Abstract
A great number of researches over the last years are allocated to know cancer reasons, prevention and treatment strategies. Bacterial infections are one of the promoting factors in cancer development. The present study was carried out to study effects of heat-killed bacteria on cancer cell lines MCF7 and HT-29. To this purpose, four bacterial strains including Salmonella typhi, Staphylococcus epidermidis, Escherichia coli and Pseudomonas aeruginosa were assayed. Thermal inactivation method was used to kill the bacteria and preserve the bacterial surface proteins unchangeable. The concentrations of 0.01, 0.1, 0.5 and 1 mg/ml of inactivated bacteria were prepared to evaluate the effects of heat-inactivated bacterial solutions on MCF7 and HT-29 cell lines. MTT assay was used to measure the cell viability of cancer cells treated with different concentration of inactivated bacterial solutions.The MTT assay results after 48 hours showed that the heat-killed bacterial solutions were able to induce the proliferation of both cancer cell lines. In addition, the most cell viability in MCF-7 cell line was seen in samples treated with S. epidermidis, while in HT29 cells, the most one was seen in S. typhi treated samples. It was concluded that bacterial infections are cancer-deteriorating agents, and any species of bacteria is specific to certain cancerous tissue.
Key Words: Inflammatory responses, Chronic infections, MTT-assay, Heat inactivation, Colon cancer, Breast cancer
INTRODUCTION
Cancer is a refractory disease resulted from several changes in cell division-related genes. A great number of researches in recent years are allocated to know different aspects of cancer, including its reasons, prevention and treatment strategies. It is demonstrated that many factors are involved in induction of cancer-leading mutations in the germ line and somatic cells. UV light, X-rays, chemicals, tobacco products, stress and viruses are such of these factors [1-3]. Furthermore, the bacterial infections as promoting factors in cancer development have been assayed for more than two decades. The results showed that the Helicobacter pylori infection is one of the first-class carcinogens and is a very important agent in gastric cancer and mucosa-associated lymphoid tissue (MALT) lymphoma [4-7]. In addition to H. pylori, the relevance of Salmonella typhi to gallbladder cancer, Streptococcus bovis/gallolyticus to colorectal carcinoma (CRC) [8-10] and Chlamydia pneumonia to lung cancer [11-15] have been distinguished in further investigations.
In fact, contrary to previous theories that believed bacteria could cause only acute diseases, it is now proved that many bacteria are the cause of chronic infections and diseases, including cancer. It is estimated that over 15% of malignancies worldwide can be attributed to bacterial infections [7] or about 1.2 million cases per year [16]. It is suggested that the relationship between bacteria and carcinogenesis can be both causative and opportunistic [17]. In the causative relation, bacteria induce the inflammatory responses in host body constantly due to the persistence of bacteria itself or bacterial toxins and mediators which are released chronically [18-21].
The aim of this study was to analyze the effects of heat-inactivated bacteria on cancer cells in vitro to find whether a killed bacterium could have effects on cancerous cells or vitality is necessary for affecting. In literatures, to inactivate the bacterial cells and preserve their surface structures, different fixation methods has been demonstrated. Inactivation by chemical agents [22], heat [23], sonication [24] and UV irradiations [25, 26] are of these methods. Most commonly used chemical fixatives includes 2.5% glutaraldehyde, 10% formalin, 4% paraformaldehyde, methanol/acetone and ethanol/acetic acid solutions [27]. Although all of these solutions are appropriate in preserving bacterial cell morphology, aldehyde-based solutions are preferred to alcohol ones, because the existence of alcohol results in detaching of the surface ultrastructures (i.e., Pilli and flagella) [27]. Thermal inactivation is one of the most historical and important preservation methods. Microorganisms are more sensitive to wet heat than to dry heat [23]. Damage to the membrane is the main mechanisms of wet heat inactivation [28, 29], while oxidation and protein denaturation is more likely in dry heat inactivation [30]. A non-thermal alternatives to conventional thermal approaches, sonication is mostly used coupled with pressure and/or heat to inactivate the microbes. Spores of bacteria are relatively resistant to this method, thus prolonged periods of ultrasonication would be required [24]. Quantitative and qualitative researches indicate that the amount of resistance to each method is different between gram negative and gram positive bacterial cells. Furthermore, based on images acquired by atomic force microscopy (AFM), the morphology of the cells after fixation is various dependent on utilized method. In this work, based on the purpose of research, the structure of interested bacteria species and advantages and disadvantages of mentioned fixation methods, the wet heat inactivation was chosen.
MATERIALS AND METHODS
Preparation of cell lines and bacterial species: Colorectal adenocarcinoma HT-29 cell line (ATCC HTB-38) was purchased from “National Center for Genetics and Biotechnology of Iran”. Breast cancer MCF-7 cell line (ATCC HTB-22) was supplied by “The Pasteur Institute of Iran”. Four bacterial species including S. typhi (PTCC1609), S. epidermidis (PTCC1436), E. coli (ATCC 25922) and P. aeruginosa (PTCC 1074) were supplied by “The Pasteur Institute of Iran”.
Inactivation of bacteria: To inactivate the bacterial cells and preserve their surface structures, different fixation methods have been demonstrated. Inactivation by chemical agents [47], heat [48], sonication [49] and UV irradiations [50, 51] are of these methods. Most commonly used chemical fixatives include 2.5% glutaraldehyde, 10% formalin, 4% paraformaldehyde, methanol/acetone and ethanol/acetic acid solutions [52]. Although all of these solutions are appropriate in preserving bacterial cell morphology, aldehyde-based solutions are preferred to alcohol ones, because the existence of alcohol results in detachment of the surface ultrastructures (i.e., Pilli and flagella) [52]. Thermal inactivation is one of the most historical and important preservation methods. Microorganisms are more sensitive to wet heat than to dry heat [48]. Damage to the membrane is the main mechanisms of wet heat inactivation [53, 54], while oxidation and protein denaturation are more likely in dry heat inactivation [55].
A non-thermal alternatives to conventional thermal approaches, sonication is mostly used coupled with pressure and/or heat to inactivate the microbes. Spores of bacteria are relatively resistant to this method, thus prolonged period of ultrasonication is required [49].
Quantitative and qualitative researches indicate that the amount of resistance to each method is different between gram negative and gram positive bacteria cells. Furthermore, based on images acquired by atomic force microscopy (AFM), the morphology of cells after fixation is various dependent on utilized method. In this work, based on the purpose of research, the structure of interested bacteria species and advantages and disadvantages of mentioned fixation methods, the wet heat inactivation was chosen.
To prepare the bacterial culture, bacterial species were grown separately in 100 ml of nutrient broth (NB) at 37℃ for 24h with shaking rate of 160 rpm. As mentioned above, in this study, thermal inactivation was used as inactivation method to preserve the bacterial surface proteins unchangeable and to kill the bacteria cells completely. For this purpose, cultured bacteria were precipitated by centrifugation in 4000 ×g for 10 min. After discharging the supernatant, the pellet was washed three times with 2 ml of PBS. Finally, the pellet was resuspended in 1 ml PBS. Inactivation step was applied by placing the obtained solutions in the water bath at 70℃ for 40 min. To insure that all of the bacterial cells were killed, a cultured plate of bacteria was provided and incubated overnight. The solutions of bacteria were dried by freeze-drying. Different concentrations of bacterial solutions including 0.1, 1, 5 and 10 mg per 1 ml PBS were prepared (note that, in MTT assay, the final concentration of solutions in each well will be diluted 10-fold).
MTT assay: MCF-7 and HT-29 cell lines were cultured in RPMI 1640 culture media at 37℃, 5% CO2 and 95% humidity. When the confluency of cells in culture flasks reached 80 percent, cells were detached by trypsin 5% and centrifuged at 500 ×g. The pellet was resuspended in RPMI and aliquoted in 96-well plate, so that approximately 3 × 104 cells were cultured in each well. Then, 20 μl of prepared bacterial solutions were added to wells. The plate was incubated in 37℃, 5% CO2 and 95% humidity for 48 hours. Afterward, 100 μl of culture media was discarded, and 22 μl of MTT solution was added. After 2 hrs incubation, the whole culture media was discarded and 100 μl DMSO was added to each well. The optical density of viable cells was read at 492-630 nm by ELISA reader.
RESULTS
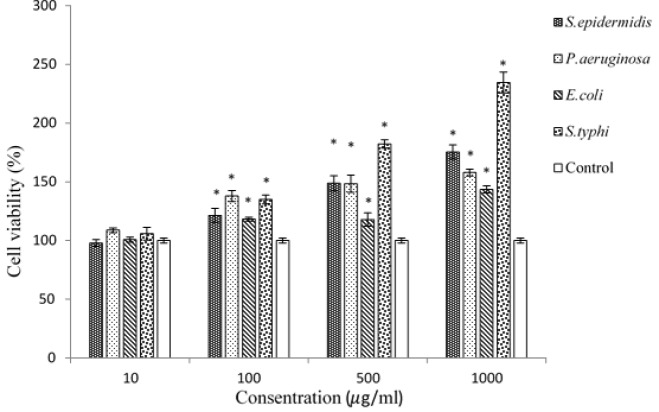
In this research, the MTT assay was performed to evaluate the effects of four different heat-inactivated bacteria strains, introduced as carcinogenic agents, on HT-29 and MCF-7 cell lines. The MTT assay results of HT-29 and MCF7 are shown in Figures 1 and 2, respectively. Furthermore, the outputs of MTT assay were analyzed by SPSS ANOVA v.21 statistical analytical software. P < 0.05 was considered to be statistically significant. According to Fig. 1, cancer-promoting effects on HT-29 cell line were heterogeneous among the four bacterial strains. The highest cancer induction activity was observed by S. typhi at the concentration of 1000, followed by S. epidermidis, P.aeruginosa and E.coli.
Figure 1.
The column graph of MTT assay results from HT-29 cell line treated with four different heat-killed bacterial strains. * Distinguishes the significant difference of samples compared to control
Figure 2.
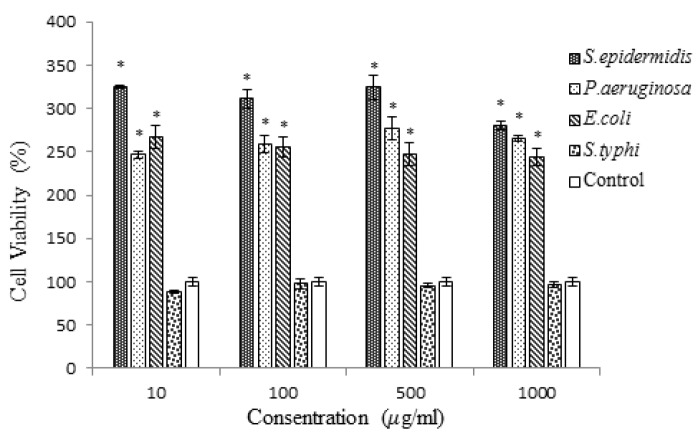
The column graph of MTT assay results from MCF7 cell line treated with four different heat-killed bacterial strains. * Distinguishes the significant difference of samples compared to control
S. typhi increased the number of cancer cells in a concentration-dependent manner up to 234.54 % compared to negative control. In contrast, the least induction effect obtained by samples treated with E. coli solution. The MTT assay showed that among all bacterial strains, along with enhancement of bacterial solutions’ concentrations from 10 to 1000, the proliferation of cancer cells and their percentage of cell viability were increased. The comparison of different strains with each other showed that the increasing effect of P. aeruginosa was more than samples treated with E. coli in all concentration. Furthermore, inactivated S. typhi induced the proliferation of cancer cells more than inactivated E. coli and S. epidermidis in all concentrations. In Fig. 2, the MTT assay results of MCF-7 cell line are shown. Three species including S. epidermidis, P. aeruginosa and E. coli had significant induction effect on cancer cells, while S. typhi had no effect on cancer cells. The most increasing activity was observed by S. epidermidis at all concentrations. S. epidermidis effect showed ascending trend by increasing the concentration of the bacterial solution from 10 to 500, but at the concentration of 1000 , a slight reduction on cell viability of cancer cells was observed. The most cell viability of MCF-7 cells, 324.24%, was obtained by samples treated with 500 of S. epidermidis.
Also, P. aeruginosa had significant induction effect on cells growth at all concentrations. The E. coli increased the proliferation of cancer cells at all concentrations. Its effect was dose-dependent with the slight negative slope.
DISCUSSION
In this work, the relationship of bacteria with cancer cells was assayed via in vitro experimental approaches. For this purpose, the effects of heat-inactivated of four different bacteria on breast and colon cancer cell lines were studied by MTT assay. Because of toxic effects of whole living bacterium on eukaryotic cells in MTT test, the bacterial solutions have to be inactivated. Consequently, the thermal inactivation method was selected to inactivate the internal metabolism of bacteria and to conserve their surface structures. The reasons have been explained in the following. On the one hand, it is distinguished that among inactivation methods for bacterial cells, inactivation with formaldehyde, paraformaldehyde and heat are better in preservation of three- dimensional structures of surface proteins [27, 31]. On the other hand, paraformaldehyde and formaldehyde are toxic to eukaryotic cells; therefore, remaining traces of them in bacterial solutions due to incomplete washing step can be problematic in obtaining true results. During recent years, a lot of researches allocated to study challenging incorporation mechanisms of bacterial cells in cancer. Some researchers believe that their relationship is more opportunistic rather than causative [32-36]. In tumor tissues due to some appropriate conditions including the existence of adequate bacterial nutrients and providing a refuge for bacteria to evade the immune system clearance [37-40], bacteria tend to enter and accumulate in tumorous tissues based on opportunism. On the contrary, it is believed by some researchers that bacteria are the causative factors in the cancer-promoting process [11, 36, 41-45], because they induce the chronic inflammatory response in host cells. Furthermore, it is investigated that microbial flora of cancerous tissues is not the same as one in normal tissue. Some researches demonstrate that microbial flora of breast cancer patients is different from normal persons, such that bacteria belonging to three genera, Enterobacter, Pseudomonas and Staphylococcus, were extracted from breast cancer patients [46, 47]. Also, Cantwell and et al. extracted S. epidermidis from breast cancer tissues [48].
In the present research, the results of MTT assay of S. epidermidis, P. aeruginosa and S. typhi on MCF-7 and HT-29 cell lines showed that presence of these bacteria in tumorous tissues, especially in breast cancer tissues, even after becoming cancerous, can exacerbate the growth of cancer cells. In other words, the taken changes in the bacterial flora of the tissues will end in favor of cancerous cells.
In the MTT assay, the MCF-7 cell line was more influenced by bacterial treatment than HT-29 cells. The most cell growth and cell viability in MCF-7 cell line were seen in samples treated with S. epidermidis, P. aeruginosa and E. coli, respectively, and no significant cell growth was seen in S. typhi treated wells (Figure 2); however, in HT-29 cell line the most cell viability was seen in samples treated with S. typhi (Figure 1). Presumably, it is because of specific interactions between bacterial species and eukaryotic cell surface proteins. It has been indicated that S. typhi recognizes a specific glycosylation pattern, GlcNAcβ1-4GlcNAcβ-N/Gly, on colon cancer cells and makes a connection with these cells [49]. Because the mentioned glycosylation pattern is not detected on breast cancer cells [50], S. typhi cannot make a junction with these cancer cell receptors. It is supposed that this weak connection is the reason of having no significant effect of S. typhi solutions on MCF-7 cells in this research.
Acknowledgements:
Support of this work by the University of Isfahan is highly acknowledged.
Conflict of Interest:
The authors declare that they have no conflict of interest.
References
- 1.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 2.Irigaray P, Newby JA, Clapp R, Hardell L, Howard V, Montagnier L, Epstein S, Belpomme D. Lifestyle-related factors and environmental agents causing cancer: an overview. Biomed Pharmacother. 2007;61:640–658. doi: 10.1016/j.biopha.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Hausen Hz. Papillomaviruses Causing Cancer: evasion from host-cell control in early events in carcinogenesis. J Natl Cancer Inst. 2000;92:690–698. doi: 10.1093/jnci/92.9.690. [DOI] [PubMed] [Google Scholar]
- 4.Cover TL, Blaser MJ. Helicobacter pylori in health and disease. Gastroenterology. 2009;136:1863–1873. doi: 10.1053/j.gastro.2009.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polk DB, Peek RM. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10:403–414. doi: 10.1038/nrc2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faguet GB. A brief history of cancer: age‐old milestones underlying our current knowledge database. Int J Cancer. 2015;136:2022–2036. doi: 10.1002/ijc.29134. [DOI] [PubMed] [Google Scholar]
- 7.Le Bars P, Matamoros S, Montassier E, Le Vacon F, Potel G, Soueidan A, Jordana F, de La Cochetière MF. The oral cavity microbiota: between health, oral disease, and cancers of the aerodigestive tract. Canadian journal of microbiology. 2017;63:475–492. doi: 10.1139/cjm-2016-0603. [DOI] [PubMed] [Google Scholar]
- 8.Biarc J, Nguyen IS, Pini A, Gosse F, Richert S, Thierse D, Van Dorsselaer A, Leize-Wagner E, Raul F, Klein JP, Schöller-Guinard M. Carcinogenic properties of proteins with pro-inflammatory activity from Streptococcus infantarius (formerly Sbovis) Carcinogenesis. 2004;25:1477–1484. doi: 10.1093/carcin/bgh091. [DOI] [PubMed] [Google Scholar]
- 9.Gold JS, Bayar S, Salem RR. Association of Streptococcus bovis bacteremia with colonic neoplasia and extracolonic malignancy. Arch Surg. 2004;139:760–765. doi: 10.1001/archsurg.139.7.760. [DOI] [PubMed] [Google Scholar]
- 10.Zarkin BA, Lillemoe KD, Cameron JL, Effron PN, Magnuson TH, Pitt HA. The triad of Streptococcus bovis bacteremia, colonic pathology, and liver disease. Ann Surg. 1990;211:786–791; discussion 791-782. doi: 10.1097/00000658-199006000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson LA, Wang SP, Nazar-Stewart V, Grayston JT, Vaughan TL. Association of Chlamydia pneumoniae immunoglobulin A seropositivity and risk of lung cancer. Cancer Epidem Biomar. 2000;9:1263–1266. [PubMed] [Google Scholar]
- 12.Zhan P, Suo LJ, Qian Q, Shen XK, Qiu LX, Yu LK, Song Y. Chlamydia pneumoniae infection and lung cancer risk: a meta-analysis. Eur J Cancer. 2011;47:742–747. doi: 10.1016/j.ejca.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Kocazeybek B. Chronic Chlamydophila pneumoniae infection in lung cancer, a risk factor: a case–control study. J Med Microbiol. 2003;52:721–726. doi: 10.1099/jmm.0.04845-0. [DOI] [PubMed] [Google Scholar]
- 14.Koyi H, Brandén E, Gnarpe J, Gnarpe H, Steen B. An association between chronic infection with Chlamydia pneumoniae and lung cancer A prospective 2‐year study. Apmis. 2001;109:572–580. doi: 10.1034/j.1600-0463.2001.d01-177.x. [DOI] [PubMed] [Google Scholar]
- 15.Chanudet E, Adam P, Nicholson AG, Wotherspoon AC, Ranaldi R, Goteri G, Pileri SA, Ye H, Müller-Hermelink HK, Du MQ. Chlamydiae and Mycoplasma infections in pulmonary MALT lymphoma. Brit J Cancer. 2007;97:949–951. doi: 10.1038/sj.bjc.6603981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mager DL. Bacteria and cancer: cause, coincidence or cure? A review. J Transl med. 2006;4:14. doi: 10.1186/1479-5876-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cummins J, Tangney M. Bacteria and tumours: causative agents or opportunistic inhabitants? Infect Agent Cancer. 2013;8 doi: 10.1186/1750-9378-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lax AJ, Thomas W. How bacteria could cause cancer: one step at a time. Trends Microbiol. 2002;10:293–299. doi: 10.1016/s0966-842x(02)02360-0. [DOI] [PubMed] [Google Scholar]
- 19.Karin M, Greten FR. NF-κB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 20.Travaglione S, Fabbri A, Fiorentini C. The Rho-activating CNF1 toxin from pathogenic E coli: A risk factor for human cancer development? Infect Agents Cancer. 2008;3:4. doi: 10.1186/1750-9378-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nath G, Gulati AK, Shukla VK. Role of bacteria in carcinogenesis, with special reference to carcinoma of the gallbladder. World J Gastroenterology. 2010;16:5395–5404. doi: 10.3748/wjg.v16.i43.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu BY, Zhang GM, Li XL, Chen H. Effect of glutaraldehyde fixation on bacterial cells observed by atomic force microscopy. Scanning. 2012;34:6–11. doi: 10.1002/sca.20269. [DOI] [PubMed] [Google Scholar]
- 23.Smelt JP, Brul S. Thermal inactivation of microorganisms. Cric Rev Food Sci Nutr. 2014;54:1371–1385. doi: 10.1080/10408398.2011.637645. [DOI] [PubMed] [Google Scholar]
- 24.Piyasena P, Mohareb E, McKellar RC. Inactivation of microbes using ultrasound: a review. Int J Food Microbiol. 2003;87:207–216. doi: 10.1016/s0168-1605(03)00075-8. [DOI] [PubMed] [Google Scholar]
- 25.Rose LJ, O'Connell H. UV light inactivation of bacterial biothreat agents. Appl Environ Microbiol. 2009;75:2987–2990. doi: 10.1128/AEM.02180-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomb RM, White TA, Coia JE, Anderson JG, MacGregor SJ, Maclean M. Review of the Comparative Susceptibility of Microbial Species to Photoinactivation Using 380‐480 nm Violet‐blue Light. Photochem Photobiol. 2018;94:445–458. doi: 10.1111/php.12883. [DOI] [PubMed] [Google Scholar]
- 27.Chao Y, Zhang T. Optimization of fixation methods for observation of bacterial cell morphology and surface ultrastructures by atomic force microscopy. Appl Microbiol Biotechnol. 2011;92:381–392. doi: 10.1007/s00253-011-3551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coleman WH, Chen D, Li YQ, Cowan AE, Setlow P. How moist heat kills spores of Bacillus subtilis. J Bacteriol. 2007;189:8458–8466. doi: 10.1128/JB.01242-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang P, Kong L, Setlow P, Li YQ. Characterization of wet-heat inactivation of single spores of Bacillus species by dual-trap Raman spectroscopy and elastic light scattering. Appl Environ Microbiol. 2010;76:1796–1805. doi: 10.1128/AEM.02851-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Setlow B, Setlow P. Heat killing of Bacillus subtilis spores in water is not due to oxidative damage. Appl Environ Microbiol. 1998;64:4109–4112. doi: 10.1128/aem.64.10.4109-4112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu Z-X, Zhang Z-L, Shi W-L, Pang D-W, Xie Z-X, Sheng P. Heat-fixation method used in an atomic force microscopy study of cell morphology. Anal Sci. 2008;24:257–260. doi: 10.2116/analsci.24.257. [DOI] [PubMed] [Google Scholar]
- 32.Glover T. Progress in Cancer Research. Canada Lancet and Practitioner. 1926:67. [Google Scholar]
- 33.Livingston W-C. Neoplastic Infections of Man and Animals. J Am Med Womens Asso. 1953;9:7–12. [Google Scholar]
- 34.Morrissey D, O'Sullivan GC, Tangney M. Tumour targeting with systemically administered bacteria. Curr Gene Ther. 2010;10:3–14. doi: 10.2174/156652310790945575. [DOI] [PubMed] [Google Scholar]
- 35.Toso JF, Gill VJ, Hwu P, Marincola FM, Restifo NP, Schwartzentruber DJ, herry RM, Topalian SL, Yang JC, Stock F, Freezer LJ, Morton KE, Seipp C, Haworth L, Mavroukakis S, White D, MacDonald S, Mao J, Sznol M, Rosenberg SA. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. Jpn J Clin Oncol. 2002;20:142–152. doi: 10.1200/JCO.2002.20.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Apostolou P, Tsantsaridou A, Papasotiriou I, Toloudi M, Chatziioannou M, Giamouzis G. Bacterial and fungal microflora in surgically removed lung cancer samples. J Cardiothorac Surg. 2011;6:137. doi: 10.1186/1749-8090-6-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cummins J, Tangney M. Bacteria and tumours: causative agents or opportunistic inhabitants? Infect Agents Cancer. 2013;8:11. doi: 10.1186/1750-9378-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei MQ, Ellem KA, Dunn P, West MJ, Bai CX, Vogelstein B. Facultative or obligate anaerobic bacteria have the potential for multimodality therapy of solid tumours. Eur J Cancer. 2007;43:490–496. doi: 10.1016/j.ejca.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Baban CK, Cronin M, O’Hanlon D, O’Sullivan GC, Tangney M. Bacteria as vectors for gene therapy of cancer. Bioeng Bugs. 2010;1:385–394. doi: 10.4161/bbug.1.6.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sznol M, Lin SL, Bermudes D, Zheng L-m, King I. Use of preferentially replicating bacteria for the treatment of cancer. J Clin Invest. 2000;105:1027. doi: 10.1172/JCI9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rogers MB. Mycoplasma and cancer: in search of the link. Oncotarget. 2011;2:271. doi: 10.18632/oncotarget.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nath G, Singh H, Shukla V. Chronic typhoid carriage and carcinoma of the gallbladder. Eur J Cancer. 1997;6:557–559. doi: 10.1097/00008469-199712000-00011. [DOI] [PubMed] [Google Scholar]
- 43.De Martel C, Plummer M, Parsonnet J, Van Doorn L, Franceschi S. Helicobacter species in cancers of the gallbladder and extrahepatic biliary tract. Brit J Cancer. 2009;100:194–199. doi: 10.1038/sj.bjc.6604780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 45.Hong SN, Lee SM, Kim JH, Lee TY, Kim JH, Choe WH, Lee SY, Cheon YK, Sung IK, Park HS, Shim CS. Helicobacter pylori infection increases the risk of colorectal adenomas: cross-sectional study and meta-analysis. Dig Dis Sci. 2012;57:2184–2194. doi: 10.1007/s10620-012-2245-x. [DOI] [PubMed] [Google Scholar]
- 46.Urbaniak C, Gloor GB, Brackstone M, Scott L, Tangney M, Reid G. The microbiota of breast tissue and its association with breast cancer. Appl Environ Microb. 2016;82:5039–5048. doi: 10.1128/AEM.01235-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Urbaniak C, Cummins J, Brackstone M, Macklaim JM, Gloor GB, Baban CK, Scott L, O'Hanlon DM, Burton JP, Francis KP, Tangney M, Reid G. Microbiota of human breast tissue. Appl Environ Microbiol. 2014;80:3007–3014. doi: 10.1128/AEM.00242-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cantwell AR, Kelso DW. Microbial findings in cancers of the breast and in their metastases to the skin. J Dermatol Surg Oncol. 1981;7:483–491. doi: 10.1111/j.1524-4725.1981.tb00682.x. [DOI] [PubMed] [Google Scholar]
- 49.Chandrasekaran E, Xue J, Neelamegham S, Matta KL. The pattern of glycosyl-and sulfotransferase activities in cancer cell lines: a predictor of individual cancer-associated distinct carbohydrate structures for the structural identification of signature glycans. Carbohyd Res. 2006;341:983–994. doi: 10.1016/j.carres.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 50.Wang CZ, Kazmierczak RA, Eisenstark A. Strains, mechanism, and perspective: Salmonella-Based Cancer Therapy. Int J Microbiol. 2016:2016. doi: 10.1155/2016/5678702. [DOI] [PMC free article] [PubMed] [Google Scholar]