Abstract
During social interaction, the brain has the enormous task of interpreting signals that are fleeting, subtle, contextual, abstract, and often ambiguous. Despite the signal complexity, the human brain has evolved to be highly successful in the social landscape. Here, we propose that the human brain makes sense of noisy dynamic signals through accumulation, integration, and prediction, resulting in a coherent representation of the social world. We propose that successful social interaction is critically dependent on a core set of highly connected hubs that dynamically accumulate and integrate complex social information and, in doing so, facilitate social tuning during moment-to-moment social discourse. Successful interactions, therefore, require adaptive flexibility generated by neural circuits composed of highly integrated hubs that coordinate context-appropriate responses. Adaptive properties of the neural substrate, including predictive and adaptive coding, and neural reuse, along with perceptual, inferential, and motivational inputs, provide the ingredients for pliable, hierarchical predictive models that guide our social interactions.
Keywords: dynamic-integration theory, adaptive flexibility, temporal dynamics, social interaction, prediction
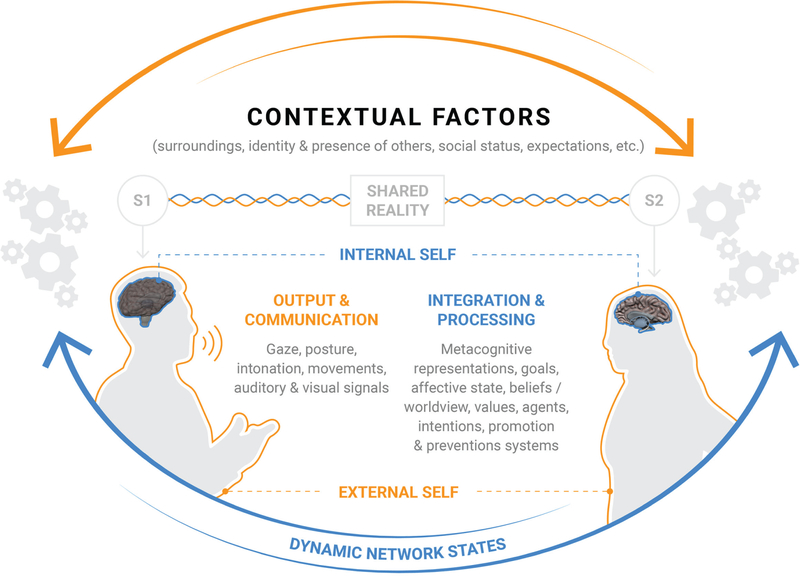
Consider a typical day-to-day social interaction between one speaker (Speaker 1) and another speaker (Speaker 2; Fig. 1). Speaker 1’s actions depend on the identity of Speaker 2 (e.g., the boss or intern), the context (e.g., work or a bar), and Speaker 1’s emotional state (e.g., nervous or excited). During these interactions, a complex exchange occurs: Speaker 1 and Speaker 2 play a game of social chess, in which Speaker 2 infers what Speaker 1 is thinking or intending, what Speaker 2 believes Speaker 1 believes Speaker 2 is thinking, and the social norms that indicate what Speaker 2 should be thinking. Speaker 1 and Speaker 2 continually integrate multiple signals while attempting to produce coherent responses. These signals are conveyed via multiple channels, such as vocal intonation, posture, expression, and eye direction, and the symmetry of this information determines the basis for understanding others. Speaker 1 and Speaker 2 may hide the contents of undesirable thoughts from one another or may suggest the existence of beliefs while strategizing to promote a desired image. These and possibly many other parallel processes initially occur subconsciously on the millisecond timescale, are subject to conscious adjustments, and involve complex integration and accumulation of multiple dynamic channels of information that result in the emergence of coherent social behavior.
Fig. 1.
Representation of components and processes during a conventional social exchange. Speaker 1 (S1), the sender, provides multimodal information in the form of sensory cues such as intonation, language, intensity, timing of verbal exchange and movements, posture, and gaze as signals. Speaker 2 (S2), the receiver, must recruit perceptual, inferential, learning, and other domain-general systems to generate and operate on an accurate interpretation of the signals. External influences such as context and internal influences, including goals and affective and motivational states, modulate processing in domain-general and social-cognitive systems.
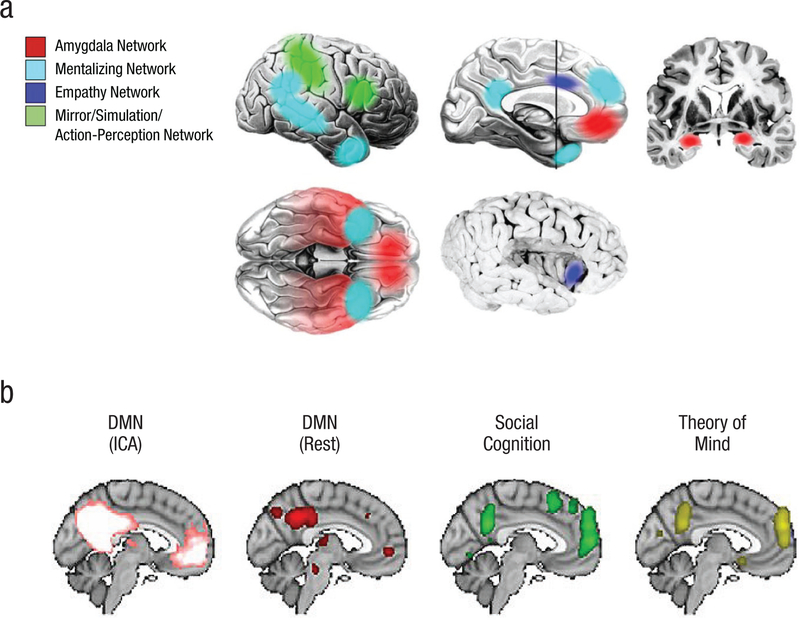
For two decades, research in the field of social neuroscience has mapped out a set of consistent brain regions involved in social cognition (Frith & Frith, 2010; Stanley & Adolphs, 2013). To date, more than a dozen discrete brain areas have been implicated in various aspects of social cognition (Fig. 2). Social processing occurs through coalitions of structures involved in sensory aspects of stimuli (e.g., fusiform face area, or FFA, superior temporal sulcus, or STS), emotional status of the social stimulus (e.g., amygdala), inferences concerning internal states and motives of agents (e.g., temporal parietal junction, or TPJ, ventromedial prefrontal cortex, or vmPFC), and social context (e.g., temporal pole). Medial prefrontal cortex (mPFC) may play a special role in social cognition, guiding, computing, and directing moment-to-moment aspects of social interaction. However, no theory currently exists to explain how these social brain areas work together to produce coherent social discourse.
Fig. 2.
Brain networks proposed to underlie separate processes that characterize the social brain, including affective, simulation, empathy, and mentalizing processes (a), and overlap of social networks with nodes of the default mode network (DMN) during active social cognition, contemplation of social interaction, and theory-of-mind processing (b). In (b), colors represent activation in the labeled networks. ICA = independent component analysis. Panel (a) reprinted with permission from Stanley and Adolphs (2013) and panel (b) reprinted with permission from Mars et al. (2012).
We propose a dynamic-integration theory (DIT) to describe the social brain. DIT proposes that the neural systems supporting social cognition reflect dynamic integration of inferential and sensory social information. The theory is based on three key elements. First, social behavior involves complex interaction between multiple neural circuits operating in parallel, involving extensively connected brain hubs, and social tuning reflects integration of dynamic interactions among these circuits toward metastable states (e.g., context-dependent stability that persists longer than most states but is shorter than a stable state) that map onto those of an interaction partner. Second, hyperconnected brain hubs play a role in orchestrating network flexibility that underwrites adaptive behaviors. Third, generative models govern a dynamic prediction process using internal knowledge, states, motivations, and goals that integrate with external cues to support context-apposite interaction, including the generation of inferences used to respond during social discourse. We move beyond the discrete mapping of the social brain to an understanding of how the network machinery might work during real-time social interactions.
Toward a DIT of the Social Brain
The social brain accumulates and integrates dynamic signals in real time through use of hyperconnected (the capacity to functionally connect with a significant number of nodes), flexible nodes with the capacity to dynamically reconfigure into different circuits. Activity over these circuits relays signals that produce time-sensitive and appropriate social behaviors. We highlight two problems the brain must overcome during real-time social discourse: (a) integration of internal states and external sensory information and (b) rapid social tuning.
Combining sensory and inferential systems
Social communication and perception require robust recognition and inferential systems, including FFA and STS, to integrate often subtle and disparate cues. The social sensory landscape is multimodal and sensitive to facial, vocal, postural, and gestural cues that may generate different interpretations depending on temporal occurrences during social interaction, depending on context, and across cultures (e.g., nodding vs. shaking of the head to indicate approval). Humans developed finely tuned cognitive-perceptual-inferential systems to discern “differences that make a difference” (Tononi, 2012, p. 293) and extract meaning from information by integrating external signals in the environment with internal knowledge, internal states, and ongoing predictions to form an integrated representation (Tononi, 2012; Tononi & Koch, 2015). This ability depends on highly sensitive and tuned neural machinery, including TPJ, vmPFC, posterior cingulate cortex (PCC)/precuneus, and temporal pole, that can efficiently and instantaneously categorize information for adaptive use in ongoing social interactions via mentalizing and simulation. Our model predicts that the integration of sensory information with internal representational and predictive models leads to convergence on a set of prioritized network transitions that evolve into a set of metastable states as an individual learns the social environment. Consistent network transition trajectories in response to similar cues and social situations produce reliable, context-appropriate behavioral output.
Social tuning, shared reality, and coherent social discourse
Effective social interaction involves communication of goals and motives that can be quickly and accurately understood (i.e., the individuals are “on the same page”). It also depends on social tuning, that is, the convergence of attitudes or more broadly an agreeable focus of attention (Shteynberg, 2010). Tuning is a prerequisite for achieving shared reality, a state in which individuals are motivated and successful in achieving shared and meaningful understanding, which should produce correlations with respect to synchronization or coupling of neuronal firing between such individuals. Shared-reality theory hinges on the perception of mutual sharing and the ability to communicate an experience or information about a target via motivationally aligned internal states (Echterhoff, Higgins, & Levine, 2009). Individuals with greater neuronal synchronization during shared reality are predicted to share more similar memories of events and detail, compared with individuals with less synchronization (Chen et al., 2017). Shared reality reinforces the confidence in perceptions and the underlying reality of both the social and physical worlds, leading to an increased likelihood of cohesive social interactions (McNally, Brown, & Jackson, 2012).
Three Principles of a DIT of the Social Brain
Social hubs as hyperconnected networks
Anatomical models describing the social brain reveal several regions that overlap with the default mode network (DMN), forcing a reconsideration of the functions of these nodes. The DMN is traditionally described as a network active in the absence of performing tasks. Some of these extensively connected overlapping hubs include PCC; precuneus; retrosplenial cortex; temporal pole (TP); inferior parietal lobule (IPL), including TPJ; and mPFC, many of which are active during mind wandering, self-processing, and examining the contents of episodic memory (Corbetta, Patel, & Shulman, 2008; Mars et al., 2012; Schilbach, Eickhoff, Rotarska-Jagiela, Fink, & Vogeley, 2008). These social hubs reduce their connectivity when internal attention is reoriented toward external objects or events. Densely connected nodes may direct or govern information gating and flow by tuning oscillatory activity, coupling field potentials, and establishing channels of synchrony (Fingelkurts & Fingelurts, 2017). The brain exploits phase locking, or synchrony, to efficiently communicate information across the brain. Synchrony is also observed between individuals, and is a reliable predictor of successful tuning (Stephens, Silbert, & Hasson, 2010). PCC/precuneus, mPFC, and IPL are strongly connected, but less connected with hippocampus and TP. Precuneus/PCC stands alone as the hub that interacts with all major DMN nodes, situating it as a major integrative center of high-order processing and a potential conductor of neural traffic associated with self-related processing and subjective self-awareness (Fransson & Marrelec, 2008). Precuneus/PCC forms a network with the amygdala associated with emotional states, which bear on the perceptions and orientation of an agent toward other social agents and may significantly drive the content and outcome of social interaction (Fang et al., 2013).
The tendency of these circuits to be active in social contexts, to display dense interconnectivity, and to shift toward a frontal bias (as found by Mars et al., 2012) suggests that intrinsic dynamics in the human brain are skewed toward considerations of the self and, in particular, self–other relations. Processing underwriting these capabilities is complex, especially in humans, with differences in cortical properties, including neuronal density and connectivity strength, corresponding to variation in high-level cognitive processes and social understanding (Lewis, Rezaie, Brown, Roberts, & Dunbar, 2011). TPJ and inferior parietal areas are critical components of networks that facilitate links and understanding between the signaler and the environment, given these regions’ putative role in mentalizing, action understanding, and interpretation (Ramsey, Cross, & Hamilton, 2011).
State transitions: context, states, and flexibility
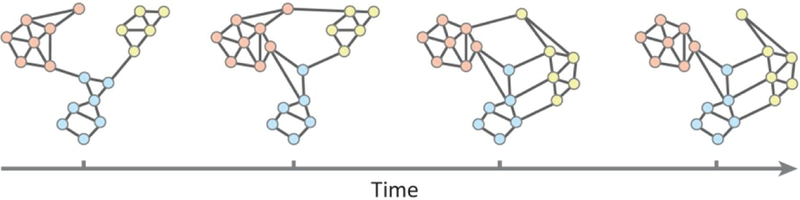
Signals, contexts, and motivational and affective states determine the orientation to social information and guide organization and tuning of neural activity. These processes result in a sequence of states that promote flexible social behaviors (Fig. 3). Accurate real-time prediction requires dynamic integration of multimodal information that is fed into an efficient simulation system capable of generating swift representations available for conscious inspection (e.g., to form hypothetical scenarios and counterfactuals to aid in developing optimal responses and enhance learning rates). Under this framework, context dictates the appropriateness of the same behavior in different circumstances, for example, inhibiting physical contact during a business meeting while facilitating contact at social events.
Fig. 3.
Reconfiguration of network modules: schematic illustrating how patterns of connectivity can change over time as someone learns. Each circle represents a node, and different node colors indicate membership to different modules. For example, network modules can separate (as the orange and yellow modules do) or coalesce (as the blue and yellow modules do). Reconfiguration can also occur at the level of single nodes, which might initially be part of one module, and then change to be part of another module (as indicated by the change in the node from the second to third frames, which starts off being affiliated with the orange module and ends being affiliated with the yellow module). Figure adapted with permission from Bassett and Mattar (2017).
Brain-state transitions require goal-oriented organizational principles that drive transition probabilities into transient, metastable states in network space. Some researchers have proposed psychological dimensions that map onto brain networks—such as valence, social domain, human specific, arousal, and agentic versus affiliative—and subdimensions including social warmth, competence, aggressiveness, self-focus, and empathy, among others (Fiske, Cuddy, & Glick, 2007; Grodin & White, 2015; Tamir, Thornton, Contreras, & Mitchell, 2016). Dimensional theories do not incorporate effects of dynamic contextual factors, which may contribute to state transitions, providing selection constraints on possible states. The organizational principals that govern adaptive and flexible transitions derive from relationships between an adaptively flexible system capable of processing a diverse range of inputs and affordances perceived in the environment subject to constraints imposed by available processing resources and current goals (Smith & Thelen, 2003).
Social interactions demand a distinct kind of adaptive flexibility from nonsocial complex cognitive operations as a result of the nature of the inputs processed. Predicting (often irrational) human beings is more complex than predicting physical (nonhuman) systems that consistently obey the same rules and the laws of physics, thus the processes necessary to successfully interact with human beings are more complex. We propose the added layer required to account for motivations and intentions of agents recruits additional nodes not recruited for other processes such as complex nonsocial decision making. Further, the inability to access the contents of other minds, the enormous state space of possible configurations and real-time reconfiguration of brain states presents a prediction challenge unique to social interaction. However, we propose nothing magical about the principles underlying social processing, as the flexible hub account has been stipulated for language (Fedorenko & Thompson-Schill, 2014) and cognition (Cole et al., 2013), more generally suggesting that certain principles are conserved across disparate functions.
Generative models, prediction, and learning in the social brain
Contemporary accounts of social learning increasingly refer to model-based processes, which assume explicit, cognitive understanding of the structure and causal mechanisms of an environment (Dunne, D’Souza, & O’Doherty, 2016). Recent evidence indicates that social learning is mediated by different neuromodulatory systems, which interact to improve the accuracy of a cognitive model. These systems contribute to hierarchical updating processes by representing prediction errors used by higher order cortical areas to assess ongoing binary true/false judgments and comprehensive traits such as trustworthiness (Diaconescu et al., 2017). While the model-based account is more effective than competing constructs, in its conventional form it will likely fall short as an adequate model when applied to ecological social situations. This is because internal states, including emotion, motivation, and ongoing processing more generally, are not always explicitly modeled.
We propose that model-based social learning can be described in terms of a generative model. Generative models can generate data and are based on Bayesian principles, updating a model of joint probabilities, possible outcomes, and input using a hierarchical and iterative process. Under this formulation, predictive coding is based on prediction errors signaled and integrated back into the model as part of a dynamic and flexible recalibration process to achieve greater accuracy. The model takes into account episodic information and is continuously updated on the basis of observed contingencies and exploratory outcomes via simulation. Humans are capable of “simulated prediction” in the absence of external sensory input, instead using internally defined inputs to generate novel outputs that in humans can be consciously inspected, manipulated, and fed back into generative models to make different meta simulated predictions. These predictions aid in the uniquely difficult task of online social decision making.
Social interactions are essentially social decision-making problems. These decision processes differ from nonsocial decision processes in that the consequences, or outcomes are of a different character than decisions for which the certainty of outcomes may near unity. For example, deciding which car to buy among several choices involves explicit calculations that yield specific and consistent results. If we provide the same inputs into the decision-making problem today, tomorrow, and the next day, we will invariably arrive at the same answer. This is not necessarily true about social interaction. The same behavioral and perceptual inputs (e.g., a similar interaction with another agent) may probabilistically yield a range of responses, resulting in a more complex decision system that involves moving targets for which the brain attempts to compensate. The two-way interaction with another agent (a) requires real-time updating of value based on feedback and (b) a mechanism to account for and dynamically predict the effects one’s behavioral output has on another agent.
How Do Dynamic Interactions Between Social Brain Circuits Tune Social Interaction?
What are the fundamental mechanisms by which the brain dynamically reconfigures to create social meaning? We suggest that social tuning depends on synchrony among interaction partners underwritten by dynamic and flexible interactions between large-scale neural circuits, a process made mathematically concrete using the emerging conceptual framework of network neuroscience (Bassett & Sporns, 2017). In this view, brain regions are treated as nodes in a graph, connected by edges that encode structural links (via white matter) or functional connections (estimated by similarity in time-varying patterns of activity measured by functional MRI, or fMRI; Bullmore & Bassett, 2011). A common and highly reproducible finding in these network representations of brain structure and function is the presence of network modules: groups of brain regions that tend to be connected to one another in fMRI-measured functional circuits that perform specific types of processes. Examples of network modules present at rest include the visual, auditory, motor, default mode, fronto-parietal, cingulo-opercular, salience, and dorsal/ ventral attention systems. Network modules present during tasks can differ from those present during rest, and indeed, different tasks can be associated with different levels of integration or segregation between resting-state modules (Mattar, Cole, Thompson-Schill, & Bassett, 2015).
Recent work has extended this general notion to track brain networks over time, as patterns of connectivity change in response to the external world (Medaglia, Lynall, & Bassett, 2015) or to internal reflections (Hutchison et al., 2013). The mathematics of temporal networks is used to describe the evolution of these networks (Holme & Saramaki, 2012; Mucha, Richardson, Macon, Porter, & Onnela, 2010). Network flexibility is mathematically defined as the frequency with which brain regions switch allegiance to different network modules, or putative cognitive systems, by changing their pattern of fMRI-measured functional connections (Bassett et al., 2011). Individual differences in network flexibility are associated with attention (Shine et al., 2016), working memory (Braun et al., 2015), learning (Bassett et al., 2011; Bassett et al., 2013), linguistic processing (Chai, Mattar, Blank, Fedorenko, & Bassett, 2016), and mood, arousal, and fatigue (Betzel, Satterthwaite, Gold, & Bassett, 2017) and can be modulated by task (Telesford et al., 2016), drugs, and disease (Braun et al., 2016). Network flexibility is also positively correlated with individual differences in cognitive flexibility (Braun et al., 2015), suggesting a potential role in the state transitions that promote adaptive social behaviors.
Intuitively, a flexible network is capable of integrating complex information in a dynamic manner to enable adaptive functions. This capability, underwritten in part by neural reuse, or the idea that a brain node or region participates in multiple functions depending on context and availability, is critically necessary for successfully navigating the complexities of social interactions (Anderson, 2010). Across nonsocial task contexts, flexible regions are largely located in association areas (particularly in frontal cortex) and subcortical structures (Bassett et al., 2011) thought to be critical for large-scale cognitive computations beyond simple task performance (Bassett et al., 2013). Flexible regions may form a dynamic “periphery” of domain-general areas that change patterns of coactivation with other regions depending on task demands, while rigid regions form a dynamic “core” of domain-specific areas that consistently coactivate with each other across tasks (Fedorenko & Thompson-Schill, 2014), While these approaches and theories have yet to be applied to neuroimaging data acquired during the performance of tasks requiring social cognition, they offer simple and appropriate putative mechanisms for brain dynamics supporting social meaning and tuning, which could be directly tested in future experiments.
Concluding Remarks
The brain is not a passive processing machine like a computer waiting for input but rather an ongoing simulating, searching, and predicting machine that refines its structural and functional architecture to optimize learning and predictive processes according to environmental demands. We propose that during social interaction, neural circuits must be dynamic, predictive, and contextually nuanced. They must include core systems and processes including motivation systems, metacognitive awareness, the ability to signal internal states, and the ability to guide decision processes that determine when to socially tune or antitune. The inputs to these systems shape its development and optimization parameters, and include cultural, familial, and interpersonal environments and relationships that act as strong filters with respect to social information processing. Specifically in humans, social reasoning and circuit recruitment appears to be context dependent: whether or not the subject of an interaction possesses or is perceived to possess relevance for current or future social actions (Carter, Bowling, Reeck, & Huettel, 2012).
Gaining a better understating of the dynamics of the social brain might lead to a further understanding of the social impairments observed in autisms disorders. For example, central-coherence theory proposes that people diagnosed with autism tend to fragment the world into small parts and “cannot see the forest for the trees” (Frith, 1989; Happe, 1999). According to DIT, the central hubs of connectivity and stable nodes are different from those of normal individuals, manifesting greater coherence within local nodes, providing enhanced processing within the local domains such as spatial, numerical, and abstract computation, at the cost of the ability to efficiently integrate information from other more remote nodes to generate (e.g., theory of mind). The inability to update in proportion to information content results in impairments in cognitive flexibility manifest as difficulty in shifting resources and adapting to dynamic environments that require coordinated activity in attention, representation, and planning circuits (Dajani & Uddin, 2015; Yahata et al., 2016). Too much flexibility as observed in schizophrenia, characterized by inappropriate or abnormal network connectivity and switching dynamics during working memory, results in aberrant interpretation of both external and internal inputs and inability to exert cognitive control, leading to inappropriate social (and other) behavioral profiles (Braun et al., 2016).
Footnotes
Declaration of Conflicting Interests
The author(s) declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
References
- Anderson ML (2010). Neural reuse: A fundamental organizational principle of the brain [Target article and discussion]. Behavioral and Brain Sciences, 33, 245–313. doi: 10.1017/S0140525X10000853 [DOI] [PubMed] [Google Scholar]
- Bassett DS, & Mattar MG (2017). A network neuroscience of human learning: Potential to inform quantitative theories of brain and behavior. Trends in Cognitive Sciences, 21, 250–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, & Sporns O (2017). Network neuroscience. Nature Neuroscience, 20, 353–364. doi: 10.1038/nn.4502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Wymbs NF, Porter MA, Mucha PJ, Carlson JM, & Grafton ST (2011). Dynamic reconfiguration of human brain networks during learning. Proceedings of the National Academy of Sciences, USA, 108, 7641–7646. doi: 10.1073/pnas.1018985108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Wymbs NF, Rombach MP, Porter MA, Mucha PJ, & Grafton ST (2013). Task-based coreperiphery organization of human brain dynamics. PLOS Computational Biology, 9(9), Article e1003171. doi: 10.1371/journal.pcbi.1003171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzel RF, Satterthwaite TD, Gold JI, & Bassett DS (2017). Positive affect, surprise, and fatigue are correlates of network flexibility. Scientific Reports, 7, Article 520. doi: 10.1038/s41598-017-00425-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U, Schafer A, Bassett DS, Rausch F, Schweiger JI, Bilek E, … Tost H (2016). Dynamic brain network reconfiguration as a potential schizophrenia genetic risk mechanism modulated by NMDA receptor function. Proceedings of the National Academy of Sciences, USA, 113, 12568–12573. doi: 10.1073/pnas.1608819113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U, Schafer A, Walter H, Erk S, Romanczuk-Seiferth N, Haddad L, … Bassett DS (2015). Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proceedings of the National Academy of Sciences, USA, 112, 11678–11683. doi: 10.1073/pnas.1422487112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore ET, & Bassett DS (2011). Brain graphs: Graphical models of the human brain connectome. Annual Review Clinical Psychology, 7, 113–140. doi: 10.1146/annurev-clinpsy-040510-143934 [DOI] [PubMed] [Google Scholar]
- Carter RM, Bowling DL, Reeck C, & Huettel SA (2012). A distinct role of the temporal-parietal junction in predicting socially guided decisions. Science, 337, 109–111. doi: 10.1126/science.1219681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai LR, Mattar MG, Blank IA, Fedorenko E, & Bassett DS (2016). Functional network dynamics of the language system. Cerebral Cortex, 26, 4148–4159. doi: 10.1093/cercor/bhw238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Leong YC, Honey CJ, Yong CH, Norman KA, & Hasson U (2017). Shared memories reveal shared structure in neural activity across individuals. Nature Neuroscience, 20, 115–125. doi: 10.1038/nn.4450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, & Braver TS (2013). Multi-task connectivity reveals flexible hubs for adaptive task control. Nature Neuroscience, 16, 1348–1355. doi: 10.1038/nn.3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Patel G, & Shulman GL (2008). The reorienting system of the human brain: From environment to theory of mind. Neuron, 58, 306–324. doi: 10.1016/j.neuron.2008.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajani DR, & Uddin LQ (2015). Demystifying cognitive flexibility: Implications for clinical and developmental neuroscience. Trends in Neurosciences, 38, 571–578. doi: 10.1016/j.tins.2015.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaconescu AO, Mathys C, Weber LAE, Kasper L, Mauer J, & Stephan KE (2017). Hierarchical prediction errors in midbrain and septum during social learning. Social Cognitive & Affective Neuroscience, 12, 618–634. doi: 10.1093/scan/nsw171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne S, D’Souza A, & O’Doherty JP (2016). The involvement of model-based but not model-free learning signals during observational reward learning in the absence of choice. Journal of Neurophysiology, 115, 3195–3203. doi: 10.1152/jn.00046.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echterhoff G, Higgins ET, & Levine JM (2009). Shared reality: Experiencing commonality with others’ inner states about the world. Perspectives on Psychological Science, 4, 496–521. doi: 10.1111/j.1745-6924.2009.01161.x [DOI] [PubMed] [Google Scholar]
- Fang Z, Zhu S, Gillihan SJ, Korczykowski M, Detre JA, & Rao H (2013). Serotonin transporter genotype modulates functional connectivity between amygdala and PCC/PCu during mood recovery. Frontiers in Human Neuroscience, 7, Article 704. doi: 10.3389/fnhum.2013.00704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, & Thompson-Schill SL (2014). Reworking the language network. Trends in Cognitive Sciences, 18, 120–126. doi: 10.1016/j.tics.2013.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingelkurts AA, & Fingelurts AA (2017). Information flow in the brain: Ordered sequences of metastable states. Information, 8(1), Article 22. doi: 10.3390/info8010022 [DOI] [Google Scholar]
- Fiske ST, Cuddy AJC, & Glick P (2007). Universal dimensions of social cognition: Warmth and competence. Trends in Cognitive Sciences, 11, 77–83. doi: 10.1016/j.tics.2006.11.005 [DOI] [PubMed] [Google Scholar]
- Fransson P, & Marrelec G (2008). The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. NeuroImage, 42, 1178–1184. doi: 10.1016/j.neuroimage.2008.05.059 [DOI] [PubMed] [Google Scholar]
- Frith U (1989). Autism: Explaining the enigma Oxford, England: Basil Blackwell. [Google Scholar]
- Frith U, & Frith C (2010). The social brain: Allowing humans to boldly go where no other species has been. Philosophical Transactions of the Royal Society B: Biological Sciences, 365, 165–176. doi: 10.1098/rstb.2009.0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodin EN, & White TL (2015). The neuroanatomical delineation of agentic and affiliative extraversion. Cognitive, Affective, and Behavioral Neuroscience, 15, 321–334. doi: 10.3758/s13415-014-0331-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happe F (1999). Autism: Cognitive deficit or cognitive style? Trends Cognitive Sciences, 3, 216–222. [DOI] [PubMed] [Google Scholar]
- Higgins ET (1998). Promotion and prevention: Regulatory focus as a motivational principle. In Zanna MP (Ed.), Advances in experimental social psychology (Vol. 30, pp. 1–46). San Diego, CA: Academic Press. [Google Scholar]
- Holme P, & Saramaki J (2012). Temporal networks. Physics and Society, 519, 97–125. [Google Scholar]
- Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, … Chang C (2013). Dynamic functional connectivity: Promise, issues, and interpretations. NeuroImage, 80, 360–378. doi: 10.1016/j.neuroimage.2013.05.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PA, Rezaie R, Brown R, Roberts N, & Dunbar RI (2011). Ventromedial prefrontal volume predicts understanding of others and social network size. NeuroImage, 57, 1624–1629. doi: 10.1016/j.neuroimage.2011.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Neubert F-X, Noonan MP, Sallet J, Toni I, & Rushworth MFS (2012). On the relationship between the “default mode network” and the “social brain.” Frontiers in Human Neuroscience, 6, Article 189. doi: 10.3389/fnhum.2012.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattar MG, Cole MW, Thompson-Schill SL, & Bassett DS (2015). A functional cartography of cognitive systems. PLOS Computational Biology, 11(12), Article e1004533. doi: 10.1371/journal.pcbi.1004533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally L, Brown SP, & Jackson AL (2012). Cooperation and the evolution of intelligence. Proceedings of the Royal Society B: Biological Sciences, 279, 3027–3034. doi: 10.1098/rspb.2012.0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medaglia JD, Lynall ME, & Bassett DS (2015). Cognitive network neuroscience. Journal of Cognitive Neuroscience, 27, 1471–1491. doi: 10.1162/jocn_a_00810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucha PJ, Richardson T, Macon K, Porter MA, & Onnela JP (2010). Community structure in time-dependent, multiscale, and multiplex networks. Science, 328, 876–878. doi: 10.1126/science.1184819 [DOI] [PubMed] [Google Scholar]
- Ramsey R, Cross ES, & Hamilton AF (2011). Eye can see what you want: Posterior intraparietal sulcus encodes the object of an actor’s gaze. Journal of Cognitive Neuroscience, 23, 3400–3409. doi: 10.1162/jocn_a_00074 [DOI] [PubMed] [Google Scholar]
- Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, & Vogeley K (2008). Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Consciousness and Cognition, 17, 457–467. doi: 10.1016/j.concog.2008.03.013 [DOI] [PubMed] [Google Scholar]
- Shine JM, Bissett PG, Bell PT, Koyejo O, Balsters JH, Gorgolewski KJ, … Poldrack RA (2016). The dynamics of functional brain networks: Integrated network states during cognitive task performance. Neuron, 92, 544–554. doi: 10.1016/j.neuron.2016.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shteynberg G (2010). A silent emergence of culture: The social tuning effect. Journal of Personality and Social Psychology, 99, 683–689. doi: 10.1037/a0019573 [DOI] [PubMed] [Google Scholar]
- Smith LB, & Thelen E (2003). Development as a dynamic system. Trends in Cognitive Sciences, 7, 343–348. [DOI] [PubMed] [Google Scholar]
- Stanley DA, & Adolphs R (2013). Toward a neural basis for social behavior. Neuron, 80, 816–826. doi: 10.1016/j.neuron.2013.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens GJ, Silbert LJ, & Hasson U (2010). Speaker-listener neural coupling underlies successful communication. Proceedings of the National Academy of Sciences, USA, 107, 14425–14430. doi: 10.1073/pnas.1008662107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir DI, Thornton MA, Contreras JM, & Mitchell JP (2016). Neural evidence that three dimensions organize mental state representation: Rationality, social impact, and valence. Proceedings of the National Academy of Sciences, USA, 113, 194–199. doi: 10.1073/pnas.1511905112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telesford QK, Lynall ME, Vettel J, Miller MB, Grafton ST, & Bassett DS (2016). Detection of functional brain network reconfiguration during task-driven cognitive states. NeuroImage, 142, 198–210. doi: 10.1016/j.neuroimage.2016.05.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G (2012). Integrated information theory of consciousness: An updated account. Archives Italiennes de Biologie, 150, 293–329. [PubMed] [Google Scholar]
- Tononi G, & Koch C (2015). Consciousness: Here, there and everywhere? Philosophical Transactions of the Royal Society B: Biological Sciences, 370(1668). doi: 10.1098/rstb.2014.0167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahata N, Morimoto J, Hashimoto R, Lisi G, Shibata K, Kawakubo Y, … Kawato M (2016). A small number of abnormal brain connections predicts adult autism spectrum disorder. Nature Communications, 7, Article 11254. doi: 10.1038/ncomms11254 [DOI] [PMC free article] [PubMed] [Google Scholar]
Recommended Reading
- Anderson ML (2011, February 23). The dynamic brain: What your brain is doing when you’re not doing anything. Psychology Today Retrieved from https://www.psychologytoday.com/blog/after-phrenology/201102/the-dynamicbrain. An accessible and revolutionary exposé arguing for the brain as an ongoing process and not a static, passive computerlike machine waiting for input.
- Dikker S, Wan L, Davidesco I, Kaggen L, Oostrik M, McClintock J, … Poeppel D (2017). Brain-to-brain synchrony tracks real-world dynamic group interactions in the classroom. Current Biology, 27, 1375–1380. Realworld evidence of social tuning as a prerequisite for enhanced communication and understanding. [DOI] [PubMed] [Google Scholar]
- Hari R, Henriksson L, Malinen S, & Parkkonen L (2015). Centrality of social interaction in human brain function. Neuron, 88, 181–193. A review highlighting the need for and benefits of studying social interaction in dynamic environments. [DOI] [PubMed] [Google Scholar]
- Seth AK (2014). A predictive processing theory of sensorimotor contingencies: Explaining the puzzle of perceptual presence and its absence in synesthesia. Cognitive Neuroscience, 5, 97–118. A thorough discussion regarding the fit of generative models to not just perception but prediction and simulation. [DOI] [PMC free article] [PubMed] [Google Scholar]