Abstract
Tissue engineering and regenerative medicine rely extensively on biomaterial scaffolds to support cell adhesion, proliferation, and differentiation physically and chemically in vitro and in vivo. Changes to the surface characteristics of the scaffolds have the greatest impact on cell response. Here, we discuss five dominant surface modification approaches used to biomimetically improve the most common scaffolds for tissue engineering, those based on aliphatic polyesters. Scaffolds of aliphatic polyesters such as poly(L-lactic acid), poly(L-lactic-co-glycolic acid), and poly(ε-caprolactone) are often used in tissue engineering because they provide desirable, tunable properties such as ease of manufacturing, good mechanical properties, and nontoxic degradation products. However, cell–surface interactions necessary for tissue engineering are limited on these materials by their smooth postfabrication surfaces, hydrophobicity, and lack of recognizable biochemical binding sites. The surface modification techniques that have been developed for synthetic polymer scaffolds reduce initial barriers to cell adhesion, proliferation, and differentiation. Topographical modification, protein adsorption, mineral coating, functional group incorporation, and biomacromolecule immobilization each contribute through varying mechanisms to improving cell interactions with aliphatic polyester scaffolds. Furthermore, rational combination of methods from these categories can provide nuanced, specific environments for targeted tissue development.
Keywords: biomaterial, cell response, functionalization, nanofibers, protein adsorption, RGD, simulated body fluid, topography
1 |. INTRODUCTION
Tissue engineering focuses on the creation of artificial tissues and organs for transplant. Tissue engineers use cells, biomaterials, biochemical factors, and physical factors to maintain, improve, or replace biological tissues (Lanza, Langer, & Vacanti, 2014). In a typical tissue engineering model, cells are harvested from an organism and grown in cell culture media, often with solid scaffolding constructs providing structure and support in vitro.
Research in this area creates scaffolds to grow and sculpt tissues in three dimensions. Scaffolds used in tissue engineering mimic the natural extracellular matrix (ECM) and provide support for cell adhesion, migration, proliferation, tissue generation, and 3D organization. The scaffold needs to be completely biodegradable so that the scaffold dis-integrates entirely and nontoxically as it is replaced by tissue.
Scaffolds are made from natural and synthetic biomaterials. Natural scaffold biomaterials include collagen, chitosan, and decellularized tissues. Synthetic biomaterials include polymers such as poly(lactic acid) (PLA), poly(L-lactic-co-glycolic acid) (PLGA), and poly(ε-caprolactone) (PCL). Although natural biomaterials are often used as scaffolds, they suffer from batch-to-batch variation. Synthetic polymers are more easily and consistently reproduced using manufactured specifications. However, they are often hydrophobic and less accurately mimic the in vivo microenvironment of cells (Lanza et al., 2014; Zhang, Ortiz, Goyal, & Kohn, 2014).
Tissue engineering relies on producing microenvironments that support cell growth and controlled differentiation into mature cells capable of regenerating the desired tissues, such as bone, skin, or spinal cord tissue. Most often, undifferentiated cells are cultured on a natural or synthetic biomaterial, which acts as a surrogate scaffold before and during differentiation and tissue development. Through cell–surface interactions, the biomaterial can support the cell mechanically and provide chemical cues (Boyan, Hummert, Dean, & Schwartz, 1996). Because scaffolds can extensively affect cell fate, biomaterials and tissue engineering researchers have focused on developing biomimetic scaffolds that provide biochemical and physical signals mimicking the target microenvironment.
Aliphatic polyesters are a group of synthetic polymers that can provide a viable base material for scaffolds due to their nontoxic biochemical degradation and adaptability. Aliphatic polyesters were first used in the medical field as sutures in 1971 (Frazza & Schmitt, 1971). Since then, their medical use has expanded to include use as tissue engineering scaffolds. Aliphatic polyesters include PLA, PLGA, and PCL (Jafari et al., 2017; Liu & Ma, 2004). Although other aliphatic polyesters see use as tissue engineering scaffolds, PLA, PLGA, and PCL have seen the most extensive application of surface modification techniques.
Poly(glycolic acid) (PGA) is often used as mesh scaffolding for blood vessel tissue as well as cartilage and neuronal tissue, but its relatively fast degradation period (2 weeks to 5 months) limits its use in other tissues (Gong & Niklason, 2008; Niklason et al., 1999; Quint, Arief, Muto, Dardik, & Niklason, 2012; Seal, Otero, & Panitch, 2001). However, PGA’s copolymers with lactic acid, PLGA, form the most widely used synthetic scaffold material in tissue engineering. Because PGA has a quick degradation rate and PLA typically takes over a year to degrade fully, copolymers with varying ratios can be used to tune the scaffold degradation rate (Peter, Miller, Yasko, Yaszemski, & Mikos, 1998).
PLA’s role as a chiral molecule with different properties for each enantiomer contributes to its versatility as a scaffolding material. The most common form of PLA is a mixture of its enantiomers, poly(D,L-lactic acid) (PDLLA). In tissue engineering scaffolds, however, poly(L-lactic acid) (PLLA) is more frequently used. PDLLA forms an amorphous polymer used in drug delivery, whereas PLLA forms a semicrystalline structure with high mechanical strength and toughness, which is more often appropriate for tissue engineering (Ramot, Haim-Zada, Domb, & Nyska, 2016; Saltzman & Kyriakides, 2014).
PCL degrades more slowly than PLA or PLGA, but its toughness and biocompatibility make it an effective scaffolding material, especially for bone tissue engineering (Abedalwafa, Wang, Wang, & Li, 2013; Saltzman & Kyriakides, 2014). PCL can be shaped in many of the same ways as PLLA and PLGA to produce nanofibers, films, or 3D scaffolds. It also shares many ways to be surface modified, including grafting, adsorption, and plasma treatment.
PLA, PLGA, and PCL have several mechanical and chemical properties in common that make them excellent scaffold biomaterials. All three, in their unmodified forms, are biocompatible, demonstrating no significant toxicity to cells cultured on their surfaces and degrading into nontoxic monomers such as lactic acid (Jafari et al., 2017; Ramot et al., 2016). They are also highly consistent synthetic materials with readily controlled polymeric properties, unlike natural and naturally derived scaffolding biomaterials. They are easily shaped using techniques such as solvent-casting, particulate-leaching, molding, and fused-deposition modeling to create specific architectures for three-dimensional scaffolds (Serra, Mateos-Timoneda, Planell, & Navarro, 2013; Tadmor, 2006; Williams et al., 2005). Their mechanical properties can be tuned to imitate multiple tissue types, such as the bone or tendon tissue. They can support adherent cells even when subject to significant fluid shear forces found in the body (Alvarez-Barreto et al., 2011; Alvarez-Barreto & Sikavitsas, 2007; Trachtenberg et al., 2017). Furthermore, with respect to the initial cell adhesion and proliferation time scales relevant to surface modification, degradation of synthetic aliphatic polymers does not play a significant role (Rohman, Pettit, Isaure, Cameron, & Southgate, 2007).
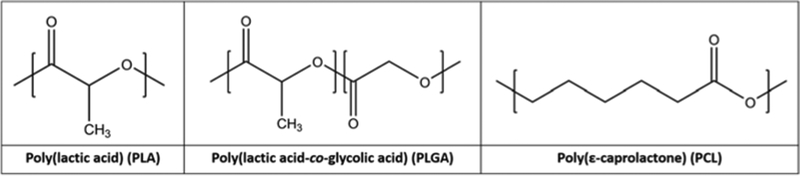
However, these synthetic polymers cannot compare with natural biomaterials in terms of biochemical activity. In many medical applications, the biological inactivity of PLA, PLGA, and PCL is advantageous, but not in tissue engineering. Although many anchorage-dependent cells can adhere to their surfaces and grow, they expend additional energy producing ECM. Natural biomaterials, such as collagen and gelatin, provide the basic constituents of ECM, accelerating cell growth and development (García & Reyes, 2005). Beyond lacking ECM constituents, PLA, PLGA, and PCL contain no bioactive moieties such as amine and thiol groups that could conjugate with ECM molecules (Figure 1). Aliphatic polyesters also suffer from low hydrophilicity due to hydrocarbon backbones, limiting cell contact spreading prior to ECM development (Shin, Jo, & Mikos, 2003; Thanki, Dellacherie, & Six, 2006; Yang, Leong, Du, & Chua, 2001). Biomimetic approaches in synthetic polymer scaffold design seek to unite the consistency, manufacturability, and mechanical properties of synthetic polymers with the cell-signaling potential found in native tissues.
FIGURE 1.
Repeating unit chemical structure of three aliphatic polyester polymers commonly used for tissue engineering scaffolds. These polymers are highly hydrophobic and provide limited functional group availability for chemical reactivity with cells
Notably, hydrogels based on synthetic polymers are highly popular alternatives to aliphatic polyester scaffolds. Hydrogels are cross-linked, insoluble polymers that absorb large amounts of water. These polymers have a high degree of chemical design flexibility, supporting a variety of biomimetic systems (Clegg, Wechsler, & Peppas, 2017; De Witte, Fratila-Apachitei, Zadpoor, & Peppas, 2018; Neves et al., 2017). In fact, hydrogels actively respond to the environment based on mechanisms such as pH-dependent swelling and molecular sensing (Clegg et al., 2017; Peppas & Van Blarcom, 2016). Thus, hydrogels hold great promise as the next generation of advanced scaffolds for tissue engineering.
However, hydrogels are limited in certain applications of tissue engineering by their lower mechanical strength, deformation under flow perfusion conditions, and differences in cell adhesion behavior compared with aliphatic polyester scaffolds. Due to the difficulty of comparing surface modification techniques between hydrogels and hydrophobic polymer scaffolds, we do not directly address them here.
For 3D scaffolds, porosity and pore structure play an important role in cell penetration and mechanical forces. For bone tissue engineering, scaffolds have been designed with trabecular bone-like porosity or through experimental trials to determine optimal porosity for cell development (Fernandez-Yague et al., 2015). Other scaffolds, such as for vascular tissue engineering, based their porosity and structure on factors such as permeability requirements (Flemming, Murphy, Abrams, Goodman, & Nealey, 1999).
Yet the greatest opportunity for biomimetic modifications occurs in the choice and design of the biomaterial itself. Physicochemical properties intrinsic to each material greatly influence cell adhesion, proliferation, and differentiation on the scaffold (Murphy, McDevitt, & Engler, 2014; Stevens & George, 2005). Improvements in cell adhesion lead to a greater fraction of cells surviving the seeding process and developing ECM on the scaffold. Faster proliferation increases cell density on the surface, and greater control of differentiation improves the chances of successful grafting upon implantation. All three factors accelerate the tissue engineering process, supporting the goal of viable engineered tissue implants in clinical practice. The choice of biomaterial and its subsequent treatments play a critical role in this process, but there are many approaches to modifying biomaterials, and each has its advantages and disadvantages for their intended tissue.
For aliphatic polyesters and other biomaterials, there are two main approaches to modification: bulk and surface modifications. Bulk modification changes the entire biomaterial, even the parts not exposed to cells. Surface modification treatments focus on changing only the surfaces where cells interact, maintaining the integrity of the material’s backbone (Ikada, 1994).
Bulk modifications tend to alter structural and mechanical properties, and many require a complete reevaluation of all the material’s properties. With functional bulk modifications, a large portion of the functionality remains unused because the cells only interact with the material’s surface. However, as the material degrades, exposure of cryptic functional groups sustains bioactivity.
Surface modification, by contrast, sees greater effect per added functionality because all function is concentrated on the cell–surface interface, but more rapidly loses their effectiveness as the surface begins to degrade. This can be advantageous in tissue engineering because cells need the greatest support and guidance during their earliest phases. Once matured and ensconced in appropriate ECM, cells will tend to produce their own differentiation and growth signals. Surface modifications do not usually change the underlying material, resulting in a bioactive change that does not sacrifice mechanical properties (Chen & Su, 2011; Goddard & Hotchkiss, 2007). For that reason, surface modifications are especially effective for biomimetic synthetic polymer scaffolds.
Here, we address the dominant approaches to modifying the surface of aliphatic polyester constructs to create biomimetic scaffolds for tissue engineering.
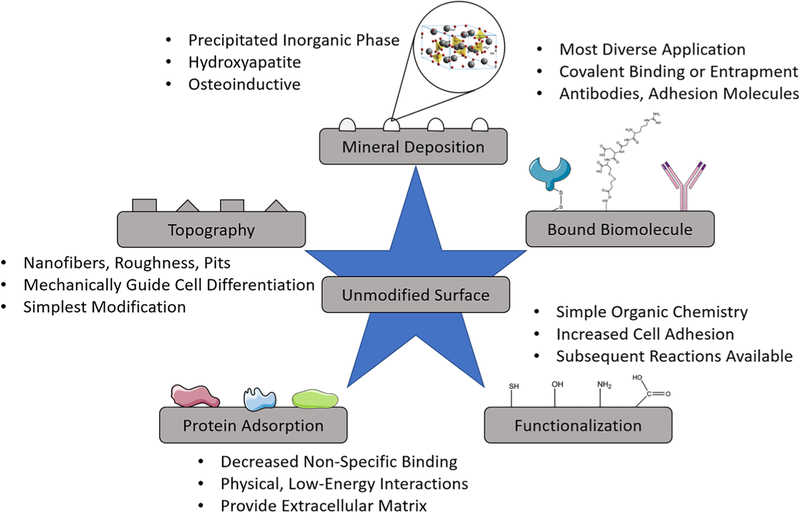
Five major groups of surface modifications are identified, and their development, objectives, and unique features are discussed to justify their categorization as distinct regimes of scaffold surface modification (Figure 2). Although each group individually contributes significantly to cell–surface interactions, it is proposed that most or all cellular mechanisms activated by the different groups are used in the natural tissue microenvironment, and therefore, all groups and their combinations warrant further study. In brief, this review addresses topographical modifications, protein adsorption, inorganic mineral deposition, functional group addition, and conjugation or entrapment of complex biomolecules. By presenting these groups in a united review, we aim to introduce a comprehensive perspective on scaffold surface modification that facilitates further comparison and rational combination of the techniques.
FIGURE 2.
Tissue engineering scaffold surface modification techniques. Mineral deposition subsection represents hydroxyapatite
2 |. TOPOGRAPHICAL MODIFICATION
Topographical modifications alter cell response through changes in the geometry of their microenvironment. Because this modification approach aims to alter the physical state of the scaffold surface without changing chemical properties, it is highly translatable across materials. Topographical modification techniques have been developed for PLA (Yang et al., 2003), PLGA (Brown et al., 2005), and PCL (Cassidy et al., 2014) as well as for titanium (Zinger et al., 2005), polystyrene (Yim, Darling, Kulangara, Guilak, & Leong, 2010), silicon (Fiedler et al., 2013), and other hard biomaterials (Itälä, Koort, Ylänen, Hupa, & Aro, 2003).
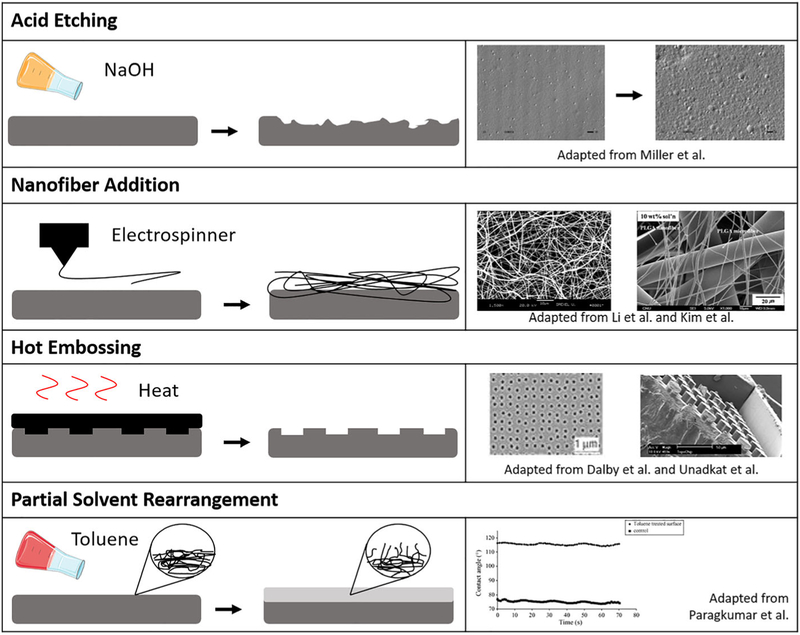
Topographical modifications are largely restricted to low-micron and nanometer ranges that adherent cells can recognize (Kantawong et al., 2010; Kim et al., 2012; Stevens & George, 2005). Modifications range from random, simple, and homogeneous to highly specific, ordered treatments (Figure 3). Basic treatments aim primarily to increase the surface roughness of the material, increasing surface area for cell interaction and increasing hydrophilicity of the surface. Alternatively, basic topographical treatments can be used to increase hydrophobicity, reducing unwanted cell adhesion (Alves et al., 2009). Advanced topographical treatments tend to elicit more specific cell responses, such as directing mobility and striation or governing differentiation timing and pathways. Both basic and advanced approaches on any material contribute to an understanding of how cells respond to surface physical factors, an important consideration in any scaffold design.
FIGURE 3.
Varying approaches in topographical modifications for guiding cell response. Figures adapted from Dalby et al. (2007), S. J. Kim, Jang, Park, and Min (2010), W. J. Li, Laurencin, Caterson, Tuan, and Ko (2002), Miller, Thapa, Haberstroh, and Webster (2004), Thanki et al. (2006), and Unadkat et al. (2011), with permission from Elsevier, Springer Nature, and Wiley
The simplest approach to aliphatic polyester topographical modification, alkali acid etching, increases the surface roughness of the scaffold and hydrophilicity. Alkali acid polyester etching has been used to modify scaffolds for several tissue types, including the bone (Smith, Niziolek, Haberstroh, Nauman, & Webster, 2007), cartilage (Park, Pattison, Park, & Webster, 2005), blood vessels (Miller et al., 2004), and bladders (Pattison, Wurster, Webster, & Haberstroh, 2005; Thapa, Miller, Webster, & Haberstroh, 2003). Acid etching relies on hydroxide nucleophilic attack of the ester bonds, often facilitated by organic compounds such as ethanol or citric acid that assist in solubilizing the hydrophobic polymer surface. Alkali acid etching has been shown to reduce the water contact angle of PLLA by more than 20° and improve cell affinity (Guo, Xiang, & Dong, 2015). PLGA treatments resulted in 500% increase in nanoscale surface roughness and enhanced vascular cell attachment and chondrocyte development (Park et al., 2005). However, alkali acid etching acts by eroding the biomaterial, so it is not viable for thin films or nanofibers.
The incorporation of nanofibers has recently emerged as one of the most popular forms of topographical modifications (Kim et al., 2010; Woo et al., 2007). Electrospinning of polymers produces nano-fibers, resulting in a nanoporous mesh that mimics fibrous ECM. PLGA and PLA nanofibers for tissue engineering scaffolds display improved cell culture properties when compared with smooth materials (W. J. Li et al., 2002). Molded 3D nanofibrous PLLA scaffolds with controllable macroporosity outperform solid PLLA 3D scaffolds in both cell proliferation and osteogenesis (Chen, Smith, & Ma, 2006). Similar scaffolds have been developed and tested for nerve tissue and epithelial tissue (H. S. Koh, Yong, Chan, & Ramakrishna, 2008; Leong, Chian, Mhaisalkar, Ong, & Ratner, 2009; Zamani, Amani-Tehran, Latifi, & Shokrgozar, 2013).
In addition to supporting differentiation goals, nanotopographical modifications on PCL have been used to support lasting MSC pluripotency over 8 weeks. Dalby and colleagues demonstrated that stem cells cultured on PCL with regularly distributed nanopits formed by hot embossing maintained stem cell phenotype for a period of 8 weeks (McMurray et al., 2011). Furthermore, these cells readily performed osteogenic or adipogenic differentiation when transferred to different scaffolds and subject to differentiation-signal media. When cultured on the ordered pits with osteogenic media, cells exhibited reduced differentiation compared to flat PCL, suggesting competitive signaling between the surface morphology and the media components. When pits were displaced into irregular position, cellular differentiation increased (Cassidy et al., 2014). On polymethylmethacrylate, nanopits were used to demonstrate that nanotopographical cues can induce osteoblastic differentiation without osteogenic media (Dalby et al., 2007). This set of experiments reveals the versatile and critical role of nanotopography in governing cell response.
Solvents and partial solvents of PLLA and PLGA significantly affect surface hydrophobicity (Thanki et al., 2006). Toluene, acetone, and ethyl acetate act as partial solvents of PLLA and PLGA, swelling the usually hard material like a hydrogel without dissolving the polymer. Temporary exposure to these weak solvents leaves lasting effects on the surface, significantly increasing water contact angle. Further study of the films indicated that hydrophobic methyl groups tend to orient toward an air-interface surface, with a time-dependent change in water contact angle suggesting restructuring of the surface when exposed to water. These conclusions present possibilities for surface entrapment approaches and important considerations for wet chemistry treatments.
Computational studies using high-throughput methods have validated a relationship between topographical cues and cell response (Unadkat et al., 2011). Machine learning algorithms discovered a Fourier-based parameter correlating spatial distribution of topographical microstructures to cell proliferation and osteogenesis.
Topography also affects protein response to surfaces. Smooth PLGA induces greater conformational change on fibrinogen than PLGA with nanoscale topographical features (Koh, Rodriguez, & Venkatraman, 2010). This result elucidates how minor topographical changes to the surface produce cascading effects on cell response because the activity of ECM proteins such as fibronectin mediates cell adhesion.
Topographical surface modifications have made impressive improvements to the cellular compatibility of polyester scaffold surfaces and contributed to an understanding of physical influences on cell fate. Biggs, Richards, and Dalby (2010) further discuss the influence of nanotopography on cell adhesion with emphasis on the mechanism of integrin-mediated focal adhesions. However, topography modification is just one of several tools to induce biomimicry in tissue engineering scaffolds. Presentation of proteins, functional groups, and biomacromolecules on the surface drives further specific cell responses. Because aliphatic polyesters in tissue engineering are valued for their mechanical and physical properties, they have greater opportunity for improvement of their chemical and biological properties.
3 |. PROTEIN ADSORPTION
Noncovalent adsorption of ECM proteins and other bioactive molecules influences cell response to surfaces. Typical adsorption bonds are weak, depending on forces such as electrostatic interactions, van der Waals forces, and ligand–receptor pairings. These treatments are more susceptible to degradation and washing effects. Due to the transient nature of adsorption, effectiveness of these treatments can be challenging to quantify. Additionally, cell response to adsorbed proteins is difficult to isolate, especially because cells are regularly cultured in serum-containing media that facilitates uncontrolled protein adsorption to surfaces.
Protein adsorption plays a large role in in vivo response because immunogenic response depends on adsorbed proteins for targeting and signaling (Kao, 1999; Li et al., 2012). Interfacial events occur quickly in vivo, requiring preimplant scaffold treatments to mitigate macrophage response. Efforts in scaffold surface adsorption have focused on either improving adsorption of desirable molecules or reducing adsorption of unwanted proteins. Protein adsorption is also studied as a form of intermediate test between physicochemical evaluation and cell-response testing (Jiao et al., 2009; Jiao, Liu, Shao, & Zhou, 2012; Leong et al., 2009; Manso, Valsesia, Ceccone, & Rossi, 2004; Sarapirom, Yu, Boonyawan, & Chaiwong, 2014). Protein adsorption techniques are more commonly used on scaffolds of other polymers, such as poly(ethylene glycol), but PLGA and other aliphatic polyesters have seen viable modifications, often facilitated by preliminary surface treatments.
Protein adsorption studies with aliphatic polyesters have contributed to better understanding protein–surface interactions and their influence on cell response to a biomaterial. For a concise assessment of protein interactions with biomaterial surfaces, we suggest two reviews by Szott and Horbett (2011a, 2011b). Rabe, Verdes, and Seeger (2011) provide a more extensive review of the general phenomenon.
Protein adsorption is moderated by external factors, such as solution pH, temperature, ion concentration, and buffer composition (Rabe et al., 2011). Adsorption also depends on the properties of both the protein itself and the material to which it adsorbs. Controllable factors for the material include surface energy, polarity, charge, and morphology. Proteins, except for glycoproteins, generally see increased adsorption on hydrophobic surfaces. This results from most proteins’ tendency to form an amphiphilic interface between the hydrophobic surface and the aqueous solution.
When studying protein adsorption, one must consider that proteins are not simple molecules, and the adsorption process may significantly change protein activity. Protein bioactivity can be tested using cell response to protein-adsorbed surfaces with and without targeted antibody treatment, as demonstrated by Qin et al. (2005) on tenocytes’ increased binding to fibronectin- and collagen-coated PLGA surfaces, with integrin-binding-site antibodies nullifying the effect.
Researchers have compared the effectiveness of protein adsorption techniques with other common surface modification methods, such as covalent cross-linking. Results vary on their advantageousness. Bosetti et al. (2014) showed that polypeptide grafting reduces protein adsorption, and although fibronectin- and laminin-adsorbed scaffolds saw greater cell adhesion and proliferation than unmodified scaffolds, grafted polypeptides were far more effective. However, X.B. Yang et al. (2001) demonstrated that adsorbed fibronectin exceeded poly(L-lysine) (PLL)-grafted arginine-glycine-aspartic acid (RGD) in promoting cell spreading, even with both techniques at optimized concentration. This dissonance may be due to confounding factors such as unlinked PLL disrupting cell–surface interactions, but the optimized fibronectin-coated surface demonstrated comparable results to the positive control, tissue culture polystyrene.
Kakinoki and Yamaoka (2010) proved that adsorption of collagen-like synthetic polypeptides supported neurite outgrowth on PLA films. However, in a comparison of bulk, adsorption, and covalent binding techniques for laminin on PLLA, H. S. Koh et al. (2008) found the bulk modification to be the simplest and most successful in peripheral nerve regeneration. Continued study of protein adsorption in context and comparison with other scaffold treatment methods is needed to qualify its usefulness, especially regarding in vivo immunogenic response.
Several groups have studied protein adsorption changes when other bioactive particles are introduced, drawing connections between biomolecule presence, protein adsorption, and cell adhesion, proliferation, and differentiation. Jiao et al. (2009) grafted chitosan to a PLLA surface and then observed fibronectin and generic protein adsorption and desorption kinetics using radiolabeled proteins, confirming the competitive nature of protein adsorption on polymer surfaces and concluding that although protein adsorption alone can give some indication of biological response to a surface, it cannot accurately dictate a cell’s reaction to the surface.
Li et al. (2012) created nanospun fibers from PCL with and without hyaluronan to characterize its effect on protein adsorption, concluding that the presence of hyaluronan decreased in vivo nonspecific protein adsorption, reducing immunogenic response to the scaffolding biomaterial upon implantation. PLLA scaffolds surface modified with hyaluronan, heparin, or chondroitin sulfate exhibited reduced protein adhesion in serum and increased cytocompatibility (Jiao et al., 2012). However, bulk hydroxyapatite incorporation on PLLA facilitated increased adsorption of relevant ECM proteins and peptides, which in turn supported increased cell density (Woo, Seo, Zhang, & Ma, 2007). Biochemical analysis of the integrin expression from cells on hydroxyapatite-loaded surfaces linked the enhanced adsorption of adhesion proteins to suppression of apoptosis.
Plasma treatments, topographical modifications, and surface charge manipulation modify protein adsorption rates and specificity. Although plasma treatments alone improve cell adhesion, a coating using fibronectin-rich serum prior to seeding significantly assisted in cell proliferation and differentiation (Brown et al., 2005; Yildirim et al., 2010). Scaffolds with fibronectin serum and no prior plasma exposure exhibited results averaging between the control unmodified scaffold and plasma treatment with no fibronectin, suggesting that the plasma treatment and fibronectin adsorption have a cooperative or at least nondestructive relationship. Scaffolds without plasma treatment saw higher protein adsorption, presumably due to higher hydrophobicity, but this was not necessarily advantageous in terms of cell response. Using ammonia plasma on PLA decreased in vivo protein adsorption, confirming in vitro observations and arguing for an increase in cellular binding due to a reduced surface energy barrier and the presence of amine groups (Sarapirom et al., 2014). As an alternative to common basic and plasma-facilitated protein adsorption, H. Zhu, Ji, and Shen (2004) demonstrated protein adsorption potential using positively charged surface layers and gelatin. Gelatin content increased linearly with number of charged bilayers, and chondrocyte viability increased significantly over the control surface.
Exposure to specified serums affects protein adhesion and subsequent cell response. A specially designed serum for protein deposition, named growth factor-rich plasma, caused tenocytes cultured on treated polymer weaves to adhere, proliferate, and develop a greater fibrin matrix than serum-treated and untreated scaffolds (Visser et al., 2010). Proteins adsorbed from osteogenic cell-conditioned media also supported stem cell growth and differentiation (Lin, Chang, Hung, Lee, & Lin, 2017). Mohan et al. (2017) studied fibrinogen, bovine serum albumin, and immunoglobulin coatings on PCL scaffolds for their interactions with primary endothelial cells in vitro. Fibrinogen produced better metabolic activity and cell spreading than other treatments, whereas immunoglobulin reduced cell metabolism and distribution. Mei et al. (2005) correlated 3D PCL scaffold chitosan adsorption efficiency to pore size, with rates varying nearly linearly from 40- to 125-micron pore sizes. Subsequent cell-based analysis demonstrated that adsorbed chitosan improves fibroblast adhesion and proliferation.
A particularly insightful study by Chastain, Kundu, Dhar, Calvert, and Putnam (2006) shows how generalizing assumptions about materials and modifications may have unintended consequences. This study confirmed that PLGA and PCL adsorb fibronectin and vitronectin at different ratios in serum-based media, and those differences affect the osteogenic differentiation profile of adherent MSCs.
Although the addressed studies validate the basic usefulness of protein adsorption in scaffold biomimetics, questions remain on the mechanisms by which these treatments interact with adherent cells. Tests with highly specified media without generic serum should allow further in vitro discoveries, and greater study of immunogenic response to these treatments may provide new criteria for biomimetic necessities.
4 |. MINERAL DEPOSITION
Mineral-based surface modification contributes primarily to bone tissue engineering as a method for introducing calcium phosphate structures to scaffolds without altering bulk properties of the underlying biomaterial. Mineral–ion deposition occurs through nucleation of inorganic crystalline particles onto scaffold surfaces from ion-rich solution. This approach uses simulated body fluid (SBF) and its derivatives to produce hydroxyapatite-like nodules on scaffold surfaces.
The use of SBF as a mineral coating technique began with Kokubo, Kushitani, Sakka, Kitsugi, and Yamamuro (1990) subjecting bioglasses and ceramics to ionic solutions to produce biomimetic substrates. SBF mimics the ion concentrations in interstitial fluid, especially the mineral components required for bone formation. Originally developed to utilize the surface properties of bioglass for apatite deposition (Hata, Kokubo, Nakamura, & Yamamuro, 1995; Li et al., 1992), the SBF coating technique has transferred to several scaffolding substrates including titanium (Tas & Bhaduri, 2004), collagen (Al-Munajjed et al., 2009), silk (Lin et al., 2008), and polyesters (Table 1).
TABLE 1.
Simulated body fluid mineral coatings on aliphatic polyester scaffolds
| Reference | SBF magnificationa | Base material | Coating period | Cell type | Scaffold structure | Additional treatment | Major result |
|---|---|---|---|---|---|---|---|
| Choong, Triffitt, & Cui (2004) | 1.5x | PCL | 14 days | hMSC | FDM 3D | NaOH pretreatment, SiO2 pretreatment | Deposited mineral structure and ion ratios are similar to hydroxyapatite. Coating improved biological response in vitro and in vivo |
| Oyane et al. (2005) | 1x | PCL | 24 hr | None | FDM 3D, film | O2 plasma pretreatment, ion dipping pretreatment | SBF treatment achieved formation of a bone-like apatite layer on PCL scaffolds |
| Oyane et al. (2005) | lx | PCL | 24 hr | None | FDM 3D, film | NaOH pretreatment, ion dipping pretreatment | SBF treatment achieved formation of a bone-like apatite layer on PCL scaffolds |
| Jiao, Liu, Cui, & Zhou (2007) | lx | PLLA | 7, 14, 21 days | None | Porogen-leached 3D, film | NaOH pretreatment | Hydrolysis pretreatment quantitatively increases precipitation from SBF solution |
| F. Yang, Wolke, & Jansen (2008) | 10x, lx | PCL | 2–6 hr | None | Electrospun fiber mesh, film | Argon plasma pretreatment | 6 hr 10x SBF incubation lost all porous structure |
| Beşkardeş & Gümüşderelioğlu (2009) | 10x | PCL | 6, 24 hr | MC3T3 | Film | None | 6- and 24-hr coating periods with SBF affect cell proliferation and osteogenesis |
| Mavis, Demirtaş, Gümüşderelioğlu, Gündüz, & Çolak (2009) | 10x | PCL | 2, 4, 6, 15 hr | MC3T3 | Electrospun fiber mesh | None | Changes in phosphate and carbonate concentrations removed the need for pretreatments |
| Suárez-González et al. 2011) | 2x | PCL | 7 days | None | SLS 3D | NaOH pretreatment, VEGF and BMP2 adsorption posttreatment | SBF-coated scaffolds bound BMP2 and VEGF, promoting sustained growth factor release |
| Choi & Murphy (2012) | 2–5x | PLGA | 2 days (2x) + 4 days (2–5x) |
hMSC | Film | NaOH pretreatment | SBF ion ratio and concentration variations created distinctive morphological regimes affecting cell response. |
| J. H. Lee et al. (2014) | 2x, 1x | PCL | 4 days (2x) + 2 days (lx) | rMSC | PCL-gelatin blend fiber mesh | FN-OCN fusion protein adsorption posttreatment | Advanced biointerfacial system improved stem cell adhesion and osteogenic differentiation |
Abbreviations: BMP2, bone morphogenetic protein-2; FDM 3D, fused deposition modeling three-dimensional scaffold; FN-OCN, fibronectin-osteocalcin fusion protein; hMSC, human mesenchymal stem cell; MC3T3, mouse calvaria osteoblast precursor cell line; NaOH, sodium hydroxide, O2, oxygen; PCL, poly(ε-caprolactone); PLGA, poly (lactic acid-co-glycolic acid); PLLA, poly(L-lactic acid); rMSC, rat-derived primary mesenchymal stem cells; SBF, simulated body fluid; SiO2, silicon dioxide; VEGF, vascular endothelial growth factor.
Fold change in concentration of SBF components from original physiological formulation
The relevance of hydroxyapatite and other bone-like minerals has been studied primarily through bulk modification techniques (Lee, Rim, Jung, & Shin, 2010; Nyberg, Rindone, Dorafshar, & Grayson, 2017; Rezwan, Chen, Blaker, & Boccaccini, 2006; Wang, Cui, & Bei, 2005). Because calcium phosphate takes forms of varying complexity, bulk materials provide a convenient method of studying their effects in tissue engineering. However, bulk modifications also significantly affect mechanical properties of the scaffold, usually increasing hardness and brittleness (Rodriguez, Dias, d’Ávila, & Bártolo, 2013). They also require more specialized manufacturing techniques because the crystalline particles degrade typical polyester 3D printing and molding devices. These shortcomings, coupled with the previously discussed general advantages of surface modification and the translatability of SBF across material types, suggest a forthcoming increase in surface modification studies for scaffold mineralization.
SBF formulations for polyester scaffolds began in the early 2000s and have advanced over time to accelerate precipitation rate and improve cell response to the resulting calcium phosphate layer (Table 1). Hydrolysis pretreatment of PLLA allows critical development of hydroxyapatite on interior surfaces of PLLA scaffolds, whereas untreated scaffolds only exhibit precipitation on the outer surface (Jiao et al., 2007). The slow formation of hydroxyapatite from traditional SBF over multiple weeks prompted efforts to increase mineral concentrations and accelerate the precipitation process. Modified SBF solutions with 10 times the natural ion concentration (10× SBF) have seen increasing use due to their ability to induce homogeneous deposition in as little as two hours.
Beşkardeş and Gümüşderelioğlu (2009) noted that apatite nucleation sites formed via 6-hr incubation of 10× SBF enhanced differentiation of MC3T3 cells, whereas 24-hr treated surfaces developed larger calcium phosphate microparticles that provided a lesser differentiation stimulus to cultured cells. The authors believe this to be a result of the 6-hr precipitates contributing both calcium phosphate chemistry and nanotopography to the cell–surface interface. Yang et al. (2008) used 10× SBF to coat PCL nanofiber meshes. They demonstrated that 2-hr incubation induced mineralization, whereas 6-hr incubation led to such extensive precipitation that porosity was lost. They also concluded that the coating treatments improved scaffold hydrophilicity. However, their evaluations are limited to physical and chemical tests, suggesting that the material may benefit from more refinement based on cell response. In contrast to the results obtained by Yang et al. (2008), Mavis et al. (2009) indicated only mild reduction in the porosity of PCL nanofiber scaffolds at 2- and 6-hr of 10× SBF immersion. Mavis et al. (2009) also thoroughly addressed cell response to the modified scaffolding, confirming increased cell proliferation and differentiation on both 2- and 6-hr SBF-treated scaffolds compared with pure PCL. In evaluating both the material properties and cell response of varying treatments, Mavis et al. (2009) provide a highly compelling argument for the relevance of SBF apatite coatings in bone tissue engineering scaffolding.
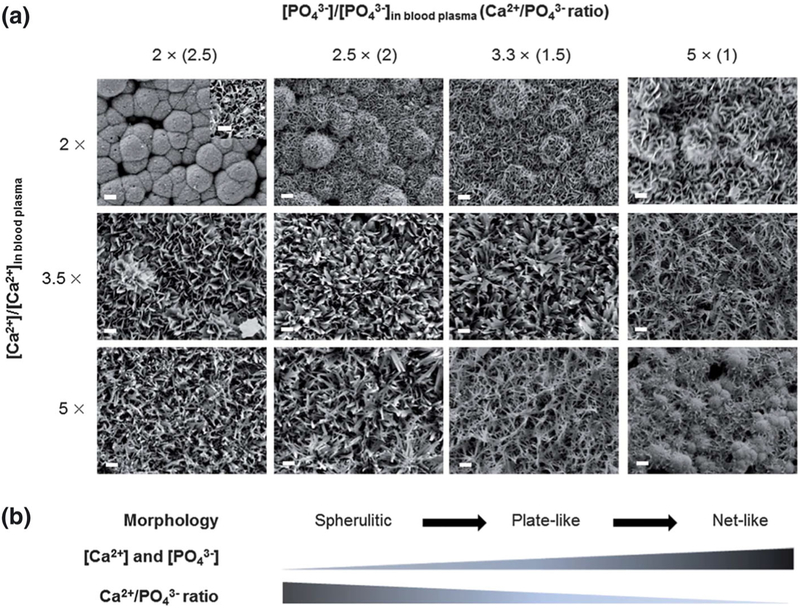
Recent work with SBF focuses on refined control of the deposited layers and their chemical and physical interactions with other biomolecules and cells. Zhao, Lemaître, and Bowen (2017) describe interactions of SBF-coated biomaterials with proteins. By varying ion ratios in SBF, Choi and Murphy (2012) control the morphology of deposited apatite, from spherulitic to plate-like to net-like, with minor changes in deposited calcium phosphate ratio, crystallinity, and dissolution rate (Figure 4). Mineral micromorphology had a direct influence on stem cell response, with spherulitic surfaces causing the greatest improvement in cell attachment and expansion.
FIGURE 4.
Scanning electron micrographs relate simulated body fluid (SBF) ion concentration to surface topography. Scale bar = 10 μm. Inset scale bar = 1 μm. The deposited mineral composition remains similar, suggesting the solution concentration primarily affects structure, with ratio-dependent transitions from spherulitic to plate-like to net-like. Importantly, cell adhesion rates were highest on the spherulitic structure. Previous publications in SBF coating techniques increased ion concentrations to accelerate precipitation without addressing effects on surface morphology. Reproduced from (Choi & Murphy, 2012) with permission from The Royal Society of Chemistry
Suárez-González et al. (2011) used SBF-based mineral coatings as controlled release factors for VEGF and BMP2. Both proteins have known binding properties with hydroxyapatite, but Suárez-González et al. (2011) used varying bicarbonate anion concentrations in their SBF solution to produce mineralization that degrades at different rates, dictating the release kinetics of the bound proteins. Lee et al. (2014) expanded on the technique of protein binding to hydroxyapatite surface coatings, immobilizing a fibronectin–osteocalcin fusion protein and acquiring in vitro and in vivo results. The biomimetic presence of bulk-modified gelatin and surface-modified hydroxyapatite and fibronectin on a PCL backbone facilitated adhesion, proliferation, and osteogenic differentiation, with the inclusion of the surface-tethered fusion protein significantly improving response both in vitro and in vivo. Lee’s study represents one of the most thoroughly designed and specialized implantable biomimetic scaffolds using surface mineralization.
Although mineral deposition may appear specifically appropriate for bone and cartilage tissue engineering, the liquid–solid ion exchange and the ability to bind with various proteins through inorganic interactions suggest the possibility for applications in other areas of tissue engineering such as controlling calcification in nonbone tissue. For further discussion of mineral deposition and SBF, we suggest the review of K. Shin, Acri, Geary, and Salem (2017).
5 |. FUNCTIONALIZATION
The surface of basic PLA, PLGA, and PCL scaffolds are functionally inactive, with their only noncarbon or hydrogen element, oxygen, used to create the polyester bond. Techniques that modify the chemical properties of a scaffold often require presence of a functional group, such as amines, carboxylic acids, or thiols on the surface. These groups provide direct bioactivity for cell binding, or they can be used to cross-link larger polypeptides, whole proteins, glycosaminoglycans, or other targeted molecules. This section will discuss methods for introducing functional groups to the surface and their varying effects on cells and subsequently tethered biomacromolecules. Common methods for functionalizing surfaces include plasma deposition, physical entrapment of small functional molecules, aminolysis, and hydrolysis.
In plasma-enhanced chemical vapor deposition (PECVD), ionized gas particles react rapidly with the surfaces of polyester films and scaffolds. For tissue engineering applications, PECVD adds functional groups to the surfaces of polymeric scaffolds to improve biocompatibility or to covalently bind bioactive molecules.
Primary factors affecting PECVD are the plasma composition, exposure time, and generator power (Chim, Ong, Schantz, Hutmacher, & Agrawal, 2003; Jordá-Vilaplana, Fombuena, García-García, Samper, & Sánchez-Nácher, 2014). Although quantitative analysis of exposure time and generator power are beyond the scope of this review, we briefly asses the qualitative effects of plasma composition on functional group formation for their applications in tissue engineering scaffold modification.
Oxygen plasma treatment produces hydroxyl, carbonyl, and carboxyl group enrichment (Chim et al., 2003). Ammonia plasma is used more frequently than oxygen plasma because it results in chemically distinct terminal amine groups that improve cell adhesion and provide convenient sites for subsequent cross-linking (Table 2). Nitrogen and argon gases behave as etchants, pretreatments for sterilization or radical creation, and carriers for other gas or solid targets (Table 2). Finally, air-based plasma is used as a cost-effective, nonspecific surface activator (Table 2).
TABLE 2.
Cross-linking surface modifications on aliphatic polyester scaffolds
| Reference | Polymer | Scaffold structure | Functionalization | Cross-linker | Target | Cell type | Major results |
|---|---|---|---|---|---|---|---|
| Y. Zhu, Gao, Liu, & Shen (2002) | PCL | Film | Aminolysis (HD) | Glutaraldehyde | Gelatin, chitosan, collagen | HUVEC | HUVECs retained vWF secreting ability and healthy cell morphology on modified scaffolds. |
| Hu, Winn, Krajbich, & Hollinger (2003) | PLA | Film, lyophilized foam | NH3 plasma | Glutaraldehyde | RGD, glycine, PLL | hOPC | RGD increases osteogenic precursor attachment and differentiation. |
| Y. Zhu, Gao, Liu, He, & Shen (2004) | PLLA | Film | Aminolysis (HD) | Glutaraldehyde | Gelatin, chitosan, collagen | HUVEC | Both aminolyzed and biomacromolecule-immobilized surfaces improved cell proliferation and cell activity. |
| Y. Zhu, Gao, Liu, & Shen (2004) | PLLA | Film | Aminolysis (HD), photografting (MAA) | Glutaraldehyde, EDC | Collagen, chondroitin sulfate | HUVEC | Collagen modification improves cell response over functionalized intermediates. |
| Z. Cheng & Teoh (2004) | PCL | Film | Ar plasma, UV/AA | EDC | Collagen | hDF, hMB | Increased hydrophilicity and roughness from treatments. Improved cell attachment and proliferation. |
| Croll, Connor, Stevens, & Cooper-White (2004) | PLGA | Film | hydrolysis, aminolysis (ED) | EDC/NHS | Chitosan | None | High ethylenediamine concentrations limit hydrolysis in favor of aminolysis. Hydrolysis and aminolysis facilitate subsequent biomacromolecule cross-linking. |
| Yoon, Song, Lee, & Park (2004) | PLGA | Porogen leached | Bulk | EGS | RGD | rMSC | Cell adhesion and differentiation increase with increased surface coating of RGD, controlled by bulk amine concentration. |
| Ma, Gao, Gong, & Shen (2005) | PLLA | Film, porogen leached | H2O2/UV, MAA | EDC | Collagen, bFGF | Rabbit chondrocyte | Stable collagen and growth factor immobilization improved chondrocyte spreading and growth. |
| Ma, He, Yong, & Ramakrishna (2005) | PCL | Electrospun mesh | Air plasma | EDC/NHS | Gelatin | HCAEC | Aligned gelatin grafted nanofibers controlled endothelial cell orientation. |
| Y. Zhu, Leong, Ong, Chan-Park, & Chian (2007) | PLLA-CL | Electrospun mesh | Aminolysis (HD) | Glutaraldehyde | Fibronectin | Porcine epithelial | Modified scaffold promoted esophageal epithelium regeneration. |
| Alvarez-Barreto, Shreve, Deangelis, & Sikavitsas (2007) | PLLA | Film, porogen leached | Amine entrapment | SPDP | RGD | rMSC | RGD bound to a 3D PLLA scaffold via amine entrapment and cross-linking improves cell adhesion. |
| Alvarez-Barreto & Sikavitsas (2007) | PLLA | Porogen leached | Amine entrapment | SPDP | RGD | rMSC | RGD-linked 3D scaffold under oscillatory flow perfusion improves cell seeding efficiency. |
| Kiss, Kutnyánszky, & Bertóti (2010) | PLGA | Film | Aminolysis (ED) | Glutaraldehyde | PEG | None | Linear and star-like PEG increased surface hydrophilicity and reduced protein adsorption. |
| Alvarez-Barreto et al. (2011) | PLLA | Porogen leached | Amine entrapment | SPDP | RGD | rMSC | RGD-linked 3D scaffold under oscillatory flow perfusion improves osteoblastic differentiation of MSCs. |
| J. P. Chen & Su (2011) | PLLA | Electrospun mesh | O2 plasma | EDC | Gelatin | Rabbit chondrocyte | Gelatin coating supported proliferation, chondrocyte phenotype, and ECM development. |
| Wulf et al. (2011) | PCL | Film | NH3 plasma, O2 plasma/APTES, hydrolysis/MDI | DSC | VEGF, ASA | Mouse fibroblast | NH3 plasma was most efficient for ASA loading, but O2 plasma/APTES treatment was most effective for VEGF immobilization. |
| Z. Yang et al. (2012) | PLLA-CL | Porogen leached | Aminolysis (HD) | Glutaraldehyde | Chitosan | hMSC | Modification increased cell compatibility and cartilage development. |
| Desmet, Poleunis, Delcorte, & Dubruel (2012) | PCL | Film | Ar plasma, AEMA | EDC | Gelatin, fibronectin | None | XPS and ToF-SIMS confirmed homogeneous grafting of target molecules to the surface. |
| Petersen et al. (2014) | PLLA | Film | NH3 plasma, aminolysis (HD), O2 plasma and APTES | EDC/NHS and Boc-(GGAP)4-COOH | Anti-CD34 | HUVEC | Fewer anchoring sites on the antibody produced greater surface coupling efficiencies. GGAP polytetrapeptide spacer improved immobilized antibody capture capacity. |
| Bosetti et al. (2014) | PLLA | Film | Hydrolysis | EDC/NHS | RGD, SIKVAV | hOB, hMSC | Adsorbed laminin and fibronectin promoted short-term cell adhesion. Bound RGD improved cell proliferation, but bulk incorporation of vitamin D3 was needed to induce osteogenesis. |
| Guex et al. (2014) | PCL | Electrospun mesh | CO2, C2H4 plasma and HD or DTOD | EDC/NHS | VEGF | HUVEC | Bound VEGF remained active and promoted cell proliferation. |
| Krok-Borkowicz et al. (2015) | PLGA | Film | Ar plasma, AEMA | EDC | Gelatin | MG63 | Gelatin-coated films increased cell growth and homogeneity. |
| Stoleru et al. (2016) | PLA | Film | N2 plasma, gamma irradiation | EDC/NHS | Lactoferrin | Mouse fibroblast | Lactoferrin conjugation provides an antioxidative, antibacterial, noncytoxic coating to PLA. |
| Swilem et al. (2016) | PLA | Film | Air plasma, AA | EDC | Glucosamine | Mouse fibroblast | Conjugation of glucosamine produces a time-stable surface with increased cell adhesion and biocompatibility. |
| Dolci et al. (2016) | PLLA | Electrospun mesh | Hydrolysis, air plasma | EDC/NHS | Ab-FITC, anti-CD10 | None | Wet chemistry and air plasma functionalization schemes produced similar antibody conjugation efficiency. |
| Song, Song, Yeo, Kim, & Lee (2017) | PLA | Woven film | NH3 plasma | Glutaraldehyde | Trypsin | None | Conjugated trypsin retained 55% of its activity after 20 days. |
Abbreviations: AA, acrylic acid; Ab-FITC; antibody-conjugated fluorescein; AEMA, 2-aminoethyl methacrylate hydrochloride; anti-CD10, antibody for CD10 lymphoma marker protein; anti-CD34, antibody for CD34 transmembrane marker protein; APTES, (3-aminopropyl)triethoxysilane; Ar, argon; ASA, acetylsalicylic acid; Boc-(GGAP)4-COOH, boc-protected polytetrapeptide of glycine-glycine-alanine-proline; C2H4, ethane; CO2, carbon dioxide; DSC, N,N-disuccinimidyl carbonate; DTOD, 11-diamino-3,6,9-trioxaudecane; ECM, extracellular matrix; ED, ethylenediamine; EDC, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide; EGS, ethyleneglycol-bissuccinimidylsuccinate; HCAEC, human coronary artery endothelial cells; HD, hexanediamine; hMSC, human-derived mesenchymal stem cell; H2O2, hydrogen peroxide; hOB, human osteoblast; hOPC, human osteoblast precursor cells; hDF, human dermal fibroblasts; hMB, human myoblasts; HUVEC, human umbilical vein endothelial cells; MAA, methacrylic acid; MDI, 4,4′-methylenebis(phenyl isocyanate); MG63, osteoblast-like cell line; N2, nitrogen; NH3, ammonia; NHS, N-hydroxysuccinimide; O2, oxygen; PCL, poly(ε-caprolactone); PEG, poly(ethylene glycol); PLA, poly(lactic acid); PLGA, poly(lactic acid-co-glycolic acid); PLL, poly(L-lysine); PLLA, poly(L-lactic acid); PLLA-CL, poly(L-lactic acid-co-ε-caprolactone); RGD, arginine-glycine-aspartic acid; rMSC, rat-derived mesenchymal stem cell; SIKVAV, hexapeptide of serine-isoleucine-lysine-valine-alanine-valine; SPDP, succinimidyl 3-(2-pyridyldithio)propionate; Tof-SIMS, time of flight secondary ion mass spectrometry; UV, ultraviolet light treatment; VEGF, vascular endothelial growth factor; vWF, von Willebrand Factor; XPS, X-ray photoelectron spectroscopy
Although PECVD is highly effective in modifying flat surfaces, results vary on its effectiveness in modifying interior surfaces of 3D polymer scaffolds. Hu et al. (2003) established that the ammonia plasma reaction occurred inside porous scaffolds, but the reaction rate inside scaffolds was much slower than that on uncovered surfaces. Seo et al. (2013) used helium plasma treatment to promote fibronectin adsorption on the interior surfaces of a 3D electrospun PLLA scaffold, with effective penetration increasing over 10 min of plasma treatment.
Similar to PECVD, sputter coating of aliphatic polyester scaffolds uses plasma and a titanium oxide or hydroxyapatite target. Sputter coating of titanium oxide on PLGA films significantly increases hydro-philicity and improves human dermal fibroblast adhesion and proliferation under standard culture conditions (Ryu et al., 2005).
Titanium oxide sputter coating on PCL microfiber scaffolds increased oxygen and titanium surface presence, and cell density increased with treatment time up to 6 min, but 9 min produced significant cytotoxicity (Barbarash et al., 2016). Hydroxyapatite sputter coating on PLLA and PCL films created a surface with increased wettability and nanotopography (Bolbasov et al., 2014). Human hybrid endothelial cells saw enhanced attachment. A subsequent study by an associated group confirmed calcium phosphate deposition using X-ray fluorescence and demonstrated increased bone marrow stem cell viability on the treated scaffolds (Tverdokhlebov et al., 2015). However, sputter coating has severely limited penetration in 3D structures, so it is less viable than PECVD or other methods for surface modification of complex scaffolds.
Predating plasma treatments in surface functionalization is the use of wet chemistry to replace ester bonds with functional groups near the surface. Wet treatment causes hydrolysis or aminolysis, depending on the resulting functional group. As with PECVD, amines provide a more distinctive target for subsequent cross-linking.
Aminolysis is often performed using 1,6-hexanediamine or ethylenediamine (Table 2). Janorkar, Fritz, Burg, Metters, and Hirt (2007) used ultraviolet-assisted wet chemical functionalization with 4,4′-diaminobenzophenone to create branching architectures of primary amines on the surface of PLLA, increasing surface amine concentration with subsequent grafting iterations. Fibroblast viability and density increased with amine concentration. Similar increases in cell attachment and proliferation occurred using plasma-induced amine groups (Cheng et al., 2017).
Unlike aminolysis, hydrolysis occurs naturally through exposure to air and water but can be accelerated using etching solvents as described in the topographical modification section. Due to the ubiquity of hydrolysis in aliphatic polyesters, it is not optimal for controlled modification but often serves as a precursor for general coatings because it tends to increase the overall reactivity and roughness of the surface.
In addition to plasma deposition and wet chemistry, physical entrapment of small functional molecules serves to functionalize scaffold surfaces. Partial solvation entrapment of primary amine-rich poly(ε-Cbz-lysine) supports subsequent cross-linking of bioactive molecules in 3D PLLA scaffolds (Alvarez-Barreto et al., 2007; Alvarez-Barreto et al., 2011; Alvarez-Barreto & Sikavitsas, 2007). Entrapment via partial solubilization and reformation of the surface provides an alternative functionalization approach without the penetration restrictions of plasma treatment or the degradation effects of aminolysis and hydrolysis. However, it is dependent on the liquid–solid interface behavior of the small functional molecules and highly susceptible to hydrophobicity and solubility. The partial solubilization technique developed by Alvarez-Barreto et al. is also prone to nonspecific incorporation of undesirable residues during the reformation phase. Although this approach holds promise as a surface modification technique, it requires further study to compare its effectiveness to plasma deposition and wet chemistry modifications.
Although functionalized surfaces directly affect cell response, most functionalized surfaces are used as intermediates in the most advanced and diverse group of surface modifications, biomolecule immobilization. Functionalization methodology controls several aspects of biomolecule immobilization, including which functional groups are present for cross-linking, the density of functional groups on the surface, and the patterns of the groups on the surface.
6 |. BIOMACROMOLECULE CROSS-LINKING AND ENTRAPMENT
Biomolecule immobilization techniques semipermanently incorporate larger bioactive molecules into a surface, from short bioactive polypeptides to ECM proteins, enzymes, and antibodies. Although stable and repetitive molecules like collagen and glycosaminoglycans can be immobilized using direct entrapment methods, most biomacromolecules must be cross-linked to the surface using mild chemical reactions that do not alter intrinsic properties such as protein conformation. Because biomacromolecules vary widely in function, this surface modification group represents the greatest diversity in effects on cell response, and it is the most commonly used surface modification group in tissue engineering biomaterials design (Baldwin & Kiick, 2010; Goddard & Hotchkiss, 2007). Full discussion of this group of surface modification techniques exceeds the scope of this review. Notably, significant recent advances have been made in polyphenol and polydopamine coating systems (Lee et al., 2018; Xu, Neoh, & Kang, 2018).
As with the functionalization through entrapment described previously, direct entrapment uses a solvent/nonsolvent mixture to swell the surface of the material before addition of the desired molecule in excess nonsolvent solution. Cui and colleagues used 70% acetone in water solution followed by aqueous gelatin or chitosan solution to entrap bioactive gelatin and chitosan molecules on the surface (Cui et al., 2003; Cui et al., 2003). As a positive control, both molecules were immobilized using hydrolysis and cross-linking. Chemical analysis confirmed the effectiveness of all modifications. For both ECM molecules, direct entrapment was as effective in promoting chondrocyte cell adhesion, proliferation, and functionality as the cross-linking technique.
In a similar study, gelatin was entrapped on PLLA films using dioxane and water partial solvation (Liu, Won, & Ma, 2005). An adsorbed gelatin control demonstrated the comparative longevity of entrapped molecules because the control lost gelatin content with subsequent washings and the entrapped gelatin remained. Both the adsorbed and entrapped gelatin was cross-linked to itself on the surface using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and n-hydroxysulfosuccinimide (NHS). Entrapped gelatin content increased with an increased dioxane to water ratio and with longer incubation periods. The entrapped and cross-linked gelatin coating resulted in significantly increased seeding efficiency and proliferation over controls.
In addition to improving cell response, direct entrapment can reduce cell interaction with a surface. Quirk, Briggs, Davies, Tendler, and Shakesheff (2001) and Quirk, Davies, Tendler, and Shakesheff (2000) used a 2,2,2-trifluoroethanol/water solvent/nonsolvent mixture to entrap poly(ethylene glycol) (PEG) and PLL on a PLA surface. In studying cell response to the engineered surfaces, they achieved a 95% reduction in attachment of bovine aortic cell to PEG-treated surfaces. Surfaces treated with a combination of PEG and PLL cross-linked to RGD exhibited intermediate cell attachment. The 2,2,2-trifluoroethanol solvent has also been used to directly entrap heparin, resulting in PLA with increased blood compatibility (Meng, Wang, Cui, Dong, & Yu, 2004).
Cross-linking of biomolecules to a surface covalently binds a desired molecule using a pendant functional group. In practice, terminal amines on the surface are often cross-linked using glutaraldehyde (Table 2). Terminal carboxylic acids and other products of hydrolysis use EDC with and without NHS, although EDC and NHS can also be applied in reverse to cross-link primary amine groups (Table 2).
Other cross-linking techniques increase specificity, resulting in alternative functionalization methods, cross-linker-level density and pattern control, and binding of less-stable target molecules. To cross-link entrapped amine groups to thiol available on a bioactive RGD-cysteine polypeptide, Alvarez-Barreto et al. used succinimidyl 3-(2-pyridyldithio)propionate, a cross-linker with an intrinsic spacer arm to extend the target molecule from the material surface (Table 2). The amine-thiol cross-linking approach provided a highly specific conjugation and kept the cross-linking process from affecting the bioactive RGD sequence of the polypeptide. When functionalization is initiated using argon plasma to create active radicals, cross-linking is initiated using ultraviolet light and acrylic acid or 2-aminoethyl-methacrylate to produce carboxylic acid groups prior to EDC cross-linking (Table 2). As research in cross-linking chemistry and surface functionalization proceeds, opportunities will arise to create surfaces capable of binding multiple different bioactive molecules in useful patterns and varying frequencies, better simulating natural ECM and reflecting the current trends in advanced tissue engineering hydrogel design.
Ultimately, the function of immobilizing bioactive molecules depends on the added molecule. Previously published reviews summarize the utility of binding many different bioactive molecules to scaffolding materials. Many studies immobilize gelatin as a simple, reliable proof of concept for improved cellular response. Gelatin immobilization has been demonstrated on PLLA, PCL, and PLGA, consistently resulting in increased cell adhesion and proliferation while maintaining phenotypic activity for preosteoblasts, endothelial cells, and chondrocytes (Table 2). In addition to gelatin, structural ECM molecules are often immobilized, including collagen, fibronectin, chitosan, heparin, and active subunits of these molecules, such as the integrin-binding polypeptide RGD (Bellis, 2011; Quirk, Briggs, et al., 2001; Table 2). These molecules behave as passive biomimetic signaling for cells, promoting cell adhesion, proliferation, and occasionally differentiation with varying effectiveness based on cell and tissue type. In addition to these common target molecules, PEG, PLL, vascular endothelial growth factor, and antibodies have been covalently bound to aliphatic polyester surfaces (Table 2). These modifications produce more specific cell responses than incorporation of ECM molecules, such as directed differentiation with growth factors, specific cell-type binding with antibodies, and overall increase or decrease of cell attachment using PEG and PLL, respectively.
7 |. CONCLUSION
Surface modification of aliphatic polyester tissue engineering scaffolds creates biomimetic surfaces with improved cell response compared with unmodified aliphatic polyesters surfaces. Aliphatic polyesters are an important group in tissue engineering surface modifications due to their safety for biological study, consistent properties, and widespread availability. Due to the diversity of surface modification techniques that have been developed, this review describes five prominent classes for modification types so more advanced surfaces can be developed through an improved understanding of the effects of the various modifications and the ways the different methods can be usefully combined. Topographical modifications change the surface physically to increase surface area and provide nonchemical cues. Protein adsorption techniques alter how native proteins interact with the surface, affecting immune response and mechanisms of cell attachment. Mineral deposition introduces inorganic moieties to the surface, promoting osteogenic and chondrogenic differentiation. Functionalization activates the surface for increased biological interaction or cross-linking sites by creating terminal functional groups. Biomolecule immobilization binds ECM molecules, growth factors, and antibodies to the surface, significantly increasing the biochemical activity of the surface for improved and specific cell response. Although further fundamental understanding of the mechanisms of each approach will improve their efficiency in promoting tissue development, each approach described here contributes in distinct ways toward creating biomimetic scaffolds. Furthermore, the trend toward increasing combination of these methods has produced advanced scaffolds with greater impact in cell response, specific tissue development, and understanding of cell–material interactions.
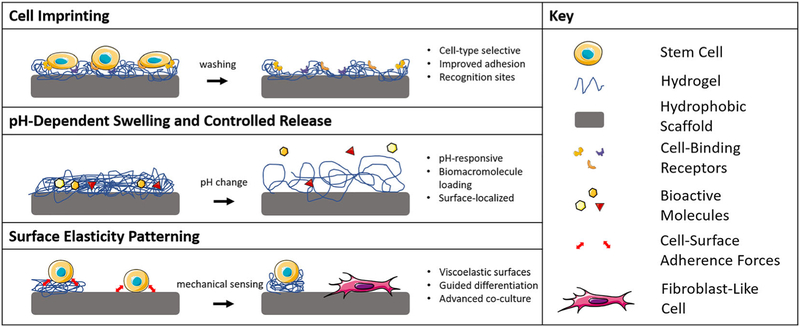
To emphasize that the five approaches for surface modification described in this review summarize the current literature in the field but do not restrict further innovation, we suggest that another major approach of hard polymer scaffold surface modification may soon emerge with significant application. The range of advanced biomaterial characteristics currently available in hydrogels may translate to novel scaffold surface modifications (Figure 5). These advanced characteristics include environmentally responsive hydrogels, gels that can present varying degrees of surface elasticity, molecularly imprinted polymers, cellularly imprinted polymers, and more (Clegg, Wechsler, & Peppas, 2017; Culver, Clegg, & Peppas, 2017; Engler, Sen, Sweeney, & Discher, 2006; Peppas & Clegg, 2016). Such modifications will provide opportunities for more finely tuned characterization of cell responses to properties such as dynamic mechanical moduli and advanced controlled release of growth factors. However, such combinations of hard polymers and hydrogels will need additional techniques to facilitate stable conjugations and rigorous characterization to assess their dynamic behavior, possibly explaining why advanced hydrogel surface modifications have not proceeded significantly past basic static coatings. We suggest that investigation into advanced hydrogel surface modification techniques for aliphatic polyester scaffolds will expand the array of useful cell–scaffold interactions available to tissue engineers, supporting improved tissue regeneration.
FIGURE 5.
Potential hydrogel-derived responsive surface modifications
ACKNOWLEDGEMENTS
The authors wish to thank Montana Minnis (Univ. Oklahoma) and John Clegg (Univ. Texas) for discussions and suggestions. This work was supported in part by the Stephenson School of Biomedical Engineering at the University of Oklahoma. The material is based upon work supported by the National Science Foundation Graduate Research Fellowship under Grant No. DGE-1610403 awarded to NRR. We acknowledge financial support from the National Institue of Health (R01-EB022025 awarded to NAP).
Funding information
Stephenson School of Biomedical Engineering at the University of Oklahoma; National Science Foundation Graduate Research Fellowship, Grant/Award Number: DGE-1610403; National Institue of Health, Grant/Award Number: R01-EB022025
Footnotes
CONFLICT OF INTEREST
No competing financial interests exist.
REFERENCES
- Abedalwafa M, Wang F, Wang L, & Li C (2013). Biodegradable poly-epsilon-caprolactone (PCL) for tissue engineering applications: A review. Reviews on Advanced Materials Science, 34, 123–140. [Google Scholar]
- Al-Munajjed AA, Plunkett NA, Gleeson JP, Weber T, Jungreuthmayer C, Levingstone T, … Brien FJ (2009). Development of a biomimetic collagen-hydroxyapatite scaffold for bone tissue engineering using a SBF immersion technique. Journal of Biomedical Materials Research, 90(2), 584–591. 10.1002/jbm.b.31320 [DOI] [PubMed] [Google Scholar]
- Alvarez-Barreto JF, Landy B, Vangordon S, Place L, Deangelis PL, & Sikavitsas VI (2011). Enhanced osteoblastic differentiation of mesenchymal stem cells seeded in RGD-functionalized PLLA scaffolds and cultured in a flow perfusion bioreactor. Journal of Tissue Engineering and Regenerative Medicine, 5(6), 464 10.1002/term.338-475 [DOI] [PubMed] [Google Scholar]
- Alvarez-Barreto JF, Shreve MC, Deangelis PL, & Sikavitsas VI (2007). Preparation of a functionally flexible, three-dimensional, biomimetic poly(l-lactic acid) scaffold with improved cell adhesion. Tissue Engineering, 13(6), 1205–1217. 10.1089/ten.2006.0330 [DOI] [PubMed] [Google Scholar]
- Alvarez-Barreto JF, & Sikavitsas VI (2007). Improved mesenchymal stem cell seeding on RGD-modified poly(l-lactic acid) scaffolds using flow perfusion. Macromolecular Bioscience, 7(5), 579–588. 10.1002/mabi.200600280 [DOI] [PubMed] [Google Scholar]
- Alves NM, Shi J, Oramas E, Santos JL, Tomás H, & Mano JF (2009). Bioinspired superhydrophobic poly(l-lactic acid) surfaces control bone marrow derived cells adhesion and proliferation. Journal of Biomedical Materials Research, 91(2), 480–488. 10.1002/jbm.a.32210 [DOI] [PubMed] [Google Scholar]
- Baldwin AD, & Kiick KL (2010). Polysaccharide-modified synthetic polymeric biomaterials. Biopolymers, 94(1), 128–140. 10.1002/bip.21334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarash LS, Bolbasov EN, Antonova LV, Matveeva VG, Velikanova EA, Shesterikov EV, … Tverdokhlebov SI (2016). Surface modification of poly-ε-caprolactone electrospun fibrous scaffolds using plasma discharge with sputter deposition of a titanium target. Materials Letters, 171, 87–90. 10.1016/j.matlet.2016.02.062 [DOI] [Google Scholar]
- Bellis SL (2011). Advantages of RGD peptides for directing cell association with biomaterials. Biomaterials, 32(18), 4205–4210. 10.1016/j.biomaterials.2011.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beşkardeş IG, & Gümüşderelioğlu M (2009). Biomimetic apatite-coated PCL scaffolds: Effect of surface nanotopography on cellular functions. Journal of Bioactive and Compatible Polymers, 24(6), 507–524. 10.1177/0883911509349311 [DOI] [Google Scholar]
- Biggs MJP, Richards RG, & Dalby MJ (2010). Nanotopographical modification: A regulator of cellular function through focal adhesions. Nanomedicine: Nanotechnology, Biology, and Medicine, 6(5), 619–633. 10.1016/j.nano.2010.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolbasov EN, Rybachuk M, Golovkin AS, Antonova LV, Shesterikov EV, Malchikhina AI, … Tverdokhlebov SI (2014). Surface modification of poly(l-lactide) and polycaprolactone bioresorbable polymers using RF plasma discharge with sputter deposition of a hydroxyapatite target. Materials Letters, 132, 281–284. 10.1016/j.matlet.2014.06.115 [DOI] [Google Scholar]
- Bosetti M, Fusaro L, Nicolì E, Borrone A, Aprile S, & Cannas M (2014). Poly-L-lactide acid-modified scaffolds for osteoinduction and osteoconduction. Journal of Biomedical Materials Research, 102(10), 3531–3539. 10.1002/jbm.a.35016 [DOI] [PubMed] [Google Scholar]
- Boyan BD, Hummert TW, Dean DD, & Schwartz Z (1996). Role of material surfaces in regulating bone and cartilage cell response. Biomaterials, 17(2), 137–146. 10.1016/0142-9612(96)85758-9 [DOI] [PubMed] [Google Scholar]
- Brown DA, Beygui RE, Maclellan WR, Laks H, Dunn JCY, & Wu BM (2005). Modulation of gene expression in neonatal rat cardiomyocytes by surface modification of polylactide-co-glycolide substrates. Journal of Biomedical Materials Research, 74(3), 419–429. 10.1002/jbm.a.30344 [DOI] [PubMed] [Google Scholar]
- Cassidy JW, Roberts JN, Smith CA, Robertson M, White K, Biggs MJ, … Dalby MJ (2014). Osteogenic lineage restriction by osteoprogenitors cultured on nanometric grooved surfaces: The role of focal adhesion maturation. Acta Biomaterialia, 10(2), 651–660. 10.1016/j.actbio.2013.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastain SR, Kundu AK, Dhar S, Calvert JW, & Putnam AJ (2006). Adhesion of mesenchymal stem cells to polymer scaffolds occurs via distinct ECM ligands and controls their osteogenic differentiation. Journal of Biomedical Materials Research, 78(1), 73–85. 10.1002/jbm.a.30686 [DOI] [PubMed] [Google Scholar]
- Chen JP, & Su CH (2011). Surface modification of electrospun PLLA nanofibers by plasma treatment and cationized gelatin immobilization for cartilage tissue engineering. Acta Biomaterialia, 7(1), 234–243. 10.1016/j.actbio.2010.08.015 [DOI] [PubMed] [Google Scholar]
- Chen VJ, Smith LA, & Ma PX (2006). Bone regeneration on computer-designed nano-fibrous scaffolds. Biomaterials, 27(21), 3973–3979. 10.1016/j.biomaterials.2006.02.043 [DOI] [PubMed] [Google Scholar]
- Cheng KY, Chang CH, Yang YW, Liao GC, Liu CT, & Wu JS (2017). Enhancement of cell growth on honeycomb-structured polylactide surface using atmospheric-pressure plasma jet modification. Applied Surface Science, 394, 534–542. 10.1016/j.apsusc.2016.10.093 [DOI] [Google Scholar]
- Cheng Z, & Teoh SH (2004). Surface modification of ultra thin poly (ε-caprolactone) films using acrylic acid and collagen. Biomaterials, 25(11), 1991–2001. 10.1016/j.biomaterials.2003.08.038 [DOI] [PubMed] [Google Scholar]
- Chim H, Ong JL, Schantz JT, Hutmacher DW, & Agrawal CM (2003). Efficacy of glow discharge gas plasma treatment as a surface modification process for three-dimensional poly (d,l-lactide) scaffolds. Journal of Biomedical Materials Research, 65(3), 327–335. 10.1002/jbm.a.10478 [DOI] [PubMed] [Google Scholar]
- Choi S, & Murphy WL (2012). The effect of mineral coating morphology on mesenchymal stem cell attachment and expansion. Journal of Materials Chemistry, 22(48), 25288 10.1039/c2jm33354f-25295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choong C, Triffitt JT, & Cui ZF (2004). Polycaprolactone scaffolds for bone tissue engineering: Effects of a calcium phosphate coating layer on osteogenic cells. Food and Bioproducts Processing, 82(2), 117–125. 10.1205/0960308041614864 [DOI] [Google Scholar]
- Clegg JR, Wechsler ME, & Peppas NA (2017). Vision for functionally decorated and molecularly imprinted polymers in regenerative engineering. Regenerative Engineering and Translational Medicine, 3(3), 166–175. 10.1007/s40883-017-0028-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg JR, Zhong JX, Irani AS, Gu J, Spencer DS, & Peppas NA (2017). Characterization of protein interactions with molecularly imprinted hydrogels that possess engineered affinity for high isoelectric point biomarkers. Journal of Biomedical Materials Research, 105(6), 1565–1574. 10.1002/jbm.a.36029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croll TI, Connor AJ, Stevens GW, & Cooper-White JJ (2004). Controllable surface modification of poly (lactic-co-glycolic acid) (PLGA) by hydrolysis or aminolysis I: Physical, chemical, and theoretical aspects. Biomacromolecules, 5(2), 463–473. 10.1021/bm0343040 [DOI] [PubMed] [Google Scholar]
- Cui YL, Hou X, Qi AD, Wang XH, Wang H, Cai KY, … Yao KD (2003). Biomimetic surface modification of poly(l-lacticacid) with gelatin and its effects on articular chondrocytes in vitro. Journal of Biomedical Materials Research, 66(4), 770–778. 10.1002/jbm.a.10071 [DOI] [PubMed] [Google Scholar]
- Cui YL, Qi AD, Liu WG, Wang XH, Wang H, Ma DM, & Yao KD (2003). Biomimetic surface modification of poly(l-lactic acid) with chitosan and its effects on articular chondrocytes in vitro. Biomaterials, 24(21), 3859–3868. 10.1016/s0142-9612(03)00209-6 [DOI] [PubMed] [Google Scholar]
- Culver HR, Clegg JR, & Peppas NA (2017). Analyte-responsive hydrogels: Intelligent materials for biosensing and drug delivery. Accounts of Chemical Research, 50(2), 170–178. 10.1021/acs.accounts.6b00533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, … Oreffo ROC (2007). The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nature Materials, 6(12), 997–1003. 10.1038/nmat2013 [DOI] [PubMed] [Google Scholar]
- De Witte TM, Fratila-Apachitei LE, Zadpoor AA, & Peppas NA (2018). Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regenerative Biomaterials, 5, 197–211. 10.1093/rb/rby013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmet T, Poleunis C, Delcorte A, & Dubruel P (2012). Double protein functionalized poly-ε-caprolactone surfaces: In depth ToF–SIMS and XPS characterization. Official Journal of the European Society for Biomaterials, 23(2), 293–305. 10.1007/s10856-011-4527-9 [DOI] [PubMed] [Google Scholar]
- Dolci LS, Liguori A, Merlettini A, Calzà L, Castellucci M, Gherardi M, … Focarete ML (2016). Antibody immobilization on poly(l-lactic acid) nanofibers advantageously carried out by means of a non-equilibrium atmospheric plasma process. Journal of Physics D: Applied Physics, 49(27), 274003 10.1088/0022-3727/49/27/274003 [DOI] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, & Discher DE (2006). Matrix elasticity directs stem cell lineage specification. Cell, 126(4), 677–689. 10.1016/j.cell.2006.06.044 [DOI] [PubMed] [Google Scholar]
- Fernandez-Yague MA, Abbah SA, McNamara L, Zeugolis DI, Pandit A, & Biggs MJ (2015). Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Advanced Drug Delivery Reviews, 84, 1–29. 10.1016/j.addr.2014.09.005 [DOI] [PubMed] [Google Scholar]
- Fiedler J, Özdemir B, Bartholomä J, Plettl A, Brenner RE, & Ziemann P (2013). The effect of substrate surface nanotopography on the behavior of multipotnent mesenchymal stromal cells and osteoblasts. Biomaterials, 34(35), 8851–8859. 10.1016/j.biomaterials.2013.08.010 [DOI] [PubMed] [Google Scholar]
- Flemming RG, Murphy CJ, Abrams GA, Goodman SL, & Nealey PF (1999). Effects of synthetic micro- and nano-structured surfaces on cell behavior. Biomaterials, 20(6), 573–588. 10.1016/s0142-9612(98)00209-9 [DOI] [PubMed] [Google Scholar]
- Frazza EJ, & Schmitt EE (1971). A new absorbable suture. Journal of Biomedical Materials Research, 5(2), 43–58. 10.1002/jbm.820050207 [DOI] [PubMed] [Google Scholar]
- García AJ, & Reyes CD (2005). Bio-adhesive surfaces to promote osteoblast differentiation and bone formation. Journal of Dental Research, 84(5), 407–413. 10.1177/154405910508400502 [DOI] [PubMed] [Google Scholar]
- Goddard JM, & Hotchkiss JH (2007). Polymer surface modification for the attachment of bioactive compounds. Progress in Polymer Science, 32(7), 698–725. 10.1016/j.progpolymsci.2007.04.002 [DOI] [Google Scholar]
- Gong Z, & Niklason LE (2008). Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs). The FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 22(6), 1635–1648. 10.1096/fj.07-087924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guex AG, Hegemann D, Giraud MN, Tevaearai HT, Popa AM, Rossi RM, & Fortunato G (2014). Covalent immobilisation of VEGF on plasma-coated electrospun scaffolds for tissue engineering applications. Colloids and Surfaces B: Biointerfaces, 123, 724–733. 10.1016/j.colsurfb.2014.10.016 [DOI] [PubMed] [Google Scholar]
- Guo C, Xiang M, & Dong Y (2015). Surface modification of poly (lactic acid) with an improved alkali-acid hydrolysis method. Materials Letters, 140, 144–147. 10.1016/j.matlet.2014.10.099 [DOI] [Google Scholar]
- Hata K, Kokubo T, Nakamura T, & Yamamuro T (1995). Growth of a bonelike apatite layer on a substrate by a biomimetic process. Journal of the American Ceramic Society, 78(4), 1049–1053. 10.1111/j.1151-2916.1995.tb08435.x [DOI] [Google Scholar]
- Hu Y, Winn SR, Krajbich I, & Hollinger JO (2003). Porous polymer scaffolds surface-modified with arginine-glycine-aspartic acid enhance bone cell attachment and differentiation in vitro. Journal of Biomedical Materials Research, 64(3), 583–590. 10.1002/jbm.a.10438 [DOI] [PubMed] [Google Scholar]
- Ikada Y (1994). Surface modification of polymers for medical applications. Biomaterials, 15(10), 725–736. 10.1016/0142-9612(94)90025-6 [DOI] [PubMed] [Google Scholar]
- Itälä A, Koort J, Ylänen HO, Hupa M, & Aro HT (2003). Biologic significance of surface microroughing in bone incorporation of porous bioactive glass implants. Journal of Biomedical Materials Research, 67(2), 496–503. 10.1002/jbm.a.10501 [DOI] [PubMed] [Google Scholar]
- Jafari M, Paknejad Z, Rad MR, Motamedian SR, Eghbal MJ, Nadjmi N, & Khojasteh A (2017). Polymeric scaffolds in tissue engineering: A literature review. Journal of Biomedical Materials Research, 105(2), 431–459. 10.1002/jbm.b.33547 [DOI] [PubMed] [Google Scholar]
- Janorkar AV, Fritz EW, Burg KJL, Metters AT, & Hirt DE (2007). Grafting amine-terminated branched architectures from poly(l-lactide) film surfaces for improved cell attachment. Journal of Biomedical Materials Research, 81(1), 142–152. 10.1002/jbm.b.30647 [DOI] [PubMed] [Google Scholar]
- Jiao Y, Liu Z, Cui F, & Zhou C (2007). Effect of hydrolysis pretreatment on the formation of bone-like apatite on poly(l-lactide) by mineralization in simulated body fluids. Journal of Bioactive and Compatible Polymers, 22(5), 492–507. 10.1177/0883911507082161 [DOI] [Google Scholar]
- Jiao Y, Liu Z, Shao X, & Zhou C (2012). Protein adsorption and cytocompatibility of poly(l-lactic acid) surfaces modified with biomacromolecules. Journal of Applied Polymer Science, 125(S2), E501–E510. 10.1002/app.36976 [DOI] [Google Scholar]
- Jiao Y, Zhou C, Li L, Ding S, Lu L, Luo B, & Li H (2009). Protein adsorption on the poly(l-lactic acid) surface modified by chitosan and its derivatives. Chinese Science Bulletin, 54(18), 3167–3173. 10.1007/s11434-009-0266-4 [DOI] [Google Scholar]
- Jordá-Vilaplana A, Fombuena V, García-García D, Samper MD, & Sánchez-Nácher L (2014). Surface modification of polylactic acid (PLA) by air atmospheric plasma treatment. European Polymer Journal, 58, 23–33. 10.1016/j.eurpolymj.2014.06.002 [DOI] [Google Scholar]
- Kakinoki S, & Yamaoka T (2010). Stable modification of poly (lactic acid) surface with neurite outgrowth-promoting peptides via hydrophobic collagen-like sequence. Acta Biomaterialia, 6(6), 1925–1930. 10.1016/j.actbio.2009.12.001 [DOI] [PubMed] [Google Scholar]
- Kantawong F, Burgess KEV, Jayawardena K, Hart A, Riehle MO, Oreffo RO, … Burchmore R (2010). Effects of a surface topography composite with puerariae radix on human STRO-1-positive stem cells. Acta Biomaterialia, 6(9), 3694–3703. 10.1016/j.actbio.2010.02.038 [DOI] [PubMed] [Google Scholar]
- Kao WJ (1999). Evaluation of protein-modulated macrophage behavior on biomaterials: Designing biomimetic materials for cellular engineering. Biomaterials, 20(23), 2213–2221. 10.1016/s0142-9612(99)00152-0 [DOI] [PubMed] [Google Scholar]
- Kim HN, Jiao A, Hwang NS, Kim MS, Kang DH, Kim DH, & Suh KY (2012). Nanotopography-guided tissue engineering and regenerative medicine. Advanced Drug Delivery Reviews, 65, 536–558. 10.1016/j.addr.2012.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Jang DH, Park WH, & Min BM (2010). Fabrication and characterization of 3-dimensional PLGA nanofiber/microfiber composite scaffolds. Polymer, 51(6), 1320–1327. 10.1016/j.polymer.2010.01.025 [DOI] [Google Scholar]
- Kiss E, Kutnyánszky E, & Bertóti I (2010). Modification of poly (lactic/glycolic acid) surface by chemical attachment of poly(ethylene glycol). Langmuir, 26(3), 1440 10.1021/la903373g-1444 [DOI] [PubMed] [Google Scholar]
- Koh HS, Yong T, Chan CK, & Ramakrishna S (2008). Enhancement of neurite outgrowth using nano-structured scaffolds coupled with laminin. Biomaterials, 29(26), 3574–3582. 10.1016/j.biomaterials.2008.05.014 [DOI] [PubMed] [Google Scholar]
- Koh LB, Rodriguez I, & Venkatraman SS (2010). Conformational behavior of fibrinogen on topographically modified polymer surfaces. Physical Chemistry Chemical Physics, 12(35), 10301 10.1039/c001747g-10308. [DOI] [PubMed] [Google Scholar]
- Kokubo T, Kushitani H, Sakka S, Kitsugi T, & Yamamuro T (1990). Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W 3. Journal of Biomedical Materials Research, 24(6), 721–734. 10.1002/jbm.820240607 [DOI] [PubMed] [Google Scholar]
- Krok-Borkowicz M, Musial O, Kruczala P, Dobrzynski P, Douglas TEL, Vlierberghe SV, … Pamula E (2015). Biofunctionalization of poly(l-lactide-co-glycolide) by post-plasma grafting of 2-aminoethyl methacrylate and gelatin immobilization. Materials Letters, 139, 344–347. 10.1016/j.matlet.2014.10.100 [DOI] [Google Scholar]
- Lanza RP, Langer R, & Vacanti J (2014). Principles of tissue engineering(4th ed.). London: Elsevier/Academic Press. [Google Scholar]
- Lee JH, Park JH, El-Fiqi A, Kim JH, Yun YR, Jang JH, … Kim HW (2014). Biointerface control of electrospun fiber scaffolds for bone regeneration: Engineered protein link to mineralized surface. Acta Biomaterialia, 10(6), 2750–2761. 10.1016/j.actbio.2014.01.021 [DOI] [PubMed] [Google Scholar]
- Lee JH, Rim NG, Jung HS, & Shin H (2010). Control of osteogenic differentiation and mineralization of human mesenchymal stem cells on composite nanofibers containing poly[lactic-co-(glycolic acid)] and hydroxyapatite. Macromolecular Bioscience, 10(2), 173–182. 10.1002/mabi.200900169 [DOI] [PubMed] [Google Scholar]
- Lee SJ, Lee H-J, Kim S-Y, Seok JM, Lee JH, Kim WD, … Park SA (2018). In situ gold nanoparticle growth on polydopamine-coated 3D-printed scaffolds improves osteogenic differentiation for bone tissue engineering applications: In vitro and in vivo studies. Nanoscale, 10(33), 15447–15453. 10.1039/C8NR04037K [DOI] [PubMed] [Google Scholar]
- Leong MF, Chian KS, Mhaisalkar PS, Ong WF, & Ratner BD (2009). Effect of electrospun poly(d,l-lactide) fibrous scaffold with nanoporous surface on attachment of porcine esophageal epithelial cells and protein adsorption. Journal of Biomedical Materials Research, 89(4), 1040–1048. 10.1002/jbm.a.32061 [DOI] [PubMed] [Google Scholar]
- Li L, Qian Y, Jiang C, Lv Y, Liu W, Zhong L, … Yang L (2012). The use of hyaluronan to regulate protein adsorption and cell infiltration in nanofibrous scaffolds. Biomaterials, 33(12), 3428–3445. 10.1016/j.biomaterials.2012.01.038 [DOI] [PubMed] [Google Scholar]
- Li P, Ohtsuki C, Kokubo T, Nakanishi K, Soga N, Nakamura T, & Yamamuro T (1992). Apatite formation induced by silica gel in a simulated body fluid. Journal of the American Ceramic Society, 75(8), 2094–2097. 10.1111/j.1151-2916.1992.tb04470.x [DOI] [Google Scholar]
- Li WJ, Laurencin CT, Caterson EJ, Tuan RS, & Ko FK (2002). Electrospun nanofibrous structure: A novel scaffold for tissue engineering. Journal of Biomedical Materials Research, 60(4), 613–621. 10.1002/jbm.10167 [DOI] [PubMed] [Google Scholar]
- Lin CH, Chang MC, Hung SC, Lee SY, & Lin YM (2017). Bioactive surface modification of polycaprolactone using MG63-conditioned medium can induce osteogenic differentiation of mesenchymal stem cells. Journal of Materials Science Letters, 52(7), 3967–3978. 10.1007/s10853-016-0659-0 [DOI] [Google Scholar]
- Lin F, Li Y, Jin J, Cai Y, Wei K, & Yao J (2008). Deposition behavior and properties of silk fibroin scaffolds soaked in simulated body fluid. Materials Chemistry and Physics, 111(1), 92–97. 10.1016/j.matchemphys.2008.03.019 [DOI] [Google Scholar]
- Liu X, & Ma PX (2004). Polymeric scaffolds for bone tissue engineering. Annals of Biomedical Engineering, 32(3), 477–486. 10.1023/B:ABME.0000017544.36001.8e [DOI] [PubMed] [Google Scholar]
- Liu X, Won Y, & Ma PX (2005). Surface modification of interconnected porous scaffolds. Journal of Biomedical Materials Research, 74(1), 84–91. 10.1002/jbm.a.30367 [DOI] [PubMed] [Google Scholar]
- Ma Z, Gao C, Gong Y, & Shen J (2005). Cartilage tissue engineering PLLA scaffold with surface immobilized collagen and basic fibroblast growth factor. Biomaterials, 26(11), 1253–1259. 10.1016/j.biomaterials.2004.04.031 [DOI] [PubMed] [Google Scholar]
- Ma Z, He W, Yong T, & Ramakrishna S (2005). Grafting of gelatin on electrospun poly (caprolactone) nanofibers to improve endothelial cell spreading and proliferation and to control cell orientation. Tissue Engineering, 11(7–8), 1149 10.1089/ten.2005.11.1149-1158. [DOI] [PubMed] [Google Scholar]
- Manso M, Valsesia A, Ceccone G, & Rossi F (2004). Activation of PCL surface by ion beam treatment to enhance protein adsorption. Journal of Bioactive and Compatible Polymers, 19(4), 287–300. 10.1177/0883911504045174 [DOI] [Google Scholar]
- Mavis B, Demirtaş TT, Gümüşderelioğlu M, Gündüz G, & Çolak Ü (2009). Synthesis, characterization and osteoblastic activity of polycaprolactone nanofibers coated with biomimetic calcium phosphate. Acta Biomaterialia, 5(8), 3098–3111. 10.1016/j.actbio.2009.04.037 [DOI] [PubMed] [Google Scholar]
- McMurray RJ, Gadegaard N, Tsimbouri PM, Burgess KV, McNamara LE, Tare R, … Dalby MJ (2011). Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nature Materials, 10(8), 637–644. 10.1038/nmat3058 [DOI] [PubMed] [Google Scholar]
- Mei N, Chen G, Zhou P, Chen X, Shao ZZ, Pan LF, & Wu CG (2005). Biocompatibility of poly (epsilon-caprolactone) scaffold modified by chitosan—The fibroblasts proliferation in vitro. Journal of Biomaterials Applications, 19(4), 323–339. 10.1177/0885328205048630 [DOI] [PubMed] [Google Scholar]
- Meng B, Wang XH, Cui FZ, Dong HY, & Yu F (2004). A new method of heparinizing PLLA film by surface entrapment. Journal of Bioactive and Compatible Polymers, 19, 131–143. 10.1177/0883911504042644 [DOI] [Google Scholar]
- Miller DC, Thapa A, Haberstroh KM, & Webster TJ (2004). Endothelial and vascular smooth muscle cell function on poly (lactic-co-glycolic acid) with nano-structured surface features. Biomaterials, 25(1), 53–61. 10.1016/s0142-9612(03)00471-x [DOI] [PubMed] [Google Scholar]
- Mohan T, Niegelhell K, Nagaraj C, Reishofer D, Spirk S, Olschewski A, … Kargl R (2017). Interaction of tissue engineering substrates with serum proteins and its influence on human primary endothelial cells. Biomacromolecules, 18(2), 413–421. 10.1021/acs.biomac.6b01504 [DOI] [PubMed] [Google Scholar]
- Murphy WL, McDevitt TC, & Engler AJ (2014). Materials as stem cell regulators. Nature Materials, 13(6), 547 10.1038/nmat3937-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves MI, Wechsler ME, Gomes ME, Reis RL, Granja PL, & Peppas NA (2017). Molecularly imprinted intelligent scaffolds for tissue engineering applications. Tissue Engineering, 23(1), 27–43. 10.1089/ten.TEB.2016.0202 [DOI] [PubMed] [Google Scholar]
- Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, & Langer R (1999). Functional arteries grown in vitro. Science, 284(5413), 489–493. 10.1126/science.284.5413.489 [DOI] [PubMed] [Google Scholar]
- Nyberg E, Rindone A, Dorafshar A, & Grayson WL (2017). Comparison of 3D-printed poly-ε-caprolactone scaffolds functionalized with tricalcium phosphate, hydroxyapatite, bio-oss, or decellularized bone matrix. Tissue Engineering, 23(11–12), 503–514. 10.1089/ten.TEA.2016.0418 [DOI] [PubMed] [Google Scholar]
- Oyane A, Uchida M, Choong C, Triffitt J, Jones J, & Ito A (2005). Simple surface modification of poly(ε-caprolactone) for apatite deposition from simulated body fluid. Biomaterials, 26(15), 2407–2413. 10.1016/j.biomaterials.2004.07.048 [DOI] [PubMed] [Google Scholar]
- Oyane A, Uchida M, Yokoyama Y, Choong C, Triffitt J, & Ito A (2005). Simple surface modification of poly(ϵ-caprolactone) to induce its apatite-forming ability. Journal of Biomedical Materials Research, 75A(1), 138–145. 10.1002/jbm.a.30397 [DOI] [PubMed] [Google Scholar]
- Park GE, Pattison MA, Park K, & Webster TJ (2005). Accelerated chondrocyte functions on NaOH-treated PLGA scaffolds. Biomaterials, 26(16), 3075–3082. 10.1016/j.biomaterials.2004.08.005 [DOI] [PubMed] [Google Scholar]
- Pattison MA, Wurster S, Webster TJ, & Haberstroh KM (2005). Three-dimensional, nano-structured PLGA scaffolds for bladder tissue replacement applications. Biomaterials, 26(15), 2491–2500. 10.1016/j.biomaterials.2004.07.011 [DOI] [PubMed] [Google Scholar]
- Peppas NA, & Clegg JR (2016). The challenge to improve the response of biomaterials to the physiological environment. Regenerative Biomaterials, 3(2), 67–71. 10.1093/rb/rbw012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppas NA, & Van Blarcom DS (2016). Hydrogel-based biosensors and sensing devices for drug delivery. Journal of Controlled Release, 240, 142–150. 10.1016/j.jconrel.2015.11.022 [DOI] [PubMed] [Google Scholar]
- Peter SJ, Miller MJ, Yasko AW, Yaszemski MJ, & Mikos AG (1998). Polymer concepts in tissue engineering. Journal of Biomedical Materials Research, 43(4), 422–427. [DOI] [PubMed] [Google Scholar]
- Petersen S, Strohbach A, Busch R, Felix SB, Schmitz KP, & Sternberg K (2014). Site-selective immobilization of anti-CD34 antibodies to poly(l-lactide) for endovascular implant surfaces. Journal of Biomedical Materials Research, 102(2), 345–355. 10.1002/jbm.b.33012 [DOI] [PubMed] [Google Scholar]
- Qin TW, Yang ZM, Wu ZZ, Xie HQ, Qin J, & Cai SX (2005). Adhesion strength of human tenocytes to extracellular matrix component-modified poly(dl-lactide-co-glycolide) substrates. Biomaterials, 26(33), 6635–6642. 10.1016/j.biomaterials.2005.04.023 [DOI] [PubMed] [Google Scholar]
- Quint C, Arief M, Muto A, Dardik A, & Niklason LE (2012). Allogeneic human tissue-engineered blood vessel. Journal of Vascular Surgery, 55(3), 790–798. 10.1016/j.jvs.2011.07.098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk RA, Briggs D, Davies MC, Tendler SJB, & Shakesheff KM (2001). Characterization of the spatial distributions of entrapped polymers following the surface engineering of poly(lactic acid). Surface and Interface Analysis, 31(1), 46–50. 10.1002/sia.951 [DOI] [Google Scholar]
- Quirk RA, Davies MC, Tendler SJB, & Shakesheff KM (2000). Surface engineering of poly(lactic acid) by entrapment of modifying species. Macromolecules, 33(2), 258–260. 10.1021/ma9916133 [DOI] [Google Scholar]
- Rabe M, Verdes D, & Seeger S (2011). Understanding protein adsorption phenomena at solid surfaces. Advances in Colloid and Interface Science, 162(1), 87–106. 10.1016/j.cis.2010.12.007 [DOI] [PubMed] [Google Scholar]
- Ramot Y, Haim-Zada M, Domb AJ, & Nyska A (2016). Biocompatibility and safety of PLA and its copolymers. Advanced Drug Delivery Reviews, 107, 153–162. 10.1016/j.addr.2016.03.012 [DOI] [PubMed] [Google Scholar]
- Rezwan K, Chen QZ, Blaker JJ, & Boccaccini AR (2006). Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials, 27(18), 3413–3431. 10.1016/j.biomaterials.2006.01.039 [DOI] [PubMed] [Google Scholar]
- Rodriguez G, Dias J, d’Ávila MA, & Bártolo P (2013). Influence of hydroxyapatite on extruded 3D scaffolds. Procedia Engineering, 59, 263–269. 10.1016/j.proeng.2013.05.120 [DOI] [Google Scholar]
- Rohman G, Pettit JJ, Isaure F, Cameron NR, & Southgate J (2007). Influence of the physical properties of two-dimensional polyester substrates on the growth of normal human urothelial and urinary smooth muscle cells in vitro. Biomaterials, 28(14), 2264–2274. 10.1016/j.biomaterials.2007.01.032 [DOI] [PubMed] [Google Scholar]
- Ryu GH, Yang WS, Roh HW, Lee IS, Kim JK, Lee GH, … Park JC (2005). Plasma surface modification of poly(D,l-lactic-co-glycolic acid) (65/35) film for tissue engineering. Surface & Coatings Technology, 193(1), 60–64. 10.1016/j.surfcoat.2004.07.062 [DOI] [Google Scholar]
- Saltzman WM, & Kyriakides TR (2014). Chapter 20—Cell interactions with polymers In Lanza RP, Langer R, & Vacanti J (Eds.), Principles of tissue engineering (4th ed.) (p. 385, 406). Cambridge, MA: Academic Press. [Google Scholar]
- Sarapirom S, Yu LD, Boonyawan D, & Chaiwong C (2014). Effect of surface modification of poly(lactic acid) by low-pressure ammonia plasma on adsorption of human serum albumin. Applied Surface Science, 310, 42–50. 10.1016/j.apsusc.2014.03.141 [DOI] [Google Scholar]
- Seal BL, Otero TC, & Panitch A (2001). Polymeric biomaterials for tissue and organ regeneration. Materials Science & Engineering R, 34(4), 147–230. 10.1016/s0927-796x(01)00035-3 [DOI] [Google Scholar]
- Seo HJ, Lee MH, Kwon BJ, Kim HL, Lee SJ, Kim BJ, … Park JC (2013). Plasma treatment induces internal surface modifications of electrospun poly(l-lactic) acid scaffold to enhance protein coating. Journal of Applied Physics, 114(7), 073304 10.1063/1.4818914 [DOI] [Google Scholar]
- Serra T, Mateos-Timoneda MA, Planell JA, & Navarro M (2013). 3D printed PLA-based scaffolds: A versatile tool in regenerative medicine. Organogenesis, 9(4), 239–244. 10.4161/org.26048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Jo S, & Mikos AG (2003). Biomimetic materials for tissue engineering. Biomaterials, 24(24), 4353–4364. 10.1016/s0142-9612(03)00339-9 [DOI] [PubMed] [Google Scholar]
- Shin K, Acri T, Geary S, & Salem AK (2017). Biomimetic mineralization of biomaterials using simulated body fluids for bone tissue engineering and regenerative medicine. Tissue Engineering Part a, 23(19–20), 1169–1180. 10.1089/ten.tea.2016.0556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LL, Niziolek PJ, Haberstroh KM, Nauman EA, & Webster TJ (2007). Decreased fibroblast and increased osteoblast adhesion on nanostructured NaOH-etched PLGA scaffolds. International Journal of Nanomedicine, 2(3), 383–388. [PMC free article] [PubMed] [Google Scholar]
- Song JE, Song WS, Yeo SY, Kim HR, & Lee SH (2017). Covalent immobilization of enzyme on aminated woven poly(lactic acid) via ammonia plasma: Evaluation of the optimum immobilization conditions. Textile Research Journal, 87(10), 1177–1191. 10.1177/0040517516648514 [DOI] [Google Scholar]
- Stevens MM, & George JH (2005). Exploring and engineering the cell surface interface. Science, 310(5751), 1135–1138. 10.1126/science.1106587 [DOI] [PubMed] [Google Scholar]
- Stoleru E, Zaharescu T, Hitruc EG, Vesel A, Ioanid EG, Coroaba A, … Vasile C (2016). Lactoferrin-immobilized surfaces onto functionalized PLA assisted by the gamma-rays and nitrogen plasma to create materials with multifunctional properties. ACS Applied Materials & Interfaces, 8(46), 31902 10.1021/acsami.6b09069-31915 [DOI] [PubMed] [Google Scholar]
- Suárez-González D, Barnhart K, Migneco F, Flanagan C, Hollister SJ, & Murphy WL (2011). Controllable mineral coatings on PCL scaffolds as carriers for growth factor release. Biomaterials, 33(2), 713–721. 10.1016/j.biomaterials.2011.09.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swilem AE, Lehocký M, Humpolíček P, Kucekova Z, Junkar I, Mozetič M, … Novák I (2016). Developing a biomaterial interface based on poly(lactic acid) via plasma-assisted covalent anchorage of D-glucosamine and its potential for tissue regeneration. Colloids and Surfaces B: Biointerfaces, 148, 59–65. 10.1016/j.colsurfb.2016.08.046 [DOI] [PubMed] [Google Scholar]
- Szott LM, & Horbett TA (2011a). Protein interactions with surfaces: Cellular responses, complement activation, and newer methods. Current Opinion in Chemical Biology, 15(5), 677–682. 10.1016/j.cbpa.2011.04.021 [DOI] [PubMed] [Google Scholar]
- Szott LM, & Horbett TA (2011b). Protein interactions with surfaces: Computational approaches and repellency. Current Opinion in Chemical Biology, 15(5), 683–689. 10.1016/j.cbpa.2011.04.016 [DOI] [PubMed] [Google Scholar]
- Tadmor Z (2006). Principles of polymer processing (2nd ed.). Hoboken, N.J.:Hoboken, N.J: : Wiley-Interscience. [Google Scholar]
- Tas AC, & Bhaduri SB (2004). Rapid coating of Ti6Al4V at room temperature with a calcium phosphate solution similar to 10× simulated body fluid. Journal of Materials Research, 19(09), 2742–2749. 10.1557/jmr.2004.0349 [DOI] [Google Scholar]
- Thanki PN, Dellacherie E, & Six JL (2006). Surface characteristics of PLA and PLGA films. Applied Surface Science, 253(5), 2758–2764. 10.1016/j.apsusc.2006.05.047 [DOI] [Google Scholar]
- Thapa A, Miller DC, Webster TJ, & Haberstroh KM (2003). Nano-structured polymers enhance bladder smooth muscle cell function. Bio-materials, 24(17), 2915–2926. 10.1016/s0142-9612(03)00123-6 [DOI] [PubMed] [Google Scholar]
- Trachtenberg JE, Santoro M, Williams C, Piard CM, Smith BT, Placone JK, … Mikos AG (2017). Effects of shear stress gradients on Ewing sarcoma cells using 3D printed scaffolds and flow perfusion. ACS Biomaterials Science & Engineering, 28, 532–554. 10.1021/acsbiomaterials.6b00641 [DOI] [PubMed] [Google Scholar]
- Tverdokhlebov SI, Bolbasov EN, Shesterikov EV, Antonova LV, Golovkin AS, Matveeva VG, … Anissimov YG (2015). Modification of polylactic acid surface using RF plasma discharge with sputter deposition of a hydroxyapatite target for increased biocompatibility. Applied Surface Science, 329, 32–39. 10.1016/j.apsusc.2014.12.127 [DOI] [Google Scholar]
- Unadkat HV, Hulsman M, Cornelissen K, Papenburg BJ, Truckenmüller RK, Carpenter AE, … de Boer J (2011). An algorithm-based topographical biomaterials library to instruct cell fate. Proceedings of the National Academy of Sciences, 108(40), 16565–16570. 10.1073/pnas.1109861108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser LC, Arnoczky SP, Caballero O, Kern A, Ratcliffe A, & Gardner KL (2010). Growth factor-rich plasma increases tendon cell proliferation and matrix synthesis on a synthetic scaffold: An in vitro study. Tissue Engineering, 16(3), 1021–1029. 10.1089/ten.tea.2009.0254 [DOI] [PubMed] [Google Scholar]
- Wang S, Cui W, & Bei J (2005). Bulk and surface modifications of polylactide. Analytical and Bioanalytical Chemistry, 381(3), 547–556. 10.1007/s00216-004-2771-2 [DOI] [PubMed] [Google Scholar]
- Williams JM, Adewunmi A, Schek RM, Flanagan CL, Krebsbach PH, Feinberg SE, … Das S (2005). Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Bio-materials, 26(23), 4817–4827. 10.1016/j.biomaterials.2004.11.057 [DOI] [PubMed] [Google Scholar]
- Woo KM, Jun JH, Chen VJ, Seo J, Baek JH, Ryoo HM, … Ma PX (2007). Nano-fibrous scaffolding promotes osteoblast differentiation and biomineralization. Biomaterials, 28(2), 335–343. 10.1016/j.biomaterials.2006.06.013 [DOI] [PubMed] [Google Scholar]
- Woo KM, Seo J, Zhang R, & Ma PX (2007). Suppression of apoptosis by enhanced protein adsorption on polymer/hydroxyapatite composite scaffolds. Biomaterials, 28(16), 2622–2630. 10.1016/j.biomaterials.2007.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulf K, Teske M, Löbler M, Luderer F, Schmitz KP, & Sternberg K (2011). Surface functionalization of poly(ε-caprolactone) improves its biocompatibility as scaffold material for bioartificial vessel prostheses. Journal of Biomedical Materials Research, 98(1), 89–100. 10.1002/jbm.b.31836 [DOI] [PubMed] [Google Scholar]
- Xu LQ, Neoh K-G, & Kang E-T (2018). Natural polyphenols as versatile platforms for material engineering and surface functionalization. Progress in Polymer Science, 87, 165–196. 10.1016/j.progpolymsci.2018.08.005 [DOI] [Google Scholar]
- Yang F, Wolke JGC, & Jansen JA (2008). Biomimetic calcium phosphate coating on electropun poly (epsilon-caprolactone) scaffolds for bone tissue engineering. Chemical Engineering Journal, 137(1), 154–161. 10.1016/j.cej.2007.07.076 [DOI] [Google Scholar]
- Yang J, Wan Y, Tu C, Cai Q, Bei J, & Wang S (2003). Enhancing the cell affinity of macroporous poly(l-lactide) cell scaffold by a convenient surface modification method. Polymer International, 52(12), 1892–1899. 10.1002/pi.1272 [DOI] [Google Scholar]
- Yang S, Leong KF, Du Z, & Chua CK (2001). The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Engineering, 7(6), 679 10.1089/107632701753337645 [DOI] [PubMed] [Google Scholar]
- Yang Z, Wu Y, Li C, Zhang T, Zou Y, Hui JHP, … Lee EH (2012). Improved mesenchymal stem cells attachment and in vitro cartilage tissue formation on chitosan-modified poly(l-lactide-co-epsilon-caprolactone) scaffold. Tissue Engineering, 18(3–4), 242–251. 10.1089/ten.TEA.2011.0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim ED, Besunder R, Pappas D, Allen F, Güçeri S, & Sun W (2010). Accelerated differentiation of osteoblast cells on polycaprolactone scaffolds driven by a combined effect of protein coating and plasma modification. Biofabrication, 2(1), 014109 10.1088/1758-5082/2/1/014109 [DOI] [PubMed] [Google Scholar]
- Yim EKF, Darling EM, Kulangara K, Guilak F, & Leong KW (2010). Nanotopography-induced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cells. Biomaterials, 31(6), 1299–1306. 10.1016/j.biomaterials.2009.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JJ, Song SH, Lee DS, & Park TG (2004). Immobilization of cell adhesive RGD peptide onto the surface of highly porous biodegradable polymer scaffolds fabricated by a gas foaming/salt leaching method. Biomaterials, 25(25), 5613–5620. 10.1016/j.biomaterials.2004.01.014 [DOI] [PubMed] [Google Scholar]
- Zamani F, Amani-Tehran M, Latifi M, & Shokrgozar M (2013). The influence of surface nanoroughness of electrospun PLGA nanofibrous scaffold on nerve cell adhesion and proliferation. Official Journal of the European Society for Biomaterials, 24(6), 1551–1560. 10.1007/s10856-013-4905-6 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Ortiz O, Goyal R, & Kohn J (2014). Chapter 23—Biodegradable polymers In Lanza RP, Langer R, & Vacanti J (Eds.), Principles of tissue engineering (4th ed.) (pp. 441–473). Cambridge, MA: Academic Press. [Google Scholar]
- Zhao W, Lemaître J, & Bowen P (2017). A comparative study of simulated body fluids in the presence of proteins. Acta Biomaterialia, 53, 506–514. 10.1016/j.actbio.2017.02.006 [DOI] [PubMed] [Google Scholar]
- Zhu H, Ji J, & Shen J (2004). Biomacromolecules electrostatic self-assembly on 3-dimensional tissue engineering scaffold. Biomacromolecules, 5(5), 1933–1939. 10.1021/bm049753u [DOI] [PubMed] [Google Scholar]
- Zhu Y, Gao C, Liu X, He T, & Shen J (2004). Immobilization of biomacromolecules onto aminolyzed poly(l-lactic acid) toward acceleration of endothelium regeneration. Tissue Engineering, 10(1–2), 53–61. 10.1089/107632704322791691 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Gao C, Liu X, & Shen J (2002). Surface modification of polycaprolactone membrane via aminolysis and biomacromolecule immobilization for promoting cytocompatibility of human endothelial cells. Biomacromolecules, 3(6), 1312–1319. 10.1021/bm020074y [DOI] [PubMed] [Google Scholar]
- Zhu Y, Gao C, Liu Y, & Shen J (2004). Endothelial cell functions in vitro cultured on poly(l-lactic acid) membranes modified with different methods. Journal of Biomedical Materials Research, 69(3), 436–443. 10.1002/jbm.a.30007 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Leong MF, Ong WF, Chan-Park MB, & Chian KS (2007). Esophageal epithelium regeneration on fibronectin grafted poly(l-lactide-co-caprolactone) (PLLC) nanofiber scaffold. Biomaterials, 28(5), 861–868. 10.1016/j.biomaterials.2006.09.051 [DOI] [PubMed] [Google Scholar]
- Zinger O, Zhao G, Schwartz Z, Simpson J, Wieland M, Landolt D, & Boyan B (2005). Differential regulation of osteoblasts by substrate microstructural features. Biomaterials, 26(14), 1837–1847. 10.1016/j.biomaterials.2004.06.035 [DOI] [PubMed] [Google Scholar]