Abstract
Pancreatic ductal adenocarcinoma is projected to become the second-leading cause of cancer-related death and is largely resistant to immunotherapies. The tumor microenvironment, largely composed of heterogeneous myeloid cells, creates a physical, metabolic, and immunosuppressive barrier that prevents T cells from infiltrating cancer beds. In this issue of the JCI, Markosyan and colleagues have reported a tumor-intrinsic mechanism that excludes T cells from the vicinity of tumor cells. They showed that a receptor tyrosine kinase, ephrin-A receptor 2 (EPHA2), regulates prostaglandin endoperoxide synthase 2 (PTGS2) (encodes COX-2) expression in a TGF-β signaling–dependent manner. Genetic ablation of Epha2 or Ptgs2 in preclinical models or pharmacological inhibition of COX-2 elicited the transformation of this immunosuppressive microenvironment into a T cell–permissive milieu. Consequent T cell relocation rendered this immunoresistant malignancy responsive to combinations of checkpoint blockers and CD40 agonists. Because the association between T cell infiltration and the EPHA2/TGF-β/COX-2 axis is supported by independent clinical data, these results provide a rationale for ensuing clinical trials aimed at incorporating pancreatic cancer into the range of immunotherapy-responsive tumors.
EPHA2 expression defines T cell exclusion
Although antibodies that block immune checkpoints have revolutionized the management of some tumors with tumor-infiltrating lymphocytes (TILs), most cancer patients still fail to respond to single-agent immunotherapies. While protective immune responses depend on the intrinsic antigenicity of the tumor, they are also influenced by T cell–recruiting chemokines at tumor beds and a physically permissive milieu that allows TILs to contact antigen-expressing tumor cells. In pancreatic ductal adenocarcinoma, a unique desmoplastic reaction impedes the accessibility of activated T cells to the vicinity of tumor cells, where they should exert their effector function (1).
By combining the analysis of human pancreatic tumors with genetic studies in mouse models, Markosyan and colleagues found that ephrin-A receptor 2 (EPHA2) expression determines CD8+ T cell exclusion in pancreatic cancer (2). The authors focused on EPHA2 by analyzing links to pathways among genes inversely correlated with the expression of CD8A and markers of cytolytic T cell activity in The Cancer Genome Atlas (TCGA) data sets. Validation analyses of independent patient cohorts and mouse models with different degrees of TILs further supported the association between EPHA2 expression and the paucity of TILs. Accordingly, Epha2 ablation in murine pancreatic cancer tumors promoted TIL enrichment at the expense of immunosuppressive myeloid cells, with a prominent decrease of cells that express granulocytic lineage markers. Although the authors did not formally test the suppressive activity of these cells, granulocytic myeloid-derived suppressor cells (gMDSCs) accumulate in patients with cancer, where they coexist with conventional neutrophils and suppress the effector activity of tumor-reactive T cells (3). This includes pancreatic cancer desmoplasia, which generates a stromal compartment composed of hematopoietic cells, extracellular matrix, and fibroblasts, typically larger than the space occupied by pancreatic tumor cells. A significant component of this desmoplastic reaction is made by heterogeneous populations of immunosuppressive gMDSCs along with macrophages and immature MDSC precursors (4). Because Epha2 ablation failed to change immunosuppressive mediator expression in tumor-associated gMDSCs on a per cell basis, Markosyan and colleagues suggest that granulocyte cell accumulation, rather than immunosuppressive activity, determines whether or not the microenvironment in pancreatic cancer will be T cell permissive or exclusive (2).
EPHA2/TGF-β/COX-2 axis governs T cell exclusion
Markosyan et al. went on to demonstrate that proinflammatory mediator COX-2 (encoded by prostaglandin endoperoxide synthase 2 [Ptgs2]) is differentially expressed in Epha2+TILlo compared with Eph2–TILhi tumors (2). Subsequent analyses of additional samples from human and mouse pancreatic cancer–bearing hosts with low or high TIL density again confirmed an association between COX-2 expression and T cell exclusion. COX-2 is the inducible form of cyclooxygenase, the enzyme responsible for converting fatty acids into prostanoids (5). A role for COX-2 in pancreatic cancer progression is not surprising, as high COX-2 expression levels in both pancreatic cancer cells and stroma cells have been previously reported (6). In addition, COX-2 expression was shown to be positively regulated by TGF-β signaling; however, mediation via SMAD3 is likely indirect, as the PTGS2 promoter region lacks SMAD3-binding sites.
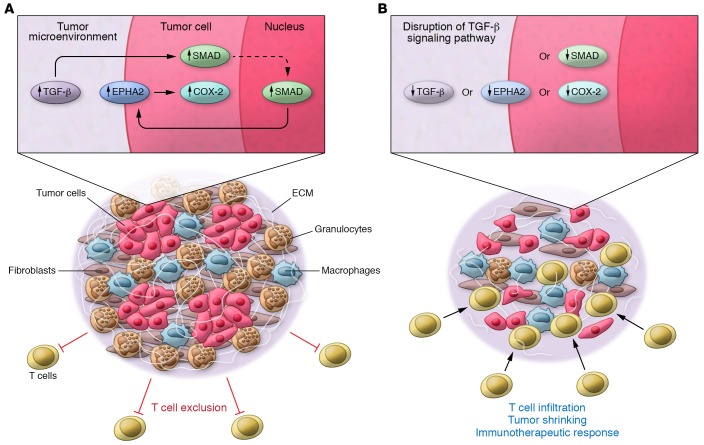
Markosyan et al. have revealed the importance of the EPHA2/TGF-β/COX-2 axis in forging the pancreatic cancer microenvironment. When tumor cells were treated with TGF-β, EPHA2 increased on the tumor cell surface. Additionally, excision of TGF-β–dependent Smad3/4 in tumor cells or ablation of Ptgs2 recapitulated the increases in T cells and decreases in granulocytes in implanted tumors similarly to what was observed upon Epha2 ablation. Furthermore, ectopic expression of COX-2 in tumor cells promoted the accumulation of granulocytes and possibly gMDSCs at the expense of antigen-presenting cells, thereby phenocopying the effects induced by spontaneous Epha2 overexpression. Collectively, these data indicate that an EPHA2/TGF-β/COX-2 axis governs the pancreatic cancer immune environment toward a myeloid-rich, T cell–poor setting (Figure 1).
Figure 1. Tumor cell–intrinsic TGF-β signaling drives the upregulation of EPHA2 on the tumor cell surface, which promotes the overexpression of COX-2.
COX-2 activity governs the orchestration of a pancreatic cancer microenvironment characterized by the accumulation of cells of the granulocytic lineage that, along with macrophages, fibroblasts, and the deposition of extracellular matrix (ECM), prevents T cell infiltration. Genetic ablation of TGF-β signaling, Epha2, or Ptgs2 (COX-2) transforms this microenvironment into one permissive to T cell trafficking, which is primarily associated with a decrease in granulocytes (likely gMDSC). Accordingly, pharmacological inhibition of COX-2 renders highly resistant pancreatic tumors sensitive to immunotherapeutic interventions.
PGE2, which is the most abundant secreted prostaglandin, shows strong tumor-promoting activities (7), including a major role in immunosuppression in cancer that acts by driving MDSC proliferation (8). Further, PGE2 may directly mediate COX-2–dependent T cell exclusion. Markosyan et al. found that minor reductions in COX-2 substantially reduced PGE2 expression. Interestingly, PGE2 also modulates chemokine production, enhancing local accumulation of both populations of MDSCs (9). While all these mechanisms can contribute to the observed effects, the results of Markosyan and colleagues point to specific determinants of the selective accumulation of granulocytes and gMDSCs at tumor beds.
An attractive candidate for driving these effects could be the chemokine family member CXCL8. CXCL8 expression is increased by PGE2 signaling in human epithelial cells (10) and could offer an additional therapeutic target for antibody blockade. Markosyan and colleagues did not find differences in the expression of the mouse counterpart of CXCL8 (Cxcl1) between WT and Epha2-KO tumors. Still, it will be interesting to determine whether a correlation between CXCL8 and EPHA2 could be identified in TCGA human pancreatic cancer data sets and other independent cohorts. Nevertheless, the results of Markosyan and colleagues suggest that EPHA2 and CXCL8 could represent different tumor cell–intrinsic mechanisms driving T cell exclusion in pancreatic cancer. Future studies will determine whether other chemokines involved in neutrophil trafficking (i.e., CXCL2) are upregulated in an EPHA2-dependent manner (11).
COX-2 inhibition could overcome resistance to immunotherapy
Strikingly, Markosyan et al. showed that treating otherwise unresponsive models of murine pancreatic cancer with celecoxib, a COX-2 inhibitor, rendered tumors sensitive to combinations of checkpoint inhibitors and CD40 agonists (2). The same group previously demonstrated that CD40 stimulation of tumor-associated macrophages is required to transform macrophages into cytotoxic killers that, unlike conventional T cells, are able to navigate the physical barriers of the pancreatic cancer stroma. However, CD40-induced stromal remodeling was insufficient, and activated T cells remained blocked from the vicinity of tumor cells (1). By reversing T cell exclusion, pharmacological inhibition of COX-2 sensitizes tumors to immunotherapy. Interestingly, in mouse models, this sensitization process can occur in the absence of strong neoepitopes (12).
COX-2 inhibitors have been previously shown to decrease stromal-dependent pancreatic cancer cell invasiveness (6), but the study by Markosyan et al. provides a mechanistic rationale for clinical trials on concurrently inhibiting COX-2 and PD-1 with existing drugs in pancreatic cancer patients. Although pancreatic cancers have relatively low mutational burdens compared with other human malignancies (13), some pancreatic cancers express cancer-associated antigens that are recognizable by bone marrow–resident T cells (14). In addition, the results presented by Markosyan et al. suggest that the immunogenicity of pancreatic cancer (and perhaps other tumors with low mutational burden) is independent of single-nucleotide mutations that are conclusively associated with immunotherapeutic responses in other tumors. Given that defects in p53 are a hallmark of this disease, gene fusions arising from genomic instability are other attractive antigenic drivers, although the role of conventional shared antigens, including cancer testis antigens, cannot be disregarded.
The work by Markosyan and colleagues underscores the importance of another appealing target for blockade, namely, TGF-β. In cancer, TGF-β suppresses CD8+ T cell effector activity (15) and TGF-β signaling drives PD-1 overexpression through a SATB1-dependent mechanism (16). In human pancreatic cancer, upregulation of TGF-β isoforms drives desmoplasia (17). However, while the results presented in this study deepen our understanding of TGF-β and SMAD signaling in antitumor immunity, long-term neutralization of TGF-β could have undesired effects; a note of caution is warranted. For example, TGF-β has positive effects in the acquisition of a tissue-resident memory (TRM) differentiation program (18). In other tumors, TRM T cells have been associated with effective protective immunity upon checkpoint blockade (19); therefore, these benefits could be lost in response to TGF-β inhibitors. On the other hand, blockade of TGF-β could synergize with other T cell–based immunotherapies
Finally, other targetable tumor cell–intrinsic drivers of T cell exclusion have been previously identified in different tumors. For instance, a CDK4/6-dependent, tumor cell–intrinsic transcriptional program associated with checkpoint inhibitors was identified in melanoma (20). The studies of Markosyan and colleagues represent an important step in determining combinatorial interventions in pancreatic cancer patients with malignant progression to revert the restrictive microenvironment into a milieu that facilitates existing and future T cell–based immunotherapies.
Acknowledgments
This study was supported by R01CA157664, R01CA124515, R01CA178687, R01CA211913, and U01CA232758 (to JRCG).
Version 1. 07/29/2019
Electronic publication
Version 2. 09/03/2019
Print issue publication
Footnotes
Conflict of interest: The author declares research support, intellectual property income, and stock options from Anixa Biosciences and Compass Therapeutics.
Copyright: © 2019, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2019;129(9):3521–3523. https://doi.org/10.1172/JCI130316.
See the related article at Tumor cell–intrinsic EPHA2 suppresses antitumor immunity by regulating PTGS2 (COX-2).
References
- 1.Beatty GL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331(6024):1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markosyan N, et al. Tumor cell–intrinsic EPHA2 suppresses antitumor immunity by regulating PTGS2 (COX-2) J Clin Invest. 2019;129(9):3594–3609. doi: 10.1172/JCI127755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou J, Nefedova Y, Lei A, Gabrilovich D. Neutrophils and PMN-MDSC: Their biological role and interaction with stromal cells. Semin Immunol. 2018;35:19–28. doi: 10.1016/j.smim.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu T, et al. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest. 2011;121(10):4015–4029. doi: 10.1172/JCI45862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahin M, Sahin E, Koksoy S. Regulatory T cells in cancer: an overview and perspectives on cyclooxygenase-2 and Foxp3 DNA methylation. Hum Immunol. 2013;74(9):1061–1068. doi: 10.1016/j.humimm.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Sato N, Maehara N, Goggins M. Gene expression profiling of tumor-stromal interactions between pancreatic cancer cells and stromal fibroblasts. Cancer Res. 2004;64(19):6950–6956. doi: 10.1158/0008-5472.CAN-04-0677. [DOI] [PubMed] [Google Scholar]
- 7.Chen EP, Smyth EM. COX-2 and PGE2-dependent immunomodulation in breast cancer. Prostaglandins Other Lipid Mediat. 2011;96(1-4):14–20. doi: 10.1016/j.prostaglandins.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prima V, Kaliberova LN, Kaliberov S, Curiel DT, Kusmartsev S. COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc Natl Acad Sci U S A. 2017;114(5):1117–1122. doi: 10.1073/pnas.1612920114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188(1):21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu Y, Chadee K. Prostaglandin E2 stimulates IL-8 gene expression in human colonic epithelial cells by a posttranscriptional mechanism. J Immunol. 1998;161(7):3746–3752. [PubMed] [Google Scholar]
- 11.Adrover JM, et al. A neutrophil timer coordinates immune defense and vascular protection. Immunity. 2019;50(2):390–402.e10. doi: 10.1016/j.immuni.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Evans RA, et al. Lack of immunoediting in murine pancreatic cancer reversed with neoantigen. 88328JCI Insight. 2016;1(14) doi: 10.1172/jci.insight.88328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellrott K, et al. Scalable open science approach for mutation calling of tumor exomes using multiple genomic pipelines. Cell Syst. 2018;6(3):271–281.e7. doi: 10.1016/j.cels.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmitz-Winnenthal FH, et al. High frequencies of functional tumor-reactive T cells in bone marrow and blood of pancreatic cancer patients. Cancer Res. 2005;65(21):10079–10087. doi: 10.1158/0008-5472.CAN-05-1098. [DOI] [PubMed] [Google Scholar]
- 15.Stephen TL, et al. Transforming growth factor β-mediated suppression of antitumor T cells requires FoxP1 transcription factor expression. Immunity. 2014;41(3):427–439. doi: 10.1016/j.immuni.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephen TL, et al. SATB1 expression governs epigenetic repression of PD-1 in tumor-reactive T Cells. Immunity. 2017;46(1):51–64. doi: 10.1016/j.immuni.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friess H, et al. Enhanced expression of transforming growth factor beta isoforms in pancreatic cancer correlates with decreased survival. Gastroenterology. 1993;105(6):1846–1856. doi: 10.1016/0016-5085(93)91084-U. [DOI] [PubMed] [Google Scholar]
- 18.Workel HH, et al. A transcriptionally distinct CXCL13. Cancer Immunol Res. 2019;7(5):784–796. doi: 10.1158/2326-6066.CIR-18-0517. [DOI] [PubMed] [Google Scholar]
- 19.Ganesan AP, et al. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat Immunol. 2017;18(8):940–950. doi: 10.1038/ni.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jerby-Arnon L, et al. A cancer cell program promotes T cell exclusion and resistance to checkpoint blockade. Cell. 2018;175(4):984–997.e24. doi: 10.1016/j.cell.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]