Summary
Vertebrate myelination is an evolutionary advancement essential for motor, sensory and higher order cognitive function. CNS myelin, a multilamellar differentiation of the oligodendrocyte plasma membrane, ensheaths axons to facilitate electrical conduction. Myelination is one of the most pivotal cell-cell interactions for normal brain development, involving extensive information exchange between differentiating oligodendrocytes and axons. The molecular mechanisms of myelination are discussed, along with new perspectives on oligodendrocyte plasticity and myelin remodeling of the developing and adult central nervous system.
Introduction
Neurologic and neurodegenerative diseases of the central nervous system (CNS) are often presented from a “neuron-centric” perspective. Pathological presentation of these diseases is primarily focused on the neuronal deficits and dysfunction that lead to glial cell reactivity and responses. The term glia refers to the historical concept that these cells are the CNS “glue”, but in the past few decades, emerging evidence has proven that glial cells are much more than the “support cells” of the CNS. Glial cells may well constitute 50–90% of the cells in the human and rodent CNS (Allen and Barres, 2009; Doetsch, 2003; Nishiyama et al., 2005; Noctor et al., 2007; Ullian et al., 2001), and understanding their involvement during development and in the adult brain is essential. A recent review by Freeman and Rowitch (2013) highlights the renewed interest in gliogenesis as an integral part of CNS development and function. Glial cells provide valuable support in axonal function, synaptic plasticity and as integral mediators of neuronal connectivity. In addition to development and aging, glial cells play crucial roles in repair and remyelination in CNS disease and disorders (Barres, 2008; Burda and Sofroniew, 2014; Gallo and Deneen, 2014; John Lin and Deneen, 2013; Nave, 2010; Schwartz et al., 2013).
This review focuses particularly on oligodendrocytes, the glial cells that generate CNS myelin, although extensive studies on peripheral nervous system (PNS) myelination by Schwann cells have provided important perspectives that will be noted. White matter deficits, both subtle and pronounced, are a common hallmark of human developmental disorders and neurodegenerative diseases (for review, see Fields, 2008). It has become increasingly clear that in order to identify the underlying mechanisms of CNS diseases, both glial cell physiology and neuronal-glial interactions must be considered.
Oligodendrocyte Development and Myelination
Developmental disorders of white matter demonstrate the importance of oligodendrocytes and developmental myelination for CNS function. In rodents, the oligodendrocyte developmental program begins with specification of oligodendrocyte progenitor cells (OPCs) derived from neural stem and progenitor cells during late embryonic gestation. The highly migratory and proliferative OPC is identified by its expression of the NG2 proteoglycan and the platelet-derived growth factor receptor alpha (PDGFRα). OPCs differentiate through a premyelinating stage to become the mature myelinating cell, which generates the myelin internode, and thereby interacts with axons to organize the nodal, paranodal and juxtaparanodal regions of myelinated axons.
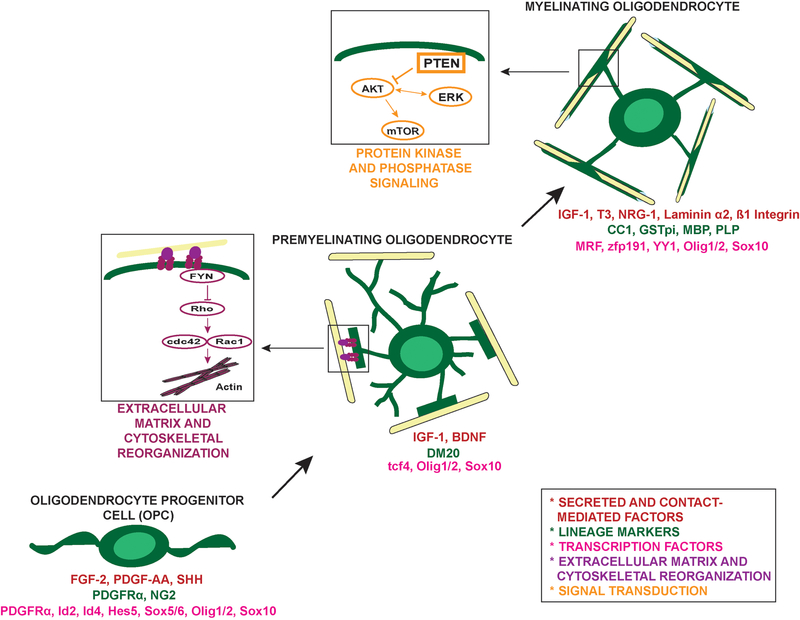
Progression through the oligodendrocyte lineage is tightly regulated by a multitude of intrinsic and extrinsic cues, which control myelination both spatially and temporally during development and after demyelination. These signals include growth factors, protein kinases, extracellular matrix molecules, which influence epigenetic modifications, transcriptional and translational regulation, and the actin cytoskeleton in oligodendrocytes (for review see Bauer et al., 2009; Emery, 2010; Kessaris et al., 2008; Miller, 2002; Mitew et al., 2013) (Figure 1). Differences in temporal expression of these factors and signals in the developing CNS result in early lineage progression and myelination in the spinal cord, and later myelination of cortical regions. Increasing evidence indicates that there are regionally diverse OPC populations that may be generated by distinct localized signaling mechanisms (Calver et al., 1998; Clarke et al., 2012; Miller et al., 1994; Richardson et al., 2006; Tsai et al., 2009; Warf et al., 1991). This is an active area of investigation, with much focus on the impact of different signaling molecules in different brain and spinal cord regions. For example, loss of mTOR and mTORC1 have differential impact on oligodendrocyte development in different CNS regions (Bercury et al., 2014; Wahl et al., 2014). Additionally, myelination in heterozygous neuregulin-1 type III mutant mice is reduced in brain while optic nerve and spinal cord myelination are normal (Taveggia et al., 2008). These differences may result from distinct developmental origins of OPC populations, unique neuronal populations to be myelinated or other local environmental signals.
Figure 1. Extrinsic and intrinsic cues regulate the oligodendrocyte program.
Progression through the oligodendrocyte lineage is mediated by numerous factors. As highlighted, signaling from the extracellular matrix (sub-figure adapted from Bauer et al., 2009) is critical in modulating the dynamic cytoskeletal reorganization of the premyelinating-myelinating oligodendrocyte transition. Additionally, signal transduction through the Akt/mTOR pathway, modified by PTEN, has been shown to be a regulator of myelin biogenesis. It should be noted that the ERK1/2 signaling pathway is also involved in regulating CNS myelination (Ishii et al., 2012), but this figure focuses on the Akt/mTOR pathway investigated in Snaidero et al. (2014).
Recent “omics” analyses of the molecular, cellular and biochemical properties of oligodendrocytes and myelin have uncovered many unknown markers of oligodendrocytes and myelin. The myelin proteome includes many more proteins than earlier studies suggested (Ishii et al., 2009; Jahn et al., 2009), and over 700 lipid moieties have now been identified in the myelin lipidome (Gopalakrishnan et al., 2013). The transcriptome of oligodendrocytes at different developmental stages has been established (Cahoy et al., 2008), and there is a searchable data base of RNAs and splice isoforms (http://web.stanford.edu/group/barres_lab/brain_rnaseq.html) for OPCs, newly formed oligodendrocytes and myelinating oligodendrocytes from P17 mouse cortex, along with comparable data for the variety of other glial and neuronal cells of the developing mouse brain (Zhang et al., 2014). Investigations into the regulation of myelination currently must consider how these RNAs, proteins and lipids are coordinately regulated to generate CNS myelin and how they are altered in animal models of CNS disease. Additionally, these databases will be a valuable resource to cross reference with deep sequencing studies of the human genome that are identifying new genetic variants and candidate genes that are disrupted in multiple developmental and degenerative diseases.
White Matter Deficits in Human Disorders and Disease
In addition to the well-known demyelinating and dysmyelinating diseases such as multiple sclerosis (MS), neuromyelitis optica and the leukodystrophies, myelin deficits resulting from altered glial structure/function and or glial/neuronal interactions are seen in human psychiatric disorders (for review, see Haroutunian et al., 2014; Nave and Ehrenreich, 2014) and developmental disorders including autism spectral disorder (ASD), sensory processing delay disorder (Owen et al., 2013), attention deficit hyperactivity disorder (ADHD) (Li et al., 2010; Wu et al., 2014) and Rett syndrome (Mahmood et al., 2010). Adult onset neurodegenerative diseases including Alzheimer’s, Parkinson’s and amyotrophic lateral sclerosis (ALS) (Cho, 2013; Defrancesco et al., 2014; Defrancesco et al., 2013; Kang et al., 2013; Kim et al., 2013; Lillo et al., 2012; Pettit et al., 2013; Philips et al., 2013) also show myelin pathology.
Genome wide association studies, fMRI imaging and molecular analyses have shown dysregulation in oligodendrocyte gene expression (Cannon et al., 2012; Haroutunian et al., 2007; Haroutunian et al., 2014; Kerns et al., 2010), reductions in white matter volume (Bakhtiari et al., 2012; Cooper et al., 2014; Frazier et al., 2012; Hong et al., 2011; Lewis et al., 2013; Prigge et al., 2013) and changes in myelin proteins (Honer et al., 1999) in these various CNS disorders and diseases. In most of these human conditions, there are significant neuronal pathologies accompanying these myelin changes that may well underlie the disease. In patients with the neurodegenerative disease multiple system atrophy (MSA), cytoplasmic inclusions that contain α-synuclein and other intracellular proteins are predominantly found in oligodendrocyte cell bodies rather than neurons (see Wong et al., 2014 for review). Thus, in neurodegenerative and psychiatric diseases involving neuron and/or oligodendrocyte pathologies, the impact of oligodendrocyte and myelin dysfunction is coming under increasing investigation. However, it has generally been unclear whether the disease and/or therapies induce changes in white matter and myelin or changes in white matter/myelin drive aspects of the disease. In order to understand the consequences of such dysfunction, an in depth understanding of myelination per se and of the function of myelin in the adult brain is necessary.
Current questions and approaches
The rapid propagation of electrical activity along an axon is physiologically essential to facilitate efficient and integrated sensory, motor and higher order cognitive function. The historical concept of myelin was as an axonal insulator that allowed faster conduction of axon potentials via saltatory conduction, which then enabled complex nervous systems to evolve within reasonable space constraints. Prevailing perspectives would suggest that its role is more multifaceted than that of a simple electrical insulator, and that it is the bidirectional communication between the myelinating cell and the axon that is essential for normal nervous system development and function. Bidirectional signaling mechanisms include direct cell-cell interactions, electrical activity, and trophic and metabolic support throughout development and adulthood.
Two crucial questions on the regulation of myelination have been addressed for decades without a definite conclusion. 1) What is the actual mechanism by which the oligodendrocyte extends its plasma membrane to wrap axons and eventually generate the multilayered compact myelin sheath around axons; and 2) How does the oligodendrocyte determine exactly how much myelin to generate for an axon of a specific diameter? Recent technological advancements in high resolution imaging, 3D electron microscopy and genetic approaches have led to new insights into the dynamics of myelination during development and provide valuable experimental evidence relevant to these questions.
Advances in Understanding Myelin Function
Elucidation of the Cellular Mechanism and Process of Active Myelination
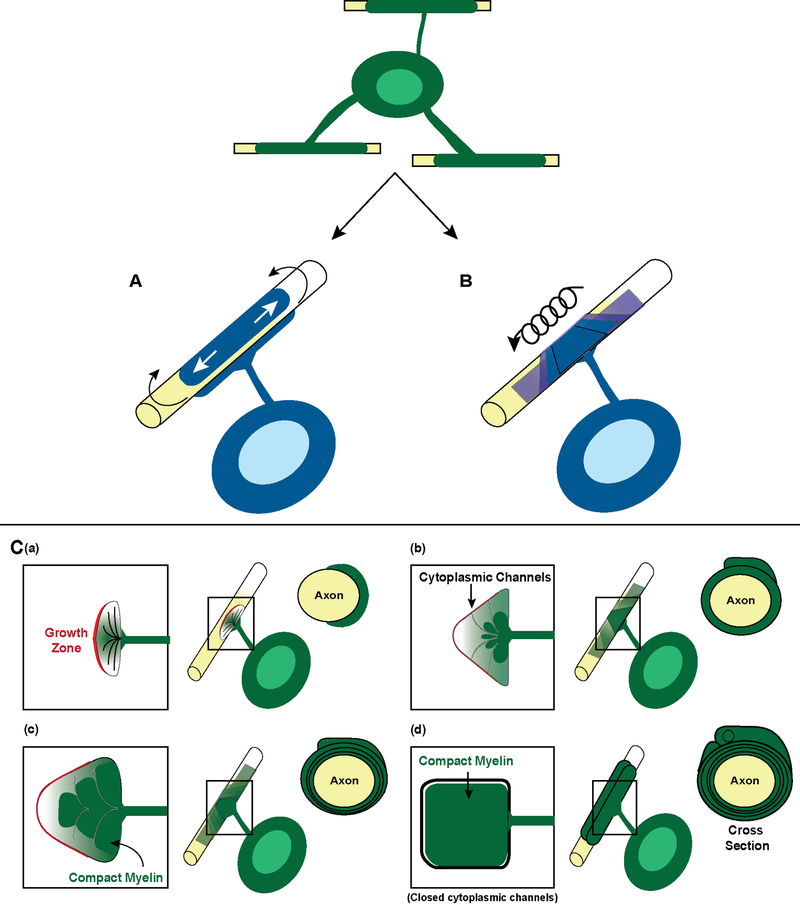
Numerous concepts of what myelin is, whether it is needed, and how it is generated have been proposed over several decades (reviewed by Rosenbluth, 1999). These working hypotheses were developed to address the fundamental question underlying the process of active myelination. Dating back to the 1950s, the advent of electron microscopy (EM) in the field of CNS and PNS myelination became a standard tool to evaluate myelin ultrastructure (Bunge et al., 1962; Bunge et al., 1967; Bunge et al., 1965). These EM analyses led to two myelination models that are particularly relevant to current studies (reviewed in Bauer et al., 2009). These include a proposed “jelly roll” structure with the Schwann cell of the PNS migrating around axons or sending an inner tongue repeatedly around the axon to generate the wraps (Geren and Schmitt, 1954) (Figure 2A). Additionally, membranous structures were seen within the oligodendrocyte in the CNS, and the EM images suggested that they eventually fused to generate myelin (De Robertis et al., 1958) The consensus from these early EM studies was that active myelination resulted from the inner turn of the oligodendrocyte membranous sheath extending in a concentric and lateral movement down the axon. In this paradigm, the polarized membranous sheath was spirally wrapping an axon as it concurrently compacted (Figure 2B). These models were of interest, but until recently, static imaging techniques were insufficient to generate conclusive experimental evidence. Live imaging of CNS myelination in EGFP-expressing mice generated the “liquid croissant” model which supports this myelination model (Sobottka et al., 2011). In these studies, oligodendrocyte processes could be imaged opening into a triangular shape in the myelin sheath, which moves in coiled turns around the axon, as the membrane spreads sideward on the axon.
Figure 2. Past and present models of myelination.
In the past, two models of myelination (blue) have been proposed to ensheath an axon. (A) Schematic of the proposed “jelly roll” model of myelination in which myelin concentrically wraps around the axon repeatedly overlapping the same internode. (B) Lateral spiral movements of an oligodendrocyte process around an axon with eventual compaction. (C) Current model of myelination adapted from (Snaidero et al., 2014). (a) The grown zone (red) of an individual oligodendrocyte process contacts the axon which it will ensheath. (b) The inner tongue of the oligodendrocyte process pushes under the outer tongue to generate the compact myelin (dark green). Cytoplasmic channels (white) allow communication between the inner and outer tongue. (c) More compact myelin is generated. (d) Cytoplasmic channels close once the appropriate number of myelin wraps per axon is generated and myelination is complete.
Elegant studies using a combination of high resolution in vivo imaging coupled with 3D reconstructions of optic nerve fixed with high pressure freezing/freeze substitution provide additional evidence (Snaidero et al., 2014). High pressure freezing facilitates microsecond fixation, prevents the nucleation of ice crystals to preserve macromolecular structures immobilized in their native state and permits vitrification of thicker samples. This enhanced methodology makes it more feasible to study the dynamic process of myelin biogenesis, even in static images. These studies show that myelination occurs through extension of the plasma membrane as an inner tongue, expanding laterally down an axon to form the paranodal loops, which is consistent with the original model proposed by Geren (Geren and Schmitt, 1954). Interestingly, cytoplasmic channels in the nascent membranes are present during development, with as much as 25% of the myelin sheath in optic nerve containing these channels during early myelination; there is rapid reduction of these channels from P10 to P14. In this same time frame, some myelin sheath outfoldings are seen, which also are resolved rapidly to generate compact myelin. The cytoplasmic channels provide communication from the outside to the inside of the developing sheath, and membranous vesicles are present within them, reminiscent of the membrane vesicles seen by deRobertis et al. (1957). These cytoplasmic channels appear to fuse into the developing membrane, as compact myelin and paranodal loops form. Figure 2C highlights elements of this current model of active myelination in the CNS. The availability of new EM approaches, such as the high pressure freezing has made it possible to image the details of myelin biogenesis in far greater depth, making a more comprehensive model of active myelination possible.
Snaidero et al. (2014) further examine the molecular mechanism underlying the myelinating process. Through manipulation of the Akt/mTOR pathways, known to increase myelination in the developing CNS (Flores et al., 2008; Goebbels et al., 2010; Narayanan et al., 2009), the substructure of the myelin was altered. When Akt/mTOR signaling was increased by genetically deleting its inhibitor PTEN, the cytoplasmic channels that would normally assemble into paranodes in control tissue were retained as channels, allowing increased production of myelin. Importantly, in adults, in which normal myelin had been generated, these cytoplasmic channels could be re-induced by deletion of PTEN, and over time the reappearance of these cytoplasmic channels was followed by increased thickness of the preexisting myelin sheath. Thus, this study proposes that myelination occurs through communication from cytoplasmic channels out to the expanding myelin membrane, and as myelination completes, these cytoplasmic channels resolve (see Figure 2d). Signaling through the Akt/mTOR pathway regulates the presence of these channels, and thereby myelination.
Visualization and Kinetics of Active Myelination
Current findings suggest that active myelination is a highly ordered and rapid process. Recently, rapid genetic manipulation to over- or under-express proteins in zebrafish along with important live imaging capability have significantly enhanced our understanding of the kinetics of myelination. In the zebrafish, there is a short five hour window during which individual oligodendrocytes produce their first and their final myelin sheaths (Czopka et al., 2013). This temporally regulated process of developmental myelination is constant among oligodendrocytes, irrespective of where in the zebrafish spinal cord they are located or when they begin myelinating. Thus, cells in different parts of the spinal cord myelinate at different times, but they all myelinate within this temporal limit of approximately 5 hours. If this short time frame for oligodendrocyte differentiation is consistent in mammalian systems, local cues that change rapidly during development could impact individual oligodendrocytes development. Single cells could be exposed to unique positive or negative cues impacting local myelination, potentially generating some of the differential regional myelination.
With the ability to visualize oligodendrocyte dynamics and active myelination, a crucial question remains: how does the oligodendrocyte determine how much myelin each axon receives? Across species, oligodendrocytes produce more wraps of myelin around larger caliber axons, and there is a relatively consistent g-ratio (the ratio of the axon diameter: myelinated fiber diameter) for axons of different diameters (Friede and Bischhausen, 1982; Hildebrand and Hahn, 1978). In the rodent PNS, axonal signaling by neuregulin III type I activates ErbB receptors on the surface of Schwann cells to regulate myelin sheath thickness (Michailov et al., 2004). In the CNS, no single factor or mechanism has been identified that regulates myelination, likely because of the complexity of myelination by oligodendrocytes, which have been estimated to generate as many as 40 internodes per oligodendrocyte in optic nerve (Peters A. and Vaughn, 1970). Recent studies in zebrafish demonstrate that oligodendrocytes are quite adaptable in generating appropriate amounts of myelin. The Mauthner axons in the zebrafish are two large diameter ventral projection axons that are myelinated by ventral oligodendrocytes. Dorsally, the spinal cord axons have far smaller diameters. Ectopic production of Mauthner axons in the dorsal spinal cord results in proper myelination of these large caliber axons by local oligodendrocytes that also appropriately myelinate the surrounding smaller caliber axons (Almeida et al., 2011). Thus, localized cues from axons can modify oligodendrocyte function, and individual oligodendrocytes respond to axons of different caliber with different amounts of myelin. These studies emphasize the synergistic relationships between the neuronal circuitry and oligodendrocytes that modify myelin plasticity in the CNS. However, while local axons clearly regulate oligodendrocyte differentiation, the actual signaling pathways regulating this in the CNS remain elusive.
Adult Myelination
Current studies have established that in the uninjured, healthy adult brain, new myelin is continually generated (Yeung et al., 2014; Young et al., 2013). This occurs in multiple CNS areas, and even the fully myelinated adult rodent optic nerve, which contains only about 1% unmyelinated axons (Dangata et al., 1996; Honjin et al., 1977), continues to generate new oligodendrocytes and new myelin in the adult. Interestingly, in the human brain, recent evidence using carbon dating has shown mature oligodendrocyte stability with only a 0.3% turnover rate after 5 years of age. Yet there persists dramatic remodeling of myelin in the adult human brain (Yeung et al., 2014). This is consistent with studies demonstrating that white matter volume can increase significantly in humans after a few weeks practicing a new skill (Bengtsson et al., 2005; Scholz et al., 2009) or can be altered upon cognitive processing such as learning a language (Schlegel et al., 2012). Thus, rapid changes in myelin occur in adults, either from genesis of new cells or new membrane production by existing cells, undoubtedly impacting the plasticity seen in the adult brain.
The relationship of myelin thickness, axonal diameter and internode length is important. Internode length would be expected to regulate axonal conduction velocity, and when Schwann cell internodes are relatively short, changes in their length impact conduction velocity (Court et al., 2004). However, in a PNS model of limb lengthening, changes in internode length occur in the adult, while the axon diameter and g-ratio remain relatively constant. Somewhat unexpectedly, axonal conduction velocity is unchanged, despite increased internodal length, but it is possible that long internodes have less impact on velocity than short internodes (Simpson et al., 2013).
While extensive data indicate that, after demyelination, remyelinated internodes are thinner and shorter than normal (Blakemore and Murray, 1981; Gledhill and McDonald, 1977), a recent study suggests that at late time points of recovery, as much as 6 months recovery, newly remyelinated fibers have comparable internode length and thickness to developmentally myelinated axons (Powers et al., 2013). This significant difference among studies may result from the extended period of remyelination used by Powers et al., (2013), from the different methodologies of analysis or from different models of demyelination/remyelination, but understanding the underlying mechanisms of myelination and remyelination in the adult brain is extremely important for our understanding of myelin repair in disease.
Discontinuous Myelination
As noted above, fundamental questions about the process of myelination itself are being answered and new studies have provided experimental evidence supporting claims made decades ago. In the past, the familiar concept that myelin internodes were uniformly dispersed down an axon became accepted in the field, but this hypothesis is now being challenged. Classically, it was thought that unmyelinated axon segments along myelinated axons resulted from damage or disruption of myelin. The general consensus was that after active myelination, the mature oligodendrocyte is in a myelin maintenance state, with subtle remodeling of localized areas of myelin, and, when necessary, remyelination by newly differentiating OPCs in demyelinated areas.
Standard transmission electron microscopy has provided high image resolution, but given the complex tissue organization of the brain, this technique could not visualize the full length of individual myelinated axons in CNS tissue without serial reconstructions of detailed electron micrographs in large tissue volumes. With high throughput automation of 3D electron microscopy and software programs that accelerate processing of high resolution reconstruction maps of these images, it is now possible to study the longitudinal distribution of myelin along individual axons in the rodent cortex. Projection neuron axonal segments have distinct myelination profiles in different cortical layers. There is a myelin gradient, with greater myelin in layers V and VI than in layers II/III. The axons of the deeper cortical layers are more uniformly myelinated, but unexpectedly, there are intermittent unmyelinated axonal segments in superficial layers of the cortex interspersed with myelinated internodes (Tomassy et al., 2014). This distinction of myelination patterns is independent of axonal caliber, i.e., neuronal soma size and axonal diameter are indistinguishable in the deep or superficial cortical layers. The availability of oligodendrocyte progenitors is also not a viable explanation, since the oligodendrocyte progenitor population is evenly dispersed throughout the brain (Tomassy et al., 2014). Furthermore, genetic manipulation of the laminar position of neurons within these cortical layers alters the distribution of mature oligodendrocytes and myelin. These studies suggest that unique features of different classes of cortical neurons regulate the differences in cortical myelination. Since myelin is critical in facilitating conduction velocity, do these unmyelinated segments have an evolutionary basis in regulating communication speed within different neuronal networks in the brain? These data would suggest that heterogeneous neuronal populations may have differential signaling patterns modulating localized oligodendrocyte myelination, and that the interaction of these neurons and oligodendrocytes may regulate some elements of plasticity in the adult brain. As noted above, new myelin is being generated in the healthy adult brain (Young et al., 2013), and adding new myelin internodes in areas of discontinuous myelination may be a mechanism for local plasticity. Altered or inadequate myelination in the adult could also be a component in some of the psychiatric or neurodegenerative disorders that involve white matter.
Synergism in Oligodendrocyte-Neuronal Interactions Regulate Axon Function and Myelination
Numerous studies have demonstrated an interdependent relationship of oligodendrocytes and the axons they myelinate (for review, see Nave, 2010; Nave and Trapp, 2008). Exciting new studies using cutting edge methodologies underscore the relevance of investigating neuronal-oligodendrocyte interactions.
Metabolic Support
While we have focused on axonal signals modulating oligodendrocyte plasticity, axons are similarly dependent on oligodendrocytes and myelin to provide support to maintain their integrity. Loss of myelin results in major axonal pathology. However, it has been known for many years that myelin can be generated in the absence of major myelin proteins such as PLP or CNP but this myelin is not normal, and myelin perturbation over time leads to axon degeneration (Edgar et al., 2009; Griffiths et al., 1998; Rasband et al., 2005). More recent work indicates that a major element of the oligodendrocyte support of axons may well be metabolic (Funfschilling et al., 2012; Lee et al., 2012; Morrison et al., 2013). Surrounded by myelin, the axon is relatively isolated from the extracellular milieu. There is ample evidence that oligodendrocytes provide essential trophic support to axons (Fruhbeis et al., 2013; Kramer-Albers et al., 2007; Lappe-Siefke et al., 2003; Rasband et al., 2005; Wilkins et al., 2003), and now studies suggest their ability also to provide metabolic support.
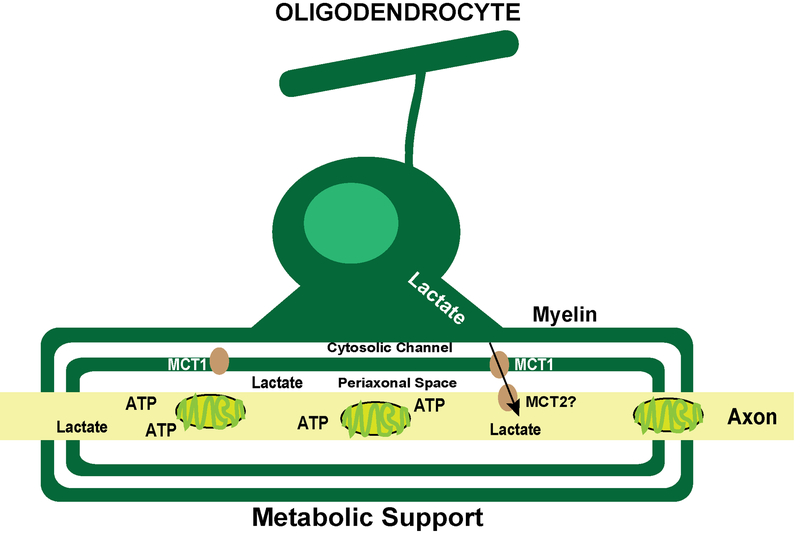
Mature oligodendrocytes that are incapable of electron transport use aerobic glycolysis, which generates lactate and pyruvate (Funfschilling et al., 2012). The lactate produced by these cells is rapidly utilized by the axons, except when neuronal function is reduced as under anesthesia, at which point lactate accumulates in the tissue. Aerobic glycolysis in oligodendrocytes is sufficient to maintain the myelin itself, as well as the structure and function of the myelinated axons. These studies generated a model for the metabolic support of myelinated axons, in which myelin delivers lactate to axons. Lactate is then metabolized in the axons, where access to other sources of energy is limited. This initial observation was supported by studies demonstrating that monocarboxylate transporter 1 (MCT1) is highly localized to oligodendrocytes and it was proposed that MCT1 transport of lactate from myelin to the underlying axons is a major source of metabolic support (Lee et al., 2012) (Figure 3).
Figure 3. Myelin metabolic support influences axonal integrity.
Model of oligodendrocyte-derived lactate delivered by the MCT1 transporter to the axon where it is metabolized and used as an energy source. Adapted from Funfschilling et al. (2012).
Extensive studies have established a major role for astrocytes in metabolic support of neurons (see Bouzier-Sore and Pellerin, 2013 for review), and it is likely that the metabolic support provided by oligodendrocytes and myelin is only part of the story (Amaral et al., 2013). Nevertheless, these studies on lactate transport from myelin to axons could substantially impact our understanding of the role of oligodendrocytes and myelin in CNS diseases. If axons are dependent on oligodendrocytes for lactate/pyruvate and there are myelin deficits, axonal degeneration, such as is seen in ALS and other neurodegenerative diseases, may occur. Recent studies suggest an active role for oligodendrocytes in ALS, which is primarily considered a neurodegenerative motor neuron disease (Kang et al., 2013; Yamanaka et al., 2008). Chimeric mice were generated in which the brains had cells that expressed mutant superoxide dismutase I (SOD1[G37R]), which induces an ALS-like disease, as well as cells that expressed wild type SOD1. The predominantly motor neuron degeneration was delayed dramatically by expression of wild type SOD1 in oligodendrocytes, despite high expression of mutant SOD1 in motor neurons (Yamanaka et al., 2008). In other studies, impaired oligodendrocyte function in gray matter oligodendrocytes in the SOD1 (G93A) mutant mouse enhances the vulnerability of motor neurons to ALS-linked genetic insults, speeding the progression of the disease (Kang et al., 2013). Enhanced glial reactivity, proliferation of NG2-positive oligodendrocyte progenitor cells and a dramatic increase in the number of oligodendrocytes occurs in this late stage SOD1 (G93A) rodent model (Kang et al., 2010). Other studies on the SOD1(G93A) mouse demonstrate dysmorphic oligodendrocytes with increased turnover of differentiating oligodendrocytes and reduced myelin basic protein (MBP), relative to wild type mice. As noted above, Lee et al. (2012) propose that MCT1 regulates lactate transport from oligodendrocytes to axons, and a significant loss of MCT1 was seen in spinal cord gray matter in these mice (Philips et al., 2013). Importantly, changes in the metabolic support of axons provided by oligodendrocytes appear likely in ALS patients as well, since (Lee et al., 2012) noted that MCT1 is downregulated by 50% in motor cortex of a cohort of ALS patients. These studies demonstrate the metabolic support by oligodendrocytes is essential for axons in animal models and suggest that the loss of oligodendrocyte metabolic support of axons may result in neurodegeneration in humans.
Neuronal Activity and Adaptive Myelination
Adaptive myelination is an evolving concept that has been investigated in the field for over a decade. This concept implies that neuronal electrical excitability modifies myelin plasticity and that myelin plasticity in turn feeds back to modulate neural activity and behavior. First demonstrated in in vitro cultures of PNS Schwann cells and dorsal root ganglion neurons, different frequencies of neural-induced firing influenced in vitro myelination through differential expression of a cell adhesion molecule L1 (Stevens et al., 1998). Studies of oligodendrocyte lineage cell/dorsal root ganglion neuron co-cultures suggested that electrical activity stimulated the localized release of neurotransmitters (ATP and vesicular glutamate) at the axo-glial synapse to influence oligodendrocyte calcium levels and modify signaling cascades that feed into local MBP protein translation (Wake et al., 2011). Recent studies demonstrate that some of the glutamate response of oligodendrocytes in myelinating cultures can be altered by signaling molecules, such as neuregulins. In studies on oligodendrocyte/dorsal root ganglion neuron co-cultures, myelination occurs independent of neuronal activity (Lundgaard et al., 2013). However, after exposure to neuregulin, myelination regulation changes, and it becomes partially dependent on neuronal excitability. Blocking NMDA channels, which initially has no impact on myelination, now reduces myelination by over 80% (Lundgaard et al., 2013).
These in vitro studies set the groundwork to investigate in vivo models in which modification of neuronal function induced changes in oligodendrocyte lineage progression and myelination. In vivo studies establish that myelination can be reduced or increased by neuronal function. Some studies suggest a critical period in which neuronal function impacts myelination, either during early or late development. Neural activity either in the medial prefrontal cortex or the barrel cortex of the somatosensory cortex impacts myelination (Barrera et al., 2013; Makinodan et al., 2012). Social isolation for as little as 2 weeks in the early post-weaning period has a dramatic effect reducing myelination in the prefrontal cortex, although motor activity is unaffected. Oligodendrocyte morphology is far simpler in these mice and myelin thickness is reduced. Reintroduction of mice to a social environment at the end of the two weeks does not improve myelination. In these studies, 30 days social isolation in the adult has little impact on myelin content (Makinodan et al., 2012). Sensory deprivation was induced by whisker trimming starting at P1 and myelination in the barrel cortex was reduced, with dramatic reduction in the density of myelinated axons. Here, however, the critical window was much later in development, consistent with the fact that active myelination of this cortical region occurs later, increasing significantly between P45 and P60 in normal animals. Nevertheless, as with social isolation, sensory deprivation in adult mice had little impact reducing myelinated axons (Barrera et al., 2013). Interestingly, in both cases, it is myelination per se that is reduced, since the number of oligodendrocytes themselves are normal in these tissues.
These investigations into the impact of neuronal function on myelination suggested the existence of a critical window of sensitivity, but other studies using social isolation in the adult indicate adults are also susceptible. Long term social isolation (8 weeks) results in significantly thinner myelin in prefrontal cortex, but not corpus callosum (Liu et al., 2012), as has also been seen in other studies on rodents maintained in isolation (Markham et al., 2009). Consistent with studies in younger animals, this again appears to be primarily an effect on myelination, since the number of oligodendrocytes in prefrontal cortex in these socially isolated mice is normal (Liu et al., 2012). As noted above, the impact of neuronal activity is also seen in humans, where extensive long term training, such as piano practice (Bengtsson et al., 2005) or learning to juggle (Scholz et al., 2009) correlates with increased white matter, in a region-specific manner depending on age (Bengtsson et al., 2005). Interestingly, white matter regions that mature in adulthood, e.g., long association fiber systems of the forebrain, are most impacted in adults by piano practicing.
The impact of neuronal activity on myelination in adult brain may result from the ubiquitous presence of OPCs throughout the adult mouse brain (Dimou et al., 2008; Kang et al., 2010; Rivers et al., 2008; Young et al., 2013). In rodent brain, adult NG2-positive OPCs are constantly migrating through the local environment, retracting and extending their branched processes (Hughes et al., 2013). Adult mouse OPCs define limited territories within which individual cells move, avoiding other adult OPCs, just as is seen for zebrafish OPCs during development (Czopka et al., 2013; Czopka and Lyons, 2011; Kirby et al., 2006; Takada and Appel, 2010), but they retain the ability to migrate to areas of damage and to effectively generate new myelin (Hughes et al., 2013).
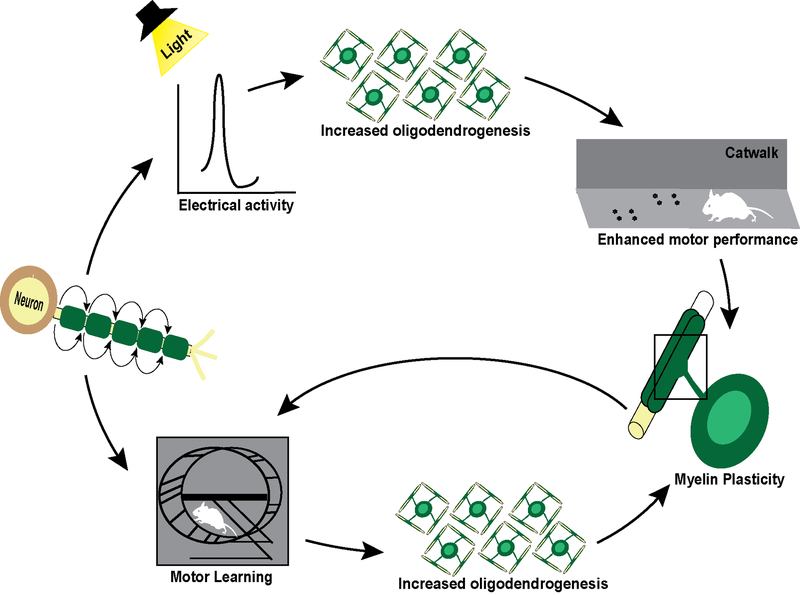
The Richardson laboratory has shown that over 30% of mature oligodendrocytes are newly formed in the adult rodent brain (Rivers et al., 2008), which would be a remarkable source of myelin plasticity throughout life. This group investigated the hypothesis that production of new myelin from these newly formed oligodendrocytes, i.e., myelin plasticity, could enhance learning in motor circuits of the brain. Myelin regulatory factor, a transcription factor essential for oligodendrocyte maturation (Emery et al., 2009), was conditionally ablated in adult mice. This blocked new myelin production, but did not impact the existing oligodendrocyte population or result in demyelination (McKenzie et al., 2014). However, the inability to generate new myelin prevented these mice from learning new motor skills on a complex running wheel (McKenzie et al., 2014) (Figure 4). This is a key observation, since this completes a feed back/feed forward mechanism: new motor skills normally increase myelin in the motor cortex but the inability of generate new myelin prevents mice from learning the new motor skills.
Figure 4. Neural activity influences oligodendrocyte plasticity.
Schematic of the neural induced modification of oligodendrogenesis and the feedback loop from myelin plasticity enhancing neural-mediated behavior. In rodents, optogenetic induced electrical activity results in enhanced oligodendrogenesis resulting in enhanced swing speed on a Catwalk apparatus. Additionally, motor learning on a complex wheel requires new oligodendrogenesis. Mouse illustration provided and used with permission from Dr. Michelle Monje.
Optogenetic studies have provided a more direct in vivo demonstration of the impact of neuronal electrical excitation in the premotor cortex on oligodendrocyte lineage cells and myelination (Gibson et al., 2014) (Figure 4). Growing in popularity, novel optogenetic techniques were developed that utilize light-reactive bacterial opsins to change the membrane voltage of cells, resulting in their excitability (Arenkiel et al., 2007; Boyden et al., 2005). The advantage of this technique is the precise and rapid excitation of distinct subpopulations of cells in a non-invasive manner in awake animals with little disruption to the native state of the tissue. In animals expressing the neuron-specific Thy1 promoter driving channelrhodopsin, light induces electrical stimulation of motor cortex layer V neurons. This protocol activated no apparent inflammation but increased OPC proliferation and survival, resulting in increased oligodendrogenesis. Mature oligodendrocyte number increased along with increased MBP protein expression and myelin sheath thickness (Gibson et al., 2014). The functional consequences of these changes in oligodendrocytes and myelin were determined by gait analysis on a Catwalk apparatus. This optogenetic manipulation and increased myelination resulted in increased swing speed of the forelimb associated with the cortical hemisphere that had received optical excitation. Subsequent studies into the mechanism regulating these changes demonstrated that neural activity directly modified oligodendrocyte differentiation and myelination.
Conclusion
The emergence and accessibility of high throughput electron microscopy and the surge in unique genetic models and optogenetic tools to study the dynamic process of CNS myelination modulating neural circuitry begin a new era investigating the complex networking in the brain. The evidence that humans undergo minimal oligodendrogenesis after early childhood but rapidly undergo myelin turnover throughout adulthood suggests that adaptive myelination may be a major element of the fine tuning of heterogeneous neural cell populations in various regions in the brain (Yeung et al., 2014). New experimental evidence about the fundamental processes of active myelination and the oligodendrocyte-neuron relationship suggest that metabolic pathways, extracellular signaling mediators and electrical activity all facilitate changes in CNS plasticity via heightened oligodendrogenesis to strengthen motor learning and function. Studies demonstrating that constant changes in white matter contribute to life-long cognitive and motor processing provide promising insight into the potential role of oligodendrocyte dysfunction as a primary contributor in CNS disorders and disease (Scholz et al., 2009; Yeung et al., 2014). The recent study demonstrating altered metabolic monocarboxylate transporter expression in human ALS cortex (Lee et al., 2012) may be just one example of the importance of oligodendrocyte metabolic and trophic support on axonal integrity.
In diseases such as multiple sclerosis, while the most obvious pathological presentation is plaques that have little myelin, there may also be elements of the disease that result in myelin dysfunction undetectable by standard approaches. For example, normal appearing white matter in multiple sclerosis tissue often has reduced axonal density, which is generally attributed to inflammation (Frischer et al., 2009). This normal appearing white matter may well have dysfunctional myelin that cannot provide the necessary trophic and metabolic support for axons. The extensive studies showing reduced white matter in disease (Bakhtiari et al., 2012; Defrancesco et al., 2014; Haroutunian et al., 2014), as well as in normal aging in humans (Haroutunian et al., 2014) suggest that altered myelin and oligodendrocyte function in human brain are major factors in neurodegeneration. The concept that myelin dysfunction could be just as important as myelin loss with respect to neurodegeneration is extremely important and may have great significance for future research directions.
References
- Allen NJ, and Barres BA (2009). Neuroscience: Glia - more than just brain glue. Nature 457, 675–677. [DOI] [PubMed] [Google Scholar]
- Almeida RG, Czopka T, Ffrench-Constant C, and Lyons DA (2011). Individual axons regulate the myelinating potential of single oligodendrocytes in vivo. Development (Cambridge, England) 138, 4443–4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral AI, Meisingset TW, Kotter MR, and Sonnewald U (2013). Metabolic aspects of neuron-oligodendrocyte-astrocyte interactions. Frontiers in endocrinology 4, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, Augustine GJ, Ehlers MD, and Feng G (2007). In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 54, 205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhtiari R, Zurcher NR, Rogier O, Russo B, Hippolyte L, Granziera C, Araabi BN, Nili Ahmadabadi M, and Hadjikhani N (2012). Differences in white matter reflect atypical developmental trajectory in autism: A Tract-based Spatial Statistics study. NeuroImage Clinical 1, 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera K, Chu P, Abramowitz J, Steger R, Ramos RL, and Brumberg JC (2013). Organization of myelin in the mouse somatosensory barrel cortex and the effects of sensory deprivation. Developmental neurobiology 73, 297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA (2008). The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60, 430–440. [DOI] [PubMed] [Google Scholar]
- Bauer NG, Richter-Landsberg C, and Ffrench-Constant C (2009). Role of the oligodendroglial cytoskeleton in differentiation and myelination. Glia 57, 1691–1705. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, and Ullen F (2005). Extensive piano practicing has regionally specific effects on white matter development. Nature neuroscience 8, 1148–1150. [DOI] [PubMed] [Google Scholar]
- Bercury KK, Dai J, Sachs HH, Ahrendsen JT, Wood TL, and Macklin WB (2014). Conditional ablation of raptor or rictor has differential impact on oligodendrocyte differentiation and CNS myelination. The Journal of neuroscience : the official journal of the Society for Neuroscience 34, 4466–4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore WF, and Murray JA (1981). Quantitative examination of internodal length of remyelinated nerve fibres in the central nervous system. Journal of the neurological sciences 49, 273–284. [DOI] [PubMed] [Google Scholar]
- Bouzier-Sore AK, and Pellerin L (2013). Unraveling the complex metabolic nature of astrocytes. Frontiers in cellular neuroscience 7, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, and Deisseroth K (2005). Millisecond-timescale, genetically targeted optical control of neural activity. Nature neuroscience 8, 1263–1268. [DOI] [PubMed] [Google Scholar]
- Bunge MB, Bunge RP, and Pappas GD (1962). Electron microscopic demonstration of connections between glia and myelin sheaths in the developing mammalian central nervous system. The Journal of cell biology 12, 448–453. [DOI] [PubMed] [Google Scholar]
- Bunge MB, Bunge RP, Peterson ER, and Murray MR (1967). A light and electron microscope study of long-term organized cultures of rat dorsal root ganglia. The Journal of cell biology 32, 439–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge RP, Bunge MB, and Peterson ER (1965). AN ELECTRON MICROSCOPE STUDY OF CULTURED RAT SPINAL CORD. The Journal of cell biology 24, 163–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda JE, and Sofroniew MV (2014). Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81, 229–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, et al. (2008). A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. The Journal of neuroscience : the official journal of the Society for Neuroscience 28, 264–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calver AR, Hall AC, Yu WP, Walsh FS, Heath JK, Betsholtz C, and Richardson WD (1998). Oligodendrocyte population dynamics and the role of PDGF in vivo. Neuron 20, 869–882. [DOI] [PubMed] [Google Scholar]
- Cannon DM, Walshe M, Dempster E, Collier DA, Marshall N, Bramon E, Murray RM, and McDonald C (2012). The association of white matter volume in psychotic disorders with genotypic variation in NRG1, MOG and CNP: a voxel-based analysis in affected individuals and their unaffected relatives. Translational psychiatry 2, e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M (2013). Oligodendrocyte failure in ALS. Nature neuroscience 16, 525. [DOI] [PubMed] [Google Scholar]
- Clarke LE, Young KM, Hamilton NB, Li H, Richardson WD, and Attwell D (2012). Properties and fate of oligodendrocyte progenitor cells in the corpus callosum, motor cortex, and piriform cortex of the mouse. The Journal of neuroscience : the official journal of the Society for Neuroscience 32, 8173–8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M, Thapar A, and Jones DK (2014). White Matter Microstructure Predicts Autistic Traits in Attention-Deficit/Hyperactivity Disorder. Journal of autism and developmental disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court FA, Sherman DL, Pratt T, Garry EM, Ribchester RR, Cottrell DF, Fleetwood-Walker SM, and Brophy PJ (2004). Restricted growth of Schwann cells lacking Cajal bands slows conduction in myelinated nerves. Nature 431, 191–195. [DOI] [PubMed] [Google Scholar]
- Czopka T, Ffrench-Constant C, and Lyons DA (2013). Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths in vivo. Developmental cell 25, 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czopka T, and Lyons DA (2011). Dissecting mechanisms of myelinated axon formation using zebrafish. Methods in cell biology 105, 25–62. [DOI] [PubMed] [Google Scholar]
- Dangata YY, Findlater GS, and Kaufman MH (1996). Postnatal development of the optic nerve in (C57BL × CBA)F1 hybrid mice: general changes in morphometric parameters. Journal of anatomy 189 (Pt 1), 117–125. [PMC free article] [PubMed] [Google Scholar]
- De Robertis E, Gerschenfeld HM, and Wald F (1958). Cellular mechanism of myelination in the central nervous system. The Journal of biophysical and biochemical cytology 4, 651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defrancesco M, Egger K, Marksteiner J, Esterhammer R, Hinterhuber H, Deisenhammer EA, and Schocke M (2014). Changes in White Matter Integrity before Conversion from Mild Cognitive Impairment to Alzheimer’s Disease. PloS one 9, e106062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defrancesco M, Marksteiner J, Deisenhammer E, Kemmler G, Djurdjevic T, and Schocke M (2013). Impact of white matter lesions and cognitive deficits on conversion from mild cognitive impairment to Alzheimer’s disease. Journal of Alzheimer’s disease : JAD 34, 665–672. [DOI] [PubMed] [Google Scholar]
- Dimou L, Simon C, Kirchhoff F, Takebayashi H, and Gotz M (2008). Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 28, 10434–10442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F (2003). The glial identity of neural stem cells. Nature neuroscience 6, 1127–1134. [DOI] [PubMed] [Google Scholar]
- Edgar JM, McLaughlin M, Werner HB, McCulloch MC, Barrie JA, Brown A, Faichney AB, Snaidero N, Nave KA, and Griffiths IR (2009). Early ultrastructural defects of axons and axon-glia junctions in mice lacking expression of Cnp1. Glia 57, 1815–1824. [DOI] [PubMed] [Google Scholar]
- Emery B (2010). Regulation of oligodendrocyte differentiation and myelination. Science (New York, NY) 330, 779–782. [DOI] [PubMed] [Google Scholar]
- Emery B, Agalliu D, Cahoy JD, Watkins TA, Dugas JC, Mulinyawe SB, Ibrahim A, Ligon KL, Rowitch DH, and Barres BA (2009). Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell 138, 172–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD (2008). White matter in learning, cognition and psychiatric disorders. Trends in neurosciences 31, 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores AI, Narayanan SP, Morse EN, Shick HE, Yin X, Kidd G, Avila RL, Kirschner DA, and Macklin WB (2008). Constitutively active Akt induces enhanced myelination in the CNS. The Journal of neuroscience : the official journal of the Society for Neuroscience 28, 7174–7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier TW, Keshavan MS, Minshew NJ, and Hardan AY (2012). A two-year longitudinal MRI study of the corpus callosum in autism. Journal of autism and developmental disorders 42, 2312–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MR, and Rowitch DH (2013). Evolving concepts of gliogenesis: a look way back and ahead to the next 25 years. Neuron 80, 613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friede RL, and Bischhausen R (1982). How are sheath dimensions affected by axon caliber and internode length? Brain research 235, 335–350. [DOI] [PubMed] [Google Scholar]
- Frischer JM, Bramow S, Dal-Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M, Laursen H, Sorensen PS, and Lassmann H (2009). The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain : a journal of neurology 132, 1175–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruhbeis C, Frohlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS, Kirchhoff F, Mobius W, Goebbels S, Nave KA, et al. (2013). Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS biology 11, e1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, et al. (2012). Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485, 517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, and Deneen B (2014). Glial development: the crossroads of regeneration and repair in the CNS. Neuron 83, 283–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geren BB, and Schmitt FO (1954). THE STRUCTURE OF THE SCHWANN CELL AND ITS RELATION TO THE AXON IN CERTAIN INVERTEBRATE NERVE FIBERS. Proceedings of the National Academy of Sciences of the United States of America 40, 863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, et al. (2014). Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science (New York, NY) 344, 1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gledhill RF, and McDonald WI (1977). Morphological characteristics of central demyelination and remyelination: a single-fiber study. Annals of neurology 1, 552–560. [DOI] [PubMed] [Google Scholar]
- Goebbels S, Oltrogge JH, Kemper R, Heilmann I, Bormuth I, Wolfer S, Wichert SP, Mobius W, Liu X, Lappe-Siefke C, et al. (2010). Elevated phosphatidylinositol 3,4,5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination. The Journal of neuroscience : the official journal of the Society for Neuroscience 30, 8953–8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan G, Awasthi A, Belkaid W, De Faria O Jr., Liazoghli D, Colman DR, and Dhaunchak AS (2013). Lipidome and proteome map of myelin membranes. Journal of neuroscience research 91, 321–334. [DOI] [PubMed] [Google Scholar]
- Griffiths I, Klugmann M, Anderson T, Yool D, Thomson C, Schwab MH, Schneider A, Zimmermann F, McCulloch M, Nadon N, et al. (1998). Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science (New York, NY) 280, 1610–1613. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Katsel P, Dracheva S, Stewart DG, and Davis KL (2007). Variations in oligodendrocyte-related gene expression across multiple cortical regions: implications for the pathophysiology of schizophrenia. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 10, 565–573. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Katsel P, Roussos P, Davis KL, Altshuler LL, and Bartzokis G (2014). Myelination, oligodendrocytes, and serious mental illness. Glia 62, 1856–1877. [DOI] [PubMed] [Google Scholar]
- Hildebrand C, and Hahn R (1978). Relation between myelin sheath thickness and axon size in spinal cord white matter of some vertebrate species. Journal of the neurological sciences 38, 421–434. [DOI] [PubMed] [Google Scholar]
- Honer WG, Falkai P, Chen C, Arango V, Mann JJ, and Dwork AJ (1999). Synaptic and plasticity-associated proteins in anterior frontal cortex in severe mental illness. Neuroscience 91, 1247–1255. [DOI] [PubMed] [Google Scholar]
- Hong S, Ke X, Tang T, Hang Y, Chu K, Huang H, Ruan Z, Lu Z, Tao G, and Liu Y (2011). Detecting abnormalities of corpus callosum connectivity in autism using magnetic resonance imaging and diffusion tensor tractography. Psychiatry research 194, 333–339. [DOI] [PubMed] [Google Scholar]
- Honjin R, Sakato S, and Yamashita T (1977). Electron microscopy of the mouse optic nerve: a quantitative study of the total optic nerve fibers. Archivum histologicum Japonicum = Nihon soshikigaku kiroku 40, 321–332. [DOI] [PubMed] [Google Scholar]
- Hughes EG, Kang SH, Fukaya M, and Bergles DE (2013). Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nature neuroscience 16, 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii A, Dutta R, Wark GM, Hwang SI, Han DK, Trapp BD, Pfeiffer SE, and Bansal R (2009). Human myelin proteome and comparative analysis with mouse myelin. Proceedings of the National Academy of Sciences of the United States of America 106, 14605–14610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii A, Fyffe-Maricich SL, Furusho M, Miller RH, and Bansal R (2012). ERK1/ERK2 MAPK signaling is required to increase myelin thickness independent of oligodendrocyte differentiation and initiation of myelination. The Journal of neuroscience : the official journal of the Society for Neuroscience 32, 8855–8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn O, Tenzer S, and Werner HB (2009). Myelin proteomics: molecular anatomy of an insulating sheath. Molecular neurobiology 40, 55–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John Lin CC, and Deneen B (2013). Astrocytes: the missing link in neurologic disease? Seminars in pediatric neurology 20, 236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SH, Fukaya M, Yang JK, Rothstein JD, and Bergles DE (2010). NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 68, 668–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SH, Li Y, Fukaya M, Lorenzini I, Cleveland DW, Ostrow LW, Rothstein JD, and Bergles DE (2013). Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nature neuroscience 16, 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns D, Vong GS, Barley K, Dracheva S, Katsel P, Casaccia P, Haroutunian V, and Byne W (2010). Gene expression abnormalities and oligodendrocyte deficits in the internal capsule in schizophrenia. Schizophrenia research 120, 150–158. [DOI] [PubMed] [Google Scholar]
- Kessaris N, Pringle N, and Richardson WD (2008). Specification of CNS glia from neural stem cells in the embryonic neuroepithelium. Philosophical transactions of the Royal Society of London Series B, Biological sciences 363, 71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Kim SJ, Kim HS, Choi CG, Kim N, Han S, Jang EH, Chung SJ, and Lee CS (2013). Alterations of mean diffusivity in brain white matter and deep gray matter in Parkinson’s disease. Neuroscience letters 550, 64–68. [DOI] [PubMed] [Google Scholar]
- Kirby BB, Takada N, Latimer AJ, Shin J, Carney TJ, Kelsh RN, and Appel B (2006). In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nature neuroscience 9, 1506–1511. [DOI] [PubMed] [Google Scholar]
- Kramer-Albers EM, Bretz N, Tenzer S, Winterstein C, Mobius W, Berger H, Nave KA, Schild H, and Trotter J (2007). Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: Trophic support for axons? Proteomics Clinical applications 1, 1446–1461. [DOI] [PubMed] [Google Scholar]
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, and Nave KA (2003). Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nature genetics 33, 366–374. [DOI] [PubMed] [Google Scholar]
- Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, et al. (2012). Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487, 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD, Theilmann RJ, Fonov V, Bellec P, Lincoln A, Evans AC, and Townsend J (2013). Callosal fiber length and interhemispheric connectivity in adults with autism: brain overgrowth and underconnectivity. Human brain mapping 34, 1685–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Sun J, Guo L, Zang Y, Feng Z, Huang X, Yang H, Lv Y, Huang M, and Gong Q (2010). Increased fractional anisotropy in white matter of the right frontal region in children with attention-deficit/hyperactivity disorder: a diffusion tensor imaging study. Neuro endocrinology letters 31, 747–753. [PubMed] [Google Scholar]
- Lillo P, Mioshi E, Burrell JR, Kiernan MC, Hodges JR, and Hornberger M (2012). Grey and white matter changes across the amyotrophic lateral sclerosis-frontotemporal dementia continuum. PloS one 7, e43993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, Vialou V, Lobo MK, Dietz DM, Nestler EJ, et al. (2012). Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nature neuroscience 15, 1621–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgaard I, Luzhynskaya A, Stockley JH, Wang Z, Evans KA, Swire M, Volbracht K, Gautier HO, Franklin RJ, Attwell D, et al. (2013). Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS biology 11, e1001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood A, Bibat G, Zhan AL, Izbudak I, Farage L, Horska A, Mori S, and Naidu S (2010). White matter impairment in Rett syndrome: diffusion tensor imaging study with clinical correlations. AJNR American journal of neuroradiology 31, 295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinodan M, Rosen KM, Ito S, and Corfas G (2012). A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science (New York, NY) 337, 1357–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Herting MM, Luszpak AE, Juraska JM, and Greenough WT (2009). Myelination of the corpus callosum in male and female rats following complex environment housing during adulthood. Brain research 1288, 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie IA, Ohayon D, Li H, de Faria JP, Emery B, Tohyama K, and Richardson WD (2014). Motor skill learning requires active central myelination. Science (New York, NY) 346, 318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, and Nave KA (2004). Axonal neuregulin-1 regulates myelin sheath thickness. Science (New York, NY) 304, 700–703. [DOI] [PubMed] [Google Scholar]
- Miller RH (2002). Regulation of oligodendrocyte development in the vertebrate CNS. Progress in neurobiology 67, 451–467. [DOI] [PubMed] [Google Scholar]
- Miller RH, Zhang H, and Fok-Seang J (1994). Glial cell heterogeneity in the mammalian spinal cord. Perspectives on developmental neurobiology 2, 225–231. [PubMed] [Google Scholar]
- Mitew S, Hay CM, Peckham H, Xiao J, Koenning M, and Emery B (2013). Mechanisms regulating the development of oligodendrocytes and central nervous system myelin. Neuroscience. [DOI] [PubMed] [Google Scholar]
- Morrison BM, Lee Y, and Rothstein JD (2013). Oligodendroglia: metabolic supporters of axons. Trends in cell biology 23, 644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan SP, Flores AI, Wang F, and Macklin WB (2009). Akt signals through the mammalian target of rapamycin pathway to regulate CNS myelination. The Journal of neuroscience : the official journal of the Society for Neuroscience 29, 6860–6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave KA (2010). Myelination and support of axonal integrity by glia. Nature 468, 244–252. [DOI] [PubMed] [Google Scholar]
- Nave KA, and Ehrenreich H (2014). Myelination and oligodendrocyte functions in psychiatric diseases. JAMA psychiatry 71, 582–584. [DOI] [PubMed] [Google Scholar]
- Nave KA, and Trapp BD (2008). Axon-glial signaling and the glial support of axon function. Annual review of neuroscience 31, 535–561. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Yang Z, and Butt A (2005). Astrocytes and NG2-glia: what’s in a name? Journal of anatomy 207, 687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, and Kriegstein AR (2007). Contribution of intermediate progenitor cells to cortical histogenesis. Archives of neurology 64, 639–642. [DOI] [PubMed] [Google Scholar]
- Owen JP, Marco EJ, Desai S, Fourie E, Harris J, Hill SS, Arnett AB, and Mukherjee P (2013). Abnormal white matter microstructure in children with sensory processing disorders. NeuroImage Clinical 2, 844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A and Vaughn JE (1970). Morphology and development of the myelin sheath, in Myelination (Springfield, Illinois: Charles C. Thomas; ). [Google Scholar]
- Pettit LD, Bastin ME, Smith C, Bak TH, Gillingwater TH, and Abrahams S (2013). Executive deficits, not processing speed relates to abnormalities in distinct prefrontal tracts in amyotrophic lateral sclerosis. Brain : a journal of neurology 136, 3290–3304. [DOI] [PubMed] [Google Scholar]
- Philips T, Bento-Abreu A, Nonneman A, Haeck W, Staats K, Geelen V, Hersmus N, Kusters B, Van Den Bosch L, Van Damme P, et al. (2013). Oligodendrocyte dysfunction in the pathogenesis of amyotrophic lateral sclerosis. Brain : a journal of neurology 136, 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers BE, Sellers DL, Lovelett EA, Cheung W, Aalami SP, Zapertov N, Maris DO, and Horner PJ (2013). Remyelination reporter reveals prolonged refinement of spontaneously regenerated myelin. Proceedings of the National Academy of Sciences of the United States of America 110, 4075–4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge MB, Lange N, Bigler ED, Merkley TL, Neeley ES, Abildskov TJ, Froehlich AL, Nielsen JA, Cooperrider JR, Cariello AN, et al. (2013). Corpus Callosum Area in Children and Adults with Autism. Research in autism spectrum disorders 7, 221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN, Tayler J, Kaga Y, Yang Y, Lappe-Siefke C, Nave KA, and Bansal R (2005). CNP is required for maintenance of axon-glia interactions at nodes of Ranvier in the CNS. Glia 50, 86–90. [DOI] [PubMed] [Google Scholar]
- Richardson WD, Kessaris N, and Pringle N (2006). Oligodendrocyte wars. Nature reviews Neuroscience 7, 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, and Richardson WD (2008). PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nature neuroscience 11, 1392–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluth J (1999). A brief history of myelinated nerve fibers: one hundred and fifty years of controversy. Journal of neurocytology 28, 251–262. [DOI] [PubMed] [Google Scholar]
- Schlegel AA, Rudelson JJ, and Tse PU (2012). White matter structure changes as adults learn a second language. Journal of cognitive neuroscience 24, 1664–1670. [DOI] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TE, and Johansen-Berg H (2009). Training induces changes in white-matter architecture. Nature neuroscience 12, 1370–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M, Kipnis J, Rivest S, and Prat A (2013). How do immune cells support and shape the brain in health, disease, and aging? The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 17587–17596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson AH, Gillingwater TH, Anderson H, Cottrell D, Sherman DL, Ribchester RR, and Brophy PJ (2013). Effect of limb lengthening on internodal length and conduction velocity of peripheral nerve. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 4536–4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaidero N, Mobius W, Czopka T, Hekking LH, Mathisen C, Verkleij D, Goebbels S, Edgar J, Merkler D, Lyons DA, et al. (2014). Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue. Cell 156, 277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobottka B, Ziegler U, Kaech A, Becher B, and Goebels N (2011). CNS live imaging reveals a new mechanism of myelination: the liquid croissant model. Glia 59, 1841–1849. [DOI] [PubMed] [Google Scholar]
- Stevens B, Tanner S, and Fields RD (1998). Control of myelination by specific patterns of neural impulses. The Journal of neuroscience : the official journal of the Society for Neuroscience 18, 9303–9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada N, and Appel B (2010). Identification of genes expressed by zebrafish oligodendrocytes using a differential microarray screen. Developmental dynamics : an official publication of the American Association of Anatomists 239, 2041–2047. [DOI] [PubMed] [Google Scholar]
- Taveggia C, Thaker P, Petrylak A, Caporaso GL, Toews A, Falls DL, Einheber S, and Salzer JL (2008). Type III neuregulin-1 promotes oligodendrocyte myelination. Glia 56, 284–293. [DOI] [PubMed] [Google Scholar]
- Tomassy GS, Berger DR, Chen HH, Kasthuri N, Hayworth KJ, Vercelli A, Seung HS, Lichtman JW, and Arlotta P (2014). Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science (New York, NY) 344, 319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HH, Macklin WB, and Miller RH (2009). Distinct modes of migration position oligodendrocyte precursors for localized cell division in the developing spinal cord. Journal of neuroscience research 87, 3320–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, and Barres BA (2001). Control of synapse number by glia. Science (New York, NY) 291, 657–661. [DOI] [PubMed] [Google Scholar]
- Wahl SE, McLane LE, Bercury KK, Macklin WB, and Wood TL (2014). Mammalian target of rapamycin promotes oligodendrocyte differentiation, initiation and extent of CNS myelination. The Journal of neuroscience : the official journal of the Society for Neuroscience 34, 4453–4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Lee PR, and Fields RD (2011). Control of local protein synthesis and initial events in myelination by action potentials. Science (New York, NY) 333, 1647–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warf BC, Fok-Seang J, and Miller RH (1991). Evidence for the ventral origin of oligodendrocyte precursors in the rat spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience 11, 2477–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins A, Majed H, Layfield R, Compston A, and Chandran S (2003). Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: a novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. The Journal of neuroscience : the official journal of the Society for Neuroscience 23, 4967–4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JH, Halliday GM, and Kim WS (2014). Exploring myelin dysfunction in multiple system atrophy. Experimental neurobiology 23, 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YH, Gau SS, Lo YC, and Tseng WY (2014). White matter tract integrity of frontostriatal circuit in attention deficit hyperactivity disorder: association with attention performance and symptoms. Human brain mapping 35, 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K, Boillee S, Roberts EA, Garcia ML, McAlonis-Downes M, Mikse OR, Cleveland DW, and Goldstein LS (2008). Mutant SOD1 in cell types other than motor neurons and oligodendrocytes accelerates onset of disease in ALS mice. Proceedings of the National Academy of Sciences of the United States of America 105, 7594–7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung MS, Zdunek S, Bergmann O, Bernard S, Salehpour M, Alkass K, Perl S, Tisdale J, Possnert G, Brundin L, et al. (2014). Dynamics of oligodendrocyte generation and myelination in the human brain. Cell 159, 766–774. [DOI] [PubMed] [Google Scholar]
- Young KM, Psachoulia K, Tripathi RB, Dunn SJ, Cossell L, Attwell D, Tohyama K, and Richardson WD (2013). Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron 77, 873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, and Guarnieri P (2014). An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. 34, 11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]