Abstract
Purpose:
Physical activity improves outcomes across a broad spectrum of cardiovascular disease. The safety and effectiveness of exercise-based interventions in patients with implantable cardioverter-defibrillators (ICDs) including cardiac resynchronization devices (CRT-Ds) remains poorly understood.
Methods:
We identified clinical studies using the following search terms: “implantable cardioverter-defibrillators”; “ICD”; “cardiac resynchronization therapy”; “CRT”; and any one of the following: “activity”; “exercise”; “training”; or “rehabilitation”; from 1/1/2000 – 10/1/2015. Eligible studies were evaluated for design and clinical endpoints.
Results:
A total of 16 studies were included: 8 randomized controlled trials, 5 single-arm trials, 2 observational cohort trials, and 1 randomized crossover trial. A total of 2547 patients were included (intervention groups = 1215 patients, control groups = 1332 patients). Exercise interventions varied widely in character, duration (median 84 d, range 23 – 168 d), and follow-up time (median 109 d, range 23 d – 48 mo). Exercise performance measures were the most common primary endpoints (87.5%), with most studies (81%) demonstrating significant improvement. ICD shocks were uncommon during active exercise intervention with 6 shocks in 635 patients (0.9%). ICD shocks in follow-up were less common in patients receiving any exercise intervention (15.6% vs 23%, OR 0.68, 95% CI: 0.48–0.80, P < .001). VO2 peak improved significantly in patients receiving exercise intervention (1.98 vs 0.36 mL/kg/min, P < .001).
Conclusion:
In conclusion, exercise interventions in patients with ICDs and CRT-Ds appear safe and effective. Lack of consensus on design and endpoints remains a barrier to broader application to this important patient population.
We performed a systematic review and meta-analysis of exercise-based interventions in patients with implantable cardioverter-defibrillators including cardiac resynchronization devices. After review of 649 articles, 16 studies with 2547 patients were included. Most studies demonstrated significant improvement in exercise performance. Device shocks were less common in patients receiving any exercise intervention.
Keywords: Exercise, implantable cardioverter defibrillator, review, meta-analysis
Implantable cardioverter defibrillators (ICDs) including those with cardiac resynchronization therapy capability (CRT-Ds) are recommended for selected patients at risk for ventricular arrhythmias.1,2 Approximately 80% of patients receive devices for primary prevention, usually based on a history of systolic heart failure despite optimal medical therapy, while the remainder includes survivors of sudden cardiac arrest.3,4 In the United States, approximately 100 000 ICDs are implanted annually, and more than 1 million patients are living with these devices.5
For patients with cardiovascular disease including heart failure and coronary artery disease, aerobic exercise provides significant benefits with improvement in exercise capacity and quality of life.6,7 However, despite guidelines recommending exercise training, there is significant underutilization of this important therapy.8 Patients with ICDs introduce several concerns regarding exercise, including increased burden of ventricular arrhythmias, ICD shocks, and sudden death. In some cases, physical activity can precipitate ventricular arrhythmias or supraventricular arrhythmias that result in ICD therapy.9 The risk for cardiovascular complications during exercise testing and training may be higher in patients with a history of life-threatening arrhythmias or cardiac arrest.10 However, regular exercise, such as cardiac rehabilitation, may exert a protective effect for patients whose cardiac substrate supports indications for ICDs and CRT-Ds.11,12 Accordingly, we performed a systematic review and meta-analysis of trial-level data with the purpose of evaluating exercise interventions in patients with ICDs and CRT-Ds in order to characterize study design, safety, and effectiveness of exercise in affected patients.
METHODS
Data Sources and Searches
A protocol for the systematic review was developed prospectively and registered with the International Prospective Register of Systematic Reviews (PROSPERO) at http:/www.crd.york.ac.uk/prospero/, registration number CRD42015027422. Following PRISMA guidelines13 for systematic reviews, we performed a systematic literature search of the MEDLINE/PubMed, Google Scholar, and Embase, and Cochrane Library databases for articles using the following terms: “implantable cardioverter-defibrillators”; “ICDs”; “cardiac resynchronization therapy”; “CRT”; AND any one of the following: “activity”; “exercise”; “training”; or “rehabilitation.” We limited dates to 1/1/2000 – 10/1/2015 to evaluate contemporary practice. No language requirement was placed on the search.
After the initial sample was obtained, we evaluated study titles and abstracts to identify potentially relevant studies and then obtained the full text articles to confirm which studies would be included in the systematic review. When the literature search was complete, we engaged in manual reference mining of our sample of articles.
Study Selection
Prespecified inclusion criteria involved characteristics of the studies themselves and the data presented. Studies included must have reported empirical data regarding an intervention or program targeting physical activity, rehabilitation programs, or exercise training specifically intended for patients with ICDs. Both clinical trials and observational studies were eligible for inclusion. Case studies, editorials, opinion pieces, commentaries, and reviews or meta-analyses without original data or analysis were excluded. We excluded trials which examined device-guided exercise optimization (eg, atrial-ventricular delay optimization during exercise) or studies which evaluated exercise capacity without a cardiovascular training component.
Data Extraction and Analysis
Two authors (DAS and DBK) independently reviewed the initial list of eligible studies, with any disagreements resolved with consultation among all 4 authors. We noted the methodology and results, with a focus on the sample size, intervention, and assessment of endpoints. Then the major limitations of each study were formally assessed. Possible sources of bias in the included studies were noted, including funding sources, methodological limitations (including lack of detail reporting of methods). Qualitative analysis was then performed on these studies to evaluate the type and duration of exercise intervention, type of primary and secondary endpoints, and outcomes. Data was evaluated at a trial-level.
The primary quantitative outcome for this review is ICD shock during follow-up. This outcome was assessed in all studies which reported the outcome in both the intervention and control arm as well as in the subset of studies with a RCT design. ICD shock was categorized as a dichotomous variable and evaluated in a standard 2×2 table with Fisher’s exact test using STATA version 12.1 software (StataCorp). Odds ratio (OR) and 95% confidence intervals (CI) were generated from these analyses. Change in peak was analyzed in all studies which reported the outcome and evaluated with the t-test statistic. A sensitivity analysis was performed.
RESULTS
Study Selection and Evaluation
The primary literature search yielded 649 studies (Figure 1). After evaluating study titles, 75 study abstracts were screened. Of these, 25 full-text articles were assessed for eligibility. We excluded 9 studies which did not meet our primary entry criteria (ie, focus was not rehabilitation program or did not provide description of intervention). Of the remaining 16 studies, 8 were RCTs, 5 were single-arm studies, 2 were observational cohort trials, and 1 was a randomized crossover trial. These 16 studies were included for qualitative analysis. For quantitative analysis, 9 of these 16 studies were excluded as they did not report patient level ICD shock data. Of the remaining 7 studies, 5 were RCTs, and 2 were observational cohort studies. Table 1 describes the trial design, study locations, intervention, patient population, primary and secondary endpoints, primary and secondary outcomes, and funding sponsor.
Figure 1.
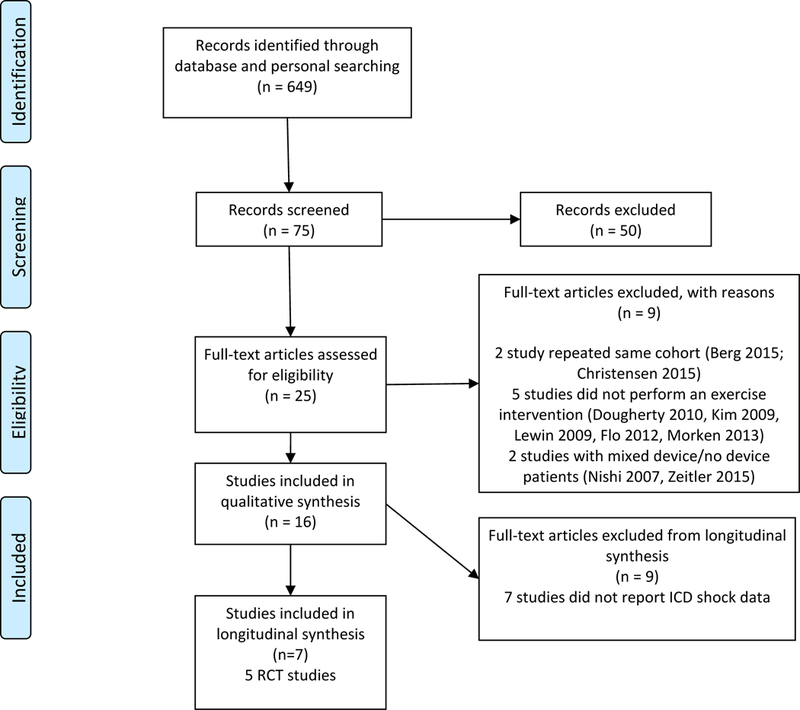
PRISMA flow diagram of study selection process. Abbreviations: ICD, implantable cardioverter defibrillator.
Table 1.
Design and Interventions of Included Studies
| Study (Author, Year) |
Design | Intervention | Patient Population |
Location | Funding Sponsor |
|---|---|---|---|---|---|
| Vanhees et al (2001)14 | Single-arm, with historical control | Supervised exercise training program for 90 min, 3 times/wk for 3 mo consisting of cycling, running, arm ergometry, rowing, isotonic calisthenics, and relaxation | 8 ICD patients, compared with historical control of 16 patients | Belgium | Not specified |
| Fitchet et al (2003)15 | Randomized crossover | Cardiac rehab for 2 hr, 2 times/wk, individually prescribed aerobic exercise with education and cognitive behavioral intervention | 16 ICD patients | UK | British Heart Foundation |
| Kamke (2003)16 | Single-arm | Observational series of patients undergoing monitored ergometry and inpatient rehabilitation program | 118 ICD patients | Germany | Not specified |
| Vanhees (2004)17 | Single-arm, with historical control | Supervised exercise training program offered for 90 min, 3 times/wk for 3 mo consisting of cycling, running, arm ergometry, rowing, calisthenics, strength training, and relaxation | 92 ICD patients, compared with a matched control group of 473 patients without ICD | Belgium | Supported by grants from the Flemish Fund for Scientific Research and the Research Council of the University of Leuven |
| Davids (2005)18 | Observational cohort | OCR participation, exercise not further specified | 82 patients receiving new ICDs; 28 (34% participated in OCR | US | Not stated |
| Belardinelli (2006)19 | RCT | Supervised exercise training program for 1 hr 3 times/wk for 8 wk at 60% peak | 52 men, NYHA class II and III, ischemic CM with defibrillator (± CRT); 30 intervention (15 ICD, 15 CRT-D) and 22 control patients (12 ICD, 10 CRT-D) | Italy and US | None |
| Conraads et al (2007)20 | RCT | Supervised exercise training program for 1 hr, 3 times/wk for 4 mo consisting of cycling and walking | 17 patients receiving CRT (13 CRT-P, 4 CRT-D), with NYHA class III-IV, ischemic and nonischemic CM; 8 intervention, 9 control; matched HF cohort, 9 intervention, 10 control | Belgium | None reported |
| Dougherty et al (2008)21 | Single-arm | Supervised exercise training program for 3 hr/wk and home walking 2 hr/wk for 8 wk | 10 patients with new ICDs within previous 6 mo | US | NIH, NINR, K01 NR07989 and the Research & Intramural Funding Program at the University of Washington, School of Nursing |
| Patwala et al (2009)22 | RCT | Supervised exercise training program for 30 min, 3x weekly for 3 mo consisting of treadmill walking and cycling | 50 patients undergoing new CRT | UK | Not specified |
| Piccini et al (2013)23 | RCT (post hoc analysis) | Supervised exercise training program for 15–30 min, 3x weekly for 6 sessions, increased to 30–35 min, 3 times/wk for 30 sessions, with home-based exercise 40 minute sessions 5 times/ wk after completing 18 supervised sessions; treadmill walking and cycling | 1053 outpatients with NYHA class II-IV, LVEF ≤35%, who had ICDs (at baseline for primary endpoint and all ICDs in sensitivity analysis) | US | HF-ACTION trial funded by NIH, analysis funded by a grant from Boston Scientific |
| Smialek et al (2013)24 | Single-arm | Comprehensive cardiac rehabilitation and supervised exercise training program consisting of 2-wk inpatient phase 1 and 12-wk outpatient phase 2, interval endurance training, resistance training, and respiratory muscle exercises | 45 consecutive patients selected for a comprehensive cardiac rehabilitation program 6 wk after ICD implantation | Poland | Not specified |
| Berg et al (2015)25 | RCT | Comprehensive cardiac rehabilitation and supervised exercise training program for 12 wk; 1 yr of psycho-educational counseling | 196 patients with new ICDs | Denmark | Novo Nordisk Foundation; the Oticon Foundation; the Danish Heart Foundation; the AP Møller and Chastine Mc-Kinney Møller Foundation; Helsefonden; the Tryg Foundation; the Augustinus Foundation; Krista and the Viggo Petersens Foundation; The Danish Cardiovascular Research School (DaCRA); King Christian X Foundation and Copenhagen University Hospital, Rigshospitalet |
| Dougherty et al (2015)26 | RCT | Home aerobic training program for 1 hr, 5 times/wk for 8 wk, followed by home aerobic maintenance program for 150 min/wk for 16 wk | 160 patients with new ICDs | US | NIH National Heart, Lung, and Blood Institute grant 5R01 HL084550–01A2 |
| Smolis-Bak et al (2015)27 | RCT | Exercise training in hospital rehab unit for 3 wk on average with home telemonitored training program 5times/wk for 8 wk (vs hospital rehabilitation alone); isometric, respiratory and range-of-motion exercises | 52 patients with NYHA class III HF and CRT-D | Poland | Not specified |
| Piotrowicz et al (2015)28 | RCT | Home telemonitored exercise training 5 times/wk for 25–60 min for 8 wk; Nordic walking | 111 patients with NYHA class II-III, LVEF ≤40%; 72 patients had ICD or CRT-D | Poland | National Science Centre, Poland (grant number NN404 107936). |
| Isaksen et al (2015)29 | Observational cohort | Supervised exercise training program 3 x weekly for 60 min for 12 wk; aerobic interval training | 38 patients with ICD or CRT-D | Norway | Western Norway Regional Health Authority, Norway (grant number 911748) and by a limited grant from Diacor, Oslo (Guidant/Boston Scientific). |
Abbreviations: CM, cardiomyopathy; CRT-D, cardiac resynchronization therapy defibrillator; HF, heart failure; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; NIH, National Institutes of Health; NINR, National Institute of Nursing Research; NYHA, New York Heart Association; OCR, outpatient cardiac rehabilitation; RCT, randomized controlled trial; UK, United Kingdom; US, United States; , oxygen uptake.
Patient Characteristics
A total of 2547 patients (median sample size 52, range 24 – 1053) were included with 1215 patients receiving exercise interventions and 1332 control patients. Patients predominately had New York Heart Association (NYHA) class II and III heart failure. The mean ages of patients in the studies ranged from 52–69 yr (median 60), a high percentage were males (82.7%), and mean left ventricular ejection fractions ranged from 24–43% (median 33%).
Intervention Characteristics
Exercise interventions varied widely in character (Table 1). Studies included both inpatient and outpatient training with varied methods including aerobic exercise (walking, running, cycling, rowing, arm ergometry, calisthenics, and Nordic walking), strength training, stretching, and psychoeducational counselling including cognitive behavioral therapy. Home telemonitoring was also used in several studies. Exercise programs generally consisted of several weekly sessions (predominately 3 times weekly) for 25 to 90 min/session, offered over several wk. Most studies also described methods of avoiding ICD interventions which generally included targeting a maximal heart rate during exercise of 10 to 30 beats/min lower than the ICD therapy rate threshold, or alternatively, adjusting the ICD therapy rate to be higher than the maximal achieved heart rate during exercise. Of note, in the largest trial,23 patients were excluded if the ICD tachycardia detection limit was set below the target heart rate for exercise training (determined by 70% of heart rate reserve [peak heart rate during exercise testing minus resting heart rate times a percent]). The median duration of exercise intervention was 84 d (range of 23–168 d) and the median total follow-up was 109 d (range 23 d - 48 mo).
Qualitative Analysis
Exercise performance measures were the most common primary endpoint (87.5%) with a majority of studies reporting peak (75%) (Table 2). Other primary outcome measures included median intensity of exercise in metabolic equivalents (METs) as estimated from patient reported activity and frequency (12.5%), improvement in exercise test time during treadmill exercise testing (6.25%), functional class (12.5%), quality of life measures (6.3%), and ICD shocks (12.5%). The primary endpoint was found to be statistically significantly improved in the majority of studies (81%). Most studies reported ICD shocks and/or anti-tachycardia pacing (ATP) during exercise intervention (81%). In these studies, ICD interventions were uncommon during exercise with 6 ICD shocks and 2 antitachycardia pacing (ATP) events in 635 patients. Only 1 ICD shock was described as inappropriate (not further described) with all other shocks and ATP were appropriate for VT.
Table 2.
Primary and Secondary Outcomes of Included Studies
| Study (Author, Year) |
Primary Endpoint |
Secondary Endpoints |
Primary Outcome | Statistically Significant Results (P < .05) |
Secondary Outcomes |
|---|---|---|---|---|---|
| Vanhees et al (2001)14 | Peak | None specified | Peak increased by 3.2±4.3 mL/kg/min (20±32%) | No, P=.08 | None |
| Fitchet et al (2003)15 | Change in exercise test time, frequency of ICD discharges, frequency of ventricular arrhythmias, and change in scale scores for anxiety and depression | None specified | Mean exercise time (min:sec) increased by 16% (from 9:55±2:33 to 11:11±2:17) and maintained at 12 wk; no episodes of ICD discharges during exercise; 2 ICD discharges during study; 3 episodes of ATP (in 2 patients); improvement in anxiety and depression scale scores | Yes, P=.001 | None |
| Kamke et al )2003)16 | Incidence of ICD shocks during exercise program | None specified | 21 ICD therapies in 19 patients – 7 appropriate shocks, 1 inappropriate shock, 7 appropriate ATP, 6 inappropriate ATP | Not evaluated | None |
| Vanhees et al, (2004)17 | Peak | None specified | Peak by 2.6±3.5 mL/kg/min (15±20%) | Yes, P=.001 | None |
| Davids et al (2005)18 | Median intensity of regular exercise levels (METS); OCR participation; both determined by telephone survey | ICD Shocks | OCR participation associated with higher average METs 5.3 (IQR 3.5–6.0) vs 3.5 (IQR 3.5–5) | Yes, P<.02 | OCR participation associated with fewer patients with shocks, appropriate shock, inappropriate shock, shocks during exercise, appropriate shocks during exercise, and inappropriate shocks during exercise |
| Belardinelli et al (2006)19 | Peak | Functional capacity, QOL, hospital readmission | Peak higher in exercise group, 18.9±2.7 vs 16.1±2.2 mL/kg/min | Yes, P<.001 | Work rate, QOL significantly improved in exercise trained CRT-D group, with no significant improvement in exercise trained ICD group; hospital readmission lower in exercise trained groups. |
| Conraads et al (2007)20 | Peak | Maximal workload, circulatory power, LVEF, intraventricular delay, QOL | Peak higher in exercise group, 19.3±.2 vs 13.8±0.9 mL/kg/min | Yes, P=.005 | Maximal workload and circulatory power improved. No significant effect on LVEF, intraventricular conduction delay, and QOL |
| Dougherty et al (2008)21 | Cardiopulmonary fitness and activity (exercise time, peak , METs) | Heart rate variability, QOL, hsCRP | Peak increased from 25.59±9.29 to 26.0±11.3 mL/kg/min (P=.78); METs increased from 6.63±2.61 to 7.0±2.94 mL/kg/min (P=.33); mean exercise time (min:sec) increased from 9:10±5:18 to 10:15±5:56 (P=.04), | Varied (see previous cell) | Heart rate variability improved (predominately non-significant); QOL results mixed; hsCRP non-significantly reduced at 8 wk |
| Patwala et al (2009)22 | Peak | NYHA class, exercise duration, QOL, several other measures of cardiopulmonary fitness | Exercise training improved peak by 1.37±2.49 vs −0.01±1.49 | Yes, P=.022 | NYHA class, exercise duration, cardiopulmonary fitness, and QOL significantly improved |
| Piccini et al (2013)23 | Incidence of all-cause ICD shocks | Composite endpoint of death, myocardial infarction, or worsening HF | No change in incidence of all-cause shocks, 20% vs 22% (Cox proportional hazards HR 0.90 [0.69–1.18], P=.45) | No, P=.45 | Exercise training did not significantly improve incidence of composite endpoint of death, myocardial infarction, or worsening HF |
| Smialek et al (2013)24 | Evaluate safety and effectiveness of early cardiac rehab program; NYHA status, echo parameters, treadmill spiroergometric exercise test, Polish version of the SF-36c(QOL), and Beck Depression Inventory | No distinction between primary and secondary endpoints | No deaths, no complications or adverse events during rehabilitation program and exercise; several events during home phase, not during exercise (7 patients with non-sustained VT without intervention, 2 patients with VF and appropriate shock, 1 patient with inappropriate ICD shock for AF); improvement in several hemodynamic measures LVEF (30.09±12.75 vs 35.43±13.4%; P=.002), peak (21.3±9.2 vs 24.2±10.3 mL/kg/min; P=.007), and duration of exercise (9.14±3.7 vs 9.53±3.8 min; P<.05); improved depression score (14.81±9.27 vs. 12.83±10.75; P=.020); improved QOL (P<.05), and improved NYHA class (P<.001) | Varied (see previous cell) | No distinction between primary and secondary endpoints |
| Berg et al (2015)25 | Peak , QOL | ICD shocks | Peak higher in exercise group, 23.0±8.9 vs 20.9±7.8 mL/kg/min (P=.004, corrected P=.015); QOL score higher in exercise group at 6 mo 66.7±22.0 vs 61.9±24.1; and 12 mo 63.5±23.7 vs 62.1±24.4 (P=.015, corrected P=.059) | Varied (see previous cell) | No significant difference in ICD shocks or ATP; No shocks during exercise training or testing. |
| Dougherty et al (2015)26 | Peak at 8 and 24 weeks | ICD therapies, hospitalizations, and musculoskeletal symptoms | Peak higher in exercise group at 8 wk 26.7±7.0 vs 23.9±6.6 (P=.002), and 24 wk 26.9±7.7 vs 23.4±6.0 (P<.001) | Yes (see previous cell) | No significant differences in ICD shocks or ATP; ATP during exercise in 1 patient in intervention group; No significant difference in frequency of hospitalizations; minor musculoskeletal symptoms in 5 patients |
| Smolis-Bak et al (2015)27 | Peak , peak , and treadmill test duration; echocardiographic measures | QoL measures | Peak , peak and treadmill test duration improved in exercise group at 3–4 mo (P<.05) but differences did not persist at 12 mo; echocardiographic parameters including LV end systolic and end diastolic dimension, and LV ejection fraction improved in both groups with no significant difference between groups. | Yes (see previous cell) | Significant improvement in QOL for both groups with no significant difference between groups; No significant differences in ICD interventions, mortality, or hospitalization rates between the groups |
| Piotrowicz et al (2015)28 | Peak | Workload duration on CPET, 6MWT distance, QOL (SF-36), safety, adherence and acceptance of NW | Peak higher in exercise group 18.4±4.1 vs 17.2±3.4 mL/kg/min | Yes, P=.0004 | Significant improvement in workload duration and 6MWT; No significant difference in QOL |
| Isaksen et al (2015)29 | Peak | Other measures of exercise capacity, endothelial function | Peak higher in exercise group 18.4±3 vs 16.2±2.7 mL/kg/min | Yes, P<.05 | Significant improvement in maximal workload in exercise group; significant improvement in flow-mediated vasodilation in exercise group. |
Abbreviations: AF, atrial fibrillation; ATP, antitachycardia pacing; CM, cardiomyopathy; CPET, cardiopulmonary exercise test; CRT-D, cardiac resynchronization therapy defibrillator; HF, heart failure; HR, hazard ratio; hsCRP, high sensitivity C-reactive protein; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; METs, metabolic equivalent; NYHA, New York Heart Association; OCR, outpatient cardiac rehabilitation; SF-36, Short Form Health Questionnaire; QOL, quality of life; RCT, randomized controlled trial; SF-36, short form , carbon dioxide production; , oxygen uptake; VT, ventricular tachycardia; 6MWT, 6-min walk test.
Cochrane risk of bias assessment tool was used to evaluate the quality of publications (SDC Figure). Among the RCTs, 2 studies reported random sequence generation while 2 other studies reported blinding of outcome assessment. There was no selective or incomplete reporting. There was no significant other bias noted in the studies.
Quantitative Analysis
A total of 7 studies reported the burden of ICD shocks during follow-up in both an intervention and control arm 18,19,23,26–29 (Table 3). From these studies, ICD shocks were less common in patients receiving any exercise intervention (15.6% vs 23%, odds ratio [OR] 0.68; 95% CI, 0.48–0.80, P < .001, Figure 2). Among this group, 5 RCTs were included for evaluation of ICD shock rates.19,23,26–28 During the follow-up period of RCT trials, patients receiving exercise interventions had a lower rate of ICD shocks (15.2% vs 20.1%, OR 0.70; 95% CI, 0.53–0.92, P = .013, Figure 3). The rate of ATP was not consistently reported and was therefore not included in the quantitative analysis.
Table 3.
Frequency of ICD Shocks by Study
| Study (Author, Year) |
Study Type | N | Intervention, n | Control, n | ICD Shocks Intervention Group, % (n) |
ICD Shocks Control Group, % (n) |
|---|---|---|---|---|---|---|
| Davids et al (2005)18 | Observational cohort | 82 | 28 | 54 | 25% (7) | 50% (27) |
| Isaksen et al (2015)29 | Observational cohort | 35 | 24 | 11 | 16.7% (4) | 45.5% (5) |
| Belardinelli et al (2006)19 | RCT | 52 | 30 | 22 | 0% (0) | 36.4% (8) |
| Piccini et al (2013)23 | RCT (post-hoc) | 1053 | 546 | 507 | 19.8% (108) | 22.3% (113) |
| Dougherty et al (2015)26 | RCT | 160 | 84 | 76 | 3.6% (3) | 5.3% (4) |
| Smolis-Bak et al (2015)27 | RCT | 52 | 26 | 26 | 3.8% (1) | 19.2% (5) |
| Piotrowicz et al (2015)28 | RCT | 72 | 56 | 16 | 1.8% (1) | 12.5% (2) |
Abbreviations: ICD, implantable cardiac defibrillator; RCT, randomized controlled trial.
Figure 2.
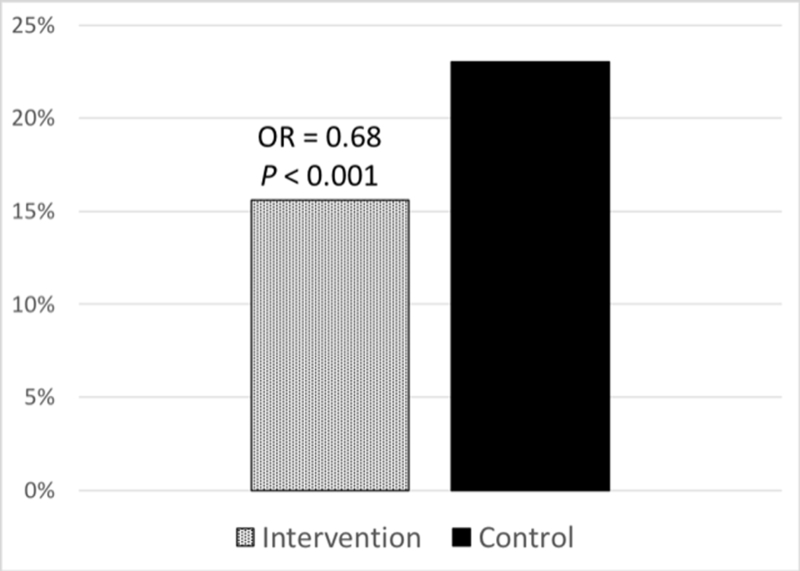
Frequency of shocks in all trials reporting shock outcomes. Abbreviation, OR, odds ratio.
Figure 3.
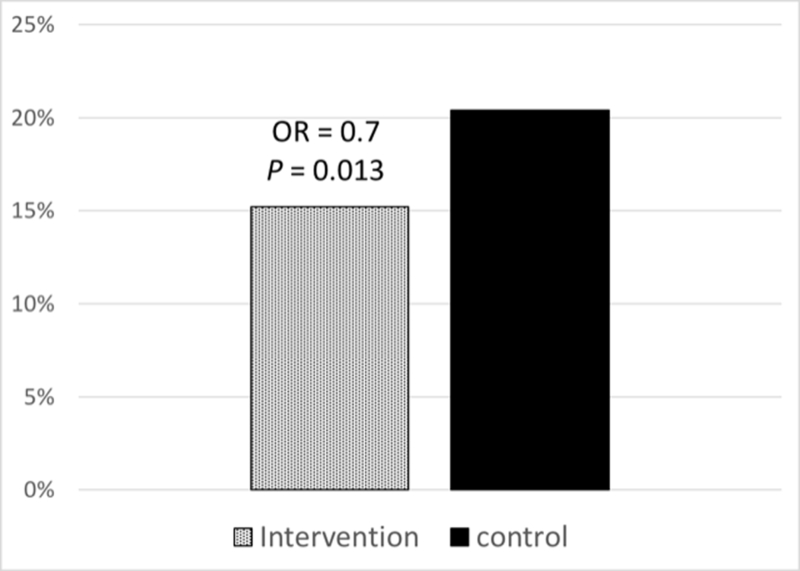
Frequency of shocks in randomized controlled trials reporting shock outcomes. Abbreviation: OR, odds ratio.
Change in peak with exercise intervention compared to control (usual care) was reported in 7 studies. peak improved significantly in patients receiving exercise intervention (1.98 vs 0.36 mL/kg/min, P < .001). Within this group, 6 studies were RCTs and demonstrated significant improvement in peak with exercise intervention (2.15 vs 0.54 mL/kg/min, P < .001).
Since data from Piccini et al23 provided a significant proportion of patients, sensitivity analysis was performed excluding these data. After this exclusion, there were no significant changes in the above outcomes.
DISCUSSION
This systematic review and meta-analysis found that exercise training appears to be safe and effective for patients with ICDs and CRT-Ds. A significant strength of this review is the wide search criteria and large number of papers screened for inclusion. Exercise interventions in the evaluated studies varied widely, but all included regular cardiovascular activity, with a reassuring safety profile specifically regarding ICD shocks. While the primary endpoint definition varied, most studies found improvements using objective measurements such as peak Taken together, these findings support broader application of exercise training among patients with ICDs, though standardization of protocols is necessary for more rigorous future study.
These conclusions extend prior reviews in this area. A review by Isaksen et al identified nine studies of exercise training in a total of 1889 patients with ICDs.30 They report a low burden of ICD therapies during exercise training as well as an improvement in aerobic fitness with exercise training. One limitation of this review was that the results from the large HF-ACTION trial23 had not yet been published. The more recent analysis from Pandey et al included the HF-ACTION trial results and evaluated a total of 6 trials (5 RCT, 1 non-RCT).31 They found that exercise training in patients with heart failure and ICDs improved cardiorespiratory fitness and was associated with a lower likelihood of ICD shocks. While both trials had similar findings to our study, our current review provides a more comprehensive evaluation of the available literature by assessing all available trials, including parallel-arm and single-arm studies.
Though fear of ICD therapies is a natural concern in this patient population, our review illustrates that, with appropriate programming and patient monitoring, ICD discharges were extremely rare both during exercise activity and in follow up in the entire cohort. In fact, patients receiving an exercise intervention had fewer device shocks compared with those who did not engage in an exercise program. Exercise training may decrease both appropriate and inappropriate shocks by modifying autonomic tone with subsequent reductions in ventricular arrhythmia, sinus tachycardia, rapid atrial fibrillation, and other supraventricular tachycardias. While catecholamine levels are higher during exercise, chronic exercise blunts this effect.32,33 Exercise also increases resting parasympathetic tone which protects against ventricular arrhythmias 34,35 and sudden death.36 Furthermore, guidelines for ICD programming have also advanced significantly in recent years, which should reduce the likelihood of both inappropriate therapies and appropriate shocks for self-limited arrhythmia during exercise programs.
Importantly, exercise interventions that improve exercise capacity and decrease ICD shock burden can have significant benefits for patients’ quality of life. Patients receiving ICD shocks have been shown to have significant decrease in mental health and physical functioning. 37 ICD shocks are associated with decreased physical activity, 38 as well as increased anxiety and depression.39 Furthermore, a decrease in ICD shocks and ventricular arrhythmias may reduce myocardial and cerebral ischemia by limiting exposure to hypotensive events. 40–43 There are several potential benefits of exercise training following ICD or CRT-D implantation including familiarization with the device, instruction about physical activity, psychological support, and improvement in exercise capacity. 8
Consensus documents suggest that patients perform a symptom-limited cardiopulmonary exercise stress test or similar evaluation (eg, conventional exercise test or 6-min walk test) prior to initiation of an exercise training program.8,44 Pre-exercise testing allows for evaluation of the chronotropic response to exercise, effectiveness of medications, and the risk of reaching a heart rate in the ICD intervention zone. We recognize that formal exercise testing prior to initiating an exercise program may not always be practical given resource and time constraints, and we would emphasize that providers should be aware of a patient’s programmed ICD intervention zone in order to provide a safe exercise prescription. The American Heart Association statement suggests that the exercise prescription for patients with defibrillators should be limited to a maximal heart rate that is at least 10 to 15 beats/min lower than the intervention zone for the defibrillator. Heart rate monitoring during the exercise program can help avoid any inappropriate interventions.
Our analysis includes several potential limitations. First, study design varied greatly and made direct comparison difficult, with limited options for quantitative analysis. The large number of observational and nonrandomized studies provided an overall relatively low quality of included studies. Confounding by indication, where healthier individuals would be more likely to be enrolled in exercise programs, in the observational studies may overestimate the benefits of exercise training and underestimate the risks of device shocks. Additionally, selection bias may be present as only published studies were able to be included, though lack of consistent endpoint definition made formal analysis of publication bias difficult.
In conclusion, exercise training in patients with ICDs and CRT appears safe and effective based on our review of the relatively scant available literature. However, lack of consensus on design and endpoints limits broader application in this important patient population.
Supplementary Material
Cochrane risk of bias assessment of included studies.
Acknowledgements
Dr. Lubitz is supported by NIH grants K23HL114724 and a Doris Duke Charitable Foundation Clinical Scientist Development Award #2014105. Dr. Noseworthy is supported by the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery. Dr. Kramer is supported by a Paul Beeson Career Development Award (NIH-NIA K23AG049563) and the Greenwall Faculty Scholars Program in Bioethics.
Footnotes
Disclosures: Dr. Steinhaus has consulted for Abbott Laboratories and Boston Scientific, and has a family member who is an executive for Medtronic. Dr. Lubitz receives sponsored research support from Bayer HealthCare, Biotronik, and Boehringer Ingelheim, and has consulted for St. Jude Medical and Quest Diagnostics. Dr. Noseworthy has no disclosures to report regarding this publication. Dr. Kramer has consulted to the Baim Institute for Clinical Research for clinical trials of medical devices.
All authors have read and approved the manuscript.
REFERENCES
- 1.Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117(21):e350–408. [DOI] [PubMed] [Google Scholar]
- 2.Epstein AE, DiMarco JP, Ellenbogen KA, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2013;61(3):e6–75. [DOI] [PubMed] [Google Scholar]
- 3.Kramer DB, Kennedy KF, Noseworthy PA, et al. Characteristics and outcomes of patients receiving new and replacement implantable cardioverter-defibrillators: results from the NCDR. Circ Cardiovasc Qual Outcomes. 2013;6(4):488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borne RT, Peterson PN, Greenlee R, et al. Temporal trends in patient characteristics and outcomes among Medicare beneficiaries undergoing primary prevention implantable cardioverter-defibrillator placement in the United States, 2006–2010. Results from the National Cardiovascular Data Registry’s Implantable Cardioverter-Defibrillator Registry. Circulation. 2014;130(10):845–853. [DOI] [PubMed] [Google Scholar]
- 5.Kurtz SM, Ochoa JA, Lau E, et al. Implantation trends and patient profiles for pacemakers and implantable cardioverter defibrillators in the United States: 1993–2006. Pacing Clin Electrophysiol. 2010;33(6):705–711. [DOI] [PubMed] [Google Scholar]
- 6.O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flynn KE, Pina IL, Whellan DJ, et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piepoli MF, Conraads V, Corra U, et al. Exercise training in heart failure: from theory to practice. A consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Heart Fail. 2011;13(4):347–357. [DOI] [PubMed] [Google Scholar]
- 9.Hong RA, Bhandari AK, McKay CR, Au PK, Rahimtoola SH. Life-threatening ventricular tachycardia and fibrillation induced by painless myocardial ischemia during exercise testing. JAMA. 1987;257(14):1937–1940. [PubMed] [Google Scholar]
- 10.Young DZ, Lampert S, Graboys TB, Lown B. Safety of maximal exercise testing in patients at high risk for ventricular arrhythmia. Circulation. 1984;70(2):184–191. [DOI] [PubMed] [Google Scholar]
- 11.Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Exercise type and intensity in relation to coronary heart disease in men. JAMA. 2002;288(16):1994–2000. [DOI] [PubMed] [Google Scholar]
- 12.Lemaitre RN, Siscovick DS, Raghunathan TE, Weinmann S, Arbogast P, Lin DY. Leisure-time physical activity and the risk of primary cardiac arrest. Arch Intern Med. 1999;159(7):686–690. [DOI] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–94. [DOI] [PubMed] [Google Scholar]
- 14.Vanhees L, Schepers D, Heidbuchel H, Defoor J, Fagard R. Exercise performance and training in patients with implantable cardioverter-defibrillators and coronary heart disease. Am J Cardiol. 2001;87(6):712–715. [DOI] [PubMed] [Google Scholar]
- 15.Fitchet A, Doherty PJ, Bundy C, Bell W, Fitzpatrick AP, Garratt CJ. Comprehensive cardiac rehabilitation programme for implantable cardioverter-defibrillator patients: a randomised controlled trial. Heart. 2003;89(2):155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamke W, Dovifat C, Schranz M, Behrens S, Moesenthin J, Voller H. Cardiac rehabilitation in patients with implantable defibrillators. Feasibility and complications. Zeitschrift fur Kardiologie. 2003;92(10):869–875. [DOI] [PubMed] [Google Scholar]
- 17.Vanhees L, Kornaat M, Defoor J, et al. Effect of exercise training in patients with an implantable cardioverter defibrillator. Eu Heart J. 2004;25(13):1120–1126. [DOI] [PubMed] [Google Scholar]
- 18.Davids JS, McPherson CA, Earley C, Batsford WP, Lampert R. Benefits of cardiac rehabilitation in patients with implantable cardioverter-defibrillators: a patient survey. Arch Phys Medicine Rehabil. 2005;86(10):1924–1928. [DOI] [PubMed] [Google Scholar]
- 19.Belardinelli R, Capestro F, Misiani A, Scipione P, Georgiou D. Moderate exercise training improves functional capacity, quality of life, and endothelium-dependent vasodilation in chronic heart failure patients with implantable cardioverter defibrillators and cardiac resynchronization therapy. Eur J Cardiovasc Prev Rehabil. 2006;13(5):818–825. [DOI] [PubMed] [Google Scholar]
- 20.Conraads VM, Vanderheyden M, Paelinck B, et al. The effect of endurance training on exercise capacity following cardiac resynchronization therapy in chronic heart failure patients: a pilot trial. Eur J Cardiovasc Prev Rehabil. 2007;14(1):99–106. [DOI] [PubMed] [Google Scholar]
- 21.Dougherty CM, Glenny R, Kudenchuk PJ. Aerobic exercise improves fitness and heart rate variability after an implantable cardioverter defibrillator. J Cardiopulm Rehabil Prev. 2008;28(5):307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patwala AY, Woods PR, Sharp L, Goldspink DF, Tan LB, Wright DJ. Maximizing patient benefit from cardiac resynchronization therapy with the addition of structured exercise training: a randomized controlled study. J Am Coll Cardiol. 2009;53(25):2332–2339. [DOI] [PubMed] [Google Scholar]
- 23.Piccini JP, Hellkamp AS, Whellan DJ, et al. Exercise training and implantable cardioverter-defibrillator shocks in patients with heart failure: results from HF-ACTION (Heart Failure and A Controlled Trial Investigating Outcomes of Exercise TraiNing). JACC Heart Fail. 2013;1(2):142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smialek J, Lelakowski J, Majewski J. Efficacy and safety of early comprehensive cardiac rehabilitation following the implantation of cardioverter-defibrillator. Kardiol Pol. 2013;71(10):1021–1028. [DOI] [PubMed] [Google Scholar]
- 25.Berg SK, Pedersen PU, Zwisler AD, et al. Comprehensive cardiac rehabilitation improves outcome for patients with implantable cardioverter defibrillator. Findings from the COPE-ICD randomised clinical trial. Eur J Cardiovasc Nurs. 2015;14(1):34–44. [DOI] [PubMed] [Google Scholar]
- 26.Dougherty CM, Glenny RW, Burr RL, Flo GL, Kudenchuk PJ. Prospective randomized trial of moderately strenuous aerobic exercise after an implantable cardioverter defibrillator. Circulation. 2015;131(21):1835–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smolis-Bak E, Dabrowski R, Piotrowicz E, et al. Hospital-based and telemonitoring guided home-based training programs: Effects on exercise tolerance and quality of life in patients with heart failure (NYHA class III) and cardiac resynchronization therapy. A randomized, prospective observation. Int J Cardiol. 2015;199:442–447. [DOI] [PubMed] [Google Scholar]
- 28.Piotrowicz E, Zielinski T, Bodalski R, et al. Home-based telemonitored Nordic walking training is well accepted, safe, effective and has high adherence among heart failure patients, including those with cardiovascular implantable electronic devices: a randomised controlled study. Eur J Prev Cardiol. 2015;22(11):1368–1377. [DOI] [PubMed] [Google Scholar]
- 29.Isaksen K, Munk PS, Valborgland T, Larsen AI. Aerobic interval training in patients with heart failure and an implantable cardioverter defibrillator: a controlled study evaluating feasibility and effect. Eur J Prev Cardiol. 2015;22(3):296–303. [DOI] [PubMed] [Google Scholar]
- 30.Isaksen K, Morken IM, Munk PS, Larsen AI. Exercise training and cardiac rehabilitation in patients with implantable cardioverter defibrillators: a review of current literature focusing on safety, effects of exercise training, and the psychological impact of programme participation. Eur J Prev Cardiol. 2012;19(4):804–812. [DOI] [PubMed] [Google Scholar]
- 31.Pandey A, Parashar A, Moore C, et al. Safety and efficacy of exercise training in patients with an implantable cardioverter-defibrillator: a meta-analysis. JACC: Clinical Electrophysiology. 2017;3(2):117–126. [DOI] [PubMed] [Google Scholar]
- 32.Ehsani AA, Heath GW, Martin WH 3rd,Hagberg JM, Holloszy JO. Effects of intense exercise training on plasma catecholamines in coronary patients. J Appl Physiol Respir Environ Exerc Physiol. 1984;57(1):155–159. [DOI] [PubMed] [Google Scholar]
- 33.Peronnet F, Nadeau RA, de Champlain J, Magrassi P, Chatrand C. Exercise plasma catecholamines in dogs: role of adrenals and cardiac nerve endings. Am J Physiol. 1981;241(2):H243–247. [DOI] [PubMed] [Google Scholar]
- 34.Kolman BS, Verrier RL, Lown B. The effect of vagus nerve stimulation upon vulnerability of the canine ventricle: role of sympathetic-parasympathetic interactions. Circulation. 1975;52(4):578–585. [DOI] [PubMed] [Google Scholar]
- 35.Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS Jr, , Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res. 1991;68(5):1471–1481. [DOI] [PubMed] [Google Scholar]
- 36.La Rovere MT, Bersano C, Gnemmi M, Specchia G, Schwartz PJ. Exercise-induced increase in baroreflex sensitivity predicts improved prognosis after myocardial infarction. Circulation. 2002;106(8):945–949. [DOI] [PubMed] [Google Scholar]
- 37.Schron EB, Exner DV, Yao Q, et al. Quality of life in the antiarrhythmics versus implantable defibrillators trial: impact of therapy and influence of adverse symptoms and defibrillator shocks. Circulation. 2002;105(5):589–594. [DOI] [PubMed] [Google Scholar]
- 38.Burgess ES, Quigley JF, Moran G, Sutton FJ, Goodman M. Predictors of psychosocial adjustment in patients with implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 1997;20(7):1790–1795. [DOI] [PubMed] [Google Scholar]
- 39.Hegel MT, Griegel LE, Black C, Goulden L, Ozahowski T. Anxiety and depression in patients receiving implanted cardioverter-defibrillators: a longitudinal investigation. Int J Psychiatry Med. 1997;27(1):57–69. [DOI] [PubMed] [Google Scholar]
- 40.Hurst TM, Hinrichs M, Breidenbach C, Katz N, Waldecker B. Detection of myocardial injury during transvenous implantation of automatic cardioverter-defibrillators. J Am Coll Cardiol. 1999;34(2):402–408. [DOI] [PubMed] [Google Scholar]
- 41.Joglar JA, Kessler DJ, Welch PJ, et al. Effects of repeated electrical defibrillations on cardiac troponin I levels. Am J Cardiol. 1999;83(2):270–272, A276. [DOI] [PubMed] [Google Scholar]
- 42.de Vries JW, Bakker PF, Visser GH, Diephuis JC, van Huffelen AC. Changes in cerebral oxygen uptake and cerebral electrical activity during defibrillation threshold testing. Anesth Analg. 1998;87(1):16–20. [DOI] [PubMed] [Google Scholar]
- 43.Murkin JM, Baird DL, Martzke JS, Yee R. Cognitive dysfunction after ventricular fibrillation during implantable cardiovertor/defibrillator procedures is related to duration of the reperfusion interval. Anesth Analg. 1997;84(6):1186–1192. [DOI] [PubMed] [Google Scholar]
- 44.Fletcher GF, Ades PA, Kligfield P, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013;128(8):873–934. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cochrane risk of bias assessment of included studies.


