Abstract
Background
In older adults, hip fractures have been described to peak in cooler months. Seasonal differences in patient vulnerability to fracture and social/behavioural factors might contribute to these trends.
Methods
Using linked health-care databases in Ontario Canada, we examined monthly variation in hip fracture hospitalizations in those > 65 years (2011–2015). We stratified results by age category (66–79, ≥80 years). We then examined for variation in the demographic and comorbidity profiles of patients across the months, and as an index of contributing social/behavioural factors, noted variation in health-care behaviours.
Results
There were 47,971 and 52,088 hospitalizations for hip fracture in those 66–79, and ≥80 years, respectively. There was strong seasonality in fractures in both groups. Peaks occurred in October and December when patients appeared most vulnerable. Rates fell in the summer in those 66–79 years, and in the late winter in those ≥80 years (when health-care utilization also declined). A smaller peak in fractures occurred in May in both groups.
Conclusions
Hip fractures peak in the autumn, early winter, and spring in Canada. A dip in fractures occurs in the late winter in the oldest old. Environmental factors might play a role, but seasonal vulnerability to fracture and winter isolation might also be influential.
Keywords: hip fractures, trends, variation, elderly
Introduction
Hip fractures can have catastrophic outcomes for older adults. (1) They lead to pain, loss of function, institutionalization, and even death.(2,3) With our aging population, hip fractures will continue to be a population health problem.
In Canada, there have been increased efforts to both prevent and improve the care and outcomes of patients with hip fractures.(4,5) In this setting, a thorough understanding of the risk factors for these fractures remains important.
The seasonality of health outcomes can provide insights about the etiology of disease, as well as help care professionals and policy makers anticipate and prepare for outcomes.(6) Where seasonal trends in hip fractures have been described across geographic regions previously, peaks were observed in cooler months.(7–9) Slippery surfaces due to freezing temperatures, and vitamin D deficiency due to lack of sunlight, have been proposed as contributors to these trends.(7,9–11) However, non-weather factors might also partly explain hip fracture trends. People might be more vulnerable to falls at different times of year due to underlying illness or comorbidities such as infections. Seasonal variation in fracture rates might also be driven by temporal differences in social or behavioural factors.(6) Patients, for example, might avoid or be unable to receive health care during particular times of the year (e.g., the holidays),(12) which could impact their susceptibility to health outcomes. Additionally, if people travel outside a region at different times of the year (i.e., seasonal migration from cold to warm climates),(12) their fractures might not be ascertainable in research studies.
There has been no recent evaluation of monthly variation in hip fractures in older adults in Canada. In the current study we provide an updated assessment of fracture trends in those >65 years in the province of Ontario. We hypothesized that hip fractures would be most common during the cooler months. To examine potential reasons for seasonal variation in hip fractures, we ascertained the demographic characteristics and comorbidity profiles of patients with fractures over the months. To determine whether social or behavioural factors might influence fracture trends, we also examined variations in health-care utilization including visits to health-care professionals, blood tests, and prescription medication dispensing.
METHODS
Study Design and Setting
We conducted a population-based study of adults age >65 years in Ontario, Canada from January 1 2011 until December 31 2015. We divided our study period into one-month intervals.
Ontario is a northern region between the 42nd and 57th latitudes, with cold winters and subzero temperatures. We have over 13 million residents who have universal coverage for hospitalizations, physician visits, and diagnostic testing. (13) People aged ≥65 years have universal access to prescription medications. Information on their health-care utilization is maintained in databases held at ICES. Databases are linked using unique, encoded identifiers. We report our study using the RECORD recommendations (Appendix A).(14)
Our study was approved by the research ethics board at Sunnybrook Health Sciences Centre (Toronto, Ontario) and was analyzed at ICES according to a pre-specified protocol. Participant consent was not necessary as ICES is a named entity under Ontario’s Personal Health Information Protection Act, and is able to receive and use health information to examine the province’s health-care system.
Patients
We considered for inclusion all adults >65 years with a hospitalization for a hip fracture over the study period (defined as at least one code for a hip fracture during an inpatient hospital stay). Our coding algorithm for hip fracture included diagnostic and medical billing codes,(15) similar to one validated previously (positive predictive value 83%, sensitivity 99%) (16) (Appendix B).
Prior to each study interval, we excluded the records of those: 1) with a missing age, sex or an invalid identification number, 2) an extreme or invalid age (negative age or age >105), 3) who died on or before the study interval, or 4) who were not permanent residents of Ontario (i.e., did not have an Ontario residential postal code at the time of hospitalization). If patients had more than one encounter for a hip fracture over the study interval (i.e., month), we included only their first hospitalization.
Data Sources
We used the records of several databases as our data sources. A full description of each database is included in Appendix C. In brief, the Registered Persons Database of Ontario provided demographic information for people who had been issued an Ontario health card. We used the Yearly Ontario Intercensal and Postcensal Population Estimates and Projection database to determine population denominators (Ontario Ministry of Health and Long-Term Care: IntelliHEALTH Ontario). We used the Ontario Drug Benefit (ODB) and the Drug Identification Number (DIN) databases to ascertain prescription medications. Prescription records are accurately maintained within the ODB with an error rate < 1%.(17) We assessed baseline comorbidities using the Ontario Diabetes Database (ODD),(18) the Ontario Chronic Obstructive Pulmonary Disease,(19) Rheumatoid Arthritis,(20) and Crohn’s and Colitis Datasets.(21) Home care visits were captured using the Home Care Database, and physician visits were ascertained using the ICES Physician Database. We used the Canadian Institute for Health Information’s Discharge Abstract Database (CIHI-DAD) and the National Ambulatory Care Reporting System (NACRS) Database to collect diagnostic and procedural information captured during hospital admissions and emergency department visits, respectively. We additionally used the Ontario Health Insurance Plan (OHIP) Database to capture patient diagnoses and procedures.
Covariates and outcomes were ascertained by a patient’s presence in a derived database (e.g., the ODD), or administrative codes (Appendix D). Administrative codes are entered into databases including the CIHI-DAD and NACRS by trained personnel, based upon diagnoses recorded in the medical record by the health-care team. We used International Classification of Diseases 10th Revision (ICD-10), the enhanced Canadian version of the 10th Revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10 CA), Canadian Classification of Health Interventions (CCI, post-2002), and OHIP fee and diagnostic codes.
Outcomes
Our primary outcome was monthly variation in hip fracture hospitalizations in those >65 years. Because the risk of osteoporosis and hip fracture is highest in the oldest old (i.e., ≥80 years),(22) we stratified trends by age group (i.e., 66–79, ≥80 years).
We performed several additional analyses. We noted monthly variation in the demographic characteristics, comorbidity profiles, and health-care utilization of patients with fractures between September 2012 and August 2013 (study midpoint). We also examined trends in prescription medication dispensing for statins, as statins are the most commonly prescribed medication to older adults in Canada.(23) Finally, to evaluate if seasonality in hip fractures might be due to older adults leaving our region over particular months of the year (i.e., ‘snowbirding’),(24) we examined monthly variation in travel supplies for statins (i.e., a prescription dispensed for >100 tablets), over the study period.(25)
Statistical Analysis
Numerators were the number of patients with at least one hospital encounter with a hip fracture over the study period, stratified by age. Denominators for all months were the estimated population of Ontario on July 1st of the relevant year in the age strata. To consider population changes over time, we calculated monthly encounter rates and their associated 95% confidence intervals (CI).
Based upon prior methods,(6) we used time series analyses to assess for seasonality in hip fractures, as well as the strength of the relationship. We first visualized the raw data and then fitted regression lines to detect trends. We then used differencing methods to ensure data stationarity. Next, we used spectral analyses to detect statistically significant seasonality, the Fisher Kappa Test to determine if there was a major sinusoidal component, and the Bartlett Kolmogorov Smirnov Test to examine departures from the null hypothesis of pure white noise. We also examined spectral plots of the data (i.e., spectral density vs. data frequency) to identify the cyclic structure of the series, and we conducted multitaper spectral analyses. To examine the strength of the seasonal relationships, we generated R2 autoregression coefficients, which are the coefficients of determination of the autoregressive regression models fitted to the data. Coefficients of 0 to < 0.4 represent weak seasonality, 0.4 to < 0.7 moderate to strong, and 0.7 to 1 very strong to perfect seasonality.(6,26)
We used descriptive statistics to summarize the characteristics, comorbidities, and health-care patterns of patients with a hip fracture from September 2012 until August 2013. If they had more than one encounter that year, we ascertained characteristics at the time of their first encounter only. We used Chi-squared analyses, one-way ANOVA, and Kruskal-Wallis tests to examine differences in covariates across the calendar months. For our prescription dispensing analysis, numerators were the number of patients 66–79 and ≥80 years with at least one statin prescription during the study interval, and denominators were the estimated population on July 1st of the relevant year in the age strata. We calculated monthly prescription rates and their associated 95% CIs. To evaluate for the possibility of snowbirding, we examined the maximum day supply of a statin per patient each month, and determined the number with a day supply of statins >100 days. The denominator for this analysis was the total number of people who filled a statin prescription within the calendar year, across the two age groups. All analyses were conducted in SAS version 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
Prior to each study interval, we excluded 12–51 (0.5–2.2%) records based upon an invalid identification number or sex, 141–220 (5.8–9.5%) records that had an invalid age, <6 records with a death recorded on or before the study interval, and <6 records for those who were not permanent residents of Ontario.
We included a total of 47,971 and 52,088 hip fracture hospitalizations in those 66–79 and ≥80 years between 2011–2015 in Ontario, Canada (42,694 and 47,648 unique patients, respectively).
Variation in Hip Fractures
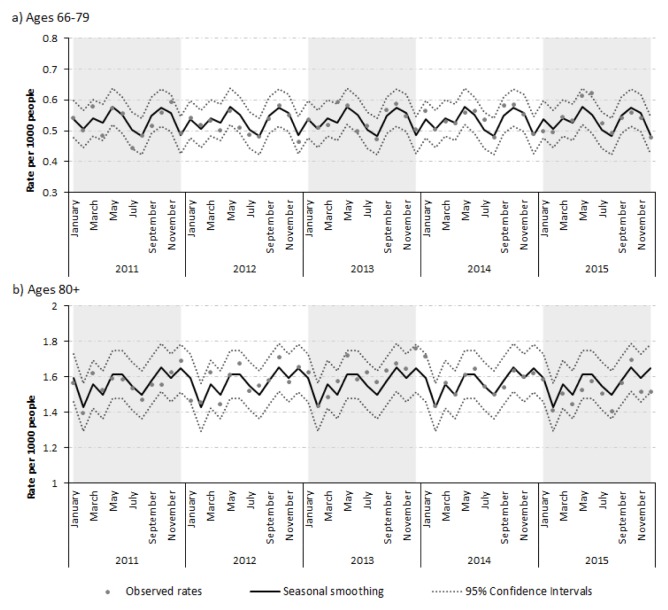
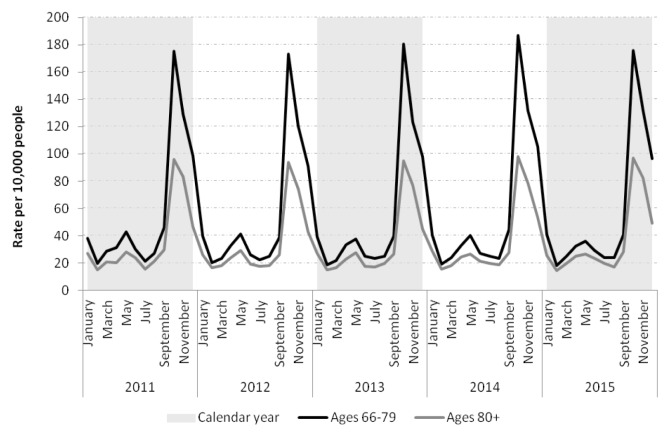
Hip fracture rates appeared stable over time across groups. Strong seasonality was apparent in both age groups (Table 1). Fractures peaked in October and December, with a smaller peak in May. In those ≥80 years, fractures fell to a minimum in February. Fracture rates appeared to drop to a minimum in August in those 66–79 (Figure 1).
TABLE 1.
Statistical summary of seasonality of hip fracture encounters
| Age Group | Fisher Kappa | BKS (p-value) | Autoregression r2 Ccoefficienta | Cycles (months) |
|---|---|---|---|---|
| 66–79 | 5.714 | 0.2791 (0.022) | 0.625 | 6, 4 |
| ≥80 | 4.460 | 0.2799 (0.021) | 0.645 | 6, 3, 12 |
| All ages | 5.317 | 0.2965 (0.012) | 0.725 | 6, 4, 3 |
An r2 coefficient of 0 to < 0.4 represents weak seasonality, 0.4 to < 0.7 moderate to strong seasonality, and 0.7 to 1 very strong to perfect seasonality.
BKS = Barlett Kolmogorov Smirnov
FIGURE 1.
Rates of hip fracture encounters in patients 66–79 and ≥80 years in Ontario from 2011–2015
Seasonal Characteristics of Fracture Patients
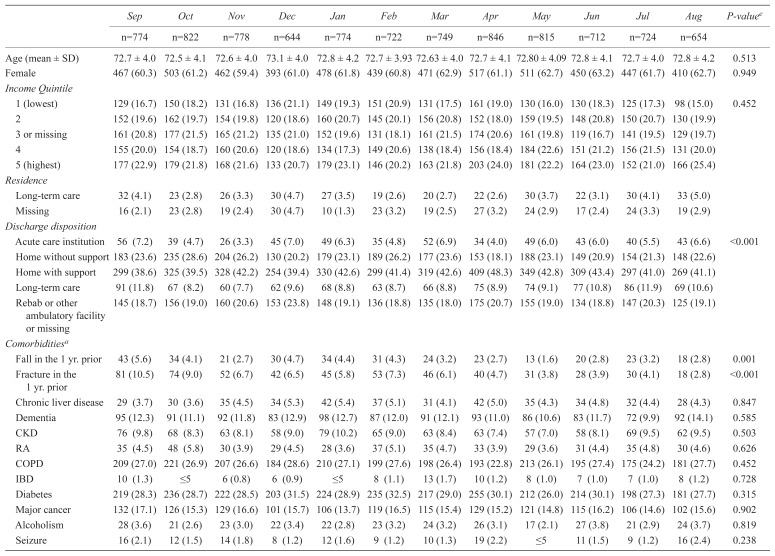
The monthly characteristics of patients with hip fractures are presented in Tables 2 and 3. Compared with those who fractured during other months, patients with an autumn and winter fracture were more likely to have experienced a fall or fracture in the year prior. Those who were 66–79 years also appeared to have more comorbidities (i.e., higher Charlson comorbidity index), and used more unique medications than those who fractured at other times of the year.
TABLE 2.
Characteristics of patients aged 66–79 with hip fractures from September 2012 to August 2013 (N=9014)
| Sep | Oct | Nov | Dec | Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | P-valuee | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| n=774 | n=822 | n=778 | n=644 | n=774 | n=722 | n=749 | n=846 | n=815 | n=712 | n=724 | n=654 | ||
| Age (mean ± SD) | 72.7 ± 4.0 | 72.5 ± 4.1 | 72.6 ± 4.0 | 73.1 ± 4.0 | 72.8 ± 4.2 | 72.7 ± 3.93 | 72.63 ± 4.0 | 72.7 ± 4.1 | 72.80 ± 4.09 | 72.8 ± 4.1 | 72.7 ± 4.0 | 72.8 ± 4.2 | 0.513 |
| Female | 467 (60.3) | 503 (61.2) | 462 (59.4) | 393 (61.0) | 478 (61.8) | 439 (60.8) | 471 (62.9) | 517 (61.1) | 511 (62.7) | 450 (63.2) | 447 (61.7) | 410 (62.7) | 0.949 |
| Income Quintile | |||||||||||||
| 1 (lowest) | 129 (16.7) | 150 (18.2) | 131 (16.8) | 136 (21.1) | 149 (19.3) | 151 (20.9) | 131 (17.5) | 161 (19.0) | 130 (16.0) | 130 (18.3) | 125 (17.3) | 98 (15.0) | 0.452 |
| 2 | 152 (19.6) | 162 (19.7) | 154 (19.8) | 120 (18.6) | 160 (20.7) | 145 (20.1) | 156 (20.8) | 152 (18.0) | 159 (19.5) | 148 (20.8) | 150 (20.7) | 130 (19.9) | |
| 3 or missing | 161 (20.8) | 177 (21.5) | 165 (21.2) | 135 (21.0) | 152 (19.6) | 131 (18.1) | 161 (21.5) | 174 (20.6) | 161 (19.8) | 119 (16.7) | 141 (19.5) | 129 (19.7) | |
| 4 | 155 (20.0) | 154 (18.7) | 160 (20.6) | 120 (18.6) | 134 (17.3) | 149 (20.6) | 138 (18.4) | 156 (18.4) | 184 (22.6) | 151 (21.2) | 156 (21.5) | 131 (20.0) | |
| 5 (highest) | 177 (22.9) | 179 (21.8) | 168 (21.6) | 133 (20.7) | 179 (23.1) | 146 (20.2) | 163 (21.8) | 203 (24.0) | 181 (22.2) | 164 (23.0) | 152 (21.0) | 166 (25.4) | |
| Residence | |||||||||||||
| Long-term care | 32 (4.1) | 23 (2.8) | 26 (3.3) | 30 (4.7) | 27 (3.5) | 19 (2.6) | 20 (2.7) | 22 (2.6) | 30 (3.7) | 22 (3.1) | 30 (4.1) | 33 (5.0) | |
| Missing | 16 (2.1) | 23 (2.8) | 19 (2.4) | 30 (4.7) | 10 (1.3) | 23 (3.2) | 19 (2.5) | 27 (3.2) | 24 (2.9) | 17 (2.4) | 24 (3.3) | 19 (2.9) | |
| Discharge disposition | |||||||||||||
| Acute care institution | 56 (7.2) | 39 (4.7) | 26 (3.3) | 45 (7.0) | 49 (6.3) | 35 (4.8) | 52 (6.9) | 34 (4.0) | 49 (6.0) | 43 (6.0) | 40 (5.5) | 43 (6.6) | <0.001 |
| Home without support | 183 (23.6) | 235 (28.6) | 204 (26.2) | 130 (20.2) | 179 (23.1) | 189 (26.2) | 177 (23.6) | 153 (18.1) | 188 (23.1) | 149 (20.9) | 154 (21.3) | 148 (22.6) | |
| Home with support | 299 (38.6) | 325 (39.5) | 328 (42.2) | 254 (39.4) | 330 (42.6) | 299 (41.4) | 319 (42.6) | 409 (48.3) | 349 (42.8) | 309 (43.4) | 297 (41.0) | 269 (41.1) | |
| Long-term care | 91 (11.8) | 67 (8.2) | 60 (7.7) | 62 (9.6) | 68 (8.8) | 63 (8.7) | 66 (8.8) | 75 (8.9) | 74 (9.1) | 77 (10.8) | 86 (11.9) | 69 (10.6) | |
| Rebab or other ambulatory facility or missing | 145 (18.7) | 156 (19.0) | 160 (20.6) | 153 (23.8) | 148 (19.1) | 136 (18.8) | 135 (18.0) | 175 (20.7) | 155 (19.0) | 134 (18.8) | 147 (20.3) | 125 (19.1) | |
| Comorbiditiesa | |||||||||||||
| Fall in the 1 yr. prior | 43 (5.6) | 34 (4.1) | 21 (2.7) | 30 (4.7) | 34 (4.4) | 31 (4.3) | 24 (3.2) | 23 (2.7) | 13 (1.6) | 20 (2.8) | 23 (3.2) | 18 (2.8) | 0.001 |
| Fracture in the 1 yr. prior | 81 (10.5) | 74 (9.0) | 52 (6.7) | 42 (6.5) | 45 (5.8) | 53 (7.3) | 46 (6.1) | 40 (4.7) | 31 (3.8) | 28 (3.9) | 30 (4.1) | 18 (2.8) | <0.001 |
| Chronic liver disease | 29 (3.7) | 30 (3.6) | 35 (4.5) | 34 (5.3) | 42 (5.4) | 37 (5.1) | 31 (4.1) | 42 (5.0) | 35 (4.3) | 34 (4.8) | 32 (4.4) | 28 (4.3) | 0.847 |
| Dementia | 95 (12.3) | 91 (11.1) | 92 (11.8) | 83 (12.9) | 98 (12.7) | 87 (12.0) | 91 (12.1) | 93 (11.0) | 86 (10.6) | 83 (11.7) | 72 (9.9) | 92 (14.1) | 0.585 |
| CKD | 76 (9.8) | 68 (8.3) | 63 (8.1) | 58 (9.0) | 79 (10.2) | 65 (9.0) | 63 (8.4) | 63 (7.4) | 57 (7.0) | 58 (8.1) | 69 (9.5) | 62 (9.5) | 0.503 |
| RA | 35 (4.5) | 48 (5.8) | 30 (3.9) | 29 (4.5) | 28 (3.6) | 37 (5.1) | 35 (4.7) | 33 (3.9) | 29 (3.6) | 31 (4.4) | 35 (4.8) | 30 (4.6) | 0.626 |
| COPD | 209 (27.0) | 221 (26.9) | 207 (26.6) | 184 (28.6) | 210 (27.1) | 199 (27.6) | 198 (26.4) | 193 (22.8) | 213 (26.1) | 195 (27.4) | 175 (24.2) | 181 (27.7) | 0.452 |
| IBD | 10 (1.3) | ≤5 | 6 (0.8) | 6 (0.9) | ≤5 | 8 (1.1) | 13 (1.7) | 10 (1.2) | 8 (1.0) | 7 (1.0) | 7 (1.0) | 8 (1.2) | 0.728 |
| Diabetes | 219 (28.3) | 236 (28.7) | 222 (28.5) | 203 (31.5) | 224 (28.9) | 235 (32.5) | 217 (29.0) | 255 (30.1) | 212 (26.0) | 214 (30.1) | 198 (27.3) | 181 (27.7) | 0.315 |
| Major cancer | 132 (17.1) | 126 (15.3) | 129 (16.6) | 101 (15.7) | 106 (13.7) | 119 (16.5) | 115 (15.4) | 129 (15.2) | 121 (14.8) | 115 (16.2) | 106 (14.6) | 102 (15.6) | 0.902 |
| Alcoholism | 28 (3.6) | 21 (2.6) | 23 (3.0) | 22 (3.4) | 22 (2.8) | 23 (3.2) | 24 (3.2) | 26 (3.1) | 17 (2.1) | 27 (3.8) | 21 (2.9) | 24 (3.7) | 0.819 |
| Seizure | 16 (2.1) | 12 (1.5) | 14 (1.8) | 8 (1.2) | 12 (1.6) | 9 (1.2) | 10 (1.3) | 19 (2.2) | ≤5 | 11 (1.5) | 9 (1.2) | 16 (2.4) | 0.238 |
| Other Conditions at Time of Encounterb | |||||||||||||
| Acute kidney injury | 11 (1.4) | 12 (1.5) | 11 (1.4) | 14 (2.2) | 12 (1.6% | 11 (1.5) | ≤5 | 13 (1.5) | 9 (1.1) | 16 (2.2) | 13 (1.8) | 14 (2.1) | 0.469 |
| Sepsis | ≤5 | 7 (0.9) | ≤5 | ≤5 | ≤5 | ≤5 | ≤5 | 6 (0.7) | ≤5 | ≤5 | ≤5 | ≤5 | 0.347 |
| Rhabdomyolysis | ≤5 | ≤5 | ≤5 | ≤5 | ≤5 | ≤5 | ≤5 | 7 (0.8) | ≤5 | ≤5 | ≤5 | 6 (0.9) | 0.655 |
| AMI | 11 (1.4) | 12 (1.5) | 12 (1.5) | 10 (1.6) | 8 (1.0) | 10 (1.4) | 11 (1.5) | 9 (1.1) | 10 (1.2) | 9 (1.3) | 11 (1.5) | ≤5 | 0.978 |
| Charlson Comorbidity Index | |||||||||||||
| Mean ± SD | 1.2 ± 1.9 | 1.1 ± 1.9 | 1.0 ± 1.7 | 1.3 ± 1.8 | 1.0 ± 1.6 | 1.2 ± 1.8 | 1.10 ± 1.82 | 1.0 ± 1.8 | 0.9 ± 1.6 | 1.1 ± 1.7 | 1.0 ± 1.8 | 1.00± 1.7 | 0.009 |
| Median (IQR) | 0 (0–2) | 0 (0–2) | 0 (0–1) | 1 (0–2) | 0 (0–1) | 0 (0–2) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | <0.001 |
| 0 (no hospitalization) | 428 (55.3) | 467 (56.8) | 457 (58.7) | 317 (49.2) | 434 (56.1) | 378 (52.4) | 407 (54.3) | 490 (57.9) | 487 (59.8) | 393 (55.2) | 441 (60.9) | 386 (59.0) | 0.006 |
| 1 | 132 (17.1) | 147 (17.9) | 150 (19.3) | 135 (21.0) | 153 (19.8) | 151 (20.9) | 165 (22.0) | 155 (18.3) | 156 (19.1) | 144 (20.2) | 121 (16.7) | 109 (16.7) | |
| 2 | 80 (10.3) | 86 (10.5) | 71 (9.1) | 81 (12.6) | 90 (11.6) | 81 (11.2) | 76 (10.1) | 85 (10.0) | 76 (9.3) | 69 (9.7) | 66 (9.1) | 77 (11.8) | |
| 3+ | 134 (17.3) | 122 (14.8) | 100 (12.9) | 111 (17.2) | 97 (12.5) | 112 (15.5) | 101 (13.5) | 116 (13.7) | 96 (11.8) | 106 (14.9) | 96 (13.3) | 82 (12.5) | |
| Medicationsc | |||||||||||||
| Statins | 332 (42.9) | 352 (42.8) | 365 (46.9) | 277 (43.0) | 348 (45.0) | 328 (45.4) | 339 (45.3) | 376 (44.4) | 355 (43.6) | 299 (42.0) | 318 (43.9) | 282 (43.1) | 0.827 |
| Bisphosphonate (1 yr) | 147 (19.0) | 135 (16.4) | 154 (19.8) | 129 (20.0) | 151 (19.5) | 127 (17.6) | 128 (17.1) | 144 (17.0) | 140 (17.2) | 131 (18.4) | 135 (18.6) | 112 (17.1) | 0.667 |
| Denosumab (1 yr) | ≤5 | 7 (0.9) | ≤5 | 6 (0.9) | 10 (1.3) | ≤5 | 6 (0.8) | 9 (1.1) | 13 (1.6) | 14 (2.0) | 11 (1.5) | 12 (1.8) | 0.075 |
| Glucocorticoid (1 yr) | 74 (9.6) | 68 (8.3) | 79 (10.2) | 55 (8.5) | 69 (8.9) | 68 (9.4) | 54 (7.2) | 63 (7.4) | 68 (8.3) | 65 (9.1) | 64 (8.8) | 65 (9.9) | 0.650 |
| PPI | 270 (34.9) | 287 (34.9) | 255 (32.8) | 211 (32.8) | 260 (33.6) | 228 (31.6) | 231 (30.8) | 275 (32.5) | 222 (27.2) | 234 (32.9) | 237 (32.7) | 217 (33.2) | 0.128 |
| Anticonvulsant | ≤5 | ≤5 | 7 (0.9) | ≤5 | ≤5 | ≤5 | ≤5 | ≤5 | ≤5 | ≤5 | ≤5 | ≤5 | 0.040 |
| Antineoplastic | 13 (1.7) | 11 (1.3) | 7 (0.9) | 6 (0.9) | ≤5 | 11 (1.5) | 8 (1.1) | 8 (0.9) | 7 (0.9) | 10 (1.4) | ≤5 | 13 (2.0) | 0.278 |
| Number of Unique Drugs | |||||||||||||
| Mean ± SD | 8.8 ± 6.4 | 8.8 ± 6.3 | 8.3 ± 6.1 | 8.3 ± 5.9 | 8.5 ± 5.8 | 8.5 ± 5.9 | 8.3 ± 6.0 | 7.9 ± 5.8 | 7.6 ± 5.7 | 8.2 ± 6.2 | 8.3 ± 6.1 | 8.5 ± 6.4 | 0.005 |
| Median (IQR) | 8 (4–12) | 7 (4–12) | 7 (4–11) | 8 (4–11) | 7 (5–11) | 8 (4–12) | 7 (4–12) | 7 (4–11) | 7 (3–11) | 7 (4–12) | 7 (4–12) | 8 (4–12) | 0.007 |
| Health-care Visitsd | |||||||||||||
| At least one GP visit | 606 (78.3) | 665 (80.9) | 662 (85.1) | 506 (78.6) | 593 (76.6) | 566 (78.4) | 584 (78.0) | 660 (78.0) | 652 (80.0) | 565 (79.4) | 553 (76.4) | 488 (74.6) | <0.001 |
| Any blood test | 190 (24.5) | 201 (24.5) | 167 (21.5) | 139 (21.6) | 179 (23.1) | 186 (25.8) | 160 (21.) | 190 (22.5) | 199 (24.4) | 168 (23.6) | 159 (22.0) | 157 (24.0) | 0.558 |
| Any specialist visit | 640 (82.7) | 710 (86.4) | 672 (86.4) | 543 (84.3) | 649 (83.9) | 621 (86.0) | 616 (82.2) | 743 (87.8) | 677 (83.1) | 594 (83.4) | 623 (86.0) | 530 (81.0) | 0.005 |
| Home-care visit | 207 (26.7) | 210 (25.5) | 196 (25.2) | 142 (22.0) | 191 (24.7) | 167 (23.1) | 208 (27.8) | 216 (25.5) | 193 (23.7) | 180 (25.3) | 161 (22.2) | 165 (25.2) | 0.32 |
Unless indicated, data represents number (percent); cell sizes <6 are not presented for privacy.
Unless specified, comorbidities were ascertained in the 5 years prior.
Conditions present at the time of the encounter were those coded during the hip fracture hospitalization.
Unless specified, medication use was ascertained in the 180 days prior.
Unless specified, health-care visits were ascertained in the 30 days prior.
We used Chi-squared analyses, one-way ANOVA, and Kruskal-Wallis tests to determine if there were differences in covariates across the calendar months.
CKD = chronic kidney disease; RA = rheumatoid arthritis; COPD = chronic obstructive pulmonary disease; IBD = inflammatory bowel disease; AMI = acute myocardial infarction; PPI = proton pump inhibitor; GP = general practitioner.
TABLE 3.
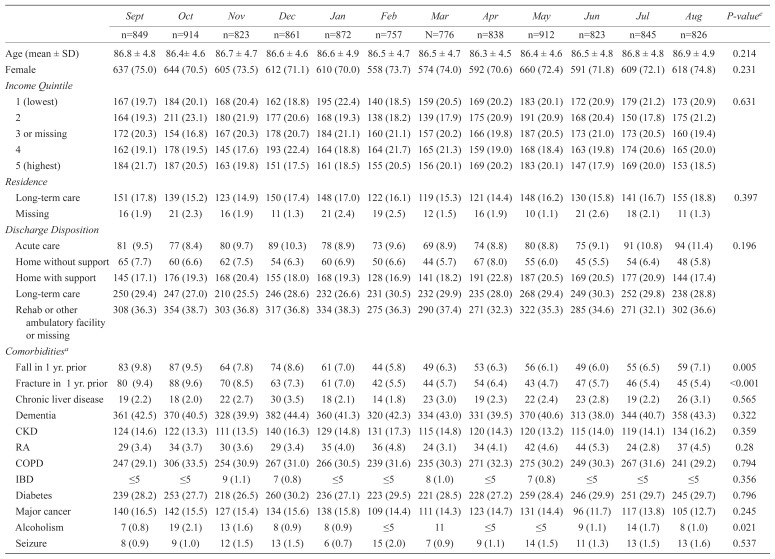
Characteristics of patients aged 80+ with hip fractures from September 2012 to August 2013 (N=10,096)
| Sept | Oct | Nov | Dec | Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | P-valuee | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| n=849 | n=914 | n=823 | n=861 | n=872 | n=757 | N=776 | n=838 | n=912 | n=823 | n=845 | n=826 | ||
| Age (mean ± SD) | 86.8 ± 4.8 | 86.4± 4.6 | 86.7 ± 4.7 | 86.6 ± 4.6 | 86.6 ± 4.9 | 86.5 ± 4.7 | 86.5 ± 4.7 | 86.3 ± 4.5 | 86.4 ± 4.6 | 86.5 ± 4.8 | 86.8 ± 4.8 | 86.9 ± 4.9 | 0.214 |
| Female | 637 (75.0) | 644 (70.5) | 605 (73.5) | 612 (71.1) | 610 (70.0) | 558 (73.7) | 574 (74.0) | 592 (70.6) | 660 (72.4) | 591 (71.8) | 609 (72.1) | 618 (74.8) | 0.231 |
| Income Quintile | |||||||||||||
| 1 (lowest) | 167 (19.7) | 184 (20.1) | 168 (20.4) | 162 (18.8) | 195 (22.4) | 140 (18.5) | 159 (20.5) | 169 (20.2) | 183 (20.1) | 172 (20.9) | 179 (21.2) | 173 (20.9) | 0.631 |
| 2 | 164 (19.3) | 211 (23.1) | 180 (21.9) | 177 (20.6) | 168 (19.3) | 138 (18.2) | 139 (17.9) | 175 (20.9) | 191 (20.9) | 168 (20.4) | 150 (17.8) | 175 (21.2) | |
| 3 or missing | 172 (20.3) | 154 (16.8) | 167 (20.3) | 178 (20.7) | 184 (21.1) | 160 (21.1) | 157 (20.2) | 166 (19.8) | 187 (20.5) | 173 (21.0) | 173 (20.5) | 160 (19.4) | |
| 4 | 162 (19.1) | 178 (19.5) | 145 (17.6) | 193 (22.4) | 164 (18.8) | 164 (21.7) | 165 (21.3) | 159 (19.0) | 168 (18.4) | 163 (19.8) | 174 (20.6) | 165 (20.0) | |
| 5 (highest) | 184 (21.7) | 187 (20.5) | 163 (19.8) | 151 (17.5) | 161 (18.5) | 155 (20.5) | 156 (20.1) | 169 (20.2) | 183 (20.1) | 147 (17.9) | 169 (20.0) | 153 (18.5) | |
| Residence | |||||||||||||
| Long-term care | 151 (17.8) | 139 (15.2) | 123 (14.9) | 150 (17.4) | 148 (17.0) | 122 (16.1) | 119 (15.3) | 121 (14.4) | 148 (16.2) | 130 (15.8) | 141 (16.7) | 155 (18.8) | 0.397 |
| Missing | 16 (1.9) | 21 (2.3) | 16 (1.9) | 11 (1.3) | 21 (2.4) | 19 (2.5) | 12 (1.5) | 16 (1.9) | 10 (1.1) | 21 (2.6) | 18 (2.1) | 11 (1.3) | |
| Discharge Disposition | |||||||||||||
| Acute care | 81 (9.5) | 77 (8.4) | 80 (9.7) | 89 (10.3) | 78 (8.9) | 73 (9.6) | 69 (8.9) | 74 (8.8) | 80 (8.8) | 75 (9.1) | 91 (10.8) | 94 (11.4) | 0.196 |
| Home without support | 65 (7.7) | 60 (6.6) | 62 (7.5) | 54 (6.3) | 60 (6.9) | 50 (6.6) | 44 (5.7) | 67 (8.0) | 55 (6.0) | 45 (5.5) | 54 (6.4) | 48 (5.8) | |
| Home with support | 145 (17.1) | 176 (19.3) | 168 (20.4) | 155 (18.0) | 168 (19.3) | 128 (16.9) | 141 (18.2) | 191 (22.8) | 187 (20.5) | 169 (20.5) | 177 (20.9) | 144 (17.4) | |
| Long-term care | 250 (29.4) | 247 (27.0) | 210 (25.5) | 246 (28.6) | 232 (26.6) | 231 (30.5) | 232 (29.9) | 235 (28.0) | 268 (29.4) | 249 (30.3) | 252 (29.8) | 238 (28.8) | |
| Rehab or other ambulatory facility or missing | 308 (36.3) | 354 (38.7) | 303 (36.8) | 317 (36.8) | 334 (38.3) | 275 (36.3) | 290 (37.4) | 271 (32.3) | 322 (35.3) | 285 (34.6) | 271 (32.1) | 302 (36.6) | |
| Comorbiditiesa | |||||||||||||
| Fall in 1 yr. prior | 83 (9.8) | 87 (9.5) | 64 (7.8) | 74 (8.6) | 61 (7.0) | 44 (5.8) | 49 (6.3) | 53 (6.3) | 56 (6.1) | 49 (6.0) | 55 (6.5) | 59 (7.1) | 0.005 |
| Fracture in 1 yr. prior | 80 (9.4) | 88 (9.6) | 70 (8.5) | 63 (7.3) | 61 (7.0) | 42 (5.5) | 44 (5.7) | 54 (6.4) | 43 (4.7) | 47 (5.7) | 46 (5.4) | 45 (5.4) | <0.001 |
| Chronic liver disease | 19 (2.2) | 18 (2.0) | 22 (2.7) | 30 (3.5) | 18 (2.1) | 14 (1.8) | 23 (3.0) | 19 (2.3) | 22 (2.4) | 23 (2.8) | 19 (2.2) | 26 (3.1) | 0.565 |
| Dementia | 361 (42.5) | 370 (40.5) | 328 (39.9) | 382 (44.4) | 360 (41.3) | 320 (42.3) | 334 (43.0) | 331 (39.5) | 370 (40.6) | 313 (38.0) | 344 (40.7) | 358 (43.3) | 0.322 |
| CKD | 124 (14.6) | 122 (13.3) | 111 (13.5) | 140 (16.3) | 129 (14.8) | 131 (17.3) | 115 (14.8) | 120 (14.3) | 120 (13.2) | 115 (14.0) | 119 (14.1) | 134 (16.2) | 0.359 |
| RA | 29 (3.4) | 34 (3.7) | 30 (3.6) | 29 (3.4) | 35 (4.0) | 36 (4.8) | 24 (3.1) | 34 (4.1) | 42 (4.6) | 44 (5.3) | 24 (2.8) | 37 (4.5) | 0.28 |
| COPD | 247 (29.1) | 306 (33.5) | 254 (30.9) | 267 (31.0) | 266 (30.5) | 239 (31.6) | 235 (30.3) | 271 (32.3) | 275 (30.2) | 249 (30.3) | 267 (31.6) | 241 (29.2) | 0.794 |
| IBD | ≤5 | ≤5 | 9 (1.1) | 7 (0.8) | ≤5 | ≤5 | 8 (1.0) | ≤5 | 7 (0.8) | ≤5 | ≤5 | ≤5 | 0.356 |
| Diabetes | 239 (28.2) | 253 (27.7) | 218 (26.5) | 260 (30.2) | 236 (27.1) | 223 (29.5) | 221 (28.5) | 228 (27.2) | 259 (28.4) | 246 (29.9) | 251 (29.7) | 245 (29.7) | 0.796 |
| Major cancer | 140 (16.5) | 142 (15.5) | 127 (15.4) | 134 (15.6) | 138 (15.8) | 109 (14.4) | 111 (14.3) | 123 (14.7) | 131 (14.4) | 96 (11.7) | 117 (13.8) | 105 (12.7) | 0.245 |
| Alcoholism | 7 (0.8) | 19 (2.1) | 13 (1.6) | 8 (0.9) | 8 (0.9) | ≤5 | 11 | ≤5 | ≤5 | 9 (1.1) | 14 (1.7) | 8 (1.0) | 0.021 |
| Seizure | 8 (0.9) | 9 (1.0) | 12 (1.5) | 13 (1.5) | 6 (0.7) | 15 (2.0) | 7 (0.9) | 9 (1.1) | 14 (1.5) | 11 (1.3) | 13 (1.5) | 13 (1.6) | 0.537 |
| Other Conditions at Time of Encounterb | |||||||||||||
| Acute kidney injury | 24 (2.8) | 38 (4.2) | 29 (3.5) | 28 (3.3) | 26 (3.0) | 30 (4.0) | 25 (3.2) | 31 (3.7) | 34 (3.7) | 34 (4.1) | 25 (3.0) | 28 (3.4) | 0.886 |
| Sepsis | 7 (0.8) | ≤5 | ≤5 | 11 (1.3) | ≤5 | ≤5 | 6 (0.8) | ≤5 | 6 (0.7) | ≤5 | 8 (0.9) | ≤5 | 0.335 |
| Rhabdomyolysis | ≤5 | 6 (0.7) | 7 (0.9) | ≤5 | 12 (1.4) | ≤5 | 6 (0.8) | ≤5 | 12 (1.3) | 8 (1.0) | 9 (1.1) | 14 (1.7) | 0.054 |
| AMI | 36 (4.2) | 40 (4.4) | 25 (3.0) | 31 (3.6) | 30 (3.4) | 34 (4.5) | 26 (3.4) | 27 (3.2) | 29 (3.2) | 28 (3.4) | 28 (3.3) | 20 (2.4) | 0.56 |
| Charlson Comorbidity Index | |||||||||||||
| Mean ± SD | 1.5 ± 1.8 | 1.4 ± 1.7 | 1.7 ± 1.8 | 1.5 ± 1.9 | 1.4 ± 1.8 | 1.5 ± 1.7 | 1.4 ± 1.9 | 1.5 ± 1.8 | 1.3 ± 1.7 | 1.5 ± 1.8 | 1.5± 1.8 | 1.4 ± 1.7 | 0.586 |
| Median (IQR) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 0.246 |
| 0 (no hospitalization) | 314 (37.0) | 356 (38.9) | 328 (39.9) | 322 (37.4) | 354 (40.6) | 274 (36.2) | 324 (41.8) | 331 (39.5) | 389 (42.7) | 297 (36.1) | 332 (39.3) | 321 (38.9) | 0.186 |
| 1 | 234 (27.6) | 245 (26.8) | 223 (27.1) | 232 (26.9) | 228 (26.1) | 199 (26.3) | 183 (23.6) | 200 (23.9) | 225 (24.7) | 235 (28.6) | 192 (22.7) | 204 (24.7) | |
| 2 | 105 (12.4) | 130 (14.2) | 116 (14.1) | 119 (13.8) | 126 (14.4) | 126 (16.6) | 114 (14.7) | 125 (14.9) | 123 (13.5) | 123 (14.9) | 143 (16.9) | 133 (16.1) | |
| 3+ | 196 (23.1) | 183 (20.0) | 156 (19.0) | 188 (21.8) | 164 (18.8) | 158 (20.9) | 155 (20.0) | 182 (21.7) | 175 (19.2) | 168 (20.4) | 178 (21.1) | 168 (20.3) | |
| Medicationsc | |||||||||||||
| Statins | 347 (40.9) | 366 (40.0) | 302 (36.7) | 346 (40.2) | 350 (40.1) | 346 (45.7) | 304 (39.2) | 350 (41.8) | 352 (38.6) | 345 (41.9) | 354 (41.9) | 340 (41.2) | 0.091 |
| Bisphosphonate (1 yr) | 232 (27.3) | 244 (26.7) | 239 (29.0) | 234 (27.2) | 234 (26.8) | 200 (26.4) | 219 (28.2) | 222 (26.5) | 238 (26.1) | 205 (24.9) | 195 (23.1) | 202 (24.5) | 0.331 |
| Denosumab (1 yr) | 12 (1.4) | 13 (1.4) | 10 (1.2) | 12 (1.4) | 19 (2.2) | 19 (2.5) | 12 (1.5) | 17 (2.0) | 18 (2.0) | 20 (2.4) | 22 (2.6) | 27 (3.3) | 0.072 |
| Glucocorticoid (1 yr) | 60 (7.1) | 69 (7.5) | 62 (7.5) | 73 (8.5) | 74 (8.5) | 56 (7.4) | 46 (5.9) | 69 (8.2) | 60 (6.6) | 76 (9.2) | 58 (6.9) | 53 (6.4) | 0.316 |
| PPI | 319 (37.6) | 333 (36.4) | 291 (35.4) | 300 (34.8) | 305 (35.0) | 266 (35.1) | 281 (36.2) | 303 (36.2) | 322 (35.3) | 294 (35.7) | 315 (37.3) | 312 (37.8) | 0.963 |
| Anticonvulsant | ≤5 | ≤5 | ≤5 | ≤5 | ≤5 | ≤5 | ≤5 | ≤5 | ≤5 | ≤5 | ≤5 | ≤5 | 0.243 |
| Antineoplastic | 13 (1.5) | 23 (2.5) | 20 (2.4) | 11 (1.3) | 19 (2.2) | 8 (1.1) | 19 (2.4) | 9 (1.1) | 12 (1.3) | 7 (0.9) | 12 (1.4) | 9 (1.1) | 0.023 |
| Number of Unique Drugs | |||||||||||||
| Mean ± SD | 10.2 ± 6.1 | 9.8 ± 6.0 | 9.6 ± 6.2 | 10.3 ± 6.2 | 10.0 ± 6.3 | 10.0 ± 6.5 | 10.0 ± 6.4 | 10.2 ± 6.3 | 9.9 ± 6.2 | 10.1 ± 6.5 | 10.0 ± 6.3 | 10.3 ± 6.4 | 0.442 |
| Median (IQR) | 9 (6–14) | 9(5–13) | 9(5–13) | 9 (6–14) | 9 (5–14) | 9 (5–13) | 9 (5–14) | 10 (6–14) | 9 (5–14) | 9 (5–14) | 9 (5–14) | 10 (6–14) | 0.307 |
| Health-care Visitsd | |||||||||||||
| At least one GP visit | 640 (75.4) | 725 (79.3) | 678 (82.4) | 673 (78.2) | 666 (76.4) | 561 (74.1) | 583 (75.1) | 633 (75.5) | 676 (74.1) | 620 (75.3) | 653 (77.3) | 594 (71.9) | <0.001 |
| Any blood test | 257 (30.3) | 276 (30.2) | 253 (30.7) | 270 (31.4) | 227 (26.0) | 228 (30.1) | 218 (28.1) | 231 (27.6) | 283 (31.0) | 240 (29.2) | 247 (29.2) | 227 (27.5) | 0.329 |
| Any specialist visit | 694 (81.7) | 745 (81.5) | 682 (82.9) | 702 (81.5) | 697 (79.9) | 616 (81.4) | 637 (82.1) | 715 (85.3) | 734 (80.5) | 681 (82.7) | 702 (83.1) | 669 (81.0) | 0.316 |
| Home care visit | 263 (31.0) | 278 (30.4) | 253 (30.7) | 291 (33.8) | 280 (32.1) | 239 (31.6) | 264 (34.0) | 261 (31.1) | 297 (32.6) | 260 (31.6) | 267 (31.6) | 258 (31.2) | 0.906 |
Unless indicated, data represents number (percent); cell sizes <6 are not presented for privacy.
Unless specified, comorbidities were ascertained in the 5 years prior.
Conditions present at the time of the encounter were those coded during the hip fracture hospitalization
Unless specified, medication use was ascertained in the 180 days prior.
Unless specified, healthcare utilization was ascertained in the 30 days prior.
We used Chi-squared analyses, one-way ANOVA, and Kruskal-Wallis tests to determine if there were differences in covariates across the calendar months.
CKD = chronic kidney disease; RA = rheumatoid arthritis; COPD = chronic obstructive pulmonary disease; IBD = inflammatory bowel disease; AMI = acute myocardial infarction; PPI = proton pump inhibitor; GP = general practitioner.
Health-care Utilization
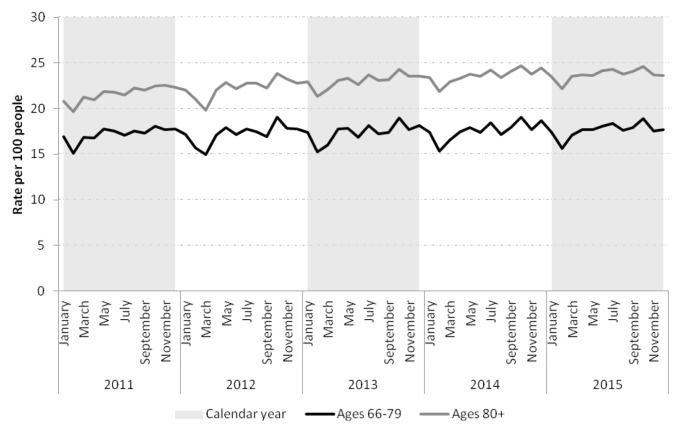
The health-care utilization of patients varied monthly. Primary care visits appeared less frequent across groups in the late winter and, in the oldest old, blood tests and specialist visits also appeared slightly less frequent (Tables 2 and 3). Statin prescription rates declined across both age groups in the late winter (Figure 2). Both groups showed evidence of snowbirding over the winter months (i.e., higher rate of travel prescription fills in the late fall), but this appeared more common in those 66–79 years than in the oldest old (Figure 3).
FIGURE 2.
Statin prescription rates per 100 people aged 66–79 and ≥80 in Ontario
FIGURE 3.
Rate of statin prescriptions with greater than 100 days supply per 10,000 people in Ontario, categorized by age groups, 66–79 and ≥80 years
DISCUSSION
Main Findings
In older adults in Ontario, hip fractures are most common in the late autumn, early winter, and spring. In the oldest old, hip fracture rates appeared lowest in the late winter.
As previously suggested, environmental factors might contribute to these trends. With exposure to ice and snow in the early winter (perhaps more so during the holidays),(8,27,28) older people might be predisposed to slips and falls. A systematic review of studies that examined the association between hip fractures and at least one weather variable across 18 locations found that snow and ice were positively correlated with hip fractures at all latitudes.(7) As hip fractures also peak in regions with no ice or snow,(29,30) colder temperatures (especially the acute lowering of temperature) might also contribute to monthly variation. When exposed to the cold, people might experience hypothermia and poor coordination. They might also dress in layers which could lead to trips and falls.(7) An acute drop in temperature from a warmer summer to a cooler autumn might have contributed to the fracture peak we saw in October, especially if people had not yet adapted to the temperature change.(31)
Vitamin D deficiency could have also contributed to fracture trends.(8,10,11,32) Vitamin D is typically lowest at the end of the winter when parathyroid hormone is high.(10) During winter months, bone turnover also increases and bone density declines.(11,33) These changes might have predisposed individuals to fracture. Vitamin D deficiency can also impair muscle strength and coordination.(34) A darker winter might have also impaired visual acuity.(35)
Beyond environmental influences, we found evidence that patient characteristics, comorbidities, and social/behavioural factors might contribute to hip fracture trends in older adults. Older adults who had a fracture appeared to be in poorer health in the autumn and winter. During these seasons, infections are more common,(6) and patients might have been predisposed to falls and fracture while they were ill.
Respiratory illnesses peak in the spring in the older age groups,(36) when we noted a second peak in fracture rates. This peak might have also been due to people becoming active again outside. In our study, people who had a hip fracture in the spring appeared healthier than those who fractured at other times of the year. Previous studies have noted that healthy active people are at higher risk of outdoor falls while engaging in vigorous activities.(37,38)
Of particular interest, we observed that in the oldest old, fractures appeared less common in the late winter. During the late winter it is coldest in Canada(39) and older adults might simply avoid the outdoors, especially if they have comorbidities, are immobilized, or institutionalized.(9,40,41) Aligned with this, their health care utilization also appeared to decline with fewer primary care visits and a lower rate of prescription medication dispensing. Although there was evidence of snowbirding in both age groups, the trend seemed most apparent in those 66–79 years. This trend wouldn’t sufficiently explain the decline in health-care utilization in the oldest old, suggesting that older seniors might avoid going outside during the late winter.
Comparison with Previous Literature
Hip fractures have been described to peak in the autumn and winter in older Canadian studies from Montreal.(9,27) Outside of Canada, in a Spanish study of people >45 years (mean age 80 years), there was also an increased risk of fracture during these months.(29) Similar trends have been described in Taiwan,(8) as well as across the United States,(42) Israel,(28) Scotland, Hong Kong, New Zealand,(30) and Norway.(43)
The decline in fractures in those ≥80 in the late winter has not been thoroughly described. While most studies note a trough in fracture rates over the summer months,(41,44) our extreme winter climate where temperatures can drop below −30°C in areas, might partly explain our findings.
Strengths and Weaknesses
There are several strengths to the current study. It is a large, up-to-date, population-based study of 100,059 hip fracture encounters (89,913 unique individuals) in our province over a five-year period. Rather than simply describing fracture variation, we evaluated trends within two age strata, and examined variation in patient comorbidities and health services utilization.
There are some limitations to discuss. We could not exclude high-velocity fractures (i.e., from motor vehicle collisions) or pathological hip fractures, although it is estimated that over 95% of hip fractures are due to a fall.(45) We also included procedural codes for hip fractures, and thus, double counting of encounters could have occurred. However, we minimized this risk by limiting to one hip fracture encounter per month. We used administrative data to ascertain our outcomes and this data were not specifically created to address our research question. However, our data can minimize the bias associated with self-report and surveys.(16) Changes in coding definitions might have occurred over the study period and may have affected our temporal trend analyses. We also could not determine the location of the hip fractures (i.e., indoors vs. outdoors). Our study population also included a mix of both community and long-term care residents.
Due to the size and duration of our study, we were only able to ascertain the characteristics and comorbidities of patients over one year. We were unable to evaluate factors, including frailty and balance, which can impact fracture risk. Importantly, this was an ecological study and we cannot infer causation at an individual level. Our results are only fully generalizable to those living in Ontario.
Implications
Our study has implications for practice, policy, and research. First, given the possible link between hip fractures and environmental factors, patients should be advised to be cautious while traversing snow and ice, and to wear proper footwear. (46) Even if indoors during the cooler months, they might be warned of tripping hazards, especially when layering for the colder weather. When people are in poorer health, they might be cautioned about the risk of falls. Further, physicians might replace vitamin D and ensure adequate calcium intake during the winter months, especially in the frail and institutionalized. (34,47,48) A role for UV light exposure in the winter has also been suggested.(8)
From a policy standpoint, health administrators might plan resources accordingly. Operating times for hip fractures might be opened in the cooler seasons, and hospitals might be prepared for more consultations. Awareness of fracture peaks might promote timely assessments and treatment.(4) Troughs in fracture rates and health services utilization over the winter might raise the possibility of social isolation; as a result, ensuring that home-based resources are available might be of importance (e.g., home care services, exercise programs, food delivery). This might be especially needed by those who are too frail to leave their residence.(35) For city workers and planners, timely ice and snow removal also remains important.(35)
Finally, from a research perspective, researchers might better consider snowbirding when conducting trend analyses, to ensure accurate denominators.
CONCLUSIONS
In our region, the oldest old have a unique pattern of hip fracture hospitalizations across the months. Beyond environmental influences, complex patient, social, and behavioural factors might contribute to observed trends.
ACKNOWLEDGEMENTS
This project was conducted at ICES Western, and was funded by an Innovations Grant from the Academic Medical Organization of Southwestern Ontario (AMOSO). ICES is funded by an annual grant from the Ontario Ministry of Health and Long-term Care (MOHLTC). Core funding for ICES Western is provided by AMOSO, the Schulich School of Medicine and Dentistry (SSMD), Western University, and the Lawson Health Research Institute (LHRI). Parts of this material are based upon data and information compiled and provided by the Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions and statements expressed herein are those of the authors, and not necessarily those of CIHI.
APPENDICES
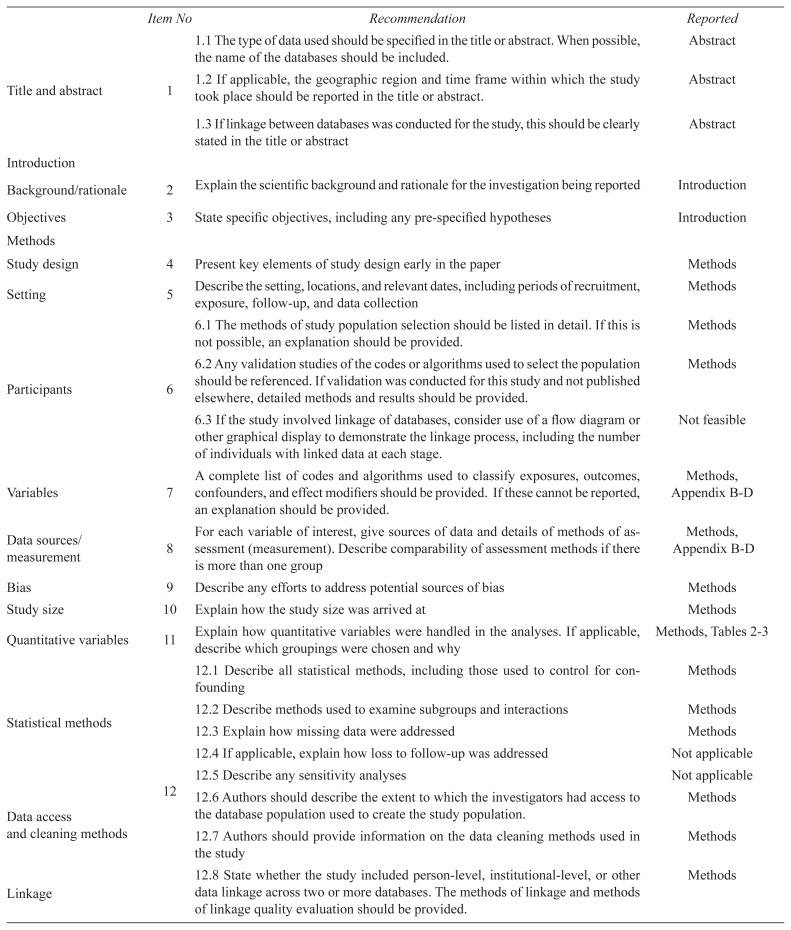
APPENDIX A. RECORD checklist of recommendations for the reporting of studies conducted using routinely collected health data
| Item No | Recommendation | Reported | |
|---|---|---|---|
| Title and abstract | 1 | 1.1 The type of data used should be specified in the title or abstract. When possible, the name of the databases should be included. | Abstract |
| 1.2 If applicable, the geographic region and time frame within which the study took place should be reported in the title or abstract. | Abstract | ||
| 1.3 If linkage between databases was conducted for the study, this should be clearly stated in the title or abstract | Abstract | ||
| Introduction | |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | Introduction |
| Objectives | 3 | State specific objectives, including any pre-specified hypotheses | Introduction |
| Methods | |||
| Study design | 4 | Present key elements of study design early in the paper | Methods |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | Methods |
| Participants | 6 | 6.1 The methods of study population selection should be listed in detail. If this is not possible, an explanation should be provided. | Methods |
| 6.2 Any validation studies of the codes or algorithms used to select the population should be referenced. If validation was conducted for this study and not published elsewhere, detailed methods and results should be provided. | Methods | ||
| 6.3 If the study involved linkage of databases, consider use of a flow diagram or other graphical display to demonstrate the linkage process, including the number of individuals with linked data at each stage. | Not feasible | ||
| Variables | 7 | A complete list of codes and algorithms used to classify exposures, outcomes, confounders, and effect modifiers should be provided. If these cannot be reported, an explanation should be provided. | Methods, Appendix B–D |
| Data sources/measurement | 8 | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | Methods, Appendix B–D |
| Bias | 9 | Describe any efforts to address potential sources of bias | Methods |
| Study size | 10 | Explain how the study size was arrived at | Methods |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | Methods, Tables 2–3 |
| Statistical methods | 12 | 12.1 Describe all statistical methods, including those used to control for confounding | Methods |
| 12.2 Describe methods used to examine subgroups and interactions | Methods | ||
| 12.3 Explain how missing data were addressed | Methods | ||
| 12.4 If applicable, explain how loss to follow-up was addressed | Not applicable | ||
| 12.5 Describe any sensitivity analyses | Not applicable | ||
| Data access and cleaning methods | 12.6 Authors should describe the extent to which the investigators had access to the database population used to create the study population. | Methods | |
| 12.7 Authors should provide information on the data cleaning methods used in the study | Methods | ||
| Linkage | 12.8 State whether the study included person-level, institutional-level, or other data linkage across two or more databases. The methods of linkage and methods of linkage quality evaluation should be provided. | Methods | |
| Results | |||
| Participants | 13 | 13.1 Describe in detail the selection of the persons included in the study (i.e. study population selection), including filtering based on data quality, data availability, and linkage. The selection of included persons can be described in the text and/or by means of the study flow diagram. | Results |
| Descriptive data | 14 | 14.1 Give characteristics of study participants (e.g. demographic, clinical, social) and information on exposures and potential confounders | Results, Tables 2–3 |
| 14.2 Indicate number of participants with missing data for each variable of interest | Tables 2–3 | ||
| 14.3 Summarize follow-up time (e.g. average and total amount) | Results | ||
| Outcome data | 15 | Report numbers of outcome events or summary measures over time | Results, Figure 1 |
| Main results | 16 | 16.1 Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (e.g. 95% confidence interval). Make clear which confounders were adjusted for and why they were included | Results |
| 16.2 Report category boundaries when continuous variables were categorized | Tables 2–3 | ||
| 16.3 If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | Not applicable | ||
| Other analyses | 17 | Report other analyses done—e.g. analyses of subgroups and interactions, and sensitivity analyses | Not applicable |
| Discussion | |||
| Key results | 18 | Summarize key results with reference to study objectives | Discussion |
| Limitations | 19 | Discuss the implications of using data that were not created or collected to answer the specific research question(s). Include discussion of misclassification bias, unmeasured confounding, missing data and changing eligibility over time, as they pertain to the study being reported. | Discussion |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | Discussion |
| Generalizability | 21 | Discuss the generalizability (external validity) of the study results | Discussion |
| Other information | |||
| Funding | 22 | 22.1 Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | Acknowledgments |
| 22.2 Authors should provide information on how to access any supplemental information such as the study protocol, raw data, or programming code. | The data set from this study is held securely in coded form at ICES. While data sharing agreements prohibit ICES from making the data set publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS. | ||
| Accessibility of protocol, raw data and programming code | |||
APPENDIX B. Hip fracture coding algorithm
| Variable | Database | Codes |
|---|---|---|
| Hip fracture | CIHI-DAD | ICD 10/ICD 10-CA: S720, S721 CCI: 1VA73, 1VC73, 1VA74, 1VA53, 1VC74, 1VA80 |
CIHI-DAD = Canadian Institute for Health Information’s-Discharge Abstract Database; CCI = Canadian Classification of Health Interventions; ICD-10/ICD 10-CA = International Classification of Diseases 10th Revision/Enhanced Canadian version of the 10th Revision of the International Statistical Classification of Diseases and Health Related Problems.
APPENDIX C. Description of ICES databases
| Database | Details |
|---|---|
| Registered Persons Database of Ontario | Contains vital statistics for all residents who have been issued an Ontario health card. |
| Yearly Ontario Intercensal and Postcensal Population Estimates and Projection Database | Contains Ontario population estimates by sex, age and geographic areas. |
| Ontario Drug Benefits Database | Contains prescription medication for adults ≥65 years and those using social assistance. |
| Drug Identification Number Database | Contains Drug Identification Numbers used in Canada from 1990. |
| Ontario Diabetes Database | Contains all patients with diabetes identified since 1991 (based upon hospitalization codes and physician diagnoses). |
| Ontario Chronic Obstructive Pulmonary Disease Database | Contains all patients with COPD identified since 1991 (based upon hospitalization codes, physician services claims). |
| Ontario Rheumatoid Arthritis Database | Contains all patients with rheumatoid arthritis identified since 1991 (based upon hospitalization codes, physician services claims). |
| Ontario Crohn’s and Colitis Cohort Database | Includes all Ontarians with Crohn’s or colitis identified since 1991 (defined by hospitalization codes, prescription medications, and physicians’ services claims). |
| Home Care Database | Contains information about home care services acquired from the Ontario Association of Community Care Access Centres. |
| ICES Physicians Database | Contains information about all physicians in Ontario (ascertained from the Ontario Health Insurance Plan and the Ontario Physician Human Resource Data Centre). |
| Canadian Institute for Health Information’s Discharge Abstract Database | Contains diagnostic and procedural information coded during inpatient hospitalizations. |
| National Ambulatory Care Reporting System Database | Contains diagnostic and procedural information coded during emergency room encounters. |
| Ontario Health Insurance Plan Database | Contains physician diagnostic and billing codes. |
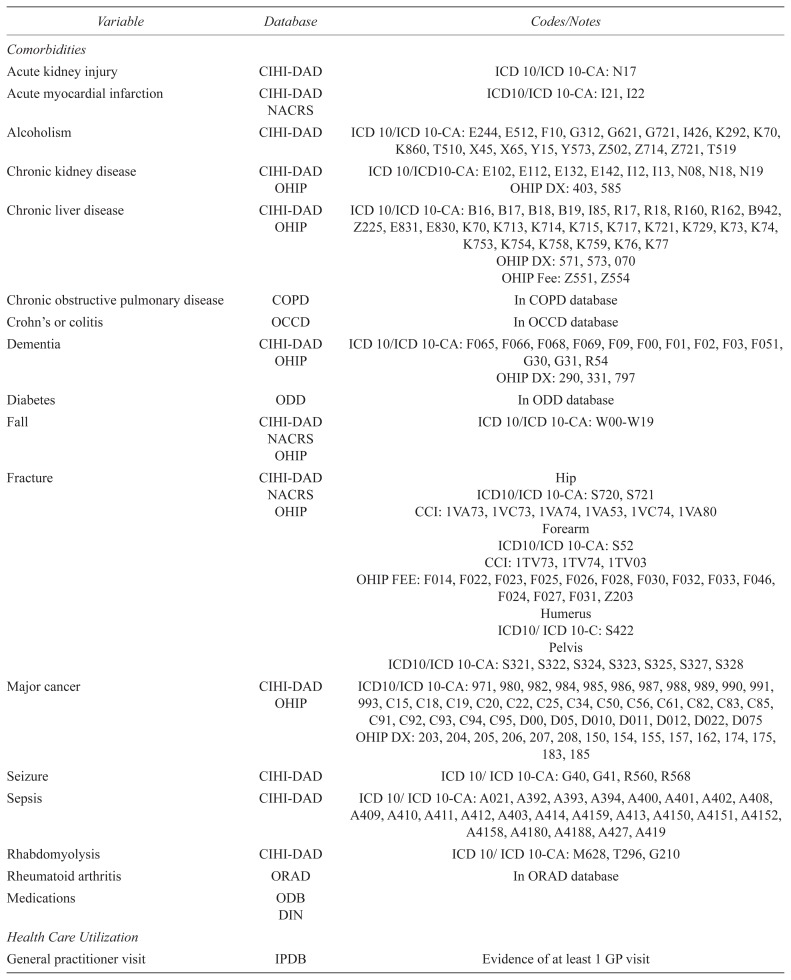
Appendix D. Variable definitions
| Variable | Database | Codes/Notes |
|---|---|---|
| Comorbidities | ||
| Acute kidney injury | CIHI-DAD | ICD 10/ICD 10-CA: N17 |
| Acute myocardial infarction | CIHI-DAD | ICD10/ICD 10-CA: I21, I22 |
| NACRS | ||
| Alcoholism | CIHI-DAD | ICD 10/ICD 10-CA: E244, E512, F10, G312, G621, G721, I426, K292, K70, K860, T510, X45, X65, Y15, Y573, Z502, Z714, Z721, T519 |
| Chronic kidney disease | CIHI-DAD | ICD 10/ICD10-CA: E102, E112, E132, E142, I12, I13, N08, N18, N19 |
| OHIP | OHIP DX: 403, 585 | |
| Chronic liver disease | CIHI-DAD | ICD 10/ICD 10-CA: B16, B17, B18, B19, I85, R17, R18, R160, R162, B942, Z225, E831, E830, K70, K713, K714, K715, K717, K721, K729, K73, K74, K753, K754, K758, K759, K76, K77 |
| OHIP | ||
| OHIP DX: 571, 573, 070 | ||
| OHIP Fee: Z551, Z554 | ||
| Chronic obstructive pulmonary disease | COPD | In COPD database |
| Crohn’s or colitis | OCCD | In OCCD database |
| Dementia | CIHI-DAD | ICD 10/ICD 10-CA: F065, F066, F068, F069, F09, F00, F01, F02, F03, F051, G30, G31, R54 |
| OHIP | ||
| OHIP DX: 290, 331, 797 | ||
| Diabetes | ODD | In ODD database |
| Fall | CIHI-DAD | ICD 10/ICD 10-CA: W00-W19 |
| NACRS | ||
| OHIP | ||
| Fracture | CIHI-DAD | Hip |
| NACRS | ICD10/ICD 10-CA: S720, S721 | |
| OHIP | CCI: 1VA73, 1VC73, 1VA74, 1VA53, 1VC74, 1VA80 | |
| Forearm | ||
| ICD10/ICD 10-CA: S52 | ||
| CCI: 1TV73, 1TV74, 1TV03 | ||
| OHIP FEE: F014, F022, F023, F025, F026, F028, F030, F032, F033, F046, F024, F027, F031, Z203 | ||
| Humerus | ||
| ICD10/ICD 10-C: S422 | ||
| Pelvis | ||
| ICD10/ICD 10-CA: S321, S322, S324, S323, S325, S327, S328 | ||
| Major cancer | CIHI-DAD | ICD10/ICD 10-CA: 971, 980, 982, 984, 985, 986, 987, 988, 989, 990, 991, 993, C15, C18, C19, C20, C22, C25, C34, C50, C56, C61, C82, C83, C85, C91, C92, C93, C94, C95, D00, D05, D010, D011, D012, D022, D075 |
| OHIP | ||
| OHIP DX: 203, 204, 205, 206, 207, 208, 150, 154, 155, 157, 162, 174, 175, 183, 185 | ||
| Seizure | CIHI-DAD | ICD 10/ICD 10-CA: G40, G41, R560, R568 |
| Sepsis | CIHI-DAD | ICD 10/ICD 10-CA: A021, A392, A393, A394, A400, A401, A402, A408, A409, A410, A411, A412, A403, A414, A4159, A413, A4150, A4151, A4152, A4158, A4180, A4188, A427, A419 |
| Rhabdomyolysis | CIHI-DAD | ICD 10/ICD 10-CA: M628, T296, G210 |
| Rheumatoid arthritis | ORAD | In ORAD database |
| Medications | ODB | |
| DIN | ||
| Health Care Utilization | ||
| General practitioner visit | IPDB | Evidence of at least 1 GP visit |
| Any blood test | OHIP | At least one OHIP lab test |
| Specialist visit | IPDB | Evidence of at least one specialist visit |
| Home care visit | HCD | Evidence of at least one home care visit |
CIHI-DAD = Canadian Institute for Health Information’s-Discharge Abstract Database; CCI = Canadian Classification of Health Interventions; COPD = Chronic Obstructive Pulmonary Disease Dataset; ICD 10/ICD 10-CA = International Classification of Diseases 10th Revision/Enhanced Canadian version of the 10th Revision of the International Statistical Classification of Diseases and Health Related Problems; OCCD = Ontario Crohn’s and Colitis Dataset; OHIP DX = Ontario Health Insurance Plan Diagnostic; OHIP Fee = Ontario Health Insurance Plan Fee; ORAD = Ontario Rheumatoid Arthritis Dataset; RPDB = Registered Persons Database of Ontario.
Footnotes
Conflict of Interest Disclosures
KC received a Diabetes Canada Junior Investigator Award sponsored by Astra Zeneca. She has also attended conferences sponsored by Merck. There are no other conflicts of interest to disclose and no endorsement by ICES, AMOSO, SSMD, LHRI or the MOHLTC is intended or should be inferred.
References
- 1.Carnevale V, Fontana A, Scillitani A, et al. Incidence and all-cause mortality for hip fracture in comparison to stroke, and myocardial infarction: a fifteen years population-based longitudinal study. Endocrine [Internet] 2017 Sep 20;58(2):320–31. doi: 10.1007/s12020-017-1423-1. [cited 2017 Oct 26] Available from: http://www.ncbi.nlm.nih.gov/pubmed/28933053. [DOI] [PubMed] [Google Scholar]
- 2.Papadimitropoulos EA, Coyte PC, Josse RG, et al. Current and projected rates of hip fracture in Canada. CMAJ [Internet] 1997 Nov 15;157(10):1357–63. [cited 2017 Jul 4] Available from: http://www.ncbi.nlm.nih.gov/pubmed/9371065. [PMC free article] [PubMed] [Google Scholar]
- 3.Morin S, Lix LM, Azimaee M, et al. Institutionalization following incident non-traumatic fractures in community-dwelling men and women. Osteoporos Int [Internet] 2012 Sep 19;23(9):2381–86. doi: 10.1007/s00198-011-1815-7. [cited 2017 Jul 4] Available from: http://www.ncbi.nlm.nih.gov/pubmed/22008882. [DOI] [PubMed] [Google Scholar]
- 4.Health Quality Ontario. Hip fracture: care for people with fragility fractures [Internet] Toronto: Health Quality Ontario; 2017. Available from: http://www.hqontario.ca/portals/0/documents/evidence/quality-standards/qs-hip-fracture-clinical-guide-en.pdf. [Google Scholar]
- 5.Osteoporosis Canada. Osteoporosis: toward a fracture free future [Internet] Toronto: Osteoporosis Canada; 2011. [cited 2018 Dec 20]. Available from: http://fls.osteoporosis.ca/wp-content/uploads/white-paper-march-2011.pdf. [Google Scholar]
- 6.Upshur REG, Moineddin R, Crighton E, et al. Simplicity within complexity: seasonality and predictability of hospital admissions in the province of Ontario 1988–2001, a population-based analysis. BMC Health Serv Res [Internet] 2005 Feb 4;5(1):13. doi: 10.1186/1472-6963-5-13. [cited 2015 Jun 12] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=549216&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Román Ortiz C, Tenías JM, Estarlich M, et al. Systematic review of the association between climate and hip fractures. Int J Biometeorol [Internet] 2015 Oct 13;59(10):1511–22. doi: 10.1007/s00484-014-0945-y. [cited 2017 Jul 4] Available from: http://link.springer.com/10.1007/s00484-014-0945-y. [DOI] [PubMed] [Google Scholar]
- 8.Lin H-C, Xiraxagar S. Seasonality of hip fractures and estimates of season-attributable effects: a multivariate ARIMA analysis of population-based data. Osteoporos Int [Internet] 2006 May 11;17(6):795–806. doi: 10.1007/s00198-005-0060-3. [cited 2017 Jul 4] Available from: http://link.springer.com/10.1007/s00198-005-0060-3. [DOI] [PubMed] [Google Scholar]
- 9.Modarres R, Ouarda TBMJ, Vanasse A, et al. Modeling seasonal variation of hip fracture in Montreal, Canada. Bone [Internet] 2012 Apr;50(4):909–16. doi: 10.1016/j.bone.2012.01.004. [cited 2017 Jul 4] Available from: http://linkinghub.elsevier.com/retrieve/pii/S8756328212000129. [DOI] [PubMed] [Google Scholar]
- 10.Vecino-Vecino C, Gratton M, Kremer R, et al. Seasonal variance in serum levels of vitamin d determines a compensatory response by parathyroid hormone: study in an ambulatory elderly population in Quebec. Gerontology [Internet] 2006 Jan 27;52(1):33–39. doi: 10.1159/000089823. Available from: www.karger.com/Article/Abstract/89823. [DOI] [PubMed] [Google Scholar]
- 11.Storm D, Eslin R, Porter ES, et al. Calcium supplementation prevents seasonal bone loss and changes in biochemical markers of bone turnover in elderly New England women: a randomized placebo-controlled trial. J Clin Endocrinol Metab [Internet] 1998 Nov;83(11):3817–25. doi: 10.1210/jcem.83.11.5289. [cited 2017 Jul 4] Available from: https://academic.oup.com/jcem/article-lookup/doi/10.1210/jcem.83.11.5289. [DOI] [PubMed] [Google Scholar]
- 12.Walker NJ, Van Woerden HC, Kiparoglou V, et al. Identifying seasonal and temporal trends in the pressures experienced by hospitals related to unscheduled care. BMC Health Serv Res [Internet] 2016 Dec 26;16(1):307. doi: 10.1186/s12913-016-1555-7. [cited 2018 Dec 23] Available from: http://bmchealthservres.biomedcentral.com/articles/10.1186/s12913-016-1555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Statistics Canada. Population by sex and age group, by province and territory (Number, both sexes) Ottawa: Government of Canada, Statistics Canada; 2014. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000501. [Google Scholar]
- 14.Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med [Internet] 2015 Oct;12(10):e1001885. doi: 10.1371/journal.pmed.1001885. [cited 2016 Jan 22] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4595218&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lix LM, Yogendran MS, Leslie WD, et al. Using multiple data features improved the validity of osteoporosis case ascertainment from administrative databases. J Clin Epidemiol [Internet] 2008 Dec;61(12):1250–60. doi: 10.1016/j.jclinepi.2008.02.002. [cited 2017 Jul 4] Available from: http://linkinghub.elsevier.com/retrieve/pii/S0895435608000504. [DOI] [PubMed] [Google Scholar]
- 16.Jean S, Candas B, Belzile E, et al. Algorithms can be used to identify fragility fracture cases in physician-claims databases. Osteoporos Int [Internet] 2012 Feb 19;23(2):483–501. doi: 10.1007/s00198-011-1559-4. [cited 2017 Jul 4] Available from: http://link.springer.com/10.1007/s00198-011-1559-4. [DOI] [PubMed] [Google Scholar]
- 17.Levy AR, O’Brien BJ, Sellors C, et al. Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. Can J Clin Pharmacol. 2003;10(2):67–71. [PubMed] [Google Scholar]
- 18.Hux JE, Ivis F, Flintoft V, et al. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;25(3):512–16. doi: 10.2337/diacare.25.3.512. [DOI] [PubMed] [Google Scholar]
- 19.Gershon AS, Wang C, Guan J, et al. Identifying individuals with physcian diagnosed COPD in health administrative databases. COPD: J Chronic Obstr Pulm Dis [Internet] 2009 Jan 8;6(5):388–94. doi: 10.1080/15412550903140865. [cited 2019 Jan 7] Available from: http://www.tandfonline.com/doi/full/10.1080/15412550903140865. [DOI] [PubMed] [Google Scholar]
- 20.Widdifield J, Bombardier C, Bernatsky S, et al. An administrative data validation study of the accuracy of algorithms for identifying rheumatoid arthritis: the influence of the reference standard on algorithm performance. BMC Musculoskelet Disord [Internet] 2014 Jun 23;15:216. doi: 10.1186/1471-2474-15-216. [cited 2019 Jan 7] Available from: http://www.ncbi.nlm.nih.gov/pubmed/24956925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benchimol EI, Guttmann A, Mack DR, et al. Validation of international algorithms to identify adults with inflammatory bowel disease in health administrative data from Ontario, Canada. J Clin Epidemiol [Internet] 2014 Aug;67(8):887–96. doi: 10.1016/j.jclinepi.2014.02.019. [cited 2019 Jan 7] Available from: http://www.ncbi.nlm.nih.gov/pubmed/24774473. [DOI] [PubMed] [Google Scholar]
- 22.Odén A, McCloskey EV, Johansson H, et al. Assessing the impact of osteoporosis on the burden of hip fractures. Calcif Tissue Int [Internet] 2013 Jan 8;92(1):42–49. doi: 10.1007/s00223-012-9666-6. [cited 2018 Dec 20] Available from: http://link.springer.com/10.1007/s00223-012-9666-6. [DOI] [PubMed] [Google Scholar]
- 23.Rotermann M, Sanmartin C, Hennessey D, et al. Prescription medication use by Canadians aged 6–79 [Internet] Ottawa: Statistics Canada (archived); 2015. [cited 2017 Jul 4]. Available from: http://www.statcan.gc.ca/pub/82-003-x/2014006/article/14032-eng.htm. [Google Scholar]
- 24.Migration and Geographic Distribution—Snowbirds [Internet] [cited 2017 Jul 4]. Available from: http://medicine.jrank.org/pages/1164/Migration-Geographic-Distribution-Snowbirds.html.
- 25.The Ontario Drug Benefit (ODB) Program: If you travel.[Internet] Toronto: MOHLTC; [cited 2018 Dec 20]. Available from: http://health.gov.on.ca/en/public/programs/drugs/programs/odb/opdp_travel_supply.aspx. [Google Scholar]
- 26.Moineddin R, Upshur RE, Crighton E, et al. Aggression as a means of assessing the strength of seasonality in a time series. Popul Health Metr [Internet] 2003 Dec 15;1(1):10. doi: 10.1186/1478-7954-1-10. [cited 2015 Jun 12] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=317382&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy AR, Bensimon DR, Mayo NE, et al. Inclement weather and the risk of hip fracture. Epidemiology [Internet] 1998 Mar;9(2):172–77. doi: 10.1097/00001648-199803000-00012. [cited 2017 Jul 4] Available from: http://www.ncbi.nlm.nih.gov/pubmed/9504286. [DOI] [PubMed] [Google Scholar]
- 28.Koren L, Barak A, Norman D, et al. Effect of seasonality, weather and holidays on the incidence of proximal hip fracture. Isr Med Assoc J [Internet] 2014 May;16(5):299–302. [cited 2017 Jul 4] Available from: http://www.ncbi.nlm.nih.gov/pubmed/24979835. [PubMed] [Google Scholar]
- 29.Tenías JM, Estarlich M, Fuentes-Leonarte V, et al. Short-term relationship between meteorological variables and hip fractures: an analysis carried out in a health area of the Autonomous Region of Valencia, Spain (1996–2005) Bone [Internet] 2009 Oct;45(4):794–98. doi: 10.1016/j.bone.2009.06.022. [cited 2017 Jul 4] Available from: http://linkinghub.elsevier.com/retrieve/pii/S8756328209016706. [DOI] [PubMed] [Google Scholar]
- 30.Douglas S, Bunyan A, Chiu KH, et al. Seasonal variation of hip fracture at three latitudes. Injury [Internet] 2000 Jan;31(1):11–19. doi: 10.1016/S0020-1383(99)00192-8. [cited 2017 Jul 4] Available from: http://www.ncbi.nlm.nih.gov/pubmed/10716045. [DOI] [PubMed] [Google Scholar]
- 31.Lee M, Nordio F, Zanobetti A, et al. Acclimatization across space and time in the effects of temperature on mortality: a time-series analysis. Environ Health [Internet] 2014;13:89. doi: 10.1186/1476-069X-13-89. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4271464&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costa AG, Wyman A, Siris ES, et al. Harvey N, editor. When, where and how osteoporosis-associated fractures occur: an analysis from the Global Longitudinal Study of Osteoporosis in Women (GLOW) PLoS One [Internet] 2013. Dec 11, p. e83306. [cited 2017 Jul 4] Available from: http://dx.plos.org/10.1371/journal.pone.0083306. [DOI] [PMC free article] [PubMed]
- 33.Rosen CJ, Morrison A, Zhou H, et al. Elderly women in northern New England exhibit seasonal changes in bone mineral density and calciotropic hormones. Bone Miner [Internet] 1994 May;25(2):83–92. doi: 10.1016/S0169-6009(08)80250-4. [cited 2017 Nov 1] Available from: http://www.ncbi.nlm.nih.gov/pubmed/8086854. [DOI] [PubMed] [Google Scholar]
- 34.Laird E, Ward M, McSorley E, et al. Vitamin D and bone health: potential mechanisms. Nutrients [Internet] 2010;2(7):693–724. doi: 10.3390/nu2070693. [cited 2017 Nov 1] Available from: http://www.ncbi.nlm.nih.gov/pubmed/22254049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bulajic-Kopjar M. Seasonal variations in incidence of fractures among elderly people. Inj Prev [Internet] 2000 Mar;6(1):16–19. doi: 10.1136/ip.6.1.16. [cited 2017 Oct 26] Available from: http://www.ncbi.nlm.nih.gov/pubmed/10728535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crighton EJ, Moineddin R, Mamdani M, et al. Influenza and pneumonia hospitalizations in Ontario: a time-series analysis. Epidemiol Infect [Internet] 2004 Dec;132(6):1167–74. doi: 10.1017/S0950268804002924. [cited 2017 Jul 4] Available from: http://www.ncbi.nlm.nih.gov/pubmed/15635976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelsey JL, Procter-Gray E, Hannan MT, et al. Heterogeneity of falls among older adults: implications for public health prevention. Am J Public Health [Internet] 2012 Nov;102(11):2149–56. doi: 10.2105/AJPH.2012.300677. [cited 2017 Oct 26] Available from: http://www.ncbi.nlm.nih.gov/pubmed/22994167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Speechley M, Tinetti M. Falls and injuries in frail and vigorous community elderly persons. J Am Geriatr Soc [Internet] 1991 Jan;39(1):46–52. doi: 10.1111/j.1532-5415.1991.tb05905.x. [cited 2017 Oct 26] Available from: http://www.ncbi.nlm.nih.gov/pubmed/1987256. [DOI] [PubMed] [Google Scholar]
- 39.Environment & Natural Resources. Station Results – 1981–2010 Climate Normals and Averages (for Ontario) [Internet] Ottawa: Government of Canada; 2010. Available from: http://climate.weather.gc.ca/climate_normals/station_select_1981_2010_e.html?searchType=stnProv&lstProvince=ON. [Google Scholar]
- 40.Mondor L, Charland K, Verma A, et al. Weather warnings predict fall-related injuries among older adults. Age Ageing [Internet] 2015 May 1;44(3):403–08. doi: 10.1093/ageing/afu199. [cited 2017 Jul 4] Available from: https://academic.oup.com/ageing/article-lookup/doi/10.1093/ageing/afu199. [DOI] [PubMed] [Google Scholar]
- 41.Bischoff-Ferrari HA, Orav JE, Barrett JA, et al. Effect of seasonality and weather on fracture risk in individuals 65 years and older. Osteoporos Int [Internet] 2007 Sep 21;18(9):1225–33. doi: 10.1007/s00198-007-0364-6. [cited 2017 Jul 4] Available from: http://link.springer.com/10.1007/s00198-007-0364-6. [DOI] [PubMed] [Google Scholar]
- 42.Mirchandani S, Aharonoff GB, Hiebert R, et al. The effects of weather and seasonality on hip fracture incidence in older adults. Orthopedics [Internet] 2005 Feb;28(2):149–55. doi: 10.3928/0147-7447-20050201-17. [cited 2017 Jul 4] Available from: http://www.ncbi.nlm.nih.gov/pubmed/15751369. [DOI] [PubMed] [Google Scholar]
- 43.Diamantopoulos AP, Rohde G, Johnsrud I, et al. Incidence rates of fragility hip fracture in middle-aged and elderly men and women in southern Norway. Age Ageing [Internet] 2012 Jan 1;41(1):86–92. doi: 10.1093/ageing/afr114. [cited 2017 Jul 4] Available from: http://www.ncbi.nlm.nih.gov/pubmed/21896555. [DOI] [PubMed] [Google Scholar]
- 44.Emaus N, Olsen LR, Ahmed LA, et al. Hip fractures in a city in Northern Norway over 15 years: time trends, seasonal variation and mortality. The Harstad Injury Prevention Study. Osteoporos Int [Internet] 2011 Oct 20;22(10):2603–10. doi: 10.1007/s00198-010-1485-x. [cited 2017 Jul 4] Available from: http://link.springer.com/10.1007/s00198-010-1485-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parkkari J, Kannus P, Palvanen M, et al. Majority of hip fractures occur as a result of a fall and impact on the greater trochanter of the femur: a prospective controlled hip fracture study with 206 consecutive patients. Calcif Tissue Int [Internet] 1999 Sep;65(3):183–87. doi: 10.1007/s002239900679. [cited 2019 Jan 7] Available from: http://www.ncbi.nlm.nih.gov/pubmed/10441647. [DOI] [PubMed] [Google Scholar]
- 46.Kelsey JL, Procter-Gray E, Uyen-Sa Nguyen, et al. Footwear and falls in the home among older individuals in the MOBILIZE Boston study. Footwear Sci [Internet] 2010 Sep;2(3):123–29. doi: 10.1080/19424280.2010.491074. [cited 2017 Oct 26] Available from: http://www.tandfonline.com/doi/abs/10.1080/19424280.2010.491074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, et al. Effect of vitamin D on falls: a meta-analysis. JAMA [Internet] 2004 Apr 28;291(16):1996–2006. doi: 10.1001/jama.291.16.1999. [cited 2017 Jul 4] Available from: http://www.ncbi.nlm.nih.gov/pubmed/15113819. [DOI] [PubMed] [Google Scholar]
- 48.Avenell A, Gillespie WJ, Gillespie LD, et al. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database System Rev [Internet] 2009 Apr.15(2):CD000227. doi: 10.1002/14651858.CD000227.pub3. [cited 2017 Jul 4] Available from: http://www.ncbi.nlm.nih.gov/pubmed/19370554. [DOI] [PubMed] [Google Scholar]