Abstract
Impaired osteoblast and osteocyte maturation contribute to mineralization defects and excess FGF23 expression in CKD bone. Vitamin D sterols decrease osteoid accumulation and increase FGF23 expression; these agents also increase osteoblast maturation in vitro but a link between changes in bone cell maturation, bone mineralization, and FGF23 expression in response to vitamin D sterols has not been established. We evaluated unmineralized osteoid accumulation, osteocyte maturity markers (FGF23:early osteocytes; sclerostin: late osteocytes), and osteocyte apoptosis in iliac crest of 11 pediatric dialysis patients before and after 8 months of doxercalciferol therapy. We then evaluated the effect of 1,25(OH)2vitamin D on in vitro maturation and mineralization of primary osteoblasts from dialysis patients. Unmineralized osteoid accumulation decreased while numbers of early (FGF23-expressing) increased in response to doxercalciferol. Osteocyte apoptosis was low but increased with doxercalciferol. Bone FGF23 expression correlated with numbers of early, FGF23-expressing, osteocytes (r=0.83, p<0.001). In vitro, 1,25(OH)2vitamin D increased expression of the mature osteoblast marker osteocalcin (BGLAP) but only very high (100 nM) concentrations affected in vitro osteoblast mineralization. High doses (10 and 100 nM) of 1,25(OH)2vitamin D also increased the ratio of RANKL/OPG expression in CKD osteoblasts. Vitamin D sterols directly stimulate osteoblast maturation. They also increase osteocyte turnover and increase osteoblast expression of osteoclast differentiation factors, thus likely modulating osteoblast/osteoclast/osteocyte coupling. By increasing numbers of early osteocytes, vitamin D sterols increase FGF23 expression in CKD bone.
Keywords: osteoblasts, osteocytes, vitamin D, chronic kidney disease
INTRODUCTION
Chronic kidney disease (CKD) is associated with changes in mineral ion homeostasis which have implications for both skeletal health and cardiovascular outcomes (1). Secondary hyperparathyroidism causes high turnover bone disease which results in severe bone deformities, bone pain, and fractures (2). Skeletal mineralization defects are also common in children with CKD but the etiology of these defects, which can be identified even in children with normal calcium, phosphorus, and PTH concentrations (3, 4), is incompletely understood. We have recently demonstrated that CKD bone is characterized by impaired osteocyte maturation; primary osteoblasts derived from CKD patients also display a maturation defect in vitro (5). This delay associates with defective mineralization of CKD osteoblasts ex vivo and may contribute to the skeletal mineralization defects which are widely prevalent in the pediatric CKD population (3, 4).
Active vitamin D sterols are effective at treating secondary hyperparathyroidism; they also directly affect bone cell physiology. 1,25(OH)2vitamin D stimulates osteoblast maturation in vitro (6) and also regulates skeletal hormone expression. Expression of the osteocyte-specific hormone fibroblast growth factor 23 (FGF23), which is markedly increased in CKD bone, increases further in response to 1,25(OH)2vitamin D (3). We have recently demonstrated that FGF23 is a marker of osteocytes in an early phase of secondary mineralization (7). However, whether 1,25(OH)2vitamin D increases FGF23 expression by increasing the number of early osteocytes in CKD bone or whether it stimulates per-osteocyte FGF23 expression in CKD bone remains unknown.
In order to investigate the effects of active vitamin D sterols on osteoblast and osteocyte maturation in CKD patients, we evaluated osteoid accumulation, osteocyte maturation markers, and osteocyte apoptosis in bone biopsy specimens from CKD patients before and after 8 months of therapy with the active vitamin D sterol doxercalciferol. We then used primary human osteoblasts from pediatric dialysis patients, which, as we have previously demonstrated (5), maintain intrinsic impairments in maturation and mineralization when removed from the uremic milieu, to evaluate the direct effects of 1,25(OH)2vitamin D on CKD-mediated osteoblast maturation and mineralization impairments in vitro.
MATERIALS AND METHODS
Study Subjects
Full thickness iliac crest bone biopsy samples were obtained after double tetracycline labeling from eleven pediatric dialysis patients (7 male and 4 female/ 9 Hispanic, 1 white, and 1 black). The average age of the patients from whom bone biopsies were obtained was 15.8 ± 0.8 years; these patients had been on dialysis for an average of 1.1 ± 0.4 years. At baseline, patients had evidence of secondary hyperparathyroidism; namely, increased bone turnover and/or bone marrow fibrosis. They were treated for 8 months with doxercalciferol, an active vitamin D analogue, and the phosphate binder sevelamer carbonate, after which time they underwent a second double tetracycline-labeled biopsy. Doxercalciferol and sevelamer were titrated during the treatment period according to a protocol which considered serum PTH, calcium, and phosphate concentrations (3). During the course of therapy, subjects received an average of 19.3 ± 3.8 mcg of doxercalciferol per week. Circulating values of calcium, phosphorus, and alkaline phosphatase values were assessed at the time of bone biopsy using an Olympus AU5400 analyzer (Olympus America Incorporated, Center Valley, PA). PTH concentrations in EDTA plasma were measured by the 1st generation immunometric assay (Quidel, San Diego, California, normal range: 10-65 pg/ml) and FGF23 levels were determined in EDTA plasma by a 2nd generation C-terminal assay (Quidel). Circulating 1,25(OH)2vitamin D levels were measured at baseline and in the middle (month 3 to 4) of therapy to monitor medication compliance. As previously reported, calcitriol levels increased by 10.9 ± 5.0 pg/ml and doxercalciferol doses correlated with the change in circulating calcitriol concentration (r=0.55, p=0.08) (8). The study was approved by the UCLA Human Subject Protection Committee and informed consent was obtained from all patients and/or parents.
Characterization of bone histomorphometry, immunohistochemistry and apoptosis
Histomorphometric analysis was performed in un-decalcified bone from all pediatric CKD patients and healthy controls using the OsteometricsR system and standard measures of bone turnover, mineralization, and volume were measured and calculated (9). The results were previously described (10). A “mineralization defect” was defined by the presence of excess osteoid volume in combination with a prolonged osteoid maturation rate. Immunohistochemistry was performed to assess bone FGF23 (225-244) (Qidel), DMP1 (LFMb31(62-513) (Santa Cruz Biotechnology), and sclerostin (R&D Systems) expression in un-decalcified sections of iliac crest. Immunoreactivity for these proteins was quantified using the Ariol scanning system and values were previously reported (11). For the current analysis, numbers of FGF23-expressing osteocytes were also counted. These were normalized to trabecular bone area (11).
Apoptosis was also assessed in non-decalcified bone sections via in situ TUNEL reaction in iliac crest samples from 5 healthy controls and 11 pediatric dialysis patients before and after doxercalciferol therapy using Klenow terminal deoxynucleotidyl transferase per manufacturer's instructions (Oncogene Research Products). Positive staining for apoptosis was detected by peroxidase streptavidin conjugated and 3,3’diaminobenzidine. Sections were counter-stained with methyl green to indentify live (green staining) osteocytes. Sections incubated with vehicle alone served as negative controls and a positive control was generated by treating one of the samples with DNAse I.
Primary osteoblast maturation and mineralization potential and gene analysis
Primary osteoblasts were obtained at the time of bone biopsy as previously described (5). Osteoblasts from 5 adolescent CKD patients and from 3 healthy adolescent controls were used for these experiments. The baseline mineralization characteristics of the cells from these patients have previously been reported (7). All CKD patients were treated with maintenance dialysis and end stage kidney disease was due to congenital anomalies of the kidneys and urinary tract (n=2) and glomerulonephritis (n=3). Bone and cells from healthy adolescent controls were obtained at the time of surgery for idiopathic scoliosis or for maxillofacial surgery requiring grafting. Mineralization potential was assessed in primary osteoblasts plated in 12 well plates at equal density (1 × 104 cells per well). Primary osteoblasts were grown to confluence in the presence of DMEM, 10% fetal bovine serum, and 100 μg/ml ascorbic acid and then stimulated to mineralize by adding 10 mM β-glycero-phosphate and 10−8 M dexamethasone and varying (0 nM, 1 nM, 10 nM, and 100 nM) concentrations of 1,25(OH)2vitamin D. Mineralization was quantified by staining cultures at weekly intervals with 1% (w/v) solution of Alizarin red S (pH 6.4) (Sigma-Aldrich). Dye was extracted from the cell layer and the supernatant was analyzed at 490 nm. Three technical replicates were evaluated at each timepoint and the coefficient of variation between technical replicates was less than 6%. Cell number was estimated by staining one parallel well at each timepoint with 0.05% Crystal Violet; staining was quantified by extracting the dye from the cell layer by incubation in methanol for 30 min and then analyzing the supernatant at 605 nm.
RNA was isolated from parallel cultures and quantitative real-time PCR amplification was performed using QuaniTect ® Probe PCR kit (Qiagen, Hilden, Germany). Taqman assays were used to quantify the expression of osteocalcin (BGLAP), Runx2 (RUNX), alkaline phosphatase (ALPL), receptor activator of nuclear factor kappa-B ligand (RANKL), and osteoprotegerin (OPG) along with the housekeeping gene GAPDH. Relative quantification studies of threshold cycle were performed with Sequence Detector software (Applied Biosystems, Foster City, CA). Samples from each individual patient were assayed in triplicate at each time point; the coefficient of variation between these technical replicates was less than 4% for each gene at each time point.
In vitro evaluation of osteocyte apoptosis
The effects of 1,25(OH)2vitamin D on osteocyte apoptosis were evaluated in immortalized murine osteocytes (MLO-Y4) which were cultured to 80% confluence. After overnight serum deprivation, cells were cultured in either normal (3 mM phosphate) or phosphate enriched (10 mM phosphate) media in the presence or absence of 1,25(OH)2vitamin D3 (100 nM). Apoptosis was assessed visually under fluorescent microscopy (510/540 nm excitation filters) by acridine orange/ethidium bromide with trypan blue exclusion staining at 24 hours. Using this technique, live cells appear uniformly green; early apoptotic cells stain green and contain bright green nuclear dots (indicating chromatin condensation and nuclear fragmentation); late apoptotic cells stain orange and also display bright green nuclear dots. Apoptosis activation was also assessed by caspase-3 activity (Biovision, Milpitas, CA) in cell lysates after 24 hours in culture with phosphate (10 nM), 1,25(OH)2vitamin D3 (100 nM), or both (as above).
Statistical analysis
Measurements are reported as median (interquartile range) or mean ± standard error. The Mann-Whitney U and the Wilcoxon Signed Rank tests were used to assess between-group and from-baseline differences, respectively. Spearman correlation coefficients were used to assess relationships between variables. All statistical analyses were performed using SAS software (SAS Institute Inc.) and all tests were two-sided. A probability of type I error less than 5% was considered statistically significant and ordinary p values are reported.
RESULTS
Doxercalciferol increases FGF23 expression by increasing numbers of early osteocytes in CKD bone
As previously shown, although circulating calcitriol concentrations correlated with prescribed doxercalciferol dose and increased with therapy (8), PTH levels did not change in response to doxercalciferol (Supplemental Table). By contrast, osteoid volume decreased. Marrow fibrosis area also tended to decrease (from 0.23 (0.11, 0.45) % to 0.07 (0.01, 0.21) %; p=0.06 from baseline). These findings confirm previous data suggesting that vitamin D sterols directly affect bone, confounding the relationship between circulating biomarkers and bone histology (3, 12).
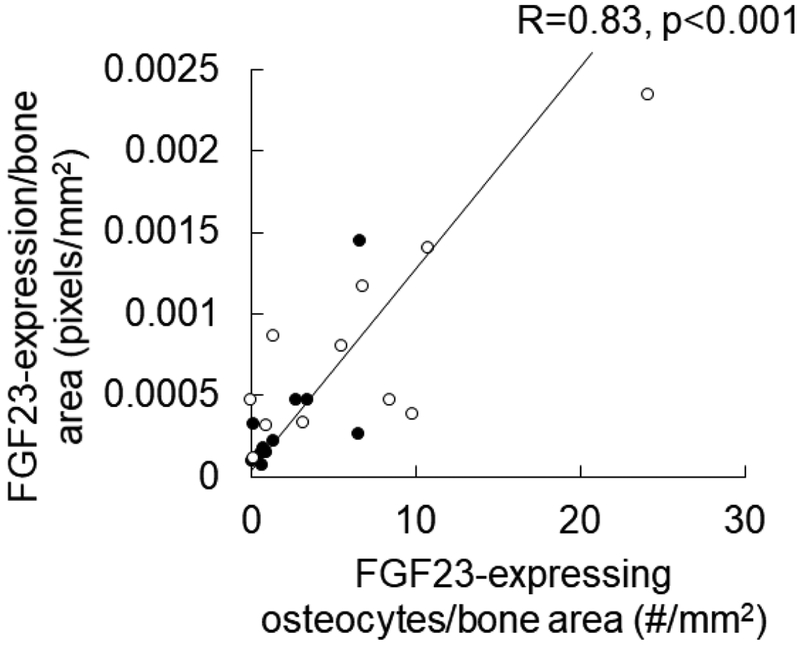
We have previously demonstrated that FGF23 protein expression, as determined by Ariol scanning of immunohistochemical detection of FGF23 in trabecular bone, increases in response to doxercalciferol treatment (8). In order to evaluate whether these changes in FGF23 expression are due to increased numbers of FGF23-expressing osteocytes, we counted numbers of FGF23-expressing osteocytes in the trabecular bone of dialysis patients before and after 8 months of doxercalciferol therapy. Circulating FGF23 levels increased in response to doxercalciferol, as did numbers of FGF23-expressing osteocytes (Supplemental Table). As shown in Figure 1, numbers of FGF23-expressing osteocytes correlated closely with bone FGF23 protein expression (r=0.83, p<0.001). In a subset of 6 patients who had pre/post therapy biopsies adequate for RNA extraction, FGF23 mRNA increased by 226 (124, 320)% (p<0.05 from baseline) while numbers of FGF23-expressing osteocytes increased by 226 (83, 440)% in this same subset. The relationship between FGF23 RNA message, FGF23 protein, and numbers of FGF23-expressing osteocytes suggests that much of the doxercalciferol-mediated increase in FGF23 expression is due to increased numbers of FGF23-expressing osteocytes in CKD bone. The previously demonstrated co-localization of FGF23 with e11/gp38 (7) suggests that the increase in numbers of FGF23-expressing osteocytes reflects an increase in early osteocyte numbers.
Figure 1: Numbers of FGF23-expressing osteocytes increase in response to active vitamin D sterols.
Scatterplot demonstrating the tight (r=0.83; p<0.001) relationship between numbers of FGF23-expressing osteocytes (X-axis) and quantification of FGF23 immunostaining in bone. Closed circles represent values in bone biopsies prior to doxercalciferol therapy and open circles represent values in samples from the same patients treated with doxercalciferol.
Doxercalciferol stimulates osteocyte turnover in vivo
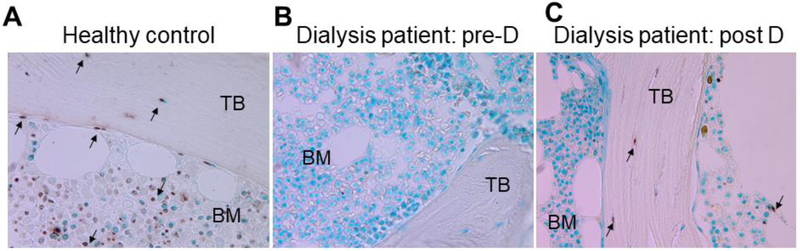
We have previously demonstrated that CKD is characterized by stagnant osteoblast and osteocyte maturation, with nearly undetectable osteocytes apoptosis in CKD bone (7). We have also previously shown an increase in expression of the mature osteocyte marker sclerostin in response to doxercalciferol (8). In order to determine whether this increase in mature osteocytes is associated with an overall increase in osteocyte turnover in response to active vitamin D sterols, we performed TUNEL staining on iliac crest bone biopsies before and after 8 months of doxercalciferol therapy. Methyl green staining demonstrated a predominance of live osteocytes with minimal numbers of empty lacunae. Apoptosis was apparent in cortical bone, trabecular bone and bone marrow of normal controls. Cortical and trabecular osteocyte apoptosis and marrow apoptosis were markedly decreased in CKD bone prior to doxercalciferol therapy. Only 27% of dialysis patients had any apoptotic cortical osteocytes and only one dialysis patient (i.e. 9%) displayed any apoptotic trabecular osteocytes at baseline. After doxercalciferol, 100% of patients had evidence of apoptotic cortical osteocytes (p<0.05) and 91% had apopotic trabecular osteocytes (p<0.05) (Figure 2); these values were similar to apoptotic osteocytes observed in healthy adolescent bone. Increased numbers of early osteocytes, increased numbers of mature osteocytes, and restoration of osteocyte apoptosis suggest that doxercalciferol increases new osteocyte recruitment and osteocyte turnover in advanced CKD.
Figure 2: Osteocyte apoptosis is decreased in advanced CKD but increases in response to active vitamin D sterols.
TUNEL staining of iliac crest in a normal control (A) and in a dialysis patient before (B) and after (C) 8 months of therapy with doxercalciferol (“D”) and phosphate binders. Arrows point to apoptotic cells.
Increased numbers of early osteocytes is associated with decreases in osteoid accumulation in response to doxercalciferol.
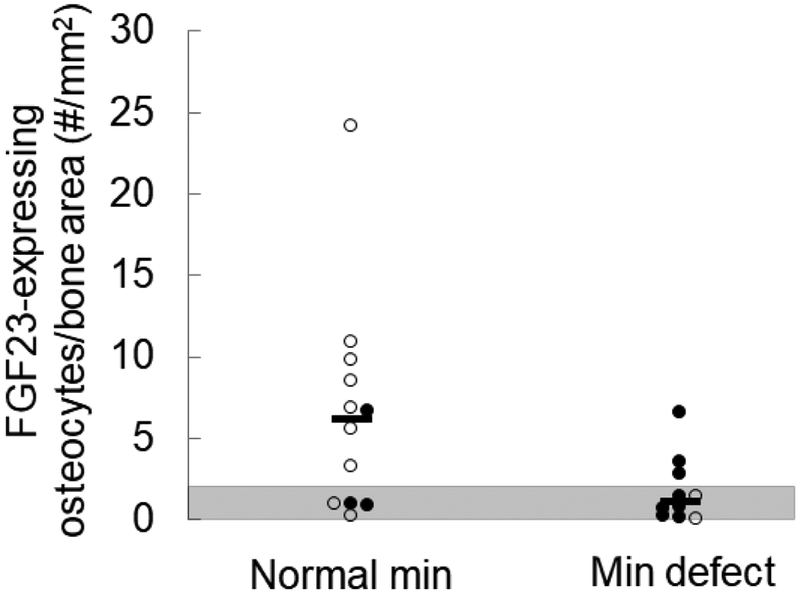
We have previously shown that FGF23-expressing osteocytes occupy packets of bone that have completed primary mineralization and have entered an early phase of secondary mineralization (7). We have also previously shown that numbers of FGF23-expressing osteocytes correlate inversely with osteoid accumulation in CKD bone (11). To evaluate whether the increase in numbers of early, FGF23-expressing, osteocytes reflects a shift in the mineralization state of peripheral trabecular bone or whether vitamin D sterols stimulate an increase in numbers of early osteocytes, independent of their effect on bone mineralization, we evaluated the relationship between numbers of FGF23-expressing osteocytes and the presence of a mineralization defect (defined by excess osteoid accumulation in combination with a prolonged osteoid maturation time) in iliac crest before and after doxercalciferol therapy. As previously shown (8), osteoid accumulation decreased in response to doxercalciferol (Supplemental Table); however, as many as 36% of patients had a persistent mineralization defect after doxercalciferol therapy. When all biopsies (both before and after doxercalciferol treatment) were considered together, biopsies with normal mineralization parameters had greater numbers of early, FGF23-expressing, osteocytes than those with mineralization defects (p<0.05 between groups), despite similar bone formation rates between groups (13.1 (12.5, 15.7) versus 12.2 (11.5, 19.8) um3/um2/day for patients with normal versus abnormal mineralization, respectively) (Table 1). Moreover, the relationship between FGF23-expressing osteocytes and mineralization defects was consistent regardless of whether the measurements were obtained before or after doxercalciferol therapy (Figure 3), suggesting that active vitamin D sterols do not alter the relationship between numbers of FGF23-expressing osteocytes and skeletal mineralization parameters. Rather, increased numbers of FGF23-expressing osteocytes appear to co-occur with increasing amounts of peripheral trabecular bone undergoing secondary mineralization (7).
Table 1:
Biochemical variables and numbers of FGF23-expressing osteocytes in patients with normal mineralization versus mineralization defects.
| Normal mineralization (n=12; 3 initial biopsies) |
Mineralization defects (n=10; 8 initial biopsies) |
|
|---|---|---|
| Biochemical values | ||
| Calcium (mg/dL) | 8.8 (8.6, 9.3) | 8.7 (8.1, 9.1) |
| Phosphorus (mg/dL) | 5.8 (5.3, 6.8) | 6.8 (5.2, 7.4) |
| PTH (pg/mL) | 458 (377, 660) | 772 (535, 1068) |
| Alkaline phosphatase (IU/L) | 165 (123, 245) | 381 (287, 543)* |
| FGF23 (RU/mL) | 1826 (683, 4069) | 522 (348, 793)* |
| 25(OH)vitamin D (ng/mL) | 23.9 (19.0, 29.8) | 25.9 (19.4, 30.1) |
| Numbers of FGF23-expressing osteocytes | ||
| FGF23-expressing osteocytes/bone area (FGF23/B.Ar) (#/mm2) | 6.11 (0.95, 8.84) | 0.33 (1.06, 2.45)* |
p<0.05 between groups
Figure 3: Normal osteoid accumulation is associated with increased numbers of FGF23-expressing osteocytes in CKD bone.
Numbers of FGF23-expressing osteocytes, normalized to trabecular bone area (FGF23/B.Ar) in iliac crest sections with normal mineralization (“normal min”) parameters versus those with evidence of a mineralization defect (“min defect”), defined by an excess in osteoid accumulation in combination with a prolonged osteoid maturation rate. Closed circles indicate pre-doxercalciferol and open circles indicate post-doxercalciferol specimens. A difference (p<0.04) was noted in numbers of FGF23-expressing osteocytes between biopsies with normal mineralization indices and those with mineralization defects.
1,25(OH)2vitamin D increases the maturation and mineralization of primary CKD osteoblasts in vitro
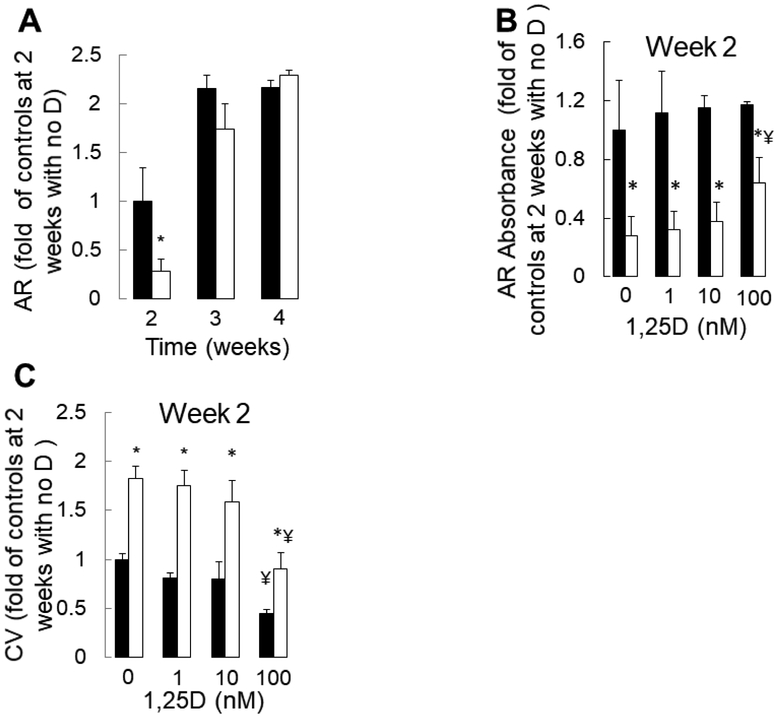
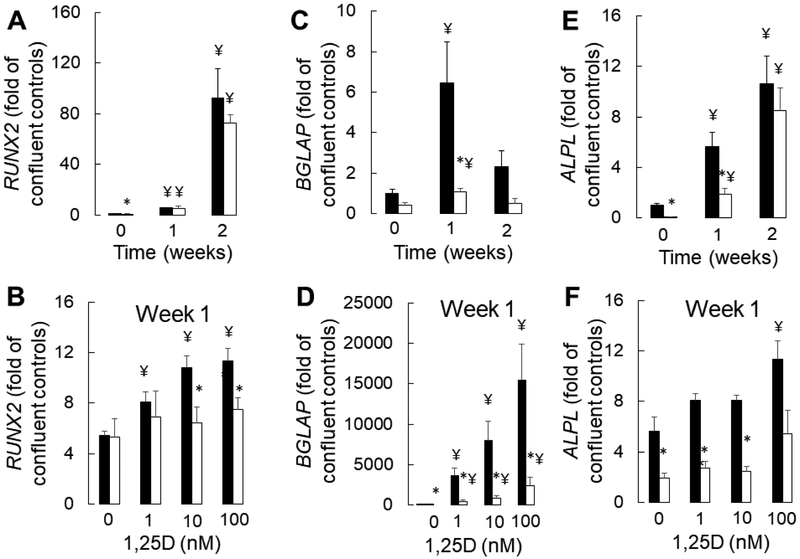
Primary CKD osteoblasts removed from the uremic milieu are highly proliferative, mineralize slowly, and have increased expression of the early osteoblast markers (5). We recently demonstrated that this phenotype is consistent with an impairment in osteoblast maturation due to CKD that persists ex vivo (5). We also demonstrated that impaired osteocyte maturation contributes to increased numbers of early, FGF23-expressing osteocytes in CKD bone. Since doxercalciferol decreases osteoid accumulation but increases numbers of early, FGF23-expressing osteocytes in CKD bone, we postulated that active vitamin D sterols might improve, although not completely rescue, the underlying CKD-mediated maturation defect observed in osteoblast lineage cells. We thus evaluated the effects of 1,25(OH)2vitamin D on CKD osteoblast maturation in vitro. As shown in Figure 4a, mineralization, as assessed by alizarin red S staining, occurred more rapidly and completely in healthy control cells than in CKD cells. No dose of 1,25(OH)2vitamin D altered the mineralization of control cells and high doses (100 nM) decreased cell viability in control cultures (Figure 4b). By contrast, cell numbers were persistently higher and mineralization rates were lower in CKD osteoblast cultures. Although no dose of 1,25(OH)2vitamin D normalized CKD osteoblast mineralization, the highest concentrations of 1,25(OH)2vitamin D (100 nM) did decrease excessive cell numbers (Figure 4c) and improve mineralization in CKD cultures. RNA expression analysis revealed that 1,25(OH)2vitamin D increased expression of the early osteoblast marker RUNX2 (RUNX2) to a minor degree in healthy control, although not in CKD, osteoblasts. The minor increase in RUNX2 expression in response to 1,25(OH)2vitamin D was dwarfed by the effect of time on this early osteoblast marker (Figure 5a). By contrast, 1,25(OH)2vitamin D markedly stimulated expression of the mature osteoblast marker osteocalcin (BGLAP) in both CKD and control cultures alike (Figure 5b). 1,25(OH)2vitamin D had very little effect on alkaline phosphatase (ALPL) expression in either control or CKD osteoblasts (Figure 5c). Together, these data suggest that although 1,25(OH)2vitamin D stimulates RNA expression of mature osteoblast markers, it has very little effect on osteoblast mineralization and does not rescue the mineralization defect intrinsic to CKD osteoblasts.
Figure 4: Mineralization of primary CKD osteoblasts is delayed but increases in response to high doses of 1,25(OH)2vitamin D.
(A) Absorbance of extracted alizarin red S dye at 405 nm, in healthy control osteoblasts (closed bars) and in CKD osteoblasts (open bars) cultured for 4 weeks under pro-mineralizing conditions in the absence of 1,25(OH)2vitamin D (“D”). (B) Absorbance of extracted alizarin red S dye at 405 nm, in healthy control osteoblasts and in CKD osteoblasts after 2 weeks of growth under pro-mineralizing conditions with varying concentrations of 1,25(OH)2vitamin D. (C) Relative live cell numbers over time, as assessed by the absorbance of extracted crystal violet (CV) staining at 605 nm, in healthy control (closed bars) and CKD (open bars) primary osteoblasts cultured under standard pro-mineralizing conditions. The asterisk (*) indicates a difference (p<0.05) between healthy control cells and CKD cells at each timepoint or dosage. The ¥ indicates a difference (p<0.05) from control (no added 1,25(OH)2vitamin D) conditions. 1,25D: 1,25(OH)2vitamin D; CV: crystal violet.
Figure 5: 1,25(OH)2vitamin D stimulates expression of the mature osteoblast marker, osteocalcin (BGLAP).
(A) RUNX2 expression over time under standard pro-mineralizing conditions (A) over time and (B) with increasing concentrations 1,25(OH)2vitamin D. (C) BGLAP expression over time under standard pro-mineralizing conditions and (D) with increasing concentrations of 1,25(OH)2vitamin D (E) ALPL expression over time under standard pro-mineralizing conditions and (D) with increasing concentrations of 1,25(OH)2vitamin D. Expression is displayed as the multiple (fold) of expression identified in confluent healthy control osteoblasts which have not been stimulated to mineralize and have not been exposed to vitamin D (“confluent controls”). The asterisk indicates a difference (p<0.05) between CKD and control osteoblasts; the ¥ indicates a difference from the control (time 0 for A,C, and E and from the no added 1,25(OH)2vitamin D condition for B, D, and F). Healthy control osteoblasts are depicted by closed bars and CKD osteoblasts by open bars. 1,25D: 1,25(OH)2vitamin D.
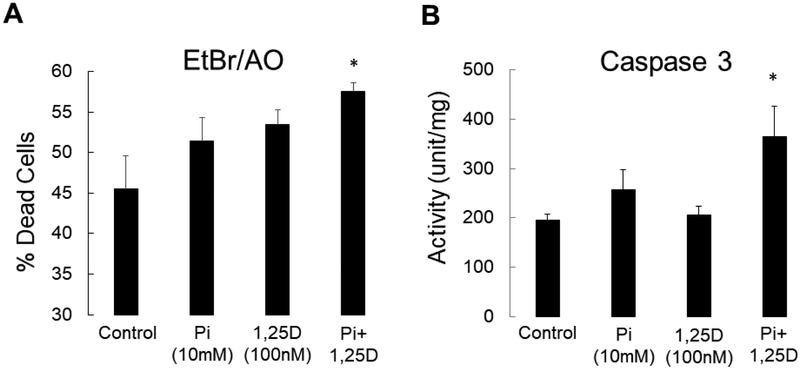
Bone biopsy data suggest that active vitamin D sterols increase osteocyte maturation and turnover and primary osteoblast cultures suggest that vitamin D directly enhances maturation of cells of the osteoblast lineage. In order to confirm that 1,25(OH)2vitamin D also directly enhances osteocyte apoptosis, we cultured immortalized murine long bone osteocyte (MLO-Y4) cells with 1,25(OH)2vitamin D in vitro. As shown in Figure 6a, 1,25(OH)2vitamin D, particularly in the presence of high phosphate, increased the number of dead and apoptotic MLO-Y4 cells at 48 hours in culture. Activity of caspase 3, an enzyme responsible for the execution of apoptosis, simultaneously increased (Figure 6b), suggesting that active vitamin D sterols directly stimulate osteocyte apoptosis, particularly in the context of hyperphosphatemia (13).
Figure 6: 1,25(OH)2vitamin D and phosphate directly increases osteocyte apoptosis in vitro.
A) The percentage of dead cells, as defined by the percentage of cells staining with ethidium bromide (Et.Br) relative to the total number of live cells staining with acridine orange (Ac.O), after 48 hours in culture. Values represent the average ± standard error for n=8 replicates. B) Caspase 3 activity in MLO-Y4 cells exposed to phosphate and 1,25(OH)2vitamin D. The asterisk indicates a difference (p<0.01) from cells treated under control (3mM Pi with no added 1,25(OH)2vitamin D) conditions. 1,25D: 1,25(OH)2vitamin D; Pi: phosphate.
Activation of osteoclasts may also indirectly contribute to vitamin D-mediated osteoblast maturation.
Since osteocyte senescence contributes to osteoclast activation which in turn stimulates osteoblastogenesis in vivo (14, 15), we evaluated the effect of doxercalciferol on histomorphometric measures of eroded surface and osteoclast number in CKD patient bone cores before and after doxercalciferol therapy. As previously shown and is reported in the Supplemental Table, number of osteoclasts/tissue area decreased by −0.23 (−1.28, 0.28)/mm2 while eroded surface decreased by −1.1 (−4.4, 0.52)% in response to doxercalciferol therapy (NS for both). While osteoclast numbers and eroded surface correlated closely (r=0.87, p<0.001), there was only a modest, non-significant, correlation between the change in circulating PTH level and the change in eroded surface in response to therapy (r=0.45, p=0.16).
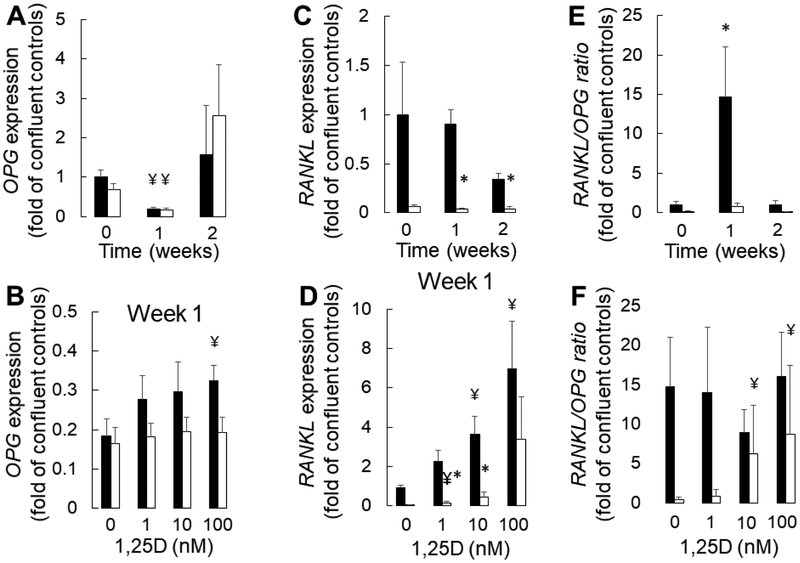
Given the imperfect correlation between PTH and osteoclast activation, we evaluated whether 1,25(OH)2vitamin D exerts a direct effect on osteoblast-specific factors known to regulate osteoclastogenesis. As shown in Figure 7, there was lower expression of receptor activator of nuclear factor kappa-B ligand (RANKL) expression in CKD than in control osteoblasts, particularly after one week in pro-mineralizing conditions. RANKL expression increased with 10 and 100 nM concentrations of 1,25(OH)2vitamin D in controls although a more muted and variable increase was observed in CKD osteoblasts. Expression of osteoprotegerin (OPG), the decoy receptor for RANKL, did not differ between confluent control and CKD osteoblasts prior to exposure to pro-mineralizing conditions. OPG expression decreased in CKD and control osteoblasts after one week of culture in standard pro-mineralizing conditions, although expression by two weeks was similar to baseline levels. OPG expression increased with the highest doses of 1,25(OH)2vitamin D in osteoblasts from healthy controls, although no change in OPG expression was observed with 1,25(OH)2vitamin D in CKD cells. These changes in OPG and RANKL expression translated to an increasing RANKL/OPG ratio by one week under pro-mineralizing conditions in control, although not in CKD, osteoblasts. 10 nM and 100 nM doses of 1,25(OH)2vitamin D, however, increased the ratio of RANKL/OPG in CKD osteoblasts. Together, these data suggest that 1,25(OH)2vitamin D may play an additional role in osteoblast maturation by regulating osteoclast-osteoblast coupling in CKD bone.
Figure 7: 1,25(OH)2vitamin D stimulates osteoclastogenesis.
(A) OPG expression over time under standard pro-mineralizing conditions (A) over time and (B) with increasing concentrations 1,25(OH)2vitamin D. (C) RANKL expression over time under standard pro-mineralizing conditions and (D) with increasing concentrations of 1,25(OH)2vitamin D (E) RANKL/OPG expression over time under standard pro-mineralizing conditions and (D) with increasing concentrations of 1,25(OH)2vitamin D. Expression is displayed as the multiple (fold) of expression identified in confluent healthy control osteoblasts which have not been stimulated to mineralize and have not been exposed to vitamin D (“confluent controls”). The asterisk indicates a difference (p<0.05) between CKD and control osteoblasts; the ¥ indicates a difference from the control (time 0 for A,C, and E and from the no added 1,25(OH)2vitamin D condition for B, D, and F). Healthy control osteoblasts are depicted by closed bars and CKD osteoblasts by open bars. 1,25D: 1,25(OH)2vitamin D.
DISCUSSION
We have previously demonstrated that CKD is associated with stagnant osteocyte maturation, characterized by an increase number of early osteocytes and a decrease in osteocyte apoptosis and that primary osteoblasts from CKD patients have intrinsic impairments in maturation and mineralization (5). Our current data further these observations, demonstrating an interplay between intrinsic maturation defects in cells of the osteoblast/osteocyte lineage and mineral ion homeostasis in the context of CKD-mediated bone disease. Here we confirm that active vitamin D sterols directly increase the maturation and turnover of cells in the osteoblast/osteocyte lineage. This effect on cell maturation contributes to increased numbers of early osteocytes which constitutively express FGF23 in CKD bone (7). In vitro studies confirm that 1,25(OH)2vitamin D directly increases the maturation of primary osteoblast lineage cells although it fails to rescue the mineralization impairments intrinsic to primary CKD osteoblasts.
In this study, despite clear changes in bone histology and osteocyte-specific protein expression, PTH levels did not change in response to doxercalciferol. This finding is consistent with previous data demonstrating an increase in skeletal PTH-resistance in patients treated with active vitamin D sterols (3). However, treatment with active vitamin D sterols did, as has been previously demonstrated (3, 8), increase circulating levels and bone expression of FGF23. The current data demonstrate that these increases are largely due to increased numbers of FGF23-expressing osteocytes—which are early osteocytes (7)—in CKD bone. Doxercalciferol likewise increased osteocyte apoptosis to levels observed in healthy control subjects, suggesting a vitamin-D mediated increase in osteocyte maturation and turnover which is linked to in vivo vitamin-D mediated improvements in skeletal mineralization indices. In vitro, 1,25(OH)2vitamin D stimulated primary osteoblast maturation, increasing expression of the mature osteoblast marker osteocalcin (BGLAP) in both healthy control and CKD osteoblasts. 1,25(OH)2vitamin D also stimulated the expression of the osteoclast differentiation factor RANKL. Particularly in CKD osteoblasts, high doses of 1,25(OH)2vitamin D increased the ratio of RANKL to OPG, suggesting that 1,25(OH)2vitamin D may also indirectly stimulate osteoblast and osteocyte maturation through improved osteoblast/osteoclast/osteocyte coupling. Interestingly, 1,25(OH)2vitamin D did not induce a corresponding improvement in primary osteoblast mineralization in vitro. Mineralization improved only CKD osteoblasts exposed to the highest (100 nM) dose of 1,25(OH)2vitamin D—a dose which far exceeds the concentrations around 1 nM which are achieved in dialysis patients receiving high doses of calcitriol (16) and which was toxic to the primary osteoblasts evaluated in this study. The discrepancy between maturation markers and primary osteoblast mineralization characteristics may be explained by previous data demonstrating that 1,25(OH)2vitamin D, while inducing the osteogenic differentiation of osteoblast precursors (17), stimulates pyrophosphate expression, thereby inhibiting in vitro mineralization of primary rodent osteoblasts (18).
The discrepancies between the effects of active vitamin D sterols on skeletal mineralization in vivo and on isolated osteoblast mineralization in vitro may be in part explained by the effects of vitamin D on systemic mineral metabolism and on osteocyte/osteoclast/osteoblast coupling. Vitamin D sterols suppress PTH and increase intestinal calcium and phosphorus absorption. Numbers of osteoblast precursors increase—and their apoptosis decreases—in mice treated with PTH (19). In dialysis patients, the removal of PTH via parathyroidectomy increases bone expression of the mature osteocyte marker sclerostin, although it does not alter numbers of new, FGF23-expressing osteocytes (20). Serum phosphate levels are associated with increased osteoblast/osteocyte maturation and with osteoblast and osteocyte apoptosis (7). Thus, the effects of vitamin D-mediated changes in mineral ion and hormone concentrations, in addition to renal osteodystrophy therapies and intrinsic alterations in osteoblast lineage cell maturation characteristics, likely combine to contribute to abnormal osteocyte-specific hormone expression and skeletal mineralization in the context of chronic kidney disease. This interplay is particularly striking when comparing differences in osteocyte maturation and turnover between uremic mice, in whom preserved circulating 1,25(OH)2vitamin D levels and hyperphosphatemia co-occur with increase rates of osteocyte apoptosis (21), and dialysis patients, in whom low circulating 1,25(OH)2vitamin D levels may contribute to low osteocyte apoptosis rates prior to vitamin D sterol therapy (7). In addition, the contribution of differentiation-inducing signals from osteoclasts and osteocytes, which play critical roles in osteoblast maturation (14, 15), are lost in insolated cultures of primary osteoblasts, likely obscuring the true effect of 1,25(OH)2vitamin D on osteoblast maturation.
While common in children (4, 10), skeletal mineralization defects have more variably been reported in the adult population, in part due to variation in how different investigators define mineralization defects (22, 23). Despite these discrepancies, the prevalence of mineralization defects, defined by the presence of increased osteoid accumulation in combination with prolonged mineralization time, appears to be substantially more prevalent in children than in adults with CKD (4, 10). The reason behind this difference is not entirely clear but may speak to differences in biology between young people who are both forming and remodeling bone and adults who are maintaining and remodeling it. Thus, it is possible that skeletal mineralization defects resulting from CKD-mediated osteoblast and osteocyte maturation failure may be a uniquely pediatric problem. Further studies are warranted to determine whether agents that increase bone cell maturation and stimulate osteoclast recruitment, such as active vitamin D sterols, may have different degrees of benefit for bone health in children as compared to adults.
Active vitamin D sterols have previously been shown to increase circulating FGF23 in dialysis patients (3) and circulating FGF23 levels correlate closely with number of FGF23-expressin osteocytes in CKD patients (11). Circulating level of FGF23 have been linked to infection (24), cardiovascular disease (25), and early mortality (26) in dialysis patients. Thus, by improving bone mineralization and overall osteocyte turnover, active vitamin D sterols may contribute to off-target co-morbidities of FGF23. It is important to note, however, that pharmacologic manipulation of FGF23 have not been shown to alter mortality; thus, the systemic benefits of active vitamin D sterol treatment—and its potential adverse effect on FGF23 excess in the CKD population—remain an open question
We acknowledge certain limitations in the current study. Namely, while valuable in its use of a rare library of human bone samples, this study is, by its rarity, limited in sample size. These samples were obtained from children and young adults; generalizability to the adult population thus remains unknown. However, FGF23 expression is increased and skeletal mineralization defects have also been identified in adult CKD bone biopsy studies (22), consistent with data from the pediatric population (3, 4). In addition, the skeletal site of primary osteoblast origin may affect osteocalcin and alkaline phosphatase expression (27) and, while 1,25(OH)2vitamin D has previously been shown to affect maturation and mineralization of osteoblasts, its effects may depend on the stage of maturation of the cells in this lineage (6). Furthermore, the current data, in five select patients with low (n=2) and high (n=3) bone turnover show lower baseline ALPL and RUNX expression than was previously reported in a cohort of 24 primary osteoblast samples from CKD patients (5). Two of the CKD “lines” used for the current analysis were from the original cohort; three of the others (obtained in the same manner) were obtained subsequently. While there has been variability in the ALPL and RUNX expression amongst cells, lower osteocalcin expression has been consistently low in the CKD osteoblasts, consistent with delayed in maturation in CKD osteoblasts in general (5, 7). Finally, it should be noted that while doxercalciferol (1α-(OH)2vitamin D2)—a prohormone that is converted by the liver to 1,25(OH)2vitamim D2—was used for the in vivo portion of this study while calcitriol (1,25(OH)2vitamin D3) was used in vitro. While it is possible that the two hormones exert different effects on osteoblast and osteocyte maturation, previous in vivo data suggest that doxercalciferol and calcitriol have similar effects on PTH, bone turnover, bone mineralization, and circulating FGF23 levels (3).
In summary, this study demonstrates that 1,25(OH)2vitamin D directly increases maturation of osteoblast lineage cells. By stimulating osteocyte apoptosis and increasing RANKL/OPG expression, 1,25(OH)2vitamin D may also play a role in modulating osteoblast/osteoclast/osteocyte coupling. The overall effects of active vitamin D sterols are to increase the number of osteocytes in a relatively early phase of maturation which constitutively express FGF23 and to also increase in osteocyte turnover. RNA data further demonstrates that CKD osteoblasts, as compared to healthy control osteoblasts, maintain biological differences which are not due to vitamin D deficiency and which may contribute to the persistence of skeletal disease in the CKD population, despite optimal treatment for renal osteodystrophy. Further studies are needed to define the mechanism by which this maturation failure occurs.
Supplementary Material
Highlights.
The overall effects of active vitamin D sterols on CKD bone are to increase the number of osteocytes in a relatively early phase of maturation, to increase numbers of late osteocytes, and to increase osteocyte turnover.
1,25(OH)2vitamin D directly increases maturation of osteoblast lineage cells.
By stimulating osteocyte apoptosis and increasing RANKL/OPG expression, 1,25(OH)2vitamin D may play a role in modulating osteoblast/osteoclast/osteocyte coupling.
CKD osteoblasts, as compared to healthy control osteoblasts, maintain biological differences which are not due to vitamin D deficiency and which may contribute to the persistence of skeletal disease in the CKD population, despite optimal treatment for renal osteodystrophy.
Active vitamin D sterols increase FGF23 expression in bone by increasing the number of early osteocytes which constitutively express this hormone.
Acknowledgments
FUNDING: This work was supported in part by the National Institutes of Health [grant numbers 1R21-AR073977, DK-67563, DK-35423, DK-080984 and CTSI grant UL1 TR-000124] and by the UCLA Children’s Discovery and Innovation Institute.
Glossary
- CKD
chronic kidney disease
- PTH
parathyroid hormone
- FGF23
fibroblast growth factor 23
- RUNX2
runt related transcription factor 2
- BGLAP
bone gamma-carboxyglutamate protein; osteocalcin
- ALPL
tissue non-specific alkaline phosphatase
- MLO-Y4
murine osteocyte-like cell line-Y4
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.(2009) KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl S1–130 [DOI] [PubMed] [Google Scholar]
- 2.Chesney RW, Moorthy AV, Eisman JA, Jax DK, Mazess RB, and DeLuca HF (1978) Increased growth after long-term oral 1alpha,25-vitamin D3 in childhood renal osteodystrophy. N Engl J Med 298, 238–242 [DOI] [PubMed] [Google Scholar]
- 3.Wesseling-Perry K, Pereira RC, Sahney S, Gales B, Wang HJ, Elashoff R, Juppner H, and Salusky IB (2011) Calcitriol and doxercalciferol are equivalent in controlling bone turnover, suppressing parathyroid hormone, and increasing fibroblast growth factor-23 in secondary hyperparathyroidism. Kidney Int 79, 112–119 [DOI] [PubMed] [Google Scholar]
- 4.Wesseling-Perry K, Pereira RC, Tseng CH, Elashoff R, Zaritsky JJ, Yadin O, Sahney S, Gales B, Jüppner H, and Salusky IB (2012) Early skeletal and biochemical alterations in pediatric chronic kidney disease. Clin J Am Soc Nephrol 7, 146–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pereira RC, Delany AM, Khouzam NM, Bowen RE, Freymiller EG, Salusky IB, and Wesseling-Perry K (2015) Primary osteoblast-like cells from patients with end-stage kidney disease reflect gene expression, proliferation, and mineralization characteristics ex vivo. Kidney Int 87, 593–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lohmann CH, Bonewald LF, Sisk MA, Sylvia VL, Cochran DL, Dean DD, Boyan BD, and Schwartz Z (2000) Maturation state determines the response of osteogenic cells to surface roughness and 1,25-dihydroxyvitamin D3. J Bone Miner Res 15, 1169–1180 [DOI] [PubMed] [Google Scholar]
- 7.Pereira RC, Salusky IB, Roschger P, Klaushofer K, Yadin O, Freymiller EG, Bowen R, Delany AM, Fratzl-Zelman N, and Wesseling-Perry K (2018) Impaired osteocyte maturation in the pathogenesis of renal osteodystrophy. Kidney Int 94, 1002–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pereira RC, Juppner H, Gales B, Salusky IB, and Wesseling-Perry K (2015) Osteocytic protein expression response to doxercalciferol therapy in pediatric dialysis patients. PLoS One 10, e0120856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Misof BM, Dempster DW, Zhou H, Roschger P, Fratzl-Zelman N, Fratzl P, Silverberg SJ, Shane E, Cohen A, Stein E, Nickolas TL, Recker RR, Lappe J, Bilezikian JP, and Klaushofer K (2014) Relationship of bone mineralization density distribution (BMDD) in cortical and cancellous bone within the iliac crest of healthy premenopausal women. Calcif Tissue Int 95, 332–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakkaloglu SA, Wesseling-Perry K, Pereira RC, Gales B, Wang HJ, Elashoff RM, and Salusky IB (2010) Value of the new bone classification system in pediatric renal osteodystrophy. Clin J Am Soc Nephrol 5, 1860–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pereira RC, Juppner H, Azucena-Serrano CE, Yadin O, Salusky IB, and Wesseling-Perry K (2009) Patterns of FGF-23, DMP1, and MEPE expression in patients with chronic kidney disease. Bone 45, 1161–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bacchetta J, Wesseling-Perry K, Kuizon B, Pereira RC, Gales B, Wang HJ, Elashoff R, and Salusky IB (2013) The skeletal consequences of growth hormone therapy in dialyzed children: a randomized trial. Clin J Am Soc Nephrol 8, 824–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rojas E, Carlini RG, Clesca P, Arminio A, Suniaga O, De EK, Weisinger JR, Hruska KA, and Bellorin-Font E (2003) The pathogenesis of osteodystrophy after renal transplantation as detected by early alterations in bone remodeling. Kidney Int 63, 1915–1923 [DOI] [PubMed] [Google Scholar]
- 14.Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL, Negley BA, Sfeir JG, Ogrodnik MB, Hachfeld CM, LeBrasseur NK, Drake MT, Pignolo RJ, Pirtskhalava T, Tchkonia T, Oursler MJ, Kirkland JL, and Khosla S (2017) Targeting cellular senescence prevents age-related bone loss in mice. Nat Med 23, 1072–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis HM, Valdez S, Gomez L, Malicky P, White FA, Subler MA, Windle JJ, Bidwell JP, Bruzzaniti A, and Plotkin LI (2019) High mobility group box 1 protein regulates osteoclastogenesis through direct actions on osteocytes and osteoclasts in vitro. J Cell Biochem [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leaf DE, Raed A, Donnino MW, Ginde AA, and Waikar SS (2014) Randomized controlled trial of calcitriol in severe sepsis. Am J Respir Crit Care Med 190, 533–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lou YR, Toh TC, Tee YH, and Yu H (2017) 25-Hydroxyvitamin D3 induces osteogenic differentiation of human mesenchymal stem cells. Sci Rep 7, 42816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lieben L, Masuyama R, Torrekens S, Van Looveren R, Schrooten J, Baatsen P, Lafage-Proust MH, Dresselaers T, Feng JQ, Bonewald LF, Meyer MB, Pike JW, Bouillon R, and Carmeliet G (2012) Normocalcemia is maintained in mice under conditions of calcium malabsorption by vitamin D-induced inhibition of bone mineralization. J Clin Invest 122, 1803–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balani DH, Ono N, and Kronenberg HM (2017) Parathyroid hormone regulates fates of murine osteoblast precursors in vivo. J Clin Invest 127, 3327–3338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pires GO, Vieira IO, Hernandes FR, Teixeira AL, Oliveira IB, Dominguez WV, Dos Reis LM, Montenegro FM, Moyses RM, Carvalho AB, and Jorgetti V (2019) Effects of parathyroidectomy on the biology of bone tissue in patients with chronic kidney disease and secondary hyperparathyroidism. Bone 121, 277–283 [DOI] [PubMed] [Google Scholar]
- 21.Dussold C, Gerber C, White S, Wang X, Qi L, Francis C, Capella M, Courbon G, Wang J, Li C, Feng JQ, Isakova T, Wolf M, David V, and Martin A (2019) DMP1 prevents osteocyte alterations, FGF23 elevation and left ventricular hypertrophy in mice with chronic kidney disease. Bone Res 7, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graciolli FG, Neves KR, Barreto F, Barreto DV, Dos Reis LM, Canziani ME, Sabbagh Y, Carvalho AB, Jorgetti V, Elias RM, Schiavi S, and Moyses RMA (2017) The complexity of chronic kidney disease-mineral and bone disorder across stages of chronic kidney disease. Kidney Int 91, 1436–1446 [DOI] [PubMed] [Google Scholar]
- 23.Malluche HH, Mawad HW, and Monier-Faugere MC (2011) Renal osteodystrophy in the first decade of the new millennium: analysis of 630 bone biopsies in black and white patients. J Bone Miner Res 26, 1368–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossaint J, Oehmichen J, Van Aken H, Reuter S, Pavenstadt HJ, Meersch M, Unruh M, and Zarbock A (2016) FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J Clin Invest 126, 962–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scialla JJ, Lau WL, Reilly MP, Isakova T, Yang HY, Crouthamel MH, Chavkin NW, Rahman M, Wahl P, Amaral AP, Hamano T, Master SR, Nessel L, Chai B, Xie D, Kallem RR, Chen J, Lash JP, Kusek JW, Budoff MJ, Giachelli CM, and Wolf M (2013) Fibroblast growth factor 23 is not associated with and does not induce arterial calcification. Kidney Int 83, 1159–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Juppner H, and Wolf M (2008) Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Eng J Med 359, 584–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez P, Moreno I, De Miguel F, Vila V, Esbrit P, and Martinez ME (2001) Changes in osteocalcin response to 1,25-dihydroxyvitamin D(3) stimulation and basal vitamin D receptor expression in human osteoblastic cells according to donor age and skeletal origin. Bone 29, 35–41 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.