Abstract
Throughout evolutionary time, all organisms and species on Earth evolved with an adaptation to consistent oscillations of sunlight and darkness, now recognized as ‘circadian rhythm.’ Single-cellular to multi-system organisms use circadian biology to synchronize to the external environment and provide predictive adaptation to changes in cellular homeostasis. Dysregulation of circadian biology has been implicated in numerous prevalent human diseases, and subsequently targeting the circadian machinery may provide innovative preventative or treatment strategies. Discovery of ‘peripheral circadian clocks’ unleashed widespread investigations into the potential roles of clock biology in cellular, tissue, and organ function in healthy and diseased states. Particularly, oxygen-sensing pathways (e.g. hypoxia inducible factor, HIF1), are critical for adaptation to changes in oxygen availability in diseases such as myocardial ischemia. Recent investigations have identified a connection between the circadian rhythm protein Period 2 (PER2) and HIF1A that may elucidate an evolutionarily conserved cellular network that can be targeted to manipulate metabolic function in stressed conditions like hypoxia or ischemia. Understanding the link between circadian and hypoxia pathways may provide insights and subsequent innovative therapeutic strategies for patients with myocardial ischemia. This review addresses our current understanding of the connection between light-sensing pathways (PER2), and oxygen-sensing pathways (HIF1A), in the context of myocardial ischemia and lays the groundwork for future studies to take advantage of these two evolutionarily conserved pathways in the treatment of myocardial ischemia.
Keywords: PER2, hypoxia, HIF1, circadian biology, circadian disruption, myocardial ischemia
Evolutionary Significance of Light- and Oxygen-Sensing Pathways
Cyanobacteria and The Great Oxygenation Event
The evolutionary link between light- and oxygen-sensing pathways provides insight into molecular and cellular adaptation for resilience to adverse changes in the environment. The history of these two pathways may aid our understanding of adaptation to environmental changes within tissue and organ systems. When the sun formed about 4.5 billion years ago and the appearance of oxygen on Earth following suit, our planet experienced what was undoubtedly the most dramatic environmental changes during evolution. A crucial factor in the evolution of adaptation to sunlight and oxygen was cyanobacteria. These prokaryotes were some of the first organisms to appear on Earth approximately 2.7 billion years ago [1]. When cyanobacteria appeared on Earth, these photosynthetic organisms had to adapt to consistent oscillations of sunlight and darkness. In doing so, cyanobacteria that developed an internal clock were preferentially selected throughout evolutionary time; cyanobacteria express photosynthetic genes in anticipation of a light phase to optimally target metabolic processes during a time when solar resources would be available compared to an inopportune time when it would be energetically wasteful to express genes involved in photosynthesis [2, 3]. The photosynthetic property of cyanobacteria led to the by-product accumulation of oxygen in the atmosphere, which at the time contained minimal to no oxygen. After free oxygen was absorbed by the oceans, rock, and land, oxygen gas accumulated in the atmosphere to unprecedented levels ~850 million years ago, triggering The Great Oxygenation Event [1]. Here, all anaerobic organisms unable to adapt to this oxygen presence, those that experienced oxygen as a toxin, were killed off. Subsequently, all organisms that appeared on Earth thereafter were required to use oxygen for fundamental cellular processes. In unison, organisms developed the ability to sense predictable environmental changes like oscillations of day and night, and the requirement to use oxygen for cellular energy metabolism. As a result, almost all organisms on this planet to this day are equipped with light- and oxygen-sensing pathways [1–5]. While in the present-day oxygen is unquestionably therapeutic as it is frequently used in the clinical setting, not as much attention has been given to light exposure.
Light-Sensing Pathways: Mammalian Circadian Feedback Loop
Light exposure is a primary mechanism to synchronize a circadian system to the environment (circadian entrainment) [6]. Sunlight is a ‘Zeitgeber’ (external cue for circadian rhythm) and the primary mechanism of entrainment. Without it, biological systems revert to their internal ‘free-running’ clock, which is generally slightly shorter or slightly longer than the 24-hour day. Zeitgebers like sunlight serve to synchronize the internal circadian clock to the environmental clock and maintain a 24-hour oscillation. In mammalian systems, photic stimuli enter the retina and travel via the retinohypothalamic tract to the suprachiasmatic nucleus (SCN) in the brain, where the signals are transduced to the molecular clockwork [7, 8]. Blue wavelengths of light are explicitly detected by melanopsin receptors in retinal ganglion cells that leads to the transcriptional induction of PER2 in the SCN and concomitant entrainment. Blue light at 470 nm shifts the circadian phase in a melanopsin-dependent manner [9]. Only light with an intensity >180 LUX can synchronize the human circadian system [10], whereas intense light (>10,000 LUX) is most effective. Chronic disruption of circadian oscillators is considered a significant predisposing factor in many prevalent diseases including sleep disorders [11–13], bipolar affective disorder or schizophrenia [14, 15], cardiovascular disease [16–22], onset of myocardial infarction (MI) [6, 23–27] [28], cardiac arrest [29], stroke [30], immune response or sepsis [31, 32], cancer [33–40] (reviewed by [41]), diabetes, and metabolic syndromes (obesity, diabetes, endocrine, and hormonal function) [42–46]. The broad impact of circadian disruption on human health compounded by the lack of understanding regarding circadian mediated mechanisms is a continuing endeavor for many researchers. Deciphering mechanisms of circadian disruption may shed light into preventative or treatment strategies for human diseases.
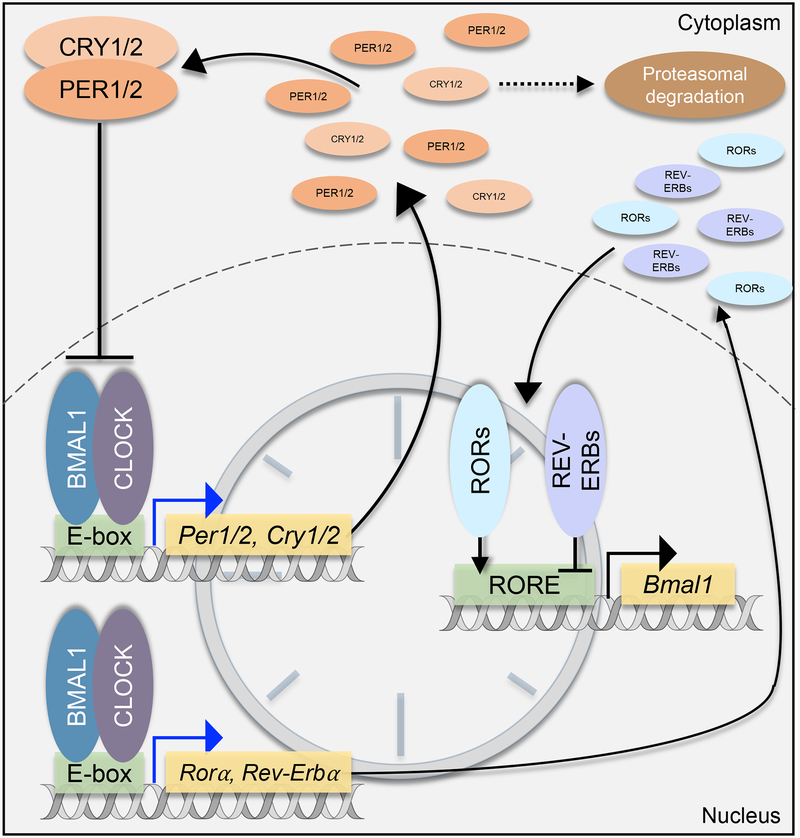
The evolution of molecular and cellular light-sensing pathways was an adaptive mechanism to coordinate with the environment’s consistent oscillations of sunlight and darkness. At the cellular level in mammals, circadian rhythm consists of a molecular negative feedback loop and four core clock proteins (Figure 1): circadian locomotor output cycle kaput (CLOCK, CLOCK), aryl hydrocarbon receptor nuclear translocator-like protein 1 (BMAL1, ARNTL), period 1/2 (PER1/2, PER1/2), and cryptochrome 1/2 (CRY1/2, CRY1/2) [47]. In an approximately 24-hour cycle, PER1/2 and CRY1/2 are expressed with protein levels accumulating in the cytoplasm gradually throughout the day, reaching a peak in the late afternoon. In the cytoplasm, PER1/2 and CRY1/2 interact with casein kinases (CK) 1ẟ and CK1ε. Phosphorylation of the clock proteins is considered to regulate subcellular localization and stability with a unique ability to lengthen the circadian phase [48–50]. In the evening, PER1/2 and CRY1/2 form a heterodimer and translocate back into the nucleus where PER1/2 and CRY1/2 act as repressors of BMAL1 and CLOCK transcriptional activity [47]. The anti-phase clock proteins BMAL1 and CLOCK form a heterodimer in the nucleus and bind to E-box response elements in the promoter regions of PER1/2 and CRY1/2 genes, driving their expression. The BMAL1 and CLOCK heterodimer also serve as transcriptional activators of the nuclear receptors ROR (retinoic acid receptor-related orphan receptors) and REV-ERB (nuclear receptor subfamily 1 group D member 1/2), which are rhythmically expressed. RORs and REV-ERBs competitively bind at the RORE (ROR binding element) in the promoter region of genes including BMAL1, CLOCK, RORA/G, and NR1D1/NR1D2 [51, 52]. REV-ERBα/β are transcriptional repressors and inhibit RORs from binding to promoter region ROREs. Besides, REV-ERBs repress BMAL1 gene expression. RORα/γ are transcriptional activators of BMAL1 and NR1D1/2 (gene for REV-ERBα/β) expression. REV-ERBs even can autoregulate since their promoter region contains ROREs [47, 51, 52]. This template of the primary negative feedback loop occurs in organisms from cyanobacteria to plants to humans [47] with many factors feeding into this 24-hour oscillation to regulate input and output functions. Such factors include the aforementioned nuclear receptors and post-translational modifiers, and additional components include light exposure, secondary signaling molecules, and oxygen fluctuation. As discussed in ensuing sections, elements of the clock function closely with oxygen-sensing pathways.
Figure 1. The circadian clock transcription-translation feedback loop functions in an approximate 24 hour cycle.
All cells have an approximate 24-hour negative feedback loop of core circadian proteins, PER1/2, CRY1/2, BMAL1, and CLOCK. PERs and CRYs, expressed upon BMAL1 and CLOCK binding to the E-box in their promoter regions, accumulate in the cytoplasm during the day. PERs and CRYs form a heterodimer and translocate back to the nucleus where they inhibit their transcriptional activators, BMAL1 and CLOCK, thereby inhibiting their own expression. Nuclear receptors like RORs and REV-ERBs regulate BMAL1 expression by either acting as activators or repressors, respectively.
Oxygen-Sensing Pathways: Hypoxia Inducible Factors
Oxygen-sensing pathways evolved after The Great Oxygenation Event and preserved the adaptive ability to sense environmental oxygen levels in the form of hypoxia inducible factors (HIFs; Figure 2) [53–56]. Physiologic hypoxia and the function of proteins essential in hypoxia have a wide variety of functions including necessity for embryonic development and cell differentiation, yet also a driver of tumor cell proliferation and tumor cell metabolism [57]. Hypoxia is, therefore, a crucial component of normal cellular function, but the machinery can be hijacked under certain circumstances leading to disease in humans. The ability of cells to adapt to hypoxia is an evolutionarily conserved, innate, and adaptive response that is advantageous for almost all organisms to rely on when environmental conditions demand the cells do so for survival.
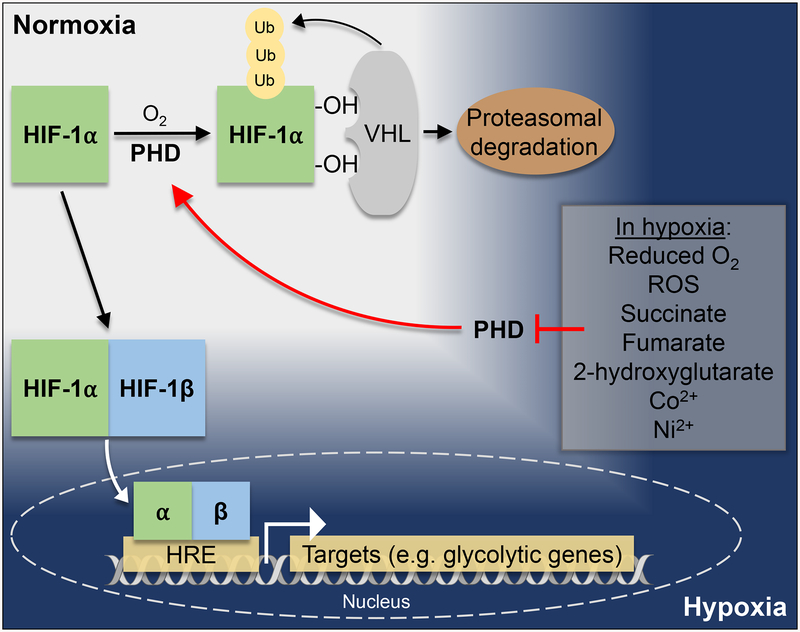
Figure 2. HIF1A is stabilized in hypoxia to dimerize with HIF1A, translocate to the nucleus, and regulate downstream targets important in adaptation to low oxygen availability.
In normoxia, HIF1A is targeted for proteasomal ubiquitination by PHDs and VHLs. In hypoxic conditions, PHDs are inhibited and therefore HIF1A stabilization is achieved. HIF1A forms a dimer with constitutively active HIF1β and translocates into the nucleus where it binds to HREs in the promoter region of target genes (adapted from [211] [212]). In hypoxia, reduced O2 availability, accumulating ROS, succinate, fumarate, 2-hydroxyglutarate, Co2+, and Ni2+ inhibit PHDs and thereby prevent HIF1A degradation.
HIFs consist of two subunits, an oxygen-regulated subunit (HIF1A or HIF2A) and a constitutively active subunit HIF1β/ARNT (in complex collectively referred to as HIF, HIF1, or HIF2). HIF1A is regulated by the oxygen sensing prolyl hydroxylase domain proteins (PHDs). In normal oxygen environments (normoxia), PHDs sense the oxygen status of the cell by using iron as a co-factor to reduce oxygen to carbon dioxide in the prolyl hydroxylation of HIF1A. This post-translational modification allows for HIF1A ubiquitination by von Hippel-Lindau tumor suppressor (VHL) and subsequent HIF1A degradation. Due to the iron-dependent nature of PHDs, lack of oxygen inhibits the functionality of PHDs, leading to HIF1A stabilization. In hypoxia, stabilized HIF1 enters the nucleus where it binds to hypoxia response elements (HREs, consensus sequence 5’-RCGTG-3’) in the promoter of genes that are crucial for adaptation to hypoxia. Many of these targets include genes expressing metabolic enzymes important for regulating metabolic balance in low oxygen conditions and in fact, specific metabolites and reactive oxygen species (ROS) produced by metabolic pathways in hypoxia inhibit PHDs [58–60]. HIF1A dependent hypoxia-sensing pathways are evolutionarily essential for environmental adaptability to changes in oxygen availability and redox status of the cell. Understanding HIF1A pathways may be a beneficial approach to overt diseases that arise from hypoxia [53, 54, 56, 61–70]. One way in which oxygen-sensing pathways link to light-sensing pathways is through the fact that the respective mediators of these pathways are part of the same superfamily of proteins: PAS domain-containing proteins.
PAS Domains: Evolutionary Link Between Circadian and Hypoxia Pathways
Linking light- and oxygen-sensing pathways begins on the cellular level in mammals [54, 60, 64, 71, 72]. HIF1A belongs to the same protein family as the core circadian proteins, the PAS domain superfamily of signal sensors for oxygen, light, or metabolism [54, 60, 64, 72, 73]. PAS domain-containing proteins are present in all kingdoms of life including Bacteria, Archaea, and Eucarya, and are present in a vast array of proteins and enzymes including kinases, chemo- and photoreceptors, circadian clock proteins, ion channels, cyclic nucleotide phosphodiesterases, hypoxia response proteins, and proteins involved in embryological central nervous system development [72]. Originally named for the first group of proteins found to contain these domains (PER, ARNT, and SIM), PAS domains are 250 – 300 amino acids in length, homologous in sequence, and the proteins containing PAS domains are a versatile group capable of sensing light, oxygen, redox status, small ligands, and energy demands of a cell. PER originates from the Drosophila PER gene identified as a key circadian rhythm regulator, ARNT was named for the aryl hydrocarbon receptor nuclear translocator protein (ARNT gene) known as the HIF1β subunit, and SIM comes from the Drosophila single-minded locus discovered for its role in regulating midline cell lineage [60]. PAS domain proteins contain basic helix-loop-helix (bHLH) motifs and are involved in a variety of cellular processes including signal transduction pathways [72] and DNA binding as transcription factors [60]. Furthermore, some PAS domains can be used for protein-protein interactions. In particular, the PAS domains in PER proteins were found to be involved in protein-protein interactions by forming homodimers or heterodimers with other PAS domain-containing proteins, such as SIM and ARNT [60, 64, 67, 73]. The shared PAS domains between PER and HIF1A suggest that their functions may be similar and perhaps have an overlapping role (Figure 3).
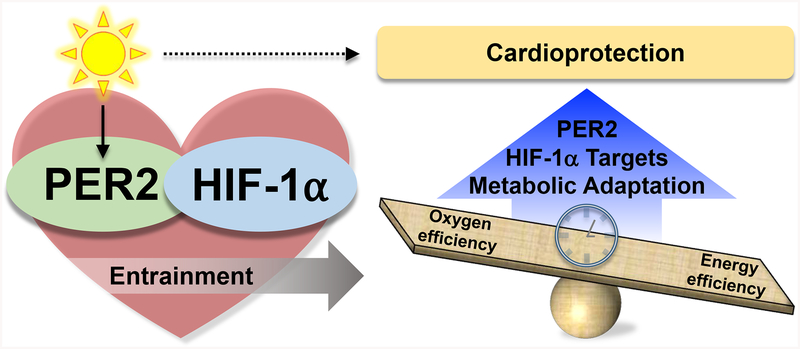
Figure 3. The circadian-hypoxia link may provide cardioprotection via PER2 and HIF1A.
PER2 and HIF1A link light- and oxygen-sensing pathways that may provide a cardioprotective effect by metabolic adaptation to low oxygen availability by mediating oxygen- versus energy-efficient metabolic pathways.
Functional Adaptation to Low Oxygen Environments
Metabolic Adaptation to Low Oxygen
The balance of energy metabolism is a critical regulatory component during hypoxia since the ability to switch between oxygen-consuming metabolic pathways and pathways that do not require oxygen is vital for adaptation to and survival from low oxygen availability. Glucose and fatty acids, two cellular fuel substrates, have strikingly different oxygen demands and high-energy phosphate yield, in the form of ATP, from catabolism. Glucose metabolism generates pyruvate for either oxidation in the mitochondria (aerobic glycolysis under normoxic conditions) or lactate (anaerobic glycolysis in hypoxia, or ‘oxygen-efficient’ since it does not require oxygen). Six moles of oxygen are used for oxidation of one mole of glucose and generates 38 high-energy phosphate bonds [74]. Conversely, 31 moles of oxygen are used for oxidation of the fatty acid palmitate and generates 129 high-energy phosphate bonds [75]. This results in glucose oxidation creating 6.3 high-energy phosphate bonds per mole of oxygen while fatty acid oxidation generates 4.1 high-energy phosphate bonds per mole of oxygen. Glucose oxidation therefore produces 53.7% more ATP for each mole of oxygen consumed than the oxidation of the fatty acid palmitate [75]. Data consistently point toward a higher yield of high-energy phosphates per mole of oxygen through glucose oxidation compared to palmitate oxidation, although some discrepancies exist: an independent study found a 12% increase in ATP between the two metabolic pathways [75] [74]. Nonetheless, the key takeaway from these studies is in the comparison of ATP generated per gram of glucose versus per gram of palmitate: fatty acid metabolism yields significantly more ATP per gram of palmitate compared to the ATP generated per gram of glucose. When oxygen availability is plentiful, fatty acid metabolism is, therefore, more energy efficient, but when oxygen availability is limited, a fast and efficient balance between metabolic pathways is beneficial, if not necessary [75]. Balancing metabolic pathways in hypoxia is of dire importance due to oxygen being used as a terminal electron acceptor in oxidative phosphorylation [76]. Electrons are shuttled through the mitochondrial membrane during respiration and excessive ROS production during periods of hypoxia in cells that are not adequately adapted will quickly become detrimental [77, 78]. Taken together, the balance between glucose or fatty acid oxidation will depend on oxygen availability and is therefore critically important for organs like the heart to manipulate during periods of hypoxia or ischemia.
Fuel source preference is cell-type specific and metabolic pathway usage is generally determined by the cell’s function. Cardiac myocytes rely primarily on fatty acids to fuel oxidative phosphorylation, so it’s beneficial for the ischemic heart to adapt by optimizing metabolic pathways when oxygen availability is limited. Cardiac myocytes are not the most abundant cell type by number, but instead have the highest volume compared to other cell types that make up the heart [79, 80], which include fibroblasts, endothelial cells, stem cells, and inflammatory cells [79]. Notably, endothelial cells are considered to have the primary role in myocardial ischemia reperfusion (IR)-injury [81–84] and are considered oxygen sensors due to their ability to tolerate a range of oxygen tensions and their high sensitivity to IR-injury [82, 85–88]. Additionally, endothelial cells have the ability in hypoxia to generate oxygen-independent ATP and use the Pentose Phosphate Pathway (PPP, an anabolic pathway branched off of the glycolytic pathway) to generate NADPH and nucleic acid precursors [89]. Endothelial cells are indeed flexible and adaptive in their metabolic pathway usage to meet cellular needs with regards to energy demand and redox status, and differences persist between endothelia based on oxygen exposure, which varies between arterial, venous, and microvascular endothelial cells [90]. While there exists a debate in the field as to whether endothelial cells or fibroblasts make up the majority of the heart’s cellular composition, this discrepancy is most likely due to limitations in detection methods with estimates ranging from ~60% endothelial cells and ~20% fibroblasts to ~6% endothelial cells and ~64% fibroblasts [79, 80]. Regardless of total composition, most researchers agree that cardiac fibroblasts are key factors in fibrosis, remodeling, and arrhythmogenesis [80] while endothelial cells are directly correlated with overall cardiac function outcomes after myocardial IR-injury [82, 91, 92]. Managing cellular metabolism when oxygen availability is limited serves as a critical mechanism for survival, and many studies point toward the interplay between circadian and oxygen pathways to be the core of this regulation.
HIF1A Mediated Metabolic Adaptation to Hypoxia
HIFs are well established for their cardioprotective role in hypoxia or ischemia, through key pathways including metabolic adaptation, angiogenesis, and the stem cell niche. For example, HIF1A stabilization via PHD gene deletion increases angiogenesis and post-MI cardiac function through pathways including VEGF proangiogenic signaling and the endothelial nitric oxide synthase pathway [93–95] (reviewed by[96]). Additionally, stabilization of HIF1A by hypoxia or PHD inhibition in cardiosphere-derived cells (from cardiac explants) or endothelial cells revealed a potential role for HIF1A in the adult stem cell niche, which is inherently hypoxic, including regulating cardiac stem cell marker genes, genes involved in proliferation, or telomerase (TERT) gene expression (involved in pluripotency state of stem cells) [97, 98]. Importantly, the hypoxic stem cell niche must regulate cellular metabolism to maintain appropriate stem-ness or drive proliferative capacity and differentiation, which ties in HIF as an important mediator [99]. Our understanding of HIF1A in metabolic adaptation to low oxygen environments, whether in the stem cell niche or during a cardiac ischemic event, may shed light on the benefits of targeting HIF1A and metabolic pathways during IR-injury.
Metabolic adaptation via HIF1A to hypoxia or ischemia is well-researched in the field and several mechanisms are identified in how HIF1A mediates the balance between glycolytic and oxidative metabolism. Cells adapt to acute or prolonged hypoxic environments by regulating metabolic pathways and activating HIF1A. HIF1A stabilization and function is determined by oxygen availability, and in turn, HIF1 promotes cellular homeostasis in terms of redox potential and energy production from metabolic pathways [100]. While the tricarboxylic acid cycle (TCA cycle) isn’t a direct consumer of oxygen, it is tightly coupled to oxidative phosphorylation, and HIF1 regulates flux into the TCA cycle to mediate downstream oxygen consumption during hypoxia. In normoxia, the TCA cycle reduces NAD+ and FAD to NADH and FADH2, which are oxidized by oxidative phosphorylation. However, during hypoxia, a strain on mitochondrial respiration prevents NADH and FADH2 from being readily oxidized. Therefore, when oxygen availability is limited, one mechanism of HIF1 mediated metabolic adaptation involves shuttling metabolites away from the mitochondria to regulate TCA cycle flux and oxidative phosphorylation to overall reduce ROS production. In concordance, mouse embryonic fibroblasts subjected to long-term hypoxia revealed HIF1 binding at an HRE in the promoter region the gene encoding pyruvate dehydrogenase kinase 1, which is responsible for phosphorylating and inactivating the catalytic subunit of pyruvate dehydrogenase, thereby inactivating the conversion of pyruvate to Acetyl-CoA [101]. In general, HIF1 is best described for upregulating its downstream gene targets including those involved in glucose metabolic pathways and glucose transporters to promote an increased flux of glucose reliance [101]. Essentially, HIF1 works to shift the balance between metabolic pathways by inhibiting pyruvate from entering the TCA cycle and subsequent oxidative phosphorylation. This potentially counteracts ROS that would be produced from the overuse of mitochondrial respiration in hypoxia [102].
Another mechanism of HIF1 mediated adaptation to hypoxia is by targeting oxidative phosphorylation. Complex IV (COX4, cytochrome c oxidase subunit 4) of oxidative phosphorylation has a HIF1 inducible subunit that is necessary for adaptation to low oxygen availability. In normoxia, COX4.1 is the dominant subunit, but in hypoxia, COX4.2 is upregulated by HIF1 via an HRE in the promoter region. Additionally, the COX4.1 subunit is degraded in a HIF1 dependent manner by the HIF1 mediated upregulation of the mitochondrial protease LON [103]. The significance of HIF1 mediated “subunit switching” between COX4.1 and COX4.2 to optimize oxidative phosphorylation in hypoxia is most likely because mitochondrial respiration cannot completely shut down, but rather optimizes how it uses oxygen. COX4 is the primary oxygen consumer and site of electron transfer to oxygen, but the mitochondrial strain in hypoxia can drive electron transfer to oxygen prematurely at Complex I or Complex III, which significantly increases ROS production [103]. This suggests regulating and optimizing mitochondrial respiration at the level of COX4 as another critical target for hypoxic adaptation.
HIF1A Converges with Light-Sensing Pathways via PER2
Ischemic Preconditioning of the Heart Links PER2 and HIF1A to Myocardial Ischemia Adaptation Mechanisms
In this review, we address the intersection of PER2 and HIF1A in the context of myocardial ischemia. Cardiovascular disease is the leading cause of death worldwide, causing approximately one death every 40 seconds in the United States, and therefore demands innovative research initiatives to develop novel therapeutic strategies for prevention or treatment [104]. A key target in the prevention of myocardial ischemia, which may provide impactful long-term effects on cardiovascular health, is a metabolic adaptation to low oxygen availability. In this regard, oxygen deficiency resulting from myocardial ischemia requires the heart to metabolically adapt to balance energy production with oxygen consumption.
Years before HIF1A was linked to cardiac PER2, Dr. Robert Berne proposed the 1960’s ‘Adenosine Hypothesis’ that postulated adenosine to be cardioprotective by balancing oxygen supply and oxygen demand during myocardial ischemia [105]. This hypothesis was initially received with skepticism until the 1970s, when adenosine receptors were identified and characterized, elucidating potential cardioprotective effects of adenosine [106]. Subsequently, in the 1980s, Dr. Charles Murry found that brief (5 min) intervals of ischemia and reperfusion (a procedure termed ischemic preconditioning (IPC)) were cardioprotective from the traditional myocardial ischemia and reperfusion (IR)-injury model [107]. Within the last decade, our lab revealed that the ectonucleotidases CD39 and CD73, the enzymes that break down extracellular ATP to AMP and AMP to adenosine, are essential for the cardioprotective effect of IPC [108] [109]. Furthermore, investigations into adenosine signaling during IPC identified a unique role for the adenosine A2B receptor to be necessary for cardioprotection [109]. It wasn’t until 2012 that studies diving into the mechanisms of cardioprotection through adenosine signaling at the A2B receptor uncovered the circadian rhythm protein Period2 (PER2) as a downstream target through both an upregulation of cAMP, phospho-CREB, and PER2 transcript, and an inhibition of PER2 protein degradation via Cullen deneddylation [110]. Indeed, peripheral tissues like the heart display oscillations in PER2 expression similar to those of the brain [110, 111] and are thought to be secreted through neurohormonal signaling molecules [7, 112, 113] or adenosine, as circulating 5’-AMP and adenosine were found to be diurnal in nature [112] with an established role for adenosine signaling in peripheral tissues like the heart [110].
Mechanistically, PER2 was found necessary for HIF1A stabilization in hypoxia [110]. Due to the known role of HIF1A in metabolic adaptation to hypoxia, subsequent studies determined PER2 to be a regulator of carbohydrate metabolism and to be cardioprotective during in situ myocardial (IR)-injury [110]. The critical role of HIF1A in cardioprotection from ischemic preconditioning (IPC) has been elucidated by numerous investigators and sheds light on additional mechanisms of adaptation to low oxygen availability resulting from ischemia. For example, one research group took isolated perfused hearts from mice that were exposed to a preconditioning protocol of no-flow ischemia for 5 minutes and 5 minutes of reperfusion and found protection from a prolonged IR phase that was abolished in the hearts from HIF1A+/− mice [114]. These findings were supported by cardiac small interfering RNAs for HIF1A expression that also inhibited cardioprotection during IPC [115]. Mechanisms into HIF1A mediated cardioprotection uncovered that the loss in cardioprotection from IPC in HIF1A+/− mouse hearts can be rescued by adenosine perfusion during IPC [114]. Additionally, gene expression of the ecto-5’-nucleotidase CD73 is under the control of HIF1A in hypoxia [116] and CD73 deficient mice do not demonstrate cardioprotection from IPC [109]. Because adenosine perfusion provides cardioprotection from IR-injury in HIF1A+/− mouse hearts [114], these data suggest that adenosine production is dependent on HIF1A [102] and may provide additional mechanisms and pathway targets for optimizing HIF1A dependent cardioprotection from low oxygen conditions by manipulating circadian PER2.
PER2 and HIF1A Regulation: A Bi-Directional Relationship
Studies deciphering the connection between PER2 and HIF1A confirmed the hypothesis that PER2 is an upstream regulator of HIF1A. PER2−/− mice express less HIF1A mRNA, which resembles less HIF1A gene expression found in ADORA2B−/− mice compared to wildtype. Furthermore, HIF1A failed to be stabilized in ADORA2B−/− mice upon oxygen depletion and these mice were unable to induce gene expression of glycolytic enzymes during IPC, an effect that similarly resembles that seen in PER2−/− mice [110]. Specifically, cardiac HIF1A protein oscillates in a circadian pattern along with protein levels of two HIF1A downstream transcriptional targets in the glycolytic pathway, pyruvate dehydrogenase kinase and lactate dehydrogenase. Notably, PER2 was necessary for this HIF1A oscillation and without PER2, HIF1A is not stabilized during IPC of the heart [110]. These prior experiments together suggest that: 1) PER2 is dependent on A2B signaling, 2) HIF1A is dependent on PER2 for glycolytic gene expression, and 3) PER2 may be a master upstream regulator of metabolic adaptation to low oxygen conditions [110].
In support of a circadian oscillation pattern of cardiac HIF1A protein levels dependent on PER2 [110], another research group reported the necessity of the circadian clock for the ability of HIF1A to sense cellular oxygen status [117]. In fact, circadian clockwork has been found to regulate HIF1A nuclear protein level localization and oscillation in mouse brain and kidney [73]. In a direct link between circadian and hypoxia pathways, HIF1A was found to work in conjunction with BMAL1 at the HRE in the PER2 promoter and the binding enhanced the amplitude of PER2 oscillation in skeletal muscle [117]. Additionally, investigational ChIP-seq studies revealed an E-box in the promoter for the HIF1A gene that was transcriptionally driven by binding of the BMAL1-CLOCK complex [118]. There is a growing body of evidence supporting a bi-directional link between circadian and hypoxia pathways, including PER2 dependent HIF1A binding to HREs in promoter regions of hypoxic target genes from in vitro experiments in HeLa cells [119] to zebrafish experiments discovering hypoxia mediated expression of the PER2 gene through regulating HIF1A binding at E-box regions in promoters [120]. Moreover, investigations into this circadian-hypoxia link revealed daily rhythmic oxygen levels in the blood and tissue of rodents, within a physiologic range (5 – 8 % oxygen), that peaked during the dark phase compared to the light phase. Mimicking rhythmic oxygenation fluctuations in vitro was sufficient to reset the circadian clock by synchronization in a HIF1A-dependent fashion [72, 121]. Together, these studies support the hypothesis that PER2 is an effector protein for HIF1A and suggest a role for HIF1A in the core circadian loop and a bi-directional relationship between light- and oxygen-sensing pathways.
PER2 and HIF1A Link Light- and Oxygen-Sensing Pathways in Metabolic Regulation
The circadian clockwork is widely appreciated for its intricate role balancing metabolic pathways and reciprocally, metabolic by-products serve as signaling cues for the circadian machinery. Many investigations into the innerworkings of the circadian system and metabolic homeostasis have revealed circadian biology as a key regulator of energy metabolism in many organ systems and tissues, including circadian metabolomics studies in humans [122–126]. In a mouse model of circadian misalignment where mice were housed in non-24-hour light:dark (L:D) cycles, researchers found significant physiological disturbances and in particular, poorer metabolic efficiency, altered substrate utilization, and a greater depression of cardiac function [127]. In mouse liver, high temporal resolution metabolite profiling revealed more than 50% of detected metabolites were regulated by the clock [128]. Furthermore, circadian gene expression oscillation is in part controlled by cellular redox status and NADPH, and in reverse, redox status and the Pentose Phosphate Pathway (PPP) are sufficient to maintain circadian amplitude. These data indicate a bi-directional feedback system between the circadian clock and metabolic homeostasis, rather than a hierarchy of single-directional downstream effects [129].
Metabolic Regulation via Sirtuins and PER2
The NAD+-dependent deacetylases, sirtuins (SIRT), provide a direct connection between metabolic pathways and the circadian clock. While sirtuins were first described by their nuclear targets for silencing gene expression by histone deacetylation, sirtuins are now appreciated for more than their nuclear role and in fact have broad applications in many cellular processes considering they are also cytoplasmic and mitochondrial proteins. In mammals, there are seven members of the sirtuin family, named SIRT1–7. Amongst them, they differ in tissue expression, subcellular localization, enzymatic activity, and target proteins (SIRT1, 6, and 7 in the nucleus, SIRT2 in the cytoplasm, and SIRT3, 4, and 5 in the mitochondria) [130]. Nuclear sirtuins (SIRT1,6,7) are responsible for rhythmic histone acetylation, representing circadian control of sirtuin activity [131]. In turn, nuclear sirtuins regulate circadian clock gene expression. Specifically, the promoter regions of PER1, PER2, and CRY1 have rhythmic histone-3 acetylation and subsequent rhythmic RNA polymerase II binding [131]. This may be due in part to the competitive inhibition of sirtuins by nicotinamide, the product of NAD+ transformation by sirtuins, indicating a negative feedback loop for its own regulation [132]. In addition, as a cofactor for sirtuins, NAD+ regulates sirtuin enzyme activity. Considering NAD+ levels increase during cellular stress, sirtuins are considered an adaptive mechanism by regulating downstream gene targets. NAD+ biosynthesis is rhythmic and oxidative enzymes in the mitochondria are subject to rhythmic acetylation [133, 134]. Furthermore, biosynthesis of NAD+ is performed by the rate limiting enzyme NAMPT, which also oscillates in a circadian manner. Inhibiting NAMPT reduces SIRT1 suppression of the CLOCK-BMAL1 complex and results in an increase in PER2 oscillation. To complete this loop, CLOCK transcriptionally regulates NAMPT gene expression, suggesting that NAMPT and NAD+ are both part of the core circadian feedback loop [135]. In the cytoplasm, the circadian rhythm protein PER2 undergoes rhythmic lysine acetylation and deacetylation by SIRT1, which regulates robust circadian oscillation of core clock genes and their protein activity [136]. The circadian deacetylase activity of SIRT1 is dependent on the metabolic by-product and circadian controlled NAD+ and SIRT1 deacetylation of PER2 results in its degradation in a circadian manner, establishing a relationship between PER2 and cellular metabolism [76, 136].
Mitochondrial SIRT3 is expressed mostly highly in tissues that are very metabolically active, including the brain, heart, liver, brown adipose tissue, and skeletal muscle [132, 137–139]. In particular, SIRT3 targets the TCA cycle and mitochondrial enzymes to deacetylate and regulate their activity [140–144]. These mitochondrial enzymes are involved in fatty acid oxidation, amino acid metabolism, oxidative phosphorylation, and antioxidant defenses [132]. SIRT3 deacetylates and therefore activates both isocitrate dehydrogenase 2 (IDH2) and succinate dehydrogenase (SDH), the latter of which is functional in both the TCA cycle and oxidative phosphorylation [132, 143]. To protect against cellular ROS, SIRT3 depends on IDH2 [142]. Importantly, SIRT3 is the only mitochondrial sirtuin responsible for mitochondrial protein deacetylation, as lack of SIRT3 in mice results in hyperacetylation of mitochondrial proteins that is not exhibited in SIRT4 or SIRT5 deficient mice. In addition, the SIRT3 deficient mice, but not SIRT4 or SIRT5 deficient mice, presented with dire downstream metabolic defect [137, 145]. These metabolic defects in SIRT3 deficient mice include metabolic syndrome, cancer, and cardiac failure [132, 146, 147]. Lastly, one study found a cardioprotective role of SIRT3 in an isolated ischemia and reperfusion injury model [145] and that SIRT3 protects cardiomyocytes from oxidative stress [148].
PER2 and Nuclear Receptors
Genetic PER2 deficient mice provide insight into metabolic defects and the role of PER2 in metabolic pathways. PER2 appears to be a key regulator of white versus brown adipose tissue, the latter of which relies heavily on oxidative metabolism: PER2−/− mice have reduced adiposity and the lack of PER2 affects gene expression such that white adipose tissue begins expressing genes involved in metabolic pathways that are usually only expressed in brown adipose tissue. These data suggest that without PER2, the adipose tissue now more closely resembling oxidative brown adipose tissue runs oxidative metabolism at a higher rate [149–151]. This phenotype may be due to the interaction of PER2 with the nuclear receptors PPARγ, PPARα, and REV-ERBα, which regulate cellular metabolism in white adipose tissue, liver, and the heart [51, 150]. PER2 directly inhibits the nuclear receptor PPARγ, which has a known role in adipogenesis and insulin sensitivity, by preventing it from reaching the promoter and thereby decreasing transcriptional activity of its targets [150]. Additionally, the REV-ERBα targets in the liver include genes involved in glucose metabolism. Furthermore, the interaction of PER2 with REV-ERBα results in rhythmic expression of these genes [51, 152, 153]. Lastly, PER2 was found to regulate gluconeogenesis and glycogen catabolism by working in concert with nuclear receptors, a mechanism seen in both liver and the heart [51, 154, 155].
PER2 and Cardiac Metabolism
Recent investigations found a necessity of PER2 in cardioprotection by regulating metabolic pathways. Studies from in vivo stable isotope glucose tracers during baseline, myocardial ischemia, or myocardial IR-injury, revealed the inability of PER2−/− mice to rely on glycolysis during ischemia, as indicated by 13C-fructose-1,6-bisphosphate, 13C-pyruvate, and 13C-lactate concentrations. Additionally, PER2−/− mice had an increase in flux of the TCA cycle during ischemia whereas wildtype mice attenuated TCA cycle flux, confirming the role of PER2 in metabolic reliance in low oxygen environments [110]. In a comparison between PER2−/− and wildtype mouse hearts by high-throughput gene array analysis during IPC [156], lipid metabolism pathways were found to be predominately dysregulated. This finding was supported by nuclear magnetic resonance imaging identifying altered fatty acid accumulation in the heart of PER2−/− mice [157] and electron microscopy analysis of PER2−/− mouse hearts that revealed mitochondrial swelling and glycogen accumulation, indicators of severe metabolic defects [110]. Investigations into potential PER2 mediated pathways began with gene array analysis of PER2−/− mouse hearts compared to wildtype mouse hearts during myocardial IR-injury and revealed dysregulated protein phosphatase 1, a key regulator of blood-glucose levels and glycogen metabolism [158], and enoyl-CoA hydratase, the enzyme in the latter end of β-oxidation that makes the precursor to Acetyl-CoA for the TCA cycle [157, 159, 160]. In addition, PER2−/− mouse hearts exhibited a reduction in long chain fatty acids and increases in carnitine palmitoyltransferase 1 protein during myocardial ischemia, indicating that PER2 is an important regulator of enyol-CoA hydratase and therefore fatty acid metabolism in the heart [110]. Furthermore, PER2−/− mice maintained elevated levels of cardiac glycogen and elevated glycogen synthase 1 protein compared to wildtype mice prior to ischemia, but the PER2−/− mice were unable to restore the glycogen stores following ischemia [110]. Together, these data suggest PER2 is an important regulator of glucose and lipid metabolism and open the door to further investigation into the role of PER2 in downstream pathways for regulating the metabolic balance in low oxygen conditions [110, 157].
An additional level to the bi-directional relationship between hypoxia and circadian clockwork includes a connection to sirtuins. SIRT3 was found to be a key regulator of mitochondrial enzymes so researchers began investigating its function in more detail. These studies found SIRT3 to be a crucial regulator of the Warburg effect (glycolysis in the presence of abundant oxygen availability) in cancer cells and it does so by destabilizing HIF1A. Without SIRT3, there’s an increase in ROS and subsequently HIF1A stabilization [161]. This was found in human breast cancer cells that have a reduced level of SIRT3 expression correlated with an increase in HIF1A regulated genes. SIRT3 overexpression thereby was found to repress glycolysis in these tumor cells and subsequent breast cancer cell proliferation, which may be a mechanism for regulating tumor suppression [161]. Considering SIRT3 and its co-factor NAD+ are under circadian control, regulating metabolic pathways may be at the crossroad of the PER2-HIF1A connection. Indeed, the circadian-hypoxia link is intertwined in a reciprocal relationship with cellular energy metabolism, which is thought to provide optimal and advantageous adaptive mechanisms for changes in the cellular environment throughout evolutionary time. Understanding these mechanisms may provide insights into mechanistic targets for prevention or treatment from conditions of low oxygen availability like myocardial ischemia.
The reciprocal relationship between PER2 and HIF1A suggests that while the circadian clockwork controls metabolic pathways, by-products of these metabolic pathways are sensed by the circadian machinery for adaptation [162]. Because circadian machinery is manipulated by light exposure, regulating light may target downstream light-sensing pathways. In fact, the timing of light exposure markedly affects circadian profiles and specifically PER2 protein concentrations in the hearts of rats [111] and accordingly, our initial investigations addressed the possibility that light exposure could function to attenuate myocardial IR-injury by increasing cardiac PER2 levels. Indeed, we observed a time-dependent induction of cardiac PER2 with significant reduction in infarct size and troponin-I release following daylight exposure in our IR-injury model in mice [110, 156]. These findings led us to investigate mechanisms of PER2 mediated cardioprotection in hypoxia or ischemia. Our results contribute to a growing body of work suggesting an evolutionarily conserved and advantageous bi-directional relationship between light-sensing (circadian) and oxygen-sensing (hypoxia) driven pathways [120] and may provide clinical benefits to improve outcomes for patients with myocardial ischemia. Here, we present our findings from circadian PER2 mediated metabolic adaptation to low oxygen availability and concomitant cardioprotection from hypoxia or ischemia.
Circadian Disruption at the Root of Disease and Circadian Considerations in Therapeutic Strategies
Circadian Disruption is Associated with a Multitude of Prevalent Diseases, Including Cardiovascular Disease
Seasonal changes in daylight exposure is a mechanism of circadian rhythmicity yet can also be a mechanism of disruption. One such study of found that humans differentially adjust to daylight savings time based on chronotype with an overall inability to sufficiently adjust from spring to fall [163]. Chronobiologists propose that our modern-day society removes the natural seasonal nature of humans (human behavior tends to be seasonal, like reproduction, mortality, and suicides [164, 165]), resulting in disruption of and dissociation from circadian rhythmicity [163]. Furthermore, studies of our modern-day light exposure found delays in circadian rhythmicity, with a significant circadian disruption following even just one weekend of late-night light exposure, a common trend for those balancing work or school in the typical work-week [166]. Differences in light exposure based on latitude location revealed that individuals furthest away from the equator tend fit into the late-chronotype category [167]. Because intense light (>10,000 LUX) is most effect at entraining the circadian system [10], those further away from the equator may have dampening of circadian amplitude compared to those who receive strong light exposure closer to the equator and most likely a more robust circadian amplitude [167]. Additionally, dampening of the circadian oscillator is part of aging and associated with multiple aging-related diseases [168].
Another common mechanism of circadian disruption is shift-work or social-jetlag. In the present day, approximately 22% of the population is engaged in some form of shift-work, which includes any variation of work outside the typical work day, such as night-shift, multiple shifts, and frequent shift in times of work [169, 170]. Those who participate in shift-work for long periods of time are heavily associated with overall poor health outcomes and are at a significantly higher risk for many diseases, not only the aforementioned cardiovascular disease and metabolic syndromes, but including ulcers, cancer, and obstructive sleep apnea [16, 42–46, 127, 169–174]. The significant association between shift-workers and disease emphasizes the importance in understanding circadian biology to hopefully identify targets for prevention or treatment of those with disrupted circadian rhythm.
In particular, the onset of myocardial infarction (MI) has a distinct circadian pattern and recent findings suggest light exposure, the foundation of circadian rhythms, as a potential strategy for treating myocardial ischemia and preventing MI [6, 23–27]. Furthermore, epidemiological studies revealed that MIs occur more frequently in the early morning hours than later in the day, are more prevalent within the first three hours after waking up in the morning than any other time of the day, and infarctions occurring in the morning are more severe than those that do present in the evening [24, 25, 175, 176]. Additional epidemiological studies revealed that there is a similar increase in MIs across all 50 states in the USA during the darker winter months [175, 176]. Experimental studies found circadian oscillations of neutrophil recruitment to heart, which determines infarct size, healing, and cardiac function after MI. Neutrophil recruitment to the heart occurs more preferentially during the active phase and heart attacks during this time have significantly higher cardiac neutrophil infiltration. MI during this time (active phase) has higher neutrophilic inflammatory response and worse cardiac repair. By limiting the neutrophil recruitment during the heart in the active phase, there was a reduction in infarct size and increase in improvement of cardiac function [177]. However, the mechanism underlying the circadian component of MIs and the role of circadian biology in cardioprotection is only recently under intense investigation.
Maintaining a Robust Circadian Amplitude through Entrainment
A form of circadian disruption is dampening of circadian amplitude, which is observed as part of the aging process [168]. Therefore, researchers believe that a robust circadian timekeeping system is important for human health and well-being [4, 5, 174, 178–180]. Indeed, approaches to increase entrainment and thereby the robustness of the clock timing process have been found to be beneficial in certain disease states. One study found that restricted feeding increased the circadian amplitude and could prevent mice from becoming obese when exposed to a high fat diet [181]. Another study found using short term caloric restricted feeding in mice to be cardioprotective. In addition, cardiac microRNA profiling of short term caloric restricted feeding was associated with the circadian clock [182]. Similarly, an independent study tested whether activity alters or could rescue a disrupted circadian system. Here, they examined the effects of wheel access on vasointestinal polypeptide (VIP)-deficient mice, a model that exhibits circadian deficits [183]. Indeed, voluntary scheduled exercise increased the amplitude of PER2 expression and rescued the disrupted circadian system in VIP deficient mice. Some studies on blue enriched light exposure on rats found similar effects on circadian rhythmicity and amplitude. In addition, marked positive effect on the circadian regulation of neuroendocrine, metabolic, and physiologic parameters associated with the promotion of animal health and wellbeing was observed [184]. Similarly, one study demonstrated that day/night rhythms play a critical role in compensatory remodeling of cardiovascular tissue, and disruption of day/night rhythms exacerbated disease pathophysiology [185]. Here, they used a murine model of pressure overload cardiac hypertrophy in a rhythm-disruptive 20-hour versus 24-hour environment. Echocardiographic studies revealed increased left ventricular end-systolic and -diastolic dimensions and reduced contractility in rhythm-disturbed animals exposed to transverse aortic constriction. Considering these findings, it is compelling that light exposure, restricted feeding, or scheduled exercise could also be cardioprotective through strengthening the circadian clock. Since microRNAs can be regulated by external cues such as light, without involvement of the circadian rhythm proteins, it is probable that light exposure also improves a disrupted circadian system through enhancing the amplitude of microRNAs and optimizing their function.
Circadian Disruption in the Clinical Environment
Unfortunately, patients in the intensive care unit (ICU) are subjected to severe circadian disruption, which we are well aware promotes disease. The ICU closely mimics a full 24 hours of light exposure and circadian disruption in the ICU is known to be detrimental to human health [186–190]. ICU patients are noted to have significant mitochondrial and endothelial dysfunction accompanied by defects in nitric oxide synthesis and pyruvate dehydrogenase activity, functions regulated by circadian proteins [5, 113, 191]. Furthermore, recent epidemiological studies revealed that ICU patients who received cardiac surgery in the morning have significantly worse outcomes than those who receive surgery in the afternoon as measured by troponin levels and long-term adverse cardiac events [192]. Many investigators have begun manipulating light exposure in mouse models to determine downstream effects of circadian disruption. The most commonly used lighting in the vivarium setting used to be cool white fluorescent lights, but many institutions have begun switching to light-emitting diodes (LED) for their numerous economic advantages. However, LED lighting has high blue light emissions and its effects on laboratory animal’s circadian system and physiology needs to be considered. Recently, one group found significant positive effects on circadian-regulated neuroendocrine, metabolic, and physiologic outcomes in rats exposed to daylight LED [184], indicating a need to consider housing conditions in animal studies. In fact, housing mice in constant light is sufficient to dampen and delay PER2 oscillation in the SCN, eventually losing complete rhythmicity [193]. These studies emphasize the need to consider light exposure and the circadian system for not only experimental animal studies but also for patients in the ICU and people in the general population, considering circadian biology and circadian disruption is tightly associated with physiological function and dysfunction.
Lastly, another mechanism of circadian disruption is through general anesthesia. Anesthesia-mediated circadian disruption is a recognized phenomenon, however its role has not been fully elucidated yet [4, 5]. Interestingly, several medications used in a daily clinical setting can disrupt the well-coordinated expression of the circadian rhythm network. As such, it has been found that anesthetics disrupt the expression of the circadian proteins including PER2, which could lead to sleep disturbances following general anesthesia [194, 195]. Studies have shown that propofol anesthesia in humans affects the circadian period, leading to increased resting activities during the day [194]. Animal studies found ketamine to reduce the amplitude of circadian rhythm gene expression [196]. Other studies using sevoflurane, a volatile anesthetic similar to isoflurane, found suppression of PER2 in the brain of rodents [197]. However, research on the influence of anesthetics on circadian rhythm protein or PER2 expression in specifically the heart is in its infancy. Understanding anesthesia mediated disruption in the brain may provide insights into circadian disruption in peripheral tissues. GABA (γ-aminobutyric acid) is the principal neurotransmitter in the SCN and studies identifying GABA to be responsible for phase shifting and synchronizing cells of the SCN indicate that GABA is responsible for synchronizing the clock in the whole animal and peripheral tissues [198]. GABA is primarily known as an inhibitory neurotransmitter in the central nervous system, however it is diurnal in nature. GABA is an inhibitory neurotransmitter during the night, which decreases the firing frequency, but GABA is an excitatory neurotransmitter during the day, which increases the firing frequency. This dual role of GABA happens through GABAA receptors and due to the daily oscillations of intracellular chloride concentrations through chloride-permeable ion channels opening and closing [199, 200]. This indicates that the inhibitory or excitatory nature of GABA is regulated by the time of day and perhaps boosts neural rhythm amplitude to drive SCN outputs to the peripheral organs [199] [200]. That being said, anesthetic GABA-mediated disruption may have detrimental effects on peripheral organs like the heart.
Pharmacological Timing and Targeting of the Circadian System (Time-of-Day-Dependent Role of Cardiovascular Drugs)
The notion that disrupted circadian rhythm can affect all aspects of physiologic homeostasis is supported by researchers who identified a significant portion of the genome – and therefore a variety of cellular processes – to be under circadian control. Recent investigations into the 24-hour transcriptome suggest that much of the mammalian genome is under circadian control. RNA sequencing of major tissues and brain regions of baboons, a primate closely related to humans, revealed 81.7% of the protein-coding genome is under circadian control, as indicated by rhythmic gene expression. These gene products are in fact part of a diverse range of biochemical and cellular functions [201]. Furthermore, the transcriptome of 64 tissues with sampling every 2 hours over a 24-hour period, tissue-specific rhythmic expression was noted, and rhythmic genes tended to peak around early morning (dawn) and sunset (dusk). There was also an inactive or “quiescent period” in the beginning hours of the night [201]. In parallel, another research group found by RNA-seq and DNA arrays of 12 mouse organs over 24 hours that 43% of protein coding genes are expressed in a circadian pattern. In general, oscillating genes tended to peak just preceding dawn and dusk, supporting the previously described results [201, 202]. Further investigations found that more than 1,000 noncoding RNAs oscillate in a circadian day, as well, suggesting more than just messenger RNA has a circadian pattern [202]. Taken together, while there are many ways to disrupt circadian biology, these mechanisms may prove to be targets for preventative or therapeutic strategies in diseases arising from desynchrony that ultimately target the circadian clockwork.
With a significant portion of the genome evidently under control of the circadian machinery, there have been many investigations into the human chronobiome to potentially develop time-of-day targeted therapies. A pilot study gathered data on diurnal variations of cardiovascular and behavioral phenotypes from healthy human volunteers and measured “omics” outputs. This study revealed a time-of-day dependent variation in blood pressure, activity level, light exposure, and food consumption associated with time-of-day dependent variation in the metabolome, proteome, and transcriptome [202, 203]. Recent studies using RNA-seq from 632 human samples including 13 different tissues revealed several common pharmacological treatments, like antihypertensive drugs (calcium channel and β-blockers) target gene products that are rhythmically expressed in the cardiovascular system [204]. Together, these studies strongly suggest the consideration of circadian biology in clinical treatment strategies.
In a small molecule screen for circadian clock modifiers, many potential pharmacological molecules were identified with targets anywhere in the pathway, whether intracellularly, within the feedback loop, or in metabolic processes. These compounds were found to alter the period, phase, or amplitude of the circadian clock [205]. There are many factors that feed into the molecular circadian feedback loop that are targets for regulating downstream effects. The polymethoxylated flavone nobiletin, originally identified as the naturally occurring compound in citrus peels, is a clock amplitude-enhancing small molecule. It was identified as a small molecular target of circadian amplitude enhancement and PER2 target through this high-throughput chemical screen [205, 206]. After its identification, nobiletin was found to be protective against metabolic disease by acting as an agonist for the ROR nuclear receptor thereby reprogramming circadian mediated metabolic gene expression [205–207]. In fact, ROR nuclear receptors are key for stabilizing the molecular oscillating loop [206] and nobiletin enhances the amplitude of circadian rhythm and regulates energy homeostasis in terms of body weight, food intake, body composition, and VO2 max. Nobiletin also regulates sleep behavior in a clock dependent manner and with respect to metabolism, nobiletin improves glucose and lipid homeostasis in mice, which is under circadian clock control [206]. Nobiletin increases RORα/γ transcriptional activity by binding directly to this nuclear receptor by competitive inhibition and NOB dose dependently increases BMAL1 promoter driven luciferase reporter activity and dependent upon RORE elements (DNA binding site) [206]. Nobiletin, as a target of nuclear receptors, is a potential mechanism to manipulate circadian clock function. Indeed, our group found that nobiletin induces cardiac Per2 and provides a cardioprotective effect from myocardial IR injury in a Per2-dependent manner. Furthermore, we demonstrated that the deleterious effects on the heart of the commonly-prescribed benzodiazepine midazolam can be rescued when nobiletin is administered prior to myocardial IR-injury [208]. Our studies are in line with other groups targeting nuclear receptors, in particular REV-ERBs, as a promising strategy in the context of cardiac IR-injury. For example, REV-ERB agonists administered to mice prior to MI model yielded improved left ventricular function and survival compared to controls, potentially through mediating the inflammatory response and remodeling [209]. Conversely, cohort studies in humans suggest REV-ERBα antagonism may be cardioprotective strategy [192], albeit with debatable interpretation of time-of-day gene expression analyses [210]. Together, these studies indicate a need for further investigation of REV-ERB modulation in cardioprotective strategies.
Conclusion
The evolutionary link between light- and oxygen-sensing pathways provides insight into molecular and cellular adaptation for resilience to adverse changes in the environment. Insights gained from an advanced understanding of the evolutionarily conserved relationship between light- and oxygen-sensing pathways, will ultimately provide new therapeutic opportunities to treat conditions of low oxygen availability, such as myocardial ischemia.
Source of financial support for the review:
National Heart, Lung, and Blood Institute (NIH-NHLBI) 5R01HL122472 Grant to T.E.
American Heart Association (AHA) Pre-doctoral Fellowship 16PRE30510006 and Colorado Clinical & Translational Sciences Institute (CCTSI) Pre-Doctoral Fellowship TL1 TR001081 to C.M.B.
Footnotes
Conflict of interest: The authors declare no competing interests
REFERENCES
- [1].Zerkle AL, Poulton SW, Newton RJ, Mettam C, Claire MW, Bekker A, Junium CK. Onset of the aerobic nitrogen cycle during the Great Oxidation Event. Nature, 2017; 542: 465–467. [DOI] [PubMed] [Google Scholar]
- [2].Gaudana SB, Krishnakumar S, Alagesan S, Digmurti MG, Viswanathan GA, Chetty M, Wangikar PP. Rhythmic and sustained oscillations in metabolism and gene expression of Cyanothece sp. ATCC 51142 under constant light. Front Microbiol, 2013; 4: 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Saha R, Liu D, Hoynes-O’Connor A, Liberton M, Yu J, Bhattacharyya-Pakrasi M, Balassy A, Zhang F, Moon TS, Maranas CD, Pakrasi HB. Diurnal Regulation of Cellular Processes in the Cyanobacterium Synechocystis sp. Strain PCC 6803: Insights from Transcriptomic, Fluxomic, and Physiological Analyses. MBio, 2016; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brainard J, Gobel M, Bartels K, Scott B, Koeppen M, Eckle T. Circadian rhythms in anesthesia and critical care medicine: potential importance of circadian disruptions. Semin Cardiothorac Vasc Anesth, 2015; 19: 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Brainard J, Gobel M, Scott B, Koeppen M, Eckle T. Health implications of disrupted circadian rhythms and the potential for daylight as therapy. Anesthesiology, 2015; 122: 1170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jakubcakova V, Oster H, Tamanini F, Cadenas C, Leitges M, van der Horst GT, Eichele G. Light entrainment of the mammalian circadian clock by a PRKCA-dependent posttranslational mechanism. Neuron, 2007; 54: 831–43. [DOI] [PubMed] [Google Scholar]
- [7].Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet, 2008; 9: 764–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau KW, Hattar S. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature, 2008; 453: 102–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pilorz V, Tam SK, Hughes S, Pothecary CA, Jagannath A, Hankins MW, Bannerman DM, Lightman SL, Vyazovskiy VV, Nolan PM, Foster RG, Peirson SN. Melanopsin Regulates Both Sleep-Promoting and Arousal-Promoting Responses to Light. PLoS Biol, 2016; 14: e1002482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science, 1980; 210: 1267–9. [DOI] [PubMed] [Google Scholar]
- [11].Toh KL, Jones CR, He Y, Eide EJ, Hinz WA, Virshup DM, Ptacek LJ, Fu YH. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science, 2001; 291: 1040–3. [DOI] [PubMed] [Google Scholar]
- [12].Ptacek LJ, Jones CR, Fu YH. Novel insights from genetic and molecular characterization of the human clock. Cold Spring Harb Symp Quant Biol, 2007; 72: 273–7. [DOI] [PubMed] [Google Scholar]
- [13].He Y, Jones CR, Fujiki N, Xu Y, Guo B, Holder JL, Jr., Rossner MJ, Nishino S, Fu YH. The transcriptional repressor DEC2 regulates sleep length in mammals. Science, 2009; 325: 866–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Albrecht U Circadian clocks and mood-related behaviors. Handb Exp Pharmacol, 2013: 227–39. [DOI] [PubMed] [Google Scholar]
- [15].Cuninkova L, Brown SA. Peripheral circadian oscillators: interesting mechanisms and powerful tools. Ann N Y Acad Sci, 2008; 1129: 358–70. [DOI] [PubMed] [Google Scholar]
- [16].van Amelsvoort LG, Schouten EG, Kok FJ. Impact of one year of shift work on cardiovascular disease risk factors. J Occup Environ Med, 2004; 46: 699–706. [DOI] [PubMed] [Google Scholar]
- [17].Kivimaki M, Virtanen M, Elovainio M, Vaananen A, Keltikangas-Jarvinen L, Vahtera J. Prevalent cardiovascular disease, risk factors and selection out of shift work. Scand J Work Environ Health, 2006; 32: 204–8. [DOI] [PubMed] [Google Scholar]
- [18].Harma M Shift work and cardiovascular disease--from etiologic studies to prevention through scheduling. Scand J Work Environ Health, 2001; 27: 85–6. [DOI] [PubMed] [Google Scholar]
- [19].Boggild H, Knutsson A. Shift work, risk factors and cardiovascular disease. Scand J Work Environ Health, 1999; 25: 85–99. [DOI] [PubMed] [Google Scholar]
- [20].Akerstedt T, Knutsson A, Alfredsson L, Theorell T. Shift work and cardiovascular disease. Scand J Work Environ Health, 1984; 10: 409–14. [DOI] [PubMed] [Google Scholar]
- [21].Akerstedt T, Knutsson A. Cardiovascular disease and shift work. Scand J Work Environ Health, 1997; 23: 241–2. [DOI] [PubMed] [Google Scholar]
- [22].Tuchsen F, Hannerz H, Burr H. A 12 year prospective study of circulatory disease among Danish shift workers. Occup Environ Med, 2006; 63: 451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Staels B When the Clock stops ticking, metabolic syndrome explodes. Nat Med, 2006; 12: 54–5; discussion 55. [DOI] [PubMed] [Google Scholar]
- [24].Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, Poole WK, Passamani E, Roberts R, Robertson T, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med, 1985; 313: 1315–22. [DOI] [PubMed] [Google Scholar]
- [25].Suarez-Barrientos A, Lopez-Romero P, Vivas D, Castro-Ferreira F, Nunez-Gil I, Franco E, Ruiz-Mateos B, Garcia-Rubira JC, Fernandez-Ortiz A, Macaya C, Ibanez B. Circadian variations of infarct size in acute myocardial infarction. Heart, 2011; 97: 970–6. [DOI] [PubMed] [Google Scholar]
- [26].Braunwald E On circadian variation of myocardial reperfusion injury. Circ Res, 2012; 110: 6–7. [DOI] [PubMed] [Google Scholar]
- [27].Cohen MC, Rohtla KM, Lavery CE, Muller JE, Mittleman MA. Meta-analysis of the morning excess of acute myocardial infarction and sudden cardiac death. Am J Cardiol, 1997; 79: 1512–6. [DOI] [PubMed] [Google Scholar]
- [28].Tofler GH, Brezinski D, Schafer AI, Czeisler CA, Rutherford JD, Willich SN, Gleason RE, Williams GH, Muller JE. Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. N Engl J Med, 1987; 316: 1514–8. [DOI] [PubMed] [Google Scholar]
- [29].Jeyaraj D, Haldar SM, Wan X, McCauley MD, Ripperger JA, Hu K, Lu Y, Eapen BL, Sharma N, Ficker E, Cutler MJ, Gulick J, Sanbe A, Robbins J, Demolombe S, Kondratov RV, Shea SA, Albrecht U, Wehrens XH, Rosenbaum DS, Jain MK. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature, 2012; 483: 96–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Panza JA, Epstein SE, Quyyumi AA. Circadian variation in vascular tone and its relation to alpha-sympathetic vasoconstrictor activity. N Engl J Med, 1991; 325: 986–90. [DOI] [PubMed] [Google Scholar]
- [31].Eckle T, Eltzschig HK. Toll-like receptor signaling during myocardial ischemia. Anesthesiology, 2011; 114: 490–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Silver AC, Arjona A, Walker WE, Fikrig E. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity, 2012; 36: 251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ballesta A, Dulong S, Abbara C, Cohen B, Okyar A, Clairambault J, Levi F. A combined experimental and mathematical approach for molecular-based optimization of irinotecan circadian delivery. PLoS Comput Biol, 2011; 7: e1002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Innominato PF, Focan C, Gorlia T, Moreau T, Garufi C, Waterhouse J, Giacchetti S, Coudert B, Iacobelli S, Genet D, Tampellini M, Chollet P, Lentz MA, Mormont MC, Levi F, Bjarnason GA, Chronotherapy Group of the European Organization for R, Treament of C. Circadian rhythm in rest and activity: a biological correlate of quality of life and a predictor of survival in patients with metastatic colorectal cancer. Cancer Res, 2009; 69: 4700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Innominato PF, Giacchetti S, Bjarnason GA, Focan C, Garufi C, Coudert B, Iacobelli S, Tampellini M, Durando X, Mormont MC, Waterhouse J, Levi FA. Prediction of overall survival through circadian rest-activity monitoring during chemotherapy for metastatic colorectal cancer. Int J Cancer, 2012; 131: 2684–92. [DOI] [PubMed] [Google Scholar]
- [36].Innominato PF, Roche VP, Palesh OG, Ulusakarya A, Spiegel D, Levi FA. The circadian timing system in clinical oncology. Ann Med, 2014; 46: 191–207. [DOI] [PubMed] [Google Scholar]
- [37].Innominato PF, Spiegel D, Ulusakarya A, Giacchetti S, Bjarnason GA, Levi F, Palesh O. Subjective sleep and overall survival in chemotherapy-naive patients with metastatic colorectal cancer. Sleep Med, 2015; 16: 391–8. [DOI] [PubMed] [Google Scholar]
- [38].Ortiz-Tudela E, Iurisci I, Beau J, Karaboue A, Moreau T, Rol MA, Madrid JA, Levi F, Innominato PF. The circadian rest-activity rhythm, a potential safety pharmacology endpoint of cancer chemotherapy. Int J Cancer, 2014; 134: 2717–25. [DOI] [PubMed] [Google Scholar]
- [39].Ortiz-Tudela E, Innominato PF, Rol MA, Levi F, Madrid JA. Relevance of internal time and circadian robustness for cancer patients. BMC Cancer, 2016; 16: 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Levi F, Dugue PA, Innominato P, Karaboue A, Dispersyn G, Parganiha A, Giacchetti S, Moreau T, Focan C, Waterhouse J, Spiegel D, Group AC. Wrist actimetry circadian rhythm as a robust predictor of colorectal cancer patients survival. Chronobiol Int, 2014; 31: 891–900. [DOI] [PubMed] [Google Scholar]
- [41].Ballesta A, Innominato PF, Dallmann R, Rand DA, Levi FA. Systems Chronotherapeutics. Pharmacol Rev, 2017; 69: 161–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kawakami N, Takatsuka N, Shimizu H. Sleep disturbance and onset of type 2 diabetes. Diabetes Care, 2004; 27: 282–3. [DOI] [PubMed] [Google Scholar]
- [43].Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol, 2009; 5: 253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Suwazono Y, Dochi M, Sakata K, Okubo Y, Oishi M, Tanaka K, Kobayashi E, Kido T, Nogawa K. A longitudinal study on the effect of shift work on weight gain in male Japanese workers. Obesity (Silver Spring), 2008; 16: 1887–93. [DOI] [PubMed] [Google Scholar]
- [45].Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet, 1999; 354: 1435–9. [DOI] [PubMed] [Google Scholar]
- [46].Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med, 2004; 1: e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet, 2017; 18: 164–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lee C, Etchegaray JP, Cagampang FR, Loudon AS, Reppert SM. Posttranslational mechanisms regulate the mammalian circadian clock. Cell, 2001; 107: 855–67. [DOI] [PubMed] [Google Scholar]
- [49].Gallego M, Virshup DM. Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol, 2007; 8: 139–48. [DOI] [PubMed] [Google Scholar]
- [50].Eng GWL, Edison, Virshup DM. Site-specific phosphorylation of casein kinase 1 delta (CK1delta) regulates its activity towards the circadian regulator PER2. PLoS One, 2017; 12: e0177834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Schmutz I, Ripperger JA, Baeriswyl-Aebischer S, Albrecht U. The mammalian clock component PERIOD2 coordinates circadian output by interaction with nuclear receptors. Genes Dev, 2010; 24: 345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Welch RD, Flaveny CA. REV-ERB and ROR: therapeutic targets for treating myopathies. Phys Biol, 2017; 14: 045002. [DOI] [PubMed] [Google Scholar]
- [53].Semenza GL. Life with oxygen. Science, 2007; 318: 62–4. [DOI] [PubMed] [Google Scholar]
- [54].McIntosh BE, Hogenesch JB, Bradfield CA. Mammalian Per-Arnt-Sim proteins in environmental adaptation. Annu Rev Physiol, 2010; 72: 625–45. [DOI] [PubMed] [Google Scholar]
- [55].Semenza GL. Hypoxia and human disease-and the Journal of Molecular Medicine. J Mol Med (Berl), 2007; 85: 1293–4. [DOI] [PubMed] [Google Scholar]
- [56].Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol (1985), 2000; 88: 1474–80. [DOI] [PubMed] [Google Scholar]
- [57].Liu W, Shen SM, Zhao XY, Chen GQ. Targeted genes and interacting proteins of hypoxia inducible factor-1. Int J Biochem Mol Biol, 2012; 3: 165–78. [PMC free article] [PubMed] [Google Scholar]
- [58].Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P, Giallongo A. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem, 1996; 271: 32529–37. [DOI] [PubMed] [Google Scholar]
- [59].Wenger RH, Gassmann M. Oxygen(es) and the hypoxia-inducible factor-1. Biol Chem, 1997; 378: 609–16. [PubMed] [Google Scholar]
- [60].Gu YZ, Hogenesch JB, Bradfield CA. The PAS superfamily: sensors of environmental and developmental signals. Annu Rev Pharmacol Toxicol, 2000; 40: 519–61. [DOI] [PubMed] [Google Scholar]
- [61].Semenza GL. Regulation of tissue perfusion in mammals by hypoxia-inducible factor 1. Exp Physiol, 2007; 92: 988–91. [DOI] [PubMed] [Google Scholar]
- [62].Semenza GL. Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem J, 2007; 405: 1–9. [DOI] [PubMed] [Google Scholar]
- [63].Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE, 2007; 2007: cm8. [DOI] [PubMed] [Google Scholar]
- [64].Hogenesch JB, Gu YZ, Jain S, Bradfield CA. The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc Natl Acad Sci U S A, 1998; 95: 5474–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Huang LE, Arany Z, Livingston DM, Bunn HF. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J Biol Chem, 1996; 271: 32253–9. [DOI] [PubMed] [Google Scholar]
- [66].Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem, 1997; 272: 22642–7. [DOI] [PubMed] [Google Scholar]
- [67].Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A, 1998; 95: 11715–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Haase C, Yu L, Eisenbarth G, Markholst H. Antigen-dependent immunotherapy of non-obese diabetic mice with immature dendritic cells. Clin Exp Immunol, 2010; 160: 331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Haase VH. The sweet side of HIF. Kidney Int, 2010; 78: 10–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Isoe T, Makino Y, Mizumoto K, Sakagami H, Fujita Y, Honjo J, Takiyama Y, Itoh H, Haneda M. High glucose activates HIF-1-mediated signal transduction in glomerular mesangial cells through a carbohydrate response element binding protein. Kidney Int, 2010; 78: 48–59. [DOI] [PubMed] [Google Scholar]
- [71].Edgar RS, Green EW, Zhao Y, van Ooijen G, Olmedo M, Qin X, Xu Y, Pan M, Valekunja UK, Feeney KA, Maywood ES, Hastings MH, Baliga NS, Merrow M, Millar AJ, Johnson CH, Kyriacou CP, O’Neill JS, Reddy AB. Peroxiredoxins are conserved markers of circadian rhythms. Nature, 2012; 485: 459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Taylor BL, Zhulin IB. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol Mol Biol Rev, 1999; 63: 479–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Button EL, Bersten DC, Whitelaw ML. HIF has Biff - Crosstalk between HIF1a and the family of bHLH/PAS proteins. Exp Cell Res, 2017; 356: 141–145. [DOI] [PubMed] [Google Scholar]
- [74].Apstein CS, Taegtmeyer H. Glucose-insulin-potassium in acute myocardial infarction: the time has come for a large, prospective trial. Circulation, 1997; 96: 1074–7. [DOI] [PubMed] [Google Scholar]
- [75].Kessler G, Friedman J. Metabolism of fatty acids and glucose. Circulation, 1998; 98: 1351. [PubMed] [Google Scholar]
- [76].O’Neill JS, Feeney KA. Circadian redox and metabolic oscillations in mammalian systems. Antioxid Redox Signal, 2014; 20: 2966–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Aon MA, Cortassa S, Marban E, O’Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem, 2003; 278: 44735–44. [DOI] [PubMed] [Google Scholar]
- [78].Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem, 2000; 275: 25130–8. [DOI] [PubMed] [Google Scholar]
- [79].Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA, Tallquist MD. Revisiting Cardiac Cellular Composition. Circ Res, 2016; 118: 400–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Zhou P, Pu WT. Recounting Cardiac Cellular Composition. Circ Res, 2016; 118: 368–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Yang Q, He GW, Underwood MJ, Yu CM. Cellular and molecular mechanisms of endothelial ischemia/reperfusion injury: perspectives and implications for postischemic myocardial protection. Am J Transl Res, 2016; 8: 765–77. [PMC free article] [PubMed] [Google Scholar]
- [82].Singhal AK, Symons JD, Boudina S, Jaishy B, Shiu YT. Role of Endothelial Cells in Myocardial Ischemia-Reperfusion Injury. Vasc Dis Prev, 2010; 7: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Lefer AM, Tsao PS, Lefer DJ, Ma XL. Role of endothelial dysfunction in the pathogenesis of reperfusion injury after myocardial ischemia. FASEB J, 1991; 5: 2029–34. [DOI] [PubMed] [Google Scholar]
- [84].Lefer AM, Lefer DJ. Endothelial dysfunction in myocardial ischemia and reperfusion: role of oxygen-derived free radicals. Basic Res Cardiol, 1991; 86 Suppl 2: 109–16. [DOI] [PubMed] [Google Scholar]
- [85].Davidson SM, Duchen MR. Endothelial mitochondria: contributing to vascular function and disease. Circ Res, 2007; 100: 1128–41. [DOI] [PubMed] [Google Scholar]
- [86].Quintero M, Colombo SL, Godfrey A, Moncada S. Mitochondria as signaling organelles in the vascular endothelium. Proc Natl Acad Sci U S A, 2006; 103: 5379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Ricci JE, Waterhouse N, Green DR. Mitochondrial functions during cell death, a complex (I-V) dilemma. Cell Death Differ, 2003; 10: 488–92. [DOI] [PubMed] [Google Scholar]
- [88].Mattson MP, Kroemer G. Mitochondria in cell death: novel targets for neuroprotection and cardioprotection. Trends Mol Med, 2003; 9: 196–205. [DOI] [PubMed] [Google Scholar]
- [89].Bierhansl L, Conradi LC, Treps L, Dewerchin M, Carmeliet P. Central Role of Metabolism in Endothelial Cell Function and Vascular Disease. Physiology (Bethesda), 2017; 32: 126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Eelen G, de Zeeuw P, Simons M, Carmeliet P. Endothelial cell metabolism in normal and diseased vasculature. Circ Res, 2015; 116: 1231–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Fedalen PA, Piacentino V 3rd, Jeevanandam V, Fisher C, Greene J, Margulies KB, Houser SR, Furukawa S, Singhal AK, Goldman BI. Pharmacologic pre-conditioning and controlled reperfusion prevent ischemia-reperfusion injury after 30 minutes of hypoxia/ischemia in porcine hearts. J Heart Lung Transplant, 2003; 22: 1234–44. [DOI] [PubMed] [Google Scholar]
- [92].Mohara J, Aguilera I, Goldman BI, Fisher CA, Gaughan JP, Libonati JR, Furukawa S, Singhal AK. Effects of nutrient and hemoglobin enriched cell free perfusates upon ex vivo isolated rat heart preparation. ASAIO J, 2005; 51: 288–95. [DOI] [PubMed] [Google Scholar]
- [93].Oriowo B, Thirunavukkarasu M, Selvaraju V, Adluri RS, Zhan L, Takeda K, Fong GH, Sanchez JA, Maulik N. Targeted gene deletion of prolyl hydroxylase domain protein 3 triggers angiogenesis and preserves cardiac function by stabilizing hypoxia inducible factor 1 alpha following myocardial infarction. Curr Pharm Des, 2014; 20: 1305–10. [DOI] [PubMed] [Google Scholar]
- [94].Adluri RS, Thirunavukkarasu M, Dunna NR, Zhan L, Oriowo B, Takeda K, Sanchez JA, Otani H, Maulik G, Fong GH, Maulik N. Disruption of hypoxia-inducible transcription factor-prolyl hydroxylase domain-1 (PHD-1−/−) attenuates ex vivo myocardial ischemia/reperfusion injury through hypoxia-inducible factor-1alpha transcription factor and its target genes in mice. Antioxid Redox Signal, 2011; 15: 1789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Wang J, Hong Z, Zeng C, Yu Q, Wang H. NADPH oxidase 4 promotes cardiac microvascular angiogenesis after hypoxia/reoxygenation in vitro. Free Radic Biol Med, 2014; 69: 278–88. [DOI] [PubMed] [Google Scholar]
- [96].Selvaraju V, Parinandi NL, Adluri RS, Goldman JW, Hussain N, Sanchez JA, Maulik N. Molecular mechanisms of action and therapeutic uses of pharmacological inhibitors of HIF-prolyl 4-hydroxylases for treatment of ischemic diseases. Antioxid Redox Signal, 2014; 20: 2631–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Tan SC, Gomes RS, Yeoh KK, Perbellini F, Malandraki-Miller S, Ambrose L, Heather LC, Faggian G, Schofield CJ, Davies KE, Clarke K, Carr CA. Preconditioning of Cardiosphere-Derived Cells With Hypoxia or Prolyl-4-Hydroxylase Inhibitors Increases Stemness and Decreases Reliance on Oxidative Metabolism. Cell Transplant, 2016; 25: 35–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Yang D, Wang J, Xiao M, Zhou T, Shi X. Role of Mir-155 in Controlling HIF-1alpha Level and Promoting Endothelial Cell Maturation. Sci Rep, 2016; 6: 35316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].De Miguel MP, Alcaina Y, de la Maza DS, Lopez-Iglesias P. Cell metabolism under microenvironmental low oxygen tension levels in stemness, proliferation and pluripotency. Curr Mol Med, 2015; 15: 343–59. [DOI] [PubMed] [Google Scholar]
- [100].Solaini G, Baracca A, Lenaz G, Sgarbi G. Hypoxia and mitochondrial oxidative metabolism. Biochim Biophys Acta, 2010; 1797: 1171–7. [DOI] [PubMed] [Google Scholar]
- [101].Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab, 2006; 3: 177–85. [DOI] [PubMed] [Google Scholar]
- [102].Semenza GL. Hypoxia-inducible factor 1: regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim Biophys Acta, 2011; 1813: 1263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell, 2007; 129: 111–22. [DOI] [PubMed] [Google Scholar]
- [104].Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics−−2014 update: a report from the American Heart Association. Circulation, 2014; 129: e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Berne RM. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am J Physiol, 1963; 204: 317–22. [DOI] [PubMed] [Google Scholar]
- [106].Dobson GP, Arsyad A, Letson HL. The Adenosine Hypothesis Revisited: Modulation of Coupling between Myocardial Perfusion and Arterial Compliance. Front Physiol, 2017; 8: 824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation, 1986; 74: 1124–36. [DOI] [PubMed] [Google Scholar]
- [108].Kohler D, Eckle T, Faigle M, Grenz A, Mittelbronn M, Laucher S, Hart ML, Robson SC, Muller CE, Eltzschig HK. CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation, 2007; 116: 1784–94. [DOI] [PubMed] [Google Scholar]
- [109].Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, Eltzschig HK. Cardioprotection by ecto-5’-nucleotidase (CD73) and A2B adenosine receptors. Circulation, 2007; 115: 1581–90. [DOI] [PubMed] [Google Scholar]
- [110].Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, Lowes BD, Han J, Borchers CH, Buttrick PM, Kominsky DJ, Colgan SP, Eltzschig HK. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med, 2012; 18: 774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Bendova Z, Sumova A. Photoperiodic regulation of PER1 and PER2 protein expression in rat peripheral tissues. Physiol Res, 2006; 55: 623–32. [DOI] [PubMed] [Google Scholar]
- [112].Zhang J, Kaasik K, Blackburn MR, Lee CC. Constant darkness is a circadian metabolic signal in mammals. Nature, 2006; 439: 340–3. [DOI] [PubMed] [Google Scholar]
- [113].Bonney S, Hughes K, Harter PN, Mittelbronn M, Walker L, Eckle T. Cardiac period 2 in myocardial ischemia: clinical implications of a light dependent protein. Int J Biochem Cell Biol, 2013; 45: 667–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Cai Z, Zhong H, Bosch-Marce M, Fox-Talbot K, Wang L, Wei C, Trush MA, Semenza GL. Complete loss of ischaemic preconditioning-induced cardioprotection in mice with partial deficiency of HIF-1 alpha. Cardiovasc Res, 2008; 77: 463–70. [DOI] [PubMed] [Google Scholar]
- [115].Eckle T, Kohler D, Lehmann R, El Kasmi K, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation, 2008; 118: 166–75. [DOI] [PubMed] [Google Scholar]
- [116].Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP. Ecto-5’-nucleotidase (CD73)regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest, 2002; 110: 993–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Peek CB, Levine DC, Cedernaes J, Taguchi A, Kobayashi Y, Tsai SJ, Bonar NA, McNulty MR, Ramsey KM, Bass J. Circadian Clock Interaction with HIF1alpha Mediates Oxygenic Metabolism and Anaerobic Glycolysis in Skeletal Muscle. Cell Metab, 2017; 25: 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Wu Y, Tang D, Liu N, Xiong W, Huang H, Li Y, Ma Z, Zhao H, Chen P, Qi X, Zhang EE. Reciprocal Regulation between the Circadian Clock and Hypoxia Signaling at the Genome Level in Mammals. Cell Metab, 2017; 25: 73–85. [DOI] [PubMed] [Google Scholar]
- [119].Kobayashi M, Morinibu A, Koyasu S, Goto Y, Hiraoka M, Harada H. A circadian clock gene, PER2, activates HIF-1 as an effector molecule for recruitment of HIF-1alpha to promoter regions of its downstream genes. Febs j, 2017; 284: 3804–3816. [DOI] [PubMed] [Google Scholar]
- [120].Egg M, Koblitz L, Hirayama J, Schwerte T, Folterbauer C, Kurz A, Fiechtner B, Most M, Salvenmoser W, Sassone-Corsi P, Pelster B. Linking oxygen to time: the bidirectional interaction between the hypoxic signaling pathway and the circadian clock. Chronobiol Int, 2013; 30: 510–29. [DOI] [PubMed] [Google Scholar]
- [121].Adamovich Y, Ladeuix B, Golik M, Koeners MP, Asher G. Rhythmic Oxygen Levels Reset Circadian Clocks through HIF1alpha. Cell Metab, 2017; 25: 93–101. [DOI] [PubMed] [Google Scholar]
- [122].Minami Y, Kasukawa T, Kakazu Y, Iigo M, Sugimoto M, Ikeda S, Yasui A, van der Horst GT, Soga T, Ueda HR. Measurement of internal body time by blood metabolomics. Proc Natl Acad Sci U S A, 2009; 106: 9890–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Dallmann R, Viola AU, Tarokh L, Cajochen C, Brown SA. The human circadian metabolome. Proc Natl Acad Sci U S A, 2012; 109: 2625–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Kasukawa T, Sugimoto M, Hida A, Minami Y, Mori M, Honma S, Honma K, Mishima K, Soga T, Ueda HR. Human blood metabolite timetable indicates internal body time. Proc Natl Acad Sci U S A, 2012; 109: 15036–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Zamboni N, Saghatelian A, Patti GJ. Defining the metabolome: size, flux, and regulation. Mol Cell, 2015; 58: 699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Davies SK, Ang JE, Revell VL, Holmes B, Mann A, Robertson FP, Cui N, Middleton B, Ackermann K, Kayser M, Thumser AE, Raynaud FI, Skene DJ. Effect of sleep deprivation on the human metabolome. Proc Natl Acad Sci U S A, 2014; 111: 10761–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].West AC, Smith L, Ray DW, Loudon ASI, Brown TM, Bechtold DA. Misalignment with the external light environment drives metabolic and cardiac dysfunction. Nat Commun, 2017; 8: 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Krishnaiah SY, Wu G, Altman BJ, Growe J, Rhoades SD, Coldren F, Venkataraman A, Olarerin-George AO, Francey LJ, Mukherjee S, Girish S, Selby CP, Cal S, Er U, Sianati B, Sengupta A, Anafi RC, Kavakli IH, Sancar A, Baur JA, Dang CV, Hogenesch JB, Weljie AM. Clock Regulation of Metabolites Reveals Coupling between Transcription and Metabolism. Cell Metab, 2017; 25: 1206. [DOI] [PubMed] [Google Scholar]
- [129].Putker M, Crosby P, Feeney KA, Hoyle NP, Costa ASH, Gaude E, Frezza C, O’Neill JS. Mammalian Circadian Period, But Not Phase and Amplitude, Is Robust Against Redox and Metabolic Perturbations. Antioxid Redox Signal, 2018; 28: 507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun, 2000; 273: 793–8. [DOI] [PubMed] [Google Scholar]
- [131].Etchegaray JP, Lee C, Wade PA, Reppert SM. Rhythmic histone acetylation underlies transcription in the mammalian circadian clock. Nature, 2003; 421: 177–82. [DOI] [PubMed] [Google Scholar]
- [132].Kincaid B, Bossy-Wetzel E. Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration. Front Aging Neurosci, 2013; 5: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Mauvoisin D, Atger F, Dayon L, Nunez Galindo A, Wang J, Martin E, Da Silva L, Montoliu I, Collino S, Martin FP, Ratajczak J, Canto C, Kussmann M, Naef F, Gachon F. Circadian and Feeding Rhythms Orchestrate the Diurnal Liver Acetylome. Cell Rep, 2017; 20: 1729–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Peek CB, Affinati AH, Ramsey KM, Kuo HY, Yu W, Sena LA, Ilkayeva O, Marcheva B, Kobayashi Y, Omura C, Levine DC, Bacsik DJ, Gius D, Newgard CB, Goetzman E, Chandel NS, Denu JM, Mrksich M, Bass J. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science, 2013; 342: 1243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, Takahashi JS, Imai S, Bass J. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science, 2009; 324: 651–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell, 2008; 134: 317–28. [DOI] [PubMed] [Google Scholar]
- [137].Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, Guarente LP, Farese RV Jr., Weissman S, Verdin E, Schwer B. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol, 2007; 27: 8807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A, 2008; 105: 14447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Gurd BJ, Holloway GP, Yoshida Y, Bonen A. In mammalian muscle, SIRT3 is present in mitochondria and not in the nucleus; and SIRT3 is upregulated by chronic muscle contraction in an adenosine monophosphate-activated protein kinase-independent manner. Metabolism, 2012; 61: 733–41. [DOI] [PubMed] [Google Scholar]
- [140].Cui XX, Li X, Dong SY, Guo YJ, Liu T, Wu YC. SIRT3 deacetylated and increased citrate synthase activity in PD model. Biochem Biophys Res Commun, 2017; 484: 767–773. [DOI] [PubMed] [Google Scholar]
- [141].Sheng S, Kang Y, Guo Y, Pu Q, Cai M, Tu Z. Overexpression of Sirt3 inhibits lipid accumulation in macrophages through mitochondrial IDH2 deacetylation. Int J Clin Exp Pathol, 2015; 8: 9196–201. [PMC free article] [PubMed] [Google Scholar]
- [142].Yu W, Dittenhafer-Reed KE, Denu JM. SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J Biol Chem, 2012; 287: 14078–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Finley LW, Haas W, Desquiret-Dumas V, Wallace DC, Procaccio V, Gygi SP, Haigis MC. Succinate dehydrogenase is a direct target of sirtuin 3 deacetylase activity. PLoS One, 2011; 6: e23295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Cimen H, Han MJ, Yang Y, Tong Q, Koc H, Koc EC. Regulation of succinate dehydrogenase activity by SIRT3 in mammalian mitochondria. Biochemistry, 2010; 49: 304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Porter GA, Urciuoli WR, Brookes PS, Nadtochiy SM. SIRT3 deficiency exacerbates ischemia-reperfusion injury: implication for aged hearts. Am J Physiol Heart Circ Physiol, 2014; 306: H1602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV, Jr., Alt FW, Kahn CR, Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature, 2010; 464: 121–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Hirschey MD, Shimazu T, Jing E, Grueter CA, Collins AM, Aouizerat B, Stancakova A, Goetzman E, Lam MM, Schwer B, Stevens RD, Muehlbauer MJ, Kakar S, Bass NM, Kuusisto J, Laakso M, Alt FW, Newgard CB, Farese RV, Jr., Kahn CR, Verdin E. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell, 2011; 44: 177–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Sundaresan NR, Samant SA, Pillai VB, Rajamohan SB, Gupta MP. SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol Cell Biol, 2008; 28: 6384–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron, 2001; 30: 525–36. [DOI] [PubMed] [Google Scholar]
- [150].Grimaldi B, Bellet MM, Katada S, Astarita G, Hirayama J, Amin RH, Granneman JG, Piomelli D, Leff T, Sassone-Corsi P. PER2 controls lipid metabolism by direct regulation of PPARgamma. Cell Metab, 2010; 12: 509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Grimaldi B, Nakahata Y, Sahar S, Kaluzova M, Gauthier D, Pham K, Patel N, Hirayama J, Sassone-Corsi P. Chromatin remodeling and circadian control: master regulator CLOCK is an enzyme. Cold Spring Harb Symp Quant Biol, 2007; 72: 105–12. [DOI] [PubMed] [Google Scholar]
- [152].Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, Waitt GM, Parks DJ, Pearce KH, Wisely GB, Lazar MA. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science, 2007; 318: 1786–9. [DOI] [PubMed] [Google Scholar]
- [153].Le Martelot G, Claudel T, Gatfield D, Schaad O, Kornmann B, Lo Sasso G, Moschetta A, Schibler U. REV-ERBalpha participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol, 2009; 7: e1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell, 2002; 109: 307–20. [DOI] [PubMed] [Google Scholar]
- [155].Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature, 2002; 417: 78–83. [DOI] [PubMed] [Google Scholar]
- [156].Eckle T, Koeppen M, Eltzschig H. Use of a hanging weight system for coronary artery occlusion in mice. J Vis Exp, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Bonney S, Kominsky D, Brodsky K, Eltzschig H, Walker L, Eckle T. Cardiac Per2 functions as novel link between fatty acid metabolism and myocardial inflammation during ischemia and reperfusion injury of the heart. PLoS One, 2013; 8: e71493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Luo X, Zhang Y, Ruan X, Jiang X, Zhu L, Wang X, Ding Q, Liu W, Pan Y, Wang Z, Chen Y. Fasting-induced protein phosphatase 1 regulatory subunit contributes to postprandial blood glucose homeostasis via regulation of hepatic glycogenesis. Diabetes, 2011; 60: 1435–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Jaswal JS, Keung W, Wang W, Ussher JR, Lopaschuk GD. Targeting fatty acid and carbohydrate oxidation--a novel therapeutic intervention in the ischemic and failing heart. Biochim Biophys Acta, 2011; 1813: 1333–50. [DOI] [PubMed] [Google Scholar]
- [160].Lopaschuk GD, McNeil GF, McVeigh JJ. Glucose oxidation is stimulated in reperfused ischemic hearts with the carnitine palmitoyltransferase 1 inhibitor, Etomoxir. Mol Cell Biochem, 1989; 88: 175–9. [DOI] [PubMed] [Google Scholar]
- [161].Finley LW, Carracedo A, Lee J, Souza A, Egia A, Zhang J, Teruya-Feldstein J, Moreira PI, Cardoso SM, Clish CB, Pandolfi PP, Haigis MC. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1alpha destabilization. Cancer Cell, 2011; 19: 416–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Eckel-Mahan K, Sassone-Corsi P. Metabolism and the circadian clock converge. Physiol Rev, 2013; 93: 107–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Kantermann T, Juda M, Merrow M, Roenneberg T. The human circadian clock’s seasonal adjustment is disrupted by daylight saving time. Curr Biol, 2007; 17: 1996–2000. [DOI] [PubMed] [Google Scholar]
- [164].Roenneberg T, Aschoff J. Annual rhythm of human reproduction: I. Biology, sociology, or both? J Biol Rhythms, 1990; 5: 195–216. [DOI] [PubMed] [Google Scholar]
- [165].Roenneberg T, Aschoff J. Annual rhythm of human reproduction: II. Environmental correlations. J Biol Rhythms, 1990; 5: 217–39. [DOI] [PubMed] [Google Scholar]
- [166].Stothard ER, McHill AW, Depner CM, Birks BR, Moehlman TM, Ritchie HK, Guzzetti JR, Chinoy ED, LeBourgeois MK, Axelsson J, Wright KP Jr., Circadian Entrainment to the Natural Light-Dark Cycle across Seasons and the Weekend. Curr Biol, 2017; 27: 508–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Leocadio-Miguel MA, Louzada FM, Duarte LL, Areas RP, Alam M, Freire MV, Fontenele-Araujo J, Menna-Barreto L, Pedrazzoli M. Latitudinal cline of chronotype. Sci Rep, 2017; 7: 5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Schroeder AM, Colwell CS. How to fix a broken clock. Trends Pharmacol Sci, 2013; 34: 605–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Haus E, Smolensky M. Biological clocks and shift work: circadian dysregulation and potential long-term effects. Cancer Causes Control, 2006; 17: 489–500. [DOI] [PubMed] [Google Scholar]
- [170].Mosendane T, Mosendane T, Raal FJ. Shift work and its effects on the cardiovascular system. Cardiovasc J Afr, 2008; 19: 210–5. [PMC free article] [PubMed] [Google Scholar]
- [171].Segawa K, Nakazawa S, Tsukamoto Y, Kurita Y, Goto H, Fukui A, Takano K. Peptic ulcer is prevalent among shift workers. Dig Dis Sci, 1987; 32: 449–53. [DOI] [PubMed] [Google Scholar]
- [172].Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst, 2001; 93: 1557–62. [DOI] [PubMed] [Google Scholar]
- [173].Persson HE, Svanborg E. Sleep deprivation worsens obstructive sleep apnea. Comparison between diurnal and nocturnal polysomnography. Chest, 1996; 109: 645–50. [DOI] [PubMed] [Google Scholar]
- [174].Depner CM, Stothard ER, Wright KP Jr. Metabolic consequences of sleep and circadian disorders. Curr Diab Rep, 2014; 14: 507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Spencer FA, Goldberg RJ, Becker RC, Gore JM. Seasonal distribution of acute myocardial infarction in the second National Registry of Myocardial Infarction. J Am Coll Cardiol, 1998; 31: 1226–33. [DOI] [PubMed] [Google Scholar]
- [176].Zhang XW, Tan ZJ, Li YL, Wang B, Yu A, Zhang GQ. [A study on yearly and daily circadian rhythm of cardiovascular events]. Zhonghua Nei Ke Za Zhi, 2009; 48: 818–20. [PubMed] [Google Scholar]
- [177].Schloss MJ, Horckmans M, Nitz K, Duchene J, Drechsler M, Bidzhekov K, Scheiermann C, Weber C, Soehnlein O, Steffens S. The time-of-day of myocardial infarction onset affects healing through oscillations in cardiac neutrophil recruitment. EMBO Mol Med, 2016; 8: 937–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Ritchie HK, Stothard ER, Wright KP. Entrainment of the Human Circadian Clock to the Light-Dark Cycle and its Impact on Patients in the ICU and Nursing Home Settings. Curr Pharm Des, 2015; 21: 3438–42. [DOI] [PubMed] [Google Scholar]
- [179].Arble DM, Ramsey KM, Bass J, Turek FW. Circadian disruption and metabolic disease: findings from animal models. Best Pract Res Clin Endocrinol Metab, 2010; 24: 785–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Foster RG, Peirson SN, Wulff K, Winnebeck E, Vetter C, Roenneberg T. Sleep and circadian rhythm disruption in social jetlag and mental illness. Prog Mol Biol Transl Sci, 2013; 119: 325–46. [DOI] [PubMed] [Google Scholar]
- [181].Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH, Panda S. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab, 2012; 15: 848–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Noyan H, El-Mounayri O, Isserlin R, Arab S, Momen A, Cheng HS, Wu J, Afroze T, Li RK, Fish JE, Bader GD, Husain M. Cardioprotective Signature of Short-Term Caloric Restriction. PLoS One, 2015; 10: e0130658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Schroeder AM, Truong D, Loh DH, Jordan MC, Roos KP, Colwell CS. Voluntary scheduled exercise alters diurnal rhythms of behaviour, physiology and gene expression in wild-type and vasoactive intestinal peptide-deficient mice. J Physiol, 2012; 590: 6213–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Dauchy RT, Wren-Dail MA, Hoffman AE, Hanifin JP, Warfield B, Brainard GC, Hill SM, Belancio VP, Dauchy EM, Blask DE. Effects of Daytime Exposure to Light from Blue-Enriched Light-Emitting Diodes on the Nighttime Melatonin Amplitude and Circadian Regulation of Rodent Metabolism and Physiology. Comp Med, 2016; 66: 373–383. [PMC free article] [PubMed] [Google Scholar]
- [185].Martino TA, Tata N, Belsham DD, Chalmers J, Straume M, Lee P, Pribiag H, Khaper N, Liu PP, Dawood F, Backx PH, Ralph MR, Sole MJ. Disturbed diurnal rhythm alters gene expression and exacerbates cardiovascular disease with rescue by resynchronization. Hypertension, 2007; 49: 1104–13. [DOI] [PubMed] [Google Scholar]
- [186].Lucassen EA, Coomans CP, van Putten M, de Kreij SR, van Genugten JH, Sutorius RP, de Rooij KE, van der Velde M, Verhoeve SL, Smit JW, Lowik CW, Smits HH, Guigas B, Aartsma-Rus AM, Meijer JH. Environmental 24-hr Cycles Are Essential for Health. Curr Biol, 2016; 26: 1843–53. [DOI] [PubMed] [Google Scholar]
- [187].Korompeli A, Muurlink O, Kavrochorianou N, Katsoulas T, Fildissis G, Baltopoulos G. Circadian disruption of ICU patients: A review of pathways, expression, and interventions. J Crit Care, 2017; 38: 269–277. [DOI] [PubMed] [Google Scholar]
- [188].Danielson SJ, Rappaport CA, Loher MK, Gehlbach BK. Looking for light in the din: An examination of the circadian-disrupting properties of a medical intensive care unit. Intensive Crit Care Nurs, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Smolensky MH, Hermida RC, Reinberg A, Sackett-Lundeen L, Portaluppi F. Circadian disruption: New clinical perspective of disease pathology and basis for chronotherapeutic intervention. Chronobiol Int, 2016; 33: 1101–19. [DOI] [PubMed] [Google Scholar]
- [190].Durrington HJ, Clark R, Greer R, Martial FP, Blaikley J, Dark P, Lucas RJ, Ray DW. ‘In a dark place, we find ourselves’: light intensity in critical care units. Intensive Care Med Exp, 2017; 5: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Ruggieri AJ, Levy RJ, Deutschman CS. Mitochondrial dysfunction and resuscitation in sepsis. Crit Care Clin, 2010; 26: 567–75, x-xi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Montaigne D, Marechal X, Modine T, Coisne A, Mouton S, Fayad G, Ninni S, Klein C, Ortmans S, Seunes C, Potelle C, Berthier A, Gheeraert C, Piveteau C, Deprez R, Eeckhoute J, Duez H, Lacroix D, Deprez B, Jegou B, Koussa M, Edme JL, Lefebvre P, Staels B. Daytime variation of perioperative myocardial injury in cardiac surgery and its prevention by Rev-Erbalpha antagonism: a single-centre propensity-matched cohort study and a randomised study. Lancet, 2018; 391: 59–69. [DOI] [PubMed] [Google Scholar]
- [193].Sudo M, Sasahara K, Moriya T, Akiyama M, Hamada T, Shibata S. Constant light housing attenuates circadian rhythms of mPer2 mRNA and mPER2 protein expression in the suprachiasmatic nucleus of mice. Neuroscience, 2003; 121: 493–9. [DOI] [PubMed] [Google Scholar]
- [194].Dispersyn G, Pain L, Touitou Y. Circadian disruption of body core temperature and rest-activity rhythms after general (propofol) anesthesia in rats. Anesthesiology, 2009; 110: 1305–15. [DOI] [PubMed] [Google Scholar]
- [195].Lonardo NW, Mone MC, Nirula R, Kimball EJ, Ludwig K, Zhou X, Sauer BC, Nechodom K, Teng C, Barton RG. Propofol is associated with favorable outcomes compared with benzodiazepines in ventilated intensive care unit patients. Am J Respir Crit Care Med, 2014; 189: 1383–94. [DOI] [PubMed] [Google Scholar]
- [196].Rivo J, Raphael J, Drenger B, Berenshtein E, Chevion M, Gozal Y. Flumazenil mimics whereas midazolam abolishes ischemic preconditioning in a rabbit heart model of ischemia-reperfusion. Anesthesiology, 2006; 105: 65–71. [DOI] [PubMed] [Google Scholar]
- [197].Matsuo I, Iijima N, Takumi K, Higo S, Aikawa S, Anzai M, Ishii H, Sakamoto A, Ozawa H. Characterization of sevoflurane effects on Per2 expression using ex vivo bioluminescence imaging of the suprachiasmatic nucleus in transgenic rats. Neurosci Res, 2016; 107: 30–7. [DOI] [PubMed] [Google Scholar]
- [198].Liu C, Reppert SM. GABA synchronizes clock cells within the suprachiasmatic circadian clock. Neuron, 2000; 25: 123–8. [DOI] [PubMed] [Google Scholar]
- [199].Wagner S, Castel M, Gainer H, Yarom Y. GABA in the mammalian suprachiasmatic nucleus and its role in diurnal rhythmicity. Nature, 1997; 387: 598–603. [DOI] [PubMed] [Google Scholar]
- [200].Colwell CS. Circadian rhythms. Time to get excited by GABA. Nature, 1997; 387: 554–5. [DOI] [PubMed] [Google Scholar]
- [201].Mure LS, Le HD, Benegiamo G, Chang MW, Rios L, Jillani N, Ngotho M, Kariuki T, Dkhissi-Benyahya O, Cooper HM, Panda S. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science, 2018; 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A, 2014; 111: 16219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Skarke C, Lahens NF, Rhoades SD, Campbell A, Bittinger K, Bailey A, Hoffmann C, Olson RS, Chen L, Yang G, Price TS, Moore JH, Bushman FD, Greene CS, Grant GR, Weljie AM, FitzGerald GA. A Pilot Characterization of the Human Chronobiome. Sci Rep, 2017; 7: 17141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Ruben Marc D. W G, Smith David F. Schmidt Robert E. Francey Lauren J. Anafi Ron C. and Hogenesch John B.. A population-based human enCYCLOPedia for circadian medicine. 2018. [Google Scholar]
- [205].Chen Z, Yoo SH, Takahashi JS. Small molecule modifiers of circadian clocks. Cell Mol Life Sci, 2013; 70: 2985–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].He B, Nohara K, Park N, Park YS, Guillory B, Zhao Z, Garcia JM, Koike N, Lee CC, Takahashi JS, Yoo SH, Chen Z. The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab, 2016; 23: 610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Chen Z, Yoo SH, Park YS, Kim KH, Wei S, Buhr E, Ye ZY, Pan HL, Takahashi JS. Identification of diverse modulators of central and peripheral circadian clocks by high-throughput chemical screening. Proc Natl Acad Sci U S A, 2012; 109: 101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Oyama Y, Bartman CM, Gile J, Sehrt D, Eckle T. The Circadian PER2 Enhancer Nobiletin Reverses the Deleterious Effects of Midazolam in Myocardial Ischemia and Reperfusion Injury. Curr Pharm Des, 2018; 24: 3376–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Stujanna EN, Murakoshi N, Tajiri K, Xu D, Kimura T, Qin R, Feng D, Yonebayashi S, Ogura Y, Yamagami F, Sato A, Nogami A, Aonuma K. Rev-erb agonist improves adverse cardiac remodeling and survival in myocardial infarction through an anti-inflammatory mechanism. PLoS One, 2017; 12: e0189330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Bartman CM, Oyama Y, Eckle T. Daytime variations in perioperative myocardial injury. Lancet, 2018; 391: 2104. [DOI] [PubMed] [Google Scholar]
- [211].Schonenberger MJ, Kovacs WJ. Hypoxia signaling pathways: modulators of oxygen-related organelles. Front Cell Dev Biol, 2015; 3: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Wong BW, Kuchnio A, Bruning U, Carmeliet P. Emerging novel functions of the oxygen-sensing prolyl hydroxylase domain enzymes. Trends Biochem Sci, 2013; 38: 3–11. [DOI] [PubMed] [Google Scholar]