Abstract
Background
Chronic rhinosinusitis (CRS) is a significant manifestation of cystic fibrosis (CF) with a wide range of symptom and disease severity. The goal of the study was to determine clinical variables that correlate with outcome measures of disease severity.
Methods
A prospective longitudinal, observational study of 33 adults with symptomatic CRS treated in a CF-focused otolaryngology clinic was performed. Symptom severity, the presence of rhinosinusitis exacerbations, and endoscopic appearance were assessed, and regression analysis was used to determine clinical predictors of disease outcome.
Results
33 adults with CF-CRS were included in the study and followed for a mean of 15 months. Rhinosinusitis exacerbations occurred in 61% of participants during the study, and female sex increased the odds of presenting with an exacerbation visit. Sinus disease exacerbations were associated with a 2.07 odds ratio of presenting with a pulmonary exacerbation at the next visit. CF-related diabetes was found to be associated with worse symptoms and endoscopic appearance. Infection with Staphylococcus aureus predicted worsening of symptoms, while those with Pseudomonas aeruginosa improved over time. Allergic rhinitis was associated with worse endoscopic appearance, and nasal steroid use was associated with improved endoscopic appearance.
Conclusion
Sex, CF-related diabetes, sinonasal infection status, allergic rhinitis and nasal steroid use may all modulate severity of CF-CRS in adults. Sinusitis exacerbation may be a precursor to pulmonary exacerbation.
Keywords: Cystic fibrosis, sinusitis, chronic rhinosinusitis, diabetes mellitus, Pseudomonas aeruginosa, exacerbation
Introduction
Sinonasal involvement in Cystic Fibrosis (CF) is characterized by chronic rhinosinusitis with or without nasal polyposis 1. Defective ion transport due to mutations in the Cystic Fibrosis Transmembrane Regulator (CFTR) leads to the accumulation of highly viscous secretions within the sinuses, mechanical occlusion of the sinus ostia, and eventually chronic bacterial infections (reviewed in 2). Development of the paranasal sinuses is also very sensitive to CFTR dysfunction. Sinus hypoplasia or aplasia is found in 67% of adults who have two mild CF causing alleles, and with two severe CF causing alleles the rate of abnormal sinus development on CT rises to over 95% 3–5. Other radiographic findings of upper airway disease include sinus opacification, mucosal edema, mucoceles, medial bulging of the maxillary walls, polyposis, and chronic osteitis 6. As would be expected from the radiographic evidence of chronic inflammation, patients with CF-CRS exhibit mucosal edema, crusting and purulent discharge 7,8. Both radiographically and on physical exam, CF is marked by evidence of severe, chronic rhinosinusitis in nearly all individuals. While objective findings of chronic rhinosinusitis (CRS) are nearly universal, only two thirds of patients report symptoms of CRS even when specifically questioned, and only one third of individuals report that the CRS symptoms are bothersome 7,9–12. Symptoms of CRS in CF include mucopurulent drainage, nasal obstruction, headache, orbital pain and decreased sense of smell 6. A subset of adult patients have severe and chronic symptoms despite adherence to medical treatments and repeated surgical interventions.
The aim of our study is to define patient characteristics predictive of more symptomatic CF sinus disease among a cohort of adults with CRS. It is unknown what, if any, biologic and clinical variables determine the severity of symptoms. Several studies correlating CFTR genotype with patient-reported symptoms have not found an association between more severe symptoms and more severe CFTR mutations 3,7,10,13. There is no correlation between severity of radiographic disease and patient reported symptoms in multiple studies including both children and adults with CF 7,11,13. Endoscopic exam also has shown limited correlation with patient symptomatology in CF 7. The drivers of highly symptomatic CRS are unknown in CF, thus prompting us to conduct a longitudinal study on adults followed in CF-focused rhinology and pulmonary clinics. We determined which clinical covariates predicted more severe patient reported symptoms using subjective and objective measures of disease 14,15.
Methods
Enrollment
A prospective observational study was conducted from January 2015 to October 2017 of adult patients with CF-CRS in a tertiary care, CF rhinology clinic at the University of Pittsburgh Medical Center Adult CF program. In addition to a diagnosis of CF, all patients fulfilled diagnostic criteria for CRS according to the Clinical Practice Guideline of the American Academy of Otolaryngology–Head and Neck Surgery16. Adult patients with symptomatic CF-CRS and prior endoscopic sinus surgery (ESS) were eligible for enrollment. Patients without prior ESS were excluded because few are referred to ENT specialty clinic at our center and the study required endoscopic sinus sample collection. Institutional review board approval at University of Pittsburgh was obtained for this study, and informed consent was obtained from all participants prior to enrollment.
Patient demographics and clinical characteristics
The following variables were obtained by retrospective chart extraction: age on enrollment, sex, spirometry results, lung transplant status, and CFTR genotype. Diagnoses of CF-Related Diabetes (CFRD) was determined if the participant met the diagnostic criteria for CFRD within the 24 months prior to study enrollment through the end of the study 17. Allergic bronchopulmonary aspergillosis, allergic rhinitis and asthma diagnoses were determined by retrospective review of pulmonary and otolaryngology notes and relevant lab testing. Medication use was obtained retrospectively from a combination of narrative note review (to ensure nonprescription nasal steroids use was recorded) and the prescription record in the electronic medical record. A period of 13 months prior to study enrollment was included for chart extraction of medication use to capture prescriptions that are ordered quarterly with refills. Medication usage is listed in Table 1. Pulmonary exacerbations were defined by at least 2 of the 3 criteria (a) a drop in FEV1%>10%, (b) institution of new systemic antibiotics by the pulmonary team or (c) direct documentation by the treating pulmonologist that the participant was judged to have a pulmonary exacerbation in the medical record. We defined pulmonary exacerbations as being present if these criteria were met within 4 weeks of a visit. Spirometry was done per the American Thoracic Society (ATS) standards and predicted FEV1 was calculated using Morris-Polgar. There was wide variability in timing of prior ESS, with many participants having their initial procedure prior to transitioning to adult clinic or at other institutions. Only one participant had an ESS revision during the study period but the remaining patients had sinuses which were healed and accessible for study purposes.
Table 1:
Enrollment Characteristics, results are in median (IQR) unless otherwise indicated, N = 33
| Male, n (%) | 13 (39.4) |
| Age, year | 27.7 (25.8–36.2) |
| FEV1 % predicted | 65.5 (52.5–78.5) |
| FVC % predicted | 75.5 (59.5–86.5) |
| BMI | 22.1 (19.6–26.3) |
| CF Related Diabetes, n (%) | 14 (42.4) |
| ABPA, n (%) | 5 (15.5) |
| Asthma, n (%) | 2 (6) |
| Allergic Rhinitis, n (%) | 17 (48.5) |
| Lung Transplant, n (%) | 3 (9.8) |
| SNOT-22 | 41.0 (18.5–58.5) |
| mLK | 8.5 (7–10) |
| Medication Usage | |
| CFTR Corrector/modulators | 10 (30.3) |
| Azithromycin | 19 (57.6) |
| Nebulized Hypertonic Saline | 29 (87.9) |
| Nebulized Dornase alpha | 28 (84.8) |
| Nasal Steroids | 16 (48.5) |
| Inhaled tobramycin | 18 (57.6) |
| Inhaled aztreonam | 14 (42.4) |
| Inhaled colistimethate | 11 (33.3) |
| Any topical sinus antibiotic | 27 (81.8) |
| Sinonasal mupirocin | 17 (51.5) |
| Sinonasal aminoglycosides | 14 (42.4) |
| Sinonasal ciprofloxacin | 11 (33.3) |
| Sinonasal vancomycin | 4 (12.2) |
Management Approach
Our approach to medical management in CF-CRS includes use of large volume nasal saline irrigations with the use of culture-directed topical antibiotics for uncontrolled symptoms. Patients are seen every 3 months or as needed for sinusitis exacerbations for evaluation and debridement. Debridement is performed by first anesthetizing and decongesting the patient’s nose with 2% tetracaine and oxymetazoline on pledgets. Crusts and debris are removed with suction and forceps. If there is additional debris and crusting that cannot be removed with the prior procedures then irrigation of the affected sinuses is performed under direct visualization. Cultures are obtained at every visit and the topical antibiotic regimen is determined based on the sensitivity information. Systemic antibiotics are provided if the topical regimen is not effective after two weeks or if the patient has significant pulmonary symptoms. The oral antibiotic and/or IV antibiotic regimen is jointly agreed upon after discussion with the pulmonary team and is usually reserved for concomitant pulmonary exacerbation.
Rhinologic Evaluation and Sinonasal Quality of Life
Visits were coded as routine or symptom-driven by the examining physician at the time of the clinically indicated visit. Exacerbation visits were usually unscheduled sick visits, though a small number of previously scheduled were coded as exacerbation visits if the participant reported an acute increase in symptom severity. All patients underwent sinonasal endoscopy. Endoscopic appearance was rated by S.E.L according to the modified Lund-Kennedy criteria (mLK)15. The degree of discharge, edema and polyposis was scored from 0–2, for a total possible score of 6 on each side. Endoscopically obtained mucus samples were submitted for microbiologic culture. Sinus cultures were taken from a single site at any visit. Sputum was collected by spontaneous expectoration during pulmonary clinic visits. At the time of this visit, sinonasal specific symptomatology and quality of life (QOL) was evaluated using the 22-item Sino-Nasal Outcome Test (SNOT-22)14. The SNOT-22 contains 22 questions each scored 0–5 (total score range, 0–110), with higher scores representing more severe QOL impact. The mLK and SNOT-22 scores were prospectively recorded in the REDCap database 18.
Statistical Analysis
Data were presented as median (interquartile range) or frequencies. For analysis of acute exacerbaton of CRS as well as follow-up data, only subjects with more than one visit were included. We grouped subjects by whether they had ever had a CRS exacerbation during the study period. We used logistic or penalized logistic regression models to test the univariate relationship between baseline variables and presence of exacerbation status (adjusted for total study duration in months). We also used mixed effects models with restricted maximum likelihood estimation to assess unadjusted effect of predictors of SNOT-22 and mLK. Separate models including interaction between baseline varaibles and time to measure the score change were developed. For analysis of mLK predictors, we used bootstrapping to estimate the p-value. To test the relationship between sinus and pulmonary exacerbations, a generalized estimating equation model was used adjusting for study duration and including a lag time for sinus exacerbation. All statistical analysis was conducted using STATA 14.2 (StataCorp, College Station, TX).
Results
Characterization of the Study Cohort
We enrolled 33 adults with CF and symptomatic chronic rhinosinusitis. At enrollment, the median age was 27.7 years, median FEV1 65.5% predicted and the median BMI 22.1 kg/m2. All individuals were actively prescribed pancreatic enzyme replacement and 42% met the diagnostic criteria for CF-related diabetes (CFRD). Additional description of our cohort is found in Table 1. Inhaled tobramycin was the most commonly used antibiotic (57.6%), followed by aztreonam (42.4%) and colistimethate (33.3%). At any time during the study, 81.8% of participants used a sinonasal rinse containing an antibiotic (listed in Table 1). Of participants with more than one visit, 54.6% had at least one hospitalization during the study. Most hospitalizations were for CF pulmonary exacerbations, although other reasons for hospitalization included abdominal pain, lung transplant surgery and indwelling port revision. Homozygosity for F508del was the most frequent CFTR genotype (42%), with the additional participant genotypes listed in Supplemental Table 1. Sinus infection at any time during the study by Pseudomonas aeruginosa (PA) was found in 55% of participants, Staphylococcus aureus (SA) was found in 67%, and MRSA, specifically, was found in 45%. (Supplementary Table 2). Of note, after PA and SA, coagulase negative Staphylococcus, Achromobacter sp, Stenotrophomonas maltophilia and Serratia marscecens was the next most frequently isolated organism from the sinuses (full results listed in Supplemental Table 3). The mean (median) duration of follow was 14.7 (11.9) months, and 23 (70%) subjects were followed for more than one visit. Of the patients who had more than one visit, the mean follow time was 21.2 months (IQR 17.9–28.7). Following description of the cohort, we determined which patient factors predicted more symptomatic disease.
Predictors of CRS Exacerbation
Exacerbations were defined at the time of visit by the presence of increased nasal congestion, facial pain or increased sino-nasal discharge, and usually presence of an unscheduled sick visit. Of subjects with more than a single visit, 60.9% had at least one CRS exacerbation during the study. As shown in Table 2, univariate analysis revealed that female sex, as compared to male sex, was associated with an OR of 8.4 of ever having a CRS exacerbation, while increasing age was also associated with increased odds of ever having an exacerbation. We did not find evidence of new pathogen acquisition at the time of exacerbation visits by traditional microbiologic culture.
Table 2:
Predictors of CRS Exacerbators, N = 23
| Patient Characteristic | OR (95% CI) | p-value |
|---|---|---|
| Age | 1.56 (0.99–2.43) | 0.051 |
| Female Sex* | 8.35 (1.36–51.42) | 0.02 |
| FEV1 % predicted | 1.06 (0.97–1.16) | 0.22 |
| BMI | 1.30 (0.72–2.34) | 0.37 |
| CFRD | 10.22 (0.44–235) | 0.15 |
| Allergic Rhinitis | 0.07 (0.00–1.84) | 0.11 |
| Nasal Steroids | 0.08 (0.00–2.25) | 0.14 |
| P. aeruginosa in Sinus* | 0.36 (0.07–1.86) | 0.23 |
| S. aureus in Sinus | 0.58 (0.01–41) | 0.80 |
Penalized logistic regression
Temporal Association Between Sinus and Pulmonary Exacerbations
It is clinically recognized the upper and lower respiratory tract symptoms in CF may temporally relate. Using a GEE model with lag time included, presentation for a sinus exacerbation was associated with a 2.07 Odds Ratio of having the next visit coded as a a pulmonary exacerbation (p=0.059). A trend was seen towards association between pulmonary and sinus exacerbations at any single visit (odds ratio = 1.55, p=0.23).
Predictors of More Symptomatic Sinus Disease
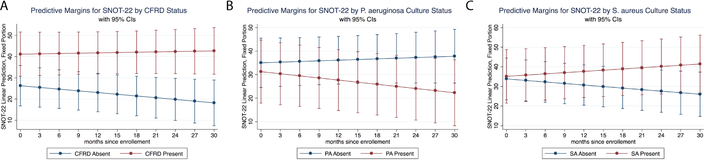
We next determined which clinical variables were associated with worse patient reported symptoms (i.e. a higher SNOT-22 score). We found that SNOT-22 scores were 4.9 points higher at exacerbation visits compared to routine visits. Over the duration of the study, higher baseline FEV1% predicted was associated with a small increase in SNOT-22, and there was trend towards higher BMI being associated with more symptomatic disease over time. We found larger effects for CFRD and sinus culture results on patient-reported symptoms. Across the panel, CFRD at each visit was associated with an average of 18.2 point higher SNOT-22 score (p=0.008). The fitted model predicting SNOT-22 changes over time by CFRD status is shown in Figure 1. Interestingly, divergent results were seen between individuals with PA vs SA infection. Those with PA had improvement overtime during the study, while those with SA worsened over time (fitted models shown in Fig1B and 1C).
Figure 1.
Fitted models for SNOT-22 change over time by (A) CFRD status, (B) presence of P aeruginosa in sinus cultures on enrollment visit, and (C) presence of Staphylococcus aureus in sinus cultures on enrollment visits. Corresponding statistics are shown in Table 3. CFRD = cystic fibrosis–related diabetes; SNOT-22 = 22-item Sino-Nasal Outcome Test.
Predictors of Worse Endoscopic Appearance
Finally, we determined which covariates were associated with worse endoscopic appearance. Exacerbation visits were associated with an average 1.3 higher mLK score. A prior diagnosis of CFRD was associated with 1.87 point increase in mLK. Allergic rhinitis and nasal steroid use had reciprocal effects, with an allergic rhinitis diagnosis being associated trend towards 1 point higher scores and nasal steroid use associated with 2.24 point lower score at each visit. At baseline, sinus culture results did not correlate with mLK. We next determined how these covariates interacted with time across the study. Over time, participants with older baseline age and allergic rhinitis tended to have higher mLK scores, while female participants had decreased mLK scores over time.
Discussion
We conducted a longitudinal study of adults with CF-CRS to determine predictors of more symptomatic disease. As CF treatments improve, more than half of individuals with CF in the US are over age 18. Furthering our understanding of CF manifestations that impair quality of life, such as CRS, will help improve patient outcomes. We describe one of the largest longitudinal cohorts to date in adult CF-CRS. The existence and behavior of CRS exacerbations is particularly difficult to describe in cross-sectional studies; thus, our study adds valuable insights to the natural history of CF exacerbation and patient characteristics that may affect outcome. Additionally, the cohort contains diversity of overall disease severity seen in contemporary adult CF clinics, with some adults having near normal spirometry, several participants were awaiting lung transplant and three participants post-lung transplant. Though this study benefits from a relatively robust and diverse patient population, the observational nature may limit the generalizability of the observed findings. Additionally, participants included were recruited from a single tertiary clinic, and only included patients that have previously undergone endoscopic sinus surgery. Seventy percent of particpants had more than one visit and are thus represented in the longitudinal analysis, and any differences between those with a single visit vs multiple visits cannot be accounted for. Our sample size limited our ability to study genotype effects because only 3 participants had at least one Class IV or V mutations or to conduct time-to-event analysis for exacerbations. Among our finding are that female sex as a risk factor for presentation with a CRS exacerbation, that CFRD is associated with both worse patient reported symptoms and worse endoscopic appearance, and that chronic infection status may portend differences in symptomatic progression over time.
In clinical practice, it has been recognized that sinus exacerbations tend to co-occur, or possibly precede, with pulmonary exacerbations. An early case series of reported an association between rhinosinusitis disease activity and drop in lung function, however this observation has not been confired in larger studies 19. Overall, symptomatic sinusitis may be protective against drop in FEV1 in adults over the age of 25 years; however, difficulty in ascertaining how sinusitis is coded in this registry study limits interpretation(20). In our longitudinal study, we saw a temporal association between sinus exacerabtions and future pulmonary exacerbation visits. The cause of CF-CRS exacerbations is unclear, although potential contributors would include new bacterial infections or shifts in the microbiome, allergic rhinitis flairs and viral upper respiratory tract infections that are slow to resolve. Many of these potential triggers could lead to both upper and lower respiratory tract disease.
We found that female sex increased the odds of presenting for an exacerbation by 8.4 compared to male sex. The definition of exacerbation used in the current study required most participants to present for an unscheduled visit, thus gender differences in medical care seeking behaviors may contribute to the effect size by this definition. However in a large, survey based study on non-CF CRS, female sex was also seen as a risk factor for exacerbation 19. We saw an increase in SNOT-22 of 4.86 points at exacerbation visits. Hopkins et al have done the most extensive validation of the SNOT-22 scale in a general rhinological surgery population in the U.K where they found the minimum clinically important difference (MCID) to be 8.9 points 14. However, this MCID was calculated for improvement, rather than worsening in symptoms, and the study was done in a non-CF population. That most exacerbation visits were unscheduled suggests that patients had burdensome enough worsening of symptoms to seek care, and also implies that the MCID for the SNOT-22 may need to be revalidated for the CF populations if the scale is to be used as an endpoint for intervention studies. The cause of CF-CRS exacerbations is unclear, although potential contributors would include new bacterial infections or shifts in the microbiome, allergic rhinitis flairs and viral upper respiratory tract infections that are slow to resolve. Understanding the triggers of CF-CRS symptom flairs is an important area for future work.
The sinuses may represent the initial site of respiratory infection with subsequent seeding of the lower airway, thereby contributing to infection. Genetically similar infections have been found between the sites, but widescale epidemiologic studies for this are limited 20,21. P. aeruginosa pulmonary isolates are highly pathoadapted and it is possible the sinuses provide the initial protective niche for pathoadaption to occur 22. Sinus and lung microbiology was concordant for both S. aureus and P. aeruginosa approximately >80% of the time in the current study, which is higher than that of previous studies in children 20,23–25. In the remaining 20%, it is unclear if the infections were intermittent, if this is due to the imperfect sensitivity of clinical testing, or if the samples collected are not representative of geographic entirety of infection. Further microbiome studies may illuminate this question. Notably, the rates of Pseudomonas aeruginosa (PA) and Staphylococcus aureus (SA), and PA/SA co-infection are approximately 2-fold higher than those reported in a recent pediatric cohort 25.
Bacterial infection affected progression of symptoms in our cohort. Over time, participants with PA symptomatically improved, while those with SA worsened. One interpretation of these findings is that our treatments for PA sinus infections (such as topical aminoglycosides and ciprofloxacin) are more effective than the more limited options for SA infections. Alternately, SA may be causing a more robust inflammatory response in the sinuses, perhaps through intra-epithelial infection nidi, or driving CRS disease progression through yet unidentified mechanism independent of treatment effects. That SA was associated with worsening of CRS symptoms is concerning given the rising prevalence of SA in the overall CF population. Further intervention studies or larger epidemiologic studies would be needed to clarify this.
CFRD was associated with a large increase in patient reported symptoms across our panel, as well as worse endoscopic findings on exam. Notably, other indicators of overall CF disease severity (BMI, age, sex, and lung function) did not consistently associate with worse sinus disease in this study. In fact, over time having a higher baseline FEV1 and higher BMI were both associated with worsening symptoms. Previously, CFRD has been identified to be a risk factor for pulmonary exacerbations, more rapid decline in lung function, the presence of symptomatic sinusitis and coinfection with PA/SA 26–28. We did not see an association between CFRD and PA/SA in the sinus cultures of our cohort as was seen in in previous studies of sputum, however this may be due to cohort size in the current study, or potentially to differences in pathophysiology by airway location 26. Why does CFRD lead to more symptomatic CRS? It has been hypothesized that elevated sputum glucose found in CFRD could be a carbon source that would support increased bacterial growth and lead to pulmonary exacerbations. In support of this hypothesis, measured sputum glucose concentrations are very low and often below detection suggesting glucose is being consumed in the sputum either by infecting microbes or the host inflammatory cells 29. The glucose concentration in sinus secretions in the context of CFRD has not been described, although similar pathophysiology could be in play. Alternately, CFRD could be altering the host inflammatory milieu, and inflammation could be driving symptomatology 30.
In the early report on nasal polypsis in CF, it was identified that a subset of patients have eosinophilic inflammation in their upper airways and treatment for allergic rhinitis has been in use in patients with CF since at least 1962 1. A full 48% of our cohort had a clinical diagnosis of allergic rhinitis, which is higher than that found in the general non-CF population. Allergic rhinitis was associated with worse endoscopic appearance and conversely the use of nasal steroids was associated with improved appearance over time. There was also a trend towards improved symptoms in the group on nasal steroids. Strong conclusions from these observations are limited by cohort size and the observational nature of the study. Additionally, there was heterogeneity in the type and route of steroids administration during the study. The heterogeneity could weaken our effect size, however determining which individuals with CF benefit from nasal steroid use and further elucidating the interplay between upper airway allergies and CF in the development of symptomatic sinus disease would be helpful for guiding clinical care.
In summary, we report a longitudinal cohort of adults with CF-CRS. Most importantly, we saw that presenting for a CRS exacerbation increased the odds of a pulmonary exacerbation at the next visit. Additionally, women have increased odds of presenting with CRS exacerbations, CFRD is predictive of worse symptoms and endoscopic disease, and S. aureus infection was associated with symptomatic worsening over time despite treatment. Identification of these risk factors gives insight into the underlying pathophysiology of CF sinus disease.
Supplementary Material
Supplementary Table 1: CFTR Genotype of Participants, N = 31*
Supplemental Table 2 – Sinus versus Sputum Microbiology, N = 33
Supplemental Table 3 – Clinical Microbiology Results, N= 33
Table 3:
Predictors of Symptom Severity (SNOT-22)
| Patient Characteristic | Panel coefficient (p-value) | Coefficient Interaction with Time*(p-value) |
|---|---|---|
| Age | 0.07 (0.94) | 0.02 (0.37) |
| Female Sex | 8.69 (0.29) | 0.06 (0.80) |
| FEV1 % predicted | −0.11 (0.39) | 0.01 (0.010) |
| BMI | −0.79 (0.29) | 0.057 (0.080) |
| CFRD | 18.22 (0.008) | 0.32 (0.11) |
| Allergic rhinitis | 10.15 (0.21) | −0.18 (0.39) |
| Nasal steroid | −9.02 (0.26) | −0.37 (0.067) |
| Azithromycin use | −1.01 (0.91) | 0.12 (0.54) |
| P. aeruginosa in Sinus | −1.19 (0.65) | −0.39 (0.053) |
| S. aureus in Sinus | −1.08 (0.65) | 0.47 (0.023) |
| CRS Exacerbation visit | 4.86 (0.017) | −0.11 (0.70) |
in months
Table 4:
Predictors of Higher mLK Score
| Patient Characteristic | Panel coefficient (p-value) | Coefficient Interaction with Time (p-value) |
|---|---|---|
| Age | −0.86 (0.059) | 0.006 (0.028) |
| Female Sex | 1.03 (0.055) | −0.15 (0.006) |
| FEV1 % predicted | −0.015 (0.46) | −0.002 (0.064) |
| BMI | −0.28 (0.07) | −0.012 (0.12) |
| CFRD | 1.87 (0.001) | 0.03 (0.49) |
| Allergic rhinitis | 1.01 (0.056) | 0.15 (0.001) |
| Nasal steroid | −2.24 (<0.001) | −0.001 (0.98) |
| Azithromycin use | 1.18 (0.082) | −0.094 (0.084) |
| P. aeruginosa in Sinus | −0.59 (0.282) | 0.07 (0.079) |
| S. aureus in Sinus | 0.63 (0.311) | 0.05 (0.27) |
| CRS Exacerbation Visit | 1.29 (0.007) | 0.03 (0.55) |
Acknowledgments
Funding
ACZ – Cystic Fibrosis Foundation Grant ZEMKE16Q0 and NIH K23HL131930
JMB – Gilead Investigator Sponsored Research Award, NIH NCATS UL1 TR0000005, R01HL123771, R61HL137077, Cystic Fibrosis Foundation Grant CFF BOMBER14G0
JMB and SEL – University of Pittsburgh Clinical and Translational Science Institute Pilot Program
JMP and MN – Cystic Fibrosis Foundation Grant PILEWS15 and NIH P30 DK072506
Footnotes
Conflict of Interest: No authors identified conflicts of interest pertaining to this manuscript.
Works Cited
- 1.Shwachman H, Kulczycki LL, Mueller HL, Flake CG. Nasal polyposis in patients with cystic fibrosis. Pediatrics. 1962;30:389–401. [PubMed] [Google Scholar]
- 2.Aanæs K Bacterial sinusitis can be a focus for initial lung colonisation and chronic lung infection in patients with cystic fibrosis. J Cyst Fibros. 2013;12 Suppl 2:S1–20. [DOI] [PubMed] [Google Scholar]
- 3.Ferril GR, Nick JA, Getz AE, et al. Comparison of radiographic and clinical characteristics of low-risk and high-risk cystic fibrosis genotypes. Int Forum Allergy Rhinol. 2014;4(11):915–920. [DOI] [PubMed] [Google Scholar]
- 4.Berkhout MC, Klerx-Melis F, Fokkens WJ, Nuijsink M, van Aalderen WM, Heijerman HG. CT-abnormalities, bacteriology and symptoms of sinonasal disease in children with Cystic Fibrosis. J Cyst Fibros. 2016;15(6):816–824. [DOI] [PubMed] [Google Scholar]
- 5.Woodworth BA, Ahn C, Flume PA, Schlosser RJ. The delta F508 mutation in cystic fibrosis and impact on sinus development. Am J Rhinol. 21(1):122–127. [DOI] [PubMed] [Google Scholar]
- 6.Gentile VG, Isaacson G. Patterns of sinusitis in cystic fibrosis. Laryngoscope. 1996;106(8):1005–1009. [DOI] [PubMed] [Google Scholar]
- 7.Casserly P, Harrison M, O’Connell O, O’Donovan N, Plant BJ, O’Sullivan P. Nasal endoscopy and paranasal sinus computerised tomography (CT) findings in an Irish cystic fibrosis adult patient group. Eur Arch Otorhinolaryngol. 2015;272(11):3353–3359. [DOI] [PubMed] [Google Scholar]
- 8.Bock JM, Schien M, Fischer C, et al. Importance to question sinonasal symptoms and to perform rhinoscopy and rhinomanometry in cystic fibrosis patients. Pediatr Pulmonol. 2017;52(2):167–174. doi: 10.1002/ppul.23613 [DOI] [PubMed] [Google Scholar]
- 9.Jorissen MB, De Boeck K, Cuppens H. Genotype-phenotype correlations for the paranasal sinuses in cystic fibrosis. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1412–1416. [DOI] [PubMed] [Google Scholar]
- 10.Berkhout MC, Van Rooden CJ, Rijntjes E, Fokkens WJ, El Bouazzaoui LH, Heijerman HGM. Sinonasal manifestations of cystic fibrosis: A correlation between genotype and phenotype? J Cyst Fibros. 2014;13(4):442–448. [DOI] [PubMed] [Google Scholar]
- 11.Rasmussen J, Aanæs K, Norling R, Nielsen KG, Johansen HK, von Buchwald C. CT of the paranasal sinuses is not a valid indicator for sinus surgery in CF patients. J Cyst Fibros. 2012;11(2):93–99. [DOI] [PubMed] [Google Scholar]
- 12.Chan DK, McNamara S, Park JS, Vajda J, Gibson RL, Parikh SR. Sinonasal Quality of Life in Children With Cystic Fibrosis. JAMA Otolaryngol Neck Surg. 2016;142(8):743. [DOI] [PubMed] [Google Scholar]
- 13.Wentzel JL, Virella-Lowell I, Schlosser RJ, Soler ZM. Quantitative sinonasal symptom assessment in an unselected pediatric population with cystic fibrosis. Am J Rhinol Allergy. 2015;29(5):357–361. [DOI] [PubMed] [Google Scholar]
- 14.Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol. 2009;34(5):447–454. [DOI] [PubMed] [Google Scholar]
- 15.Psaltis AJ, Li G, Vaezeafshar R, Cho K-S, Hwang PH. Modification of the Lund-Kennedy endoscopic scoring system improves its reliability and correlation with patient-reported outcome measures. Laryngoscope. 2014;124(10):2216–2223. [DOI] [PubMed] [Google Scholar]
- 16.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical Practice Guideline (Update): Adult Sinusitis. Otolaryngol Neck Surg. 2015;152(2_suppl):S1–S39. [DOI] [PubMed] [Google Scholar]
- 17.Moran A, Brunzell C, Cohen RC, et al. Clinical Care Guidelines for Cystic Fibrosis-Related Diabetes: A position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care. 2010;33(12):2697–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Umetsu DT, Moss RB, Lewiston NJ, King VV. Sinus disease in patients with severe cystic fibrosis: relation to pulmonary exacerbation. Lancet. 1990. [DOI] [PubMed] [Google Scholar]
- 20.Konstan MW, Wagener JS, VanDevanter DR, et al. Risk factors for rate of decline in FEV1in adults with cystic fibrosis. J Cyst Fibros. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuiper JR, Hirsch AG, Bandeen-Roche K, et al. Prevalence, severity, and risk factors for acute exacerbations of nasal and sinus symptoms by chronic rhinosinusitis status. Allergy Eur J Allergy Clin Immunol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muhlebach MS, Miller MB, Moore C, Wedd JP, Drake AF, Leigh MW. Are lower airway or throat cultures predictive of sinus bacteriology in cystic fibrosis? Pediatr Pulmonol. 2006;41(5):445–451. [DOI] [PubMed] [Google Scholar]
- 23.Johansen HK, Aanaes K, Pressler T, et al. Colonisation and infection of the paranasal sinuses in cystic fibrosis patients is accompanied by a reduced PMN response. J Cyst Fibros. 2012;11(6):525–531. [DOI] [PubMed] [Google Scholar]
- 24.Hansen SK, Rau MH, Johansen HK, et al. Evolution and diversification of Pseudomonas aeruginosa in the paranasal sinuses of cystic fibrosis children have implications for chronic lung infection. ISME J. 2012;6(1):31–45. doi: 10.1038/ismej.2011.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Godoy JM, Godoy AN, Ribalta G, Largo I. Bacterial pattern in chronic sinusitis and cystic fibrosis. Otolaryngol - Head Neck Surg. 2011;145(4):673–676. [DOI] [PubMed] [Google Scholar]
- 26.Dosanjh A, Lakhani S, Elashoff D, Chin C, Hsu V, Hilman B. A comparison of microbiologic flora of the sinuses and airway among cystic fibrosis patients with maxillary antrostomies. Pediatr Transplant. 2000;4(3):182–185 [DOI] [PubMed] [Google Scholar]
- 27.Sobin L, Kawai K, Irace AL, et al. Microbiology of the Upper and Lower Airways in Pediatric Cystic Fibrosis Patients. Otolaryngol Head Neck Surg. 2017;157(2):302–308. [DOI] [PubMed] [Google Scholar]
- 28.Limoli DH, Yang J, Khansaheb MK, et al. Staphylococcus aureus and Pseudomonas aeruginosa co-infection is associated with cystic fibrosis-related diabetes and poor clinical outcomes. Eur J Clin Microbiol Infect Dis. 2016. [DOI] [PubMed] [Google Scholar]
- 29.Marshall BC, Butler SM, Stoddard M, et al. Epidemiology of cystic fibrosis-related diabetes. J Pediatr. 2005. [DOI] [PubMed] [Google Scholar]
- 30.Koch C, Rainisio M, Madessani U, et al. Presence of cystic fibrosis-related diabetes mellitus is tightly linked to poor lung function in patients with cystic fibrosis: Data from the European epidemiologic registry of cystic fibrosis. Pediatr Pulmonol. 2001. [DOI] [PubMed] [Google Scholar]
- 31.Van Sambeek L, Cowley ES, Newman DK, Kato R. Sputum glucose and glycemic control in cystic fibrosis-related diabetes: a cross-sectional study. Kostikas K, ed. PLoS One. 2015;10(3):e0119938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mulrennan S, Baltic S, Aggarwal S, et al. The role of receptor for advanced glycation end products in airway inflammation in CF and CF related diabetes. Sci Rep. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: CFTR Genotype of Participants, N = 31*
Supplemental Table 2 – Sinus versus Sputum Microbiology, N = 33
Supplemental Table 3 – Clinical Microbiology Results, N= 33