Abstract
We analyzed the correlation between the expression of HIF-1α (hypoxia-inducible factor 1 alpha), the nuclear receptors, VDR (vitamin D receptor), RORα (retinoic acid receptor-related orphan receptor alpha), and RORγ and CYP24A1 (cytochrome P450 family 24 subfamily A member 1) and CYP27B1 (cytochrome P450 family 27 subfamily B member 1), enzymes involved in vitamin D metabolism. In primary and metastatic melanomas, VDR negatively correlated with nuclear HIF-1α expression (r=−0.2273, p=0.0302; r=−0.5081, p=0.0011). Furthermore, the highest HIF-1α expression was observed in pT3-pT4 VDR-negative melanomas. A comparative analysis of immunostained HIF-1α and CYP27B1 and CYP24A1 showed lack of correlation between these parameters both in primary tumors and melanoma metastases. In contrast, RORα expression correlated positively with nuclear HIF-1α expression in primary and metastatic lesions (r=0.2438, p=0.0175; r=0.3662, p=0.0166). Comparable levels of HIF-1α expression pattern was observed in localized and advanced melanomas. RORγ in primary melanomas correlated also positively with nuclear HIF-1α expression (r=0.2743, p=0.0129). HIF-1α expression was the lowest in localized RORγ-negative melanomas. In addition, HIF-1α expression correlated with RORγ-positive lymphocytes in melanoma metastases. We further found that in metastatic lymph nodes FoxP3 immunostaining correlated positively with HIF-1α and RORγ expression in melanoma cells (r=0.3667; p=0.0327; r=0.4208, p=0.0129). In summary, our study indicates that the expression of VDR, RORα and RORγ in melanomas is related to hypoxia and/or HIF1-α activity, which also affects FoxP3 expression in metastatic melanoma. Therefore, the hypoxia can affect tumor biology by changing nuclear receptors expression and molecular pathways regulated by nuclear receptors and immune responses.
Keywords: melanoma, vitamin D, vitamin D receptor, retinoic acid receptor-related orphan receptor, hypoxia-inducible factor
Graphical Abstract
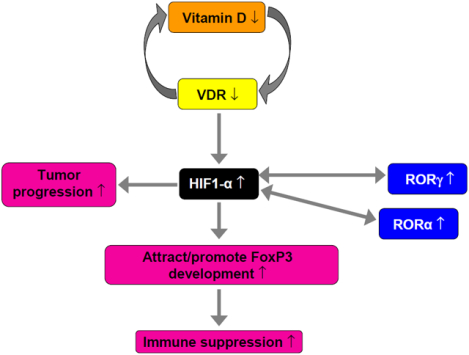
Proposed mechanism involving deregulation of HIF-1α and VDR and RORs in melanoma development and progression. In melanomas, overexpression of HIF-1α (which can be related to the vitamin D deficiency) can induce RORα and RORγ expression, and, acting through HIF-1α-related pathways, can promote tumor progression, attract/promote development of FoxP3-positive lymphocytes, contributing to immunosuppression.
Introduction
Hypoxia is an important hallmark of the tumor microenvironment and under the hypoxic conditions the transcription of numerous genes is changed through a process regulated by HIFs[1]. HIF-1α regulates transcription of many target genes related to various cellular processes such as survival, apoptosis, glucose metabolism, or angiogenesis, by binding to hypoxia response elements (HREs) in gene regulatory regions[2]. While under physiological conditions, these changes are important for regulation of local homeostasis, under pathological conditions they can promote cancer cells survival, angiogenesis and tumor progression including metastatic spread, all linked to poor clinical outcomes[3–5]. Overexpression of HIF1-α was observed in several types of human cancers, including melanoma[6–11]. Both normal and malignant melanocytes are able to synthesis the melanin pigment, which protects cells against noxious physical and chemical factors [12] and under pathologic conditions melanin can increase the resistance of melanoma cells to chemo-, radio- and immunotherapy[13–17]. Melanogenesis, by modulating the oxygen consumption and stimulation of aerobic glycolysis, affects microenvironment and can enhance hypoxia both in normal melanocytes and melanoma cells[9, 18–20]. It was also shown, that hypoxic microenvironment via HIF-1α can promote melanocyte transformation and melanoma development[21].
HIF1-α and hypoxia involvement in the regulation of T cell functions is complex and not fully understand, but it is postulated that HIF1-α is important in both T regulatory cells (Treg) and Th17 differentiation (reviewed [22]). The Th17 development is enhanced by HIF1-α via direct transcriptional activation of RORγt belonging to nuclear receptor (NR) family[23, 24]. In tumors hypoxia can promote development of immunosuppressive microenvironment by affecting the rapid differentiation of myeloid derived suppressive cells to tumor associated macrophages[25]. Furthermore hypoxic conditions can regulate T cell metabolism and differentiation and Treg (iTreg) and Th17 cell balance, and the regulation of those processes is coordinated by HIF1-α, RORγ and FoxP3[23, 26, 27].
NRs, highly conserved transcription factors, regulate a variety of biological processes under normal and pathological conditions, including cell differentiation, immune response, circadian rhythm, lipid, steroid, xenobiotic, and glucose metabolism, (reviewed in [28–30]). Vitamin D receptor (VDR) are targets for bioregulatory functions of 1,25(OH)2D3[31–33] and novel CYP11A1 derived vitamin D-hydroxyderivatives derivatived[34–36] in skin cancers. In addition, vitamin D and lumisterol hydroxyderivatives can act on RORα, RORγ as inverse agonists[34, 36, 37] and CYP11A1-derived vitamin D metabolites also acts as agonists on aryl hydrocarbon receptor (AhR)[38]. Recently several studies showed that the expression of VDR could be regulated by hypoxia[39]. In osteoclasts, vitamin D analogs, acting via VDR, can regulate HIF-1α expression[40]. Other studies showed that the expression of RORα increased in variety of cells under hypoxic conditions that is mediated by the interaction of HIF-1α to an HRE in the promoter region of RORα[41–44]. On the other hand, RORα enhances HIF-1α protein stability, its nuclear localization, transactivation functions and HIF-1α transcriptional activity[43, 45]. In addition, HIF1-α, acting via transcriptional activation of RORγt, promotes the development of Th17 cells[23].
Previously, we found that changes in the expression of VDR, RORα, RORγ, CYP27B1 and CYP24A1 in melanomas correlate with melanoma progression[46–50] and may affect the disease outcome[35, 51]. In addition, active melanogenesis stimulates the expression of HIF-1α and HIF-dependent pathways[52]. In this study, we have analyzed the correlation between the expression of HIF-1α and nuclear receptors (VDR, RORα, RORγ) and two enzymes involved in vitamin D metabolism (CYP24A1 and CYP27B1). We also analyzed the relationship between HIF-1α expression in melanomas cells and FoxP3 in lymphocytes both in primary and metastatic melanomas.
Material and methods
Patients
Cutaneous melanoma patients treated between 2001–2009 in Oncology Center, Bydgoszcz, Poland, were initially qualified into this study based on pathomorphological diagnosis. After the assessment of availability of melanoma tissues in the paraffin blocks, 77 primary melanomas (38 superficial spreading, 37 nodular and 2 acral) and 36 metastatic melanomas were included into this study. The mean age of patients (37 female, 40 male) was 60.0 yrs (25 to 100 yrs). The advancement of melanomas was evaluated according to AJCC 8th Edition TNM classification[53] and included 4 pTis, 19 pT1, 11 pT2, 14 pT3 and 29 pT4 cases; 45 pN0, 13 pN1, 10 pN2 and 6 pN2 cases (there was lack data for 3 patients); 72 pM0 and 2 pM1 cases (there was lack for 2 patients). This study was approved by the institutional review board.
Immunohistochemical (IHC) staining and IHC evaluation
Formalin-fixed paraffin-embedded 4μm section were immunostained and evaluated as previously described[9, 46–49, 54, 55]. Briefly, heat-induced antigen retrieval was performed using Tris/EDTA buffer and microwave heating for VDR immunostaining, Target Retrieval Solution, pH 9 and PT Link device (Dako, Glostrup, Denmark) for FoxP3 immunostaining and Target Retrieval Solution, pH 9 and PT Link device for other antibodies. The list of primary and secondary antibodies, antigen retrieval protocols and visualization systems are presented in Table 1. Isotopic controls served as negative controls to determine non-specific binding of antibodies. For each immunostaining run also appropriate positive controls were prepared (Table 1).
Table 1.
Characteristic of primary and secondary antibodies and visualization systems used in IHC staining.
| Antibody (vendor) | Primary antibody dilution* | Secondary antibody (vendor) | Chromogen (vendor) | Positive control |
|---|---|---|---|---|
| Mouse anti-HIF-1α (Santa Cruz Biotechnology, Santa Cruz, CA, USA, Cat# sc-53546) | 1:750** | EnVision+System HRP Labelled Polymer (Dako, Glostrup, Denmark, Cat# K4000) | Vector NovaRED HRP substrate (Vector Laboratories, Inc., Burlingame, CA, USA, Cat#SK-4800) | breast cancer |
| Rat anti-VDR (Abcam, Cambridge, MA, Cat#ab8756) |
1:75 | Vectastain ABC-AP Kit (Vector Laboratories Inc, Burlingame, CA, Cat#AK-5004) | Vectastain ABC-AP Reagent and alkaline phosphatase substrate (Vector Laboratories Inc, Burlingame, CA, USA, and SK5100). | normal skin/kidney |
| Rabbit anti-CYP27B1 (Santa Cruz Biotechnology, Santa Cruz, CA, Cat#sc-67261) | 1:75 | EnVision+System HRP Labelled Polymer (Dako, Glostrup, Denmark, Cat# K4002) | ImmPACT NovaRED (Vector Laboratories Inc., Burlingame, CA, USA, Cat#SK-4805) | kidney |
| Rabbit anti-CYP24A1 (Abcam, Cambridge, MA) |
1:40 | EnVision+System HRP Labelled Polymer (Dako, Glostrup, Denmark) | ImmPACT NovaRED (Vector Laboratories Inc., Burlingame, CA, USA) | kidney |
| Rabbit anti-RORγ (Dr Anton M. Jetten) | 1:50 | EnVision™ FLEX/HRP (Dako, Glostrup, Denmark, Cat# K4002) | Vector NovaRED HRP substrate (Vector Laboratories, Inc., Burlingame, CA, USA, Cat#SK-4800) | normal skin |
| Goat-antiROR α (Santa Cruz Biotechnology, Santa Cruz, CA, USA, Cat#sc-6062) | 1:25 | Vectastain Elite ABC Goat IgG (Vector Laboratories, Burlingame, CA, USA, Cat#PK6105) | ImmPACT™ NovaRED™ HRP substrate (Vector Laboratories, Cat#SK-4805) | normal skin |
| Mouse anti-FoxP3 (Abcam, Cambridge, UK, Cat#ab20034) | 1:100 | EnVision+System HRP Labelled Polymer (Dako, Glostrup, Denmark, Cat# K4000) | Vector NovaRED HRP substrate (Vector Laboratories, Inc., Burlingame, CA, USA, Cat#SK-4800) | lymph node |
ON – overnight;
primary antibodies were applied overnight at 4°C;
Tyramide Signal Amplification kit (Life Technologies, Carlsbad, CA, Cat#T20931) was used according the manufacturer’s protocol
An observer analyzing immunostained sections was blinded for detailed histopathological diagnosis, malignancy grade/stage and other clinical data, as previously described[9, 46–49, 54, 55]. Briefly, the staining intensity was evaluated with reference to immunostaining of epidermal cells for VDR, RORα, RORγ, CYP27B1, lymph node lymphocytes for FoxP3, kidney cells for CYP24A1, breast cancer cells for HIF-1α, scored as strong. The semiquantitative score, defined by arbitrary units (A.U.) was calculated as: SQ=mean(IR × SI)/100 for VDR, CYP27B1, CYP24A1, HIF1-α, or SQ=mean(IR × SI) for RORα, RORγ and FoxP3, where IR is the percentage of immunoreactive cells and SI is the staining intensity from 0 to 3 arbitrary units (A.U.) with 0 as negative (0), weak (1), moderate (2) and strong (3). The immunostaining cut-off points were as follows: 0–0.99 (no); 1–1.99 (low) and 2–3.0 (high) A.U. for VDR; 0.0–10.0 (no); 10.1–50.0 (low); 50.1–300.0 (high) for RORα; 0–49.99 (no); 50.0–99.99 (low); 100–300 (high) A.U. for RORγ; 0.0–0.99 (no), 1.0–1.99 (low) and 2.0–3.0 (high) A.U. for CYP27B1; 0.0–1.0 (no), 1.1–2.0 (low) and 2.1–3.0 (high) A.U. for CYP24A1; 0–0.99 (no); 1–1.99 (low) and 2–3.0 (high) A.U. for HIF-1α; 0.0–10.0 (no); 10.1–50.0 (low); 50.1–300.0 (high) for FoxP3. Primary and metastatic melanomas were considered as HIF-1α-positive or FoxP3-positive when more than 10% cells showed positive immunostaining.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software (version 5.0; GraphPad Software, La Jolla, CA, USA). Statistical analysis of differences between patient subgroups according to tested variables was performed using a t-test with P<0.05 considered as a statistically significant.
Results
Correlation between VDR and HIF-1α expression
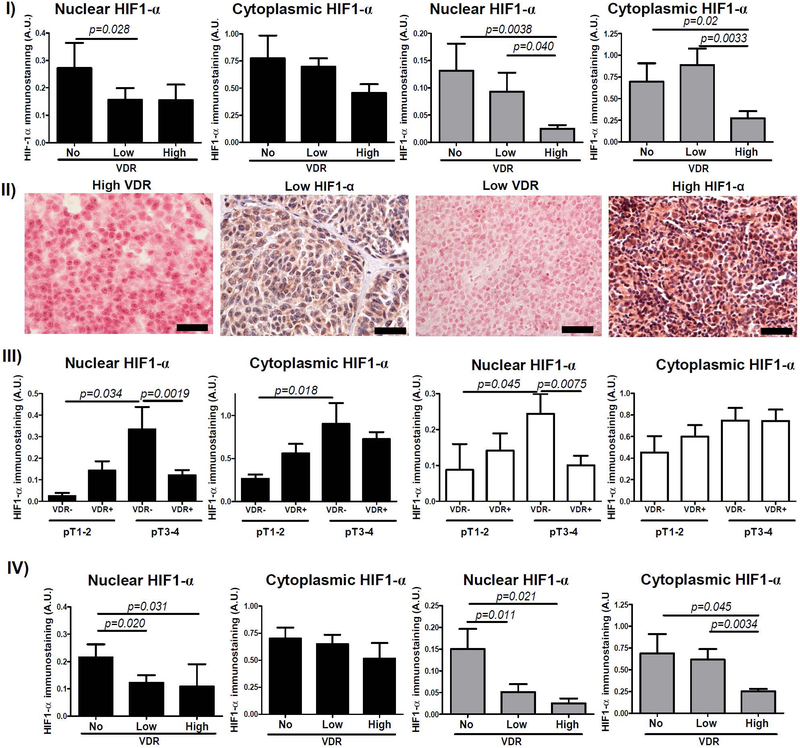
Thirty two out of 77 (42%) and 72 out of 77 (94%) primary melanomas showed nuclear and cytoplasmic HIF-1α immunostaining, respectively. In metastatic melanomas, 8 out of 36 (22%) and 31 out of 36 (86%) cases showed nuclear and cytoplasmic HIF-1α immunostaining, accordingly. In primary melanomas nuclear VDR correlated negatively with nuclear HIF-1α expression (r=−0.2273, p=0.0302). Analysis of both primary and metastatic melanomas subgrouped according to VDR expression revealed significantly lower HIF-1α immunostaining in cases with high nuclear VDR (Figure 1–I, Figure 1–II). In addition, this correlation was stronger in more advanced (pT3-pT4) VDR-negative melanomas showing significantly higher HIF-1α expression than VDR-positive cases (Figure 1–III). Statistically significant differences in HIF-1α expression were also found between pT1-pT2 and pT3-pT4 VDR-negative melanomas (Figure 1–III). pT1-pT2 melanomas with or without VDR showed similar HIF-1α expression.
Figure 1.
Relationship between HIF-1α and VDR immunostaining. I) Differences of cytoplasmic and nuclear HIF-1α immunostaining in primary (black bars) and metastatic (gray bars) melanomas in relation to nuclear VDR immunostaining. Statistically significant differences are denoted with p values as determined by Student’s t-test (upper panel). II) Representative immunostaining of VDR and HIF-1α in melanoma patient with high VDR and low HIF-1α and in melanoma patient with low VDR and high HIF-1α. Scale bars - 50μm (lower panel). III) Differences in nuclear and cytoplasmic HIF-1α immunostaining and nuclear (black bars) and cytoplasmic (white bars) VDR in pT1–2 and pT3–4 melanomas. Statistically significant differences are denoted with p values as determined by Student’s t-test. IV) Differences cytoplasmic and nuclear HIF-1α immunostaining in primary (black bars) and metastatic (gray bars) melanomas in relation to cytoplasmic VDR immunostaining. Statistically significant differences are denoted with p values as determined by Student’s t-test.
Similarly, in primary melanomas with high cytoplasmic VDR immunostaining, the HIF-1α expression was significantly lower (Figure 1–IV). In addition, nuclear and cytoplasmic VDR showed reverse correlation with HIF-1α in melanoma metastases (r=−0.5081, p=0.0011; r=−0.3932, p=0.0107), where cases without both nuclear and cytoplasmic VDR showed highest HIF-1α expression (Figure 1–I, Figure 1–IV).
Correlation between RORα and HIF-1α expression
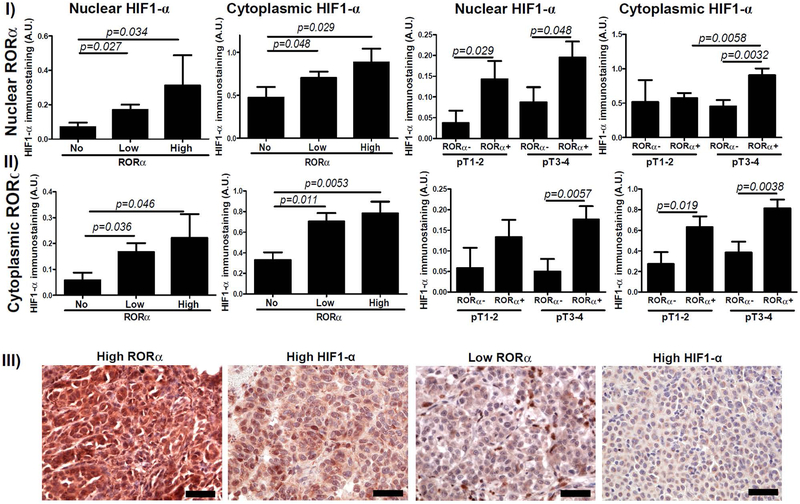
In contrast to the above, in primary and metastatic melanomas nuclear RORα expression was correlated positively with nuclear and cytoplasmic HIF-1α expression (r=0.2438, p=0.0175; r=0.3662, p=0.0166, respectively) and RORα-negative primary melanomas showed significantly lower HIF-1α immunostaining (Figure 2–I, Figure 2–III). Analysis of melanomas subgrouped with respect to pT stage revealed that both localized (pT1-pT2) and advanced melanomas (pT3-pT4) with nuclear RORα immunostaining showed high nuclear HIF-1α expression, with cytoplasmic expression being significant for pT3–T4 (Figure 2–II). Similarly, both localized and advanced melanomas with cytoplasmic RORα showed significantly higher cytoplasmic and nuclear HIF-1α (Figure 2–II).
Figure 2.
Relationship between HIF-1α and RORα immunostaining. Differences in nuclear (I) and cytoplasmic (II) HIF-1α immunostaining in relation to nuclear (upper panel) and cytoplasmic (middle panel) RORα in primary melanomas. Statistically significant differences are denoted with p values as determined by Student’s t-test. III) Representative immunostaining of RORα and HIF-1α in melanoma patient with high RORα and high HIF-1α and in melanoma patient with low RORγ and low HIF-1α. Scale bars - 50μm (lower panel).
Correlation between RORγ and HIF-1α expression
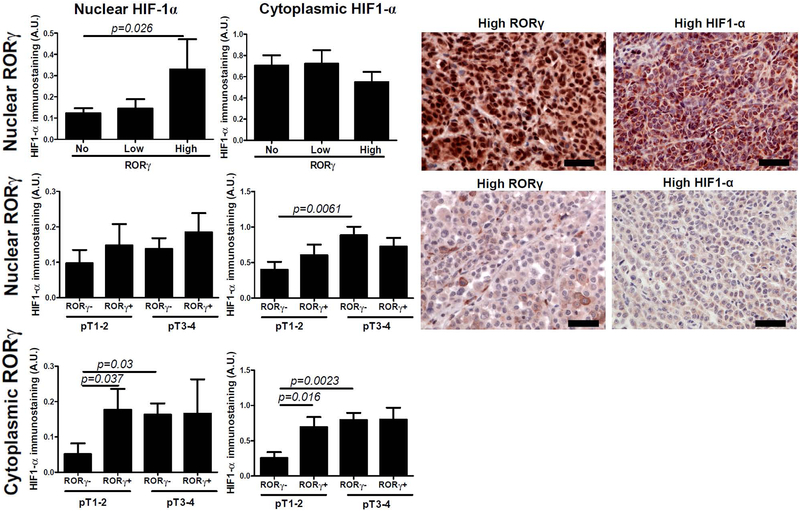
Similarly to RORα, RORγ expression in primary melanomas was correlated positively with nuclear HIF-1α expression (r=0.2743, p=0.0129) and subgroups with high nuclear RORγ staining showed higher HIF-1α expression (Figure 3). There were no statistically differences for HIF-1α expression in relation to cytoplasmic RORγ (not shown). Furthermore, analysis in relation to RORγ immunostaining and advanced tumor stage revealed lower HIF-1α expression in early (pT1-pT2) nuclear RORγ-negative melanomas (Figure 3). RORγ-positive metastatic melanoma showed higher HIF-1α expression, however, this difference did not reach the statistical significance (p=0.067, not shown)
Figure 3.
Differences in nuclear HIF1-α immunostaining and cytoplasmic HIF1-α immunostaining and nuclear (upper and middle panels) and cytoplasmic (lower panel) RORγ in primary melanomas. Statistically significant differences are denoted with p values as determined by Student’s t-test. Representative immunostaining of RORγ and HIF1-α in melanoma patient with high RORγ and high HIF1-α and in melanoma patient with low RORγ and low HIF1-α. Scale bars - 50μm.
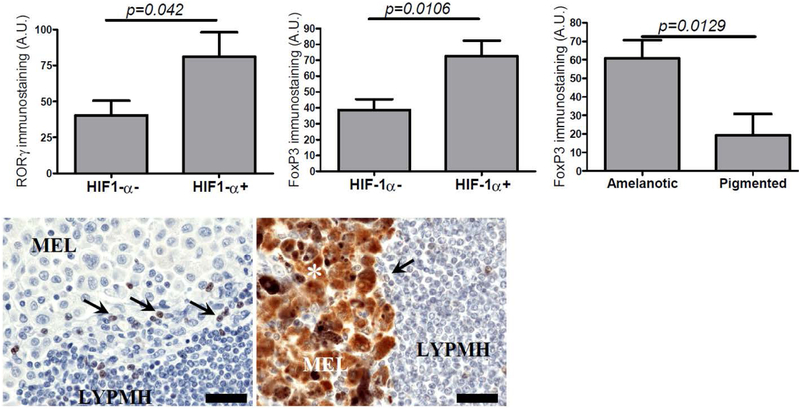
HIF-1α expression in melanoma cells also correlated with a presence of RORγ-positive lymphocytes in melanoma metastases (r=0.4599; p=0.0043) and statistically significant differences were observed between HIF-1α-positive and HIF-1α-negative cases (Figure 4).
Figure 4.
Differences in nuclear RORγ (left) and nuclear FoxP3 [15] immunostaining in metastatic lymph nodes in HIF1-α-negative and HIF1-α-positive cases. FoxP3 immunostaining in amelanotic and pigmented metastatic melanomas [72]. Statistically significant differences are denoted with p values as determined by Student’s t-test. Representative immunostaining of FoxP3 immunostaining in amelanotic (left) and pigmented metastatic mmelanomas. Asterisk – melanin, arrows – FoxP3-positive lymphocytes, MEL-melanoma cells, LYMPH-lymphocytes. Scale bars - 50μm (lower panel).
Other Parameters
FoxP3 positive Treg cells correlate with immunosuppression, we therefore analyzed FoxP3 positive lymphocytes in primary and metastatic melanomas. Sixty two percent of metastatic melanomas and 69% of primary melanomas showed the presence of FoxP3-positive lymphocytes. In metastases, immunostaining of FoxP3 in lymphocytes positively correlated with the expression of HIF-1α (r=0.3667; p=0.0327) and RORγ (r=0.4208, p=0.0129) in melanoma cells and statistically significant differences were observed between HIF1-α-negative and HIF1-α-positive cases (Figure 4). In addition, FoxP3-positive lymphocytes negatively correlated with VDR expression in melanoma cells (r=−0.3697; p=0.0264), whereas no correlation was observed of FoxP3-positive lymphocytes and RORα.
In metastatic amelanotic melanomas the percentage of FoxP3-positive lymphocytes was significantly higher than in pigmented ones (Figure 4). In primary melanomas these differences were not observed. Similarly, the expression of FoxP3 in melanoma cells did not correlate with the pigmentation of either primary or metastatic tumors.
There was no correlation between FoxP3 and other analyzed markers in primary melanomas. A comparative analysis of immunostained HIF-1α and CYP27B1 and CYP24A1 showed lack of correlation between these parameters both in primary tumors and melanoma metastases (not shown).
Discussion
Hypoxia via HIF-1α expression and HIF-1α-related pathways promotes melanoma cells survival, its growth and metastasis[56]. In the present study, we analyzed the relationship between HIF-1α expression, and the expression of nuclear receptors and enzymes involved in vitamin D metabolism. We found that in melanomas HIF-1α expression correlated with the expression of nuclear receptors, VDR (reverse correlation), RORα and RORγ (positive correlation). With respect to the VDR, this correlation was dependent on the stage of melanoma progression with the most pronounced HIF-1α expression observed in VDR-negative and advanced melanomas. Positive correlation between RORα- and RORγ and HIF-1α expression was independent of melanoma stage. Furthermore, HIF-1α-positive metastatic melanomas correlated with higher FoxP3-positive and RORγ-positive lymphocytes.
The role of vitamin D and VDR under hypoxic conditions is not clear and published papers show contradictory results. In PMA-differentiated U937 cells, 1,25(OH)2D3 significantly upregulated HIF1-α[57]. In human epithelial breast cells 1,25(OH)2D3 increased mRNA and protein HIF1-α level, while in Harvey-Ras oncogene transfected human epithelial breast cells 1,25(OH)2D3 increased only protein HIF1-α level through different regulatory mechanisms[58]. In contrast, vitamin D deficiency in mice caused HIF1-α mRNA upregulation in mesenteric perivascular adipose tissue[59]. Other authors reported that 1,25(OH)2D3 inhibited HIF1-α protein expression, its transcriptional activity and HIF1-α-target genes, with no effect on HIF1-α mRNA transcription or degradation of HIF1-α in several cancer lines[60]. Ma et al[39] found reduced expression of VDR and increased expression of CYP27B1 and CYP24A1 under hypoxic conditions. In our study, we found a negative correlation between HIF1-α and VDR expression both in primary and metastatic lesions. Furthermore, the highest HIF1-α expression was found in pT3-pT4 VDR-negative melanomas, suggesting that the effect of hypoxic conditions on VDR expression are the most pronounced in advanced melanomas.
Recent studies have shown that hypoxia induces RORα expression and that RORα expression protects keratinocytes, attenuates apoptotic potential via caspase 3/7-related pathway and promotes its survival under hypoxic conditions[43]. Furthermore, decreased expression of RORα was followed by attenuation of HIF1α-regulated expression of genes related to cell differentiation, such as involucrin, prolyl hydroxylase-3, adipose differentiation-related protein, and others[43]. Similarly, in benign and malignant tumor cells hypoxia induced RORα expression[41, 45]. Previously, we observed significant reduction of RORα expression in primary and metastatic melanomas with the lowest expression in tumors with aggressive phenotype and poor prognosis[49]. In present study, we observed higher HIF-1α expression in RORα-positive in both localized and advanced melanomas. This apparent inconsistency might be explained by a possibility that in malignant cells under conditions of oxygen depletion, RORα can regulate cell survival, as in normal cells, and by promoting cell survival RORα can also promote tumor progression, especially under hypoxia.
The role of hypoxia and immunosuppression in the induction of Treg development in tumors is not fully understood. HIF-1α may control balance between Th17 and Treg T cells by promoting Foxp3 protein degradation and inhibiting Tregs differentiation, and stabilizing the expression of RORγt and promoting Th17 development[23]. Hypoxia-induced immunosuppression can at least in part be due to a downregulation of the proliferation of CD4+ cells, reduced expression of pro-inflammatory cytokines and inhibition of Th1 differentiation under hypoxic conditions[61]. Others found hypoxia-induced expression of Foxp3-positive cells (Treg) by HIF1-α-dependent pathway[62], and FoxP3, and HIF-1α expression in metastatic gastric cancers cells were positively correlated with lack of such correlation in patients without metastasis[63]. The same study reported that supernatants from cancer cells cultured under hypoxic conditions induced the expression of Foxp3 in CD4+ T cells[63]. In addition, immunosuppression and increased number of Tregs was found in glioblastoma multiforme under hypoxic conditions[64]. Hypoxia has been reported to be required for Treg cells to induce tumor and Treg-induced angiogenesis via stimulation of chemokine (C-C motif) ligand 28 expression[65]. In Treg cells of nasal polyps the expression of RORγ and HIF-1α is increased and their levels show positive correlation to each other[66]. In present study, we observed higher level of FoxP3-positive lymphocytes in lymph nodes involved by metastatic process with higher HIF-1α expression. We also observed increased level of RORγ-positive lymphocytes in lymph nodes involved by metastatic process with high HIF-1α expression. Thus, recent identification of IL-17-producing FoxP3-positive regulatory T cells[67, 68] raises a possibility that in metastatic melanomas the hypoxic conditions can lead to differentiation towards FoxP3-positive and RORγ-positive lymphocytes. However, further studies elucidating the mechanisms of hypoxia-related changes in T lymphocytes differentiation in melanomas are needed. It must be noted that there is lack of information on the RORγ expression in cancer cells under hypoxic conditions. In our study, similarly to RORα, RORγ expression positively correlated with HIF-1α levels both in primary and metastatic melanomas. Therefore, we suggest that its expression might also be regulated by hypoxia/HIF-1α, as in normal cells[69–71].
In summary, we suggest that decreased VDR expression, which could be also related to the vitamin D deficiency in melanomas, especially in advanced melanomas, can be related to HIF1-α overexpression and, acting through HIF1-α-related pathways, can promote tumor progression. Hypoxic conditions, as identified by HIF1-α expression in metastatic melanomas cells, can attract/promote development of FoxP3-positive and/or FoxP3-positive and RORγ-positive lymphocytes that contribute to immunosuppression in metastatic melanomas. We also suggest that hypoxia, via HIF1-α, can induce RORα and RORγ, both of which can also regulate HIF1-α activities in melanomas through self-amplifying communication. Therefore, the hypoxia can affect tumor biology by changing the expression of nuclear receptors including VDR or RORα or RORγ. And we propose that by the modulation of hypoxia we can change the expression of these nuclear receptors and, in addition, its activities can be modified by different secosteroidal and lumisterol related ligands[35, 37], offering new pathways in melanoma therapy.
Acknowledgements
The research was supported in by grants 2014/15/B/NZ4/00751 from National Science Centre, Poland, 03/CM/2013 from Nicolaus Copernicus University to AAB, funds for statutory research from Collegium Medicum Nicolaus Copernicus University, NIH grants 1R01AR071189–01A1 and R01AR073004 and VA merit grant 1I01BX004293-01A1 to ATS, and the Intramural Research Program of the National Institute of Environmental Health Sciences, the National Institutes of Health [Z01-ES-100486](AMJ).
Abbreviations:
- CYP24A1
cytochrome P450 family 24 subfamily A member 1
- CYP27B1
cytochrome P450 family 27 subfamily B member 1
- HIF
hypoxia-inducible factor
- HRE
hypoxia response element
- NR
nuclear receptor
- RORα
retinoic acid receptor-related orphan receptor alpha
- RORγ
retinoic acid receptor-related orphan receptor gamma
- Treg
T regulatory cells
- VDR
vitamin D receptor
Footnotes
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wang GL, Semenza GL. J Biol Chem. 1995, 270, 1230–1237. [DOI] [PubMed] [Google Scholar]
- 2.Majmundar AJ, Wong WJ, Simon MC. Mol Cell. 2010, 40, 294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Cancer Research. 1996, 56, 4509–4515. [PubMed] [Google Scholar]
- 4.Semenza GL Oncogene. 2013, 32, 4057–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaupel P, Mayer A. Cancer Metastasis Reviews. 2007, 26, 225–239. [DOI] [PubMed] [Google Scholar]
- 6.Birner P, Schindl M, Obermair A, Plank C, Breitenecker G, Oberhuber G. Cancer Research. 2000, 60, 4693–4696. [PubMed] [Google Scholar]
- 7.Rankin EB, Giaccia AJ. Cell death and differentiation. 2008, 15, 678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rankin EB, Nam JM, Giaccia AJ. Trends in cancer. 2016, 2, 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slominski AT, Kim TK, Takeda Y, et al. , FASEB Journal: Official publication of the Federation of American Societies for Experimental Biology. 2014, 28, 2775–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valencak J, Kittler H, Schmid K, et al. , Clinical and Experimental Dermatology. 2009, 34, e962–964. [DOI] [PubMed] [Google Scholar]
- 11.Giatromanolaki A, Sivridis E, Kouskoukis C, Gatter KC, Harris AL, Koukourakis MI. Melanoma Res. 2003, 13, 493–501. [DOI] [PubMed] [Google Scholar]
- 12.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Physiological Reviews. 2004, 84, 1155–1228. [DOI] [PubMed] [Google Scholar]
- 13.Brozyna AA, Jozwicki W, Carlson JA, Slominski AT. Human Pathology. 2013, 44, 2071–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brozyna AA, Jozwicki W, Roszkowski K, Filipiak J, Slominski AT. Oncotarget. 2016, 7, 17844–17853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brozyna AA, VanMiddlesworth L, Slominski AT. Int J Cancer. 2008, 123, 1448–1456. [DOI] [PubMed] [Google Scholar]
- 16.Slominski A, Zbytek B, Slominski R. International Journal of Cancer. 2009, 124, 1470–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slominski A, Paus R, Mihm MC. Anticancer Res. 1998, 18, 3709–3715. [PubMed] [Google Scholar]
- 18.Li W, Slominski R, Slominski AT. Analytical Biochemistry. 2009, 386, 282–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scislowski PW, Slominski A, Bomirski A. The International Journal of Biochemistry. 1984, 16, 327–331. [DOI] [PubMed] [Google Scholar]
- 20.Scislowski PW, Slominski A, Bomirski A, Zydowo M. Neoplasma. 1985, 32, 593–598. [PubMed] [Google Scholar]
- 21.Bedogni B, Welford SM, Cassarino DS, Nickoloff BJ, Giaccia AJ, Powell MB. Cancer Cell. 2005, 8, 443–454. [DOI] [PubMed] [Google Scholar]
- 22.McNamee EN, Korns Johnson D, Homann D, Clambey ET. Immunologic Research. 2013, 55, 58–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dang EV, Barbi J, Yang HY, et al. , Cell. 2011, 146, 772–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jetten AM, Takeda Y, Slominski A, Kang HS. Curr Opin Toxicol. 2018, 8, 66–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar V, Gabrilovich DI. Immunology. 2014, 143, 512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsun A, Chen Z, Li B. Protein & Cell. 2011, 2, 778–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volchenkov R, Nygaard V, Sener Z, Skalhegg BS. Front Immunol. 2017, 8, 698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cook DN, Kang HS, Jetten AM. Nuclear Receptor Research. 2015, 2, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jetten AM Nuclear receptor signaling. 2009, 7, e003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jetten AM, Kang HS, Takeda Y. Frontiers in Endocrinology. 2013, 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bikle DD Can J Physiol Pharmacol. 2015, 93, 349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bikle DD, Jiang Y, Nguyen T, Oda Y, Tu CL. Front Physiol. 2016, 7, 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chagani S, Kyryachenko S, Yamamoto Y, Kato S, Ganguli-Indra G, Indra AK. J Invest Dermatol. 2016, [DOI] [PubMed] [Google Scholar]
- 34.Slominski AT, Kim TK, Hobrath JV, et al. , J Steroid Biochem Mol Biol. 2017, 173, 42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slominski AT, Brozyna AA, Skobowiat C, et al. , J Steroid Biochem Mol Biol. 2018, 177, 159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim TK, Wang J, Janjetovic Z, et al. , Molecular and Cellular Endocrinology. 2012, 361, 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slominski AT, Kim TK, Hobrath JV, et al. , Sci Rep. 2017, 7, 11434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slominski AT, Kim TK, Janjetovic Z, et al. , International Journal of Molecular Sciences. 2018, 19, [Google Scholar]
- 39.Ma R, Gu Y, Zhao S, Sun J, Groome LJ, Wang Y. American Journal of Physiology Endocrinology and Metabolism. 2012, 303, E928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato Y, Miyauchi Y, Yoshida S, et al. , PloS one. 2014, 9, e111845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chauvet C, Bois-Joyeux B, Berra E, Pouyssegur J, Danan JL. The Biochemical Journal. 2004, 384, 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chauvet C, Bois-Joyeux B, Danan JL. The Biochemical Journal. 2002, 364, 449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li H, Zhou L, Dai J. Journal of Cellular Physiology. 2018, 233, 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miki N, Ikuta M, Matsui T. J Biol Chem. 2004, 279, 15025–15031. [DOI] [PubMed] [Google Scholar]
- 45.Kim EJ, Yoo YG, Yang WK, et al. , Arteriosclerosis, Thrombosis, and Vascular Biology. 2008, 28, 1796–1802. [DOI] [PubMed] [Google Scholar]
- 46.Brozyna AA, Jochymski C, Janjetovic Z, Jozwicki W, Tuckey RC, Slominski AT. International Journal of Molecular Sciences. 2014, 15, 19000–19017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brozyna AA, Jozwicki W, Janjetovic Z, Slominski AT. Human Pathology. 2011, 42, 618–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brozyna AA, Jozwicki W, Janjetovic Z, Slominski AT. Human Pathology. 2013, 44, 374–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brozyna AA, Jozwicki W, Skobowiat C, Jetten A, Slominski AT. Oncotarget. 2016, 7, 63261–63282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brozyna AA, Jozwicki W, Slominski AT. Anticancer Res. 2014, 34, 2735–2743. [PMC free article] [PubMed] [Google Scholar]
- 51.Slominski AT, Brozyna AA, Zmijewski MA, et al. , Lab Invest. 2017, 97, 706–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slominski A, Kim TK, Brozyna AA, et al. , Arch Biochem Biophys. 2014, 563, 79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual. New Yotk: Springer International Publishing: 2018. [Google Scholar]
- 54.Brozyna AA, Jozwicki W, Jochymski C, Slominski AT. Oncology Reports. 2015, 33, 599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jozwicki W, Brozyna AA, Siekiera J, Slominski AT. International Journal of Molecular Sciences. 2015, 16, 24369–24386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bedogni B, Powell MB. Pigment Cell Melanoma Res. 2009, 22, 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee B, Kwon E, Kim Y, et al. , Immunology Letters. 2015, 163, 14–21. [DOI] [PubMed] [Google Scholar]
- 58.Jiang Y, Zheng W, Teegarden D. Cancer Letters. 2010, 298, 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pelham CJ, Drews EM, Agrawal DK. Journal of Molecular and Cellular Cardiology. 2016, 98, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ben-Shoshan M, Amir S, Dang DT, Dang LH, Weisman Y, Mabjeesh NJ. Molecular Cancer Therapeutics. 2007, 6, 1433–1439. [DOI] [PubMed] [Google Scholar]
- 61.Westendorf AM, Skibbe K, Adamczyk A, et al. , Cell Physiol Biochem. 2017, 41, 1271–1284. [DOI] [PubMed] [Google Scholar]
- 62.Ben-Shoshan J, Maysel-Auslender S, Mor A, Keren G, George J. European Journal of Immunology. 2008, 38, 2412–2418. [DOI] [PubMed] [Google Scholar]
- 63.Deng B, Zhu JM, Wang Y, et al. , PloS one. 2013, 8, e63777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wei J, Wu A, Kong LY, et al. , PloS one. 2011, 6, e16195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ren L, Yu Y, Wang L, Zhu Z, Lu R, Yao Z. Oncotarget. 2016, 7, 75763–75773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jin J, Chang DY, Kim SH, et al. , The Laryngoscope. 2014, 124, E151–159. [DOI] [PubMed] [Google Scholar]
- 67.Voo KS, Wang YH, Santori FR, et al. , Proceedings of the National Academy of Sciences of the United States of America. 2009, 106, 4793–4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ogawa C, Bankoti R, Nguyen T, et al. , Cell Rep. 2018, 25, 19–28 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shi LZ, Wang R, Huang G, et al. , J Exp Med. 2011, 208, 1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ikejiri A, Nagai S, Goda N, et al. , Int Immunol. 2012, 24, 137–146. [DOI] [PubMed] [Google Scholar]
- 71.Matsui-Hasumi A, Sato Y, Uto-Konomi A, et al. , Int Immunol. 2017, 29, 133–143. [DOI] [PubMed] [Google Scholar]