Summary
Background:
Insulin therapy is most effective if dosage titrations are done regularly and frequently, which is seldom possible for busy clinicians. The d-Nav® Insulin Guidance System was design to address the insulin titration gap in patients with type 2 diabetes. It relies on the d-Nav handheld device, which is used to measure glucose, determine the glucose patterns and automatically determine the appropriate next insulin dose. It closes the titration gap in a scalable way utilizing the support of dedicated health care professionals (HCP). This multicenter randomized controlled study tested whether the combination of the d-Nav system and HCP support (d-Nav+HCP-S) is superior to HCP support alone (HCP-S).
Methods:
181 subjects using insulin with sub-optimally controlled type 2 diabetes were randomized 1:1 to either d-Nav+HCP-S or HCP-S alone. Both groups were contacted 7 times during a 6-month follow-up. The primary outcome was to compare average change in HbA1c during the study while the secondary outcome was to compare the percent of participants who achieve HbA1c <7% (<53mmol/mol), <8% (<64mmol/mol), and >9⋅0% (>75mmol/mol) at study end. Safety was assessed by the frequency of hypoglycaemia. The Student’s t-test was used to assess the primary outcome for statistical significance.
Findings:
At baseline, HbA1c was 8⋅7%±0⋅8% (72±8⋅8mmol/mol) in the d-Nav+HCP-S group and 8⋅5%±0.8% (69mmol/mol±8⋅8mmol/mol) in the HCP-S (p=0⋅2). The mean decrease of HbA1c from baseline to 6 months was 1⋅0%±1⋅0% (11±11mmol/mol) in the d-Nav+HCP-S group, and 0⋅3%±0⋅9% (3⋅3mmol/mol±9⋅9mmol/mol) in the HCP-S group (p<0⋅0001). For the d-Nav+HCP-S group, reduction in HbA1c was achieved by 1⋅1±0⋅2 automated insulin titrations per week. The frequency of hypoglycaemia (<54 mg/dL or <3⋅0mmol/l) was similar between the groups at 0.3 hypoglycemic readings per month (p>0⋅9).
Interpretation:
The combination of automated insulin titration guidance with HCP support offers superior glycemic control to HCP support alone. Such a solution facilitated safe and effective insulin titration in a large number of patients with type 2 diabetes.
Introduction
Insulin therapy is used by more than a quarter of all patients with diabetes, but its effectiveness has been unsatisfactory. Despite advancements in technology and pharmacotherapy, average glycated hemoglobin (HbA1c) in insulin users in the Western world has been ~8⋅5% (~69mmol/mol). Most patients do not achieve the recommended HbA1c goal, with only a one-third reaching an HbA1c of <7% (<53mmol/mol) and another one-third having an HbA1c of 9% (75mmol/mol) or higher(1, 2).
There is a rising awareness that insulin therapy can be effective if titrations are done regularly and frequently to overcome intra-individual and inter-individual variations in insulin requirements(3–10). But in practice, due to limited time and medical expertise, insulin dosage adjustments are done sporadically during outpatient clinic visits every 3–6 months. Moreover, based on the units of insulin/kg body weight utilized in previous clinical trials evaluating effective insulin therapy in type 2 diabetes management, most patients using insulin are under dosed(11).
This gap in insulin titrations is addressed using hybrid closed-loop insulin delivery systems in patients with type 1 diabetes. The system includes an insulin pump, a linked continuous glucose monitor, and an algorithm in the pump or a hand-held unit that adjusts insulin doses every 5 minutes. In a recent, randomized control study, by Thabit et al. children and adults using a hybrid closed-loop insulin delivery system, reduced their HbA1c from an average of ~8⋅5% (~69mmol/mol) to ~7⋅5% (~58mmol/mol) in 12 weeks(12).
Given the cost and complexity of hybrid closed loop therapy(13, 14), it is mostly too complex and cost-prohibitive for the large population of patients with type 2 diabetes. For each individual with type 1 diabetes who uses insulin, there are 5 individuals who use insulin to manage their type 2 diabetes(15).
Hygieia Inc. has developed a scalable solution to improve the effectiveness of insulin therapy. The system automates the guidance of insulin titration. This scalable solution, can be used by the large population of patients with type 2 diabetes who use insulin(9, 16–20). It relies on d-Nav® (diabetes navigator), a handheld device that automatically titrates insulin dosage, based on glucose readings the patient is already scheduled to take with the d-Nav. Patients use the device to check their glucose level before each injection and to obtain a recommended insulin dose. By analyzing glucose patterns, d-Nav automatically adjusts insulin dosage over time without supervision to fit patients’ changing needs while working to prevent an increase in hypoglycaemia. Additional software tools are available to provide further insight regarding insulin dynamics(16).
The d-Nav device has been shown to be effective when coupled with the support of dedicated health care professionals (HCP-S). The support specialists initiate periodic telephone calls and in-person consultations multiple times per year in order to bestow user confidence, correct usage errors, and identify uncharacteristic clinical courses. The support specialists are not involved in the process of insulin dosage titration, which is handled by the device and therefore enables scalability to the growing population of patients with type 2 diabetes who use insulin. The solution has been in use in the United Kingdom since 2012(21).
The purpose of this multicenter randomized controlled study was to evaluate whether the use of d-Nav plus HCP support (d-Nav + HCP-S) for the management of insulin treated type 2 diabetes, is superior to management of insulin therapy with HCP-S alone.
Methods
Study design
This 6-month, prospective, open-label, multicenter, randomized controlled study (clinicaltrial.gov #NCT02424500) of patients with type 2 diabetes, was conducted in 3 diabetes centers in the US from February 2015 to December 2017 (see study protocol in supplementary appendix). The Centers were the International Diabetes Center at Park Nicollet, MN; Henry Ford Medical Center Endocrinology, Detroit, MI; and the Iowa Diabetes and Endocrinology Research Center, Des Moines, IA.
Participants
Key inclusion and exclusion criteria included: Adult participants (≥20 and ≤70 years of age at screening) diagnosed with type 2 diabetes with HbA1c ≥7⋅5% (≥58mmol/mol) and ≤11% (≤97mmol/mol), using the same insulin regimen for the previous 3 months, with or without other diabetes agents at a stable dosage for the last 3-months, were eligible for inclusion. Exclusion criteria included body mass index (BMI) ≥45 kg/m2; severe impairment of cardiac, hepatic, or renal functions; psychological or cognitive impairment; more than two episodes of severe hypoglycaemia in the past year; a history of hypoglycaemia unawareness; and a lack of regularly monitored blood glucose.
The study protocol was approved by the appropriate local ethics committees or institutional review boards, and was conducted according to Good Clinical Practice guidelines and the Declaration of Helsinki. All participants provided written informed consent.
Randomization and masking
Upon enrollment, subjects were randomized in a 1:1 ratio either to the d-Nav + health care professional intervention group (d-Nav + HCP-S) or to the health care professional control group (HCP-S). The trial was designed as open-label since the difference in equipment between the groups could not be blinded. Randomization was blocked within each site to ensure approximately equal distribution of treatment groups within each site.
Procedures
During the initial visit, participants who were randomized to the d-Nav + HCP-S group were provided with and trained on the use of the d-Nav device. The d-Nav device was then set up with the participant’s current insulin regimen and dosage. Participants in both groups received free testing supplies either for d-Nav (d-Nav + HCP-S) or for their glucose meters’ consumables (HCP-S). All participants were encouraged to measure their blood glucose before each insulin injection and any time they felt symptoms of hypoglycaemia.
Four insulin regimens were used: a single injection of long-acting insulin analog per day (required one glucose measurement per day); twice daily biphasic or pre-mixed insulin (required 2 glucose measurements per day); basal-bolus regimen with fixed meal doses and a correction factor (required 4 glucose measurements per day); and, basal-bolus regimen with carbohydrate counting and a correction factor (required 4 glucose measurements per day). Subjects were allowed to change insulin regimens during the study.
During the 6-month study, participants in both groups received seven provider-patient interactions (3 face-to-face and 4 phone visits) by the study team who inquired about patients’ well-being, health changes, challenges in management, and any side effects. Patients assigned to the d-Nav + HCP-S group were also asked about details pertaining to the use of the device. The study team included endocrinologists, diabetes educators, and study coordinators who were allowed to make changes in the insulin dosage if deemed necessary.
Participants were seen in the study centers at 0, 3 and 6 months during which d-Nav devices (d-Nav + HCP-S group) or glucose meters (HCP-S group) were downloaded. HbA1c levels were evaluated in each visit in a central reference laboratory (Advanced Research and Diagnostic Laboratory, University of Minnesota, MN, USA) using the high-performance liquid chromatography method. Phone visits were initiated by the study center at weeks 1, 2, 4 and 20.
All study sites were accredited specialty diabetes clinics, led by experienced diabetologists. Thus, teams were unlikely to be biased against adjusting insulin dosage in the HCP-S group.
Study cohorts
It has been recognized that some patients are particularly susceptible to hypoglycaemia (22), which may preclude safe reduction of average glucose and HbA1c. Accordingly, patients enrolled in the study were divided to two pre-defined cohorts:
Primary cohort: the group that does not have frequent hypoglycaemia. It was estimated that 90% of patients will be in this cohort.
Secondary cohort: the group that has frequent hypoglycaemia.
Frequent hypoglycaemia was defined a priori according to >42 glucose readings <65 mg/dL (<3⋅6mmol/l) through the course of this 6-month study (>85 hypoglycemic events per year) as described previously(23). Since only 2 subjects in the d-Nav + HCP-S group and 1 subject in the HCP-S group were found to qualify to the second cohort, it was decided to include all subjects in the primary cohort without segregating patients.
Outcomes
Primary outcomes:
The primary outcome was to assess whether d-Nav users (d-Nav + HCP-S group) and control patients (HCP-S group) had different average change (difference in difference) in HbA1c between baseline and 6 months.
The secondary outcomes were:
To determine the difference between the control (HCP-S) and d-Nav group (d-Nav + HCP-S) in the percent of participants who achieve HbA1c <7% (<53mmol/mol), <8% (<64mmol/mol), and >9⋅0% (>75mmol/mol) at 6 months.
Additional analyses included:
To determine the difference between the control (HCP-S) and d-Nav group (d-Nav + HCP-S) in the percent of participants who achieve HbA1c <7% (<53mmol/mol), <8% (<64mmol/mol) without a severe hypoglycaemia event at 6 months.
To determine the difference between the control (HCP-S) and d-Nav group (d-Nav + HCP-S) in the number of glucose readings <50 mg/dL (<2⋅8mmol/l), <60 mg/dL (<3⋅3mmol/l) and <70 mg/dL (<3⋅9mmol/l) (symptomatic or asymptomatic) utilizing the documented downloaded glucose values at 3 months and 6 months.
To determine the difference between the control (HCP-S) and d-Nav group (d-Nav + HCP-S) in the mean fasting glucose utilizing the documented downloaded glucose values at 3 months and 6 months.
To determine the difference between the control (HCP-S) and d-Nav group (d-Nav + HCP-S) in the standard deviation (SD) and coefficient of variation (CV) of the mean fasting glucose utilizing the documented downloaded glucose values at 3 months and 6 months.
To determine the difference between the control (HCP-S) and d-Nav group (d-Nav + HCP-S) in mean blood glucose utilizing the documented downloaded glucose values at 3 months and 6 months.
To determine the difference between the control (HCP-S) and d-Nav group (d-Nav + HCP-S) in the number of test strips used at 3 months and 6 months.
To compare type and frequency of adverse events for d-Nav group (d-Nav + HCP-S) vs. control (HCP-S).
Statistical analysis
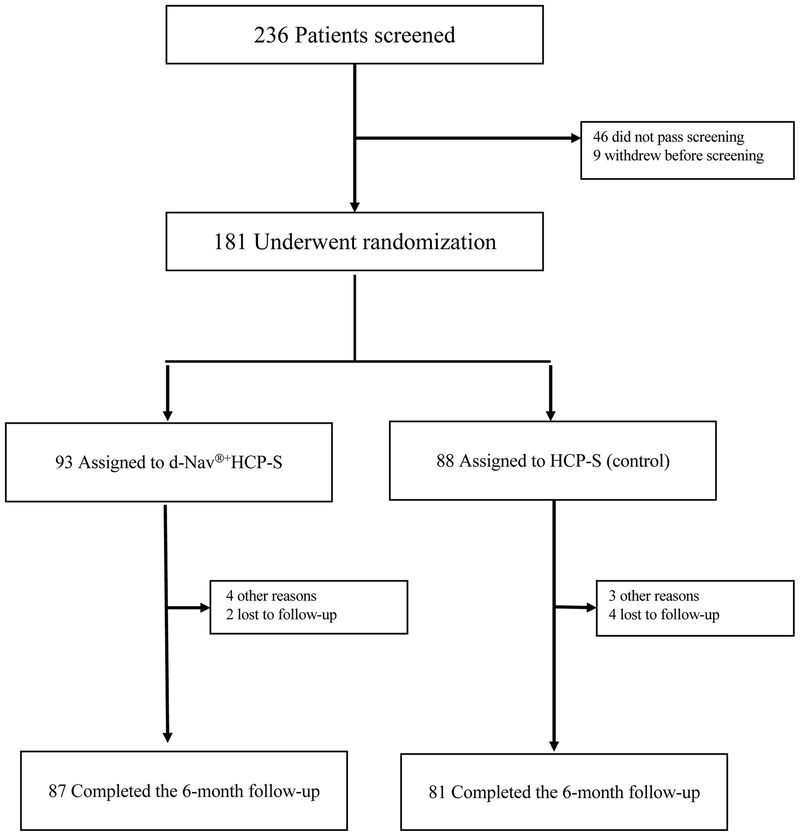
The study was powered to detect a mean HbA1c difference of 0⋅5% (5⋅5mmol/mol) between treatment groups with an estimated standard deviation of 1⋅0%. Based on a 0⋅05 level two-sample t-test, 77 patients per group would give 80% power. The HbA1c difference outcome in each patient was used to account for baseline differences including baseline HbA1c. To allow for potential attrition, 100 patients per group were chosen. Because the attrition rate was lower than expected (Figure 1), recruitment was completed after 181 participants had been enrolled.
Figure 1:
Flow of participants through the trial.
Of the subjects who did not complete the study, a total of 3 (23% of missing observations, 1.6% of recruited subjects) had partial data. An analysis using last observation carried forward did not change the primary objective and thus we report the subjects with available data from the analysis of 6-month outcomes for continuous variables, and report all available data for descriptive statistics reported at baseline. For binary outcomes such as frequency of hypoglycemic events, which offered no opportunity for a carry-forward approach, we conservatively used the total number of subjects as the denominator regardless of attrition. Although the protocol prespecified regression for analyses, in a linear regression analyses the effects of clinical site and treatment regimen were not significant. Thus, we report results pooled over site and regimen. No interim analysis was planned or performed.
Descriptive statistics for each variable included measures of central tendency and variation. Tests for continuous variables used a t-test. When the distribution of a variable did not support the use of parametric statistics, nonparametric approaches or data transformations were implemented. Tests for differences between categorical variables used the Chi-Square test. Tests for differences between repeated observations used the Wilcoxon Signed-rank test. An intention-to-treat analysis was used. Unless stated otherwise, results are presented as mean ± standard deviation (SD). Statistical significance was determined based on an alpha value of 0⋅05.
The trial was registered at clinicaltrial.gov #NCT02424500. Analyses were performed in R3.4.4 (www.r-project.org).
Role of the funding source
The funding source, National Institute of Health’s NIDDK program, award number 2R42DK085974–02A1, did not participate in the study design, data collection, analysis, interpretation, writing of the report or in the decision to submit the paper for publication.
Findings
Participants were recruited between February 2, 2015 and March 17, 2017. Of the 181 participants with type 2 diabetes enrolled in the study, 93 were randomized to the d-Nav + HCP-S group and 88 to the HCPS group and included in the intention to treat population; 87(93⋅5%) in the d-Nav + HCP-S group and 81 (92⋅0%) in the HCP-S group completed the study (Figure 1). All randomized participants were included in the analysis whether the completed the study or not. The entire study population was cumulatively followed for 83⋅2 patient-years.
Of the 6 participants who discontinued the study in the d-Nav + HCP-S group, 2 were lost to follow up, 1 discontinued due to overwhelming information, 1 because of resistance to changing their insulin regimen, 1 due to an unrelated medical concern (foot fracture), and 1 who lost their d-Nav device and decided not to continue. Of the 7 participants who discontinued the study in the HCP-S group, 4 were lost to follow-up, 1 discontinued since they did not see any benefit in participating, and 2 because of limited response to the study team phone calls. Data obtained from subjects prior to discontinuation their participation was included in analysis.
Baseline characteristics were similar in both treatment groups (Table 1). Participants in the d-Nav + HCP-S group were slightly older than participants in the HCP-S group (p=0⋅03). For other baseline demographic and clinical characteristics, no statistically significant differences were found. Characteristics of the entire study population were mean age 60⋅3 years; mean duration of diabetes 15⋅7 years; mean HbA1c 8⋅6% (70mmol/mol); and initial total daily insulin dose 0⋅7units/kg/day. The insulin regimens were similar between the groups (Table 1). Three subjects in each group changed insulin regimens during the study. In the d-Nav + HCP-S group, 2 subjects changed from long-acting insulin only to basal-bolus without carbohydrate counting and one from biphasic to basal-bolus without carbohydrate counting. In the HCP-S group, two subjects changed from long-acting insulin only regimens to basal-bolus without carbohydrate counting and one from basal bolus without carbohydrate counting to the same with carbohydrate counting. For classification in tests for regimen differences, they were counted based on the latest regimen. Information about additional anti-hyperglycemic classes can be found in Table 1.
Table 1:
Baseline demographics and clinical characteristics.
| d-Nav® +HCP-S n=93 |
HCP-S n=88 |
d-Nav® +HCP-S n=93 |
HCP-S n=88 |
d-Nav® +HCP-S n=93 |
HCP-S n=88 |
|||
|---|---|---|---|---|---|---|---|---|
| Gender: | Complications (%): | Insulin regimen (%) **: | ||||||
| Male (%) | 45 (48·4%) | 48 (54·5%) | Retinopathy | 14 (15·1%) | 10 (11·4%) | Long-acting insulin only | 31 (33·3%) | 35 (39·8%) |
| Age (years; mean±SD): * | 61·7±6·9 | 58·8±8·5 | Nephropathy/albuminuria | 12 (12·9%) | 8 (9·1%) | Biphasic/pre-mixed insulin | 12 (12·9%) | 9 (10·2%) |
| Race (%): | Neuropathy | 26 (28·0%) | 19 (21·6%) | Basal bolus therapy | 42 (45·2%) | 27 (30·7%) | ||
| American Indian/Alaska native | 2 (2·2%) | 2 (2·3%) | Coronary artery disease/congestive heart failure | 6 (6·5%) | 7 (8·0%) | Basal bolus therapy with carb counting | 8 (8·6%) | 17 (19·3%) |
| Asian | 2 (2·2%) | 5 (5·7%) | Peripheral vascular disease | 2 (2·2%) | 2 (2·3%) | Other medications (%): | ||
| African American | 19 (20·4%) | 19 (21·6%) | Comorbidities (%): | Biguanides | 29 (31·2%) | 32 (36·4%) | ||
| Native Hawaiian/other Pacific | 0 | 0 | Hypertension | 72 (77·4%) | 70 (79·5%) | Sulfonylurea | 6 (6·5%) | 9 (10·2%) |
| White | 68 (73·1%) | 57 (64·8%) | Dyslipidemia | 85 (91·4%) | 71 (80·7%) | Thiazolidinediones | 1 (1·1%) | 0 |
| More than one | 0 | 2 (2·3%) | Tobacco smoking | 42 (45·2%) | 43 (48·9%) | Meglitinides | 0 | 0 |
| Unknown | 2 (2·2%) | 3 (3·4%) | Education (%): | DPP-4 inhibitors | 2 (2·2%) | 3 (3·4%) | ||
| Ethnicity (%): | Junior high | 0 | 0 | SGLT-2 inhibitors | 2 (2·2%) | 5 (5·7%) | ||
| Hispanic | 3 (3·2%) | 5 (5·7%) | Some high school | 2 (2·2%) | 3 (3·4%) | GLP-1 agonists | 3 (3·2%) | 9 (10·2%) |
| Non-Hispanic | 76 (81·7%) | 69 (78·4%) | High school graduate | 10 (10·8%) | 9 (10·2%) | |||
| Unknown | 14 (15·1%) | 14 (15·9%) | Some college | 31 (33·3%) | 26 (29·5%) | |||
| Weight (kg): | 100·2±17·1 | 101·1±17·2 | Associate’s degree | 18 (19·4%) | 15 (17·0%) | |||
| BMI (kg/m2): | 34·7±5.1 | 34·9±5·0 | Bachelor’s degree | 19 (20·4%) | 21 (23·9%) | |||
| Duration of diabetes (years): | 16·1±7·3 | 15·3±6·0 | Post graduate degree | 13 (14·0%) | 11 (12·5%) | |||
| Duration of insulin therapy (years): | Not reported | 0 | 3 (3·4%) | |||||
| Baseline A1c: | 8·7±0·8% (72±8·8 mmol/mol) | 8·5±0·8% (69±8·8 mmol/mol) |
p=0⋅03 between groups.
In patients who changed the insulin regimens during the study, the latest regimen was used for reporting.
Abbreviations: DPP-4=dipeptidyl peptidase-4; SGLT-2-=sodium-dependent glucose cotransporter-2; GLP-1=Glucagon-like peptide-1; d-Nav®=diabetes navigator; HCP-S=healthcare professional support Abbreviations: SD=standard deviation.
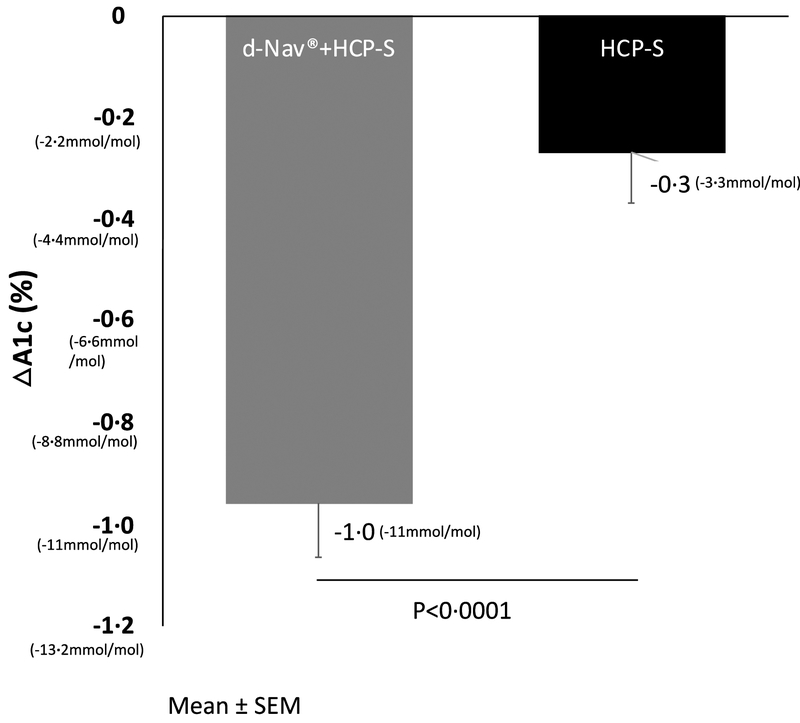
At baseline, mean HbA1c was similar in both groups (d-Nav + HCP-S, 8⋅7%±0⋅8% or 72mmol/mol±8⋅8mmol/mol; HCP-S, 8⋅5±0⋅8% or 69mmo/mol±8⋅8mmol/mol; p=0⋅2). The mean decrease of HbA1c from baseline to 6 months was 1⋅0%±1⋅0% (11mmol/mol±11mmol/mol) in the d-Nav + HCP-S group, and 0⋅3%±0⋅9% in the HCP-S group (p<0⋅0001 between groups; effect size 95%CI; 0⋅7% 0⋅4, 1⋅0 or 7⋅7 mmol/mol 4⋅7,10⋅8). In both groups, HbA1c changes during the study were statistically significant (Figure 2). In a linear regression analyses clinical site was not found to affect the differences in HbA1c changes between the groups. Mean differences was −0.688 (95%CI −0.977, −0.400) unadjusted for clinical site and −0.689 (95%CI −0.978, −0.399) when adjusted for clinical site.
Figure 2:
Average HbA1c changes during the study. Results are Mean ± SEM.
At baseline, 22⋅6% of the participants in the d-Nav + HCP-S group and 31⋅8% in the HCP-S group had HbA1c<8% (<64mmol/mol). By the end of the study, 62⋅4% of participants in the d-Nav + HCP-S group achieved HbA1c<8% (<64mmol/mol), compared to 33⋅0% in the HCP-S group (p=0⋅0001; effect size 95%CI; 29⋅4% 13⋅9, 42⋅2)(Supplementary Figure 1). Interestingly, 43⋅2% of the participants in the HCP-S experienced deterioration or lack of improvement in glycemia, compared to 14⋅9% in the d-Nav + HCP-S group (supplementary Figure 2).
Mean weekly glucose and mean fasting weekly glucose, decreased during the study in the d-Nav + HCP-S group in a statistically significant manner (p<0⋅0001) (Supplementary Figures 3,4). Mean weekly glucose and mean fasting weekly glucose remained stable in the HCP-S group (p=0⋅7 and p=0⋅3, respectively) (Supplementary Figures 3,4).
Throughout the 6-month study, the average rate of confirmed hypoglycaemia (glucose readings <54m/dl or <3mmol/l)(24), as recorded by d-Nav or glucose meters, was similar in both groups at 0⋅3±0⋅5 per month in the d-Nav + HCP-S group and 0⋅3±1⋅1 per month in the HCP-S group (p>0⋅9; effect size 95%CI; 0 −0⋅25, 0⋅25) (Table 3). When a non-parametric analysis using the Mann-Whitney test was applied, the two groups had different distributions (d-Nav + HCP-S – median 0⋅085 per month, maximum 2⋅1; HCP-S median 0 per month, maximum 8⋅7; p=0⋅003). While more patients in the d-Nav + HCP-S group experienced at least one episode of hypoglycaemia (48⋅4% vs. 21⋅6%), the values of glucose reading during a hypoglycemic event were lower in the HCP-S group than in the d-Nav + HCP-S group. As shown in Table 2, the frequency of glucose readings <50mg/dL (<2⋅8mmol/l) in the HCP-S group was higher than the frequency in d-Nav + HCP-S group (p=0⋅02). On the other hand, more readings at the ranges between ≥50 (≥2⋅8mmol/l) and <60mg/dL (<3⋅3mmol/l) (p=0⋅008) and reading between ≥60 (≥3⋅3mmol/l) and <70mg/dL (<3⋅9mmol/l) (p=0⋅001), were seen in the d-Nav + HCP-S group compared to the HCP-S group.
Table 3:
Occurrence of hypoglycemia.
| d-Nav®+HCP-S | Severity | Relation to the study | HCP-S | Effect size (95% CI) | Severity | Relation to the study | P | |
|---|---|---|---|---|---|---|---|---|
| Frequency of glucose <54mg/dL (<3mmol/l) | 0⋅29±0⋅48 per month or (0⋅07±0⋅12) per week | Not defined | Not defined | 0⋅29±1⋅12 per month or (0⋅07±0⋅28) per week | 0 (−0⋅25, 0⋅25) per month | Not defined | Not defined | P>0⋅9 |
| Severe hypoglycemia (event per 6 month): | 3 | 1. Severe 2. Moderate 3. Not defined |
1. Unrelated 2. Reasonable possibility 3. Not defined |
2 | 1. Severe 2. Severe |
1. Unrelated 2. Unrelated |
P=0⋅7 |
Severe hypoglycemia is defined as a hypoglycemic episode requiring assistance of another person to actively administer carbohydrates, glucagon, or other resuscitative action.
Abbreviations: d-Nav®=diabetes navigator; HCP-S=healthcare professional support; CI=confidence interval.
Table 2:
Primary and secondary outcomes.
| d-Nav®+HCP-S n=93 |
HCP-S n=88 |
Effect size (95% CI) | P= | |
|---|---|---|---|---|
| Primary objective: | ||||
| Reduction in HbA1c at 6-months | 1⋅0±1⋅0% (11±11mmol/mol) |
0⋅3±0⋅9% (3.3±9.9 mmol/mol) | 0⋅7%(0⋅4, 0) (7⋅7 mmol/mol (4⋅7, 10⋅8) | P<0⋅0001 |
| Secondary objectives: | ||||
| Percent of participants with A1c <7% (<53mmol/mol) at baseline | 0 | 0 | ||
| Percent of participants with A1c <7% (<53mmol/mol) at 6 months | 20 (21⋅5%) | 4 (4⋅5%) | 17⋅0% (7⋅3, 26⋅8) | P=0⋅0008 |
| Percent of participants with A1c <8% (<64mmol/mol) at baseline | 21 (22⋅6%) | 28 (31⋅8%) | 9⋅2% (−3⋅7, 21⋅8) | P=0⋅1 |
| Percent of participants with A1c <8% (<64mmol/mol) at 6 months | 58(62⋅4%) | 29 (33⋅0%) | 29⋅4% (13⋅9, 42⋅2) | P=0⋅0001 |
| Percent of participants with A1c >9% (>75mmol/mol) at baseline | 24 (25⋅8%) | 20 (22⋅7%) | 3⋅1% (−9⋅4, 15⋅4) | P=0⋅6 |
| Percent of participants with A1c >9% (>75mmol/mol) at 6 months | 10 (10⋅8%) | 7 (8⋅0%) | 2⋅8% (−6⋅2, 11⋅7) | P=0⋅5 |
| Percent of participants with A1c <7% (<53mmol/mol) without severe hypoglycemia at 6 months | 20 (21⋅5%) | 3 (3⋅4%) | 18⋅1% (8⋅7,27⋅8) | P=0⋅0003 |
| Percent of participants with A1c <8% (<64mmol/mol) without severe hypoglycemia at 6 months | 56 (60⋅2%) | 28 (31⋅8%) | 28⋅4% (13⋅9, 41⋅2) | P=0⋅0001 |
| Frequency of glucose <50mg/dL (<2⋅8mmol/l) (% of total hypoglycemic readings; % of total readings) | 0⋅1±0⋅2 per month or (0⋅03±0⋅05) per week (6⋅4%; 0⋅2%) | 0⋅2±0⋅9 per month or (0⋅05±0⋅24) per week (23⋅0%; 0⋅4%) | 0⋅1 (−0⋅1, 0⋅3) per month | P=0⋅02 |
| Frequency of glucose ≥50 (≥2–8mmol/l) and <60mg/dL (<3.3mmol/l) (% of total hypoglycemic readings; % of total readings) | 0⋅6±0⋅9 per month or (0⋅14±0⋅24)per week (29⋅0%; 0⋅8%) | 0⋅2±0⋅6 per month or (0⋅06±0⋅14) per week (26⋅6%; 0⋅5%) | 0⋅4 (0⋅2, 0⋅6) per month | P=0⋅008 |
| Frequency of glucose ≥60 (≥3⋅3mmol/l) and <70mg/dL (<3⋅9mmol/l) (% of total hypoglycemic readings; % of total readings) | 1⋅2±1.6 per month or (0⋅31±0⋅4) per week (64⋅6%; 1⋅7%) | 0⋅5±0⋅7 per month or (0⋅11±0⋅17) per week (50⋅4%; 0⋅9%) | 0⋅7 (0⋅3, 1⋅1)per month | P=0⋅001 |
| Frequency of glucose ≥54 (≥3⋅0mmoI/l) and <70mg/dL (<3.9mmol/l) | l⋅6±2⋅2 per month or (0⋅41±0⋅54) per week | 0⋅6±1⋅0 per month or (0⋅15±0⋅25)per week | 1⋅0 (0⋅5,1⋅5) per month | P=0⋅0015 |
| Difference ± SD of fasting glucose between 0 and 6 months in mg/dL (mmol/1) | −28⋅0±56–4 (−1⋅6±3⋅1) | −9⋅8±45⋅9 (−0⋅5±2⋅6) | 18⋅2 (3⋅1, 33–3) | P=0⋅00005 |
| CV of mean fasting glucose at baseline (first week on the study) | 29⋅8% | 27⋅6% | ||
| CV of mean fasting glucose at 3 months (last week before visit 2) | 35⋅7% | 25⋅1% | ||
| CV of mean fasting glucose at 6 months (last week before visit 3) | 26⋅6% | 31⋅2% |
Results are expressed as mean±SD, if applicable.
Abbreviations: SD=standard deviation; CV=coefficient of variation; NA=not-applicable; d-Nav®=diabetes navigator; HCP-S=healthcare professional support; CI=confidence interval.
Three episodes of severe hypoglycaemia (requiring the assistance of another person) were reported in the d-Nav + HCP-S group and 2 episodes in the HCP-S group (p>0⋅05) (Table 3). No fatal or near-fatal events were reported. The percent of participants who achieved HbA1c<7% (<53mmol/mol) without severe hypoglycaemia was 21⋅5% in the d-Nav + HCP-S group and 3⋅4% in the HCP-S (p=0⋅0003; effect size 95%CI; 18⋅1 8⋅7, 27⋅8). The percent of participants who achieved HbA1c<8% (<64mmol/mol) without severe hypoglycaemia was 60⋅2% in the d-Nav + HCP-S group and 31⋅8% in the HCP-S (p=0⋅0001; effect size 95%CI; 28⋅4 13⋅9, 41⋅2). No other study-related severe adverse events were reported.
On average, for subjects in the d-Nav + HCP-S group, insulin dosage adjustments were done 1⋅1+0⋅2 times per week. On average, 0⋅2+0⋅3 adjustments per week resulted in a dosage decrease. In other words, 1 out of every 6⋅5 dosage adjustments was to reduce insulin dosage. Data for the titration frequency of the HCP-S group was not available.
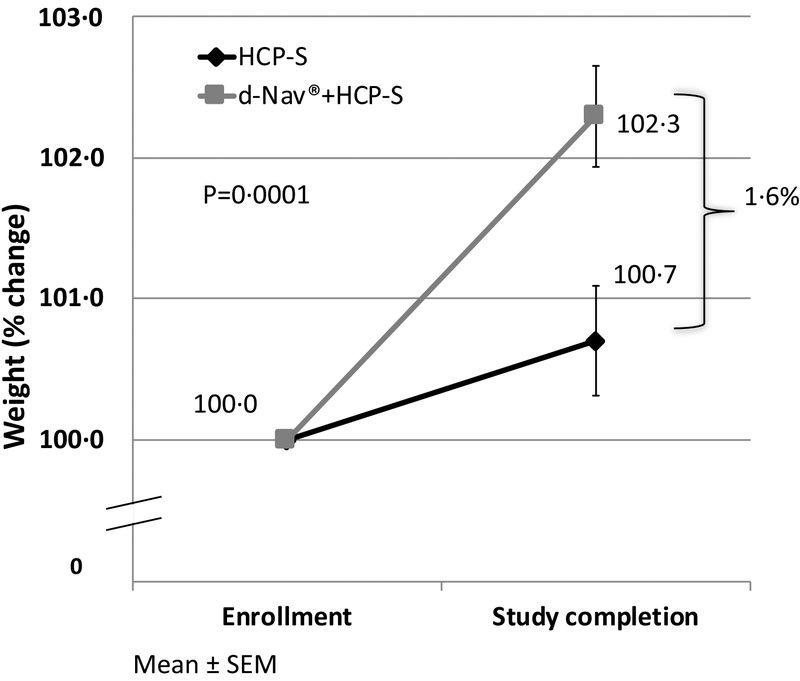
The baseline average weight in the d-Nav + HCP-S group was 100⋅2±17⋅1kg with a BMI of 34⋅7±5⋅1kg/m2. The baseline average weight in the HCP-S group was 100⋅1±17⋅2kg with a BMI of 34⋅9±5⋅01kg/m2 (p=0⋅8). Minor weight gain was seen in both groups during the study from 100⋅2±17⋅1kg to 101⋅6±18⋅0kg in the d-Nav + HCP-S group and from 101⋅1±17⋅2kg to 101⋅8±17⋅4kg in the HCP-S group. Average percentage increase in weight from baseline was 2⋅3% in the d-Nav + HCP-S group and 0⋅7% in the HCP-S (p=0⋅0001 between groups) (Figure 3).
Figure 3:
Average weight changes during the study, in percent of the initial weight. Results are Mean ± SEM. Average percentage change in weight was calculated by averaging individual percent weight change.
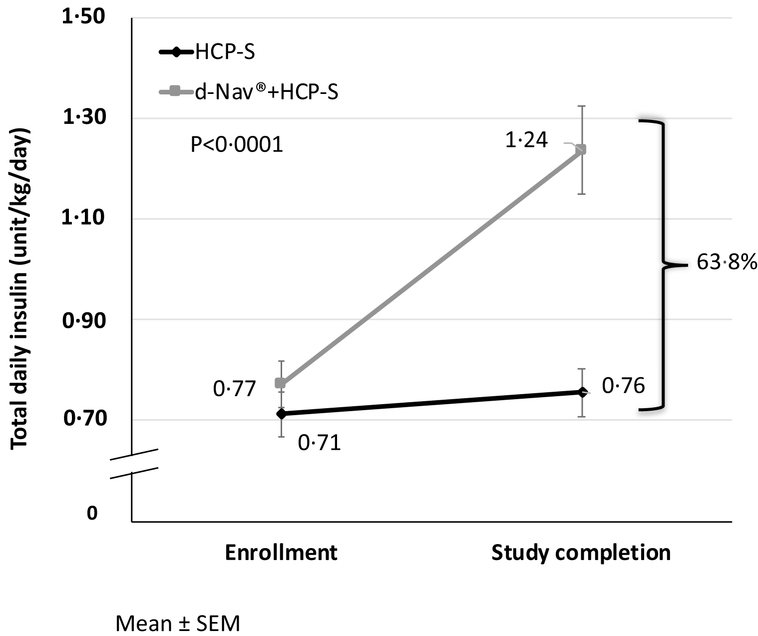
Total daily dose of insulin, normalized to body-weight, increased in both groups (Figure 4). In the d-Nav + HCP-S group, total daily dose of insulin increased from 0⋅77±0⋅4 unit/kg/day at baseline to 1⋅24±0⋅8 unit/kg/day at 6 months. In contrast, in the HCP-S group, total daily dose of insulin increased from 0⋅71±0⋅4 unit/kg/day at baseline to 0⋅76±0⋅4 units/kg/day at 6 months. The final total daily dose (normalized to body weight) was 63⋅8% higher in the d-Nav + HCP-S group compared to the HCP-S group (p=0⋅0001).
Figure 4:
Average total daily dose of insulin during the study, normalized to body weight. Results are Mean ± SEM.
Patients’ frequency of glucose measurements per week before the study was self-reported in the screening questionnaire. Frequency of glucose measurements during the study was calculated based on d-Nav or glucose meters downloads. The average expected glucose measurement per week was calculated based on 7 reading for patient using a long-acting insulin regimen, 14 for biphasic or pre-mixed regimen and 28 for basal-bolus regimen with or without carbohydrate counting. Before the study, the self-reported frequency of glucose measurements was higher than 100% of the expected in both groups. During the study, patients in the d-Nav + HCP-S group measured glucose more often than patients in the HCP-S group. In the d-Nav + HCP-S group, the lowest frequency did not decline below the recommended rate (Supplementary Figure 5).
Baseline questionnaires revealed that in both groups, for about half of the participants, diabetes was managed by primary care physicians and for the other half by endocrinologists (supplementary Table 2). In both groups, during the last year before the study, three quarters of the participants’ insulin dosage was titrated twice or less. By the end of the study, about two thirds of participants in both groups were either satisfied or very satisfied with their diabetes management during the study. About three quarters of subjects in both groups reported that they would probably or definitely agree to continue to monitor their glucose as often as needed, as dictated by their insulin regimen. In the d-Nav + HCP-S group, 39⋅3% were very comfortable having their insulin titrated by the d-Nav devices rather their providers, 31⋅0% comfortable and 20⋅2% somewhat comfortable.
Interpretation
Frequent insulin titration is a key element in effective insulin therapy(3–10). Yet the frequent titrations needed to adjust to dynamic insulin needs, coupled with the high volume of patients, prohibit clinicians from fulfilling this need. The evolving technology of closed-loop delivery systems is aiding in closing the titration gap in patients with type 1 diabetes. Yet, due to its cost and complexity, the majority of the insulin users with insulin requiring type 2 diabetes will not likely utilize closed-loop delivery systems(13, 14). In this article, we provide evidence for the superiority of a health care professional supported automated titration guidance technology over health care professional support alone. The d-Nav system streamlines the insulin titration process in what appears to be a scalable way.
The population recruited for the study was ethnically and geographically diverse. Compared to the HCP-S group, the glycemic outcomes in the d-Nav + HCP-S group were superior, as measured by a number of endpoints. When compared with the control group, the d-Nav + HCP-S group experienced a greater than 3-fold improvement in HbA1c. Patients in this group were 3 times more likely to show any glycemic improvement than those in the control group, with about 2 times as many patients reducing their HbA1c below 8% (64mmol/mol) and 6 times as many patients reducing HbA1c below 7% (53mmol/mol). The limited number of self-measured glucose readings per day is the most probable cause explaining why HbA1c levels did not precisely match the average glucose and average fasting glucose.
The average frequency of hypoglycaemia <54mg/dL (<3⋅0mmol/l), was similar in both groups. In contrast, while more patients in the d-Nav + HCP-S group experienced at least one hypoglycemic event compared to the HCP-S group, the values of low glucose readings were lower in the HCP-S group. This result corresponds to the current consensus regarding hypoglycaemia risk stratification and reporting(24). The frequency of severe hypoglycaemia was low and similar between groups. Yet, the number of patients achieving glycemic goal without severe hypoglycaemia was more than 6 times higher in the d-Nav + HCPS group. The number of overall glucose measurements in the HCP-S group was 30%−40% lower than the number in the d-Nav + HCP-S. Therefore, the reported frequency of hypoglycemia in the HCP-S group may have underestimated the actual one.
Preservation of adequate treatment safety while achieving superior glycemic control, as suggested by our results, is expected when frequent insulin titrations are implemented. Insulin requirements do not have a steady state. It has been shown that most patients experience unpredictable 39⋅9%±12⋅6% decline in insulin requirements every 1⋅3 years for a period of 10⋅0±7⋅7 weeks(9, 25). Therefore, the capacity to promptly down titrate insulin is fundamental. This feature (reduction of insulin dosage as needed) was utilized by the d-Nav devices (in the d-Nav + HCP-S group) in 15⋅4% of the titrations.
By the end of the 6-month follow up, total daily insulin (normalized to body weight) was more than 60% higher in the d-Nav + HCP-S group compared with the HCP-S group. Since the percent of weight gain was only 1⋅6% higher in the d-Nav + HCP-S group, weight changes did not explain changes in insulin needs, as previously shown(26). Weight gain is an inherent well-known phenomenon associated with insulin intensification. Also, it is known that average insulin requirement in patients with long-standing type 2 diabetes is about 1.7unit per kg body weight per day(5). Reassuringly, both phenomena have not shown to compromise the benefit of insulin replacement(27, 28).
The final dosage of 1⋅24 unit/kg/day in the d-Nav + HCP-S group is, in fact, lower than the expected age- and BMI-matched US patients(5, 29). As the authors have demonstrated, due to the small but steady nature of the d-Nav’s dosage increments, more than 6 months are needed to allow HbA1c and insulin requirements to stabilize(26). It has been previously shown that many patients with type 2 diabetes are under dosed with insulin and a wide gap exists between their prescribed dosage and their approximate requirements(5, 11, 29). For the majority of patients, insulin titrations in real-world setting are incapable of closing this gap in a reasonable timeframe.
Most patients in each group were treated with regimens that could be used for the long-term, namely basal-bolus or biphasic insulin therapy(30)(see Supplementary Table 1). Thus the use of long-acting insulin regimen was unlikely to affect the average total daily dose of insulin.
The high frequency of patient-provider communication in both groups exceeded the typical standard of care. Yet, glycemic improvement was modest in the HCP-S group. The titrations made by d-Nav in the d-Nav group were well accepted by patients. Also, subjects in the d-Nav + HCP-S group tended to measure glucose levels more frequently than in the HCP-S group. Intriguingly, this difference was seen also during the 2nd visit (3 months) during which superior average HbA1c in the d-Nav + HCP-S group has already been seen (not shown). Yet at this time subjects in both groups measured glucose more than 100% of the expected number of tests. These data imply that the difference in glucose measurement was unlikely to be the cause for the difference in glycemia between the groups, rather witnessing improved glycemia may have been the driving force for the patients in the d-Nav + HCP-S group to measure more, as previously shown(31).
There are currently a number of alternative approaches to optimizing insulin therapy. As digital communication technology advances, a multitude of data delivery systems enable real-time delivery of patients’ glucose readings from their monitoring devices to their providers(32). Many titration guidelines have been transformed into decision support systems used by providers. Telemedicine platforms allow providers to conduct video calls with patients and to make medication adjustments(33). However, such solutions still require providers—whose time is already overstretched— to adjust and convey the new insulin dosage to patients. Many titration guidelines have been transformed into apps that can be used by patients to titrate their insulin dosage, although such FDA cleared apps are prescription only devices. Yet, despite the long-term availability of insulin self-titration instructions, glycemic control in insulin treated patients has not improved for decades.
The main limitation of the study was its relatively short duration. The main strengths of the study were its large number of patients, diverse ethnical background and multiplicity of geographic locations.
In summary, health care professional support without self-titration technology did not substantially improve the care of patient using insulin. On the other hand, the combination of the automated guidance of insulin titration with HCP-S support closes the titration gap in a way that facilitates a significant improvement in glycemic control while maintaining adequate treatment safety.
Supplementary Material
Research in context.
Evidence before this study
The National Health and Nutrition Examination Survey (NHANES) collects cross-sectional, complex probability samples of the US population on a regular basis. Recently, nearly 5000 adult participants diagnosed with diabetes in the 1988–1994 and 2005–2012 NHANES cycles were compared. Investigators at Johns Hopkins evaluated this NHANES data set for trends in insulin use and diabetes control. Both the percent using any insulin (30% in 1988–1994, 29% in 2005–2012) and the percent of participants achieving an HbA1c <7% (34% in 1988–1994, 32% in 2005–2012) were remarkably constant over this approximately twenty year period. Despite advancements in technology and pharmacotherapy, most patients who use insulin do not achieve their therapy goals, increasing the risk for debilitating and costly complications. Literature review indicates that health care support and frequent and ongoing insulin dose adjustments are two key elements associated with improved glycemic control. Despite this evidence it is rare that timely and effective insulin dose titrations are made on a regular basis due in part to limited time and medical expertise. This led us to conduct a randomized control trial in individuals with type 2 diabetes on insulin, not at HbA1c goals, that compared health care professional support to the use of a new technology for insulin dose guidance along with health care professional support to see which was most effective at improving HbA1c levels while minimizing hypoglycemia.
Added value of this study
This multicenter randomized controlled study (N=181) tested whether the d-Nav® Insulin Guidance System, a handheld device that contains a glucose meter and insulin adjustment software that provides its user with a dose-by-dose insulin recommendation, together with health care professional support, is superior to a health care professional support model alone. Over a period of 6 months, the combination of d-Nav and health care professional support resulted in a lower HbA1c in patients with type 2 diabetes [HbA1c reduction (mean±SD) of 1.0%±1.0% in the d-Nav plus health care professional support group compared to an HbA1c reduction (mean±SD) of 0.3%±0.9% in the health care professional support model alone (p<0.0001)] with a similar safety profile.
Implications of all the available evidence
Insulin can be used effectively to achieve improved glycemic control if technology to provide automated insulin titration guidance is combined with health care professional support. Such an approach now needs to be evaluated across large health care systems to confirm these findings and study its cost effectiveness.
Acknowledgments
The funding source, National Institute of Health’s NIDDK program, award number 2R42DK085974–02A1. We thank the patients who participated in the study.
Funding: National-Institute of Health (2R42DK085974–02A1).
Appendix
About d-Nav®
d-Nav is designed to allow patients to enhance their insulin regimen. It does so by providing patients with dose-by-dose guidance, while preforming forced titration in the background. Rather than having the physician (HCP) “in the loop,” i.e. titrating the insulin dosage, it keeps the physician “on the loop,” allowing the health care provider to decide if the patient is using an appropriate insulin regimen knowing that d-Nav will titrate each dose.
From a patient perspective, d-Nav is primarily a dose calculator. It tells the patient how many units of insulin to inject. Dose guidance is based on a built-in insulin dosage function, similar to what a patient would be prescribed on a piece of paper. Before every scheduled injection, the patient would insert a test strip and apply blood to measure their glucose. d-Nav shows the glucose reading for a few seconds and then replaces the glucose value with an “event” screen, e.g. Breakfast. Based on the selected event and the stored dosage function d-Nav recommends a dose of insulin.
Under the hood, d-Nav continuously assesses the glucose readings and search for glucose patterns. These are used to automatically titrate the stored dosage function without HCP intervention. Different doses are adjusted independently from each other. For example, in a basal-bolus regimen: patterns in breakfast glucose are used to adjust the basal dose, patterns in lunch readings are used to adjust the breakfast dose, patterns in dinner readings are used to adjust lunch doses, and patterns in bedtime readings are used to adjust dinner doses. In a biphasic insulin regimen, patterns in breakfast glucose are used to adjust the dinner dose, patterns in dinner readings are used to adjust the breakfast dose.
It takes a full week before d-Nav is allowed to increase any doses, but doses can decrease as often as a “low glucose pattern” is detected. For example, two blood glucose readings below 65 mg/dl at lunch would immediately cause a reduction in breakfast dose. To increase a dose, four or more elevated glucose readings, for a single event such as “Breakfast,” within the last week are required. Dose adjustments are proportional to the distance between the average glucose and the target range, i.e. if the average glucose is 250 mg/dl d-Nav would increase a dose by ~20%, while if the average glucose is 150mg/dl it will increase a dose by ~10%.
We have reported elsewhere results regarding patients’ satisfaction and long-term outcomes with d-Nav (9, 16–20). It was shown that on average d-Nav titrates the patient’s dosage function every 6.4 days. Most patients respond very favorably to the simplicity of d-Nav, and often comment about the fact that it takes the guess-work out of insulin injections. It was further shown that patients were able to maintain A1c at goal for more than three years of continual use of d-Nav.
d-Nav is not designed to replace the treating physician but rather to support physicians’ ability to treat patients using desired insulin regimens without increasing the workload. For example, if a patient is prescribed with once a day basal insulin alone and they come to the clinic 6 months later with an elevated A1c it is hard to know if it’s the “wrong dose” or the “wrong regimen.” With d-Nav it becomes clear that it is not a dose question since doses are constantly optimized by the device. Yet, if the physician sees a patient has a stable dose of long-acting insulin only regimen, a close to normal fasting glucose, and an elevated A1c, they can quickly realize that it’s time to intensify the insulin therapy. The solution has been in use in the United Kingdom since 2012, supporting patient’s insulin therapy for over 6 years(21).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
EB is the chief executive officer for Hygieia Inc.; IH is a co-founder of Hygieia; SGB is an employees of Hygieia; DFK holds stocks of Hygieia; DJMI is a paid consultant for Hygieia; RMB, MJ, RP, AB, and NY have no financial interest in Hygieia.
References
- 1.Selvin E, Parrinello CM, Daya N, Bergenstal RM. Trends in Insulin Use and Diabetes Control in the U.S.: 1988–1994 and 1999–2012. Diabetes Care. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Y, Abbott S, Nguyen M, Grabner M, Quimbo R. Glycemic Control of Insulin Treated Patients Across the U.S.: Epidemiologic Analysis of a Commercially Insured Population. American Diabetes Association Meeting. 2013;2765–PO. [Google Scholar]
- 3.Riddle MC, Yki-Jarvinen H, Bolli GB, Ziemen M, Muehlen-Bartmer I, Cissokho S, et al. One year sustained glycaemic control and less hypoglycaemia with new insulin glargine 300 U/mL compared with 100 U/mL in people with type 2 diabetes using basal + meal-time insulin (EDITION 1 12-month randomized trial including 6-month extension). Diabetes Obes Metab. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davidson MB. How our current medical care system fails people with diabetes: lack of timely, appropriate clinical decisions. Diabetes Care. 2009;32(2):370–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergenstal RM, Johnson M, Powers MA, Wynne A, Vlajnic A, Hollander P, et al. Adjust to target in type 2 diabetes: comparison of a simple algorithm with carbohydrate counting for adjustment of mealtime insulin glulisine. Diabetes Care. 2008;31(7):1305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holman RR, Thorne KI, Farmer AJ, Davies MJ, Keenan JF, Paul S, et al. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med. 2007;357(17):1716–30. [DOI] [PubMed] [Google Scholar]
- 7.Herman WH, Ilag LL, Johnson SL, Martin CL, Sinding J, Al Harthi A, et al. A clinical trial of continuous subcutaneous insulin infusion versus multiple daily injections in older adults with type 2 diabetes. Diabetes Care. 2005;28(7):1568–73. [DOI] [PubMed] [Google Scholar]
- 8.Buse JB, Wolffenbuttel BH, Herman WH, Shemonsky NK, Jiang HH, Fahrbach JL, et al. DURAbility of basal versus lispro mix 75/25 insulin efficacy (DURABLE) trial 24-week results: safety and efficacy of insulin lispro mix 75/25 versus insulin glargine added to oral antihyperglycemic drugs in patients with type 2 diabetes. Diabetes Care. 2009;32(6):1007–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harper R, Donnelly R, Bi Y, Bashan E, Minhas R, Hodish I. Dynamics in insulin requirements and treatment safety. J Diabetes Complications. 2016;30(7):1333–8. [DOI] [PubMed] [Google Scholar]
- 10.Bastyr EJ 3rd, Zhang S, Mou J, Hackett AP, Raymond SA, Chang AM. Performance of an Electronic Diary System for Intensive Insulin Management in Global Diabetes Clinical Trials. Diabetes Technol Ther. 2015;17(8):571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenstock J, Ahmann AJ, Colon G, Scism-Bacon J, Jiang H, Martin S. Advancing insulin therapy in type 2 diabetes previously treated with glargine plus oral agents: prandial premixed (insulin lispro protamine suspension/lispro) versus basal/bolus (glargine/lispro) therapy. Diabetes Care. 2008;31(1):20–5. [DOI] [PubMed] [Google Scholar]
- 12.Thabit H, Tauschmann M, Allen JM, Leelarathna L, Hartnell S, Wilinska ME, et al. Home Use of an Artificial Beta Cell in Type 1 Diabetes. N Engl J Med. 2015;373(22):2129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foerster V, Severn M. A Hybrid Closed-Loop Insulin Delivery System for the Treatment of Type 1 Diabetes. CADTH Issues in Emerging Health Technologies. Ottawa (ON) 2016. p. 1–11. [PubMed] [Google Scholar]
- 14.McQueen RB, Ellis SL, Campbell JD, Nair KV, Sullivan PW. Cost-effectiveness of continuous glucose monitoring and intensive insulin therapy for type 1 diabetes. Cost Eff Resour Alloc. 2011;9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prevention CfDCa. 2018. Available from: https://www.cdc.gov/diabetes.
- 16.Bashan E, Harper R, Bi Y, Hodish I. A novel approach to optimise glycaemic control in insulin users. BMJ Case Rep. 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergenstal RM, Bashan E, McShane M, Johnson M, Hodish I. Can a Tool That Automates Insulin Titration Be a Key to Diabetes Management? Diabetes Technol Ther. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bashan E, Hodish I. Frequent insulin dosage adjustments based on glucose readings alone are sufficient for a safe and effective therapy. J Diabetes Complications. 2012;26(3):230–6. [DOI] [PubMed] [Google Scholar]
- 19.Bashan E, Herman WH, Hodish I. Are glucose readings sufficient to adjust insulin dosage? Diabetes Technol Ther. 2011;13(1):85–92. [DOI] [PubMed] [Google Scholar]
- 20.Rosenthal ES, Bashan E, Herman WH, Hodish I. The effort required to achieve and maintain optimal glycemic control. J Diabetes Complications. 2011;25(5):283–8. [DOI] [PubMed] [Google Scholar]
- 21.Donnelly RC S;Harper R Diabetes Insulin Guidance System: a real-world evaluation of new technology (d-Nav) to achieve glycaemic control in insulin-treated type 2 diabetes. Practical Diabetes. 2015;32(7):247–52. [Google Scholar]
- 22.Henderson JN, Allen KV, Deary IJ, Frier BM. Hypoglycaemia in insulin-treated Type 2 diabetes: frequency, symptoms and impaired awareness. Diabet Med. 2003;20(12):1016–21. [DOI] [PubMed] [Google Scholar]
- 23.Bergenstal RM, Bashan E, McShane M, Johnson M, Hodish I. Can a tool that automates insulin titration be a key to diabetes management? Diabetes Technol Ther. 2012;14(8):675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.International Hypoglycaemia Study G. Glucose Concentrations of Less Than 3.0 mmol/L (54 mg/dL) Should Be Reported in Clinical Trials: A Joint Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2017;40(1):155–7. [DOI] [PubMed] [Google Scholar]
- 25.Harper R, Bashan E, Bisgaier SG, Willis M, Isaman DJM, Hodish I. Temporary Reductions in Insulin Requirements Are Associated with Hypoglycemia in Type 2 Diabetes. Diabetes Technol Ther. 2018;Accepted for publication in 11/18. [DOI] [PubMed] [Google Scholar]
- 26.Holman RR, Farmer AJ, Davies MJ, Levy JC, Darbyshire JL, Keenan JF, et al. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009;361(18):1736–47. [DOI] [PubMed] [Google Scholar]
- 27.Carnethon MR, De Chavez PJ, Biggs ML, Lewis CE, Pankow JS, Bertoni AG, et al. Association of weight status with mortality in adults with incident diabetes. JAMA. 2012;308(6):581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akram K, Pedersen-Bjergaard U, Borch-Johnsen K, Thorsteinsson B. Frequency and risk factors of severe hypoglycemia in insulin-treated type 2 diabetes: a literature survey. J Diabetes Complications. 2006;20(6):402–8. [DOI] [PubMed] [Google Scholar]
- 29.Riddle MC, Bolli GB, Ziemen M, Muehlen-Bartmer I, Bizet F, Home PD, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 1). Diabetes Care. 2014;37(10):2755–62. [DOI] [PubMed] [Google Scholar]
- 30.Wang C, Mamza J, Idris I. Biphasic vs basal bolus insulin regimen in Type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabet Med. 2015;32(5):585–94. [DOI] [PubMed] [Google Scholar]
- 31.Schutt M, Kern W, Krause U, Busch P, Dapp A, Grziwotz R, et al. Is the frequency of self-monitoring of blood glucose related to long-term metabolic control? Multicenter analysis including 24,500 patients from 191 centers in Germany and Austria. Exp Clin Endocrinol Diabetes. 2006;114(7):384–8. [DOI] [PubMed] [Google Scholar]
- 32.Mora P, Buskirk A, Lyden M, Parkin CG, Borsa L, Petersen B. Use of a Novel, Remotely Connected Diabetes Management System Is Associated with Increased Treatment Satisfaction, Reduced Diabetes Distress, and Improved Glycemic Control in Individuals with Insulin-Treated Diabetes: First Results from the Personal Diabetes Management Study. Diabetes Technol Ther. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.eVisit. 2018. Available from: https://evisit.com.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.