Abstract
Rationale:
Preclinical testing of cardiotoxicity and efficacy of novel heart failure therapies faces a major limitation: the lack of an in situ culture system that emulates the complexity of human heart tissue and maintains viability and functionality for a prolonged time.
Objective:
To develop a reliable, easily reproducible, medium-throughput method to culture pig and human heart slices under physiological conditions for a prolonged period of time.
Methods and Results:
Here, we describe a novel, medium-throughput biomimetic culture system that maintains viability and functionality of human and pig heart slices (300 μm thickness) for 6 days in culture. We optimized the medium and culture conditions with continuous electrical stimulation at 1.2 Hz and oxygenation of the medium. Functional viability of these slices over 6 days was confirmed by assessing their calcium homeostasis, twitch force generation, and response to β-adrenergic stimulation. Temporal transcriptome analysis using RNAseq at day 2, 6, and 10 in culture confirmed overall maintenance of normal gene expression for up to 6 days, while over 500 transcripts were differentially regulated after 10 days. Electron microscopy demonstrated intact mitochondria and Z-disc ultra-structures after 6 days in culture under our optimized conditions. This biomimetic culture system was successful in keeping human heart slices completely viable and functionally and structurally intact for 6 days in culture. We also used this system to demonstrate the effects of a novel gene therapy approach in human heart slices. Furthermore, this culture system enabled the assessment of contraction and relaxation kinetics on isolated single myofibrils from heart slices after culture.
Conclusions:
We have developed and optimized a reliable medium-throughput culture system for pig and human heart slices as a platform for testing the efficacy of novel heart failure therapeutics and reliable testing of cardiotoxicity in a 3D heart model.
Subject Terms: Basic Science Research, Cellular Reprogramming, Contractile Function, Gene Therapy, Metabolism, Heart slice, gene therapy, cardiac contractility and energetics, cardiotoxicity, heart failure, calcium
Graphical Abstract:
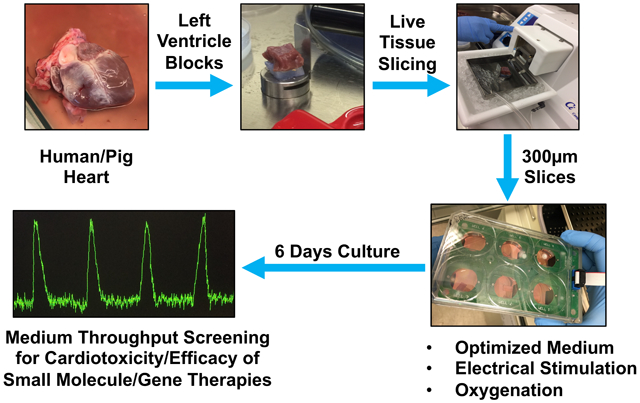
INTRODUCTION
Heart failure is the number one killer1 and drug induced cardiotoxicity is a major cause of market withdrawal2. The lack of availability of culture systems for human heart tissue that is functionally and structurally viable for more than 24 hours limits validation of novel heart failure therapies and reliable cardiotoxicity testing. Therefore, there is an urgent need to develop a reliable system for culturing human heart tissue for testing drug efficacy and toxicity. The recent move toward the use of human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) in cardiotoxicity and drug efficacy testing has provided a partial solution to address this issue; however, the immature nature of the hiPSC-CMs and loss of tissue integrity compared to multicellular heart tissue are major limitations of this technology3. A recent study has shown that this limitation can be partially overcome if cardiac tissues are formed on hydrogels from early-stage hiPSC-CMs soon after the initiation of spontaneous contractions and are subjected to gradual increase in electrical stimulation over time4. However, the electromechanical properties did not achieve the maturity seen in adult human myocardium. Moreover, heart tissue is structurally more complicated, being composed of a heterogeneous mixture of various cell types including endothelial cells and various types of stromal fibroblasts linked together with a unique mixture of extracellular matrix proteins5. This heterogeneity of the non-cardiomyocyte cell population6-8 in the adult mammalian heart is a major obstacle in modeling heart tissue using individual cell types. These major limitations highlight the importance of developing methods to enable culture of intact cardiac tissue for optimal studies involving physiological and pathological conditions4.
Culturing human heart slices is a promising model of intact human myocardium. This technology provides access to complete 3D multicellular system that is similar to the human heart tissue that reflects the human myocardium in physiological or pathological conditions, both functionally and structurally. The first culture system using an air-liquid interface was developed by9. Using, transwell system, and simplified medium (M199/ITS) for culturing heart slices. Even though the electrophysiological properties of the heart slices were maintained for 28 days, the heart slices lost over 90% of their ability to contract within 24 hours in culture9. This culture system has been used by others to culture heart slices for up to 24 hours10-12. Three recent studies have shown that electromechanical stimulation is paramount for maintaining heart slices in culture13-15. These studies represent a significant advancement in slice technology and present incontrovertible evidence that mechanical load is essential. However, these studies involved highly sophisticated bioengineered devices, which may limit adoption of the technology by other laboratories. In addition, these studies used the standard simplified medium (M199/ITS), which does not support the high metabolic demands of cardiac tissue. Therefore, more work is needed to optimize culture conditions; to simplify the method to promote accessibility; and to test applications, such as gene therapy, and isolated myofibril contractility. Here, we developed a medium-throughput, easily reproducible, heart slice culture system which does not compromise the heart slice functionality for several days. Therefore, using a commercially available apparatus (C-PACE), which is able to accommodate eight 6-well plates at once, we emulated the adult cardiac milieu by inducing electromechanical stimulation at the physiological frequency (1.2 Hz), and screened for the fundamental medium components to prolong the duration of functional human heart slices in culture. Because pig and human hearts are similar in size and anatomy16, we developed a biomimetic heart slice culture system using pig hearts and subsequently validated it in human heart slices.
METHODS
All RNAseq data and materials have been made publicly available at the NCBI GEO (GSE133723) and can be accessed at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE133723”
Heart tissue collection from pigs and humans.
To collect hearts from the 15 pigs used in the current study, all animal procedures were in accordance with the institutional guidelines of Tenaya Therapeutics and the University of Louisville and approved Institutional Animal Care and Use Committee at both places. Fresh human hearts were provided from consented donors through the Maryland Legacy Foundation (Novabiosis) and all procedures were approved by the Institutional Review Board of the University of Louisville. In this study, we used 3 de-identified human hearts from donors aged 36, 42, and 45 years old with no cardiovascular disease history. As described before in 11, 15, pig or human hearts were clamped at the aortic arch and perfused with 1 L sterile cardioplegia solution (110 mM NaCl, 1.2 mM CaCl2, 16 mM KCl, 16 mM MgCl2, 10 mM NaHCO3, 5 units/ml heparin, pH to 7.4), then the hearts were preserved on ice-cold cardioplegic solution until transported to the lab on wet ice within 12 hours.
Please see online supplemental methods section for more details regarding slicing and culture procedures for the heart slices, calcium transient assessment, contractile force assessment, MTT assay, immunofluorescence, electron microscopy, RNAseq, metabolic flux assessment, glucose utilization assay, and single myofibril isolation and contractile mechanics assessment.
Statistical analyses.
For all assays, power analyses were performed to choose the group sizes which will provide >80% power to detect a 10% absolute change in the parameter with a 5% Type I error rate. These power analyses indicated a minimum of 4 experimental replicates per group, therefore, we used a range of 5 – 15 experimental replicates per group for each assay. Special statistical consideration for RNAseq was detailed under the online supplemental methods section. Then, Kolmogorov-Smirnov (K-S) test for normality was conducted; all data sets showed normal distribution. Then, differences between 2 groups were examined for statistical significance with unpaired Student t tests. However, to compare data consisting of more than 2 groups, we performed one- or Two- way ANOVA tests followed by Bonferroni post hoc multiple comparisons to get the corrected p-value. A value of P<0.05 was regarded as significant. Error bars indicate SEM. The person who performed the analysis was blinded to the experimental groups.
RESULTS
Development and optimization of a biomimetic culture system for porcine heart slices.
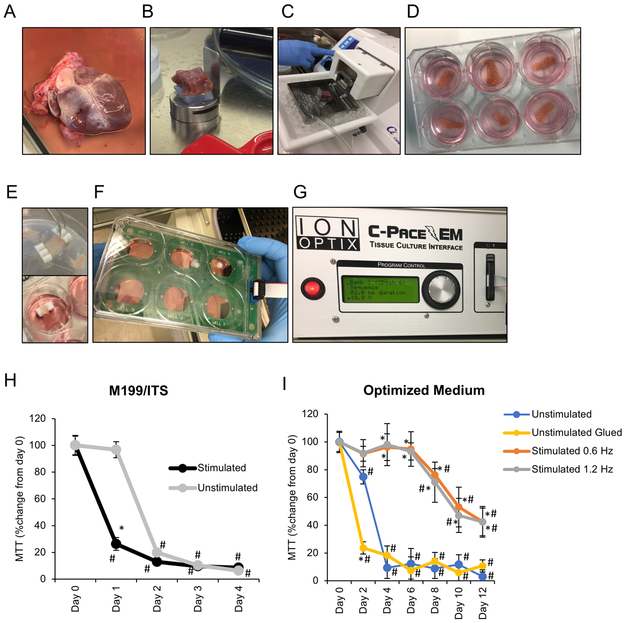
Previous reports have shown that culture systems using an air-liquid interface and M199/ITS media (Figure 1A-D) did not maintain heart slice functionality for more than 24 hours9-12. Therefore, we tested the effect of mimicking the biological conditions in the heart. First, similar to recent studies13-15 we provided continuous electrical stimulation to pig heart slices at the physiological rate (1.2 Hz) (Figure 1E-G). To provide adequate oxygen supply to the slices, the medium was changed every 8 hours with oxygenated medium. It has recently been shown that applying a physiological mechanical load is essential for the maintenance of cardiac structural, functional and transcriptional properties 15. To provide minimal mechanical load that does not require the complex bioengineered devices, and to enable heart slices to freely contract, we glued the slices to small, light-weight polyurethane supports using a Histoacryl Blue topical skin adhesive (Figure 1E). Given that cardiomyocytes are metabolically active, we used the MTT assay to quantify the activity of the mitochondrial NAD(P)H-dependent cellular oxidoreductase enzymes, as a measure of metabolic activity to assess heart slice viability. First, we optimized the MTT assay for the tissue slices to avoid signal saturation (Online Figure I). Using the MTT assay, we found that electrical stimulation alone did not extend the viability of the heart slices (Figure 1H), but instead decreased viability when applied to slices cultured in the media formulation commonly used for heart slices Medium 199, ITS, and antibiotics10-12, 14. We reasoned that medium optimization may be required to support the metabolic needs of active cardiac tissue. To address this potential need, we tested the addition of several components known to support the culture of human iPSC-CMs and induced cardiomyocytes derived from direct cardiac reprogramming17-19. These components were; FBS, FGF/VEGF, fatty acids (oleic and palmitic acids), and the basal medium, RPMI/B2718. We first implemented these different components and their combinations in the unstimulated transwell culture system and assessed how long each combination could support the viability of porcine heart slices. In this condition, the optimal formulation contained M199 as basal medium, 1x ITS, 10% FBS, and FGF/VEGF which showed significantly higher viability than any other media formulation at day 2 and 3 (Online Figure II A and B). Next, we tested this new medium formulation in combination with the continuous electrical stimulation at different frequencies (0.6 Hz and 1.2 Hz). The new medium formulation, in conjunction with the continuous electrical stimulation at either frequency, maintained viable heart slices until day 6, after which viability declined progressively as assessed by MTT assay (Figure 1I). Because 6 days of viable heart slice culture is adequate to support testing of efficacy or acute toxicity of most therapeutic agents, we considered this to be our baseline protocol and refer to this heart slice culture technique as the biomimetic stimulated culture system.
Figure 1. Optimization of pig heart slice biomimetic culture conditions.
Once a full pig heart is obtained (A), the left ventricle is dissected into 1-2 cm3 cubes and placed on a holder with the epicardium facing down so the cutting occurs in alignment with the cardiac myofibril orientation (B). (C) Setup for slicing 300 μm thick heart slices in ice-cold bath using a vibrating microtome Model 7000smz-2 from Campden Instruments Ltd. (D) Example of the transwell culture setup using air-liquid interface. (E) Example of the heart slice trimmed and glued to light polyurethane supports and submerged in the medium in a 6-well plate, which is covered by the C-Dish cover containing graphite electrodes (F) and connected to the C-Pace EM stimulator from IonOptix Ltd (G). Quantification of heart slice viability overtime using MTT assay in either stimulated or unstimulated culture in standard medium (M199/ITS) (H) or optimized medium (I) (n=5 pig hearts each in triplicate, Two-Way ANOVA test was conducted to compare between groups; *p<0.05 compared to the unstimulated culture at the same time point, and #p<0.05 compared to fresh heart slices at day 0). Unstimulated: transwell culture, Unstimulated Glued: transwell culture with slices glued to the transwell.
Functional assessment of pig heart slices.
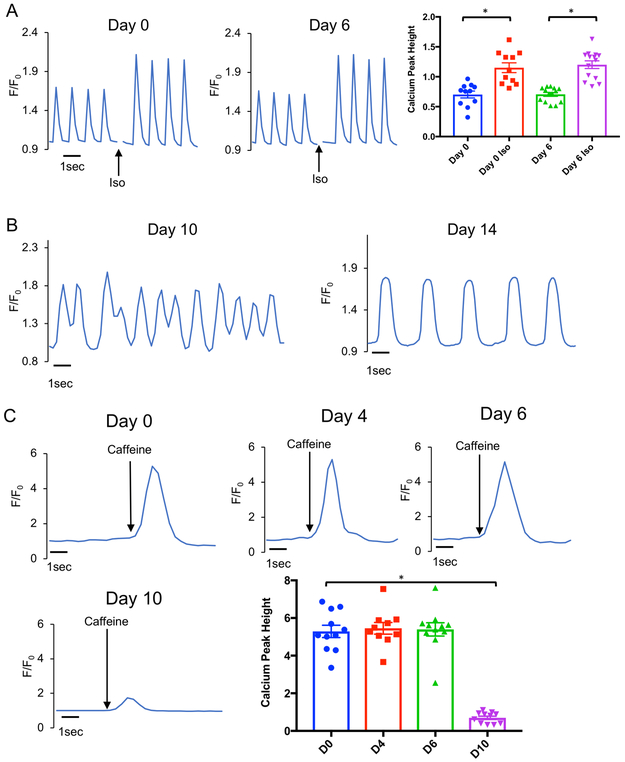
The major functional characteristic of heart tissue is contraction, which is preceded by induction of calcium transients upon electrical stimulation. To test these functions in heart slices cultured in our biomimetic stimulated culture system, we first assessed the induction of calcium transients over time. We observed that, within the first 6 days of culture in the biomimetic stimulated culture system, the slices perform similarly to fresh heart slices in that they did not exhibit any spontaneous calcium transients except upon electrical stimulation (Online Video I). In addition, on day 6 the heart slices responded to isoproterenol stimulation in a manner similar to fresh heart slices (Figure 2A). Due to the slight beat-to-beat variation in calcium amplitude within the cell calcium recording, we performed the analysis on the average of 10 consecutive beats from each cell. Exemplary traces and quantification are shown (Online Figure III A, B). However, by day 7, some cardiomyocytes showed spontaneous calcium transients (Online Video II). Nevertheless, the rest of the tissue still responded to electrical stimulation. This phenomenon of spontaneous calcium transients propagates quickly and included the entire heart slice on days 10 and 14 (Online Videos III & IV, Figure 2B). The timing of this change aligns with the MTT viability data and reflects a variation in functional characteristics of the heart slice at this time point. Next, we assessed the calcium content of the sarcoplasmic reticulum (SR) following caffeine stimulation to release SR calcium stores. The SR calcium reserves during the first 6 days of culture were similar to those of fresh heart slices. However, there was a significant reduction in SR calcium content on day 10 in culture (Figure 2C).
Figure 2. Calcium homeostasis in heart slices remains stable for 6 days in the biomimetic stimulated culture system.
(A) Representative calcium signal trace from a fresh heart slice (Day 0) and after 6 days in culture (Day 6) with and without 1 μM isoproterenol (Iso) stimulation. Transients were recorded after loading the heart slices with Fluo-4 calcium dye and using 1 Hz/20 V electrical stimulation at the time of recording. Quantification of calcium signal amplitude with and without stimulation with isoproterenol shows a similar patterns of calcium transients at day 0 and day 6 in culture (n= 4 pig hearts, 10-15 cells analyzed in each group, Two-Way ANOVA test was conducted to compare between groups; *p<0.05 comparing baseline to Iso stimulation at the same time point, p-value D0 vs D6=0.95, and p-value D0 Iso vs D6 Iso=0.93). (B) Examples of irregular calcium transients from heart slices in culture for 10 (left) or 14 (right) days with no synchronization with electrical stimulation. (C) Representative traces and quantification of calcium amplitude following caffeine stimulation of heart slices as an indicator of the SR calcium contents in fresh heart slices (Day 0), 4, 6, and 10 days after culture (n=3 pig hearts each, 10-15 cells were analyzed from each group, One-Way ANOVA test was conducted to compare between groups; *p<0.05 compared to D0, p-value D0 vs D4=0.97, and p-value D0 vs D6=0.95).
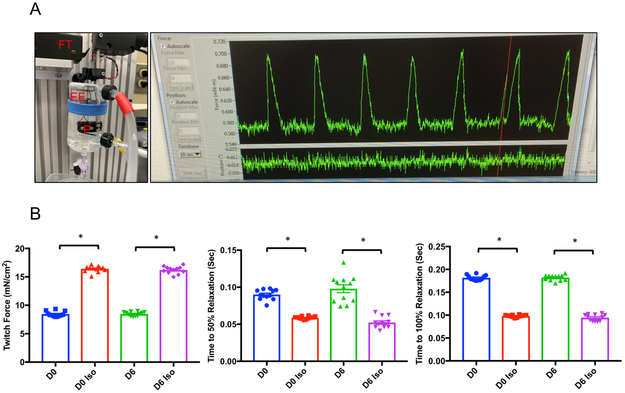
Finally, we assessed the ability of the heart slices to generate contractile force before and after culture using an Aurora Scientific Isolated Muscle System (Figure 3A). Until 6 days in the biomimetic stimulated culture system, the heart slices produced contractile force similar to that of fresh slices and showed normal inotropic response to isoproterenol treatment (Figure 3B). By day 10, contractile force was no longer detected in any heart slice (data not shown).
Figure 3. Contractile force generated by heart slices after 6 days in culture is comparable to fresh heart slices.
(A) Contractile force assessment setup; the heart slice is hung between a force transducer (FT) and a stable post (P) between two electric electrodes (EE) for electrical stimulation. Upon stimulation, the slice contracts and the contraction is recorded using the designated software. (B) Bar graphs show the quantification of contractile force parameters in the presence or absence of isoproterenol (Iso) generated by fresh heart slices (Day 0) and after 6 days in culture (n=4 pig hearts each in triplicate, Two-Way ANOVA test was conducted to compare between groups; *p<0.05 comparing baseline to Iso stimulation at the same time point, twitch force: p-value D0 vs D6=0.96, p-value D0 Iso vs D6 Iso=0.81; Time to 50% relaxation: p-value D0 vs D6=0.47, p-value D0 Iso vs D6 Iso=0.43; time to 100% relaxation: p-value D0 vs D6=0.91, p-value D0 Iso vs D6 Iso=0.36).
Transcriptome and metabolic profiling of pig heart slices under optimized conditions.
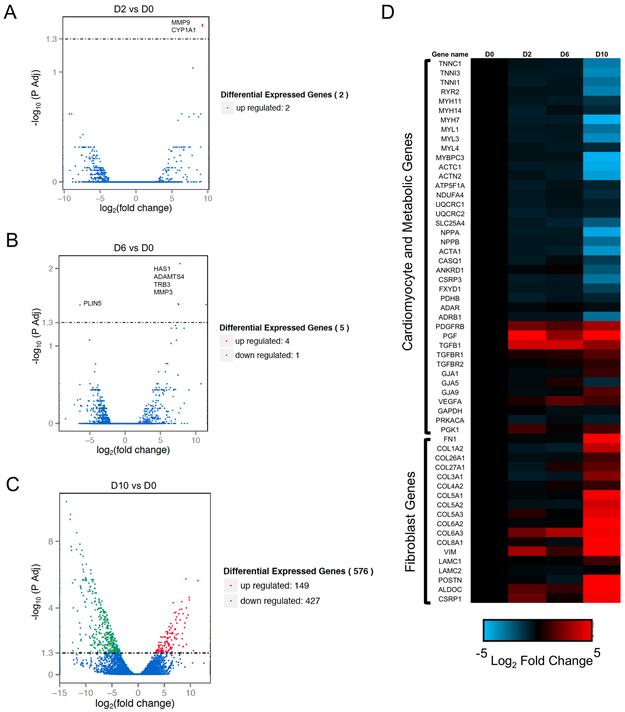
To assess changes in gene expression during culture, we performed a full transcriptome analysis of the heart slices before culture and compared these data to those of slices cultured in the biomimetic stimulated culture system for 2, 6, and 10 days. Only 2 and 5 transcripts were significantly differentially expressed after 2 and 6 days, respectively (Figure 4 A&B). However, after 10 days in culture, there were significant changes in the expression of over 500 transcripts (Figure 4C). The gene ontology (GO) terms of the genes downregulated after 10 days in culture were related to cardiac muscle, while the GO terms representing the upregulated genes focused on fibroblasts, extracellular matrix, and inflammation. These GO terms are indicative of the deterioration of myocyte health by day 10 in culture and the overrepresentation of fibroblasts and inflammatory cells (Online Figure IV). Furthermore, a heatmap of the expression of cardiac and fibroblast genes shows that downregulation of cardiac gene expression and upregulation of fibroblast gene expression are evident at day 10, but not before (Figure 4D). A few exceptional genes were upregulated as early as day 2 (e.g. PDGFRB, PGF, TGFB1), which is likely a response to the presence of FGF and VEGF in the culture medium.
Figure 4. Temporal transcriptome analysis of heart slices shows no significant changes in gene expression for up to day 6 in culture.
Volcano plots show significant changes in gene expression in heart slices. Fresh heart slices (D0) are compared to 2 days (A), 6 days (B) and 10 days (C) in our stimulated biomimetic culture. (D) Heat map showing log2 fold change in gene expression of representative cardiac, metabolic and fibroblast genes on days 2, 6, and 10 in culture compared to fresh heart slices (n=3 biological replicates from 3 different pig hearts).
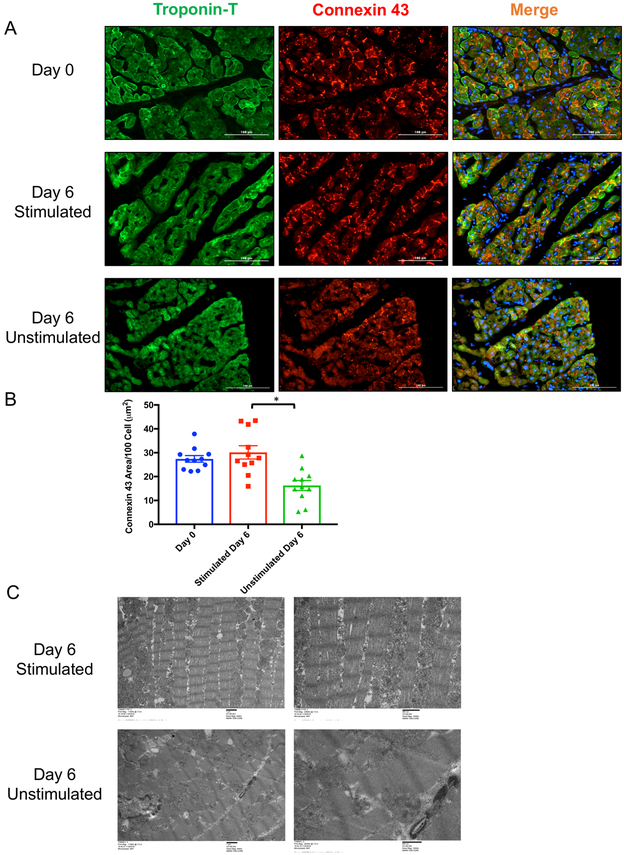
Connexin 43 is the major conductance protein in the heart localized at gap junctions between cardiomyocytes to propagate the electrical signal through cardiac tissue. We found that over the first 6 days of culture in the biomimetic stimulated culture system, connexin 43 expression remained intact at the gap junctions and is similar to that in fresh heart slices. However, unstimulated transwell culture for 6 days resulted in distortion of the connexin 43 expression at the gap junction (Figure 5A and B). Furthermore, electron microscopy showed that mitochondrial and Z-disc alignment and structure remained intact for 6 days in the biomimetic stimulated culture system but was completely distorted in unstimulated cultures (Figure 5C). Furthermore, the biomimetic stimulated culture was suitable for culturing pig heart slices from the border zone of infarcted hearts. The slice viability and connexin 43 expression was maintained for 6 days, just as we observed with slices from the healthy myocardium (Online Figure V). The one early change noticed in our biomimetic stimulated culture system was a shift towards energetic reliance on glycolysis. As early as day 2 in culture, the heart slices utilized significantly less oxygen, yet maintained glycolytic activity. By day 8 however, oxygen utilization was nearly undetectable and glucose catabolism was significantly diminished (Online Figure VI). These interesting findings raised the question of how each component of the medium contributes to the metabolic state of the heart slice and whether the addition of fatty acids would improve the metabolic health of the myocardial slice. Therefore, we tested 4 different media formulations; M1: M199/ITS, M2: M199/ITS/FBS, M3: M199/ITS/FBS/VEGF/FGF, and M4: M199/ITS/FBS/VEGF/FGF/Fatty acids (Online Figure VII A). Only heart slices cultured with M3 maintained full viability over 6 days and the addition of fatty acids deteriorated viability as assessed by MTT assay (Online Figure VII B). Interestingly, we found that the addition of fatty acids to the culture medium significantly reduced connexin 43 expression (Online Figure VII C), likely due to their mild solubilization effects on membrane proteins 20. By assessing the metabolic flux in these heart slices over time, we found that M3 was the only medium that maintained the glycolytic capacity of the slices up to day 6 (Online Figure VIII A-C), which is likely to be the reason underlying their more prolonged viability. Collectively, these data suggest that VEGF and FGF in heart slice medium maintains glycolytic capacity and that the addition of fatty acids does not improve heart slice function or viability.
Figure 5. Biomimetic stimulated culture maintains the connexin 43 and myofilament ultrastructure for 6 days compared to unstimulated culture.
(A) Representative immunofluorescence images showing expression of connexin 43 (red) in cardiomyocytes (green) in cross sections from either fresh heart slices (top panel), slices cultured for 6 days under biomimetic stimulated culture conditions (middle panel), or slices cultured for 6 days under unstimulated transwell conditions (bottom panel), (scale bar, 100 μm). (B) Quantification of the area occupied by connexin 43 in fresh heart slices compared to heart slices cultured for 6 days either under biomimetic stimulated culture or unstimulated culture (n=4 pigs, 3 replicates of each, One-Way ANOVA test was conducted to compare between groups; *p<0.05 compared to stimulated day 6 group, p-value day 0 vs stimulated day 6=0.65). (C) Electron microscopy images from stimulated culture (top panel) and unstimulated culture (bottom panel) for 6 days, show that the mitochondrial and Z-disc alignment and structure in biomimetic stimulated culture are not evident in unstimulated culture.
Human heart slices perform similarly to porcine heart slices in the biomimetic stimulated culture system.
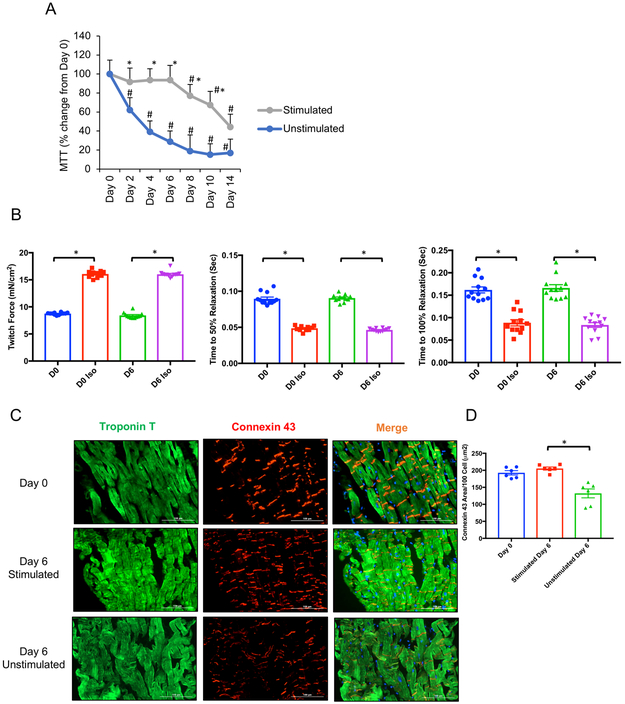
Our ultimate goal in developing a heart slice culture system is to enable efficacy and toxicity testing of various therapeutics on human heart tissue. Based on the enhanced viability and functionality of pig heart slice provided by our biomimetic stimulated heart slice culture system, we next tested its application to human heart slices. Heart slices derived from donors whose hearts were disqualified for transplantation, because of drug abuse, were received and processed within 12 hours of death. The slices from these healthy hearts performed in a manner similar to the pig heart slices in our biomimetic stimulated culture system. The slices showed 100% viability, similar to fresh slices, for up to day 6 in culture, after which viability declined. By comparison, using the transwell unstimulated culture system, human heart slice viability started to decline as early as day 2 in culture (Figure 6A). Functionally, the human heart slices cultured for 6 days in the biomimetic stimulated culture system produced twitch force and responses to isoproterenol similar to fresh slices (Figure 6B). Furthermore, the slices kept in the biomimetic stimulated culture for 6 days showed integrity of connexin 43 at the gap junctions similar to fresh human heart slices. In contrast, slices cultured for 6 days using the transwell culture showed disruption and significant decrease in connexin 43 expression (Figure 6C and D).
Figure 6: Human heart slices are functionally viable for 6 days in biomimetic stimulated culture.
(A) Quantification of heart slice viability over time using MTT assay in either stimulated or unstimulated culture system in optimized medium (n=3 human hearts each in triplicate, Two-Way ANOVA test was conducted to compare between groups; *p<0.05 compared to the unstimulated culture at the same time point, and #p<0.05 compared to fresh heart slices at day 0). (B) Bar graph shows the quantification of the contractile force in the presence or absence of isoproterenol (Iso) generated by fresh human heart slices (Day 0) and after 6 days in biomimetic culture (n=2 human hearts each in triplicate, Two-Way ANOVA test was conducted to compare between groups; *p<0.05 comparing baseline to Iso stimulation at the same time point, twitch force: p-value D0 vs D6=0.39, p-value D0 Iso vs D6 Iso=0.95; time to 50% relaxation: p-value D0 vs D6=0.96, p-value D0 Iso vs D6 Iso=0.69; time to 100% relaxation: p-value D0 vs D6=0.96, p-value D0 Iso vs D6 Iso=0.95). (C) Representative immunofluorescence images showing expression of connexin 43 (red) in cardiomyocytes (green) in longitudinal sections from either fresh heart slices (top panel), slices cultured for 6 days under biomimetic stimulated culture conditions (middle panel), or slices cultured for 6 days under unstimulated transwell conditions (bottom panel), scale bar, 100 μm. (D) Quantification of the area occupied by connexin 43 in fresh heart slices, and heart slices cultured for 6 days either under biomimetic stimulated culture or unstimulated culture (n=2 human hearts, 3 replicates of each, One-Way ANOVA test was conducted to compare between groups; *p<0.05 compared to stimulated day 6 group, p-value day 0 vs stimulated day 6=0.58).
Genetic manipulation of heart slices to test gene therapy for heart failure.
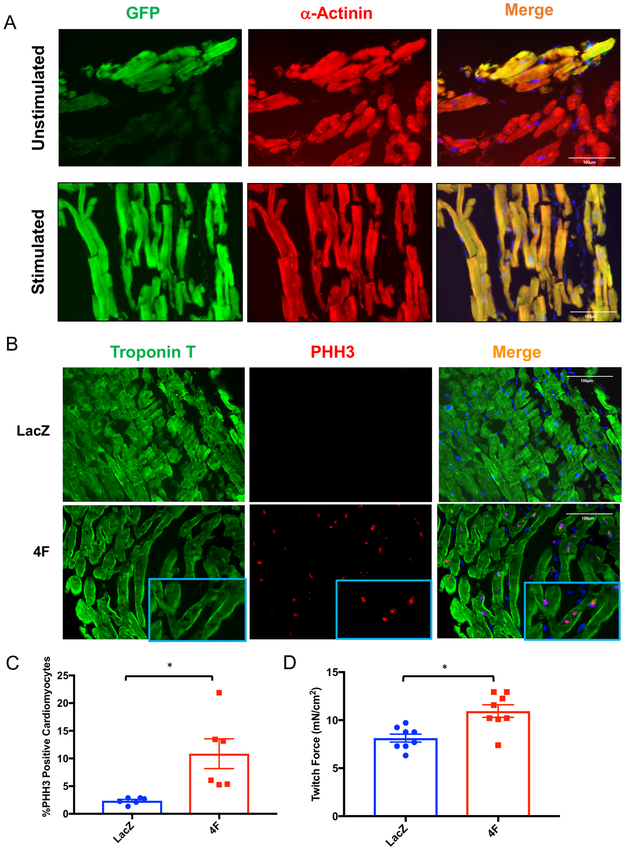
One of the major limitations of existing heart slice technologies is the inability to genetically manipulate them using viral infection. This is mainly due to the nature of the transwell culture and the unavailability of a submerged culture system. To determine whether our biomimetic stimulated culture system would overcome this obstacle, we infected heart slices with adenovirus encoding GFP. We observed only superficial virus penetration in the transwell culture system compared to complete infection in the biomimetic stimulated culture system (Figure 7A). Based on this successful infection, we performed a proof-of-principle study to assess the activity of a potential gene therapy in human heart slices. Recently, we reported that a cocktail of 4 cell cycle factors (CDK1, CDK4, Cyclin D, and Cyclin B) induced adult cardiomyocyte proliferation and functional improvement in mouse hearts18. We tested whether infecting human heart slices with adenoviruses encoding these 4 cell cycle factors could also induce human cardiomyocyte proliferation. We observed a significant increase in cardiomyocyte staining for phospho-histone H3, a marker of early proliferation, in slices infected with the 4 cell cycle factors compared to those infected with control virus (Figure 7B and C). Functionally, the heart slices infected with the 4 cell cycle factors also showed a significant increase in twitch force compared to those infected with control virus, possibly due to an increase in functional myocyte number and density (Figure 7D). This is the first demonstration of functional testing of a potential cardiac gene therapy using cultured heart slices.
Figure 7. Genetic manipulation of heart slices in culture.
(A) Representative immunofluorescence images showing expression of green fluorescent protein (GFP, green) in cardiomyocytes (red) after 72 hours of infection with adenovirus encoding GFP in unstimulated transwell (top panel) or stimulated (bottom panel) culture system. (B) Representative immunofluorescence images showing the expression of phospho-histone-H3 (PHH3) (red) in cardiomyocytes (green) 72 hours following infection with 4F adenoviruses or LacZ control virus. (C) Quantification of PHH3 positive cardiomyocytes in heart slices infected either with 4F encoding adenovirus or LacZ control (n=2 human hearts each in triplicate, unpaired Student t tests was conducted to compare between groups; *p<0.05). (D) Bar graph showing the quantification of the contractile force generated by heart slices following infection with either 4F or LacZ control adenovirus (n=4 slices each in duplicate, unpaired Student t tests was conducted to compare between groups; *p<0.05).
Assessment of myofibril mechanics isolated from cultured heart slices.
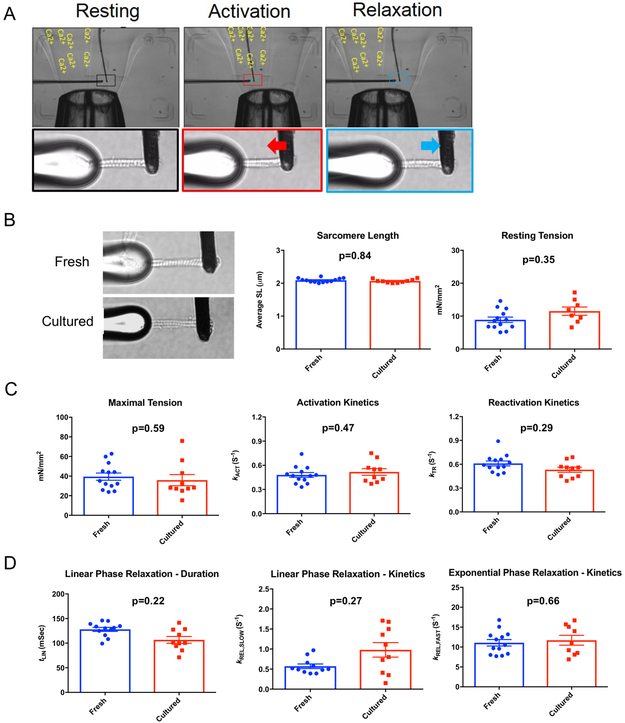
It is important to take this heart slice technology to the next level of rigor and accuracy and demonstrate their utility for determining the effect of therapeutics and toxins on single cardiac myofibrils. Therefore, we isolated single myofibrils from fresh hearts and heart slices cultured for 48 hours. While there was unexpected variability in the myofibrils isolated from the heart slices, the myofibrils that were successfully activated had similar parameters to myofibrils isolated from fresh hearts in terms of resting tension, force generation and activation/relaxation kinetics (Figure 8 A-D). This confirms the suitability of such a culture system to be combined with other technologies to support rigorous assessments that predict the efficacy and toxicity of various potential therapeutics.
Figure 8. Myofibril mechanics in cultured heart slices are similar to fresh heart slices.
(A) Image of double barrel perfusion pipette delivering different concentrations of calcium. Myofibrils are mounted between a force probe (calibrated to detect force in μN/μm) and a supporting stretcher. (B) Representative images for myofibrils isolated from fresh as well as cultured pig heart slices (left panel). Quantification of resting sarcomere length and resting tension for myofibrils isolated from fresh and cultured heart slices (n=8-13 myofibrils in each group, unpaired Student t tests was conducted to compare between groups). (C) Quantification of myofibril maximal tension, activation kinetic, and reactivation kinetics shows no significant differences between myofibrils isolated from fresh and cultured heart slices (n=10-13 myofibrils in each group, unpaired Student t tests was conducted to compare between groups). (D) Quantification of linear and exponential relaxation phases’ kinetics shows no significant difference between the myofilaments isolated from fresh and cultured pig heart slices (n=10-13 in each group, unpaired Student t tests was conducted to compare between groups).
DISCUSSION
Here we describe a novel simplified medium throughput method that enables culture of human and pig heart slices for a period sufficiently long to test cardiotoxicity and therapeutic efficacy. The proposed conditions mimic the environment of the heart, including nutrient availability, frequency of electrical stimulation, and oxygenation. We attribute the prolonged viability of heart slices in our biomimetic stimulated culture to our focus on recreating the physiological conditions experienced by the intact heart. This concept is supported by our data showing that electrical stimulation alone, without providing essential nutrients, is not sufficient to maintain heart slice viability. Identification of required medium components was based on recent knowledge gained from culturing human iPSC-CMs and induced cardiomyocytes derived through direct cardiac reprogramming17, 18, 21. We found that it is essential to include FBS in the medium to maintain the viability. This is likely due to the requirement for a variety of proteins, macromolecules, fatty acids, trace elements, enzymes, proteins, chemical components, and hormones, which are usually present in the serum and delivered to heart tissue in vivo22. Furthermore, we found that the addition of FGF and VEGF to the medium enhanced tissue viability. FGF and VEGF factors are well known angiogenic factors essential for maintenance of cultured endothelial cells23. In addition, they improve the differentiation and maintenance of directly reprogrammed cardiomyocytes19. Therefore, it is likely that FGF and VEGF are needed to maintain the endothelial cells as well as cardiomyocytes in culture to support tissue viability. Our work is the first to modify the simplified culture medium previously reported for heart slices, and it opens the possibility for further optimization of the media components in subsequent studies.
Three recent studies have demonstrated the importance of continuous electromechanical stimulation in maintaining slices in culture13-15. However, all of these studies used the basal M199/ITS medium, which is not sufficient to maintain the metabolic needs of heart slices. This led to several compromises in the slice contractility and calcium homeostasis early during culture13-15. Qiao et al.,13 have shown that using a highly sophisticated bioengineering chips, they can maintain the electrophysiological properties of the heart slices for 4 days, but no assessment of the contractile function was provided. Fischer et al.,14 have shown that failing human heart slices can be maintained in vitro for up to 4 months when cultured under sub-physiological (0.2 Hz) stimulation auxotonic loading and media agitation in a bioengineering device. However, these non-physiological conditions may induce a variety of changes in the heart slices, as reflected in their RNAseq data, which showed over 10 fold downregulation of cardiac gene expression as early as the first time point of assessment (day 8)14. Finally, Watson et al.,15 have bioengineered a low throughput culture system to demonstrate the importance of diastolic sarcomere length for the maintenance of cardiac muscle properties for 24 hours. For efficient testing of drug toxicity and efficacy of potential therapeutics, it is preferable to perform analyses in a simplified, medium-throughput system under full physiological conditions which could reflect the normal cardiac state. Our biomimetic stimulated culture system emulates the controllable conditions experienced by the heart in situ and, therefore, should provide a reliable readout of the functional and the structural outcomes of drug treatment with regard to cardiotoxicity or efficacy compared to compromised culture systems.
Our rigorous assessments show that our biomimetic stimulated culture system maintains viability and functionality of human and pig heart slices for 6 days of culture. However, starting at day 7, we observed cardiomyocytes with spontaneous calcium transients, which is the first sign of dedifferentiation; cell death ensued after day 7. The only early change that we observed in the heart slices in our biomimetic stimulated culture system was a decrease in mitochondrial respiration, leading to a higher reliance on glycolysis for energy. The decrease in oxidative phosphorylation occurred by day 2 in culture; however, glycolysis remained stable until day 8. These changes are expected, given that the culture medium contains low levels of fatty acids, supplied by FBS24. The fact that metabolic changes preceded transcriptional changes is consistent with previous findings that indicate the critical role of cellular metabolism in modulating gene expression (as reviewed in 25). It was interesting that the addition of FGF and VEGF maintained glycolytic capacity of the heart slices for up to 6 days in culture. These findings are in line with the large body of literature showing that FGF and VEGF signaling can influence glycolytic capacity in various cell types26-30. Furthermore, the addition of fatty acids to the culture medium significantly reduced connexin 43 expression; this is likely due to their mild solubilization effects on membrane proteins 20. Nevertheless, it remains possible that genetic manipulation of fatty acid metabolism enzymes or addition of specific fatty acid cocktails is important for extending viability and functionality beyond 6 days. It remains unclear whether the metabolic shift to glycolysis promotes cardiomyocyte dedifferentiation or hastens cell death. Future studies should test whether pharmacological agents that promote mitochondrial biogenesis or provision of adjuvant substrates for mitochondrial respiration (e.g., ketones, lactate, fatty acid cocktails) maintain the heart slices in culture for longer periods of time.
This new culture system addresses one of the major limitations of previous heart slice culture systems as it provides a medium-throughput platform to test novel gene therapy approaches in human heart tissue in situ. We provide the first proof of concept by demonstrating that one of our recently developed gene therapy approaches, which induces cardiomyocyte proliferation in mouse models in vivo, is also effective in activating cardiomyocyte replication in human heart slices. Furthermore, we demonstrated that this technology could be used to assess the effect of therapeutics or toxins on contractile mechanics of single myofibrils. This technology could serve in the future as a platform to test the efficacy of novel therapeutic agents for heart failure in intact 3D human heart tissue prior to initiating clinical trials. In addition, our culture platform could be used as a medium-throughput assay to test cardiotoxicity because we can simultaneously culture 8 × 6 well plates using one C-Pace device. Biochemical assays such as MTT, CK-MB, or LDH could detect overt cardiotoxicity; however, more in-depth functional assessments, such as calcium transients and force production, could be used to test for subtle undesirable drug effects on cardiac function in situ.
In conclusion, our biomimetic stimulated human and pig heart slice culture system is a novel, reliable, and easily reproducible medium-throughput method for testing acute drug cardiotoxicity or efficacy of novel heart failure therapies. This study provides several major advancements over existing methodologies including culture media composition, physiological electrical stimulation, and genetic manipulation of the heart slices. Future work should be aimed at further optimization of this biomimetic stimulated culture system with the goal of maintaining the viability and functionality of heart slices over several months. In particular, further optimization of the culture medium components is likely to be an essential factor for improvement of this technology.
Limitations of the study.
One of the major limitations of this study is lack of a statistical test for similarity. In lieu, we have used differential significance tests and assayed multiple parameters to increase the confidence in our claims of similarity. In addition, for several parameters, we performed multiple testing using one- or two-way ANOVA with a post-hoc Bonferroni correction and the non-significant p-values are ranging from 0.22–0.98. Therefore, decreasing the statistical significance threshold to correct for multiple testing will entail the same conclusions. Furthermore, our results indicate that culture-induced diminishment of oxidative phosphorylation might instigate deterioration of the heart slices. Unfortunately, we were not able to identify an optimal method to maintain oxidative phosphorylation. It is likely that enhancing fatty acid metabolism in heart slices is not as simple as adding fatty acids to the growth medium, and will require further optimization, which could be addressed in future studies. In addition, it is unclear how heart slices compensate for the shift to a less efficient ATP-producing mechanism in culture. In terms of single myofibril isolation, despite comparable kinetic parameters between myofibrils isolated from fresh hearts and myofibrils isolated from heart slices, there were significant number of non-viable myofibrils observed from heart slices. This is likely because we used previously described methods for isolating myofibrils from flash frozen tissue as well as primary cultured cardiomyocytes31, 32. Therefore, there is a need for further optimization for the myofibril isolation procedure from the heart slices to achieve 100% myofibril recovery. Last, although our culture system can emulate several physiological conditions, it cannot mimic changes in mechanical load and shear stress during the cardiac contractile cycle. Further optimization of physiological and metabolic factors could help prolong heart slice viability and function.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Heart slice viability in culture could be partially maintained for 24 hours in transwells.
Recently, sophisticated bioengineered culture devices that provide continuous electrical stimulation partially extended the heart slice functionality in culture.
What New Information Does This Article Contribute?
Demonstrate that the combination of new maintenance medium and electrical stimulation maintain the heart slices at full functionality and viability for 6 days.
Provide a simplified heart slice culture system with medium-throughput using commercially available products.
Uncovered mechanistic insights regarding the metabolic regulation of heart slices in culture.
Demonstrate the feasibility of this heart slice culture system to be used in testing gene therapy for heart failure.
All studies up to now were using sophisticated bioengineered culture devices and tried to optimize electrical stimulation to maintain the heart slice functionality in culture but did not address the importance of nutrient availability to maintain cardiac cells or tissue. Through a screening of various components, we identified a combination of essential components of the medium and the electrical stimulation conditions that maintain the heart slices at full functionality and viability similar to fresh heart tissue for 6 days. In addition, we simplified the heart slice culture system and increased the throughput to 48 slices per run using commercially available products. Thus, many laboratories should be able to use the culture system to address their scientific questions. Furthermore, we uncovered mechanistic insights regarding the metabolic regulation of heart slices in culture and uncover how addition of growth factors impact their viability. These mechanistic insights will open new avenues to manipulate the system to optimally support metabolic needs. Finally, we show that the heart slice system is suitable for testing gene therapy for heart failure. In addition, we demonstrated the ability to isolate single myofibrils from the heart slices and assess the mechanics of contractility on single isolated myofibrils after culture.
ACKNOWLEDGMENTS
The authors thank E. León, A. Sharfi, R. Nasri, and A. Elshaer for technical assistance and helpful suggestions. The authors also thank B. Turgeon for help in editing the manuscript. In addition, the authors thank the University of Louisville electron microscopy core facility. Special thanks to the human heart donor families. In addition, we would like to thank John Tomaso and Orlando Mendoza from Novabiosis for their co-ordination for the heart procurement procedures and logistics.
SOURCES OF FUNDING
TMAM is supported by NIH grant P30GM127607 and American Heart Association grant 16SDG29950012. Part of this work was performed with the assistance of the UofL Electron Microscopy Facility, which is supported by NIH P20GM103436 (KY IDeA Networks of Biomedical Research Excellence). The authors also acknowledge NIH grants P30GM127607
(SPJ, BGH), R01HL131647 (SPJ), R01HL130174 (BGH), R01ES028268 (BGH), P01HL78825 (RB, BGH, SPJ) and UM1HL113530 (RB), and the American Diabetes Association Pathway to Stop Diabetes Grant, ADA 1–16-JDF-041 (BGH). British Heart Foundation MBPhD studentship (FS/15/35/31529) (SW), BHF Centre for Regenerative Medicine (RM/17/1/33377) (CMT and FP). T.A.M. received support from the NIH [HL147558 HL116848, HL127240 and DK119594] and American Heart Association [16SFRN31400013]. Y.H.L. was supported by a fellowship from the American Heart Association [16POST30960017].
Nonstandard Abbreviations and Acronyms:
- hiPSC-CMs
human induced pluripotent stem cell-derived cardiomyocytes
- NAD(P)H
Nicotinamide adenine dinucleotide phosphate
- MTT
3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide
- FBS
Fetal bovine serum
- FGF
Fibroblast growth factor
- VEGF
Vascular endothelial growth factor
- ITS
Insulin – Transferrin – Sodium Selenite
- RPMI
Roswell Park Memorial Institute medium
- EM
Electron Microscopy
- RPMI 1640
Roswell Park Memorial Institute (RPMI) 1640 Medium
- CK-MB
Creatine kinase-muscle/brain
- LDH
Lactate dehydrogenase
Footnotes
DISCLOSURES
TMAM, KI and ZJ hold equities in Tenaya Therapeutics. The other authors report no conflicts.
REFERENCES
- 1.Writing Group M, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics-2016 update: A report from the american heart association. Circulation. 2016;133:e38–360 [DOI] [PubMed] [Google Scholar]
- 2.Onakpoya IJ, Heneghan CJ, Aronson JK. Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: A systematic review of the world literature. BMC Med. 2016;14:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robertson C, Tran DD, George SC. Concise review: Maturation phases of human pluripotent stem cell-derived cardiomyocytes. Stem Cells. 2013;31:829–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, Morikawa K, Teles D, Yazawa M, Vunjak-Novakovic G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. 2018;556:239–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA, Tallquist MD. Revisiting cardiac cellular composition. Circ Res. 2016;118:400–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, Brody MJ, SC JL, Aronow BJ, Tallquist MD, Molkentin JD. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun. 2016;7:12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu X, Khalil H, Kanisicak O, Boyer JG, Vagnozzi RJ, Maliken BD, Sargent MA, Prasad V, Valiente-Alandi I, Blaxall BC, Molkentin JD. Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J Clin Invest. 2018;128:2127–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kretzschmar K, Post Y, Bannier-Helaouet M, Mattiotti A, Drost J, Basak O, Li VSW, van den Born M, Gunst QD, Versteeg D, Kooijman L, van der Elst S, van Es JH, van Rooij E, van den Hoff MJB, Clevers H. Profiling proliferative cells and their progeny in damaged murine hearts. Proc Natl Acad Sci U S A. 2018;115:E12245–E12254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandenburger M, Wenzel J, Bogdan R, Richardt D, Nguemo F, Reppel M, Hescheler J, Terlau H, Dendorfer A. Organotypic slice culture from human adult ventricular myocardium. Cardiovasc Res. 2012;93:50–59 [DOI] [PubMed] [Google Scholar]
- 10.Perbellini F, Watson SA, Scigliano M, Alayoubi S, Tkach S, Bardi I, Quaife N, Kane C, Dufton NP, Simon A, Sikkel MB, Faggian G, Randi AM, Gorelik J, Harding SE, Terracciano CM. Investigation of cardiac fibroblasts using myocardial slices. Cardiovasc Res. 2018;114:77–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watson SA, Scigliano M, Bardi I, Ascione R, Terracciano CM, Perbellini F. Preparation of viable adult ventricular myocardial slices from large and small mammals. Nature protocols. 2017;12:2623–2639 [DOI] [PubMed] [Google Scholar]
- 12.Kang C, Qiao Y, Li G, Baechle K, Camelliti P, Rentschler S, Efimov IR. Human organotypic cultured cardiac slices: New platform for high throughput preclinical human trials. Sci Rep. 2016;6:28798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiao Y, Dong Q, Li B, Obaid S, Miccile C, Yin RT, Talapatra T, Lin Z, Li S, Li Z, Efimov IR. Multiparametric slice culture platform for the investigation of human cardiac tissue physiology. Progress in biophysics and molecular biology. 2018 [DOI] [PubMed] [Google Scholar]
- 14.Fischer C, Milting H, Fein E, Reiser E, Lu K, Seidel T, Schinner C, Schwarzmayr T, Schramm R, Tomasi R, Husse B, Cao-Ehlker X, Pohl U, Dendorfer A. Long-term functional and structural preservation of precision-cut human myocardium under continuous electromechanical stimulation in vitro. Nat Commun. 2019;10:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watson SA, Duff J, Bardi I, Zabielska M, Atanur SS, Jabbour RJ, Simon A, Tomas A, Smolenski RT, Harding SE, Perbellini F, Terracciano CM. Biomimetic electromechanical stimulation to maintain adult myocardial slices in vitro. Nat Commun. 2019;10:2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crick SJ, Sheppard MN, Ho SY, Gebstein L, Anderson RH. Anatomy of the pig heart: Comparisons with normal human cardiac structure. J Anat. 1998;193 ( Pt 1):105–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohamed TM, Stone NR, Berry EC, Radzinsky E, Huang Y, Pratt K, Ang YS, Yu P, Wang H, Tang S, Magnitsky S, Ding S, Ivey KN, Srivastava D. Chemical enhancement of in vitro and in vivo direct cardiac reprogramming. Circulation. 2017;135:978–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohamed TMA, Ang YS, Radzinsky E, Zhou P, Huang Y, Elfenbein A, Foley A, Magnitsky S, Srivastava D. Regulation of cell cycle to stimulate adult cardiomyocyte proliferation and cardiac regeneration. Cell. 2018;173:104–116 e112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamakawa H, Muraoka N, Miyamoto K, Sadahiro T, Isomi M, Haginiwa S, Kojima H, Umei T, Akiyama M, Kuishi Y, Kurokawa J, Furukawa T, Fukuda K, Ieda M. Fibroblast growth factors and vascular endothelial growth factor promote cardiac reprogramming under defined conditions. Stem Cell Reports. 2015;5:1128–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibarguren M, Lopez DJ, Escriba PV. The effect of natural and synthetic fatty acids on membrane structure, microdomain organization, cellular functions and human health. Biochim Biophys Acta. 2014;1838:1518–1528 [DOI] [PubMed] [Google Scholar]
- 21.Ang YS, Rivas RN, Ribeiro AJ, Srivas R, Rivera J, Stone NR, Pratt K, Mohamed TM, Fu JD, Spencer CI, Tippens ND, Li M, Narasimha A, Radzinsky E, Moon-Grady AJ, Yu H, Pruitt BL, Snyder MP, Srivastava D. Disease model of gata4 mutation reveals transcription factor cooperativity in human cardiogenesis. Cell. 2016;167:1734–1749 e1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franke J, Abs V, Zizzadoro C, Abraham G. Comparative study of the effects of fetal bovine serum versus horse serum on growth and differentiation of primary equine bronchial fibroblasts. BMC Vet Res. 2014;10:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vuorenpaa H, Ikonen L, Kujala K, Huttala O, Sarkanen JR, Ylikomi T, Aalto-Setala K, Heinonen T. Novel in vitro cardiovascular constructs composed of vascular-like networks and cardiomyocytes. In Vitro Cell Dev Biol Anim. 2014;50:275–286 [DOI] [PubMed] [Google Scholar]
- 24.Stoll LL, Spector AA. Changes in serum influence the fatty acid composition of established cell lines. In Vitro. 1984;20:732–738 [DOI] [PubMed] [Google Scholar]
- 25.van der Knaap JA, Verrijzer CP. Undercover: Gene control by metabolites and metabolic enzymes. Genes Dev. 2016;30:2345–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu P, Wilhelm K, Dubrac A, Tung JK, Alves TC, Fang JS, Xie Y, Zhu J, Chen Z, De Smet F, Zhang J, Jin SW, Sun L, Sun H, Kibbey RG, Hirschi KK, Hay N, Carmeliet P, Chittenden TW, Eichmann A, Potente M, Simons M. Fgf-dependent metabolic control of vascular development. Nature. 2017;545:224–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradley CA. Fgfr1 reprogrammes cell metabolism. Nat Rev Urol. 2018;15:528. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Chen G, Liu Z, Liu S, Cai Z, You P, Ke Y, Lai L, Huang Y, Gao H, Zhao L, Pelicano H, Huang P, McKeehan WL, Wu CL, Wang C, Zhong W, Wang F. Aberrant fgfr tyrosine kinase signaling enhances the warburg effect by reprogramming ldh isoform expression and activity in prostate cancer. Cancer Res. 2018;78:4459–4470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitzgerald G, Soro-Arnaiz I, De Bock K. The warburg effect in endothelial cells and its potential as an anti-angiogenic target in cancer. Front Cell Dev Biol. 2018;6:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi S, Xu J, Zhang B, Ji S, Xu W, Liu J, Jin K, Liang D, Liang C, Liu L, Liu C, Qin Y, Yu X. Vegf promotes glycolysis in pancreatic cancer via hif1alpha up-regulation. Curr Mol Med. 2016;16:394–403 [DOI] [PubMed] [Google Scholar]
- 31.Jeong MY, Lin YH, Wennersten SA, Demos-Davies KM, Cavasin MA, Mahaffey JH, Monzani V, Saripalli C, Mascagni P, Reece TB, Ambardekar AV, Granzier HL, Dinarello CA, McKinsey TA. Histone deacetylase activity governs diastolic dysfunction through a nongenomic mechanism. Sci Transl Med. 2018;10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woulfe KC, Ferrara C, Pioner JM, Mahaffey JH, Coppini R, Scellini B, Ferrantini C, Piroddi N, Tesi C, Poggesi C, Jeong M. A novel method of isolating myofibrils from primary cardiomyocyte culture suitable for myofibril mechanical study. Front Cardiovasc Med. 2019;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.