Abstract
Background
Future infertility is a significant concern for survivors of childhood and adolescent cancer. Children and adolescents may have the opportunity to undergo fertility preservation (FP) procedures (which preserve gonadal tissue or gametes for future use) prior to the cancer treatment. However, the decision is very complex, as it is often made by parents as proxy decision makers at the time of cancer diagnosis, and is time-sensitive (needing to occur before the cancer treatment begins). Furthermore, FP procedures in children and adolescents are experimental and cannot guarantee future fertility. An uninformed decision may result in future decision regret.
Objective
This study aimed to assess the acceptability, usability, and feasibility of a Web-based FP decision aid (DA) in parents of children and adolescents with cancer and clinicians. Fertility knowledge and decision regret were compared in families who reviewed the DA compared with those who did not.
Methods
The Web-based DA was developed according to the International Patient Decision Aid Standards. A cross-sectional study of parents of patients with cancer, who discussed fertility, and clinicians at a tertiary children’s hospital was undertaken. The acceptability, usability, and feasibility of the DA were assessed using a pre-post survey design. Measures included the validated Decision Regret Scale, a purpose-designed fertility-related knowledge scale, questions regarding satisfaction with the DA, and open-ended responses for additional feedback. Furthermore, clinicians involved in FP were also invited to review the DA.
Results
We enrolled 34 parents and 11 clinicians in this study. Participants who reviewed the DA (15 parents and 11 clinicians) expressed satisfaction with its content and functionality. Parents reported an improved understanding of cancer treatments, infertility, and FP procedures and did not report greater decision regret after DA review. Most parents (13/15, 86%) would recommend the DA to other parents. All clinicians had a consensus that this was a valid and relevant information source for all involved in fertility care.
Conclusions
It is an international standard of care to discuss the impact of cancer treatment on fertility before cancer treatment. This is the first fertility DA for parents of children and adolescents with cancer and is found to be relevant and acceptable by parents and clinicians. This DA has the potential to help support parents to make informed fertility-related decisions for their children and adolescents. However, future research is needed to assess the impact of the DA on prospective decision making.
Keywords: adolescent, cancer, decision aid, fertility preservation, pediatric, shared decision making, Values Clarification Exercise
Introduction
Australia has one of the highest incidences of childhood cancer worldwide, with >1500 children (0-12 years) and adolescent-young-adult patients (13-25 years) newly diagnosed annually [1,2]. Improvements in care have seen the 5-year survival rate surpass 83%, after which, the lifetime survival is comparable to that of their healthy peers [2,3]. Attention must be given to the late effects of cancer diagnosis and treatment in this growing population of survivors [2].
Common cancer treatments (alkylating chemotherapies and radiotherapy) may have gonadotoxic effects that can damage the reproductive system, resulting in infertility or sterility [4-8]. The risk to fertility is variable and difficult to exactly predict [9,10]. For some patients, this risk is negligible; for others, however, infertility may be almost certain [8,9]. Treatment regimen and dosage, sex, age at diagnosis, pubertal status, and disease are factors that may affect the risk of infertility. Survivors of childhood cancer regard infertility as one of their greatest concerns [7,8,10]. Fertility preservation (FP) procedures may be offered to patients at risk of infertility when medically appropriate [7].
Research regarding the application and efficacy of FP in humans is ongoing [8,11]. Gender and pubertal status determine the availability and accessibility of FP. For females, oocyte and embryo cryopreservation are the most effective means of preserving fertility but not possible in children [7,11]. In addition, ovarian tissue cryopreservation is available, although still considered experimental, with only 130 live births recorded to date [12]. For males, semen cryopreservation is currently the only viable FP procedure available. Preservation of immature testicular tissue is yet to be proven successful in humans [13]. Therefore, ovarian and testicular tissue harvesting procedures are usually only offered to children and adolescents under special governance [8,14].
Many young people are simply too young or feel too overburdened to make the fertility decision themselves and are glad that their parents take the initiative [15,16]. Overall, 48% of young people and 42% of parents experience posttraumatic stress symptoms around the diagnosis. Parental consent for FP decisions is usually required for all children under the age of 18 years (because of vulnerability), with only 33% of boys aged <12 years able to appropriately comprehend fertility information [17]. The importance of parental input is further highlighted that even in young adults (age≤25 years), parents contribute to fertility decisions in 82% of cases. Thus, a large weight of responsibility sits with parents. The potential procedure-related risks, time delays in cancer treatment, and the potential message of false hope regarding cancer survival or the success of FP procedures must be considered [18]. Of concern, is the potential misinterpretation of risks and unrealistic expectations of FP success by patients and their parents [11,18]. The clarification of these factors is of vital importance and may be aided through the provision of balanced and understandable information.
Much of the information regarding FP is new to parents, and the involvement of patients in the decision-making process is variable [8,19]. Patients and families have limited time to consider their options as FP procedures are best undertaken prior to the commencement of gonadotoxic cancer treatments, soon after a cancer diagnosis [8,10]. In addition, there is often no clearly preferable decision, with each FP option having its own inherent risks and benefits that need to be considered with respect to personal values [20]. Thus, the decision to forgo or to pursue FP is difficult, and in this ethically complex scenario, decision makers require decision support.
Decision aids (DA) are educational tools designed to complement clinician counseling and facilitate difficult preference-sensitive decisions [21]. DAs have been shown across a range of health care choices to reduce the decisional conflict (a measure of uncertainty), increase decision satisfaction and knowledge, and minimize future regret, without increasing harm [21]. DAs are now considered to be the “gold standard” approach to shared decision making for complex health care decisions [21,22]. Considering the complexity of FP decision making in the pediatric setting, a DA could provide standardized, evidence-based decision support for parents of pediatric patients with cancer. To the best of our knowledge currently no FP DA is available for use in this clinical setting. Thus, this study aimed to develop and assess the acceptability, usability, and feasibility of a Web-based FP DA for parents of children with cancer who had previously made a fertility discussion as part of their clinical care. In addition, this study compared fertility knowledge and decision regret around their decision in families who reviewed the DA compared with those who did not. Finally, this study aimed to assess the clinician acceptance of the DA by its perceived usefulness and whether they would recommend its use in the clinical practice.
Methods
Participants and Study Design
This study used a cross-sectional pre-post survey design. Parents of patients with cancer (aged 0-18 years) diagnosed between December 2010 and December 2015 at The Royal Children’s Hospital, Melbourne, were invited to participate. In addition, clinicians involved in oncofertility (gynecologists, endocrinologists, oncologists, ethicists, pediatric surgeons, and in vitro fertilization specialists) were invited to review the DA. All parents had previously discussed their child’s fertility with their clinical team. This retrospective study design aimed to minimize the risk to new patients, which is typical for DA pilot studies [9,23]. Ethics approval was obtained from The Royal Children’s Hospital Human Research Ethics Committee (36016A).
Participant Population
Parents were eligible for participation if their child’s cancer diagnosis occurred within 5 years before December 2015; the child was not on active treatment; they previously had an FP discussion; were proficient in English; and had consented to be contacted for future research. Families where the child was palliative or deceased were excluded. Furthermore, families were excluded if the treating oncologist felt it was clinically inappropriate for them to be contacted for research purposes. The child’s risk of infertility was classified as low (<20%), medium (20%-80%), or high (>80%), according to previously published risk tables [9].
Clinician Population
Of 24 invited clinicians, 46% (11/24) consented to participate in this study and completed a post-DA review survey. Of these, 82% (9/11) of the clinicians were involved in FP consultations and were from the disciplines of gynecology, endocrinology, urology, oncology, and clinical ethics.
Procedure
All eligible parents were provided with an invitation pack by the researcher, containing an introductory letter, information sheet, consent form, and pre-DA questionnaire. Once consenting parents (1 parent per family) completed the questionnaire, they were given access to the Web-based DA and the post-DA questionnaire.
Clinicians involved in fertility consultations and oncological care were approached either in person or through email. If clinicians consented, they either met for an informal discussion and completed the survey or reviewed the DA online and completed the survey online.
Data Analysis
Data were analyzed using SPSS V22 (IBM Corp, Armonk, NY, USA). Descriptive statistics (means, ranges, and SDs) were calculated to describe sample characteristics and response rates and to assess DA acceptability. In addition, t tests were used to compare normally distributed data. Furthermore, thematic analysis was conducted on open-ended responses.
Decision Aid
Development of the electronic DA was theoretically guided by Coulter et al [24] and the International Patient Decision Aid Standards, an evidence-based theoretical model for effective behavioral interventions [23,25]. The DA content was informed by two formalized information needs assessments, which were conducted at the Royal Children’s Hospital [26,27]; and input from FP Taskforce consumer group. The DA was developed using the WordPress Content Management System (a software application that allows users to design and manage Web-content and materials). The DA has 11 chapters with 22 pages (Multimedia Appendix 1). Where appropriate, content was divided according to the patients’ gender. Furthermore, medical illustrations and infographics were included to help quantify the risks of various outcomes and enhance patients’ understanding [28].
A novel Web-based Values Clarification Exercise (VCE) was developed for this DA. Questions were designed to help parents clarify the importance of their child’s fertility in the context of cancer diagnosis and treatment planning. Parents rated sex- and age-specific statements on a Likert-type scale with responses ranging from “strongly disagree” to “strongly agree.” Results were scored from −2 to +2. Higher scores (eg, +2) indicated that fertility was considered a priority, whereas lower scores (eg, −2) indicated that fertility was not a priority; 0 was considered neutral. Figure 1 provides an example of the VCE questions. Parents were provided with a results summary bar (Figure 2), where their mean score represented the priority of FP for the parent and SD represented the variability around that score. The mean VCE score is plotted as a percentage of 100, where “not a priority” ranges from 0% to 33%, “neutral” ranges from 34% to 67%, and “priority” ranges from 68% to 100%. Color spread is calculated using SD, adjusted to a range between 10 and 50 points, and spread from the central score in both directions.
Figure 1.

Example questions from the values clarification exercise.
Figure 2.
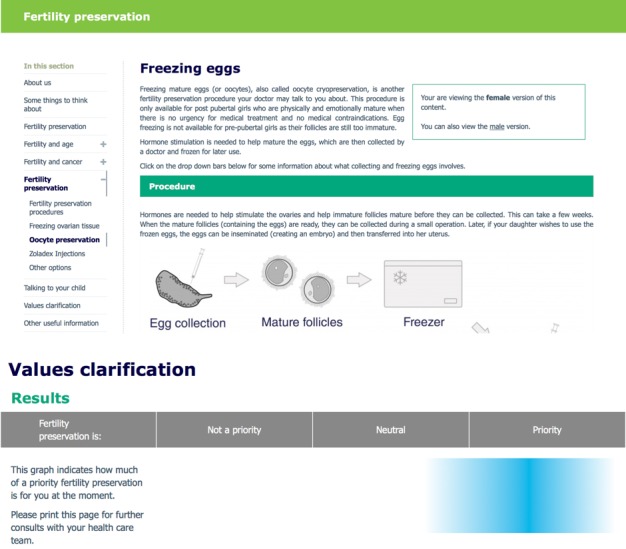
Decision aid and values clarification results bar.
Survey Measures
Questionnaires were adapted from those previously used in similar studies [25,29]. Multimedia Appendix 2 outlines the outcome measures assessed. The clinician survey included a question of whether the clinician would recommend the DA to patients and an open-ended question about future improvements and thoughts.
Results
Response Rates
In this study, 74 families were eligible for participation. Of these, 34 parents consented to participate and completed the pre-DA questionnaire (survey 1). Then, 19 parents withdrew after completing survey 1, citing time constraints (n=5), or did not respond to follow-up (n=14). Subsequently, 15 parents reviewed the DA and completed the post-DA questionnaire (Figure 3).
Figure 3.
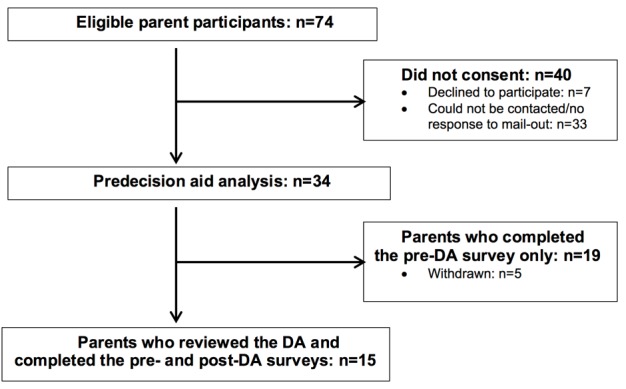
Parent participant recruitment flowchart.
Characteristics of Parents Who Reviewed the Decision Aid
The mean age of parents was 43 years. Compared with parents who only completed survey 1 (n=19), those who reviewed the DA (n=15) were more likely to be in part-time or full-time employment and have a higher level of education (Table 1). In addition, the distribution of infertility risk differed markedly between the 2 groups (Table 2). Otherwise, the 2 groups were similar with respect to demographics and concerns around their child’s future fertility.
Table 1.
Demographic characteristics of parents of children and adolescents with cancer.
| Characteristics | Total parents (N=34) | Completed the pre-DAa survey only (n=19) | Reviewed the DA and completed the pre- and post-DA surveys (n=15) | P valueb | |||||
| Age in years, mean (SD); range | 41.5 (11.1); 27-57 | 43.2 (8.1); 27-57 | 42.6 (7.0); 31-49 | .28 | |||||
| Age at child’s diagnosis in years, mean (SD); range | 39.3 (11.2); 25-55 |
40.8 (7.7); 25-55 | 40.5 (6.7); 30-47 | .19 | |||||
| Decision regret score, mean (SD); range | 15.6, (20.5); 0-95 | 16.8 (23.5); 0-95 | 16.5 (18.6); 0-50 | .74 | |||||
| Country of birth, n (%) | |||||||||
|
|
Australia | 24 (71) | 16 (67) | 8 (33) | .84 | ||||
|
|
Other | 10 (29) | 3 (30) | 7 (70) | |||||
| Primary spoken language at home, n (%) | |||||||||
|
|
English | 29 (85) | 18(62) | 11(38) | .51 | ||||
|
|
Other | 5 (15) | 1 (20) | 4 (80) | |||||
| Relationship status, n (%) |
|
||||||||
|
|
Married or de facto | 25 (74) | 12 (48) | 13 (52) | .26 | ||||
|
|
Separated or divorced | 7 (21) | 5 (71) | 2 (29) | |||||
|
|
Unknown | 2 (6) | 2 (100) | 0 (0) | |||||
| Highest level of education, n (%) | |||||||||
|
|
≤Year 10 | 6 (18) | 4 (67) | 2 (33) | .04c | ||||
|
|
Year 12 | 4 (12) | 2 (50) | 2 (50) | |||||
|
|
Technical and Further Education certificate or diploma | 4 (12) | 3 (75) | 1 (25) | |||||
|
|
Bachelor’s degree | 12 (35) | 2 (17) | 10 (83) | |||||
|
|
Postgraduate degree | 7 (21) | 7 (100) | 0 (0) | |||||
|
|
Unknown | 1 (3) | 1 (100) | 0 (0) | |||||
| Employment status, n (%) | |||||||||
|
|
Full time | 9 (26) | 2 (29) | 7 (71) | .001c | ||||
|
|
Part time | 11 (32) | 7 (64) | 4 (36) | |||||
|
|
Self employed | 2 (6) | 1 (50) | 1 (50) | |||||
|
|
Full-time or part-time student | 1 (3) | 0 (0) | 1 (100) | |||||
|
|
Unemployed | 7 (21) | 6 (86) | 1 (14) | |||||
|
|
Unknown | 4 (12) | 3 (75) | 1 (25) | |||||
| Occupation, n (%) | |||||||||
|
|
Professional | 15 (44) | 6 (40) | 9 (60) | .51 | ||||
|
|
Clerk or sales | 3 (9) | 2 (100) |
|
|||||
|
|
Home duties | 5 (15) | 4 (80) | 1 (20) | |||||
|
|
Other | 11 (32) | 7 (64) | 4 (36) | |||||
| Parity, n (SD); ranged | 2.5 (1.1); 1-6 | 2.6 (1.4);1-6 | 2.3 (0.6);1-3 | .32 | |||||
| Parents own past conception difficulties, n (%) | |||||||||
|
|
Yes | 5 (15) | 1 (20) | 4 (80) | .50 | ||||
|
|
No | 29 (85) | 18 (62) | 11 (38) | |||||
| Concerns regarding their child’s future fertility at diagnosis, n (%) | |||||||||
|
|
Yes | 23 (68) | 12 (52) | 11 (48) | .19 | ||||
|
|
No | 8 (24) | 5 (63) | 3 (37) | |||||
|
|
Unsure | 3 (9) | 2 (67) | 1 (33) | |||||
| Recalled a fertility discussion, n (%) | |||||||||
|
|
Yes | 29 (85) | 16 (55) | 13 (45) | .85 | ||||
|
|
No | 5 (15) | 3 (60) | 2 (40) | |||||
| Clinician involved in fertility discussion, n (%) | |||||||||
|
|
Oncologist | 12 (35) | 7 (58) | 5 (42) | .10 | ||||
|
|
Gynecologist | 7 (21) | 4 (57) | 3 (43) | |||||
|
|
Oncologist + gynecologist or endocrinologist or nurse | 7 (21) | 4 (57) | 3 (43) | |||||
|
|
Endocrinologist | 0 (0) | 0 (0) | 0 (0) | |||||
|
|
Nurse | 2 (6) | 1 (50) | 1 (50) | |||||
|
|
Social worker | 2 (6) | 1 (50) | 1 (50) | |||||
|
|
Unknown | 4 (12) | 2 (50) | 2 (50) | |||||
aDA: decision aid.
bt test (two-tailed) between parents who completed only the pre-DA survey only and those who completed both pre- and post-DA surveys.
cSignificant at P ≤.05.
dThe number of children the parents of patients have had.
Table 2.
Characteristics of childhood and adolescent patients with cancer.
| Characteristics |
Total children and adolescents (N=34) | Those whose parents completed the pre-DAa survey only (n=19) | Those whose parents reviewed the DA and completed the pre-and post-DA surveys (n=15) | P valueb | ||||
| Age in years, current mean (SD); range | 9.9 (6.2); 1.5-19.6 | 9.9 (6.2); 1.8-19.6 | 9.3 (6.4); 1.5-19.2 | .88 | ||||
| Age at diagnosis in years, mean (SD); range | 7.7 (5.9); 1.0-17.2 | 7.7 (6.0); 1.0-17.2 | 7.4 (5.9); 1.0-17.2 | .58 | ||||
| Pubertal status at diagnosis, n (%) | ||||||||
|
|
Prepubertal | 22 (65) | 12 (55) | 10 (45) | .63 | |||
|
|
Postpubertal | 12 (35) | 7 (58) | 5 (42) | ||||
| Diagnosis, n (%) | ||||||||
|
|
Leukemia | 10 (29) | 4 (40) | 6 (60) | .38 | |||
|
|
Rhabdomyosarcoma | 5 (15) | 2 (40) | 3 (60) | ||||
|
|
Ewing’s Sarcoma | 5 (15) | 4 (80) | 1 (20) | ||||
|
|
Central nervous system | 3 (9) | 2 (67) | 1 (33) | ||||
|
|
Hodgkin’s Disease | 2 (6) | 1 (50) | 1 (50) | ||||
|
|
Osteosarcoma | 3 (9) | 2 (67) | 1 (33) | ||||
|
|
Other solid cancers | 5 (15) | 3 (60) | 2 (40) | ||||
|
|
Non-Hodgkin’s | 1 (3) | 1 (100) | 0 (0) | ||||
| Estimated risk of infertility, n (%) | ||||||||
|
|
Low | 5 (15) | 1 (20) | 4 (80) | .04 | |||
|
|
Medium | 14 (41) | 8 (57) | 6 (43) | ||||
|
|
High | 15 (44) | 10 (67) | 5(33) | ||||
| Type of fertility preservation procedures, n (%) | ||||||||
|
|
Ovarian tissue cryopreservation | 8 (24) | 6 (75) | 2 (25) | .68 | |||
|
|
Ovarian tissue cryopreservation + gonadotropin-releasing hormone agonist + oocytes | 2 (6) | 1 (50) | 1 (50) | ||||
|
|
Testicular tissue cryopreservation | 10 (29) | 5 (50) | 5 (50) | ||||
|
|
Semen cryopreservation | 3 (9) | 2 (67) | 1 (33) | ||||
|
|
No procedure | 11 (32) | 5 (45) | 6 (55) | ||||
aDA: decision aid.
bt test (two-tailed); significant at P≤.05.
Decision Aid Assessment and Impact in Those Who Completed Surveys 1 and 2
Satisfaction With Decision Aid Design
Most parents (10/15; 67%) reported reading the DA “quite thoroughly” or “thoroughly from beginning to end,” with a median time of 25 minutes (range 15 to >60 minutes). All parents considered the length to be “about right,” 53% (8/15) reported that the DA was “very appealing” to look at, and 73% (11/15) mentioned that it was “very clearly” presented. In addition, 60% (9/15) parents were satisfied with the website format, while 33% (5/15) said they would also like a booklet, and 1 parent stated they would have liked a video.
Satisfaction With Content
The majority (13/15, 87%) of parents reported that the information in the DA was “balanced and fair,” and 13% (2/15) reported that the DA was in “favor of FP.” Most parents (12/15, 80%) felt that the information was “sufficiently detailed.” One parent found the DA to be confusing, while 87% (13/15) parents reported that it “clearly” or “very clearly” presented their child’s fertility choices. The majority (12/15, 80%) reported that the information would have been “quite” or “very” relevant when considering FP for their child.
Expectations of the Decision Aid
Overall, parents were “satisfied” (11/15, 73%) or “very satisfied” (4/15, 27%) with the DA. One parent, however, reported that the DA would not have helped them cope with their situation. The DA “met” (11/15, 73%) or “exceeded” (4/15, 27%) the expectations of all parents.
Emotional Impact of the Decision Aid
In this study, 47% (7/15) parents reported having “somewhat” thought about the information since reading the DA. In addition, 40% parents (6/15) reported feeling “a little” worried or concerned about the information. Themes emerging from open-ended responses related to concerns regarding future impacts of treatments on fertility with 1 parent commenting that she was “worried for my (child) as preservation was not an option for her” and another commenting that she was “not so much worried I guess, just sad,” indicating that worry was linked to concerns about the future impact of treatments on fertility.
Perceived Usefulness as a Decision-Making Tool
Overall, 86% (13/15) of parents reported that the DA would have been “helpful” or “very helpful” in helping them decide on their child’s treatment in general. In addition, 86% (13/15) reported that it would have been “helpful,” “very helpful,” or “extremely helpful” in making decisions about FP and would recommend the DA to other families facing an FP decision.
Of the 8 parents who completed the VCE, half reported that it would have been “satisfactory” in helping them decide, while the other half reported it would have been “very helpful.” Reasons cited for not completing the VCE included time constraints, that the parent believed it was irrelevant to their situation, or that they had technical issues with the use of the website.
Improvements in Knowledge and Understanding
In this study, 74% (11/15) parents reported that only some of the information was new to them. The remaining parents reported that either “most” (2/15; 13%) or “none” (2/15; 13%) of the information was new to them. Overall, parents reported the DA helped improve their understanding of cancer treatments, infertility, and FP procedures to some degree (Multimedia Appendix 3).
In addition, 14 parents answered the FP knowledge scale pre- and post-DA review. Prior to the review, parents answered an average of 5.21 (SD 1.66; range 1-8) out of 10 FP knowledge questions correctly. Knowledge scores improved by 1.50 to an average of 6.71 correctly answered questions after reviewing the DA (Table 3); this was a significant (P<.04) increase in the number of correct responses overall. Prior to reviewing the DA, 21% (3/14) parents scored >70% on the FP knowledge scale; this increased to 64% (9/14) after DA review.
Table 3.
Change in the parental fertility preservation-related knowledge and decision regret pre- and postdecision aid (n=15).
| Change | Pre-DAa mean score (SD); range | Post-DA mean score (SD); range | Mean change score | SE | 95% CI | t b | Degree of freedom | P value | |||||||||||
| Fertility preservation knowledge | |||||||||||||||||||
|
|
All parents (n=14) | 5.21 (1.66); 1-8 | 6.71 (1.94); 3-10 | 1.50 | 0.66 | 0.07 to 2.93 | 2.27 | 13 | .04c | ||||||||||
|
|
Parents of boys (n=6) | 5.17 (1.46); 4-8 | 6.33 (2.13); 3-10 | 1.17 | 1.20 | 0.19 to 1.90 | 0.98 | 5 | .37 | ||||||||||
|
|
Parents of girls (n=8) | 5.25 (1.79); 1-7 | 7 (1.73); 4-10 | 1.75 | 0.80 | 0.13 to 3.63 | 2.20 | 7 | .06 | ||||||||||
| Decision regret scale scores | |||||||||||||||||||
|
|
All parents (n=14) | 16.5 (18.6); 0-50 | 18.5 (19.4); 0-50 | 1.9 | 5.17 | –9.51 to 4.67 | 0.64 | 13 | .54 | ||||||||||
|
|
Parents of boys (n=6) | 5.8 (12.0); 0-30 | 10.0 (16.7); 0-40 | 4.2 | 3.75 | –13.79 to 5.46 | 1.11 | 5 | .32 | ||||||||||
|
|
Parents of girls (n=8) | 25.7 (19.0); 0-50 | 25.7 (19.7); 0-50 | 0 | 4.76 | –11.64 to 11.64 | 0.00 | 7 | 1.0 | ||||||||||
aDA: decision aid.
bt test (two-tailed).
cSignificant at P≤.05.
Expectations of the Fertility Preservation
The expectation of the FP success was asked in general, not relating specifically to their child and encompassed all FP procedures, not just experimental procedures. Notably, 11 parents reported their expectations of the future success of FP procedures. The majority “agreed” or “strongly agreed” (8/15; 73%) that FP would be successful in their lifetime. Similarly, 73% (11/15) responded that they “agreed” or “strongly agreed” that FP will be successful within the lifetime of their child. This decreased to 46% (7/15) after DA review. The change in expectations varied between parents of boys and girls (Figure 4). Expectations of success in this lifetime decreased in parents of boys and increased in parents of girls. Conversely, expectations of success in the next generation increased for parents of boys and decreased in girls.
Figure 4.
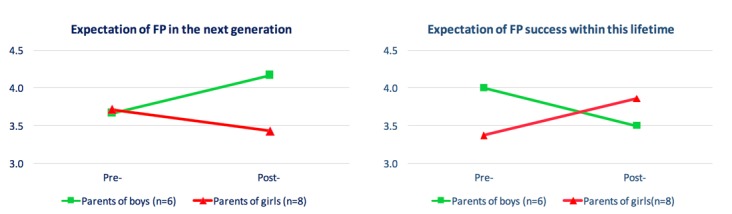
Parents' expectations of fertility preservation success within this lifetime and the lifetime of the next generation. FP: fertility preservation; 5: Strongly agree; 4: Agree; 3: Neither agree nor disagree; 2: Disagree; 1: Strongly disagree.
Decision Regret
In this study, 14 parents completed the Decision Regret Scale (Table 3). The mean regret score pre-DA review was 16.5 (SD 18.6; range 0-50), and post-DA review was 18.5 (SD 19.4; range 0-50). There was a nonsignificant increase in scores across all parents of 1.9 points (4.2-point increase in parents of boys and 0 in parents of girls; P=.52).
Clinician Review of the Decision Aid
Usability and Content Usefulness and Satisfaction of the Decision Aid Design
All clinicians reported that they would recommend the DA to patients. When asked for their thoughts on the DA, three main themes emerged from the comments: (1) the DA was well designed and easy to use; (2) the DA was a good information source; and (3) there is a need for more information and resources for patients and parents beyond the DA.
Design, Usability, and Content
Clinicians reported satisfaction with the design and usability of the DA website, commenting that it was an “excellent and well-structured” resource. In addition, the DA was regarded as a valid and relevant source of information for clinicians, patients, and their families with one clinician commenting that “I found it useful as a resource prior to meeting with a patient.”
Perceived Need for Information and Patient Resources
In this study, 36% (4/11) of the interviewed clinicians highlighted a lack of patient and parent resources regarding infertility, FP procedures, and processes. One clinician commented that she had “observed more and more adolescents, especially boys” making FP decisions, noting that there are very few resources tailored toward adolescents and parents of adolescents.
Discussion
In this pilot study, we evaluated the parental and clinical acceptance of the first FP DA for parents of children and adolescents with cancer. Our data suggest that the DA was acceptable, did not increase parental concern, and would be useful for parents making an FP decision. Furthermore, initial testing suggests the DA increases FP-related knowledge. Parents and clinicians would recommend this tool to others faced with a similar decision. However, we acknowledge the high dropout in this study. These results support further formal evaluation of the DA in a larger prospective trial, prior to the implementation of the DA as a decision-support tool in clinical practice.
The DA was positively received by parents who viewed it. Parents felt that the DA provided unbiased information in an easy-to-read format and was relevant to their situation. Of note, 13% (2/15) parents felt that the DA favored FP. The theoretical framework behind DA development is that it should not favor a particular option but provide a balanced view so that users can make fair decisions [21]. Most of the cohort had undergone an FP procedure, and our DA presented a large amount of information concerning FP procedure outcomes, which may have contributed to the impression of some parents that it favored FP.
One of the primary purposes of our DA was to increase parents’ fertility-related knowledge scores in parents despite parents already having experience with FP. These results should be interpreted cautiously because of small numbers. However, in parents with no or little FP awareness, we hope there would be a more significant impact on knowledge, as has been demonstrated in other health-related DA studies [21,29].
A key component of informed decision making is an understanding of the likelihood of the possible outcomes and the associated risks and benefits [13]. Interestingly, post-DA review parents’ expectations of FP had changed. Expectations of the success of experimental procedures “in this lifetime” decreased for parents of boys and increased for parents of girls; this change may reflect a better understanding of the technologies as they currently stand, considering that ovarian tissue cryopreservation is being used to achieve pregnancy, but testicular tissue cryopreservation has not yet been successfully used in humans. That parents had perhaps more realistic expectations of FP is important in that (1) they may have a more accurate perception of the risks and likely future successes of FP; (2) that improved perception may lead to better-quality decision making, thereby decreasing the risk of future decision regret; and (3) there may be scope for this DA to improve informed consent in clinical practice.
The DA had an emotional impact on some parents, with 40% (6/15) reporting feeling “a little” worried or concerned after reviewing the DA; this was somewhat expected as the DA was a comprehensive fertility resource and the additional information may have raised issues that were not previously discussed with parents at the time of decision making or may have since been forgotten. One participant stated that the information in the DA gave them a more realistic understanding of their child’s fertility risk; this highlights the importance of ensuring that parents are well informed at the time of diagnosis and receive adequate fertility counseling during their posttreatment care [30].
The DA did not increase decision regret in parents; this may reflect that the DA confirmed that parents made the right decision. Ultimately, it does not appear that the DA increases distress, and thus, it is likely to be suitable for use in parents of newly diagnosed children adolescents. Notably, decision regret was measured shortly after DA review. While it was not evident at that time, it is possible that regret may increase months or years later [31], depending on the state of reproductive technologies when the patient wishes to conceive; this has yet to be explored and is an area for further research.
A novel feature of this DA is the rapid feedback provided through the Web-based VCE, which provided a visual representation of the importance of FP to participants, based on the responses provided to a series of questions. To the best of our knowledge, this is the first report of a VCE that functions in this way. All parents who completed the VCE reported that it would have been helpful to some extent when making the decision. Although our data did not provide the information to ascertain whether this was a result of the ease of the click-through VCE, the visual result, or both, it does suggest that a Web-based tool may have merit. Although these data are positive, only 53% (8/15) completed the VCE. Reasons for noncompletion included time constraints, inaccessibility, and unclear instructions. A post-hoc consumer review revealed that the link name for the VCE was confusing. Other studies have reported varying rates of the VCE completion in pilot studies and suggest that what is reported in pilot studies is not reflective of what happens in prospective studies [32]; thus, prospective evaluation is needed.
Although our findings support the utility of a fertility DA for parents of children and adolescents with cancer, our study had inherent limitations. As with most small samples, care must be taken with interpretation of results, as they may not be generalizable. Parents were asked to reflect on their FP decision-making process, which could have been up to 5 years previously. It was not possible to capture the lived experience, and parents may have been biased by intervening events. In addition, study measures were limited by a retrospective sample. Therefore, it was not possible to measure the decisional conflict, a measure of uncertainty and a key factor affecting decision making [33]. However, this study design is an appropriate and necessary way to assess DA acceptability and usability prior to prospective evaluation.
Dropout in this study was higher than expected, resulting in a small sample size. While parents indicated they were keen to participate, many noted that the high time demands of the study and had limited time to engage in a detailed review of the DA. It is likely that in a study of parents making prospective decisions in real time, a higher proportion of parents would wish to actively review the DA. Previous pilot DA studies have shown similar sample sizes to be sufficient to test acceptability and usability of the tools prior to prospective evaluation [32,34].
Overall, clinicians reported satisfaction with the DA design and most importantly would recommend the DA to parents facing a fertility-preservation decision. The resource was regarded as valid and relevant, and interestingly was “a useful resource prior to meeting with a patient.” Research has shown that even with the best intentions, clinicians struggle to convey information and potential risks to patients in language that is easily comprehensible [14]. In the future, this plain language resource could be used to support clinician education and potentially improve fertility-preservation counseling. Lastly, clinicians reported a lack of resources to support children and adolescents in the shared decision-making process. Perhaps the development of an adolescent and young adult FP DA or toolkit may address this gap.
There is a growing body of evidence highlighting the importance of information provision regarding the risks to fertility from cancer treatments and potential FP options. Information provision is important at the time of diagnosis prior to treatment and the potential harm to reproductive tissues [8]. Information regarding cancer treatments, fertility, and potential FP procedures is complex and may be difficult to comprehend [35], especially given the stress of a new cancer diagnosis and the short timeframes in which patients and their parents are required to make decisions. This DA is acceptable and relevant to parents and may assist families who are actively engaged in making an FP decision. Results warrant evaluation in prospective studies, which can assess outcome measures such as decisional conflict as well as decision regret.
As the health care user and provision landscape are rapidly changing, it is increasingly important for health care tools to evolve to further improve patients’ interactions with health care systems, clinical teams, and improve participation and satisfaction with shared decisions. This novel study has developed and preliminarily assessed the first, Web-based FP DA for parents of children and adolescents with cancer. This research has shown the DA to be acceptable to parents who have previously made an FP decision for their children and adolescents with cancer without causing distress. This study adds to the growing pool of research regarding pediatric FP, DA evaluation, and parental (proxy) decision making.
Acknowledgments
Georgios I Misiakos provided design visuals and infographics for the electronic decision aid. Adam Leadoux from The Royal Children’s Hospital Educational Resource Centre designed the Web-based content management system for the electronic decision aid. YJ is supported by the Victorian Cancer Agency and is a National Health and Medical Research Council Translation of Research into Clinical Practice fellow; MP is supported by the National Breast Cancer Foundation Early Career Fellowship (grant number: ECF-015).
Abbreviations
- DA
decision aid
- FP
fertility preservation
- VCE
Values Clarification Exercise
Fertility decision aid content.
Pre- and postdecision aid survey measures.
Improved understanding of fertility preservation in parents’ postdecision aid review; n=15 (%).
Footnotes
Conflicts of Interest: None declared.
References
- 1.Australian Institute of Health and Welfare . Cancer in Australia: an overview. Canberra: AIHW; 2014. p. a. [Google Scholar]
- 2.Cancer Australia . Cancer Australia. Online: Australian Government; 2015. [2018-09-20]. Children's Cancer Statistics https://childrenscancer.canceraustralia.gov.au/about-childrens-cancer/statistics . [Google Scholar]
- 3.Youlden D, Baade P, Hallahan A, Valery P, Green A, Aitken J. Conditional survival estimates for childhood cancer in Australia, 2002-2011: A population-based study. Cancer Epidemiol. 2015 Jun;39(3):394–400. doi: 10.1016/j.canep.2015.02.008.S1877-7821(15)00044-2 [DOI] [PubMed] [Google Scholar]
- 4.Anderson R, Mitchell R, Kelsey T, Spears N, Telfer E, Wallace W. Cancer treatment and gonadal functionxperimental and established strategies for fertility preservation in children and young adults. The Lancet Diabetes & Endocrinology. 2015;3:e. doi: 10.1016/S2213-8587(15)00039-X. [DOI] [PubMed] [Google Scholar]
- 5.Debra A, Edgar D, Borg J, Archer J, Lutjen P, McBain J. Oocyte maturation, follicle rupture and luteinization in human cryopreserved ovarian tissue following xenografting. Human Reproduction. 2003;18(9):1772–81. doi: 10.1093/humrep/deg365. [DOI] [PubMed] [Google Scholar]
- 6.Levine J. Fertility preservation in children and adolescents with cancer. Minerva Pediatr. 2011 Feb;63(1):49–59.R15113642 [PubMed] [Google Scholar]
- 7.Long CJ, Ginsberg JP, Kolon TF. Fertility Preservation in Children and Adolescents With Cancer. Urology. 2016 Dec;91:190–6. doi: 10.1016/j.urology.2015.10.047.S0090-4295(15)01176-0 [DOI] [PubMed] [Google Scholar]
- 8.Knapp Caprice A, Quinn Gwendolyn P, Murphy Devin. Assessing the Reproductive Concerns of Children and Adolescents with Cancer: Challenges and Potential Solutions. J Adolesc Young Adult Oncol. 2011 Apr;1(1):31–35. doi: 10.1089/jayao.2010.0003. http://europepmc.org/abstract/MED/23610730 .10.1089/jayao.2010.0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace WHB, Anderson RA, Irvine DS. Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol. 2005 Apr;6(4):209–18. doi: 10.1016/S1470-2045(05)70092-9.S1470-2045(05)70092-9 [DOI] [PubMed] [Google Scholar]
- 10.Vassilakopoulou M, Boostandoost E, Papaxoinis G, de La Motte Rouge Thibault, Khayat D, Psyrri A. Anticancer treatment and fertility: Effect of therapeutic modalities on reproductive system and functions. Crit Rev Oncol Hematol. 2016 Jan;97:328–34. doi: 10.1016/j.critrevonc.2015.08.002.S1040-8428(15)30017-2 [DOI] [PubMed] [Google Scholar]
- 11.Levine J, Canada A, Stern CJ. Fertility preservation in adolescents and young adults with cancer. J Clin Oncol. 2010 Nov 10;28(32):4831–41. doi: 10.1200/JCO.2009.22.8312.JCO.2009.22.8312 [DOI] [PubMed] [Google Scholar]
- 12.Beckmann MW, Dittrich R, Lotz L, van der Ven K, van der Ven HH, Liebenthron J, Korell M, Frambach T, Sütterlin M, Schwab R, Seitz S, Müller A, von WM, Häberlin F, Henes M, Winkler-Crepaz K, Krüssel JS, Germeyer A, Toth B. Fertility protection: complications of surgery and results of removal and transplantation of ovarian tissue. Reprod Biomed Online. 2018 Feb;36(2):188–196. doi: 10.1016/j.rbmo.2017.10.109.S1472-6483(17)30611-9 [DOI] [PubMed] [Google Scholar]
- 13.Anger JT, Gilbert BR, Goldstein M. Cryopreservation of sperm: indications, methods and results. J Urol. 2003 Oct;170(4 Pt 1):1079–84. doi: 10.1097/01.ju.0000084820.98430.b8.S0022-5347(05)63100-X [DOI] [PubMed] [Google Scholar]
- 14.Kemertzis M, Clark H, Ho C, Gook D, Clarke G, Bourne H. Paediatric-adolescent fertility preservation: an overview of clinical practice at the Royal Children's Hospital Melbourne. Shanghai; International Society for Fertility Preservation; November; Shanghai. 2015. Nov 13, [Google Scholar]
- 15.Vetsch J, Fardell JE, Wakefield CE, Signorelli C, Michel G, McLoone JK, Walwyn T, Tapp H, Truscott J, Cohn RJ, ANZCHOG survivorship study group “Forewarned and forearmed” : Long-term childhood cancer survivors' and parents' information needs and implications for survivorship models of care. Patient Educ Couns. 2017 Dec;100(2):355–363. doi: 10.1016/j.pec.2016.09.013.S0738-3991(16)30423-2 [DOI] [PubMed] [Google Scholar]
- 16.McCarthy MC, McNeil R, Drew S, Dunt D, Kosola S, Orme L, Sawyer SM. Psychological Distress and Posttraumatic Stress Symptoms in Adolescents and Young Adults with Cancer and Their Parents. J Adolesc Young Adult Oncol. 2016 Dec;5(4):322–329. doi: 10.1089/jayao.2016.0015. [DOI] [PubMed] [Google Scholar]
- 17.Wyns C, Collienne C, Shenfield F, Robert A, Laurent P, Roegiers L, Brichard B. Fertility preservation in the male pediatric population: factors influencing the decision of parents and children. Hum Reprod. 2015 Sep;30(9):2022–30. doi: 10.1093/humrep/dev161.dev161 [DOI] [PubMed] [Google Scholar]
- 18.McDougall R. The Ethics of Fertility Preservation for Paediatric Cancer Patients: From Offer to Rebuttable Presumption. Bioethics. 2015 Nov;29(9):639–45. doi: 10.1111/bioe.12190. [DOI] [PubMed] [Google Scholar]
- 19.McCarthy MC, Campo M, Drew SE. Pediatric oncology survivorship: conveying risks and communicating information at the right time for the individual. Curr Opin Support Palliat Care. 2013 Sep;7(3):289–95. doi: 10.1097/SPC.0b013e32836395e0. [DOI] [PubMed] [Google Scholar]
- 20.Bradford BR. Chemotherapy-induced infertility in patients with testicular cancer. Oncol Nurs Forum. 2012 Jan;39(1):27–30. doi: 10.1188/12.ONF.27-30.HJ82MU82X0666Q76 [DOI] [PubMed] [Google Scholar]
- 21.O'Connor Annette M, Bennett Carol L, Stacey Dawn, Barry Michael, Col Nananda F, Eden Karen B, Entwistle Vikki A, Fiset Valerie, Holmes-Rovner Margaret, Khangura Sara, Llewellyn-Thomas Hilary, Rovner David. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2009 Jul 08;(3):CD001431. doi: 10.1002/14651858.CD001431.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Pope T, Lessler D. Revolutionizing Informed Consent: Empowering Patients with Certified Decision Aids. Patient. 2017 Oct;10(5):537–539. doi: 10.1007/s40271-017-0230-3.10.1007/s40271-017-0230-3 [DOI] [PubMed] [Google Scholar]
- 23.Elwyn Glyn, O'Connor Annette, Stacey Dawn, Volk Robert, Edwards Adrian, Coulter Angela, Thomson Richard, Barratt Alexandra, Barry Michael, Bernstein Steven, Butow Phyllis, Clarke Aileen, Entwistle Vikki, Feldman-Stewart Deb, Holmes-Rovner Margaret, Llewellyn-Thomas Hilary, Moumjid Nora, Mulley Al, Ruland Cornelia, Sepucha Karen, Sykes Alan, Whelan Tim, International Patient Decision Aids Standards (IPDAS) Collaboration Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ. 2006 Aug 26;333(7565):417. doi: 10.1136/bmj.38926.629329.AE. http://europepmc.org/abstract/MED/16908462 .bmj.38926.629329.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coulter A, Stilwell D, Kryworuchko J, Mullen PD, Ng CJ, van der Weijden T. A systematic development process for patient decision aids. BMC Med Inform Decis Mak. 2013;13 Suppl 2:S2. doi: 10.1186/1472-6947-13-S2-S2. https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/1472-6947-13-S2-S2 .1472-6947-13-S2-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wakefield C, Meiser B, Homewood J, Ward R, O'Donnell S, Kirk J, Australian GENetic testing Decision Aid Collaborative Group Randomized trial of a decision aid for individuals considering genetic testing for hereditary nonpolyposis colorectal cancer risk. Cancer. 2008 Sep 01;113(5):956–65. doi: 10.1002/cncr.23681. doi: 10.1002/cncr.23681. [DOI] [PubMed] [Google Scholar]
- 26.Hand M, Kemertzis M, Peate M, Gillam Lynn, McCarthy Maria, Orme Lisa, Heloury Yves, Sullivan Michael, Zacharin Margaret, Jayasinghe Yasmin. A Clinical Decision Support System to Assist Pediatric Oncofertility: A Short Report. J Adolesc Young Adult Oncol. 2018 Aug;7(4):509–513. doi: 10.1089/jayao.2018.0006. [DOI] [PubMed] [Google Scholar]
- 27.Kemertzis M, Ranjithakumaran H, Hand M, Peate M, Gillam L, McCarthy M, Super Leanne, McQuillan Sarah, Drew Sarah, Jayasinghe Yasmin, Orme Lisa. Fertility Preservation Toolkit: A Clinician Resource to Assist Clinical Discussion and Decision Making in Pediatric and Adolescent Oncology. J Pediatr Hematol Oncol. 2018 Apr;40(3):e133–e139. doi: 10.1097/MPH.0000000000001103. [DOI] [PubMed] [Google Scholar]
- 28.Trevena L, Zikmund-Fisher B, Edwards A, Gaissmaier W, Galesic M, Han P, King John, Lawson Margaret L, Linder Suzanne K, Lipkus Isaac, Ozanne Elissa, Peters Ellen, Timmermans Danielle, Woloshin Steven. Presenting quantitative information about decision outcomes: a risk communication primer for patient decision aid developers. BMC Med Inform Decis Mak. 2013;13 Suppl 2:S7. doi: 10.1186/1472-6947-13-S2-S7. https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/1472-6947-13-S2-S7 .1472-6947-13-S2-S7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peate M, Meiser B, Cheah B, Saunders C, Butow P, Thewes B, Hart R, Phillips K-a, Hickey M, Friedlander M. Making hard choices easier: a prospective, multicentre study to assess the efficacy of a fertility-related decision aid in young women with early-stage breast cancer. Br J Cancer. 2012 Mar 13;106(6):1053–61. doi: 10.1038/bjc.2012.61. doi: 10.1038/bjc.2012.61.bjc201261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peate M, Meiser B, Friedlander M, Zorbas H, Rovelli S, Sansom-Daly U, Sangster J, Hadzi-Pavlovic D, Hickey M. It's now or never: fertility-related knowledge, decision-making preferences, and treatment intentions in young women with breast cancer--an Australian fertility decision aid collaborative group study. J Clin Oncol. 2011 May 01;29(13):1670–7. doi: 10.1200/JCO.2010.31.2462.JCO.2010.31.2462 [DOI] [PubMed] [Google Scholar]
- 31.Brehaut JC, O'Connor AM, Wood TJ, Hack TF, Siminoff L, Gordon E, Feldman-Stewart D. Validation of a decision regret scale. Med Decis Making. 2003;23(4):281–92. doi: 10.1177/0272989X03256005. [DOI] [PubMed] [Google Scholar]
- 32.Peate M, Watts K, Wakefield CE. The 'value' of values clarification in cancer-related decision aids. Patient Educ Couns. 2013 Feb;90(2):281–3. doi: 10.1016/j.pec.2012.10.023.S0738-3991(12)00430-2 [DOI] [PubMed] [Google Scholar]
- 33.O'Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15(1):25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- 34.Wakefield CE, Watts KJ, Meiser B, Sansom-Daly U, Barratt A, Mann GJ, Lobb EA, Gaff CL, Howard K, Patel MI. Development and pilot testing of an online screening decision aid for men with a family history of prostate cancer. Patient Educ Couns. 2011 Apr;83(1):64–72. doi: 10.1016/j.pec.2010.05.025.S0738-3991(10)00318-6 [DOI] [PubMed] [Google Scholar]
- 35.Quinn G, Murphy D, Knapp C, Stearsman D, Bradley-Klug K, Sawczyn K, Clayman Marla L. Who decides? Decision making and fertility preservation in teens with cancer: a review of the literature. J Adolesc Health. 2011 Oct;49(4):337–46. doi: 10.1016/j.jadohealth.2011.01.005. http://europepmc.org/abstract/MED/21939862 .S1054-139X(11)00007-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fertility decision aid content.
Pre- and postdecision aid survey measures.
Improved understanding of fertility preservation in parents’ postdecision aid review; n=15 (%).


