Abstract
The thunder god vine (Tripterygium wilfordii Hook. F) is traditionally used for inflammation-related diseases in traditional Chinese medicine. In recent years, celastrol (a natural compound from the root of the thunder god vine) has attracted great interest for its potential anticancer activities. The free radical nitric oxide (NO) is known to play a critical role in colorectal cancer growth by promoting tumour angiogenesis. However, how celastrol influences the NO pathway and its mechanism against colorectal cancer is largely unknown. In this study, we investigated the effects and mechanism of celastrol on nitric oxide synthase (NOS) and the angiogenesis pathway in colorectal cancer. Our data show that celastrol inhibited HT-29 and HCT116 cell proliferation, migration, and NOS activity in the cytoplasm. The antiproliferation activity of celastrol was associated with the inhibition of iNOS and eNOS in colorectal cancer cells. Treatment with celastrol inhibited colorectal cancer cell growth and migration, and was associated with suppression of the expression of key genes (TYMP, CDH5, THBS2, LEP, MMP9, and TNF) and proteins (IL-1b, MMP-9, PDGF, Serpin E1, and TIMP-4) involved in the angiogenesis pathway. In addition, combinational use of celastrol with 5-fluorouracil, salinomycin, 1400 W, and L-NIO showed enhanced inhibition of colorectal cancer cell proliferation and migration. In sum, our study suggests that celastrol could suppress colorectal cancer cell growth and migration, likely through suppressing NOS activity and inhibiting the angiogenesis pathway.
Keywords: Angiogenesis, celastrol, colorectal cancer, nitric oxide synthase inhibitors
Introduction
Colorectal cancer (CRC) is a malignant tumour arising from the inner wall of the large intestine. In the USA, colorectal cancer is the third most common cancer diagnosed among men and women and the second leading cause of death from cancer. During 2018, it is estimated that 97,220 individuals will be diagnosed with colon cancer and 43,030 with rectal cancer and 50,630 will die because of this disease [1–3]. Chemotherapy is the most common systemic treatments which can extend and improve quality of life for patients who have had metastatic colorectal cancer. It can also reduce the risk of recurrence in patients with high-risk colon cancer findings at surgery. 5-Fluorouracil (5-FU) and salinomycin (Sal) are common chemotherapeutic agents used to treat with CRC or recurrence after CRC surgery [4–7]. However, these drugs usually lead to severe side effects, including hand-foot syndrome, neuropathy, and allergic or sensitivity reactions [8,9].
Aside from common chemotherapeutic agents, anti-vascular endothelial growth factor (VEGF) agents, such as bevacizumab, ziv-aflibercept, regorafenib, and ramucirumab, have all shown efficacy in the treatment of metastatic colorectal cancer (mCRC) and are currently approved by the United States Food and Drug Administration (FDA) for mCRC [10–12]. Since the introduction of antiangiogenic agents, there has been significant interest in understanding angiogenesis in CRC on a molecular basis and developing potent agents with the capability of inhibiting the angiogenesis pathway [13]. The cellular processes of angiogenesis include the following: hypoxia induces the production of nitric oxide (NO) and the expression of VEGF and angiopoietin-1 and −2 (Ang-1 and Ang-2), which interact with extracellular matrix (ECM) proteases to increase the permeability of the capillary vessel wall. Increased permeability leads to the leakage of plasma proteins and fibrinogen from capillary vessels, promoting the formation of transient ECM. The transient ECM can support the growth of new vessels and accelerate the proliferation and migration of CRC [14,15].
Nitric oxide synthases (NOSs) are a family of enzymes catalyzing the production of NO from L-arginine. NO is a well-known free radical that has been reported to be actively involved in the development of angiogenesis and cancer [16]. There is also a strong correlation between iNOS (inducible NOS) expression and tumour angiogenesis; one of the most important promoters of the NO-mediated angiogenesis pathway is VEGF [17]. The association between increased NOS activity and the presence of metastases raises the possibility that NO works as a promoter for colorectal tumour cells and suggests the inhibitors of NOS as potential anti-CRC drugs [18].
Celastrol (tripterine) is isolated from the thunder god vine (Tripterygium wilfordii Hook. F), which is a traditional Chinese medicinal material for rheumatoid arthritis [19]. Previous studies have investigated the effect of celastrol on lipopolysaccharide (LPS)-activated LP-1 human multiple myeloma cell-induced angiogenesis. Celastrol can down-regulate LPS-induced TLR4 expression and inhibit LPS-induced VEGF secretion in LP-1 cells [20]. Celastrol also targets the AKT/mTOR/P70S6K pathway, which leads to suppression of tumour growth and angiogenesis [21,22]. Recently, reports indicated that celastrol regulates HIF-1α at multiple levels that may together or individually contribute to its antitumour activity against hypoxia-induced angiogenesis and metastasis [23,24]. The effects of celastrol on endothelial cell tubulogenesis have also been tested by multiple studies. Pang and colleagues demonstrated that celastrol inhibits VEGF–induced chemotactic motility, capillary-structure formation, and cell viability of the human umbilical vein endothelial cells (HUVECs) [25]. In addition, Ke et al. showed that celastrol significantly diminishes the adhesion of HUVECs to fibronectin and inhibits HUVEC migration [26]. Celastrol has been found to protect the TGF-β1-induced endothelial-mesenchymal transition of HUVECs and inhibit the migration capacity of the transitioned endothelial cells [27]. Using an in vitro angiogenesis assay, Li et al. also observed that HUVEC migration and invasion were suppressed by celastrol-loaded nano micelles (CNMs), which also show inhibitory effects on activated macrophage-induced corneal neovascularization (CNV) in rats [28].
Although there are several studies reporting the anti-cancer and antiangiogenesis activities of celastrol, the effects and mechanism of celastrol on the angiogenesis and upstream NO-related signalling pathways in CRC is largely unknown. In this study, we have explored the effects of celastrol on CRC and its mechanism, and also investigated the effects of the combinational use of celastrol and other chemotherapeutic agents (5-FU, salinomycin, 1400 W, and L-NIO) in CRC.
Materials and methods
Cell culture, transfection and reagents
Two CRC cell lines, HT29, and HCT116 cells were obtained from ATCC. Cells were maintained in Minimum Essential Medium (MEM) (Cellgro) supplemented with 4 mM L-glutamine, 100 units/mL penicillin, 100 μg/mL streptomycin, 1% sodium pyruvate, 1% non-essential amino acids, and 10% fetal bovine serum (FBS) at 37 °C with 5% CO2. For shRNA transfection, HT29 and HCT116 cells were seeded (1 × 106/well) in 6-well plates a day before transfection and treated with iNOS (Santa Cruz) or eNOS (Santa Cruz) shRNA plasmids for 48 hours with Lipofectamine 2000 (Invitrogen, Paisley, UK) according to the manufacturer’s instructions. The iNOS or eNOS knockdown cell populations were selected with puromycin treatment for 3 days. 1400 W and L-NIO were purchased from Cayman Chemical (Ann Arbor, USA).
NOS assay
HCT116 cells with/without a stable knockdown of iNOS or eNOS, treated with celastrol or vehicle control, were analyzed for NO production with a NOS assay, using the Ultra-sensitive assay for the nitric oxide synthase kit (Oxford Biomedical Research, Oxford, MI, USA). Cell culture media were collected or cell lysates were extracted with cell lysis buffer (1% Triton X-100, 50 mM Tris-HCl pH 7.4, 5% glycerol, 100 mM NaCl) supplemented with protease inhibitor cocktail (Roche Applied Science, Indianapolis, USA) and were subjected to NOS assay. One hundred microliters of standards or samples were loaded onto a 96-well microplate in triplicate. After the colour reagents were added, the absorbance values were read at 540 nm in a microtiter plate reader (SpectraMax M5; Molecular Devices, Sunnyvale, CA, USA).
Cell viability assay
The MTS cell proliferation assay (Promega, Southampton, UK) was performed according to the manufacturer’s instructions. Briefly, cells were seeded at 8000 cells (in 100 μL medium) per well into 96-well plates, incubated overnight and exposed to treatments for the indicated time periods. Then 20 μL of CellTiter ® 96 Aqueous One Solution Reagent was added to each well. After 4-h incubation at 37 °C, the quantity of formazan product was measured by recording the absorbance at 490 nm with a 96-well plate reader (SpectraMax M5; Molecular Devices). Cell viability was calculated as a percentage of the control group (normalized to 100%).
Wound healing assay
A wound healing assay was used to assess cell migration of both the HT-29 and HCT-116 cancer cell lines upon treatment with celastrol, salinomycin, 1400 W, L-NIO or 5-FU and their combinations. HT-29 and HCT-116 cells were seeded at 1 × 106 cells per well (6-well plate). After the cells reached 90% confluence, the cells were wounded by scratching with a sterile pipette tip and subsequently washed with phosphate buffered saline (PBS) to eliminate the impaired cells. The medium was changed to medium with 1% fetal bovine serum. The cells were subjected to different treatments and observed for 24 h. The wound area was measured using Image-J software (NIH, Bethesda, MD, USA). The wound area percentage was calculated as the wound area from 24 h vs. the wound area from 0 h in each group.
Human angiogenesis PCR array
HT 29 cells were treated with drugs or vehicle control for 24 hours. RNA was then extracted from the cells and converted into complementary DNA using a cDNA synthesis kit (Invitrogen). The cDNAs were then subjected to an angiogenesis PCR array. The Human angiogenesis RT2 Profiler™ PCR Array (Qiagen, Valencia, CA, USA) profiles the expression of 84 key genes involved in modulating the biological processes of angiogenesis, which includes growth factors and their receptors, chemokines and cytokines, matrix and adhesion molecules, and proteases and their inhibitors, as well as transcription factors.
Proteome profiler human angiogenesis array
HT29 cells were treated with celastrol (CEL) or control for 24 hours. Proteins were then extracted from the cells using cell lysis buffer. Protein concentrations were analyzed with a BCA assay using a Pierce™ BCA Protein Assay Kit (Thermo Fisher). The proteins were then subjected to a proteome profiler angiogenesis array (R&D Systems, Minneapolis, USA) according to the manufacturer’s instructions. Briefly, samples were mixed with a cocktail of biotinylated detection antibodies and then incubated with the array membrane, which was spotted in duplicate with capture antibodies to specific target proteins. Captured proteins were visualized using chemiluminescent detection reagents. The image quantification was performed by Image-J software (NIH, Bethesda, MD, USA).
mRNA expression correlation analysis using TCGA database
The mRNA expression of the angiogenesis-related molecules in 636 patients with colorectal adenocarcinoma (colorectal adenocarcinoma, TCGA provisional) from the TCGA database was analyzed. The expression of TYMP was correlated with CDH5, THBS2, LEP, TNF, IL1B, PDGFB, SERPINE1, and TIMP4. Detailed information on the RNA-Seq experiments, protocols, and software used can be found at the TCGA Data Portal at https://tcga-data.nci.nih.gov/tcga/. All correlation analyses and Spearman’s correlation coefficients were generated using cbioportal for cancer genomics (http://www.cbioportal.org/).
Statistics
SPSS 23.0 statistical software was used for statistical analysis. The measurement data were expressed as mean ± standard deviation. Comparison between groups was done using analysis of variance; two pairs of comparison was done using Student’s t-test; when the variance was not uniform, comparisons were performed using a rank sum test. p < .05 for the difference was considered statistically significant.
Results
Celastrol suppresses colorectal cancer cell proliferation and migration
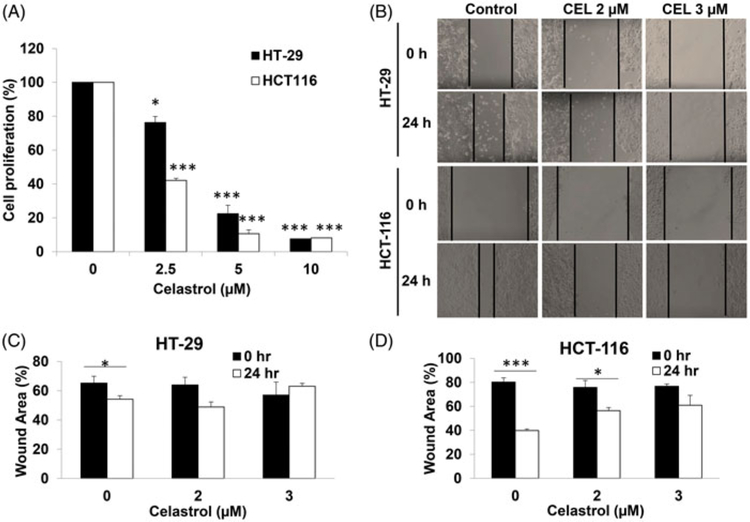
To investigate the effects of celastrol on CRC cells, a cell proliferation assay and a wound healing assay were performed on HT-29 and HCT116 cells after treatment with celastrol in different concentrations. Celastrol treatment resulted in a significant decrease in proliferation of HT-29 and HCT116 cells in a dose-dependent manner (p < .05) (Figure 1(A)).
Figure 1.
Celastrol suppresses colorectal cancer cell proliferation and migration. (A) HT-29 and HCT116 cells were treated with celastrol for 48 h at the indicated concentrations (0, 2.5, 5, 10 μM), respectively. A dosage-dependent inhibition of cell proliferation was observed by MTS assay. All data are shown as the mean ± SEM. *p < .05, ***p < .001 vs. Control group. (B) Images of the scratch on HT-29 and HCT116 cells (treated with 0, 2, 3 μM celastrol) were taken at time 0 and 24 h later. (C and D) Quantification of the wound healing assay for HT29 and HCT116 respectively. All data are shown as the mean ± SEM. *p < .05, ***p < .001 vs. the corresponding wound at 0 hours.
In the wound healing assay, after treatment of HT-29 and HCT-116 cells with celastrol over 24 h, the remaining wound areas were much bigger than those of control cells (Figure 1(B)). Quantification of the wound area suggests a statistically significant difference (p < .05) (Figure 1(C,D)), which indicates that celastrol suppresses the migration of CRC cells.
Celastrol inhibits NOS activity in CRC cells
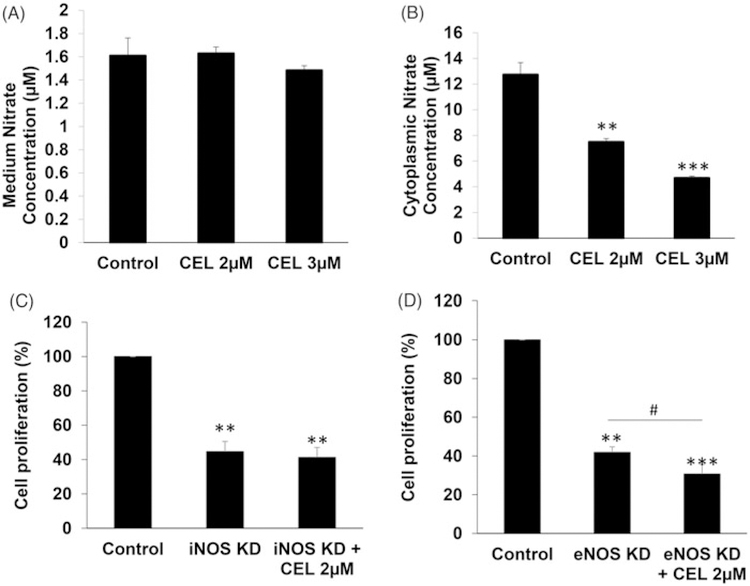
To determine whether celastrol could suppress NO synthesis, the NOS activity of HCT116 was tested by measuring the concentration of nitrite. The NOS assay results showed that there was no significant change in nitrite production in the HCT116 cell culture medium upon celastrol treatment (Figure 2(A)). However, the amount of nitrite production in HCT116 cell lysates was significantly decreased by celastrol treatment (p < .01) (Figure 2(B)).
Figure 2.
Celastrol inhibits NOS activity in CRC cells. (A) HCT116 cells were treated with celastrol (2, 3 μM) or vehicle control for 24 h. The NOS activity was tested by measuring nitrate concentration. No significant change of medium nitrate concentration after celastrol administration was observed. (B) HCT116 cells were treated with celastrol (2, 3 μM) or vehicle control for 24 h. Cell lysates were extracted with cell lysis buffer and were subjected to NOS assays. (C) HCT116 cells were treated with iNOS shRNA plasmids for 48 h, and the iNOS knockdown cell population was selected with puromycin treatment for 3 days. The cells with stable knockdown of iNOS were then treated with 2 μM celastrol or vehicle control for 24 h and cell proliferation analyzed by MTS assay. (D) The HCT116 cells with stable knockdown of eNOS were treated by adding 2 μM celastrol or vehicle control for 24 h and then the cell proliferation was analyzed by MTS assay. All data are shown as the mean ± SEM. *p < .05, **p < .01, ***p < .001 vs. control group; #p < .05 vs. eNOS knockdown group. CEL = celastrol. iNOS KD = shRNA-knockdown iNOS. eNOS KD = shRNA-knockdown eNOS.
The function of iNOS and eNOS in celastrol’s action on cell proliferation was further investigated by shRNA knockdown of iNOS (iNOS-KD) or eNOS (eNOS-KD) in HCT116 cells. iNOS-KD suppressed CRC cell proliferation by more than 50% (p < .01). In iNOS-KD cells, the celastrol treatment did not display further inhibition of cell proliferation (Figure 2(C)). The data suggest that celastrol may at least partially rely on iNOS to exert its inhibitory effects on HCT116 cell proliferation.
In addition, eNOS-KD also suppressed CRC cell proliferation by more than 50% (p < .01). In contrast, celastrol treatment enhanced the inhibitory effects of eNOS-KD on HCT116 cell proliferation (p < .05) (Figure 2(D)).
Celastrol systematically inhibits angiogenesis-related gene transcription in CRC
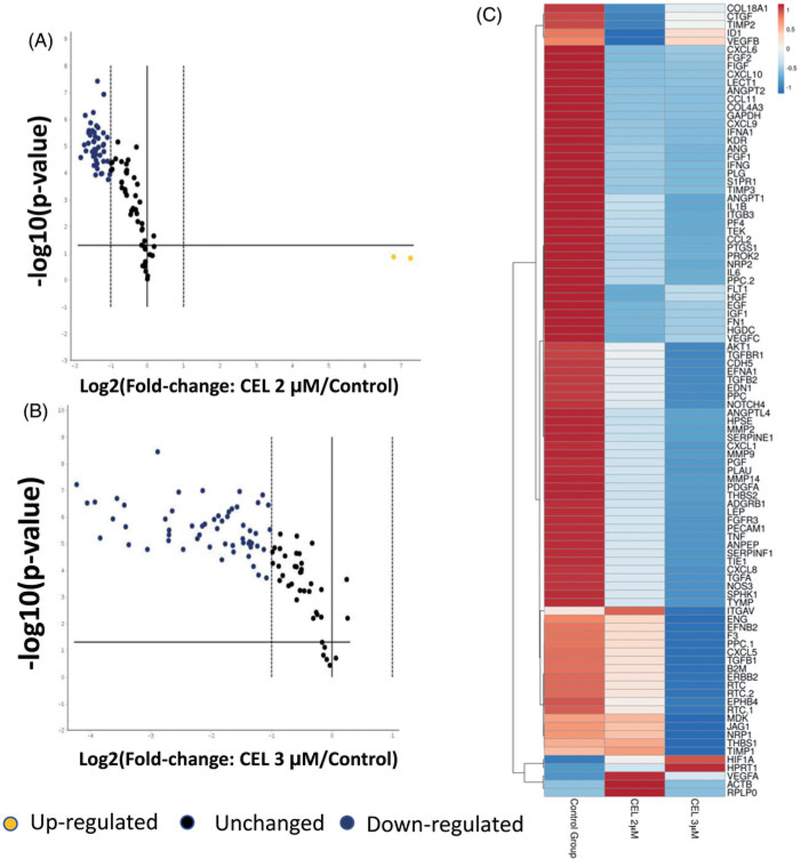
To analyze the effects of celastrol on angiogenesis, we used the PCR array to analyze the transcription of key genes in the angiogenesis pathway. Interestingly, among the 86 tested angiogenesis-related genes, a majority of them were down-regulated by celastrol treatment at the mRNA level (Figure 3(A)). Increasing the concentration of celastrol from 2 to 3 μM further shifted the gene transcriptions to lower levels (Figure 3(B)). This trend is also clearly presented for each gene by the heatmap and gene clustering in Figure 3(C).
Figure 3.
Celastrol systematically inhibits angiogenesis-related gene transcription in CRC. HT29 cells were treated with celastrol or vehicle control for 24 h. RNA was then extracted from cells and converted into complementary DNA using a cDNA synthesis kit. The cDNAs were then subjected to the angiogenesis PCR array. (A) A plot comparing the 2 μM celastrol-treated group with the control group. (B) A plot comparing the 3 μM celastrol-treated group with the control group. Among the 86 genes tested, most of the genes were down-regulated by celastrol treatment at the mRNA level. Yellow dots indicate up-regulated genes. Black dots indicate unchanged genes. Blue dots indicated down-regulated genes. (C) The heat map displays the gene expression pattern in the angiogenesis pathway of CRC cells treated with or without celastrol. CEL = celastrol.
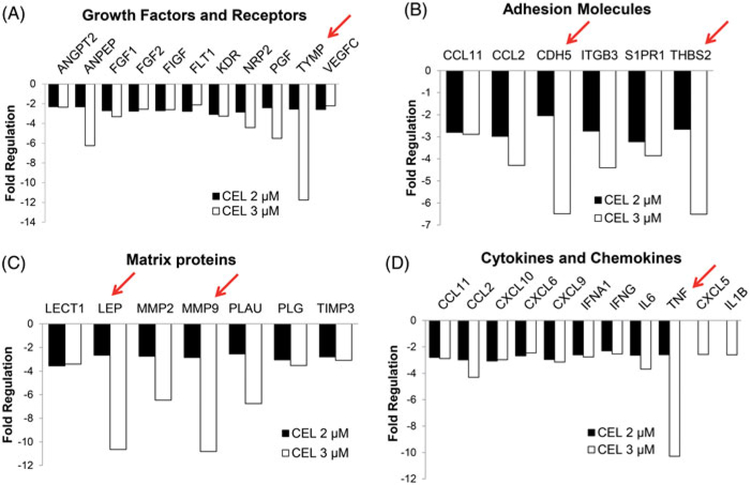
Our data show that celastrol inhibited the gene transcription of angiogenesis-related growth factors and receptors, with thymidine phosphorylase (TYMP) as the most affected gene, which was down-regulated by about 12-fold (Figure 4(A)). In addition, celastrol also inhibited the transcription of adhesion molecules, such as CDH5 and THBS2 (Figure 4(B)). The mRNA levels for matrix proteins encoding genes LEP and MMP9, which are known as important regulators of angiogenesis, were down-regulated by more than 10-folds (Figure 4(C)). Similarly, multiple cytokine- and chemokine-encoding genes, such as TNF, were down-regulated by celastrol treatment (Figure 4(D)). These results suggest that celastrol may regulate angiogenesis in CRC cells in a systematic way.
Figure 4.
Celastrol inhibits key genes involved in modulating angiogenesis. (A) Celastrol (2, 3 mM) inhibits angiogenesis-related growth factors and receptor gene transcription. The most affected gene was TYMP (down-regulated ~12 fold, marked with a red arrow). (B) Celastrol (2, 3 μM) inhibits adhesion molecules (CDH5 and THBS2 are the most affected genes, marked with red arrows). (C) Celastrol (2, 3 μM) inhibits matrix proteins, with LEP and MMP9 downregulated by more than 10-fold (marked with red arrows). (D) Celastrol (2, 3 μM) inhibits cytokines and chemokines, with the TNF gene transcription down-regulated more than 10-fold (marked with a red arrow). CEL = celastrol.
Celastrol suppresses human angiogenesis-related proteins in CRC
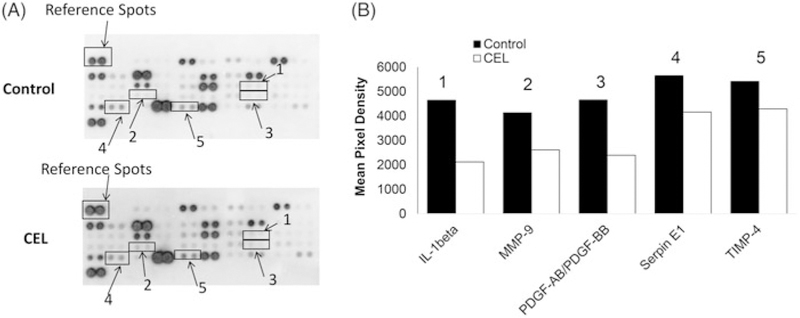
To further investigate the mechanism of celastrol on angiogenesis, the key proteins in the angiogenesis pathway have been analyzed by the proteome profiler angiogenesis array in HT29 cells. Results show that celastrol treatment significantly down-regulated the protein expression levels of various important molecules in angiogenesis pathways. The quantification proteome results indicated that IL-1β, MMP-9, PDGF, Serpin E1, and TIMP-4 were mostly down-regulated by celastrol treatment (Figure 5A and B).
Figure 5.
Celastrol suppresses human angiogenesis-related proteins in CRC. (A) The membranes of control and celastrol treated cells were analyzed by a proteome profiler human angiogenesis array. The boxes and arrows indicate the down-regulated proteins (1–5). (B) The image was analyzed by ImageJ. The pixel intensity of each spot was analyzed and normalized to the reference spots. The most affected proteins, shown in B, including IL-1β (1), MMP-9 (2), PDGF-AB/PDGF-BB (3), Serpin E1 (4), and TIMP-4 (5), were down-regulated by celastrol treatment. CEL = celastrol.
Celastrol improves the efficacy of chemotherapeutic drugs in CRC
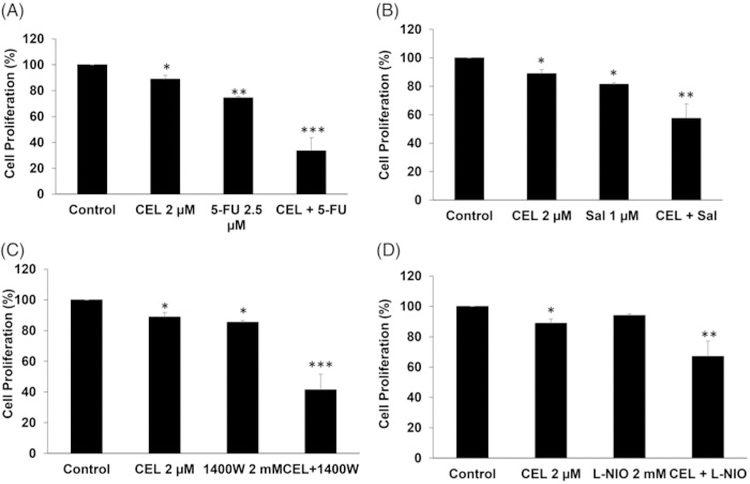
To investigate the effects of a combination of celastrol with other chemotherapeutic drugs, cell proliferation of HT29 cells was analyzed upon treatment with celastrol and 5-FU, celastrol and salinomycin, celastrol and 1400 W, and celastrol and L-NIO, respectively. Compared to celastrol (2 μM, ~10% inhibition (p < .05)) or 5-FU (2.5 μM, ~20% inhibition (p < .01)) alone, the combination of celastrol and 5-FU showed synergistic effects (~60% inhibition (p < .001)) (Figure 6(A)). The combination of celastrol and salinomycin, a cancer stem cell inhibitor, resulted in enhanced effects (~40% growth inhibition (p < .01)) compared to celastrol (~10% inhibition (p < .05)) and salinomycin (~20% (p < .05), single treatment, Figure 6(B)). 1400 W is a slow and highly selective inhibitor of inducible nitric oxide synthase. Our data show that the combination of celastrol and 1400W also resulted in enhanced effects (~60% growth inhibition (p < .001)) compared to celastrol alone (~10% inhibition (p < .05)) or 1400 W alone (~15% (p < .05), Figure 6(C)). L-NIO is another NOS inhibitor that inhibits the activity of eNOS and nNOS. Compared to celastrol (~10% inhibition (p < .05)) or L-NIO (~5% inhibition) alone, the combination of celastrol and 5 L-NIO showed slightly synergistic effects (~30% inhibition (p < .01)) (Figure 6(D)). These results suggest that celastrol could be considered for future combinational regimens with these drugs for CRC treatment.
Figure 6.
Celastrol improves the efficacy of chemotherapeutic drugs in CRC. (A) HT29 cells were treated with 2 μM celastrol and 2.5 μM 5-FU or the combination for 24 h, and cell proliferation was analyzed by MTS assay. (B) HT29 cells were treated with 2 μM celastrol and 1 μM salinomycin or the combination for 24 h, and cell proliferation was analyzed by MTS assay. (C) HT29 cells were treated with 2 μM celastrol and 2 mM 1400W or the combination for 24 h, and cell proliferation was analyzed by MTS assay. (D) HT29 cells were treated with 2 μM celastrol and 2 mM L-NIO or the combination for 24 h, and cell proliferation was analyzed by MTS assay. All data are shown as the mean ± SEM. *p<.05, **p<.01, ***p<.001 vs. control group. CEL = celastrol. Sal = Salinomycin.
Discussion
In the present study, we demonstrated that celastrol (a distinct compound from Tripterygium wilfordii Hook. F) suppressed proliferation, migration, NOS activity, and NO production in CRC cells. The results of the PCR array and proteome profiler angiogenesis array indicated that the anticancer effect of celastrol is likely associated with its inhibition of angiogenesis-related gene transcription and protein expression. In addition, celastrol also enhanced the efficacy of other chemotherapeutic drugs, including 5-FU, salinomycin, 1400 W, and L-NIO, in inhibiting CRC cell proliferation.
NOSs are a family of enzymes catalyzing the production of NO from L-arginine. NO is a retrograde neurotransmitter, which also helps modulate vascular tone, insulin secretion, airway tone, and peristalsis, and functions in angiogenesis and neural development [17]. NO is mediated in mammals by the calcium-calmodulin-controlled isoenzymes: eNOS (endothelial NOS) and nNOS (neuronal NOS). The iNOS is inducible isoform NOS, which binds calmodulin at physiological concentrations, and produces NO [18]. However, NO has been reported to exert variable effects in the multistage model of cancer. It could promote the progress of cancer via modulating different cancer-related events (such as angiogenesis, apoptosis, cell cycle, invasion, and metastasis) [16].
The possible role of iNOS in promoting VEGF-mediated angiogenesis in human tumours has been suggested in some previously published studies [15,29]. Marrogi and colleagues showed that iNOS expression is positively correlated with the expression of both MVD and VEGF in nonsmall-cell lung cancer [30]. Ambs and colleagues found that VEGF protein levels in adenomas (which have high iNOS expression) are higher than those in adjacent normal mucosa (low iNOS expression) [31–33]. These authors hypothesized that the excessive production of NO could stimulate VEGF production and angiogenesis, and even result in the vascularization of colon adenomas transitioning into adenocarcinoma [15,34]. Our study showed results consistent with previous findings that knockdown of either iNOS or eNOS impairs CRC cell proliferation. We have also, for the first time, showed a reduction in the cellular level of NO in celastrol-treated CRC cells (Figure 2). Intriguingly, in iNOS-KD cells, the celastrol treatment did not further inhibit cell proliferation, indicating that celastrol may at least partially rely on inhibiting iNOS in order to exert its inhibition on HCT116 cell proliferation. This topic warrants future investigation.
We also conducted PCR arrays and proteome profiler angiogenesis arrays to test whether celastrol treatment could affect the key molecules in the angiogenesis pathway at the gene and protein levels (Figures 3–5). Our results show that celastrol systematically inhibits angiogenesis-related gene transcription and protein levels, with the most affected genes including MMP9, TNFα, IL1β, etc. Interestingly, this effect is found to be dependent on the dosage of celastrol. The majority of the gene transcriptions were down-regulated more upon increasing the concentration of celastrol.
The systemic inhibition of angiogenesis-related genes/proteins and cytokines by celastrol was also observed in other types of cancers in previous studies. Jung and colleagues reported that the production of NO, TNFα, and IL1β in BV-2 microglial cells was largely inhibited in the presence of celastrol [35]. Similar effects were observed when celastrol incorporated into dendrimer nanocarriers was used to treat microglia [36]. The inhibition of MMP proteins by celastrol in CRCs was also observed in another recent report [21]. In our study, we observed a systemic down-regulation of angiogenesis-related molecules from celastrol treatment. Interestingly, correlations between these molecules in CRCs have been reported in some other studies. For example, the most down-regulated growth factor, TYMP (thymidine phosphorylase), identified in our PCR array was previously found to be correlated with other angiogenic factors (e.g. VEGF) and extracellular matrix modelling proteins in the prognosis of CRC by analyzing the tissue samples from 97 patients with CRC [37]. Thus, we also used RNA sequencing data from the TCGA database with 636 colorectal adenocarcinoma patient samples to analyze the correlation of the molecules, mostly down-regulated by celastrol treatment, identified by our PCR array (Figures 3 and 4) and the proteome profiler angiogenesis array (Figure 5). Interestingly, we found a significant positive correlation between most of these molecules (Supplemental Figure 1). These results suggest there are signalling interactions and transcription regulations between these molecules. The mechanism for these correlations and the related signalling would be a very interesting subject for future investigation.
One of the interesting features of celastrol is its widely observed chemosensitization activity. A number of plausible mechanisms have been proposed, although the exact mechanism is still obscure. Celastrol has been found to potentiate the apoptosis induced by TNF and chemotherapeutic agents (paclitaxel and doxorubicin) in a panel of different cancer cells [38]. It has also been shown to sensitize lung cancer cells to TRAIL-induced apoptosis via regulation of mitochondrial transmembrane potential and reactive oxygen species [39]. The enhancement of TRAIL-induced cancer cell apoptosis by celastrol was also found to be correlated with its effects on the up-regulation of death receptors 4 and 5 [40,41]. The sensitization of lung cancer cells to DNA crosslinking agents by celastrol was linked to its induction of proteasomal degradation of FANCD2 (FA Complementation Group D2) [42]. Celastrol has also been found to sensitize human prostate cancer to radiation therapy by inducing DNA damage [43]. By inhibiting HSP90, celastrol was shown to induce apoptosis in imatinib-resistant chronic myelogenous leukaemia cells harbouring the T315I mutation [44]. Celastrol was also shown to exert synergistic inhibitory effects with the BH3 mimetic drug ABT-737 by inducing ER stress [45]. In addition, celastrol synergistically enhances temozolomide cytotoxicity in melanoma cells, although the mechanism is unclear [46]. In our study, we have revealed the inhibitory effects of celastrol on the NOS activity and the angiogenesis pathway in colorectal cancer, which may also contribute to celastrol’s chemosensitization activity. Future investigations regarding the exact mechanism for the synergistic effect of celastrol with chemotherapeutic drugs would be very valuable.
Although VEGF inhibitors and other chemotherapeutic reagents are widely used in clinical treatment, drug resistance is still the major obstacle for CRC therapy. For example, insensitivity to 5-FU, a front-line chemo drug for colon cancer treatment, has been reported in many colon cancer studies [4,9]. Therefore, there is an urgent need to develop a combination treatment with 5-FU in order to improve 5-FU’s efficacy. Here we have made the first test of celastrol combined with other reagents and demonstrated that the combination of celastrol with NOS inhibitors (1400 W or L-NIO) and chemotherapeutic reagents (5-FU or salinomycin) resulted in enhanced antiproliferation effects in CRC cells compared to using these chemotherapeutic reagents alone. Our research outcomes may guide us to develop a novel combinational treatment strategy for colon cancer treatment.
Supplementary Material
Acknowledgments
Funding
This study was supported by the Science and Technology Project of Social Development of Shaanxi Province, China (No. 2012K13–01-05), the Science and Technology Project of Social Development of Shaanxi Province, China (No. 2016SF-139), the Clinical Research Award of the First Affiliated Hospital of Xi’an Jiaotong University, China (No. XJTU1AHCR2014–035) and National Cancer Institute grant R15CA195499 (S. Qian), USA.
Footnotes
Supplemental data for this article can be accessed here.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68(6):394–424.. [DOI] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- [3].Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67(3): 177–193. [DOI] [PubMed] [Google Scholar]
- [4].Chen EY, Blanke CD, Haller DG, et al. A Phase II study of celecoxib with irinotecan, 5-fluorouracil, and leucovorin in patients with previously untreated advanced or metastatic colorectal cancer. Am J Clin Oncol 2018; 41(12):1193–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gasparini G, Gattuso D, Morabito A, et al. Combined therapy with weekly irinotecan, infusional 5-fluorouracil and the selective COX-2 inhibitor rofecoxib is a safe and effective second-line treatment in metastatic colorectal cancer. Oncologist 2005;10(9):710–717. [DOI] [PubMed] [Google Scholar]
- [6].Maroun J, Marginean H, Jonker D, et al. A Phase I study of irinotecan, capecitabine (Xeloda), and oxaliplatin in patients with advanced colorectal cancer. Clin Colorectal Cancer 2018;17(2):e257–e268. [DOI] [PubMed] [Google Scholar]
- [7].Petrioli R, Francini E, Cherri S, et al. Capecitabine plus oxaliplatin and bevacizumab, followed by maintenance treatment with capecitabine and bevacizumab for patients aged > 75 years with metastatic colorectal cancer. Clin Colorectal Cancer 2018;17(4): e663–e669. [DOI] [PubMed] [Google Scholar]
- [8].Kusano M, Aoyama T, Okabayashi K, et al. A randomized phase III study of hepatic arterial infusion chemotherapy with 5-fluorouracil and subsequent systemic chemotherapy versus systemic chemotherapy alone for colorectal cancer patients with curatively resected liver metastases (Japanese Foundation for Multidisciplinary Treatment of Cancer 32). J Cancer Res Ther 2018;14(Supplement):S761–S766. [DOI] [PubMed] [Google Scholar]
- [9].Yuan L, Zhang S, Li H, et al. The influence of gut microbiota dysbiosis to the efficacy of 5-fluorouracil treatment on colorectal cancer. Biomed Pharmacother 2018;108:184–193. [DOI] [PubMed] [Google Scholar]
- [10].Noguerido A, Mulet-Margalef N, Matos I, et al. The safety of ramucirumab for the treatment of colorectal cancer. Expert Opin Drug Saf 2018;17(9):945–951. [DOI] [PubMed] [Google Scholar]
- [11].Ushida Y, Shinozaki E, Chin K, et al. Two cases of long-term survival of advanced colorectal cancer with synchronous lung metastases treated with mFOLFOX6/XELOX+bevacizumab. Case Rep Oncol 2018;11(2):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Van Cutsem E, Martinelli E, Cascinu S, et al. Regorafenib for patients with metastatic colorectal cancer who progressed after standard therapy: results of the large, single-arm, open-label Phase IIIb CONSIGN Study. Oncologist 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Griffioen AW. Therapeutic approaches of angiogenesis inhibition: are we tackling the problem at the right level? Trends Cardiovasc Med 2007;17(5):171–176. [DOI] [PubMed] [Google Scholar]
- [14].de Castro Junior G, Puglisi F, de Azambuja E, et al. Angiogenesis and cancer: a cross-talk between basic science and clinical trials (the ‘do ut des’ paradigm). Crit Rev Oncol Hematol 2006;59(1):40–50. [DOI] [PubMed] [Google Scholar]
- [15].Lin Z, Zhang Q, Luo W. Angiogenesis inhibitors as therapeutic agents in cancer: challenges and future directions. Eur J Pharmacol 2016;793:76–81. [DOI] [PubMed] [Google Scholar]
- [16].Choudhari SK, Chaudhary M, Bagde S, et al. Nitric oxide and cancer: a review. World J Surg Oncol 2013; 11:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rao CV. Nitric oxide signaling in colon cancer chemo-prevention. Mutat Res 2004;555(1–2):107–119. [DOI] [PubMed] [Google Scholar]
- [18].Fitzpatrick B, Mehibel M, Cowen RL, et al. iNOS as a therapeutic target for treatment of human tumors. Nitric Oxide 2008;19(2):217–224. [DOI] [PubMed] [Google Scholar]
- [19].Marcus DM. Comparison of Tripterygium wilfordii Hook F with methotrexate in the treatment of rheumatoid arthritis. Ann Rheum Dis 2014;73(9):e56. [DOI] [PubMed] [Google Scholar]
- [20].Xiong Y, Yan Y, Li Y. Tripterine alleviates LPS-induced inflammatory injury by up-regulation of miR-146a in HaCaT cells. Biomed Pharmacother 2018;105:798–804. [DOI] [PubMed] [Google Scholar]
- [21].Bufu T, Di X, Yilin Z, et al. Celastrol inhibits colorectal cancer cell proliferation and migration through suppression of MMP3 and MMP7 by the PI3K/AKT signaling pathway. Anticancer Drugs 2018;29(6):530–538. [DOI] [PubMed] [Google Scholar]
- [22].Mi C, Shi H, Ma J, et al. Celastrol induces the apoptosis of breast cancer cells and inhibits their invasion via downregulation of MMP-9. Oncol Rep 2014;32(6): 2527–2532. [DOI] [PubMed] [Google Scholar]
- [23].Huang L, Zhang Z, Zhang S, et al. Inhibitory action of celastrol on hypoxia-mediated angiogenesis and metastasis via the HIF-1α pathway. Int J Mol Med 2011;27(3):407–415. [DOI] [PubMed] [Google Scholar]
- [24].Ma J, Han LZ, Liang H, et al. Celastrol inhibits the HIF-1α pathway by inhibition of mTOR/p70s6K/eIF4E and ERK½ phosphorylation in human hepatoma cells. Oncol Rep 2014;32(1):235–242. [DOI] [PubMed] [Google Scholar]
- [25].Pang X, Yi Z, Zhang J, et al. Celastrol suppresses angiogenesis-mediated tumor growth through inhibition of AKT/mammalian target of rapamycin pathway. Cancer Res 2010;70(5):1951–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ke C, Jin H, Cai J. AFM studied the effect of celastrol on b1 integrin-mediated HUVEC adhesion and migration. Scanning 2013;35(5):316–326. [DOI] [PubMed] [Google Scholar]
- [27].Gong F, Zhao F, Gan XD. Celastrol protects TGF-β1-induced endothelial-mesenchymal transition. J Huazhong Univ Sci Technol Med Sci 2017;37(2): 185–190. [DOI] [PubMed] [Google Scholar]
- [28].Li Z, Li J, Zhu L, et al. Celastrol nanomicelles attenuate cytokine secretion in macrophages and inhibit macrophage-induced corneal neovascularization in rats. Int J Nanomater 2016;11:6135–6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ricciuti B, Foglietta J, Bianconi V, et al. Enzymes involved in tumor-driven angiogenesis: a valuable target for anticancer therapy. Semin Cancer Biol 2017. [DOI] [PubMed] [Google Scholar]
- [30].Marrogi AJ, Travis WD, Welsh JA, et al. Nitric oxide synthase, cyclooxygenase 2, and vascular endothelial growth factor in the angiogenesis of non-small cell lung carcinoma. Clin Cancer Res 2000;6(12): 4739–4744. [PubMed] [Google Scholar]
- [31].Ambs S, Bennett WP, Merriam WG, et al. Relationship between p53 mutations and inducible nitric oxide synthase expression in human colorectal cancer. J Natl Cancer Inst 1999;91(1):86–88. [DOI] [PubMed] [Google Scholar]
- [32].Ambs S, Merriam WG, Ogunfusika MO, et al. p53 and vascular endothelial growth factor regulate tumor growth of NOS2-expressing human carcinoma cells. Nat Med 1998;4(12):1371–1376. [DOI] [PubMed] [Google Scholar]
- [33].Ambs S, Bennett WP, Merriam WG, et al. Vascular endothelial growth factor and nitric oxide synthase expression in human lung cancer and the relation to p53. Br J Cancer 1998;78(2):233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Falcon BL, Chintharlapalli S, Uhlik MT, et al. Antagonist antibodies to vascular endothelial growth factor receptor 2 (VEGFR-2) as anti-angiogenic agents. Pharmacol Ther 2016;164:204–225. [DOI] [PubMed] [Google Scholar]
- [35].Jung HW, Chung YS, Kim YS, et al. Celastrol inhibits production of nitric oxide and proinflammatory cytokines through MAPK signal transduction and NF-κB in LPS-stimulated BV-2 microglial cells. Exp Mol Med 2007;39(6):715–721. [DOI] [PubMed] [Google Scholar]
- [36].Boridy S, Soliman GM, Maysinger D. Modulation of inflammatory signaling and cytokine release from microglia by celastrol incorporated into dendrimer nanocarriers. Nanomedicine 2012;7(8):1149–1165. [DOI] [PubMed] [Google Scholar]
- [37].Mitselou A, Ioachim E, Skoufi U, et al. Predictive role of thymidine phosphorylase expression in patients with colorectal cancer and its association with angiogenesis-related proteins and extracellular matrix components. In Vivo 2012;26(6):1057–1067. [PubMed] [Google Scholar]
- [38].Sethi G, Ahn KS, Pandey MK, et al. Celastrol, a novel triterpene, potentiates TNF-induced apoptosis and suppresses invasion of tumor cells by inhibiting NF-κB–regulated gene products and TAK1-mediated NF-κB activation. Blood 2007;109(7):2727–2735. [DOI] [PubMed] [Google Scholar]
- [39].Nazim UM, Yin H, Park S-Y. Autophagy flux inhibition mediated by celastrol sensitized lung cancer cells to TRAIL-induced apoptosis via regulation of mitochondrial transmembrane potential and reactive oxygen species. Mol Med Rep 2019;19:984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhu H, Liu XW, Ding WJ, et al. Up-regulation of death receptor 4 and 5 by celastrol enhances the anti-cancer activity of TRAIL/Apo-2L. Cancer Lett 2010;297(2): 155–164. [DOI] [PubMed] [Google Scholar]
- [41].Zhu H, Ding WJ, Wu R, et al. Synergistic anti-cancer activity by the combination of TRAIL/APO-2L and celastrol. Cancer Invest 2010;28(1):23–32. [DOI] [PubMed] [Google Scholar]
- [42].Wang GZ, Liu YQ, Cheng X, et al. Celastrol induces proteasomal degradation of FANCD 2 to sensitize lung cancer cells to DNA crosslinking agents. Cancer Sci 2015;106(7):902–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dai Y, DeSano JT, Meng Y, et al. Celastrol potentiates radiotherapy by impairment of DNA damage processing in human prostate cancer. Int J Radiat Oncol Biol Phys 2009;74(4):1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lu Z, Jin Y, Qiu L, et al. Celastrol, a novel HSP90 inhibitor, depletes Bcr–Abl and induces apoptosis in imatinib-resistant chronic myelogenous leukemia cells harboring T315I mutation. Cancer Lett 2010;290(2): 182–191. [DOI] [PubMed] [Google Scholar]
- [45].Zhu H, Yang W, He LJ, et al. Upregulating noxa by ER stress, celastrol exerts synergistic anti-cancer activity in combination with ABT-737 in human hepatocellular carcinoma cells. PLOS ONE 2012;7(12):e52333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chen M, Rose AE, Doudican N, et al. Celastrol synergistically enhances temozolomide cytotoxicity in melanoma cells. Mol Cancer Res 2009;7(12):1946–1953. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.