Abstract
Objective
To determine whether early and more frequent mobilization after stroke affects health-related quality of life.
Methods
A Very Early Rehabilitation Trial (AVERT) was an international, multicenter (56 sites), phase 3 randomized controlled trial, spanning 2006–2015. People were included if they were aged ≥18 years, presented within 24 hours of a first or recurrent stroke (ischemic or hemorrhagic), and satisfied preordained physiologic criteria. Participants were randomized to usual care alone or very early and more frequent mobilization in addition to usual care. Quality of life at 12 months was a prespecified secondary outcome, evaluated using the Assessment of Quality of Life 4D (AQoL-4D). This utility-weighted scale has scores ranging from −0.04 (worse than death) to 1 (perfect health). Participants who died were assigned an AQoL-4D score of 0.
Results
No significant difference in quality of life at 12 months between intervention (median 0.47, interquartile range [IQR] 0.07–0.81) and usual care (median 0.49, IQR 0.08–0.81) groups was identified (p = 0.86), nor were there any group differences across the 4 AQoL-4D domains. The same lack of group difference in quality of life was observed at 3 months. When cohort data were analyzed (both groups together), quality of life was strongly associated with acute length of stay, independence in activities of daily living, cognitive function, depressive symptoms, and anxiety symptoms (all p < 0.001). Quality of life in AVERT participants was substantially lower than population norms, and the gap increased with age.
Conclusions
Earlier and more frequent mobilization after stroke did not influence quality of life.
Clinical trial registration
anzctr.org.au; ACTRN12606000185561
Classification of evidence
This study provides Class II evidence that for people with stroke, earlier and more frequent mobilization did not influence quality of life over the subsequent year.
Stroke can have a major effect on quality of life (QOL),1 a multidimensional construct that incorporates physical, psychological, and social elements of well-being. The comprehensive scope of QOL means it has been viewed as the single health outcome that is most relevant to the individual,2 and thus it is important to identify interventions that improve QOL following stroke. Care in a stroke unit is one such intervention.3 A component of stroke unit care that may contribute to better outcomes is earlier mobilization, as this has been independently associated with discharge to home within 6 weeks.4 More directly, our phase 2 trial indicated that early and more frequent mobilization after stroke is associated with benefit in the “Independent Living” domain of the Assessment of Quality of Life 4D (AQoL-4D) instrument.5 Early initiation of physical rehabilitation following stroke (within 7 days) has also been associated with better subsequent QOL.6
As a multidimensional construct, QOL has a diverse array of contributing factors. Studies indicate that lower QOL after stroke is strongly related to lack of functional independence, depression, and older age.7,8 Cognitive impairment, too, has been widely linked to low QOL after stroke.9,10 The deleterious effect of stroke on QOL has been reported in both younger11 and older12 cohorts, but the important contributing factors may differ across the lifespan. In younger people with stroke, a reduced ability to concentrate was associated with lower QOL.13 Even minor stroke-related deficits can have a marked effect on QOL at this age: in a group of working-age survivors of mild stroke, the majority reported limitations in returning to leisure activities (58%) and work (52%).14 Given that an individual judges QOL in his or her own unique context, and in relation to goals and expectations, the effect of stroke may also differ according to seemingly tangential factors such as geographic region or education background.
Methods
Study design
A Very Early Rehabilitation Trial (AVERT) phase 3 was a pragmatic, parallel-group, single-blind, multicenter, randomized controlled trial with blinded assessment of outcome, conducted at 56 sites internationally.15 It was designed to investigate the efficacy of very early mobilization (earlier and more frequent out-of-bed activity), compared with usual care, in acute stroke. The primary outcome was good functional outcome (0–2 on the modified Rankin Scale [mRS]16) at 3 months. A prespecified secondary hypothesis—constituting Class II evidence—was better QOL at 12 months in the intervention than usual care group. The later time point was chosen to reflect longer-term recovery and adjustment. Given AVERT's large sample size, we also planned to determine associations between a range of demographic and clinical factors and QOL in the full cohort (both groups together). The trial had ethical approval from the relevant Human Ethics Committee at each participating site. It was stopped when the recruitment target of 2,104 was reached. Details of the study rationale, design, and statistical analysis have been published previously.17
Participants
Eligible participants were aged 18 years or older and were recruited within 24 hours of a confirmed stroke (first or recurrent, ischemic or hemorrhagic). Exclusion criteria included premorbid disability, early deterioration, palliation, other serious illness or coronary condition, and physiologic readings (blood pressure, heart rate, temperature) falling outside set limits.
Randomization
Participants were randomly assigned to receive usual stroke unit care alone or very early and more frequent mobilization in addition to usual care, stratified by hospital site and stroke severity. The randomization schedule was computer-generated, with allocation concealed and delivered via a secure, purpose-built online interface.
Intervention
For both intervention and control participants, the components of usual care were at the discretion of individual sites. The very early mobilization intervention comprised 3 crucial elements: (1) begin within 24 hours of stroke onset, (2) focus on out-of-bed activity (e.g., sitting, standing, walking), and (3) result in at least 3 out-of-bed sessions per day in addition to usual care. The intervention period lasted 14 days or until discharge from the acute stroke unit, whichever was sooner.
Outcomes
The focus of the current analysis is the secondary outcome of QOL at 12 months. The AQoL is a multiple-domain, utility-weighted measure of health-related QOL.18 We used the AQoL-4D, which has 12 items that cover 4 domains: Independent Living (self-care, household tasks, mobility), Social Relationships (relationships with others, social isolation, family role), Physical Senses (seeing, hearing, communication), and Psychological Well-being (sleep, anxiety and depression, pain). It also includes 3 items on Illness (prescribed medicines, medication and aids, medical treatment), but these do not contribute to the total AQoL-4D score. Each item has 4 response options, and the respondent is instructed to choose the alternative that best describes him or her over the last week. Raw scores are transformed into domain disutility scores using specific algorithms that are based on weights from an Australian population sample. Domain scores can then be calculated using 1 − domain disutility score. Each weighted domain score is between 0 (worst health state) to 1 (best health state). A single weighted overall utility score is calculated from the 4 domain scores, and ranges from −0.04 (worse than death) to 0 (equivalent to death) to 1 (full health). Blinded assessors administered the AQoL-4D at 3 and 12 months poststroke. Participants who had died were assigned an AQoL-4D score of 0. In cases where the participant was unable to respond, responses from proxies (relative or carer of the participant) were accepted. For participants with verbal or written communication difficulties, a modified, aphasia-friendly version was developed. Modifications included large print (16-point font), bolded key words for each question, and double paragraph spacing. Questions were presented to participants one at a time with a point response. For these participants, a proxy also completed the AQoL-4D.
Demographic details collected included age, sex, education, marital status, living arrangements (institution was defined as nursing home or other supported accommodation), employment status, premorbid physical disability (mRS), and geographic region. Stroke characteristics included stroke severity (using the NIH Stroke Scale [NIHSS]19), stroke subtype (using the Oxfordshire Community Stroke Project classification20), and length of stay in the acute hospital. Clinical outcome measures included the Irritability, Depression and Anxiety (IDA) scale,21 the Montreal Cognitive Assessment (MoCA),22 and the Barthel index.23
Statistical analysis
The full statistical analysis plan for AVERT was published prior to trial completion and database lock.17 We used bootstrapped median regression to analyze between-group differences in AQoL-4D at 12 months, adjusting for age, sex, and stroke severity. This analytic approach was then applied to each of the AQoL-4D domains. We then repeated the same series of multivariable, bootstrapped median regression analyses to determine whether there were any group differences in AQoL-4D at 3 months. Next we considered the cohort as a whole, with intervention and usual care groups together. Descriptive statistics were used to compare AQoL-4D scores in the AVERT cohort to population norms24 across the age range. To evaluate whether minor stroke-related symptoms have a relatively greater effect on QOL in younger people, we compared AQoL-4D scores in AVERT participants with a 12-months mRS score of 1 or 2 to the population norms. Bootstrapped median regression analyses, adjusted for age, sex, and stroke severity, were employed to determine the associations between QOL and stroke type, acute length of stay, independence in activities of daily living, cognitive function, depressive symptoms, and anxiety symptoms. Correlations were computed to identify whether change in QOL from 3 to 12 months was associated with change in mood symptoms over this same period. Descriptive statistics were used to outline differences in QOL between participants with varying severity of aphasia, as classified by the NIHSS. Further bootstrapped median regression analyses, adjusted for age, sex, and stroke severity, were employed to determine the associations between QOL and return to work, living arrangements, geographic region, and education. All analyses were performed using STATA version 14.0.
Data availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
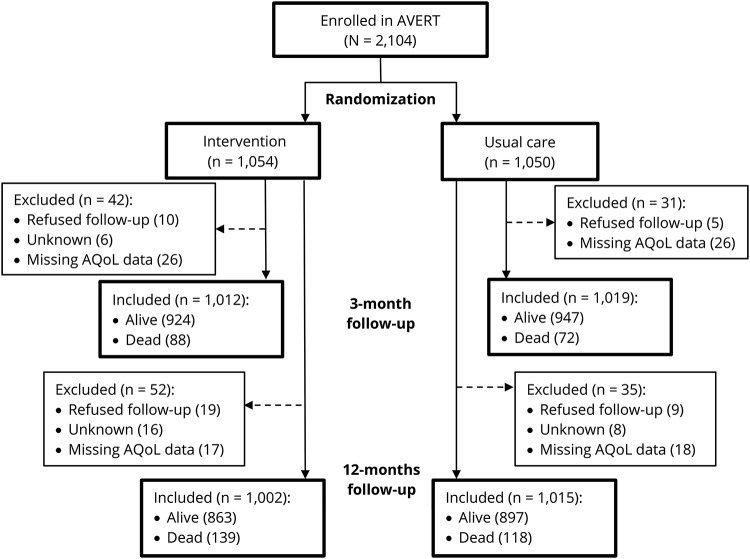
Mean age of the participants was 70.6 years (SD 12.8) and 61% were male. Characteristics of participants were similar between groups (table 1). Among 2,104 participants, AQoL-4D data at 12 months were available for 2,017 (96%), including the 257 participants who had died and were assigned a score of 0. Of the other 87, 28 (1%) refused follow-up, 24 (1%) were lost to follow-up, and 35 (2%) had missing data for one or more AQoL-4D items (figure 1). There was little evidence of attrition bias: the complete data group (n = 1,760) were 62% male and had a median baseline NIHSS of 6 (IQR 4–11), whereas the missing data group (n = 87) were 61% male and had a median baseline NIHSS of 6 (interquartile range [IQR] 3–9). Interestingly, the missing data group (mean age 65.3, SD 13.8) was younger than the complete data group (mean age 69.9, SD 12.8) (t[93] = 3.0, p = 0.003).
Table 1.
Characteristics of the 2,104 A Very Early Rehabilitation Trial (AVERT) participants
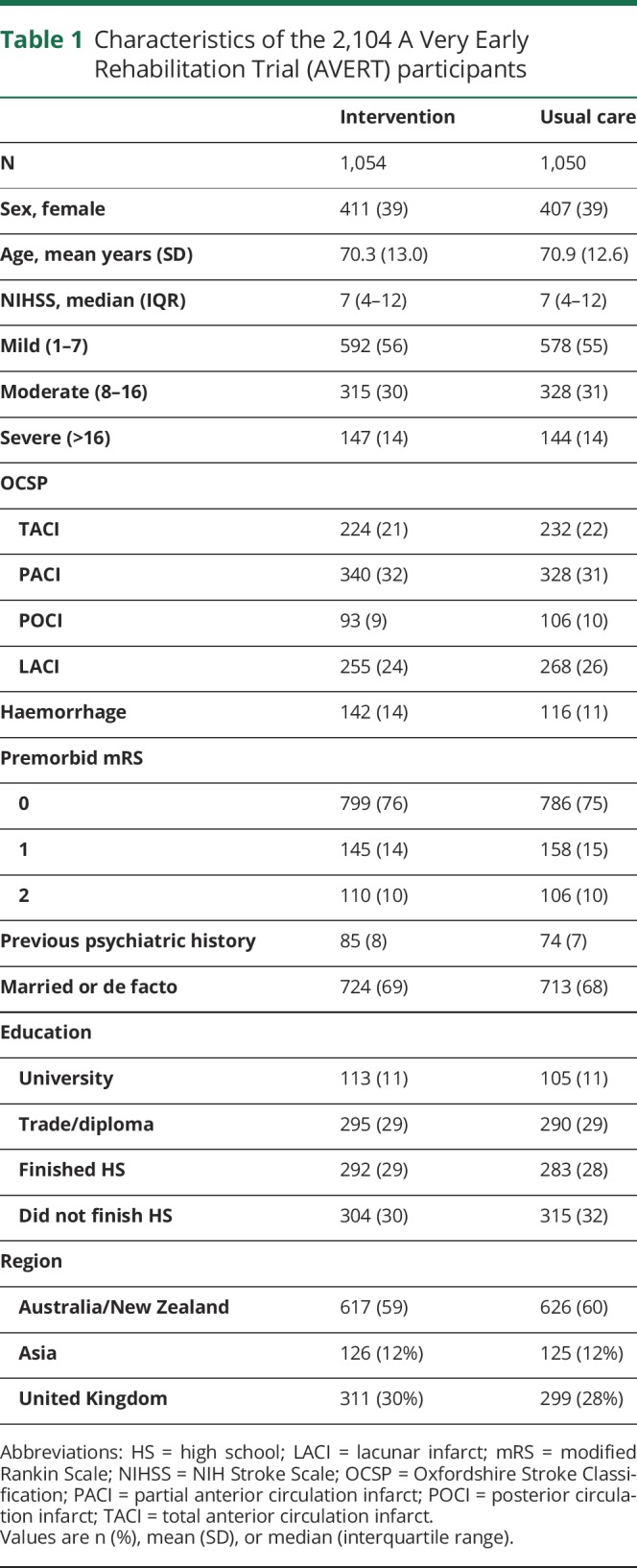
Figure 1. CONSORT flowchart.
AQoL = Assessment of Quality of Life; AVERT = A Very Early Rehabilitation Trial.
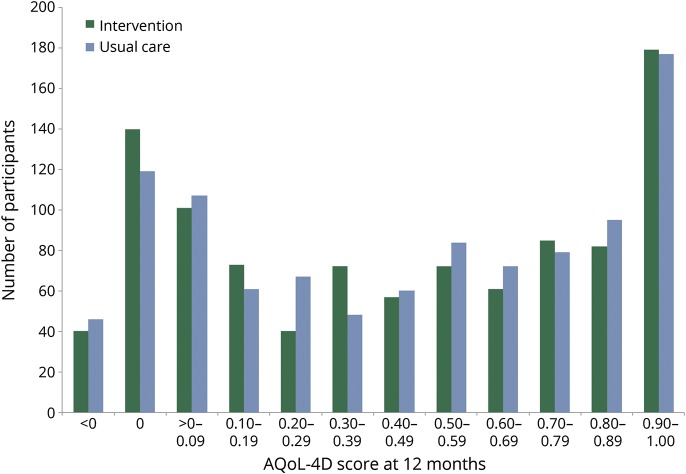
At 12 months, AQoL-4D scores were similar in the intervention (n = 1,002; median 0.47, IQR 0.07–0.81; mean 0.46, SD 0.37) and usual care (n = 1,015; median 0.49, IQR 0.08–0.81; mean 0.47, SD 0.36) groups. The distribution of AQoL-4D scores is illustrated in figure 2. Adjusted median regression indicated no significant group difference in either total AQoL-4D scores (coefficient = −0.004; 95% confidence interval [CI] −0.043, 0.036; p = 0.86) or in any of the 4 domains at 12 months: Independent Living (coefficient = −0.007; 95% CI −0.038, 0.024; p = 0.66), Social Relationships (coefficient = 0.011; 95% CI −0.007, 0.030; p = 0.24), Physical Senses (coefficient = 0.009; 95% CI −0.004, 0.023; p = 0.16), and Psychological Well-being (coefficient = 0.001; 95% CI −0.011, 0.014; p = 0.85).
Figure 2. Distribution of Assessment of Quality of Life 4D (AQoL-4D) scores at 12 months in intervention and usual care groups.
<0 Is considered “worse than death,” 0 was assigned to participants who had died, 1 is considered perfect health.
At 3 months, AQoL-4D was similar between the intervention (n = 1,012; median 0.42, IQR 0.08–0.77; mean 0.44, SD 0.36) and usual care (n = 1,019; median 0.44, IQR 0.09–0.77; mean 0.45, SD 0.35) groups, with no significant group difference on median regression (coefficient = −0.019; 95% CI −0.058, 0.019; p = 0.33). Intervention participants scored higher than usual care participants on the Physical Senses domain at 3 months (coefficient = 0.013; 95% CI 0.001, 0.025; p = 0.035), but there were no group differences in the other 3 AQoL-4D domains: Independent Living (coefficient = −0.026; 95% CI −0.064, 0.011; p = 0.17), Social Relationships (coefficient = 0.005; 95% CI −0.012, 0.023; p = 0.54), and Psychological Well-being (coefficient = −0.003; 95% CI −0.013, 0.007; p = 0.60).
Deaths excluded
When we included only surviving participants, bootstrapped median regression analysis failed to detect a significant difference between groups at 12 months; AQoL-4D was similar in intervention (n = 863; median 0.56, IQR 0.20–0.86; mean 0.53, SD 0.34) and usual care (n = 897; median 0.56, IQR 0.22–0.86; mean 0.53, SD 0.34). The group comparison was little changed when the sample was further restricted to survivors who had not experienced a serious adverse event of stroke progression or recurrent stroke over the 12 months of follow-up; AQoL-4D at 12 months was similar in intervention (n = 798; median 0.58, IQR 0.25–0.87; mean 0.55, SD 0.34) and usual care (n = 835; median 0.57, IQR 0.25–0.87; mean 0.55, SD 0.34). In all survivors at 3 months, AQoL-4D was similar in the intervention (n = 924; median 0.48, IQR 0.16–0.81; mean 0.48, SD 0.34) and usual care (n = 947; median 0.50, IQR 0.17–0.80; mean 0.49, SD 0.34) groups. Mean change in AQoL-4D between 3 and 12 months revealed comparable improvement across time in intervention (n = 852, mean 0.03, SD 0.22) and usual care (n = 885, mean 0.02, SD 0.21) groups. In the intervention group, QOL improved between 3 and 12 months in 53% of participants, declined in 38%, and was unchanged in 9%; in usual care, it improved in 51%, declined in 40%, and was unchanged in 9%.
Intervention and usual care groups combined
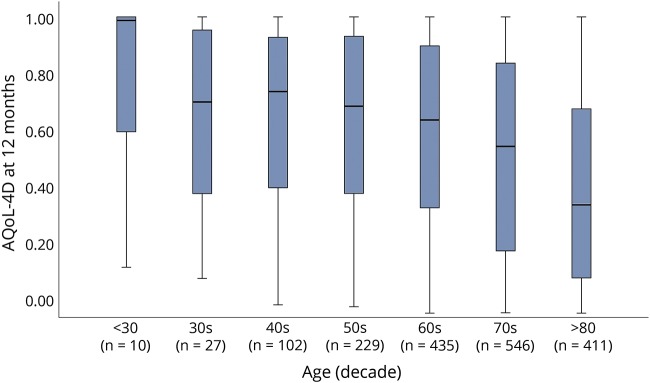
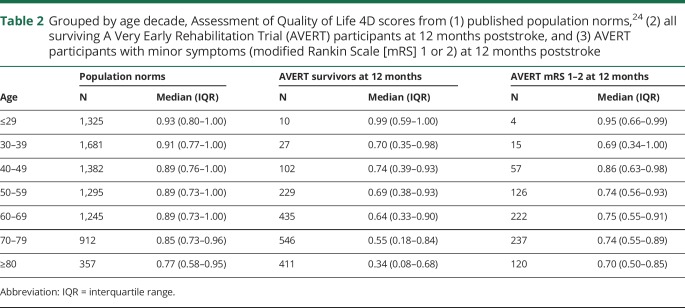
With participants grouped into age decades, we observed an inverse relationship between age and QOL (figure 3). QOL was lower in our stroke sample than in population norms,24 and this difference increased as age increased (table 2). When excluding the smaller samples of participants under 40 years old, the difference in median AQoL-4D score between our participants and the norms grew with each decade of life: 0.15 in the 40s, 0.20 in the 50s, 0.25 in the 60s, 0.30 in the 70s, and 0.43 in the over-80s. When comparing AQoL-4D scores of those with a 12-month mRS score of 1 or 2 to the population norms, we observed the opposite pattern, with the difference in medians decreasing as age increased: 0.15 in the 50s, 0.14 in the 60s, 0.11 in the 70s, and 0.07 in the over-80s (table 2).
Figure 3. Boxplots of Assessment of Quality of Life 4D (AQoL-4D) scores at 12 months in all surviving A Very Early Rehabilitation Trial (AVERT) participants, grouped by age decade.
Table 2.
Grouped by age decade, Assessment of Quality of Life 4D scores from (1) published population norms,24 (2) all surviving A Very Early Rehabilitation Trial (AVERT) participants at 12 months poststroke, and (3) AVERT participants with minor symptoms (modified Rankin Scale [mRS] 1 or 2) at 12 months poststroke
Association with clinical variables
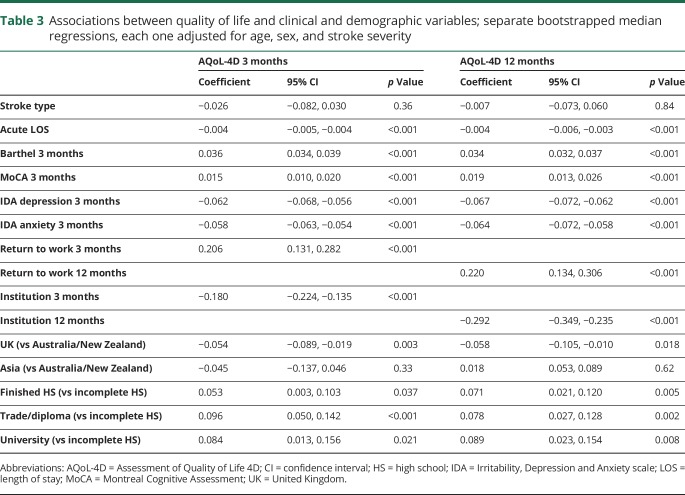
No significant difference in AQoL-4D was identified between participants with ischemic and hemorrhagic stroke, at either 3 or 12 months (table 3). Higher AQoL-4D scores at both 3 and 12 months were significantly associated with shorter acute length of stay, greater independence in activities of daily living (Barthel Index at 3 months), better cognitive function (MoCA at 3 months), and fewer depressive and anxiety symptoms (IDA subscales at 3 months). Change in AQoL-4D score between 3 and 12 months was significantly correlated with change in depressive (n = 1,555; r = −0.32, p < 0.001) and anxiety (n = 1,551; r = −0.29, p < 0.001) symptoms over the same time period. Participants identified as having aphasia (as per the NIHSS item) had lower AQoL-4D scores at 12 months than those without aphasia (table 4).
Table 3.
Associations between quality of life and clinical and demographic variables; separate bootstrapped median regressions, each one adjusted for age, sex, and stroke severity
Table 4.
Total Assessment of Quality of Life 4D (AQoL-4D) scores at 12 months in surviving participants according to language status on the NIH Stroke Scale (NIHSS) at baseline and 3 months
Association with demographic factors
There were 527 participants who were employed at the time of their stroke. At both 3 and 12 months, those who had returned to work had better QOL than those who had not returned (table 3). Participants living in an institution at 3 months (n = 130) and at 12 months (n = 148) had lower QOL than those not living in an institution at these time points. Splitting participants by geographic region, AQoL-4D at 3 months was highest in Asia (median 0.58, IQR 0.20–0.85), followed by Australia/New Zealand (median 0.50, IQR 0.16–0.79) and the United Kingdom (median 0.42, IQR 0.13–0.79). The same pattern was identified at 12 months: Asia (median 0.72, IQR 0.28–0.94), Australia/New Zealand (median 0.57, IQR 0.23–0.84), and the United Kingdom (median 0.49, IQR 0.14–0.83). Compared to Australia/New Zealand, AQoL-4D was significantly lower in the United Kingdom at both time points (table 3). In terms of education, AQoL-4D at 3 months was highest in those with a university degree (median 0.60, IQR 0.23–0.84), followed by those with a trade or diploma (median 0.54, IQR 0.22–0.84), then those who finished high school (median 0.48, IQR 0.16–0.81), and those who did not complete high school (median 0.40, IQR 0.11–0.73). The same pattern was identified at 12 months: university degree (median 0.68, IQR 0.30–0.91), trade or diploma (median 0.61, IQR 0.23–0.88), finished high school (median 0.56, IQR 0.24–0.86), did not complete high school (median 0.51, IQR 0.16–0.79). Compared to people not finishing high school, QOL was significantly higher in all 3 other education groups at both 3 and 12 months (table 3).
Proxy respondents
QOL at 3 months was poorer for participants with a proxy respondent (n = 207; median 0.05, IQR −0.01 to 0.24) than for participants who responded themselves (n = 1,664; median 0.56, IQR 0.23–0.83). Similarly, 12-month QOL was poorer for those with a proxy respondent (n = 220; median 0.07, IQR 0.00–0.30) than for those who responded themselves (n = 1,540; median 0.63, IQR 0.30–0.89). Our aphasia-friendly version of the AQoL-4D was designed to increase the chance of participants with communication difficulties responding themselves, but it was used in only 14 participants. Given such low numbers, proxy data were used for these respondents.
Discussion
AVERT included a very large cohort of stroke survivors, recruited across 56 hospital sites internationally. This, combined with the wide inclusion criteria, meant the sample was broadly representative of the general stroke population. QOL data were collected over the year following stroke, with very low rates of missing data and loss to follow-up. Our hypothesis that the intervention group would have better overall QOL than the usual care group at 12 months poststroke was not supported. Furthermore, there was no group difference in any of the 4 AQoL-4D domains at 12 months. Similarly, group comparison of total AQoL-4D score at 3 months yielded no significant difference. The only significant group difference in domain score at 3 months was in Physical Senses, where intervention participants had better QOL than usual care participants. While statistically significant, however, this difference was not clinically meaningful and was confined to the lower end of the distribution (both groups had a median utility score of 0.94 for the Physical Senses domain). The tendency towards better scores on the Independent Living domain identified in the intervention arm of AVERT phase 25 was not replicated here.
The lack of group difference in QOL contrasts with AVERT's primary outcome analysis, which indicated that fewer intervention participants had a favorable functional outcome at 3 months (mRS 0–2) than controls (46% vs 50%, adjusted odds ratio 0.73 [95% CI 0.59–0.90], p = 0.004).15 While an association between mRS outcome and QOL after stroke has been demonstrated,25 the strength of correspondence is diluted by substantial variability. At 3 months poststroke, QOL at a given mRS level is highly heterogeneous.26 Such findings have prompted attempts to develop a utility-weighted mRS,27,28 with the aim to reflect patient perception of QOL and thus enhance interpretability of the ordinal scale. It is possible that the AVERT intervention may have improved some aspects of QOL that are reflected in the AQoL-4D but not in the mRS.
When the 2 groups were considered together, QOL in the full AVERT cohort was substantially lower than population norms,24 giving support to previous findings of lower QOL in stroke survivors than in stroke-free individuals.1,11,12 At 12 months, surviving AVERT participants had a mean AQoL-4D score of 0.53, marginally higher than the mean of 0.47 reported at 2 years poststroke in the population-based NEMESIS study.29 When participants were stratified by age decade, 2 patterns were notable. First, our stroke survivors had median 12-month AQoL-4D scores >0.13 lower than population norms—reflecting a clinically meaningful difference in QOL30—across every decade (excepting the very small sample of participants under 30). Second, the difference tended to increase with age: there is a gradual decline in QOL with age in the norms, but there is a marked decline with age in the AVERT participants. This may reflect the tendency for older participants to have more severe strokes, and thus poorer QOL. Mindful of the strong influence of disability level, we investigated whether a similar decline in QOL with age was present in participants with minor stroke-related symptoms. Among participants who experienced minor symptoms (mRS 1 or 2) at 12 months, the gap in QOL to population norms appeared to decrease, rather than increase, with age. In other words, there was some evidence that mild deficits had a greater effect on QOL in younger than in older stroke survivors.
A number of the associations we identified were confirmatory: better QOL was related to greater independence in activities of daily living, having fewer symptoms of depression and anxiety, and living at home rather than in an institution. There is precedent for our findings of higher QOL in those with better cognitive function9 and those who have returned to work.31 In line with previous research,32 we failed to detect a significant difference in QOL between ischemic and hemorrhagic stroke survivors. Other findings were more novel. Better QOL was associated with a shorter length of stay in the acute hospital, and this may be partly attributable to complications experienced during the inpatient stay. Analysis of participants by region indicated that QOL was lower in the United Kingdom than it was in Australia/New Zealand or Asia. There does not appear to be a matching difference in QOL between the general populations of these countries. While no normative data for the AQoL-4D are available from the United Kingdom, a national comparison of population norms on a different scale revealed slightly higher QOL in the United Kingdom than in New Zealand.33 Data on educational background revealed that participants with higher levels of education had better QOL. There is some evidence that this association is mediated by lower levels of emotional and physical distress in the better educated, largely brought about by higher personal control over employment and economic resources.34 Whatever the explanation underlying these associations (or lack thereof), they cannot be attributed to simple confounding, as all analyses were adjusted for age, sex, and stroke severity.
Our major limitation was missing data, due to deaths and to other reasons. This is inevitable, given the inclusion of participants across the full stroke severity spectrum. The rate of missing data, however, was very low when compared to that of other poststroke QOL studies,35 and is a tribute to the work ethic of the blinded assessors. Fewer than 5% of all participants were excluded from analysis of AQoL-4D at 12 months due to refusing follow-up, being lost to follow-up, or having missing data. To minimize missing data, we allowed for proxy respondents, though we acknowledge that proxies have a tendency to overestimate impairments and underestimate QOL.36 In our sample, it is unsurprising that AQoL-4D scores for participants with proxy respondents were very low, given that these participants often had severe stroke or communication problems, or both.
Our findings indicate that providing early and more frequent mobilization after stroke does not have a marked influence on QOL over the subsequent year.
Acknowledgment
The authors thank the AVERT investigators, particularly the blinded assessors, who collected the quality of life data, and A/Prof Miranda Rose for developing an aphasia-friendly version of the AQoL-4D scale. The Florey Institute of Neuroscience and Mental Health acknowledges the support of the Victorian Government's Operational Infrastructure Support Grant.
Glossary
- AQoL-4D
Assessment of Quality of Life 4D
- AVERT
A Very Early Rehabilitation Trial
- CI
confidence interval
- IDA
Irritability, Depression and Anxiety
- IQR
interquartile range
- MoCA
Montreal Cognitive Assessment
- mRS
modified Rankin Scale
- NIHSS
NIH Stroke Scale
- QOL
quality of life
Appendix 1. Authors
Appendix 2. AVERT Trial Collaboration Group
Footnotes
Class of Evidence: NPub.org/coe
Study funding
The trial was initially supported by the National Health and Medical Research Council (NHMRC) of Australia (grants 386201, 1041401). Additional funding was received from Chest Heart and Stroke Scotland (Res08/A114), Northern Ireland Chest Heart and Stroke, Singapore Health (SHF/FG401P/2008), the UK Stroke Association (TSA2009/09), and the UK National Institute of Health Research (HTA Project 12/01/16). NHMRC fellowship funding was provided to A.G.T. (1042600), H.D. (336102), and J.B. (1058635). J.B. also received fellowship funding from the Australia Research Council (0991086) and the National Heart Foundation.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
Management Committee
Julie Bernhardt (Chair), Leonid Churilov, Janice Collier, Helen Dewey, Geoffrey Donnan, Fiona Ellery, Peter Langhorne, Richard Lindley, Marj Moodie, Brooke Parsons (Consumer Representative), Amanda Thrift.
Trial Steering Committee
Geoffrey Donnan (Co-Chair), Helen Dewey (Co-Chair), Julie Bernhardt, Peter Langhorne, Marj Moodie, Brooke Parsons (Consumer Representative), and main investigators (MIs) from all participating hospitals.
International Advisors
L. Bent Indredavik, Torunn Askim.
Data Monitoring Committee
Professor Phillip Bath (University of Nottingham, Nottingham, UK, Chair), Professor Christopher Bladin (Box Hill Hospital, Melbourne, Australia), Professor Christopher Reid (Monash University, Melbourne, Australia), Dr. Stephen Read (Royal Brisbane and Women's Hospital, Melbourne, Australia), Associate Professor Cathy Said (Austin Health, Melbourne, Australia).
Outcomes Committee
Professor Sandy Middleton (Australian Catholic University, Sydney, Australia, Chair), Dr. Judith Frayne (Alfred Hospital, Melbourne, Australia), Professor Velandai Srikanth (Monash Health, Melbourne, Australia).
Country Leaders and Grant Holders
Australia: Julie Bernhardt (NHMRC: Helen Dewey, Julie Bernhardt, Geoffrey Donnan, Amanda Thrift, Robert Carter, Richard Lindley) (NHMRC: Julie Bernhardt, Geoff Donnan, Richard Lindley, Amanda Thrift, Peter Langhorne, Marj Moodie, Helen Dewey, Leonid Churilov).
United Kingdom: Peter Langhorne (CHSS: Peter Langhorne, Olivia Wu, Julie Bernhardt, Matthew Walters Claire Ritchie, Lorraine Smith) (TSA: Peter Langhorne, Olivia Wu, Anne Ashburn, Helen Rodgers, Julie Bernhardt) (HTA: Peter Langhorne, Anne Ashburn, Julie Bernhardt, Helen Rogers, Olivia Wu).
Northern Ireland: Sheila Lennon (NICHS: Sheila Lennon, Michael Power, Julie Bernhardt).
Singapore: Shahul Hameed (Singhealth: Shahul Hameed, Ratnagopal Pavanni, Peter Lim, Julie Bernhardt, Dawn Tan).
Statistics and Data Management
Leonid Churilov, Tim Brewer, Janice Collier, Nick Haritos, Edwin Leong, Cecilia Li, Caesar NayWin, Marcus Nicol, Liudmyla Olenka, Li Chun Quang.
Health Economics
Marj Moodie, Robert Carter, Silvia Hope, Lauren Sheppard, Kiusiang Tay-Teo, Olivia Wu.
Cognition
Toby Cumming, Thomas Linden.
Trial Coordinating Centres
Florey Institute of Neuroscience and Mental Health, Melbourne, Australia: Karen Borschmann, Jan Chamberlain, Janice Collier, Toby Cumming, Fiona Ellery, Teresa Occhiodoro, Helen Palfreeman, Tara Purvis, Bernadette Sirgo, Nick Tiliacos, John Van Holsteyn, Henry Zhao.
University of Glasgow, UK: Beverly Armstrong, Louise Craig, Fiona Graham, Lynn Legg, Rosemary Morrison, Heather Moorhead, Lorraine O'Donohue, Susan Rogers, Myra Smith.
University of Central Lancashire Preston, UK: Denise Forshaw, Jane Fitzgerald.
AVERT Hospital Teams
Teams are listed by country. Figures in parentheses are the number of patients recruited by the center. MI are listed first for each site. Some investigators worked across multiple sites, but are listed for one site only. All others listed are hospital clinicians who were involved in providing interventions and collecting data.
Australia: Austin Hospital (253): E. Hibbert, R. Melling, S. Petrolo, T. Purvis, H. Williamson (MIs), P. Adams, L. Augoustakis, S. Batcheler, S. Berney, V. Cobani, B. Cohen, H. Dewey, S. Gangi, N. Giofre, C. Gordon, L. Hegarty, M. Hindson, F. Horvath, S. Kalinowski, A. Kleine, S. Kramer, J. Lawrence, S. Lindquist, N. Logan, A. Macdonell, J. Matlioski, N. McDonough, S. McLennan, M. McNamee, L. Miller, C. Nall, E. Nelson, K. Ng, Z. Nicholas, C. Nunn, K. Owen, E. Plant, L. Proud, D. Quah, K. Rodway, S. Sertori, V. Sheldon, L. Sherry, S. Speare, K. Stansfeld, N. Studden, Z. Teoh, L. Twist, G. Velupillai, L. Walker, K. Wall, A. Warwick, R. Wharrie, J. Wilson, H. Worboys, D. Young. Royal Perth Hospital (149): J. Ancliffe (MI), M. Bryant, B. Doran, M. Field, P. Fogliani, A. Haber, G. Hankey, D. Hendrie, V. Jackaman, A. Jacobsen, S. Jose, R. Lim, R. Louis, S. Nanthakumar, S. Pain, A. Power, B. Rappeport, J. Reynolds, L. Smith, S. Tombe, A. Wesseldine, T. West. The Royal Melbourne Hospital (95): K. Clarke, H. Maccanti, L. Marr, S. Plumb, J. Quiney, L. Werner (MIs), E. Abeykoon, W. Apirutvorrachod, L. Attard, S. Behanan, D. Brown, K. Buchanan, D. Butler, M. Camac, S. Davis, D. Diocera, N. Gan, C. Gendre, J. Germaine, P. Hand, L. Maurenbrecher, J. McCulloch, S. Mcritchie, M. Ong, R. Pachett, L. Pesavento, H. Power, R. Reilly, M. Sawers, G. Silva, C. Stevens, L. Taylor, T. Timms, M. Ugalde, A. Vardy, J. Wallace, S. Walsh, E. Whatley, E. Winter. Frankston Hospital (90): M. Baxter, M. Davis, L. Sundararajan (MIs), E. Butler, K. Caspers, E. Coulter, S. Shaw, F. Kent, H. Lack, F. Leavold, J. Lord, J. Martin-Francisco, R. Mohanraj, R. Nelson, T. O'Neill, R. Otto, J. Parker, V. Rees, B. Stevens. Westmead Hospital (84): R. Chen (MI), R.I. Lindley, J. Bindra, R. Dongre, N. Downey, M. Ferris, L. Gibson, R. Gonzalez, M. Kinniburgh, M. Lazaridou, D. McCormack, R. Singh, A. Stepney, Y. Tria. Geelong Hospital (74): K. Bainbridge, B. Killey, R. Sheedy (MIs), O. Aitchison, L. Bray, K. Clatworthy, S. Coghill, M. Collins, L. Cornwall, J. Dow, P. Gates, S. Gillett, N. Johnson, S. Joseph, K. Kopelke, R. Lam, R. Levy, N. Lloyd, S. Logan, G. McPherson, M. Newth, C. Parsons, K. Powles, M. Rebis, T. Samakowidic, L. Sanders, S. Savickas, J. Shrimpton, H. Smith, L. Smith, J. Spehr, J. Summers, G. Taylor, M. Thackeray, B. Wilkinson. The Alfred (42): K. Richardson (MI), J. Frayne, E. Barber, L. Bode, A. Brakey, K. Chand, P. Christin, G. Crook, D. Delrosario-Kelly, R. Descallar, A. Deutsch, S. Easo, M. Farquhar, P. Fergus, J. Ford, E. Hamson, M. Hlaing, E. Hope, J. Lacivita, J. Laurenson, K. Lock, N. Ly, K. McKay, C. Mill, K. Moloney, L. Price, T. Terry, A. Tyers, S. Willems, R. Woolstencroft. Flinders Medical Centre (40): N. Crawshaw, J. Luker, C. Wood, S. Choat (MIs), C. Archer, D. Benham, M. Billinger, M. Bronca, S. Curchin, C. Dickie, M. Dixon, D. Douglass, M. Enomoto, K. Ernst, L. Fries, S. George, E. Green, L. Hamilton, Z. Harris, T. Heard, G. Hunt, N. Jamieson, M. Mackenzie, H. McKearney, B. Oermann, C. O'Reilly, T. Pearson, N. Reid, L. Rodda, D. Scutcheon, C. Simons, R. Smith, L. Tait, J. Troake, D. Usher. Western Hospital (37): L. Mackey, T. Wijeratne (MIs), C. Abela, S. Ashoka, C. Chen, T. Cheng, V. Chong, S. Cooke, A. Fok, L. Galang, C. Grant, S. Karageorge, K. Kat, L. Keo, B. Lee, A. Luscombe, J. Mackay, M. Minett, J. Mizen, P. Nim, N. Nunlist, V. Patel, M. Pathirage, A. Paton, M. Pombuena, N. Rathnayake, L. Rhodes, M. Sequeira, S. Smart, S. Somaratne, N. Sta Maria, L. Talbot, R. Tecle. Epworth Richmond (23): M. Shannon, R. Gerraty (MIs), S. Allen, R. Boyle, N. Fatchen, N. Hendley, A. Hyde, M. Inal, P. Kalubowilage, M. Laverde, K. Lawless, A. McFadyen, K. Peters, C. Pugh, C. Qin, J. Robertson, S. Smee, R. Tomlinson, V. Wang, F. Williams, D. Woolley, R. Yawieriin. St George Hospital (23): N. Austin, S. Pomfret, M. Tinsley (MIs), L. Allport, C. Ang, L. Armitage, E. Blundell, A. Courtney, M. Dela Costa, T. Devi Thapa, P. Diwakar, M. Dulleh, J. Francis, P. Cic, G. Gellie, C. Gill, D. James, S. Lee, T. Mai, K. Majcher, C. Mawson, G. Newton, N. Qiu, E. Ragonton, L. Roberts, H. Saitamis, L. Stanwell, L. Ting, P. Xu, L. Yin. Albury Hospital (23): V. Crosby (MI), K. Broadhead, J. Church, R. Collins, K. Everitt, M. Fisher, K. Hochmuth, N. Jones, A. Lieschke, E. McCarthy, C. McGlone, D. Morey, D. Neilson, S. Spry, M. Vile. Nambour General Hospital (20): R. Grimley, D. Rowley (MIs), I. Rosbergen, E. Ahern, L. Anderson, J. Boreham, R. Devin, R. Doolan, M. Dyke, L. Griffiths, K. Guest, D. Hecita, N. Kendal, J. Koltermann, M. Lacy, S. Lebeter, D. Lloyd, M. Matthews, C. McAuley, A. Pollock, M. Pyke, T. Rogers, S. Street, G. Styles, A. Tampiyappa, J. Trinder, T. Verral, K. Walker, C. White. Sir Charles Gairdner Hospital (15): T. Beckwith, L. Cormack (MIs), J. Arriagada, C. Babenschneider, D. Blacker, S. Bennett, S. Connor, J. Cowmeadow, N. Daniel, G. Edmonds, M. Faulkner, M. Garcia-Vega, K. Kruger, B. Martial, P. McGinley, H. Mountford, V. Riley, N. Smith, F. Stepan, S. Tilley, S. Whisson. Warrnambool Base Hospital (15): P. Groot (MI), J. Bailey, K. Ballinger, C. Bell, B. Camilleri, C. Charnley, D. Crabbe, S. Crossland, N. Edirimanna, C. Fitzgerald, C. Gibbins, J. Gibbs, K. Hirst, A. Kennedy, E. Klose, K. McDowall, S. Miller, R. Morgan, A. Noonan, M. North, M. Oliver, K. Richards, T. Russell, N. Scott, A. Shlanski, A. Traynor. West Gippsland Hospital (12): S. Smith (MI), R. Adams, C. Banks, K. Burke, S. Hewat, B. McKenna, M. McKimmie, L. Polmear, M. Traumanis, S. Whiteman. St Vincent's Hospital (12): V. Bramah, R. Errey, M. Halpin, V. Molan, D. Wheelwright, N. Wilson, W. Zhang (MIs), M. Bakshi, S. Bracher, M. Bryant, W. Byrnes, T. Denton, N. DeVries, P. Fay, P. Galbraith, T. Gallaher, O. Haidar, K. Holgate, K. Hozack, N. Jackson, S. Kipps, S. Lerner, R. Markus, R. Merheb, C. Naismith, G. Nolan, R. Odelli, K. Page, P. Sangvatanakul, T. Simpson, P. van Vliet, K. Walch, S. Walker, T. Yasue. Wyong Public Hospital (11): G. Auld (MI), R. Baker, K. Cousins, M. Fairbrother, K. Hutchinson, M. Maclean, E. Maher, D. Mills, S. Ohlback, J. Sturm, M. Tooth, J. Watkins. The Wesley Hospital (9): J. Cramb (MI), P. Atkinson, J. Conrad, D. Fichera, S. Follent, C. Gilbert, M. Herzig, S. Kohler, S. McCracken, L. Nunan, S. Roberts, J. Shelley, S. Varendorff, A. Wills. Calvary Mater Newcastle Hospital (8): A. Moore, A. Robertson (MIs), J. Britton, A. Burgess, T. Coates, J. Croft, E. Greening, J. Holland, P. O'Brien, R. Strong. Wodonga Hospital (8): L. Tighe (MI), S. Bilston, J. Black, K. De Rivera, I. Dwyer, S. Gissane, K. Heckenberg, S. Jackson, A. Maclagan, L. O'Hare, H. Patel, J. Pearce, C. Scanlan, K. Seymour, M. Symington, A. Tyers, A. Waite, K. Wiesner. Belmont Hospital (5): O. Katalinic, M. Spear (MIs), P. Brown, E. Difuntorum, S. Gilbert, J. Henderson, D. James, H. Janssen, E. Lane, S. Lowndes, D. Smith, S. Thompson, D. Weaver, S. Weston, S. Wright. Wollongong Hospital (4): S. Cox, C. Tse (MIs), J. Adrian, M. Doughty, J. Kok, R. McGrath, T. Morris, A. Pickup, E. Ray, R. Richardson, M. Sims, C. Thompson, K. Trinh, N. Walton, F. Whittaker. Gosford Hospital (2): P. Andersen (MI), J. Burrows, M. Dawson, D. Griffiths, G. Harris, P. Kavalieros, B. O'Brien, K. Roberts, J. Watkins, C. Whyatt.
New Zealand: Auckland City Hospital (189): A. McRae, G. Wavish (MIs), F. Anos, J. Armstrong, E. Au, A. Barber, C. Bates, M. Bertulfo, A. Boggs, F. Burgess, K. Cassels-Brown, M. Chiu, S. Dass, N. Duff, J. Farrell, W. Foster, D. Fuertez, C. Gadhvi, S. George, A. Green, L. Harvey-Fitzgerald, L. Hau, L. Hayward, D. Holman, K. Huggins, M. Jacobs, A. John, H. Kaur, T. Lagerstedt, J. Lee, R. Llenes, L. Lyons, S. Magandi, M. Martin, S. Mathew, T. Mathew, D. McKellar, E. Moss, K.L. Nand, K. Nicol, F. Peterson, A. Prasad, K. Quick, E. Revell, S. Roy, J. Ryan, N. Samadi, B. Scrivener, J. Slow, S. Tharakan, J. Torrens, E. van Bysterveldt, C. Villaluz, S. Yang.
Malaysia: UKM Medical Centre (123): M.A. Katijjahbe (MI), G. Ai Sing, A. Azlina, M.I. Azmi, M.H. Efri, A.Z. Fadilah, H. Fathuddeen, H. Haryani, H. Hussien, M. Izuani, Z.C. Man, C. Man Ying, K.M. Mashitoh, A. Noor Azah, M.I. Norlinah, I. Norliza, K.B. Ravinder, R. Rohaizah, K. Rosnita, A. Rozita, J. Safwan, R. Sahathevan, I. Shahrul, S.M. Sharifah, H.J. Tan, W.Y. Wan Nafisah, Y.L. Yee, M.A. Zaharah, A.S. Zunaidah.
Singapore: Singapore General Hospital (128): D. Tan, M.T. Ahmad, S. Hameed (MIs), M.F.B. Bakari, J. Britto, J.J. Chen, S. Choo, M. Faizal, F.K. Fong, S. Hong, J. Ja'afar, Z. Ke, G. Koh, C.K. Lee, Y.F. Lee, P. Lim, G.M. Lim, S.H. Ninhadi, G. Ong, T. Pei Pei, V. Penero, N. Rahim, P. Ratnagopal, K. Saleh, H.C. Seow, E. Sim, C.K. Tan, P.Y. Tay, I. Teo, S. Thilarajah, P.H.J. Wong, W.P. Wong, S. Yeap.
United Kingdom: Forth Valley Royal Hospital (65): M. Macleod (MI), A. Anderson, K. Armstrong, K. Baird, D. Balfour, M. Boyd, J. Cameron, C. Carswell, C. Clanachan, L. Cuthill, I. Devoy, S. Forsyth, J. Gavin, M. Hughes, E. Marr, S. McAuley, E. McCagherty, K. McCallum, N. McDonald, C. McGhee, T.A. McIntyre, L. Noonan, A. Smart, R. Walshe. Yeovil District Hospital (61): D. Neal (MI), J. Allison, G. Ball, S. Board, H. Brunt, C. Buckley, C. Carroll, D. Hayward, T. Hutchinson, E. Jones, E. Keeling, E. Marsh, N. Mead, H. Smith, C. Vickers, B. Williams-Yesson, D. Wood. York Hospital (54): J. Coyle, M. Keeling (MIs), L. Ackroyd, C. Brown, K. Donnan, N. Dyer, H. Green, G. Kilbride, C. Nicholson, M. Porteous. Royal Victoria Infirmary (35): S. Louw (MI), A. Annamalai, A. Barkat, S. Crawford, M. Fawcett, D. Harvey, V. Hogg, A. Hughes, J. Kemp, J. Morrison, K. Storey, T. Thompson. Aberdeen Royal Infirmary (33): J. Furnace, M.J. Macleod (MIs), J. Bell, K. Bennett, M. Bruce, R. Clarke, H. Cowie, H. Gow, J. Irvine, A. Joyson, S. MacDonald, A. Macvicar, N. Murphy, J. Robertson. Royal Bournemouth Hospital (32): C. Gordon, J. Kwan, L. Redpath, K. Saunders (MIs), J. Bell, R. Burrow, C. Clarke, C. Dickson, G. Hann, M. Heath, S. Heath, A. Hewett, R. Humphrey, B. Longland, A. Orpen, C. Ovington, J. Page, E. Rogers, K. Toombs. Imperial College Healthcare, St Mary's Hospital (29): R. Howes, A. Lacey, P. Meakin (MIs), D. Ames, S. Banerjee, E. Beranova, S. Berry, M.J. Burke, V. Cassama, K. Collins, J. Crow, A. Dunne, C. Gomez, A. Hawkins, K. Hellier, S.A. Howard, A. Kar, E. Lambert, H. Lee, C. Mandri, J. Moye, E. Murtagh, J. Pushpa-Rajah, J. Richardson, T. Sachs, J. Stilwell, V. Tilley, P. Wilding, N. Wilson. Wishaw General Hospital (28): E. Feely, S. Kirk (MIs), P. Cassidy, A. Chalmers, C. Duguid, N. Hughes, J. Hutton, K. Lapsley, J. Lee, A. Murray, L. Weir, M. Whitelaw. Monklands Hospital (26): M. Barber, D. Esson (MIs), H. Armit, C. Devlin, R. Duncan, C. Forman, K. Frame, L. Hogg, P. McLeod, R. McWhinney, J. Porter, M. Purves, L. Snowball. Ulster Hospital (25): B. Wroath (MI), L. Ferson, M. Gibson, S. Gillespie, N. Ignatius, T. Kane, J. Kwant, M. Matthews, C. McCallion, C. McConville, M. McDowell, C. McNally, L. Moore, P. Murphy, A. Nesbitt, J. Newell, M. Power, E. Reid, K. Robinson. Royal Devon and Exeter Hospital (23): C. Charnley, M. James (MIs), S. Bacon, N. Booth, A. Bowring, L. Boxall, J. Burt, J. Cageao, N. Green, K. Gupwell, S. Keenan, H. Kingwell, M. Kryszkowska, J. Mortimore, B. Peace, C. Roughan. Queen Elizabeth The Queen Mother Hospital (21): G. Gunathilagan, J. Sampson, G. Thomas (MIs), T. Allen, G. Dane, K. Harris, S. Hart, S.A. Jones, M. Reader. Antrim Area Hospital (18): P. Browne, C. McGoldrick, D. Mullan (MIs), P. Adair, J. Armstrong, E. Beggs, I. Bell, C. Edwards, L. Gilligan, C. Kelly, M. Kennedy, J. Kurian, L. Leal, A. McAtamney, E. McKay, E. Rogan, M. Smyth, E. Wiseman, J. Vahidassr. Wansbeck General Hospital (18): C. Price, V. Riddell (MIs), E. Bendix, K. Craig, R. Davison, A. Harrison, A. Smith. Blackpool Hospital (17): V. Green (MI), K. Ashton, W. Barkhuizen, A. Daniel, C. Dickinson, H. Durdu, D. Eastwood, H. Goddard, R. Hodkin, J. Howard, C. Jeffs, S. Joyce, C. Kelly, G. Kerr, J. Lanes, B. Magnall, M. McMahon, M. Moody, S. Patton, R. Taylor, A. Watson. North Tyneside General Hospital (17): K. Mitchelson, L. Mokoena (MIs), L. Aird, R. Lakey, J. Murdy, K. Nelson, G. Storey. Belfast City Hospital (15): R. McGeown, S. Tauro (MIs), R. Brady, D. Holland, M. Kinnaird, L. Maltman, D. Martin, K. McCord, S. McKenna, C. Morgan, C. Shannon, A. Steele, I. Wiggam. Harrogate District Hospital (15): S. Appleby, S. Brotheridge, M. Prescott (MIs), P. Bagot, D. Baston, C. Bennett, J. Featherstone, C. Hare, A. McCluskey, S. Wade, R. Worton. St Mary's Hospital, Isle of Wight (13): E. Hakim, J. Herman, T. Norman (MIs), L. Beale, E. Buckley, K. Byrne, M. Gasior, B. Robles, C. Smallwood, S. Stevens, M. Thomas, V. Williams. Nevill Hall Hospital (12): K. Buck (MI), S. Armstrong, V. Brice, A. Edwards, S. Gething, A. Griffiths, T. Hills, D. Howells, S. Langdon, S. Moseley, G. Powell, G. Reynolds, B. Richard, E. Scott, R. White, J. Zebedee. Western Infirmary (10): M. Walters (MI), J. Alexander, L. Brand, E. Colquhoun, A. Hill, D. Macartney, H. MacDonald, B. Manak, H. Morgan, C. Ritchie. South Tyneside District Hospital (8): H. Hunter (MI), T. Blair, M. Duffy, J. Graham, J. Scott, T. Vu, P. Yorston. Calderdale Royal Hospital (7): A. Nair (MI), I. Shakir, C. Button, M. Friend, J. Greig, B. Hairsine, S. Wade, S. Williamson. St George's Hospital (7): G. Cloud (MI), T. Adedoyin, N. Dayal, S. Gawned, R. Ghatala, N. Jeyaraj, L. Kerin, L. Montague, C. Orefo, J. O'Reilly, J. Styles, S. Trippier, C. Watchurst, F. Watson. North Devon District Hospital (6): J. Hunt, R. Latif (MIs), C. Barrett, J. Cox, F. Hammonds, K. Quick, K. Robinson, A. Skinner, C. Vernon. Royal Infirmary of Edinburgh (6): S. Burgess, T. Elder-Gracie (MIs), C. Browne, W. Cameron, V. Coleman, C. Fulgencio, L. Gibson, P. Halliday, D. Heaney, L. Main, K. McGavin, G. Mead, F. Proudfoot, A. Redpath, C. Rodger, S. Scott. Hexham General Hospital (5): K. Robinson. University Hospital Crosshouse (3): K. Mason (MI), L. Baxter, A. Bryce, M. Halkett, J. Halliday, A. McAllister, M. McGuiness, M. Munro, A. Robb, A. Thompson, B. Tougher, J. Weadon, J. Young. Daisy Hill Hospital (1): C. Douglas (MI), M. McParland, S. Boyle, B. Byrne, L. Comiskey, J. Gilpin, S. Gilpin, A. Harris, S. Harshaw, J. Haughey, F. McArdle, L. McConnell, E. McEneaney, M. Millar, M. Murphy, J. Tilley.
References
- 1.Hackett ML, Duncan JR, Anderson CS, Broad JB, Bonita R. Health-related quality of life among long-term survivors of stroke: results from the Auckland Stroke Study, 1991-1992. Stroke 2000;31:440–447. [DOI] [PubMed] [Google Scholar]
- 2.Lau AL, McKenna K, Chan CC, Cummins RA. Defining quality of life for Chinese elderly stroke survivors. Disabil Rehabil 2003;25:699–711. [DOI] [PubMed] [Google Scholar]
- 3.Indredavik B, Bakke F, Slordahl SA, Rokseth R, Haheim LL. Stroke unit treatment improves long-term quality of life: a randomized controlled trial. Stroke 1998;29:895–899. [DOI] [PubMed] [Google Scholar]
- 4.Indredavik B, Bakke F, Slordahl SA, Rokseth R, Haheim LL. Treatment in a combined acute and rehabilitation stroke unit: which aspects are most important? Stroke 1999;30:917–923. [DOI] [PubMed] [Google Scholar]
- 5.Tyedin K, Cumming TB, Bernhardt J. Quality of life: an important outcome measure in a trial of very early mobilisation after stroke. Disabil Rehabil 2010;32:875–884. [DOI] [PubMed] [Google Scholar]
- 6.Musicco M, Emberti L, Nappi G, Caltagirone C. Early and long-term outcome of rehabilitation in stroke patients: the role of patient characteristics, time of initiation, and duration of interventions. Arch Phys Med Rehabil 2003;84:551–558. [DOI] [PubMed] [Google Scholar]
- 7.Ahlsio B, Britton M, Murray V, Theorell T. Disablement and quality of life after stroke. Stroke 1984;15:886–890. [DOI] [PubMed] [Google Scholar]
- 8.Jönsson AC, Lindgren I, Hallström B, Norrving B, Lindgren A. Determinants of quality of life in stroke survivors and their informal caregivers. Stroke 2005;36:803–808. [DOI] [PubMed] [Google Scholar]
- 9.Cumming TB, Brodtmann A, Darby D, Bernhardt J. The importance of cognition to quality of life after stroke. J Psychosom Res 2014;77:374–379. [DOI] [PubMed] [Google Scholar]
- 10.Patel MD, McKevitt C, Lawrence E, Rudd AG, Wolfe CD. Clinical determinants of long-term quality of life after stroke. Age Ageing 2007;36:316–322. [DOI] [PubMed] [Google Scholar]
- 11.Naess H, Waje-Andreassen U, Thomassen L, Nyland H, Myhr KM. Health-related quality of life among young adults with ischemic stroke on long-term follow-up. Stroke 2006;37:1232–1236. [DOI] [PubMed] [Google Scholar]
- 12.Clarke P, Marshall V, Black SE, Colantonio A. Well-being after stroke in Canadian seniors: findings from the Canadian Study of Health and Aging. Stroke 2002;33:1016–1021. [DOI] [PubMed] [Google Scholar]
- 13.Röding J, Glader EL, Malm J, Lindström B. Life satisfaction in younger individuals after stroke: different predisposing factors among men and women. J Rehabil Med 2010;42:155–161. [DOI] [PubMed] [Google Scholar]
- 14.Palmcrantz S, Holmqvist LW, Sommerfeld DK. Long-term health states relevant to young persons with stroke living in the community in southern Stockholm: a study of self-rated disability and predicting factors. Disabil Rehabil 2012;34:817–823. [DOI] [PubMed] [Google Scholar]
- 15.Bernhardt J, Langhorne P, Lindley R, et al. Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): a randomised controlled trial. Lancet 2015;386:46–55. [DOI] [PubMed] [Google Scholar]
- 16.Bonita R, Beaglehole R. Recovery of motor function after stroke. Stroke 1988;19:1497–1500. [DOI] [PubMed] [Google Scholar]
- 17.Bernhardt J, Churilov L, Dewey H, et al. Statistical analysis plan (SAP) for A Very Early Rehabilitation Trial (AVERT): an international trial to determine the efficacy and safety of commencing out of bed standing and walking training (very early mobilization) within 24 h of stroke onset vs. usual stroke unit care. Int J Stroke 2015;10:23–24. [DOI] [PubMed] [Google Scholar]
- 18.Hawthorne G, Richardson J, Osborne R. The Assessment of Quality of Life (AQoL) instrument: a psychometric measure of health-related quality of life. Qual Life Res 1999;8:209–224. [DOI] [PubMed] [Google Scholar]
- 19.Brott T, Adams HP, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864–870. [DOI] [PubMed] [Google Scholar]
- 20.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. A prospective study of acute cerebrovascular disease in the community: the Oxfordshire Community Stroke Project: 1981-86: 2: incidence, case fatality rates and overall outcome at one year of cerebral infarction, primary intracerebral and subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 1990;53:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snaith RP, Constantopoulos AA, Jardine MY, McGuffin P. A clinical scale for the self-assessment of irritability. Br J Psychiatry 1978;132:164–171. [DOI] [PubMed] [Google Scholar]
- 22.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 23.Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J 1965;14:61–65. [PubMed] [Google Scholar]
- 24.Hawthorne G, Korn S, Richardson J. Population norms for the AQoL derived from the 2007 Australian National Survey of Mental Health and Wellbeing. Aust NZ J Public Health 2013;37:7–16. [DOI] [PubMed] [Google Scholar]
- 25.Ali M, Fulton R, Quinn T, Brady M. How well do standard stroke outcome measures reflect quality of life? A retrospective analysis of clinical trial data. Stroke 2013;44:3161–3165. [DOI] [PubMed] [Google Scholar]
- 26.Rangaraju S, Haussen D, Nogueira RG, Nahab F, Frankel M. Comparison of 3-month stroke disability and quality of life across modified Rankin Scale categories. Interv Neurol 2017;6:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaisinanunkul N, Adeoye O, Lewis RJ, et al. Adopting a patient-centered approach to primary outcome analysis of acute stroke trials using a utility-weighted modified Rankin Scale. Stroke 2015;46:2238–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dijkland SA, Voormolen DC, Venema E, et al. Utility-weighted modified Rankin Scale as primary outcome in stroke trials: a simulation study. Stroke 2018;49:965–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sturm JW, Donnan GA, Dewey HM, et al. Quality of life after stroke: the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke 2004;35:2340–2345. [DOI] [PubMed] [Google Scholar]
- 30.Hawthorne G, Osborne R. Population norms and meaningful differences for the Assessment of Quality of Life (AQoL) measure. Aust NZ J Public Health 2005;29:136–142. [DOI] [PubMed] [Google Scholar]
- 31.Chang WH, Sohn MK, Lee J, et al. Return to work after stroke: the KOSCO Study. J Rehabil Med 2016;48:273–279. [DOI] [PubMed] [Google Scholar]
- 32.de Haan RJ, Limburg M, Van der Meulen JH, Jacobs HM, Aaronson NK. Quality of life after stroke: impact of stroke type and lesion location. Stroke 1995;26:402–408. [DOI] [PubMed] [Google Scholar]
- 33.Janssen MF, Szende A, Cabases J, Ramos-Goñi JM, Vilagut G, König HH. Population norms for the EQ-5D-3L: a cross-country analysis of population surveys for 20 countries. Eur J Health Econ 2019;20:205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ross CE, Van Willigen M. Education and the subjective quality of life. J Health Soc Behav 1997;38:275–297. [PubMed] [Google Scholar]
- 35.van Eeden M, van Heugten C, van Mastrigt GAPG, van Mierlo M, Visser-Meily JMA, Evers SMAA. The burden of stroke in the Netherlands: estimating quality of life and costs for 1 year poststroke. BMJ Open 2015;5:e008220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oczkowski C, O'Donnell M. Reliability of proxy respondents for patients with stroke: a systematic review. J Stroke Cerebrovasc Dis 2010;19:410–416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.