in patients with traumatic brain injury. Clin Rehabil 2007;21:545–53. [DOI] [PubMed] [Google Scholar]
- 69.Sakellariou VI, Grigoriou E, Mavrogenis AF, Soucacos PN, Papagelopoulos PJ. Heterotopic ossification following traumatic braininjuryandspinalcordinjury:insightintotheetiologyandpathophysiology. J Musculoskelet Neuronal Interact 2012;12:230–40. [PubMed] [Google Scholar]
- 70.Schoenfeld AJ, Laughlin MD, McCriskin BJ, Bader JO, Waterman BR, Belmont PJ Jr. Spinal injuries in United States military personnel deployed to Iraq and Afghanistan: an epidemiological investigation involving 7877 combat casualties from 2005 to 2009. Spine (Phila Pa 1976) 2013;38:1770–8. [DOI] [PubMed] [Google Scholar]
- 71.Hurvitz EA, Mandac BR, Davidoff G, Johnson JH, Nelson VS. Risk factors for heterotopic ossification in children and adolescents with severe traumatic brain injury. Arch Phys Med Rehabil 1992;73:459–62. [PubMed] [Google Scholar]
- 72.Dizdar D, Tiftik T, Kara M, Tunc H, Ersoz M, Akkus S. Risk factors for developing heterotopic ossification in patients with traumatic brain injury. Brain Inj 2013;27:807–11. [DOI] [PubMed] [Google Scholar]
- 73.Genet F, Kulina I, Vaquette C, et al. Neurological heterotopic ossification following spinal cord injury is triggered by macrophage-mediated inflammation in muscle. J Pathol 2015; 236:229–40. [DOI] [PubMed] [Google Scholar]
- 74.Kang H, Dang AB, Joshi SK, et al. Novel mouse model of spinal cord injury-induced heterotopic ossification. J Rehabil Res Dev 2014;51:1109–18. [DOI] [PubMed] [Google Scholar]
- 75.Urist MR, Mikulski A, Lietze A. Solubilized and insolubilized bone morphogenetic protein. Proc Natl Acad Sci U S A 1979; 76:1828–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takagi K, Urist MR. The reaction of the dura to bone morphogenetic protein (BMP) in repair of skull defects. Ann Surg 1982; 196:100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lounev VY, Ramachandran R, Wosczyna MN, et al. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J Bone Joint Surg Am 2009;91:652–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu X, Kang H, Shahnazari M, et al. A novel mouse model of trauma induced heterotopic ossification. J Orthop Res 2014;32: 183–8. [DOI] [PubMed] [Google Scholar]
- 79.Uchibe K, Son J, Larmour C, Pacifici M, Enomoto-Iwamoto M, Iwamoto M. Genetic and pharmacological inhibition of retinoic acid receptor gamma function promotes endochondral bone formation. J Orthop Res 2017;35:1096–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tian XB, Sun L, Yang SH, et al. Ectopic osteogenesis of mouse bone marrow stromal cells transfected with BMP 2/VEGF(165) genes in vivo. Orthop Surg 2009;1:322–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hayashi C, Hasegawa U, Saita Y, et al. Osteoblastic bone formation is induced by using nanogel-crosslinking hydrogel as novel scaffold for bone growth factor. J Cell Physiol 2009;220:1–7. [DOI] [PubMed] [Google Scholar]
- 82.Marsell R, Einhorn TA. The biology of fracture healing. Injury 2011;42:551–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dimarino AM, CaplanAI, BonfieldTL. Mesenchymal stem cells in tissue repair. Front Immunol 2013;4:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hadjiargyrou M, Lombardo F, Zhao S, et al. Transcriptional profiling of bone regeneration. Insight into the molecular complexity of wound repair. J Biol Chem 2002;277:30177–82. [DOI] [PubMed] [Google Scholar]
- 85.Convente MR, Wang H, Pignolo RJ, Kaplan FS, Shore EM. The immunological contribution to heterotopic ossification disorders. Curr Osteoporos Rep 2015;13:116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tredget EE, Levi B, Donelan MB. Biology and principles of scar management and burn reconstruction. Surg Clin North Am 2014; 94:793–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Evans KN, Forsberg JA, Potter BK, et al. Inflammatory cytokine and chemokine expression is associated with heterotopic ossification in high-energy penetrating war injuries. J Orthop Trauma 2012;26:e204–13. [DOI] [PubMed] [Google Scholar]
- 88.Salisbury E, Rodenberg E, Sonnet C, et al. Sensory nerve induced inflammation contributes to heterotopic ossification. J Cell Biochem 2011;112:2748–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Scarlett RF, Rocke DM, Kantanie S, Patel JB, Shore EM, Kaplan FS. Influenza-like viral illnesses and flare-ups of fibrodysplasia ossificans progressiva. Clin Orthop Relat Res 2004;423:275–9. [DOI] [PubMed] [Google Scholar]
- 90.Aro HT, Viljanto J, Aho HJ, Michelsson JE. Macrophages in trauma-induced myositis ossificans. APMIS 1991;99:482–6. [DOI] [PubMed] [Google Scholar]
- 91.De Benedetti F, Rucci N, Del Fattore A, et al. Impaired skeletal development in interleukin-6-transgenic mice: a model for the impact of chronic inflammation on the growing skeletal system. Arthritis Rheum 2006;54:3551–63. [DOI] [PubMed] [Google Scholar]
- 92.Kraft CT, Agarwal S, Ranganathan K, et al. Trauma-induced heterotopic bone formation and the role of the immune system: a review. J Trauma Acute Care Surg 2016;80:156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shafritz AB, Shore EM, Gannon FH, et al. Overexpression of an osteogenic morphogen in fibrodysplasia ossificans progressiva. N Engl J Med 1996;335:555–61. [DOI] [PubMed] [Google Scholar]
- 94.Ranganathan K, Agarwal S, Cholok D, et al. The role of the adaptive immune system in burn-induced heterotopic ossification and mesenchymal cell osteogenic differentiation. J Surg Res 2016;206:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Loder S, Agarwal S, Sorkin M, et al. Lymphatic contribution to the cellular Niche in heterotopic ossification. Ann Surg 2016; 264:1174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gannon FH, Glaser D, Caron R, Thompson LD, Shore EM, Kaplan FS. Mast cell involvement in fibrodysplasia ossificans progressiva. Hum Pathol 2001;32:842–8. [DOI] [PubMed] [Google Scholar]
- 97.Cheung EV, Sarkissian EJ. Complications of elbow trauma. Hand Clin 2015;31:683–91. [DOI] [PubMed] [Google Scholar]
- 98.Taly AB, Nair KP, Jayakumar PN, et al. Neurogenic heterotopic ossification: a diagnostic and therapeutic challenge in neurorehabilitation. Neurol India 2001;49:37–40. [PubMed] [Google Scholar]
- 99.Chopra S, Harjai K, Chhibber S. Potential of combination therapy of endolysin MR-10 and minocycline in treating MRSA induced systemic and localized burn wound infections in mice. Int J Med Microbiol 2016;306:707–16. [DOI] [PubMed] [Google Scholar]
- 100.Nishiguchi T, Ito I, Lee JO, Suzuki S, Suzuki F, Kobayashi M. Macrophage polarization and MRSA infection in burned mice. Immunol Cell Biol 2017;95:198–206. [DOI] [PubMed] [Google Scholar]
- 101.Maes C Signaling pathways effecting crosstalk between cartilage and adjacent tissues: Seminars in cell and developmental biology: the biology and pathology of cartilage. Semin Cell Dev Biol 2017;62:16–33. [DOI] [PubMed] [Google Scholar]
- 102.Himeno M, Enomoto H, Liu W, et al. Impaired vascular invasion of Cbfa1-deficient cartilage engrafted in the spleen. J Bone Miner Res 2002;17:1297–305. [DOI] [PubMed] [Google Scholar]
- 103.Maes C, Carmeliet P, Moermans K, et al. Impaired angiogenesis and endochondral bone formation in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Mech Dev 2002;111:61–73. [DOI] [PubMed] [Google Scholar]
- 104.Ortega N, Wang K, Ferrara N, Werb Z, Vu TH. Complementary interplay between matrix metalloproteinase-9, vascular endothelial growth factor and osteoclast function drives endochondral bone formation. Dis Model Mech 2010;3:224–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ortega N, Behonick DJ, Colnot C, Cooper DN, Werb Z. Galectin-3 is a downstream regulator of matrix metalloproteinase-9 function during endochondral bone formation. Mol Biol Cell 2005;16:3028–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Inada M, Wang Y, Byrne MH, et al. Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc Natl Acad Sci U S A 2004;101:17192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Keel M, Trentz O. Pathophysiology of polytrauma. Injury 2005; 36:691–709. [DOI] [PubMed] [Google Scholar]
- 108.Kimmel HM, Grant A, Ditata J. The presence of Oxygen in wound healing. Wounds 2016;28:264–70. [PubMed] [Google Scholar]
- 109.Mach DB, Rogers SD, Sabino MC, et al. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience 2002;113:155–66. [DOI] [PubMed] [Google Scholar]
- 110.Heffner MA, Anderson MJ, Yeh GC, Genetos DC, Christiansen BA. Altered bone development in a mouse model of peripheral sensory nerve inactivation. J Musculoskelet Neuronal Interact 2014;14:1–9. [PMC free article] [PubMed] [Google Scholar]
- 111.Madsen JE, Hukkanen M, Aune AK, et al. Fracture healing and callus innervation after peripheral nerve resection in rats. Clin Orthop Relat Res 1998;351:230–40. [PubMed] [Google Scholar]
- 112.Santavirta S, Konttinen YT, Nordstrom D, et al. Immunologic studies of nonunited fractures. Acta Orthop Scand 1992;63: 579–86. [PubMed] [Google Scholar]
- 113.Lazard ZW, Olmsted-Davis EA, Salisbury EA, et al. Osteoblasts have a neural origin in heterotopic ossification. Clin Orthop Relat Res 2015;473:2790–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci 2006;361:1545–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem 2003;72:609–42. [DOI] [PubMed] [Google Scholar]
- 116.Tomlinson RE, Li Z, Zhang Q, et al. NGF-trka signaling by sensory nerves Coordinates the vascularization and ossification of developing endochondral bone. Cell Rep 2016; 16:2723–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med 2010;16:1400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Agarwal S, Loder SJ, Sorkin M, et al. Analysisof bone-cartilagestromal progenitor populations in trauma induced and genetic models of heterotopic ossification. Stem Cells 2016;34: 1692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Miyazono K, Maeda S, Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev 2005;16:251–63. [DOI] [PubMed] [Google Scholar]
- 120.Gautschi OP, Toffoli AM, Joesbury KA, Skirving AP, Filgueira L, Zellweger R. Osteoinductive effect of cerebrospinal fluid from brain-injured patients. J Neurotrauma 2007;24: 154–62. [DOI] [PubMed] [Google Scholar]
- 121.Kluk MW, Ji Y, Shin EH, et al. Fibroregulation of mesenchymal progenitor cells by BMP-4 after traumatic muscle injury. J Orthop Trauma 2012;26:693–8. [DOI] [PubMed] [Google Scholar]
- 122.Jackson WM, Aragon AB, Onodera J, et al. Cytokine expression in muscle following traumatic injury. J Orthop Res 2011;29: 1613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Evans KN, Potter BK, Brown TS, Davis TA, Elster EA, Forsberg JA. Osteogenic gene expression correlates with development of heterotopic ossification in war wounds. Clin Orthop Relat Res 2014;472:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Grenier G, Leblanc E, Faucheux N, Lauzier D, Kloen P, Hamdy RC. BMP-9 expression in human traumatic heterotopic ossification: a case report. Skelet Muscle 2013;3:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Barfield WR, Holmes RE, Hartsock LA. Heterotopic ossification in trauma. Orthop Clin North Am 2017;48:35–46. [DOI] [PubMed] [Google Scholar]
- 126.Potter BK, Burns TC, Lacap AP, Granville RR, Gajewski DA. Heterotopic ossification following traumatic and combatrelated amputations. Prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am 2007;89:476–86. [DOI] [PubMed] [Google Scholar]
- 127.Almangour W, Schnitzler A, Salga M, Debaud C, Denormandie P, Genet F. Recurrence of heterotopic ossification after removal in patients with traumatic brain injury: a systematic review. Ann Phys Rehabil Med 2016;59:263–9. [DOI] [PubMed] [Google Scholar]
- 128.Pavey GJ, Polfer EM, Nappo KE, Tintle SM, Forsberg JA, Potter BK. What risk factors predict recurrence of heterotopic ossification after excision in combat-related amputations? Clin Orthop Relat Res 2015;473:2814–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen S, Yu SY, Yan H, et al. The time point in surgical excision of heterotopic ossification of post-traumatic stiff elbow: recommendation for early excision followed by early exercise. J Shoulder Elbow Surg 2015;24:1165–71. [DOI] [PubMed] [Google Scholar]
- 130.Chalidis B, Stengel D, Giannoudis PV. Early excision and late excision of heterotopic ossification after traumatic brain injury are equivalent: a systematic review of the literature. J Neurotrauma 2007;24:1675–86. [DOI] [PubMed] [Google Scholar]
- 131.Genet F, Ruet A, Almangour W, Gatin L, Denormandie P, Schnitzler A. Beliefs relating to recurrence of heterotopic ossification following excision in patients with spinal cord injury: a review. Spinal Cord 2015;53:340–4. [DOI] [PubMed] [Google Scholar]
- 132.Popovic M, Agarwal A, Zhang L, et al. Radiotherapy for the prophylaxisofheterotopicossification:asystematicreviewandmetaanalysis of published data. Radiother Oncol 2014;113:10–7. [DOI] [PubMed] [Google Scholar]
- 133.Burnet NG, Nasr P, Yip G, et al. Prophylactic radiotherapy against heterotopic ossification following internal fixation of acetabular fractures: a comparative estimate of risk. Br J Radiol 2014;87:20140398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Museler AC, GrasmuckeD, JansenO, et al. In-hospital outcomes following single-dose radiation therapy in the treatment of heterotopic ossification of the hip following spinal cord injury-an analysis of 444 cases. Spinal Cord 2017;55:244–6. [DOI] [PubMed] [Google Scholar]
- 135.Chidel MA, Suh JH, Matejczyk MB. Radiation prophylaxis for heterotopic ossification of the knee. J Arthroplasty 2001;16:1–6. [DOI] [PubMed] [Google Scholar]
- 136.Davis JA, Roper B, Munz JW, et al. Does postoperative radiation decrease heterotopic ossification after the Kocher-Langenbeck approach for acetabular fracture? Clin Orthop Relat Res 2016; 474:1430–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vanden Bossche LC, Van Maele G, Wojtowicz I, et al. Free radical scavengers versus methylprednisolone in the prevention of experimentally induced heterotopic ossification. J Orthop Res 2009;27:748–51. [DOI] [PubMed] [Google Scholar]
- 138.Chantaphakul H, Ruxrungtham K. Fixed-Dose combination of the inhaled corticosteroid and long-acting beta2-agonist therapy in adults with persistent asthma. Expert Opin Pharmacother 2016;17:631–42. [DOI] [PubMed] [Google Scholar]
- 139.Weinreb M, Suponitzky I, Keila S. Systemic administration of an anabolic dose of PGE2 in young rats increases the osteogenic capacity of bone marrow. Bone 1997;20:521–6. [DOI] [PubMed] [Google Scholar]
- 140.Horibe S, Tanahashi T, Kawauchi S, Mizuno S, Rikitake Y. Preventative effects of Sodium Alginate on indomethacin-induced small-intestinal injury in mice. Int J Med Sci 2016;13:653–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Romano CL, Duci D, Romano D, Mazza M, Meani E. Celecoxib versus indomethacin in the prevention of heterotopic ossification after total hip arthroplasty. J Arthroplasty 2004;19:14–8. [DOI] [PubMed] [Google Scholar]
- 142.Teasell RW, Mehta S, Aubut JL, et al. A systematic review of the therapeutic interventions for heterotopic ossification after spinal cord injury. Spinal Cord 2010;48:512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Macfarlane RJ, Ng BH, Gamie Z, et al. Pharmacological treatment of heterotopic ossification following hip and acetabular surgery. Expert Opin Pharmacother 2008;9:767–86. [DOI] [PubMed] [Google Scholar]
- 144.Kim HK, Aruwajoye O, Du J, Kamiya N. Local administration of bone morphogenetic protein-2 and bisphosphonate during non-weight-bearing treatment of ischemic osteonecrosis of the femoral head: an experimental investigation in immature pigs. J Bone Joint Surg Am 2014;96:1515–24. [DOI] [PubMed] [Google Scholar]
- 145.Gil JA, Waryasz GR, Klyce W, Daniels AH. Heterotopic ossification in neurorehabilitation. R I Med J (2013) 2015;98:32–4. [PubMed] [Google Scholar]
- 146.Orchard GR, Paratz JD, Blot S, Roberts JA. Risk factors in Hospitalized patients with burn injuries for developing heterotopic ossification–a retrospective analysis. J Burn Care Res 2015;36: 465–70. [DOI] [PubMed] [Google Scholar]
- 147.Mohedas AH, Xing X, Armstrong KA, Bullock AN, Cuny GD, Yu PB. Development of an ALK2-biased BMP type I receptor kinase inhibitor. ACS Chem Biol 2013;8:1291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pavlou G, Kyrkos M, Tsialogiannis E, Korres N, Tsiridis E. Pharmacological treatment of heterotopic ossification following hip surgery: an update. Expert Opin Pharmacother 2012;13:619–22. [DOI] [PubMed] [Google Scholar]
- 149.Cappato S, Tonachini L, Giacopelli F, et al. High-throughput screening for modulators of ACVR1 transcription: discovery of potential therapeutics for fibrodysplasia ossificans progressiva. Dis Model Mech 2016;9:685–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shimono K, Tung WE, Macolino C, et al. Potent inhibition of heterotopic ossification by nuclear retinoic acid receptor-gamma agonists. Nat Med 2011;17:454–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shimono K, Morrison TN, Tung WE, et al. Inhibition of ectopic bone formation by a selective retinoic acid receptor alpha-agonist: a new therapy for heterotopic ossification? J Orthop Res 2010;28:271–7. [DOI] [PubMed] [Google Scholar]
- 152.Zimmermann SM, Schwitter LW, Scheyerer MJ, Jentzsch T, Simmen HP, Werner CM. Prevention of heterotopic ossification: an experimental study using a plasma expander in a murine model. BMC Surg 2016;16:29. [DOI] [PMC free article] [PubMed] [Google Scholar]