Abstract
Locomotion, that is active propulsive movement of the body in space, is a vital motor function. Intensive studies of the main, for the majority of living beings, form of locomotion, forward locomotion, have revealed essential features of the organization and operation of underlying neural mechanisms. However, animals and humans are capable to locomote not only forward but also in other directions in relation to the body axis, e.g. backward, sideways, etc. Single steps in different directions are also used for postural corrections during locomotion and during standing. Recent studies of mechanisms underlying control of locomotion in different directions have greatly expanded our knowledge about locomotor system and can contribute to improvement of rehabilitation strategies aimed at restoration of locomotion and balance control in patients. This review outlines recent advances in the studies of locomotion in different directions in lower and higher vertebrates, with special attention given to the neuronal locomotor mechanisms.
Keywords: swimming, walking, backward, spinal networks, supraspinal control, reflexes
Introduction
Locomotion is an evolutionary old basic motor function. Neural mechanisms for control of the main form of locomotion, forward locomotion, have been studied in different species from simple animals to humans [1]. These studies revealed basic principles in the organization and operation of these mechanisms common for phylogenetically remote species.
Most vertebrates can locomote not only forward but also in other directions. Thus, lower vertebrates exhibiting axial locomotion can swim backward [2,3], and legged higher vertebrates can walk in any direction in relation to the body axis [4,5,6•]. These forms of locomotion are usually generated in the context of avoidance behavior (called “escape” in the lamprey and “struggling” in the zebrafish and Xenopus tadpole). Also, single steps in different directions are used for postural corrections during locomotion [7,8] and during standing [9•,10,11].
This review outlines recent progress in understanding the neural mechanisms underlying control of locomotion in different directions in lower and higher vertebrates.
Forward and backward locomotor movements
In general, backward locomotion could be considered as reversed forward locomotion. Thus, in low vertebrates during backward swimming the waves of periodic lateral body flexion propagate in a caudo-rostral direction during forward locomotion, while in a rostro-caudal - during backward locomotion [2,3]. In higher vertebrates and humans during stance and during swing phases of backward locomotion, a limb moves in directions opposite to those during forward stepping [4,5]. Although in both lower and higher vertebrates the muscle activity patterns of forward and backward locomotion differ, analysis of these patterns in higher vertebrates and humans revealed similar basic flexor-extensor synergies [6•,12,13]. This led to a suggestion that there are some common direction-independent neuronal mechanisms contributing to generation of both forward and backward locomotion.
Organization of spinal networks
In both lower and higher vertebrates, the neuronal mechanisms generating locomotion in different directions reside in the spinal cord [14,15,16,17]. The axial locomotor rhythm is generated by a chain of coupled segmental oscillators (Fig. 1a,b; [18,19,20]). Each oscillator generates rhythmically alternating bursts of activity in the right and left hemisegments. Recording of individual spinal interneurons during forward and backward swimming in the tadpole and larval zebrafish, revealed a group of excitatory interneurons with activity modulated in locomotor rhythm during swimming in both directions [3,21,22]. This suggests the presence of a core rhythmogenic kernel that is active independently of the particular direction of locomotion. In the tadpole, these neurons exhibit mutual excitation [22] and form two subpopulations preferentially active during forward and backward swimming, respectively [21]. Differences in biophysical properties of these subpopulations may explain differences in parameters of forward and backward rhythms [21,23•]
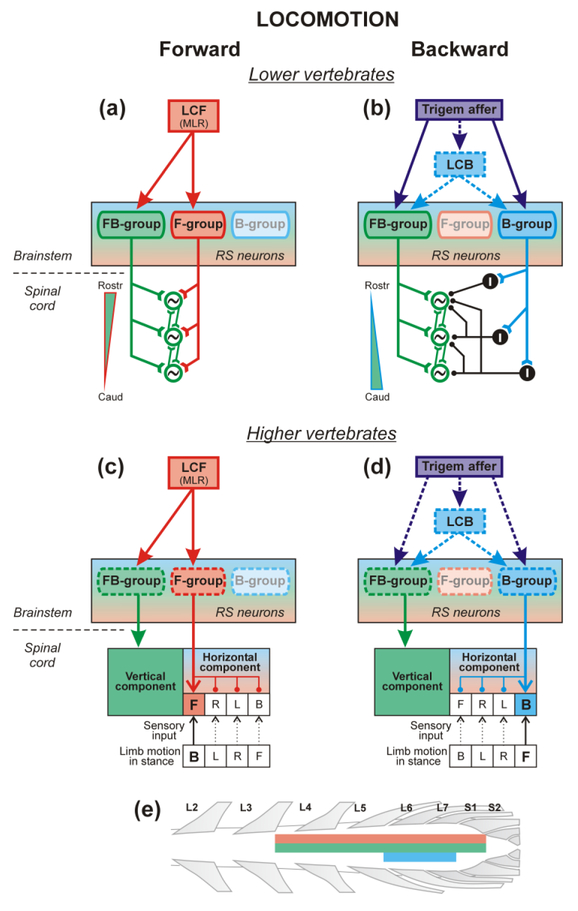
Hypothetical neural mechanisms for the control of locomotion in different directions. (a-d) Hypothetical models of the spinal locomotor network and of the descending commands controlling forward (a,c) and backward (b,d) locomotion in lower (a,b) and higher (c,d) vertebrates. (a,b) The spinal locomotor network in lower vertebrates consists of segmental oscillators (green circles) that excite one another and thus form a chain, along which the waves of activity propagate. (a) Elicitation of forward swimming. The MLR, which is a locomotor center for forward locomotion (LCF), activates reticulospinal (RS) neurons of FBgroup and F-group. FB-group activates the chain of oscillators generating the swim rhythm. F-group stabilizes an intrinsic rostro-caudal gradient of excitability of oscillators or creates this gradient (green ramp with red outline). As a result, the waves of activity propagate in the caudal direction. (b) Elicitation of backward swimming. Trigeminal afferents activate RS neurons of FB-group and B-group (possibly, through the locomotor center for backward locomotion, LCB). FB-group activates the chain of oscillators generating the swim rhythm. At the same time, neurons of B-group activate inhibitory neurons (I) that invert the gradient of excitability (green ramp with blue outline). This results in the caudo-rostral propagation of locomotor waves. (c,d) Spinal cord of higher vertebrates contains two principal mechanisms, one generating the vertical component of step (limb elevation and lowering), and the other generating the horizontal component (limb transfer from one extreme point to the other). The latter includes networks generating the horizontal component of step in different directions (for simplicity, only the networks generating steps in four directions – F, forward; B, backward; R, rightward; L, leftward are shown). These networks receive sensory input signaling the limb motion during stance. (c) Elicitation of forward stepping. The MLR activates specific populations of RS neurons (presumably, FB-group and F-group). FB-group activates a network generating the vertical component of step. At the same time, neurons of Fgroup activate a network generating the horizontal component for forward stepping. Sensory input signaling that the limb reached the extreme caudal position during stance (B) assists in initiation of the forward swing. (d) Elicitation of backward stepping. Trigeminal afferents presumably activate RS neurons of FB-group and B-group (possibly, through LCB). FBgroup activates a network generating the vertical component of step. At the same time, neurons of B-group activate a network generating the horizontal component for backward stepping. Sensory input signaling that the limb reached the extreme rostral position in stance (F), assist in initiation of the backward swing. (e) Rostro-caudal distribution in the lumbosacral enlargement of networks generating vertical component of the steps, horizontal component for forward steps, and horizontal component for backward steps, are shown schematically by thick green, red and blue lines, respectively.
Two models have been proposed to explain the change in the direction of locomotor waves. The trailing-oscillator model with symmetrical intersegmental connections [24] suggests that the direction of propagation of locomotor waves is determined by a gradient of excitability of individual oscillators along the chain. In isolated lamprey spinal cord this gradient is rostrocaudal. In the tadpole, ascending glycinergic inhibitory interneurons are involved in the locomotor wave reversal [25,26] and presumably inverse this gradient (I in Fig. 1b) [27]. Such inhibitory interneurons (active only during backward locomotion) have been found in the zebrafish [3]. A model with asymmetrical intersegmental connections explains the reverse of the wave propagation from rostro-caudal to caudo-rostral by switching from activation of the descending coupling to activation of the ascending one [28].
Functional organization of networks generating stepping in different directions was studied by analyzing locomotor movements evoked by direct unspecific activation of these networks by epidural stimulation of the spinal cord in the decerebrate cat [29••]. It was demonstrated that during stimulation of certain sites, the direction of locomotion is determined by the direction of the treadmill belt motion, and on immobile surface or in the air, in-place stepping is observed, suggesting that the locomotor system includes two principal mechanisms (Fig. 1c,d). One mechanism generates the vertical component of step (VC, limb elevation and lowering), and the other generates the horizontal component (HC, limb transfer from one extreme point to the other). The latter includes networks generating the horizontal component of step in different directions. These circuits receive sensory input signaling limb motion in stance; reaching an extreme position triggers the limb lifting and transfer in the opposite direction. One can suggest that VC-mechanism contains rhythm-generating while HCmechanism – pattern formation networks.
Analysis of kinematics and EMG patterns of single corrective steps in different directions generated in response to postural disturbances during standing, revealed their similarity with those of locomotor steps in the corresponding direction [9•]. It was proposed that a corrective step is generated by the same (VC and HC) mechanisms, which generate locomotor steps in corresponding direction, but they are activated by sensory information caused by postural disturbance and signaling deviation of the limb in relation to the trunk. This hypothesis is supported by finding that training locomotion in different directions improves balance control in spinal cord injured and stroke subjects [17,30,31].
Mapping the efficacy of epidural stimulation of different sites of the lumbosacral enlargement to evoke forward and backward locomotion combined with c-Fos immunostaining [32•] led to a suggestion that networks generating the VC of steps and the HC for forward stepping are distributed throughout the whole lumbosacral enlargement, while the network generating the HC for backward stepping is confined to a zone from caudal L5 to L7 (Fig. 1e). Recording of the same individual spinal interneurons in L5-L6 during both forward and backward locomotion, revealed neurons with activity phase in the locomotor cycle independent of the locomotion direction and those modulated during forward or during backward locomotion only, presumably belonging to the VC and corresponding HC networks, respectively [33].
Though the basic principles of organization of axial and legged locomotor networks are similar, their neuronal compositions are different. In the zebrafish, spinal glutamatergic V2a interneurons are necessary and sufficient for the locomotor rhythm generation [34]. In contrast, in mice, glutamatergic non-V2a spinal interneurons contribute to generation of locomotor rhythm [35,36•], while V2a interneurons are involved in interlimb coordination [37]. In the zebrafish, an increase in the locomotor speed is associated with recruitment of new modules of locomotor network containing only excitatory (V0v) commissural interneurons [38]. By contrast, in mice, inhibitory V0d interneurons determine the left-right limb alternation at low frequencies characteristic for slow forward walking, while excitatory V0v interneurons – at higher frequencies characteristic for both fast forward and backward locomotion [39,40,41]. These findings suggest that though some populations of genetically identified spinal interneurons are elements of both axial and legged locomotor networks, they have different specific functional role in generation of locomotion. Most likely, the legged locomotor network cannot be considered as axial network updated with some new populations of neurons, and thus the axial network does not represent the core of the legged one.
Supraspinal control
In all studied vertebrates, forward locomotion can be elicited and the speed of progression controlled from mesencephalic locomotor region (MLR), which represents a command center for forward locomotion only [29••,42]. Its activation leads to formation of reticulospinal commands, which selectively activate a part of spinal locomotor networks necessary for generation of forward locomotion (Fig. 1a,c). Recent study in mice demonstrated that activation of glutamatergic neurons of MLR located in both the cuneiform (CnF) and the pedunculopontine nucleus evokes slow, alternating-gait locomotion, whereas activation of those in the CnF – high-speed synchronous-gait locomotion [43•]. One can hypothesize that some other forms of locomotion (e.g. backward and sideward) also have their command centers.
In both lower and higher vertebrates, backward locomotion can be initiated by continuous stimulation of the skin mechanoreceptors of the head [2,3,15,41]. Signals from these receptors are transmitted by specific populations of trigeminal nerve afferents [2].
In the lamprey and tadpole, reticulospinal neurons active exclusively during forward (Fgroup) or during backward locomotion (B-group), or active both during forward and backward locomotion (FB-group), were revealed [21,27]. It was suggested that the FB-group activates segmental oscillators during forward and during backward swimming (Fig. 1a,b; [27]). In the framework of the trailing-oscillator model with symmetrical intersegmental connections [24], activation of FB-group alone (due to intrinsic rostro-caudal gradient of excitability in the chain of segmental oscillators) evokes forward swimming. The F-group can contribute to stabilization or (in the framework of the model with asymmetrical intersegmental connections [28]) creation of the rostro-caudal gradient of excitability. The Bgroup inverses the excitability gradient in the chain of segmental oscillators presumably through the ascending inhibitory interneurons (I in Fig. 1b), and thus, co-activation of Bgroup and FB-group results in backward swimming. One may hypothesize that in higher vertebrates, signals from MLR and skin mechanoreceptors activate VC mechanism via FBgroup of reticulospinal neurons, and specific HC circuits determining forward and backward direction of locomotion via F- and B-populations of reticulospinal neurons, respectively (Fig. 1c,d).
Besides populations of reticulospinal neurons contributing to activation of locomotion [44••,45,46•], reticulospinal neurons terminating forward locomotion were found in both lower and higher vertebrates [44••,47,48,49••]. In lampreys and tadpoles, glutamatergic and GABA-ergic reticulospinal neurons terminate forward locomotion, respectively. It was demonstrated that in tadpoles they do not stop backward locomotion. In mice, glutamatergic V2a reticulospinal neurons in the rostral medulla terminate forward locomotion through inhibition of interneurons of the rhythm-generating network [49••]. One can expect that they stop locomotion in other directions as well.
While supraspinal control of forward locomotion in higher vertebrates was studied in a considerable detail [1], only activity of corticospinal neurons was investigated during locomotion in different directions [50]. In intact cat, activity of almost all corticospinal neurons is phasically modulated in the rhythm of stepping during both forward and backward locomotion. However, the modulation pattern is direction-dependent. It is caused by inputs only from locomotor mechanisms of the projection girdle when this girdle is leading, and from locomotor mechanisms of both girdles when this girdle is trailing. This suggests flexibility of functional roles of individual corticospinal neurons during different forms of locomotion. Involvement of motor cortex in control of both backward and forward locomotion was also demonstrated in humans [51].
Sensory feedback
In lower vertebrates, a specific movement-related sensory feedback can be provided by intraspinal mechanoreceptors (stretch receptor neurons (SRNs) in the lamprey [52] and cerebrospinal fluid contacting neurons (CSF-cNs) in zebrafish [53]), while in higher vertebrates – by proprioceptors and cutaneous afferents [54].
Recent studies demonstrated that in the lamprey, the spinal reflex to body bending during forward swimming mediated by SRNs observed during forward locomotion is reversed during backward locomotion [55•]. This reflex reversal is aimed at reinforcement of the movements generated in each of these behaviors. It assists in initiation of contralateral bending during forward swimming and augments the body undulations amplitude during backward locomotion. A population of reticulospinal neurons transmitting commands causing modification of unilateral spinal networks processing signals from SRNs, which lead to the reflex reversal, has been revealed. They are activated by trigeminal nerve stimulation causing backward swimming and presumably belong to B-group. It was found that in the zebrafish CSF-cNs increase speed of forward locomotion [53], however, their role in control of backward swimming is unknown.
It was shown that in higher vertebrates, modulation of the efficacy of the soleus H-reflex during locomotor cycle, as well as cutaneous afferents reflex effects, which are characterized by a “reversal of actions” that depends on the step cycle phase, are similar during backward and forward locomotion [56,57], suggesting that phase-dependent changes of these reflexes most likely caused by a locomotor network common for forward and backward locomotion.
A critical point in the step cycle is onset of the swing phase. It was suggested that the swing onset in any direction is determined by the afferents signaling a critical limb deviation in the stance phase [29••,54]. One can expect that supraspinal command determining the direction of stepping, selects and activates a specific spinal network in HC-mechanism for processing this information (Fig. 1c,d).
Conclusions
The rhythm-generating part of locomotor networks, as well as neuronal mechanisms underlying left-right coordination are common and contribute to generation of locomotion in any direction. By contrast, networks determining direction of locomotion are different and specific for each direction. They contain neuronal mechanisms for specific processing of movement-related sensory feedback. A detailed analysis of these networks (including clarification of functional roles of genetically identified populations of neurons) and their interaction, is one of the major lines of future studies. Evidence of shared neuronal networks for locomotor and corrective steps in different directions provides a basis for rehabilitation strategies employing walking in different directions aimed at improvement of both locomotion and balance control in patients. Such strategies showed promising results [17,30,31]. Determining specific functions of genetically identified populations of neurons and subsequent well-controlled precisely targeted activation/inactivation of relevant populations will undoubtedly boost these efforts.
Highlights:
Vertebrates can locomote not only forward but also backward, sideways, etc.
Spinal rhythm-generating locomotor network is common for locomotion in any direction.
Spinal networks determining direction of locomotion are specific for each direction.
Each direction-determining network specifically process sensory feedback.
Different supraspinal commands activate rhythm-generating and direction-determining networks.
Acknowledgements
This work was supported by grant from Swedish Research Council (no. 2017–02944) to TGD, by NIH grant (NS100928) to TGD and PEM, by grant from Swedish Research Council (no. 21076) to PVZ, by grants from Russian Foundation for Basic Research <mi> to PEM and by Grant of President of Russian Federation (MD-1018.2017.7) to PEM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
•of special interest
••of outstanding interest
References
- 1.Orlovsky GN, Deliagina TG, Grillner S: Neuronal control of locomotion From mollusc to man. New York: Oxford UP, 1999. [Google Scholar]
- 2.Islam SS, Zelenin PV: Modifications of locomotor pattern underlying escape behavior in the lamprey. J Neurophysiol 2008, 99:297–307. [DOI] [PubMed] [Google Scholar]
- 3.Liao JC, Fetcho JR: Shared versus specialized glycinergic spinal interneurons in axial motor circuits of larval zebrafish. J Neurosci 2008, 28:12982–12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buford JA, Zernicke RF, Smith JL: Adaptive control for backward quadrupedal walking. I. Posture and hindlimb kinematics. J Neurophysiol 1990, 64:745–755. [DOI] [PubMed] [Google Scholar]
- 5.Thorstensson A: How is the normal locomotor program modified to produce backward walking. Exp Brain Res 1986, 61:664–668. [DOI] [PubMed] [Google Scholar]
- 6. •.Zelik KE, La Scaleia V, Ivanenko YP, Lacquaniti F: Can modular strategies simplify neural control of multidirectional human locomotion? J Neurophysiol 2014, 111:1686–1702.In this paper, the EMG patterns during forward, backward, sideward and in place walking in humans, is analyzed to reveal shared across locomotor behaviors muscle synergies. The functional significance of the revealed synergies and modules is thoroughly discussed.
- 7.Karayannidou A, Zelenin PV, Orlovsky GN, Sirota MG, Beloozerova IN, Deliagina TG: Maintenance of lateral stability during standing and walking in the cat. J Neurophysiol 2009, 101:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musienko PE, Deliagina TG, Gerasimenko YP, Orlovsky GN, Zelenin PV: Limb and trunk mechanisms for balance control during locomotion in quadrupeds. J Neurosci 2014, 34: 5704–5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. •.Hsu LJ, Zelenin PV, Lyalka VF, Vemula MG, Orlovsky GN, Deliagina TG: Neural mechanisms of single corrective steps evoked in the standing rabbit. Neuroscience 2017, 347:85–102.In this study, postural corrections, which include single corrective steps in different directions, have been analyzed in intact and decerebrate rabbits. It was demonstrated that a corrective step is generated by a mechanism activated by sensory signal from the displaced limb, and that integrity of the highest levels of CNS is not necessary for their generation. Results of this study allowed to propose that a single corrective step in a particular direction is generated by the same locomotor system that generates sequential stepping in the corresponding direction.
- 10.Chvatal SA, Torres-Oviedo G, Safavynia SA, Ting LH: Common muscle synergies for control of center of mass and force in nonstepping and stepping postural behaviors. J Neurophysiol 2011, 106:999–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee P-Y, Gadareh K., Bronstein AM: Forward-backward postural protective stepping responses in young and elderly adults. Human Movement Science 2014, 34:137–146. [DOI] [PubMed] [Google Scholar]
- 12.Pratt CA, Buford JA, Smith JL: Adaptive control for backward quadrupedal walking V. Mutable activation of bifunctional thigh muscles. J Neurophysiol 1996, 75:832–842. [DOI] [PubMed] [Google Scholar]
- 13.Jansen K, De Groote F, Massaad F, Meyns P, Duysens J, Jonkers I: Similar muscles contribute to horizontal and vertical acceleration of center of mass in forward and backward walking: implications for neural control. J Neurophysiol 2012, 107:3385–3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu L-J, Orlovsky GN, Zelenin PV: Different forms of locomotion in the spinal lamprey. Eur J Neurosci 2014, 39:2037–2049. [DOI] [PubMed] [Google Scholar]
- 15.Soffe SR: Triggering and gating of motor responses by sensory stimulation: behavioural selection in Xenopus embryos. Proc R Soc Lond B 1991, 246:197–203. [DOI] [PubMed] [Google Scholar]
- 16.Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, Roy RR, Sofroniew MV, Edgerton VR: Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci 2009, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah PK, Gerasimenko Y, Shyu A, Lavrov I, Zhong H. Roy RR, Edgerton VR: Variability in step training enhances locomotor recovery after spinal cord injury. Eur J Neurosci 2012, 36:2054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grillner S: Neurobiological bases of rhythmic motor acts in vertebrates. Science 1985, 228: 143–149. [DOI] [PubMed] [Google Scholar]
- 19.Sigvardt KA, Williams TL: Effects of local oscillator frequency on intersegmental coordination in the lamprey locomotor CPG: theory and experiment. J Neurophys 1996, 76:4094–4103. [DOI] [PubMed] [Google Scholar]
- 20.Tunstall MJ, Roberts A: Longitudinal coordination of motor output during swimming in Xenopus embryos. Proc R Soc Lond B 1991, 244:27–32. [DOI] [PubMed] [Google Scholar]
- 21.Li W-C, Sautois B, Roberts A, Soffe SR: Reconfiguration of a vertebrate motor network:specific neuron recruitment and context-dependent synaptic plasticity. J Neurosci 2007, 27:12267–12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W-C, Roberts A, Soffe SR: Locomotor rhythm maintenance: electrical coupling among premotor excitatory interneurons in the brainstem and spinal cord of young Xenopus tadpoles. J Physiol 2009, 587:1677–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. •.Borisyuk R, Merrison-Hort R, Soffe SR, Koutsikou S, Li W-C: To swim or not to swim: a population-level model of Xenopus tadpole decision making and locomotor behavior. BioSystems 2017, 161:3–14.The authors incorporate all previous experimental data in one model that simulates integration of different sensory signals resulting in choice of behavior (to locomote forward or backward or to halt) in the tadpole. This model is one of the most advanced and detailed among all vertebrate species.
- 24.Matsushima T, Grillner S: Neural mechanisms of intersegmental coordination in lamprey: local excitability changes modify the phase coupling along the spinal cord. J Neurophysiol 1992, 67: 373–388, 1992. [DOI] [PubMed] [Google Scholar]
- 25.Green CS, Soffe SR: Roles of ascending inhibition during two rhythmic motor patterns in Xenopus tadpoles. J Neurophys 1998, 79:2316–2328. [DOI] [PubMed] [Google Scholar]
- 26.Issberner JP, Sillar KT: The contribution of the NMDA receptor glycine site to rhythm generation during fictive swimming in Xenopus laevis tadpoles. Eur J Neurosci 2007, 26:2556–2564. [DOI] [PubMed] [Google Scholar]
- 27.Zelenin PV: Reticulospinal neurons controlling forward and backward swimming in the lamprey. J Neurophysiol 2011, 105:1361–1371. [DOI] [PubMed] [Google Scholar]
- 28.Kopell N, Ermentrout GB. Coupled oscillators and the design of central pattern generators. Math Biosci 1988, 89:14–23. [Google Scholar]
- 29. ••.Musienko PE, Zelenin PV, Lyalka VF, Gerasimenko YP, Orlovsky GN, Deliagina TG: Spinal and supraspinal control of the direction of stepping during locomotion. J Neurosci 2012, 32:17442–17453.In this study, locomotor movements evoked by stimulation of the mesencephalic locomotor region (MLR) and by epidural stimulation of the spinal cord and performed on the treadmill moving in different directions, as well as on the immobile surface or in the air, were compared. Results of this study allowed to formulate the functional model of the locomotor system generating stepping in different directions. It was also demonstrated that the MLR is a command center for forward locomotion only.
- 30.Rose DK, DeMark L, Fox EJ, Clark DJ, Wludyka P: A backward walking training program to improve balance and mobility in acute stroke: a pilot randomized controlled trial. JNPT 2018, 42:12–21. [DOI] [PubMed] [Google Scholar]
- 31.Foster H, DeMark L, Spigel PM, Rose DK, Fox EJ: The effects of backward walking training on balance and mobility in an individual with chronic incomplete spinal cord injury: a case report. Physiother Theiry Pract 2016, 32:536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. •.Merkulyeva N, Veshchitskii A, Gorsky O, Pavlova N, Zelenin PV, Gerasimenko Y, Deliagina TG, Musienko P: Distribution of spinal neuronal networks controlling forward and backward locomotion. J Neurosci 2018, 38:4695–4707.In this study, first, efficacy of epidural stimulation of different spinal segments to evoke forward and backward locomotion was tested. Second, by using c-Fos immunostaining, the distributions of spinal neuronal networks controlling forward and backward locomotion were compared. Results indicate that the neuronal networks responsible for forward locomotion are distributed broadly in the lumbosacral spinal cord, while the network determining backward direction of stepping is distributed from caudal part of L5 to caudal part of L7..
- 33.Zelenin PV, Musienko PE, Gorskii OV, Lyalka VF, Merkulyeva N, Gerasimenko YP, Orlovsky GN, Deliagina TG: Activity of individual spinal neurons during forward and backward locomotion. Soc Neurosci Abstr 2016, 535.02. [Google Scholar]
- 34.Ljunggren EE, Haupt S, Ausborn J, Ampatzis K, El Manira A: Optogenetic activation of excitatory premotor interneurons is sufficient to generate coordinated locomotor activity in larval zebrafish. J Neurosci 2014, 34:134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dougherty KJ, Zagoraiou L, Satoh D, Rozani I, Doobar S, Arber S, Jessell TM, Kiehn O: Locomotor rhythm generator linked to the output of spinal Shox2 excitatory interneurons. Neuron 2013, 80:920–933. [DOI] [PubMed] [Google Scholar]
- 36. •.Caldeira V, Dougherty KJ, Borgius L, Kiehn O: Spinal Hb9::Cre-derived excitatory interneurons contribute to rhythm generation in the mouse. Sci Rep 2017, 7:41369.Results of this study indicate that spinal excitatory Hb9 INs represent a distinct population of neurons that participates in generation of the locomotor rhythm. This study and study [35] demonstrate that in the mouse the rhythm-generating network contains neurons with different genetic identities.
- 37.Crone SA, Quinlan KA, Zagoraiou L, Droho S, Restrepo CE, Lundfald L et al. : Genetic ablation of V2a ipsilateral interneurons disrupts left-right locomotor coordination in mammalian spinal cord. Neuron 2008, 60:70–83. [DOI] [PubMed] [Google Scholar]
- 38.Björnfors R, El Manira A: Functional diversity of excitatory commissural interneurons in adult zebrafish. eLife 2016, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Talpalar AE, Bouvire J, Borgius L, Fortin G, Pierani A, Kiehn O: Dual-mode operation of networks involved in left-right alternation. Nature 2013, 500:85–88. [DOI] [PubMed] [Google Scholar]
- 40.Bellardita C, Kiehn O: Phenotypoc chracterization of speed-associated gait changes in mice reveals modular organization of locomotor networks. Curr Biol 2015, 25:1426–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vemula MDG, Lyalka VF, Talpalar AE, Kiehn O, Deliagina TG, Zelenin PV: Role of V0 commissural interneurons in control of basic motor behaviors. Soc Neurosci Abstr 2018, 151.01. [Google Scholar]
- 42.Sirota MG, Di Prisco GV, Dubuc R: Stimulation of the mesencephalic locomotor region elicits controlled swimming in semi-intact lampreys. Eur J Neurosci 2000, 12:4081–4092. [DOI] [PubMed] [Google Scholar]
- 43. •.Caggiano V, Leiras R, Goñi-Erro H, Masini D, Bellardita C, Bouvier J, Caldeira V, Fisone G, Kiehn O: Midbrain circuits that set locomotor speed and gait selection. Nature 2018, 553:455–460.With optogenetic methods, the authors demonstrate that glutamatergic neurons from different nuclei comprising the mesencephalic locomotor region play different functional roles. These results shed new light on the mechanisms of the gait control.
- 44. ••.Juvin L, Grätsch S, Trillaud-Doppia E, Gariépy JF, Büschges A, Dubuc R: A specific population of reticulospinal neurons controls the termination of locomotion. Cell Rep 2016, 15:2377–2386.In this study, three patterns of activity of reticulospinal neurons compatible with starting, maintaining, and stopping locomotion were revealed in the lamprey. Pharmacological activation and inactivation of reticulospinal neurons activated at the end of a locomotor bout showed that they determine locomotor offset. This result together with a similar result obtained in mice [49••] suggest an evolutionary conserved supraspinal system transmitting commands terminating locomotion.
- 45.Kimura Y, Satou C, Fujioka S, Shoji W, Umeda K, Ishizuka T, Yawo H, Higashijima S: Hindbrain V2a neurons in the excitation of spinal locomotor circuits during zebrafish swimming. Curr Biol 2013, 23:843–849. [DOI] [PubMed] [Google Scholar]
- 46. •.Capelli P, Pivetta C, Esposito MS, Arber S: Locomotor speed control circuits in the caudal brainstem. Nature. 2017, 551:373–377.By using optogenetic techniques, different functional roles of intermingled supraspinal neuronal subpopulations within the lateral paragigantocellular nucleus were revealed. It was demonstrated that glutamatergic neurons are essential to support high-speed locomotion, while glycinergic neurons can induce different forms of behavioural arrest. This study complements the other studies in the mouse [43•,49••] related to functional roles of different populations of brainstem neurons in control of locomotion.
- 47.Perrins R, Walford A, Roberts A: Sensory activation and role of inhibitory reticulospinal neurons that stop swimming in hatching frog tadpoles. J Neurosci 2002, 22:4229–4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li W-C, Perrins R, Walford A, Roberts A: The neuronal targets for GABAergic reticulospinal inhibition that stops swimming in hatching frog tadpoles. J Comp Physiol A 2003, 189:2937. [DOI] [PubMed] [Google Scholar]
- 49. ••.Bouvier J, Cagguano V, Leiras R, Caldeira V, Bellardita C, Balueva K, Fuchs A, Kiehn O: Descending command neurons in the brainstem that halt locomotion. Cell 2015, 163:11911203.The authors demonstrated that genetically identified population of reticulospinal neurons (V2a neurons) located in the rostral medulla transmit commands inhibiting spinal rhythm-generating networks and thus terminating locomotion.
- 50.Zelenin PV, Deliagina TG, Orlovsky GN, Karayannidou A, Stout EE, Sirota MG, Beloozerova IN: Activity of motor cortex neurons during backward locomotion. J Neurophysiol 2011, 105:2698–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kurz MJ, Wilson TW, Arpin DJ: Stride-time variability and sensorimotor cortical activation during walking. Neuroimage 2012, 59:1602–1607. [DOI] [PubMed] [Google Scholar]
- 52.Grillner S, Williams T, Lagerbäck PA: The edge cell, a possible intraspinal mechanoreceptor. Science 1984, 223:500–503. [DOI] [PubMed] [Google Scholar]
- 53.Böhm UL, Prendergast A, Djenoune L, Nunes Figueiredo S, Gomez J, Stokes C, Kaiser S, Suster M, Kawakami K, Charpentier M et al. : CSF-contacting neurons regulate locomotion by relaying mechanical stimuli to spinal circuits. Nat Commun 2016, 7:10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rossignol S, Dubuc R, Gossard JP: Dynamic sensorimotor interactions in locomotion. Physiol Rev 2006, 86: 89–154. [DOI] [PubMed] [Google Scholar]
- 55. •.Hsu LJ, Zelenin PV, Orlovsky GN, Deliagina TG: Supraspinal control of spinal reflex responses to body bending during different behaviors in lampreys. J Physiol 2017, 595:883–900.The present study has demonstrated that reflex response to body bending observed during forward swimming in lamprey is reversed during backward swimming, as well as during other forms of escape behavior. For the first time the neuronal mechanisms underlying reflex reversal in vertebrate animal have been characterized.
- 56.Ung RV, Imbeault MA, Ethier C, Brizzi L, Capaday C: On the potential role of the corticospinal tract in the control and progressive adaptation of the soleus h-reflex during backward walking. J Neurophysiol 2005, 94:1133–1142. [DOI] [PubMed] [Google Scholar]
- 57.Buford JA, Smith JL: Adaptive control for backward quadrupedal walking. III. Stunbling corrective reactions and cutaneous reflex sensitivity. J Neurophys 1993, 70:1102–1114 [DOI] [PubMed] [Google Scholar]