Abstract
Chlamydia muridarum and Chlamydia caviae have equivalent growth rates in mouse epithelial cells but only C. muridarum replicates inside mouse macrophages, while C. cavie does not. Macrophages infected with C. muridarum or C. caviae were used to address the hypothesis that the early signaling pathways initiated during infection depend on the fate of chlamydiae in the host cell. Transmission electron microscopy of C. muridarum-infected macrophages showed intact chlamydial elementary bodies and reticulate bodies 2 h post-infection in compact vacuoles. Conversely, in macrophages infected with C. caviae, chlamydiae were observed in large phagocytic vacuoles. Furthermore, C. caviae infections failed to develop into inclusions or produce viable bacteria. Expression of pro-inflammatory cytokines TNF-α, IL-1β, and MMP13 was similar in C. caviae- or C. muridarum-infected macrophages at 3 hours post-infection, indicating that chlamydial survival is not required for initiation of these responses. IL-1β secretion, dependent on inflammasome activation, occurred in C. caviae-infected macrophages despite no chlamydial growth. Conversely, IFNβ mRNA was observed only in C. muridarum- but not in C. caviae-infected macrophages. These data demonstrate that differential signaling events are initiated during a productive vs. non-productive chlamydial infection in a macrophage.
Keywords: Chlamydia, IFNβ, IL-1β, macrophages
INTRODUCTION
Chlamydia trachomatis is the major cause of genital infection in women leading to pelvic inflammatory disease. Although many host cell types are susceptible to Chlamydia infection in tissue culture, the primary target cells in vivo are the columnar epithelial cells of the genital tract, the conjunctiva or the gut epithelia. Interestingly, some of the Chlamydia spp. have the ability to infect and grow efficiently inside macrophages both in vivo and in cell culture [reviewed in1]. This includes the mouse C. muridarum and the human strains LGV and C. pneumoniae. Further, there are a few reports of C. muridarum infection at remote tissue sites, suggesting that macrophages could serve as vehicles for dissemination [reviewed in2]. Macrophages are professional phagocytes that normally internalize and degrade bacterial pathogens in their lysosomes. However, a number of bacterial pathogens, including C. muridarum, have evolved strategies to subvert phagocytosis by macrophages. Macrophages are recruited to the infection site and produce a number of cytokine/chemokines at the site of infection. Cytokine/chemokine responses to infection drive tissue pathology and the mechanism of initiation of these cytokine responses in immune cells has been an intense area of study.
We have previously shown that IFNβ expression during Chlamydia infection requires chlamydial growth and DNA-sensing by the host DNA sensor cGAS-STING pathway3, 4. We have also shown that NLRP3 and ASC are required for IL-1β secretion in resting macrophages during C. muridarum infection, while only ASC is required for high levels of IL-1β secretion during infection in LPS pre-stimulated/activated macrophages5. Finnety et al6 have also shown the requirement of DNA-sensing AIM2 inflammasome for IL-1β secretion in activated macrophages. Inhibition of bacterial growth by inhibitors of bacterial transcription and translation do not inhibit IL-1β secretion5. These studies have indicated that successful inclusion formation by chlamydiae vs. their engulfment by lysosomes could initiate differential host cytokine responses.
We hypothesize that the early intracellular fate of chlamydiae determines the initiation of specific innate responses. To address this hypothesis, two species of chlamydiae, the guinea pig strain, C. caviae, and the mouse strain, C. muridarum, which display differential growth properties in mouse macrophages, were used. C. caviae and C. muridarum grow at almost equal rates in mouse L929 cells but only C. muridarum grows in primary macrophages to form mature inclusions, while C. caviae fails to do so. Epithelial cells support growth of multiple chlamydial strains at all times under resting conditions. Therefore, differential growth of chlamydial strains in macrophages provides a better model system for studying the mechanism of initiation of cytokine responses to successful infection vs. just phagocytosis and killing of live chlamydiae. Our results show that induction of pro-inflammatory cytokine mRNAs, such as TNFα and IL-1β, occur independent of chlamydial growth in macrophages although their sustained expression required chlamydial growth. ASC-dependent inflammasome activation was observed independent of chlamydial growth. Conversely, as previously reported3, IFNβ and IFN response genes are dependent on chlamydial survival and growth in macrophages. Our data demonstrate that the intracellular niche of Chlamydia contributes to differential sensing and signaling in the host.
RESULTS
Chlamydia muridarum can replicate in mouse macrophages while C. caviae fails to do so.
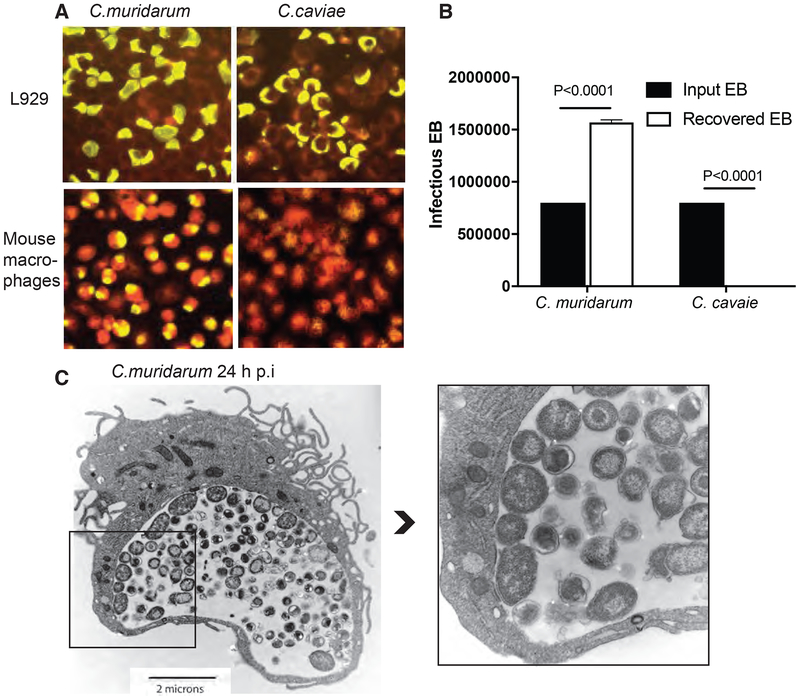
C. muridarum and C. caviae stocks were titrated to give equal percentage of infection in McCoy cells by IFU and FACS, and used for infecting primary mouse macrophages at 1 MOI of infection. C. muridarum and C. caviae grow in mouse L929 cells forming inclusions (yellow) (Fig 1A). However, in primary mouse macrophages, only C. muridarum forms mature inclusions at 24 h post-infection, with about a 40–60% infection rate, while C. caviae fails to do so (Fig 1A). Cell lysates from infected macrophages were harvested and numbers of infectious elementary bodies (EB) were measured by infecting L929 cells. Although macrophages were infected with the same numbers of EB, a three log less infectious yield was obtained from C. caviae relative to C. muridarum (data not shown). The percentage of recovered IFU showed a 200% recovery of IFU for C. muridarum at 24 h, suggesting formation of new EBs, while only 0.2% C. caviae were recovered, suggesting active killing and no growth of C. caviae in macrophages. Consistent with the IFU results, TEM images of C. muridarum-infected macrophages at 24 hours post-infection showed mature inclusions containing both the metabolically active reticulate body (RB) forms and the infectious EB forms (Fig 1C and Supplementary Fig S1). The RBs line up on the inner side of the inclusion membrane in some inclusions as predicted by Wilson et al model7. At 24 h post-infection, C. caviae-infected macrophages were indistinguishable from uninfected macrophages (data not shown).
Fig 1: C. muridarum survives and forms mature inclusion in primary mouse macrophages while C. caviae does not.
L929 cells and primary mouse macrophages were infected with C. muridarum or C. caviae for 24 h at 1 MOI and stained using Pathfinder FITC-conjugated murine anti-chlamydial mAb (yellow) with evans blue counter stain (red) (A). Cell lysates from infected macrophages (done in triplicate) were collected and used to infect L929 cells for IFU counts. Data represent means plotted to show input and recovered EB from macrophages (B). Error bars represent SD and significance determined by one-way ANOVA. TEM image of macrophages infected with C. muridarum at 24 h post-infection (C and inset). Darker smaller forms inside the inclusions are chlamydial EBs and larger less dense forms are chlamydial RBs.
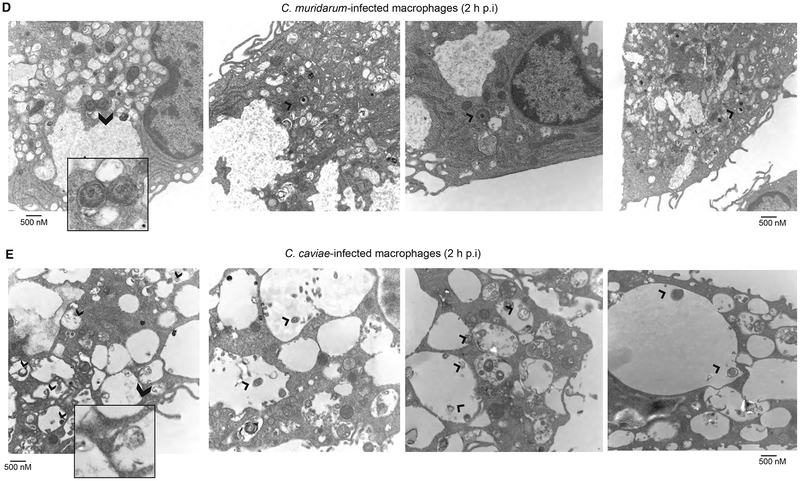
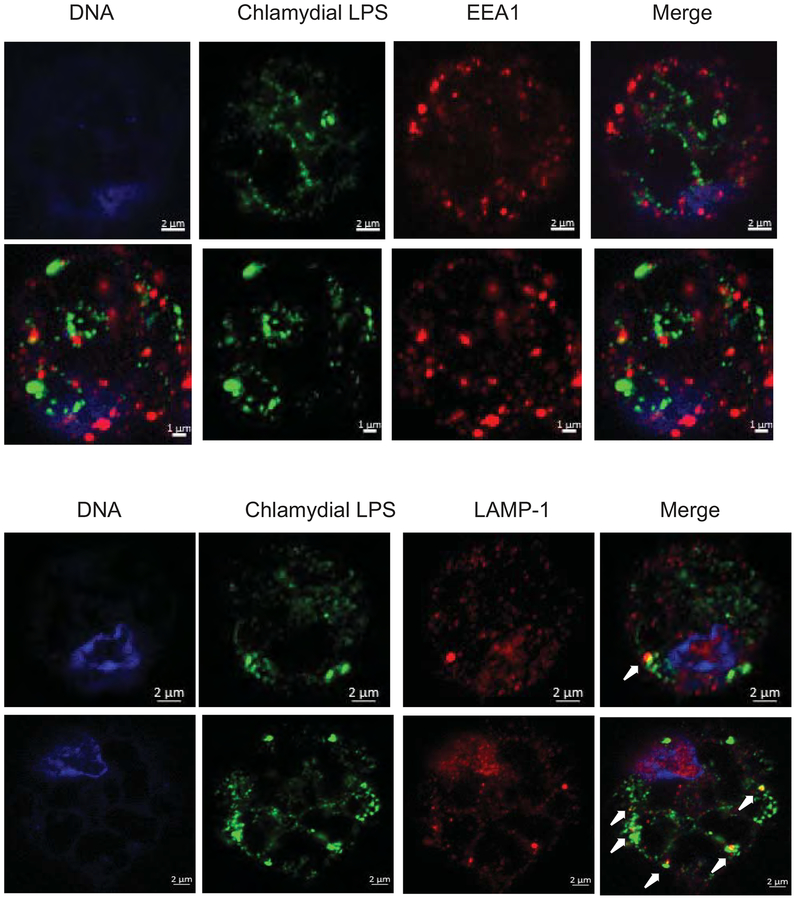
To observe the chlamydial fate and changes in host cell morphology during early stages of infection, TEM of macrophages were obtained at 2 h post-infection. C. muridarum EBs were secured inside a closed membrane bound vacuole, with chlamydial membrane in close proximity to the vacuolar membrane (Fig 2). Conversely, C. caviae EBs/RBs were found in the large mono-layered phagocytic vacuoles with no proximity of chlamydial membrane to the vacuolar membrane (Fig 2). Further, C. muridarum-infected macrophages were unperturbed with their pseudopods, while C. cavie-infected macrophages contained large vacuoles and lost their pseudopods (Figure S2). To determine the nature of the vacuoles where the chlamydiae were localized, macrophages were stained for an early endosomal marker EEA-1 and the lysosomal marker LAMP-1. Both C. muridarum and C. caviae did not co-localize with the EEA-1 suggesting that the chlamydia-containing vacuoles had already lost the characteristics of early endosomes (Fig 3 and Fig S3). At 3 h post-infection, C. muridarum did not co-localize with LAMP-1, suggesting escape from lysosomes. However, a larger proportion of C. caviae co-localized with LAMP-1, suggesting fusion of C. caviae containing vacuoles with lysosomes (Fig 3 and Fig S3). UV-killed chlamydiae did not co-localize to lysosomes at this time, suggesting differential kinetics for live vs. killed bacteria (data not shown).
Fig 2: At 2 h post-infection, C. muridarum survives in small compact vacuoles, while C. caviae are in large vacuoles.
TEM images of macrophages infected at 10 MOI for 2 h with C. muridarum (A) or C. caviae (B). Inset in each panel shows chlamydiae in small compact vacuoles (C. muridarum) or in large vacuoles (C. caviae). Arrows point to chlamydiae inside vacuoles.
Fig 3: At 2 h post-infection, both C. muridarum and C. caviae do not co-localize with early endosomes, but more numbers of C. caviae co-localize with lysosomes.
Macrophages infected with C. muridarum or C. cavaie at 1 MOI infection for 3 h p.i were fixed and immuno-stained for an early endosomal marker EEA-1 (A) or lysosomal marker LAMP-1 (B) with chlamydial LPS staining. Arrows indicate co-localization of LAMP-1 and LPS. Image shows a single cell for clarity. Larger field is shown in Fig S3. A representative image from one of two experiments is shown.
Differential gene expression in C. muridarum- and C. caviae-infected macrophages.
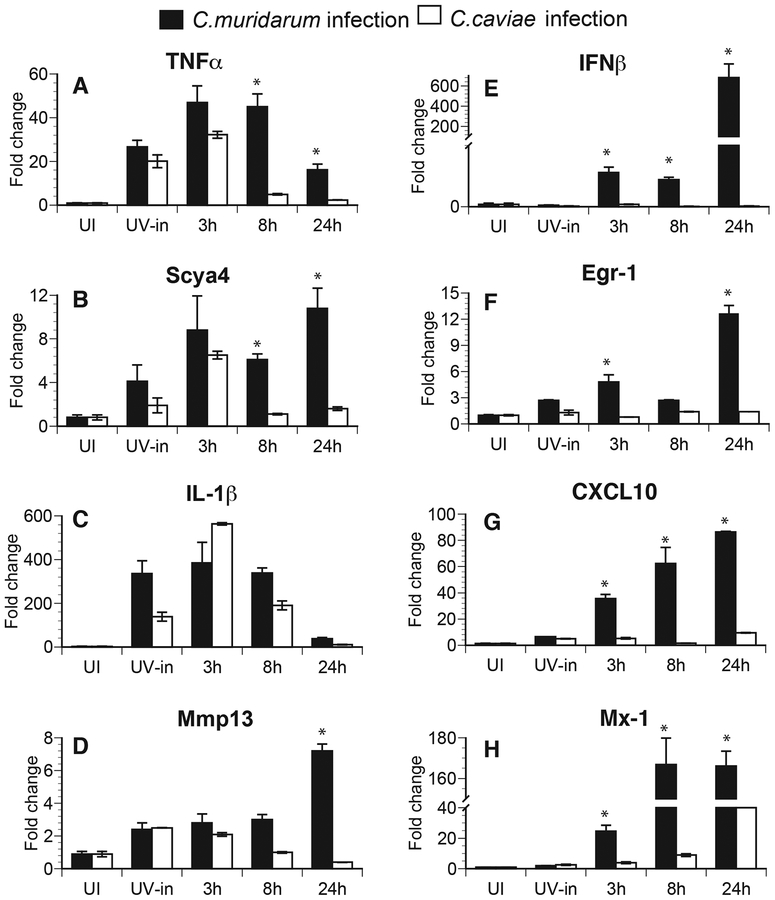
To address the hypothesis that early host signaling events are determined by chlamydial fate in the host cells, RNA was extracted from infected macrophages and mRNA from two groups of genes were analyzed, based on the activation of the essential transcription factor(s). The genes in the first group were Tnfα, Scya4, Il1β, and Mmp13, all of which require NFκB/MAPK activation for their expression. The second group included Ifnβ, and type I IFN dependent genes; Egr-1, Cxcl10, and Mx-1, which require activation of transcription factor IRF3 in addition to NF-κB. We have previously shown that CXCL-10 expression during Chlamydia infection is dependent on IFNα/β signaling8. The IFNβ-inducible nature of these genes has been reported by microarray analysis9 and was further validated at http://systemsimmunity.org/tools/shiny/IntegratedBrowser/. Infection with both C. muridarum and C. caviae led to similar induction of mRNA for Tnfα, Scya4, Il1β, and Mmp13 (Fig 4A–4D) at 3 h post-infection, although mRNA levels were significantly reduced by 8 h post-infection only in C. caviae-infected cells. Similar results were observed for Il-6 and Tnfs9 (data not shown). Additionally, infection with UV-killed chlamydiae was also able to initiate these responses at 3 h, although sustained expression was not observed at 8 h pi. These results suggest that uptake of chlamydiae by macrophages was sufficient to initiate these responses. Conversely, Ifnβ, Egr-1, Cxcl10, and Mx-1 were expressed at 3 h post-infection only in C. muridarum-infected but not in C. caviae-infected macrophages (Fig 4E–4H) and their expression increased with time in C. muridarum-infected macrophages. Similar results were also obtained with Ccl5 and GemGTPase (data not shown). UV-killed bacteria failed to initiate these responses, as previously observed for IFNβ10. These data suggest differential initiation of gene expression depend on early chlamydial fate, before the initiation of chlamydial replication.
Fig 4: Pro-inflammatory gene transcripts are induced following both C. muridarum and C. caviae infection, while IFNβ and IFN response gene transcripts are induced only during C. muridarum infection.
Quantitative RT-PCR data are expressed as fold changes compared to uninfected (UI) controls from RNA extracted from macrophages at 3, 8, and 24 h post-infection. RNA was extracted at 3 h post-infection in cells infected with UV inactivated (UV-in) Chlamydia. Individual gene expression was normalized to actin expression. TNFα (A), Scya4 (B), IL-1β (C) and Mmp13 (D) are shown on the left panel and data for IFNβ (E) and IFN-response genes, Egr-1 (F), CXL10 (G) and Mx-1 (H) are shown on the right panel. Samples were run in triplicates and data are representative of 3 independent experiments. Mean values are shown and error bars represent SD. * represents significant changes (P<0.05) by multiple t tests.
Early NFκB activation occurs independent of chlamydial growth.
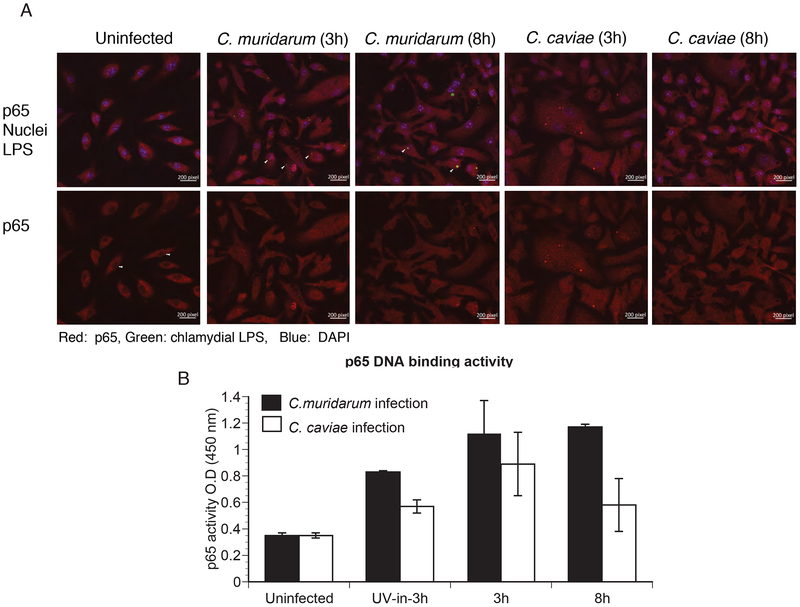
Induction of inflammatory cytokines through NFκB activation requires activation of the p65 subunit of NFκB, its nuclear localization and binding to the promoter of several inflammatory genes. Immunofluorescence staining of NF-κB p65 showed that p65 was localized to the nucleus at 3 h post-infection in macrophages infected with C. muridarum or C. caviae (Fig 5A). Infection with UV-killed bacteria also resulted in some p65 nuclear localization (data not shown). By 8 h post-infection, a reduction in nuclear localization of p65 was observed in macrophages infected with C caviae relative to those infected with C. muridarum (Fig 5B). Consistent with nuclear localization, equivalent levels of p65-DNA binding activity was observed at 3 h post-infection with both strains, although the activation level declined by 8 h in C. caviae-infected macrophages (Fig 5C).
Fig 5: p65 nuclear translocation and its promoter binding activity were similar between C. muridarum- and C. caviae- infected macrophages.
Macrophages were infected with C. muridarum or C. caviae, and stained for p65, chlamydial inclusion (LPS) and DAPI for nuclear staining at 3 and 8 h post-infection (A). Cells were fixed and visualized using a Zeiss confocal microscope. Top panel shows staining for p65 (red), chlamydial inclusion (green) and DAPI (blue), while lower panel shows the same image for p65 staining only. Red signal inside the nucleus and similar area in the cytosol for each group was quantified using Image J software. Data represented as nuclei/cytoplasm ratio (B). Data shows mean and SEM from 8 cells. Significance determined by one way ANOVA with multiple comparison tests. Nuclear extracts were prepared from infected cells at 3 h and 8 h post-infection and p65 DNA-binding activity (C) was determined as described in Methods. Data represent mean of triplicate values from one of two experiments, error bars represent SD, and significance determined by one way ANOVA. * represents <0.05, ** represents<0.01, *** represents <0.001 and ns represents “not significant”.
Chlamydia caviae infection results in equivalent levels of IL-1β secretion as C. muridarum infection in resting macrophages.
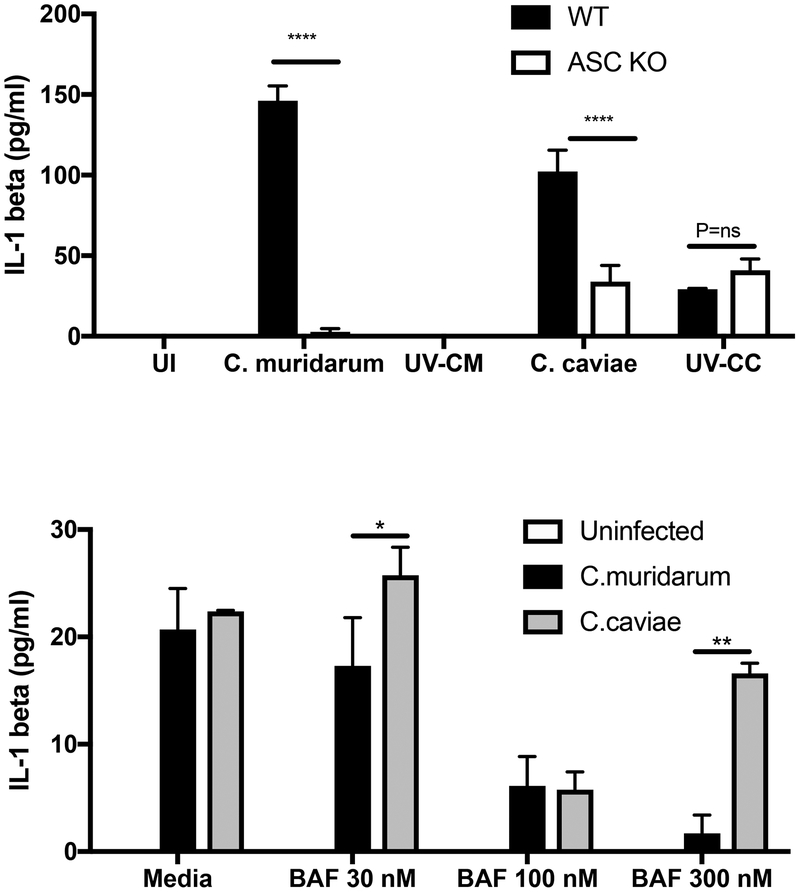
IL-1β secretion needs two signals. In the first signal, IL-1β mRNA is expressed and inactive pro-IL-1β protein is made. In the second, activation of inflammasome results in caspase-1 activation, which cleaves pro-IL-1β to mature IL-1β allowing the protein to be secreted out of the cells to initiate inflammation. Since, NFκB activation and IL-1β mRNA levels were similar in C. caviae-infected and C. muridarum-infected macrophages at 3 h post-infection, we tested inflammasome-dependent IL-1β protein secretion in the culture supernatants. Surprisingly, C. caviae infection resulted in equivalent or more IL-1β secretion, than C. muridarum infection, suggesting no alteration in inflammasome activation (Fig 6A). During C. muridarum infection, IL-1β secretion was fully dependent on the inflammasome adaptor ASC (Fig 6A) as shown before5. Although not completely abolished, IL-1β secretion was significantly reduced in ASC KO macrophages during C. caviae infection. C. caviae failed to grow in ASC macrophages, with similar numbers of IFU recovered as observed for WT macrophages (data not shown). C. caviae also co-localized to LAMP-1 at 3 h post-infection (data not shown), suggesting a minimal role for inflammasome in growth inhibition. These results reveal that C. caviae infection was able to provide both signals for IL-1β production despite no chlamydial growth. To address if an inhibitor of vacuolar ATPase, which prevents acidification of late endosomes, will alter chlamydial growth and inflammasome activation, macrophages were exposed to Bafilomycin A, a known inhibitors of endosomal acidification. Bafilomycin exposure had minimal effect on C. muridarum inclusion formation or IL-1β secretion at lower concentrations. Bafilomycin exposure did not rescue C. caviae growth (data not shown), but a significant increase in IL-1β secretion was observed for C. caviae infection, suggesting blocking this pathway increased inflammasome activation, specifically in the absence of chlamydial growth.
Fig 6: C. caviae induces inflammasome activation in resting macrophages.
(A) Supernatants from WT and ASC KO-infected macrophages were analyzed for IL-1β protein by ELISA 24 h post-infection. Data represents averages from 3 experimental replicates (B) WT macrophages treated with three different concentrations of Bafilomycin were infected with C. muridarum or C. caviae and supernatants analyzed for IL-1β by ELISA. Mean values are shown and error bars represent SD. *** indicates p<0.001 and ** indicates p<0.01 by one way ANOVA. ND=Not detected.
DISCUSSION
Chlamydia is predominantly an epithelial pathogen and its growth is initiated at the mucosal surface. However, dissemination to other tissue sites has been observed in the mouse model in the absence of IFNγR signaling11 or in the absence of B cells12. This ability to disseminate has been attributed to the growth of C. muridarum in macrophages11, 13. Growth of human chlamydial isolates in primary macrophages is variable1, 14. With the exception of LGV, most human C. trachomatis isolates do not grow well in macrophages1. Restrictive growth has been attributed to perforin-215, early phago-lysosome fusion, and late autophagy16. C. muridarum and C. caviae have different host requirements in vivo, but in in vitro cell culture both grow in epithelial cells at equal rates. However, while C. muridarum can replicate successfully in an inclusion in macrophages, C. caviae fails to do so. In this study, we have used primary mouse macrophages as a model system to study early host response to chlamydial survival or failure to do so, using C. muridarum and C. caviae. Our results indicate that IFNβ and IFN-response gene expression are initiated only when chlamydiae escape phago-lysosome fusion and survive. These responses further require chlamydial growth/replication inside an inclusion, since UV-killed C. muridarum fail to induce these responses. In contrast, early NFκB-dependent signaling in the host is initiated independent of chlamydial survival and whether or not chlamydiae are inside lysosomal vacuoles. Further, our data demonstrate that inflammasome activation in macrophages does not require chlamydial growth. These findings suggest that intracellular niches for bacterial ligands determine initiation of host-signaling events.
DNA sensing by cGAS4 and activation of STING3 are essential for IFNβ induction during infection with multiple C. trachomatis isolates4. It has also been shown that direct sensing of chlamydial second messenger cdi-AMP by STING can induce IFNβ17. IFNβ expression requires actively replicating bacteria and inhibition of bacterial growth by chloramphenicol abrogates this response18. During chlamydial entry, followed by localization to early endosomes that resist fusion with lysosomes, chlamydial DNA is unavailable for recognition by cytosolic host DNA sensors. Therefore, initiation of an IFNβ response could be a result of direct sensing of the chlamydial second messenger cdi-AMP by STING, in the host cytosol17. As C. muridarum replicates inside the inclusion, it is possible that DNA fragments released inside the inclusion escape into the cytosol and are detected by the cytosolic DNA sensor cGAS. Conversely, during C. caviae infection, fusion of early endosomes with lysosomes leads to digestion of bacteria by lysosomal enzymes preventing any cdi-AMP production. However, bacterial digestion in the lysosomes could release fragmented DNA fragments. But these are likely not sensed by cGAS as it is unable to access DNA at this niche. This could explain why no IFNβ transcripts are made during C. caviae infection versus during C. muridarum infection at 3 h post-infection. Therefore, even though exogenous DNA in the host cells is a danger signal, it may not be seen as a “viral” threat to generate an IFNβ response if the DNA is inside the lysosomes.
Previously we have shown that resting macrophages induce very low levels of IL-1β during C. muridarum infection5. One possible explanation for the poor inflammasome activation in resting macrophages during C. muridarum infection could be due to the pathogen’s ability to inhibit fusion with lysosomes rapidly and secure itself in endosomes. Consequently, the macrophage experiences minimal mitochondrial stress as observed by cell morphology with intact pseudopodia (Supplementary Fig S2) or DNA-release, required for NLRP3 or AIM2-mediated inflammasome activation19. It is interesting to note that different chlamydial preparations induce differential levels of IL-1β at similar MOI, suggesting that less pure preparation may activate phagocytic pathway and increased signal. Macrophages exposed to LPS before infection secrete high levels of IL-1β secretion5, although the numbers of C. muridarum inclusions are significantly reduced, with several trapped in lysosomes (data not shown), suggesting a minimal role of chlamydial growth in this response. Entry of C. caviae into macrophages does not inhibit lysosomal fusion and bacteria appear in several large vacuoles in macrophages 2 h post-infection (Fig 1 and Supplementary Fig S2). Since C. caviae is very effective in inducing TLR2 responses20, there is significant first signal leading to NFκB activation to generate pro-IL-1β. C. caviae-infected resting macrophages thereby secrete high levels of IL-1β without pre-stimulation. Inflammasome activation was dependent on ASC, suggesting contribution from NLRP3 and AIM2, which have been previously shown to contribute to this response. UV-killed C. muridarum fail to activate inflammasome, despite production of high levels of IL-1β mRNA, suggesting the requirement of viable bacteria for this process. However, UV-killed C. caviae infection result in low levels of IL-1β secretion, which was surprisingly ASC independent.
Considering both AIM2 and cGAS sense exogenous DNA, it is interesting that cGAS sensing occurs only when inclusions are observed as in C. muridarum infection, while inflammasome-mediated sensing is chlamydial growth-independent. Further studies are required to determine the compartmentalization of these two sensors during infection which results in differential cytokine responses. It also remains unclear why C. muridarum is successful in establishing infection in macrophages while most other C. trachomatis strains are not. Likewise, human isolate LGV has been shown to grow in macrophages14. Whether these strains have additional effectors that subvert macrophage phagocytic pathways giving them an edge remains unclear and requires independent investigation.
The antagonistic nature of IFNβ and IL-1β pathways has been described, with type I IFN inhibiting IL-1β production21 and, conversely caspase-1-mediated cleavage of cGAS22, suggesting preferential signaling in one direction versus the other. Interestingly, both IFNAR signaling23 and IL-1R signaling24 promote oviduct pathology during genital C. muridarum infection in the mouse model, suggesting that signaling pathways initiated by differential host responses does not ensure protection from disease. IFNβ expression is an antiviral response, but it is ineffective in eradicating intracellular bacteria. IFNβ has been shown to down-modulate IFNγR signaling during Listeria infection25 and Chlamydia infection23. IL-1R signaling has some contribution in controlling bacterial numbers but also contributes to neutrophil recruitment and oviduct pathology24. These findings underscore how Chlamydia is a successful pathogen despite engaging several host responses. Furthermore, our results demonstrate that sustained chlamydial growth is not necessary for certain cytokine responses such as TNFα- and IL-1- signaling, suggesting antibiotics to stop chlamydial growth or replication are not sufficient to inhibit the damage caused by some cytokine responses in the female genital tract.
METHODS
Chlamydial stocks:
C. muridarum strain “Nigg” was originally obtained in 1977 as a yolk sac preparation from the ATCC and has been passaged continually in our laboratory since that time. C. caviae was originally obtained from the late Edward Murray in the early 1970’s and has been continually passaged in Dr. Rank’s laboratory since that time. Both isolates were grown in mycoplasma-free McCoy cells as described previously26, 27. Elementary bodies were harvested from infected cells, resuspended in SPG buffer (250 mM sucrose, 10 mM sodium phosphate, and 5 mM L-glutamic acid, pH 7.2), and quantified as inclusion forming units (IFU) on McCoy cell monolayers as described previously.
Isolation of murine macrophages, in vitro infection and Bafilomycin treatment:
C57BL/6J (WT) or ASC−/− mice were injected with 3% thioglycollate and peritoneal macrophages were harvested after 3 days. Animal protocols were approved by UAMS Little Rock and UNC Chapel Hill. Macrophages were plated in complete media (RPMI media supplemented with 10% FBS, 100 mM HEPES, 1 mM sodium pyruvate, 2 mM L-glutamine, 100 μM nonessential amino acids, 100 U/ml penicillin/100 μg/ml streptomycin, 50 mM β-mercaptoethanol) for 48–72 h prior to infection at 8 × 105 cell/well in a 24 well culture plate. In vitro infection using chlamydiae were carried out as described8. To obtain similar levels of infection with C. muridarum and C. caviae in macrophages, the chlamydial stocks were used to infect McCoy cells at different MOI of the 2 chlamydial strains. The host cells were treated with 0.3% saponin and intracellular staining for chlamydial inclusions was carried out using the “Pathfinder” FITC-conjugated murine anti-chlamydial mAb (Bio-Rad). McCoy cells were analyzed by flow cytometry to determine the percentage of infected cells. MOIs that gave an equal percentage of infection in McCoy cells were used to infect macrophages. Infected macrophages on cover slips were fixed with methanol 24 hours post-infection and stained for chlamydial inclusions using the “Pathfinder” FITC-conjugated murine anti-chlamydial mAb (Bio-Rad) according to the manufacturer’s instructions. Inclusions were viewed using an Olympus fluorescent microscope. For Bafilomycin A1 exposure, the peritoneal macrophages from WT mice infected with 1MOI of C. caviae or C. muridarum were either kept in media or exposed to 30 nM, 100 nM or 300 nM of Bafilomycin A1 (Sigma Aldrich) during the period of infection. At 18 hours post-infection, supernatants were collected and assayed for IL-1β by ELISA.
Electron microscopy:
Macrophages (107) were infected with C. caviae or C. muridarum as described above in plates coated with poly-L-lysine. The infected macrophages were detached at indicated times with 10× trypsin containing EDTA, by gently scraping cells with a rubber policeman. Macrophages were washed and centrifuged at 1200 rpm for 5 min; the cell pellets were then suspended in EPON fixative and processed for transmission electron microscopy (TEM) as described earlier28.
Quantitative real time RT-PCR:
RNA was prepared using the RNeasy kit (Qiagen). Reverse transcription reaction and quantitative real time PCR (RT-PCR) were carried out as described previously8. Primers used are described in supplementary Table 1. The results are presented as fold increase in expression over uninfected samples, by comparing the threshold cycle of gene of interest to β-actin gene expression, using the ΔΔCT method29.
NFκB activity and p65 nuclear translocation:
Nuclear extracts from infected cells were prepared using Nuclear extract kit (Active Motif). TransAM NFκB kits (Active Motif) were used to determine p65 activity. For NFκB p65 staining, mouse primary macrophages grown on coverslips were infected at indicated times and fixed in methanol-free 4% paraformaldehyde (EM Bioscience) for 15 min at room temperature. Cells were rinsed with PBS three times and blocked for 1 h at room temperature in 5% normal goat serum diluted in 1× PBS and 0.3% Triton X-100. NFκB p65 Ab (C22B4, rabbit mAb #4764 Cell-signaling) was used at 1:25 in 1× PBS with 1% BSA and 0.3% Triton X-100 and incubated overnight at 4°C on a shaker. Mouse immune sera containing anti-chlamydial antibody was used to stain for Chlamydia. Coverslips were rinsed thrice in PBS for 5 min each. Secondary antibodies (anti-rabbit AF488 and anti-mouse AF568-Invitrogen) were used at 1:1500 dilution in 1% BSA and 0.3% Triton X-100 and incubated for 2 h at room temperature. Coverslips were rinsed three times in PBS for 5 min each and mounted with Prolong gold anti-fade reagent (Invitrogen), with or without DAPI for nuclear staining.
EEA1 and LAMP-1 staining:
Mouse macrophages infected with 1 MOI of C. muridarum or C. caviae were fixed with 4% PFA (Electron Microscopy Sciences, Hatfield, PA) at 3 h and 18 h post-infection. Cells were stained for either early endosomal marker EEA1 using anti-EEA1, Cat # MA5–14794 (Invitrogen, Thermo Fisher Scientific) or lysosomal marker LAMP-1 using anti-LAMP1, Cat# 21997–1-AP) (Proteintech) at 1:200 dilution, and with Chlamydial LPS (mouse monoclonal Anti-LPS, cat# MCA2718) (BioRAD) used at 1:300 dilution. The secondary antibody combination contained goat anti-mouse Alexa 488 (cat# A11017) and goat anti-rabbit Alexa 594 (cat# A11037) (Invitrogen, Thermo Fisher Scientific) at 1:1000 dilution. Cells were washed and mounted using Prolong anti-fade containing DAPI (Invitrogen). Confocal images were acquired with the 63× oil 0.8 numerical aperture objective using Zeiss confocal microscope (LSM 880) and images were analyzed using image J or Zen lite software.
IL-1β ELISA:
Macrophages from C57BL/6 and ASC knock out mice were plated (8 × 105) and incubated for 48–72 h prior to infection. Macrophages were infected at the same infection rate with C. muridarum and C. caviae as explained in above methods section. ASC KO mice peritoneal macrophages were included in the study to determine inflammasome involvement in IL-1β release during C. caviae infection. Supernatants were collected at indicated times for IL-1β protein quantitation. IL-1β ELISA was carried out as described in manufacturer’s protocol (R&D systems).
Statistics:
Infection phenotype, IFU, RNA and protein analysis were performed using at least three independent times, EEA/LAMP1 staining and p65 study were done twice and TEM was done once. Significance determined using the analysis component of graph pad software. Unpaired student T test was used for determining significance between two individual groups. For more than two treatment group with one variable, a one-way ANOVA with pairwise multiple comparison (Holm-Sidak method) was performed to determine statistically significant differences. For more than two treatment group with two variables, a 2-way ANOVA with the post-hoc Tukey test as a multiple or pair wise comparison procedure.
Supplementary Material
Supplementary Fig 1: C. muridarum forms mature inclusion in primary mouse macrophages. TEM images of C. muridarum inclusions in mouse macrophages at 24 h post-infection shown at 2 micron magnifications showing mature RBs (larger less dense chlamydial forms) and EBs (smaller dense chlamydial forms) inside large inclusions. Inset image is at 500 nm, and shows RBs in contact with each other and with the inclusion membrane.
Supplementary Fig 2: At 2 h post-infection, C. muridarum-infected macrophages retain pseudopodia while C. caviae-infected macrophages lose their pseudopodia and contain large vacuoles. TEM images of C. muridarum and C. caviae-infected macrophages 2 h post-infection shown at 2 micron magnification. C.muridarum-infected macrophages retain their pseudopodia and looks relatively undisturbed. C.caviae-infected macrophages show increased number of vacuoles and loss of pseudopodia. Arrows outside the macrophages indicate pseudopodia and arrows inside macrophages indicate internalized chlamydiae.
Supplementary Fig 3: Both C.muridarum and C.caviae do not co-localize with early endosomes, but more numbers of C. caviae co-localize with lysosomes. Macrophages infected with C. muridarum or C. cavaie at 1 MOI infection for 3 h p.i were fixed and immuno-stained for an early endosomal marker EEA-1 or lysosomal marker LAMP-1 with chlamydial LPS staining. Arrows indicate co-localization of LAMP-1 and LPS.
ACKNOWLEDGMENTS
Assistance by Dr. Laxmi Yeruva, UAMS on one of the macrophage experiments is acknowledged. Dr. Shanmugam Nagarajan’s scientific input and editing of manuscript is greatly appreciated. The study was supported using funds from NIH AI067678 to UN.
Footnotes
CONFLICT OF INTEREST
All authors have no conflict of interests
References
- 1.La Verda D, Byrne GI. Interactions between macrophages and chlamydiae. Immunol Ser 1994; 60: 381–99. [PubMed] [Google Scholar]
- 2.Gerard HC, Whittum-Hudson JA, Carter JD et al. The pathogenic role of Chlamydia in spondyloarthritis. Current Opinion in Rheumatology 2010; 22: 363–7. [DOI] [PubMed] [Google Scholar]
- 3.Prantner D, Darville T, Nagarajan UM. Stimulator of IFN gene is critical for induction of IFN-beta during Chlamydia muridarum infection. J Immunol 2010; 184: 2551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Yeruva L, Marinov A et al. The DNA sensor, cyclic GMP-AMP synthase, is essential for induction of IFN-beta during Chlamydia trachomatis infection. J Immunol 2014; 193: 2394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prantner D, Darville T, Sikes JD et al. Critical role for interleukin-1beta (IL-1beta) during Chlamydia muridarum genital infection and bacterial replication-independent secretion of IL-1beta in mouse macrophages. Infection and immunity 2009; 77: 5334–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finethy R, Jorgensen I, Haldar AK et al. Guanylate binding proteins enable rapid activation of canonical and noncanonical inflammasomes in Chlamydia-infected macrophages. Infection and immunity 2015; 83: 4740–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson DP, Timms P, McElwain DL et al. Type III secretion, contact-dependent model for the intracellular development of chlamydia. Bull Math Biol 2006; 68: 161–78. [DOI] [PubMed] [Google Scholar]
- 8.Nagarajan UM, Ojcius DM, Stahl L et al. Chlamydia trachomatis induces expression of IFN-gamma-inducible protein 10 and IFN-beta independent of TLR2 and TLR4, but largely dependent on MyD88. J Immunol 2005; 175: 450–60. [DOI] [PubMed] [Google Scholar]
- 9.Der SD, Zhou A, Williams BR et al. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc Natl Acad Sci U S A 1998; 95: 15623–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prantner D, Nagarajan UM. Role for the chlamydial type III secretion apparatus in host cytokine expression. Infection and immunity 2009; 77: 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotter TW, Ramsey KH, Miranpuri GS et al. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infection and immunity 1997; 65: 2145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li LX, McSorley SJ. B cells enhance antigen-specific CD4 T cell priming and prevent bacteria dissemination following Chlamydia muridarum genital tract infection. PLoS Pathog 2013; 9: e1003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wyrick PB, Brownridge EA. Growth of Chlamydia psittaci in macrophages. Infection and immunity 1978; 19: 1054–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuo CC. Cultures of Chlamydia trachomatis in mouse peritoneal macrophages: factors affecting organism growth. Infection and immunity 1978; 20: 439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fields KA, McCormack R, de Armas LR et al. Perforin-2 restricts growth of Chlamydia trachomatis in macrophages. Infection and immunity 2013; 81: 3045–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Zeer MA, Al-Younes HM, Lauster D et al. Autophagy restricts Chlamydia trachomatis growth in human macrophages via IFNG-inducible guanylate binding proteins. Autophagy 2013; 9: 50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barker JR, Koestler BJ, Carpenter VK et al. STING-Dependent Recognition of Cyclic di-AMP Mediates Type I Interferon Responses during Chlamydia trachomatis Infection. MBio 2013; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prantner D, Sikes JD, Hennings L et al. Interferon regulatory transcription factor 3 protects mice from uterine horn pathology during Chlamydia muridarum genital infection. Infection and immunity 2011; 79: 3922–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou R, Yazdi AS, Menu P et al. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011; 469: 221–5. [DOI] [PubMed] [Google Scholar]
- 20.Frazer LC, Darville T, Chandra-Kuntal K et al. Plasmid-cured Chlamydia caviae activates TLR2-dependent signaling and retains virulence in the guinea pig model of genital tract infection. PLoS One 2012; 7: e30747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guarda G, Braun M, Staehli F et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 2011; 34: 213–23. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Ning X, Gao P et al. Inflammasome Activation Triggers Caspase-1-Mediated Cleavage of cGAS to Regulate Responses to DNA Virus Infection. Immunity 2017; 46: 393–404. [DOI] [PubMed] [Google Scholar]
- 23.Nagarajan UM, Prantner D, Sikes JD et al. Type I interferon signaling exacerbates Chlamydia muridarum genital infection in a murine model. Infection and immunity 2008; 76: 4642–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagarajan UM, Sikes JD, Yeruva L et al. Significant Role of IL-1 Signaling, but Limited Role of Inflammasome Activation, in Oviduct Pathology during Chlamydia muridarum Genital Infection. J Immunol 2012; 188: 2866–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rayamajhi M, Humann J, Penheiter K et al. Induction of IFN-alphabeta enables Listeria monocytogenes to suppress macrophage activation by IFN-gamma. J Exp Med 2010; 207: 327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caldwell HD, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infection and immunity 1981; 31: 1161–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darville T, O’Neill JM, Andrews CW Jr. et al. Toll-like receptor-2, but not Toll-like receptor-4, is essential for development of oviduct pathology in chlamydial genital tract infection. J Immunol 2003; 171: 6187–97. [DOI] [PubMed] [Google Scholar]
- 28.Giles DK, Whittimore JD, LaRue RW et al. Ultrastructural analysis of chlamydial antigen-containing vesicles everting from the Chlamydia trachomatis inclusion. Microbes and infection/Institut Pasteur 2006; 8: 1579–91. [DOI] [PubMed] [Google Scholar]
- 29.Nagarajan UM, Bushey A, Boss JM. Modulation of gene expression by the MHC class II transactivator. Journal of Immunology 2002; 169: 5078–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig 1: C. muridarum forms mature inclusion in primary mouse macrophages. TEM images of C. muridarum inclusions in mouse macrophages at 24 h post-infection shown at 2 micron magnifications showing mature RBs (larger less dense chlamydial forms) and EBs (smaller dense chlamydial forms) inside large inclusions. Inset image is at 500 nm, and shows RBs in contact with each other and with the inclusion membrane.
Supplementary Fig 2: At 2 h post-infection, C. muridarum-infected macrophages retain pseudopodia while C. caviae-infected macrophages lose their pseudopodia and contain large vacuoles. TEM images of C. muridarum and C. caviae-infected macrophages 2 h post-infection shown at 2 micron magnification. C.muridarum-infected macrophages retain their pseudopodia and looks relatively undisturbed. C.caviae-infected macrophages show increased number of vacuoles and loss of pseudopodia. Arrows outside the macrophages indicate pseudopodia and arrows inside macrophages indicate internalized chlamydiae.
Supplementary Fig 3: Both C.muridarum and C.caviae do not co-localize with early endosomes, but more numbers of C. caviae co-localize with lysosomes. Macrophages infected with C. muridarum or C. cavaie at 1 MOI infection for 3 h p.i were fixed and immuno-stained for an early endosomal marker EEA-1 or lysosomal marker LAMP-1 with chlamydial LPS staining. Arrows indicate co-localization of LAMP-1 and LPS.