Abstract
Importance
Chemotherapy induced peripheral neuropathy is a common side effect of neurotoxic chemotherapy resulting in pain, sensory loss and decreased quality of life. Few studies have prospectively examined the relationship between sensory neuropathy symptoms, falls and fall related injuries in those receiving neurotoxic chemotherapy.
Objective
Determine the association between the symptoms of chemotherapy induced peripheral neuropathy (CIPN) and fall risk in those receiving neurotoxic chemotherapy.
Design
Patients starting neurotoxic chemotherapy with a taxane or platinum called a novel automated phone system daily for one full course of chemotherapy. The phone system, “Symptom Care @ Home” utilized a series of relevant CIPN questions to track symptoms on a 0–10 ordinal scale and contained a questionnaire about falls. Those reporting a numbness and tingling severity of 3 or greater for at least 10 days were considered to have significant CIPN symptoms and were compared to those who did not.
Setting
Ambulatory participants at an academic cancer center.
Participants
A consecutive sample of breast, ovarian and lung cancer patients beginning neurotoxic chemotherapy was recruited from oncology clinics. The study population was largely female (94%) and Caucasian (96%).
Exposures
Chemotherapy with a neurotoxic taxane or platinum agent.
Main Outcome Measure
Patient reported fall events (falls or near falls) and fall related injuries. The hypothesis was generated after data collection but prior to data analysis.
Results
32/116 patients met the predetermined criteria for CIPN symptoms. The mean duration of follow up was 62 days with 51 calls completed per participant. 74 fall events were reported. Those with CIPN symptoms were nearly 3 times more likely to have a fall event than those without (HR 2.67, CI 1.62–4.41, p<0.001). The CIPN group was more likely to obtain medical care for falls (25.0% vs. 7.1%, p=0.013).
Conclusions and Relevance
These findings suggest the sensory symptoms of CIPN are an indicator of increased fall risk and health care utilization. This study demonstrates the utility of a novel phone based system to track neuropathy symptoms. Careful monitoring and coaching of patients receiving neurotoxic chemotherapy for new sensory symptoms may facilitate more effective fall prevention strategies.
Introduction
Chemotherapy induced peripheral neuropathy (CIPN) is a common and disabling side effect of life saving neurotoxic chemotherapy.1–3 Depending on the agent, 30–70% of people receiving neurotoxic chemotherapy develop neuropathy.4,5 The taxanes, platinums, and vinca alkaloids are the agents most commonly associated with CIPN. The risk of developing CIPN increases with both the total cumulative dose and exposure to multiple neurotoxic agents.5–8 CIPN most commonly causes a length dependent sensory predominant axonal neuropathy, although the platinums and taxanes are unique in that the hands and feet can be affected simultaneously.5,9
CIPN causes pain, sensory loss, and poor dexterity that reduce patient reported quality of life. Other health outcomes such as falls have not been well explored and the influence of CIPN on fall risk is poorly understood.10 In general, patients with cancer have an elevated incidence of falls, approaching 50% in advanced malignancy. Many patients suffer fall related injuries.11 Previous large retrospective studies found higher fall rates in those treated with neurotoxic chemotherapy than those without.4 A recent CIPN treatment trial demonstrated a correlation between the severity of motor neuropathy and falls, with 12% of CIPN patients falling over a 3 month period.12 Few studies have prospectively demonstrated a significant association between sensory neuropathy symptoms and falls and none have evaluated the detailed circumstances of those falls. This prospective study was designed to closely monitor chemotherapy related symptom severity and falls in patients beginning chemotherapy.
Methods
Study Design
This is a secondary analysis of a prospective observational study. This study utilized a novel automated phone system that prospectively queried patients receiving chemotherapy regarding symptoms and falls. The phone system, named “Symptom Care @ Home”, used a series of relevant questions developed by cancer outcomes experts to track multiple chemotherapy related side effects, including CIPN.13 The phone tree began with yes/no questions about the presence or absence of 11 symptoms over the past 24 hours (numbness/ tingling, nausea/vomiting, diarrhea, fatigue, trouble thinking/ concentrating, trouble sleeping, depression, anxiety, pain, distress about changes in appearance, and sore mouth). For each “yes” response, the severity and level of distress it caused (1–10) were tracked. Participants were then asked if they have fallen or nearly fallen in the last 24 hours. A “yes” response triggered further questions regarding location, time of day, provocation, recent medication changes, ability to stop the fall, lighting, shoes, injury, and whether or not the participant obtained medical care for the fall. Fall events (falls and near falls) were the main outcome of the study. Falls and near falls were grouped as a single outcome because participants were injured in both. Participants were asked to call on the first day of the first cycle and then daily for one chemotherapy protocol or 6 months of therapy, which ever came first. If they did not call by 2:30pm they received a reminder call. On days when participants did not call, no assumptions were made about their symptoms and no symptoms scores were reported. For the purposes of this study, if a participant called less than ten times over the study period they were excluded from the analysis.
Participants
Participants were prospectively recruited from a NCI designated cancer center. Inclusion criteria included a new diagnosis of breast, ovarian, or lung cancer and planned treatment with a taxane or platinum chemotherapeutic agent. Enrollment occurred prior to the initiation of chemotherapy and extensive demographic, diagnostic, medication and past medical information was obtained. Chemotherapeutic treatment data including medications and total cumulative doses was captured from prescription records and oncology notes at multiple points throughout and at the conclusion of the study.
Classification of CIPN symptoms severity
Many neurotoxic chemotherapy agents are associated with transitory infusion related sensory syndromes.14 These symptoms are typically mild and transient, whereas CIPN symptoms are persistent. In those who do develop CIPN, the effects on quality of life, and morbidity such as falls are likely dictated by severity. In order to exclude those with transient infusion related sensory symptoms, those with a numbness and tingling severity of 3 or greater (on a 0–10 ordinal scale) for 10 or more days were considered to have significant CIPN symptoms. All other participants were categorized as “asymptomatic”. The CIPN symptom definition was determined prior to the analysis of the data. Questions about pain were part of the survey but the responses were not utilized for this analysis because the questions do not inquire about the site or characteristics of pain.
Standard protocol approvals, registrations and patient consents
The study was approved by the University of Utah IRB and each participant provided written informed consent.
Statistical analysis
Chi square or Fisher’s exact test was used to compare dichotomous or unordered categorical patient characteristics between patient groups. Two sample t tests were used to compare continuous variables between groups, or an unequal variances to sample t test, as appropriate. For reporting continuous patient characteristics, the ± notation represents mean ± standard deviation. A multivariable shared frailty Cox regression model was fitted with time varying covariates to compare fall risk between groups. In this model, multiple outcomes events were permitted and the multiple call in reports were nested within patients. Potential confounders, sex and age, were included in the model if they altered the coefficient of the primary predictor variable, which was numbness and tingling severity, by more than 10%.15 In the table describing falls and fall injuries, the data were too sparse for most table rows to use mixed effects models to account for falls nested within patients, so simple statistics, chi square and Fisher’s exact test, were reported in a descriptive fashion.
Results
A total of 165 participants were enrolled. 49 whose treatment plan was altered and were ultimately not treated with neurotoxic chemotherapy were excluded. 116 participants successfully completed the study and no patients were excluded due to an inadequate number of completed phone calls. Mean age was 55.5 (SD ± 11.9) years. 109 (94%) were female and 111 (96%) were Caucasian. 83 (72%) had breast, 23 (20%) ovarian, and 10 (8%) lung cancer. 75 (65%) received a taxane alone, 9 (8%) received a platinum, and 32 (27%) received a combination of taxane and platinum. The mean duration of follow up was 62.2 ± 36.7 days. The mean number of calls over the study period was 51.1 ± 33.0. There was no drop out in call compliance over the course of chemotherapy as 53.4% of participants had more calls in the second versus first half of the study.
Of 116 participants, 32 (28%) met the predetermined criteria for CIPN symptoms. There were no differences in demographic characteristics between those with and without CIPN symptoms (table 1.) Those with CIPN symptoms had significantly higher percent of days with any numbness/tingling (53.2% ± 32.1 vs. 9.2% ± 12.1, p<0.001), and percent of days with numbness/tingling ≥ 3 compared to the asymptomatic group (47.6% ± 30.6 vs. 4.6% ± 8.6, p<0.001). The mean severity of numbness and tingling also differed between groups (CIPN symptoms 0.3 ± 3.6 vs. 2.4 ± 1.7, p<0.001). The percent of days patients reported they were too sick to complete the entire phone tree did not differ between groups (CIPN symptoms1.8 ± 4.7 vs. 1.8 ± 5.5, p=0.967).
Table 1.
Clinical and Demographic Characteristics
| All Participants [n=116] |
No CIPN Symptoms [n=84] |
CIPN Symptoms [n=32] |
p Value | |
|---|---|---|---|---|
| Sex | ||||
| Female | 109 (94.0) | 79 (94.1) | 30 (94.0) | 0.952 |
| Mean Age | 55.5 (11.9) | 55.3 (12.3) | 55.8 (11.2) | 0.841 |
| Cancer | ||||
| Breast | 83 (71.6) | 59 (70.2) | 24 (75.0) | |
| Ovarian | 23 (19.8) | 18 (11.9) | 5 (15.6) | 0.952 |
| Lung | 10 (8.6) | 7 (8.3) | 3 (9.4) | |
| Agent | ||||
| Taxane | 75 (64.7) | 53 (63.1) | 22 (68.8) | |
| Platinum | 9 (7.8) | 9 (10.1) | 0 | 0.153 |
| Combination | 32 (27.6) | 22 (26.2) | 10 (31.2) | |
| Cancer stage | ||||
| I | 20 (17.2) | 14 (16.6) | 6 (18.8) | |
| II | 42 (33.3) | 31 (36.9) | 11 (33.4) | |
| III | 28 (24.1) | 20 (23.8) | 8 (25.0) | 0.990 |
| IV | 26 (22.4) | 19 (22.6) | 7 (21.9) | |
| Mean # of calls | 51.1 (31.4) | 44.6 (31.1) | 68.1 (32.1) | <0.001 |
Data are in n (%) or mean (SD)
Abbreviations: CIPN symptoms = the group with CIPN symptoms; No CIPN symptoms = the asymptomatic group; Combination = treatment with a taxane and platinum.
Over the course of the study, 24 participants had 74 fall events (37 full falls and 37 near falls). For analysis, full falls and near falls were combined and referred to as “falls” because both types of events resulted in injuries. The number of calls placed was not associated with fall status (fallers: 60.6 ± 41.0 vs. non fallers: 48.6 ± 30.3, p=0.113). Over a third (34.3%) of those with CIPN symptoms fell compared to 15.4% of those without (p=0.025). After controlling for age, gender, and length of follow up, those with CIPN symptoms were 2.5 times more likely to have a fall event than those without (HR 2.67, 95%CI 1.62–4.41, p<0.001). This relationship persisted if near falls were excluded from the analysis (HR=2.10, 95%CI 1.01–3.99, p=0.047). In a secondary analysis that was limited to the first fall per patient in the Cox model and Kaplan-Meier graph, the Hazard ratio was similar however the result only approached statistical significance (HR 2.14, 95%CI 0.85–5.39, p=.11)(Figure 1). Independent of neuropathy status, increasing age was associated with increased fall risk (HR per 5 year increase in age = 1.27, 95%CI 1.14–1.42, p<0.001).
Figure 1.
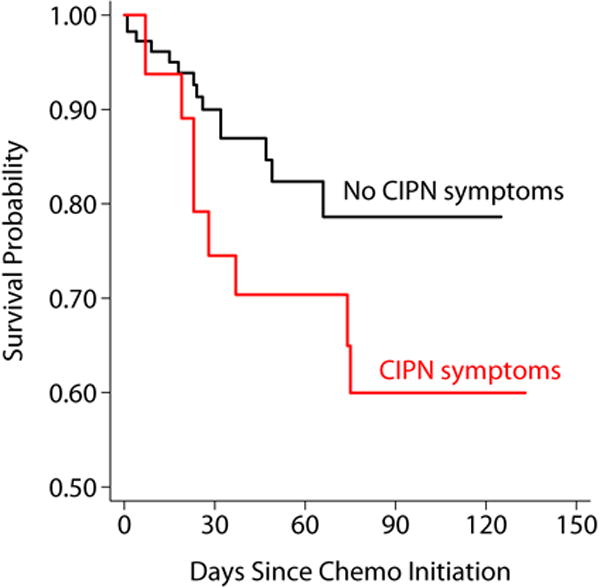
Kaplan Meier Survival Estimates, According to CIPN Symptoms. Survival represents freedom from first fall.
Over all reporting days, those who fell at any point in the study had higher mean numbness and tingling severity scores than those who did not (1.5 ± 0.4 vs. 0.7 ± 0.1, p=0.006). Fallers also had more days with any numbness and tingling (18.0 ± 5.0 vs 9.1 ± 1.7, p=0.038). The average numbness and tingling severity scores were modestly correlated with the number of falls per participant (Spearman rho = 0.20, p= 0.035). Participants that were married or living with a partner did not fall less than those who were widowed, divorced or single (17.1% vs 29.4%, p=0.135).
The details of falls and their associated injuries were cataloged (Table 2). 35% of falls occurred in low lighting situations and all but one occurred indoors. Fallers with CIPN symptoms were more likely than those without to be wearing shoes at the time of the fall (55.2% vs 11.1%, p<0.001). The most common locations of falls were on flat ground (83.8%), in the bathroom (5.4%), and getting up or using stairs (both 4.1%). There was no difference in fall location between groups. The most common cause of falls was “I lost my balance” accounting for 68.4% of falls in the group with CIPN symptoms and 50% in the group without symptoms. The overall distribution of cause did not differ between groups (p=0.305). A minority (11.1%) of all participants felt the fall might have been related to a change in their medications. In the symptomatic group, 40% of all fall events were aborted and described by participants as near falls, compared to 56.8% in the asymptomatic group (p=0.879). Regaining balance was the most common method of fall abortion in both groups (76.2 vs 62.5%, p=0.367).
Table 2.
Fall Characteristics
| All Participants [n=116] |
No CIPN Symptoms [n=84] |
CIPN Symptoms [n=32] |
P Value | |
|---|---|---|---|---|
| Wearing shoes | 25 (33.8) | 4 (13.9) | 21 (55.2) | <0.001 |
| Poorly lit area | 26 (35.1) | 13 (36.1) | 13 (34.2) | 0.864 |
| Location | 0.756 | |||
| On flat ground | 62 (83.8) | 28 (77.7) | 34 (89.5) | |
| On stairs | 3 (4.1) | 2 (5.6) | 1 (2.6) | |
| Getting up | 3 (4.1) | 2 (5.6) | 1 (2.6) | |
| In the bathroom | 4 (5.4) | 3 (8.3) | 1 (2.6) | |
| Other | 2 (2.7) | 1 (2.78) | 1 (2.6) | |
| How fall occurred | 0.305 | |||
| Lost balance | 44 (59.4) | 18 (50.0) | 26 (68.4) | |
| Felt faint | 20 (27.0) | 13 (36.1) | 7 (18.4) | |
| Legs gave way | 4 (5.4) | 1 (2.8) | 3 (7.9) | |
| Tripped | 2 (2.7) | 1 (2.8) | 1 (2.6) | |
| Fell off a chair | 1 (1.3) | 1 (2.8) | 0 | |
| Don’t know | 3 (4.1) | 2 (5.6) | 1 (2.6) | |
| Med change | 8 (11.1) | 2 (5.7) | 6 (16.2) | 0.262 |
| Body part injured | 0.006 | |||
| Wrist | 1 (7.1) | 1 (16.7) | 0 | |
| Arm | 1 (7.1) | 0 | 1 (12.5) | |
| Head | 8 (57.1) | 1 (16.7) | 7 (87.5) | |
| Hand | 2 (14.3) | 2 (33.3) | 0 | |
| Multiple injuries | 2 (14.3) | 2 (33.3) | 0 | |
| Type of care | p>.99 | |||
| ER | 3 (21.4) | 1 (16.7) | 2 (25) | |
| Instacare | 2 (12.3) | 1 (16.7) | 1 (12.5) | |
| PMD | 1 (7.1) | 0 | 1 (12.5) | |
| Oncologist | 7 (50) | 3 (50) | 4 (50) | |
| Other | 1 (7.1) | 1 (16.7) | 0 |
Data are in n (%)
Falls or fall injuries are the unit of analysis, and data are expressed as n (%).
p values are based on Fisher’s exact test or chi squared test, as appropriate. These tests assume independent events, while events were actually nested within patient and so not independent; therefore, the p values should be interpreted as descriptive instead of confirmatory.
Abbreviations: CIPN symptoms = the group with CIPN symptoms; No CIPN symptoms = the asymptomatic group; med change = fall reported due to medication change; PMD = personal medical doctor.
There was no difference in the rate of injury associated with falls between the symptomatic and asymptomatic groups (23.7 % vs. 27.8%, p=0.687). Head injuries accounted for 87.5% of injuries in the symptomatic group compared to 16.6% in asymptomatic group and the overall distribution of injuries differed between groups. (p=.006).
Over the course of the study, the group with CIPN symptoms was more likely to seek medical attention for a fall (25.0% vs 7.1%, p=0.013), although the percentage of time the participant sought medical care for an individual fall did not differ between groups (21.0% vs 16.7%, p=0.630). The most common site of fall related medical care was the oncologist’s office, accounting for 50% of post fall care in each group.
Discussion
CIPN is a common and debilitating side effect of life saving cancer treatment. The pain, sensory loss and gait disturbance of CIPN are associated with reduced quality of life.16 Little else is known about CIPN related health outcomes. While other studies have retrospectively evaluated falls, this study is the first to prospectively demonstrate that persistent CIPN sensory symptoms are significantly associated with the risk of falling in those treated with neurotoxic chemotherapy.17 Participants with sensory symptoms were 2.7 times more likely to have a fall event (fall or near fall) than those without after controlling for confounders, with an absolute fall incidence of 34% over the course of the study. This nearly 3 fold increase in risk is particularly striking given cancer patients already have an elevated fall risk.11 In this study, CIPN symptoms were defined by persistent numbness and tingling. While examination data were not available, it is likely these patients also experience objective sensory deficits involving multiple modalities including light touch and proprioception.18 Deficits in proprioception impair balance and are a well-recognized risk factor for falling. 19,20 Diabetic and idiopathic neuropathies are both strong independent predictors of fall risk.21 In these and other types of neuropathy, worsening severity is associated with greater fall risk. 22 Among patients with type 1 diabetes, those with significant sensory loss are substantially more likely to suffer a fall related injury than those with mild sensory loss.23 Accordingly, this study found numbness and tingling severity was correlated with the number of falls per participant.
Although the location, specific cause, or physical setting of falls did not differ between groups the results provide insight into fall risk factors, mechanisms and potential mitigation strategies. Fall prevention counseling often focuses on areas of high risk such as the bathroom, stairs, or poorly lit areas.24 In this study the vast majority of falls occurred indoors in a flat well-lit area, not in the bathroom or on the stairs. While falling in a bathroom with a hard surface may be more dangerous, fall risk mitigation strategies must also recognize that most falls may not occur in these areas of the home.
Almost 20% of falls resulted in injury and over the course of the study, participants with CIPN symptoms were more likely to receive medical care for a fall. The majority of the visits were to the oncologist’s office, followed by the ER and urgent care. This increased utilization of healthcare contributes to the overall cost of care for cancer patients. This finding is consistent with a large retrospective Medicare claims study demonstrating that annual health care costs are almost $20,000 more for those with CIPN than matched cancer patients without CIPN.25 Although the CIPN symptom group had a higher proportion of head injuries, this finding should be interpreted with caution because a single participant had multiple fall related head injuries.
It is probable that this study underestimates the risk and impact of falls among CIPN patients given the mean duration of follow up was just over 2 months. Many years after treatment of the initial cancer, patients often continue to experience sensory symptoms that decrease their quality of life.16 Superimposed on these uncomfortable symptoms is the fear of falling. In the geriatric population, nearly half of those who fall develop a fear of falling which contributes to a decline in independent function and decreased quality of life.26 As there are no proven preventative or therapeutic strategies for CIPN, current treatment focuses on symptom management. There is no consensus on when patients receiving potentially neurotoxic chemotherapy should be referred for physical therapy aimed at preventing falls. At a minimum, providers should strongly consider fall prevention teaching for all patients being prescribed neurotoxic chemotherapy who develop neuropathic symptoms. While there are no studies that look at balance or gait training in this population, there is strong evidence that fall prevention programs decrease fall risk and injuries in those with neuropathy and the geriatric population. 27,28
This study utilized a novel approach to neuropathy research. The use of an automated daily symptom tracking system is a promising approach to identify patients requiring early intervention. A strength of this system is its adaptability as questions and answer options can be changed over time. Since the conclusion of this study, the symptom monitoring system was further modified to provide more detailed information regarding the type, location, and severity of neuropathic symptoms for ongoing studies. This additional information will increase the ability to differentiate between paresthesias and neuropathy. Despite good compliance with phone calls, future studies may consider the use of a mobile app or web based technology to improve convenience for participants.
There are several limitations to this study including its modest sample size, female predominance (due to disproportionate representation of breast and ovarian malignancies), and limited racial and ethnic diversity. Because participants did not undergo a detailed neurological examination it was impossible to confirm a CIPN diagnosis. Neuropathy is not the only cause of neurologic symptoms in patients undergoing neurotoxic chemotherapy. Those exposed to taxanes can develop infusion related paresthesias while some platins produce cold induced dyesthesias. The prospective decision to require persistent, moderate to severe sensory symptoms mitigates but does not preclude against the risk of inclusion of patients without CIPN. Non chemotherapeutic medications can also cause persistent tingling, but paresthesias without sensory loss are unlikely to affect fall risk. While the cumulative chemotherapy dose is directly related to neuropathy development, the inclusion of multiple agents precluded against an analysis of a dose relationship with symptoms or falls. Acquisition bias is also a potential limitation of this study. The group with more numbness and tingling had more daily calls. Therefore, it is possible the sample of evaluable data was biased towards CIPN and fall risk severity if those with no or minor symptoms were less likely to call. However, it is also possible that those with severe symptoms failed to call because they felt too ill. The statistical model accounted for these potential limitations by including call frequency in the multivariate model. Finally, given the duration of the study, fall risk was only assessed in the first few months after beginning chemotherapy and long term risk was not evaluated.
Conclusions
This study demonstrates that in a sample of patients receiving neurotoxic chemotherapy, those with persistent numbness and tingling are at a substantially higher risk of a fall or near fall than those without symptoms. CIPN related falls and the associated injuries increase healthcare utilization while decreasing quality of life. Further research is needed to elucidate the best strategies to identify CIPN patients early and intervene to prevent falls in this vulnerable population.
Acknowledgments
Noah Kolb had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data in the analysis. All listed authors shared responsibility for the data analysis:
A NIH R01 CA120558 (KM) provided funding for this project but did not have a direct role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
A NIH UL1TR001067 provided funding for Noah Kolb’s, MD research time but did not have a direct role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The study was sponsored by: NIH R01 CA120558 (KM). Noah Kolb, MD’s research time was supported by NIH UL1TR001067.
Footnotes
Author contributions:
N.Kolb, MD: study design, drafting and editing the manuscript, statistical analysis, and interpretation of the data.
A.G. Smith, MD: study design, editing the manuscript, interpretation of the data.
J.R. Singleton, MD: study design, editing the manuscript, interpretation of the data.
Susan Beck, PHD, APRN, FAAN, AOCN : study design, patient enrollment/ recruitment, analysis and interpretation of the data.
G. Stoddard: study design, drafting and editing the manuscript, statistical analysis and interpretation of the data.
Summer Brown, MD: study design, editing the manuscript, interpretation of the data.
Kathi Mooney, PHD, RN, FAAN: study design, patient enrollment/ recruitment, analysis and interpretation of the data.
Relevant disclosures:
N.Kolb (MD): Dr. N. Kolb reports no disclosures.
A.G. Smith (MD): Dr. A.G. Smith reports no disclosures.
J.R. Singleton (MD): Dr. J.R. Singleton reports no disclosures.
Susan Beck (PHD, APRN, FAAN, AOCN): Dr. S. Beck reports no disclosures.
Greg Stoddard (MS): Greg Stoddard reports no disclosures.
Summer Brown, MD: Dr. S. Brown reports no disclosures.
Kathi Mooney (PHD, RN, FAAN): Dr. K. Mooney reports no disclosures.
Trial Registration
none
Contributor Information
A.G. Smith, Email: gordon.smith@hsc.utah.edu.
J.R. Singleton, Email: rob.singleton@hsc.utah.edu.
S. Beck, Email: susan.beck@nursing.utah.edu.
G.J. Stoddard, Email: greg.stoddard@hsc.utah.edu.
S. Brown, Email: summer.brown@hsc.utah.edu.
K. Mooney, Email: kathi.mooney@nurs.utah.edu.
References
- 1.Won H-H, Lee J, Park JO, et al. Polymorphic markers associated with severe oxaliplatin-induced, chronic peripheral neuropathy in colon cancer patients. Cancer. 2012;118(11):2828–2836. doi: 10.1002/cncr.26614. [DOI] [PubMed] [Google Scholar]
- 2.Raymond E, Chaney SG, Taamma A, Cvitkovic E. Oxaliplatin: a review of preclinical and clinical studies. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 1998;9(10):1053–1071. doi: 10.1023/a:1008213732429. [DOI] [PubMed] [Google Scholar]
- 3.Hertz DL, Roy S, Motsinger-Reif AA, et al. CYP2C8*3 increases risk of neuropathy in breast cancer patients treated with paclitaxel. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2013;24(6):1472–1478. doi: 10.1093/annonc/mdt018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward PR, Wong MD, Moore R, Naeim A. Fall-related injuries in elderly cancer patients treated with neurotoxic chemotherapy: A retrospective cohort study. J Geriatr Oncol. 2014;5(1):57–64. doi: 10.1016/j.jgo.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Pachman DR, Barton DL, Watson JC, Loprinzi CL. Chemotherapy-induced peripheral neuropathy: prevention and treatment. Clin Pharmacol Ther. 2011;90(3):377–387. doi: 10.1038/clpt.2011.115. [DOI] [PubMed] [Google Scholar]
- 6.Vellas BJ, Wayne SJ, Romero LJ, Baumgartner RN, Garry PJ. Fear of falling and restriction of mobility in elderly fallers. Age Ageing. 1997;26(3):189–193. doi: 10.1093/ageing/26.3.189. [DOI] [PubMed] [Google Scholar]
- 7.Lapointe NE, Morfini G, Brady ST, Feinstein SC, Wilson L, Jordan MA. Effects of eribulin, vincristine, paclitaxel and ixabepilone on fast axonal transport and kinesin-1 driven microtubule gliding: Implications for chemotherapy-induced peripheral neuropathy. Neurotoxicology. 2013;37:231–239. doi: 10.1016/j.neuro.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13(1):27–46. doi: 10.1111/j.1529-8027.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- 9.Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249(1):9–17. doi: 10.1007/pl00007853. [DOI] [PubMed] [Google Scholar]
- 10.Tofthagen C, Donovan KA, Morgan MA, Shibata D, Yeh Y. Oxaliplatin-induced peripheral neuropathy’s effects on health-related quality of life of colorectal cancer survivors. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2013 doi: 10.1007/s00520-013-1905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stone CA, Lawlor PG, Kenny RA. How to identify patients with cancer at risk of falling: a review of the evidence. J Palliat Med. 2011;14(2):221–230. doi: 10.1089/jpm.2010.0326. [DOI] [PubMed] [Google Scholar]
- 12.Gewandter JS, Fan L, Magnuson A, et al. Falls and functional impairments in cancer survivors with chemotherapy-induced peripheral neuropathy (CIPN): a University of Rochester CCOP study. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2013;21(7):2059–2066. doi: 10.1007/s00520-013-1766-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mooney KH, Beck SL, Friedman RH, Farzanfar R. Telephone-linked care for cancer symptom monitoring: a pilot study. Cancer Pract. 2002;10(3):147–154. doi: 10.1046/j.1523-5394.2002.103006.x. [DOI] [PubMed] [Google Scholar]
- 14.Argyriou AA, Bruna J, Marmiroli P, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Critical reviews in oncology/hematology. 2012;82(1):51–77. doi: 10.1016/j.critrevonc.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79(3):340–349. doi: 10.2105/ajph.79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mols F, Beijers T, Lemmens V, van den Hurk CJ, Vreugdenhil G, van de Poll-Franse LV. Chemotherapy-Induced Neuropathy and Its Association With Quality of Life Among 2- to 11-Year Colorectal Cancer Survivors: Results From the Population-Based PROFILES Registry. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(21):2699–2707. doi: 10.1200/JCO.2013.49.1514. [DOI] [PubMed] [Google Scholar]
- 17.Tofthagen C, Overcash J, Kip K. Falls in persons with chemotherapy-induced peripheral neuropathy. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2011;20(3):583–589. doi: 10.1007/s00520-011-1127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simoneau GG, Derr JA, Ulbrecht JS, Becker MB, Cavanagh PR. Diabetic Sensory Neuropathy Effect on Ankle JointMovement Perception. Archives of Physical Medicine and Rehabilitation. 1996;(77):453–460. doi: 10.1016/s0003-9993(96)90033-7. [DOI] [PubMed] [Google Scholar]
- 19.Richardson JK, Ashton-Miller JA, Lee SG, Jacobs K. Moderate Peripheral Neuropathy Impairs Weight Transfer and Unipedal Balance in the Elderly. Archives of Physical Medicine and Rehabilitation. 1996;77:1152–1156. doi: 10.1016/s0003-9993(96)90139-2. [DOI] [PubMed] [Google Scholar]
- 20.Ganz DA, Bao Y, Shekelle PG, Rubenstein LZ. Will my patient fall? JAMA. 2007;297(1):77–86. doi: 10.1001/jama.297.1.77. [DOI] [PubMed] [Google Scholar]
- 21.Richardson JK, Hurvitz EA. Peripheral neuropathy: a true risk factor for falls. J Gerontol A Biol Sci Med Sci. 1995;50(4):M211–5. doi: 10.1093/gerona/50a.4.m211. [DOI] [PubMed] [Google Scholar]
- 22.Richardson JK. Factors associated with falls in older patients with diffuse polyneuropathy. J Am Geriatr Soc. 2002;50(11):1767–1773. doi: 10.1046/j.1532-5415.2002.50503.x. [DOI] [PubMed] [Google Scholar]
- 23.Cavanagh PR, Derr JA, Ulbrecht JS, Maser RE, Orchard TJ. Problems with gait and posture in neuropathic patients with insulin-dependent diabetes mellitus. Diabet Med. 1992;9(5):469–474. doi: 10.1111/j.1464-5491.1992.tb01819.x. [DOI] [PubMed] [Google Scholar]
- 24.Rosen T, Mack KA, Noonan RK. Slipping and tripping: fall injuries in adults associated with rugs and carpets. J Inj Violence Res. 2013;5(1):61–69. doi: 10.5249/jivr.v5i1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pike CT, Birnbaum HG, Muehlenbein CE, Pohl GM, Natale RB. Healthcare Costs and Workloss Burden of Patients with Chemotherapy-Associated Peripheral Neuropathy in Breast, Ovarian, Head and Neck, and Nonsmall Cell Lung Cancer. Chemotherapy Research and Practice. 2012;2012(1):1–10. doi: 10.1634/theoncologist.6-5-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubenstein LZ, Josephson KR. The epidemiology of falls and syncope. Clin Geriatr Med. 2002;18(2):141–158. doi: 10.1016/s0749-0690(02)00002-2. [DOI] [PubMed] [Google Scholar]
- 27.Tofthagen C, Visovsky C, Berry DL. Strength and balance training for adults with peripheral neuropathy and high risk of fall: current evidence and implications for future research. Oncol Nurs Forum. 2012;39(5):E416–24. doi: 10.1188/12.ONF.E416-E424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Khoury F, Cassou B, Charles M-A, Dargent-Molina P. The effect of fall prevention exercise programmes on fall induced injuries in community dwelling older adults: systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f6234. doi: 10.1136/bmj.f6234. [DOI] [PMC free article] [PubMed] [Google Scholar]


