Abstract
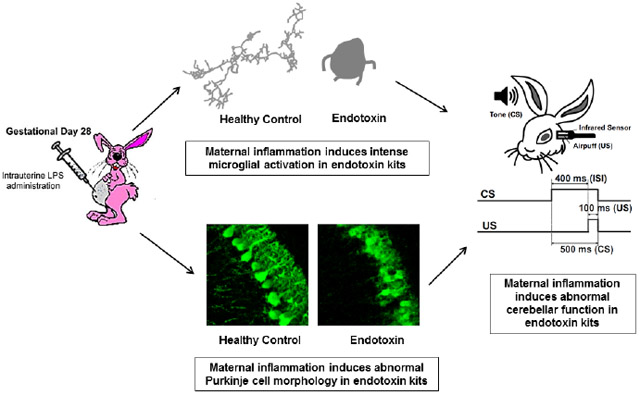
Cerebellum is involved in higher cognitive functions and plays important roles in neurological disorders. Cerebellar injury has been detected frequently in patients with preterm birth resulting in cognitive dysfunction later in life. Maternal infection and inflammation is associated with preterm birth and in neonatal brain injury. We have previously shown that intrauterine lipopolysaccharide (LPS) exposure induces white matter injury and microglial activation in the cerebral white matter tracts of neonatal rabbits, resulting in motor deficits consistent with the clinical findings of cerebral palsy (CP). Here we investigated whether intrauterine LPS exposure induced cerebellar inflammation and functional impairment. Timed-pregnant New Zealand white rabbits underwent a laparotomy on gestational day 28 (G28) and LPS (3200 EU, endotoxin group) was injected along the wall of the uterus as previously described. Controls did not receive surgical intervention. Kits born to control and endotoxin treated dams were euthanized on postnatal day (PND)1 (3 days post-injury) or PND5 (7 days post-injury) and cerebellum evaluated for presence of inflammation. The microglial morphology in cerebellar white matter areas was analyzed using Neurolucida and Neurolucida Explorer. mRNA expression of inflammatory cytokines was quantified by real-time-PCR. We found that intrauterine exposure to LPS induced intensive microglial activation in cerebellar white matter areas, as evidenced by increased numbers of activated microglia and morphological changes (amoeboid soma and retracted processes) that was accompanied by significant increases in pro-inflammatory cytokines. The Purkinje cell layer was less developed in endotoxin exposed kits than healthy controls. In kits that survived to PND 60, soma size and cell density of Purkinje cells were significantly decreased in endotoxin exposed kits compared to controls. The findings of altered Purkinje cell morphology were consistent with impaired cerebellar function as tested by eye-blink conditioning at 1 month of age. The results indicate that the cerebellum is vulnerable to perinatal insults and that therapies targeting cerebellar inflammation and injury may help in improving outcomes and function.
Keywords: Maternal infection, microglia, cerebral palsy, cerebellum, white matter, dendrimer, cytokines
Graphical Abstract
1. Introduction
Cerebral palsy (CP) is a neurodevelopmental disorder caused by a multitude of etiologies, which lead to a static encephalopathy syndrome. CP is the most common pervasive childhood motor disability with a prevalence of 2.6-2.9 per 1000 children (Maenner, Blumberg et al. 2016). The incidence of CP remains largely unchanged, in spite of the reductions in infant mortality and improvements in prenatal care, causing a significant health care economic burden (Bear and Wu 2016). Maternal infection and inflammation are implicated in neonatal brain injury resulting in not only motor deficits as seen in CP but more commonly in cognitive, learning and behavioral deficits (O'Shea, Allred et al. 2009). Maternal inflammation is known to be a common cause for preterm birth. Our previous studies showed that intrauterine LPS exposure triggers intense and prolonged microglial activation in the periventricular white matter brain regions of neonatal rabbits, resulting in white matter injury, motor deficits (Kannan, Saadani-Makki et al. 2007; Saadani-Makki, Kannan et al. 2008; Kannan, Dai et al. 2012; Balakrishnan, Dai et al. 2013), decreased neuronal counts in the thalamus, decreased dendrites and spines in the retrospenial cortex (Kannan, Dai et al. 2012; Balakrishnan, Dai et al. 2013), increased glutamate excitotoxicity (Zhang, Bassam et al. 2016), increased inflammation (Zhang, Jyoti et al. 2018), as well as a decrease in the serotonin staining thalamocortical fibers in the parietal somatosensory cortex (Kannan, Saadani-Makki et al. 2011) and dysregulation of tryptophan metabolism (Williams, Zhang et al. 2017). We have also shown that the microglial changes are associated with increased CD11b staining and pro-inflammatory cytokines till at least postnatal day 5 (Kannan, Dai et al. 2012; Zhang, Jyoti et al. 2018) and on in vivo PET imaging till PND 14 (Zhang, Jyoti et al. 2018). These changes are consistent with the phenotype of CP (Saadani-Makki, Kannan et al. 2008).
The cerebellum has long been known to be associated with motor function, however, growing evidence has shown that cerebellum is also involved in behavioral and cognitive functions (Stoodley and Schmahmann 2010; Stoodley 2012). The cerebellum is particularly vulnerable to injury since it rapidly develops in the perinatal period (Volpe 2009). Imaging studies have demonstrated volumetric decreases of the cerebellum, hemorrhage or subtle diffusion tensor changes in the cerebellum in the brain of children born prematurely. Cognitive changes such as reading impairment and abnormalities in fine moor skills and visual memory ability that are associated with cerebellar white matter microstructural changes have been demonstrated in older children and adolescents (Travis, Leitner et al. 2015; Thomas, Lacadie et al. 2017). A volumetric magnetic resonance imaging study has shown that the total brain, cerebellum, and grey matter volumes are significantly reduced in children with cerebral palsy, compared with age-matched healthy controls (Kulak, Maciorkowska et al. 2016). A recent study demonstrated that even in the absence of focal cerebellar lesions, prematurity led to an impairment in cerebellar learning determined by classical eyeblink conditioning in young adults (Tran, 2017). There is a crucial need to develop and evaluate pre-clinical models that mimic the cerebellar injury seen in patients following perinatal insults in order to design and evaluate appropriate therapeutic strategies that would attenuate this injury. In this study we investigated the effect of in utero LPS exposure on microglial activation in the cerebellar white matter region, mRNA expression of inflammatory cytokine, Purkinje cell development, and cerebellar function determined by classical eyeblink conditioning in neonatal and adolescent New Zealand white rabbits. We hypothesized that intrauterine LPS exposure will result in cerebellar neuroinflammation, abnormal Purkinje cell development and impaired cerebellar function.
2. Materials and Methods
2.1. Animals
All animal procedures were in accordance with the Animal Care and Use Committee guidelines at Johns Hopkins University and the United States Department of Agriculture. Timed-pregnant New Zealand rabbits were purchased from Robinson Services Inc. (Winston-Salem, NC). All animals were housed under ambient conditions (22°C, 50% relative humidity, and a 12-h light/dark cycle), and necessary precautions were taken throughout the study to minimize pain and stress associated with the experimental treatments.
2.2. Surgical Procedure
Time pregnant New Zealand white rabbits underwent a laparotomy at gestational day 28 (term pregnancy = 31 days) under general anesthesia (2-3% isoflurane by mask). LPS (3,200 EU, Escherichia coli was injected along the length of the uterine wall between fetuses as previously described (Saadani-Makki, Kannan et al. 2008; Zhang, Bassam et al. 2016). The dose was lower than we used previously in order to improve longer term survival. Heart rate, oxygen saturation, rectal temperature, arterial blood pressure, and end-tidal CO2 were monitored continuously throughout the procedure. Pregnant rabbits that did not receive any surgical intervention served as controls. To control the timing of delivery, pregnant rabbits were induced on gestational day 30-31 using nasal oxytocin. The kits were kept in incubators with a temperature of ~32-35°C and relative humidity of ~60-70% until euthanasia. Necessary precautions were undertaken throughout the study to minimize pain and stress associated with the experimental treatments. The in utero and immediate postnatal mortality was ~10% in the present study.
2.3. Immunohistochemistry
The kits were anesthetized and transcardially perfused with PBS at PND1 or PND5. The brains were removed and fixed in 10% formalin and cryoprotected in 30% sucrose solutions. The cerebellum was dissected from the whole brain and sectioned using a cryostat. Parasagittal cerebellar sections (30 μm of thickness, in 1:6 series) were collected and mounted on gelatincoated slides. For IBA1 (microglial marker) and calbindin (Purkinje cell marker) DAB staining, sections were pretreated with hydrogen peroxide, blocked with 3% donkey serum, and incubated with goat anti-IBA1 (1:500, Abcam, MA, USA) or mouse anti-calbindin (1:500, GeneTex, CA, USA) overnight at 4°C. The sections were washed, incubated with Biotinylated secondary antibodies (1:250, Vector Laboratory) for 4 h, followed by Vectastain’s ABC kit (Vector Laboratory) for 2 h, and color developed with DAB kit (Vector Laboratory). All the images were captured using Nikon Eclipse 90i and Stereo Investigator software (MBF Bioscience, Williston, VT, U.S.A.) with 40x magnification, keeping similar settings during image acquisition for different groups.
For IBA1 and calbindin fluorescent staining, brain sections were incubated overnight at 4°C with goat anti-IBA1 (1:250, Abcam, MA. U.S.A.) or mouse anti-calbindin (1:250, GeneTex, CA, USA). Sections were washed and incubated with fluorescent secondary antibodies (1:250; Life Technologies, MA, U.S.A.) for 2 h at room temperature followed by with DAPI (1:1000, Invitrogen) for 15 min. After washing, the slides were dried and cover slipped with mounting medium (Dako, Carpinteria, CA, USA). Confocal images (z-stack) were acquired with Zeiss ZEN LSM 710 (Zeiss, CA, U.S.A.) and processed with ZEN software.
2.4. Imaging Analysis
Microglia in the white matter tracts of the cerebellum were visualized and imaged using Nikon Eclipse 90i and Stereo Investigator software (MBF Bioscience, Williston, VT, U.S.A.). All slides were coded and analyzed by personnel blinded to the experimental design. Images (40X, 5-6 images/animal) were randomly acquired from different white matter areas. The number of activated and resting microglia and the ratio of activated to total (activated + resting) microglia were manually quantified using ImageJ software. The changes in the morphology of the microglia were analyzed using Neurolucida and Neurolucida Explorer 6.21.2 (MBF Bioscience, Williston, VT, U.S.A.). Microglia were traced that met the following criteria: (1) Cell body located in the white matter area; (2) Processes were completely contained within the slice; (3) Cells sufficiently stained to allow for tracing processes. The morphology of soma and processes of the microglia were analyzed and compared between groups.
Purkinje cells in the cerebellum were visualized and imaged using Nikon Eclipse 90i or Zeiss ZEN LSM 710 (Zeiss, CA, U.S.A.) and Stereo Investigator software (MBF Bioscience, Williston, VT, U.S.A.). All slides were coded and analyzed by personnel blinded to the experimental design. Images of Purkinje cell layer (40X, 5-6 images/animal) were randomly acquired at similar anatomical locations from control and endotoxin kits. The soma size, the mean maximal length of dendrites and linear density of Purkinje cells were analyzed using Neurolucida and Neurolucida Explorer 6.21.2 (MBF Bioscience, Williston, VT, U.S.A.). The maximal length of dendrite of each Purkinje cell was measured from the initial start point to the end of the dendrite (Figure 4C). Purkinje cells linear density was analyzed based on published protocols (Louis, Babij et al. 2013). In brief, the Purkinje cell layer length in each field of view was measured by drawing a freehand line through the center of the soma of cells in the layer. The total number of Purkinje cells divided by the Purkinje cell layer length was defined as Purkinje cell linear density. The soma size and linear density of Purkinje cells were averaged and compared between control and endotoxin groups.
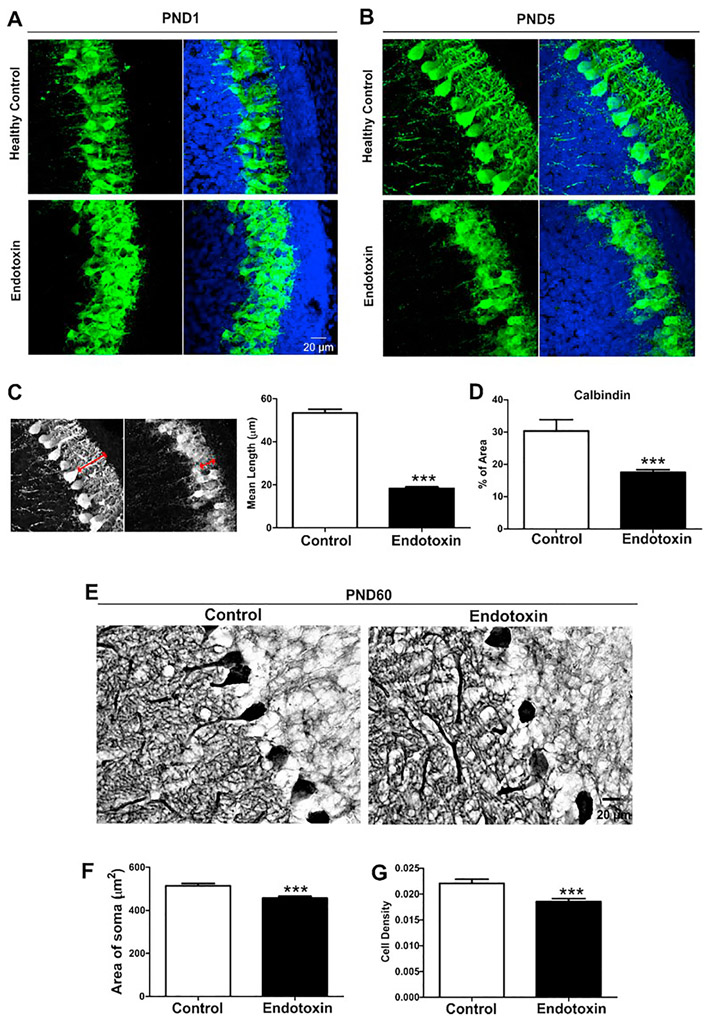
Figure 4.
Purkinje cell morphology. A, B, Representative confocal images of Purkinje cells in control and endotoxin kits at PND1 (A) and PND5 (B). Purkinje cells had less dendritic structures in endotoxin kits. C. The mean maximal dendritic length was significantly decreased in endotoxin kits at PND5, compared with healthy controls. D. The percentage of calbindin staining significantly decreased in endotoxin kits when compared with healthy controls. E. Representative images of Purkinje cells in control and endotoxin kits at PND60. F. The size of the soma of Purkinje cells in endotoxin kits was significantly smaller than controls. G. The cell density of Purkinje cells in endotoxin kits significantly decreased, compared with controls. ***p<0.001
2.5. Area measurement of calbindin
All slides and images were coded and the analysis was performed with personnel blinded to the experiments. Images (40 × 5 images/animal) were randomly acquired at similar anatomical locations from control and endotoxin kits using Zeiss ZEN LSM 710 (Zeiss, CA, U.S.A.). The percentage of area of calbindin expressions were measured using particle analysis function in ImageJ software as previously described (Zhang, Bassam et al. 2016). In brief, the images were converted to 8-bit greyscale and the threshold was manually adjusted followed by application of the ‘analyze particles’ function with the size parameters (pixel units) of 0.0001–infinity and circularity 0–1 (show outlines). The optimum parameters were identified by manual tracing using “freehand” tool on one image and systematically varying one parameter at a time while fixing all other parameters to avoid detecting artifacts.
2.6. Real Time PCR
Brains from rabbits at PND1 and PND 5 were quickly harvested and stored in RNAlater solution (Life technologies, Grand Island, NY, USA). The whole cerebella (~50 mg) were isolated and the total RNA was extracted using TRIZOL (Life Technologies, Grand Island, NY, USA) according to the manufacturer instructions. RNA samples were quantified using the Nanodrop ND-1000 Spectrophotometer. Single-stranded complementary DNA (cDNA) was first reverse transcribed from the total RNA samples (2μg) using the High Capacity cDNA Reverse Transcription Kit with RNase inhibitor (Life Technologies, Grand Island, NY, USA). Real-time PCR was performed with Power SYBR Green PCR Master Mix (Life Technology, Grand Island, NY, USA) using Fast 7500 Real-time PCR systems (Life Technologies, Grand Island, NY, USA). Amplification conditions included 30 min at 48°C, 10 min at 95°C, 40 cycles at 95°C for 15 s and 60°C for 1 min. Primers were custom designed and ordered from Integrated DNA technology (Iowa, USA). Table 1 lists primers used and their specifications. The Comparative Ct method was used to assess differential gene expressions between control and endotoxin groups at different time points. Gene expression levels for each sample were normalized to its expression level of the housekeeping gene encoding glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (ΔCt); the difference between the endotoxin group and the control group was used to determine the ΔΔCt. The 2-ΔΔCt gave the relative fold changes in gene expression.
Table 1.
Primers for real-time-PCR.
| Gene | Accession Number | Forward Primer | Reverse primer |
|---|---|---|---|
| TNF-α | AB128153.1 | TAGTAGCAAACCCGCAAGTG | CTGAAGAGAACCTGGGAGTAGA |
| IL-1β | KT279631.1 | TGCCAACCCTACAACAAGAG | AAAGTTCTCAGGCCGTCATC |
| IL-6 | AF169176.1 | CATCAAGGAGCTGAGGAAAGAG | CCTTGGAAGGTGCAGATTGA |
| IL-10 | AF068058.1 | CCTGTGGGATTTGAGTGTCTTA | GCTCGGCTTAGGAGTTAGAAAG |
| IL-4 | XM_008254869.1 | CATCCTACCCGAAGTCATCAAA | CTCTCTCTCGGTTGTGTTCTTG |
| TGF-β1 | XM_002722312.1 | TGAGAGGTGGAGAGGAAATAGA | GGAACTGATCCCGTTGATGT |
| GAPDH | NM_001082253 | TGA CGA CAT CAA GAA GGT GGT G | GAA GGT GGA GGA GTG GGT GTC |
2.7. Eye-blink Test
Cerebellum plays a major role in conditioned eye-blink associative learning (Thompson 1986; Attwell, Ivarsson et al. 2002; Christian and Thompson 2003; Ohyama, Nores et al. 2006; Bracha, Zbarska et al. 2009). In this study we used the eye-blink associative test to evaluate the effects of LPS exposure on cerebellum-dependent learning and memory (Figure 5A). Eye-blink testing was carried out using an eye-blink apparatus modified for rabbits (San Diego Instruments, San Diego, CA). A speaker, placed close to the ears of the rabbits, delivered a tone, the conditioned stimulus, CS. A lightweight device containing 1) an infrared emitter and detector for recording eye movement, and 2) a plastic nozzle for delivery of an air puff, the unconditioned stimulus, US was positioned approximately 2 cm from the rabbit’s left eye. The timing and amplitude of stimuli were controlled by San Diego Instruments software. Output was digitized, stored, and analyzed using San Diego Instruments software. Rabbits were placed in an apparatus (20.5 cm × 11.1 cm) that included adjustable head restraint bars, an adjustable back "butt" plate, stainless steel locking adjustment pin for the neck support, an adjustable formed back support to protect the back, and a non-slip floor material to provide secure animal footing and calm the animal (Plas-Labs Inc. MI, U.S.A).
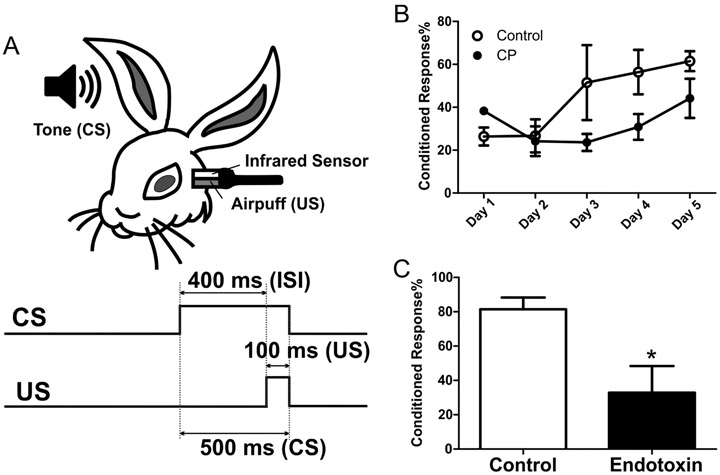
Figure 5.
Cerebellar dependent learning and memory. At 4-6 weeks of age, rabbits from control and endotoxin groups underwent eye-blink training for 5 consecutive days. A probe test was carried out on the sixth day. The cerebellar dependent learning was indicated by the percentage of conditioned responses. A. The schematic of eye-blink test. CS, conditioned stimulus; US, unconditioned stimulus; ISI, inter-stimulus interval. B. The percentage of conditioned responses in endotoxin kits was lower than controls over the 5-day training period. C. The percentage of conditioned responses in endotoxin kits was significantly lower than controls during the probe trial at day-6 (24 hours after the last training session). *p<0.05.
Rabbits were handled daily and habituated in the apparatus for 3 h per day for 2 consecutive days before the test. The eye-blink paradigm was modified from previously published protocols (Bracha, Zbarska et al. 2009; Brown and Woodruff-Pak 2012). A delayed conditioning paradigm was used in which the tone (CS, 500 ms, 1-kHz, 85-dB) preceded, overlapped, and co-terminated with the corneal airpuff (US, 100 ms, 7-8 psi) to the left eye. The duration of each trial was 1300 ms, and the first 250 ms of the trial was baseline without any stimulus. The inter-stimulus interval (ISI) between CS and US onset was 400 ms, and an average inter-trial interval was 25 sec. The rabbits received one training session (90 trials of paired CS-US) per day for 5 consecutive days. The external eyelid or nictitating membrane movement (whichever was first) was recorded and analyzed with San Diego Instruments software. A response was registered when the external eyelid or nictitating membrane movement reached at least 10% of the maximum response amplitude generated that session. During the training session, if a response exceeded threshold between 1 and 399 ms after CS onset, a conditioned response (CR) was recorded. If the response exceeded threshold after US onset (US onset was 400 ms after CS onset), an unconditioned response (UR) was recorded. Cerebellar leaning was indicated by the percentage of conditioned responses in the 90 trials of training: % of CR= number of CR/90 *100.
On the sixth day (24 hours after the last training session), cerebellar dependent memory retention was evaluated with a probe session (30 trials with only CS without US). Based on our preliminary experiments, we chose 30 trials in the probe session, which showed the most robust difference between control and endotoxin groups. During the probe session, if tone alone (CS) elicited a detectable external eyelid or nictitating membrane movement in a test trial, this trial was defined as “conditioned response (CR)”; if tone alone did not elicit a detectable eye closure in a test trial, this trial was defined as “no response”. Cerebellar memory retention was determined by the percentage of conditioned responses in the 30 tone-alone (CS) test trials: % of CR= number of CR/30 *100. A total of 4 rabbits (from 4 litters) for the healthy controls and 4 rabbits (from 4 litters) in the endotoxin group underwent 90 trials per day for 5 days for the training sessions and 30 trials on day 6 for the probe session.
2.8. Statistical Analysis
Statistical analysis of data was carried out using GraphPad Prism5. The real-time PCR data were normalized by log-transformation prior to statistical analysis to reduce the variability in the data and the effect of outliers on the final analysis (Gelman and Hill 2007). Two-tailed student t-test was used for control and endotoxin group comparisons. Differences were considered statistically significant if p < 0.05.
3. Results
3.1. Maternal inflammation induces intense microglial activation in cerebellar white matter
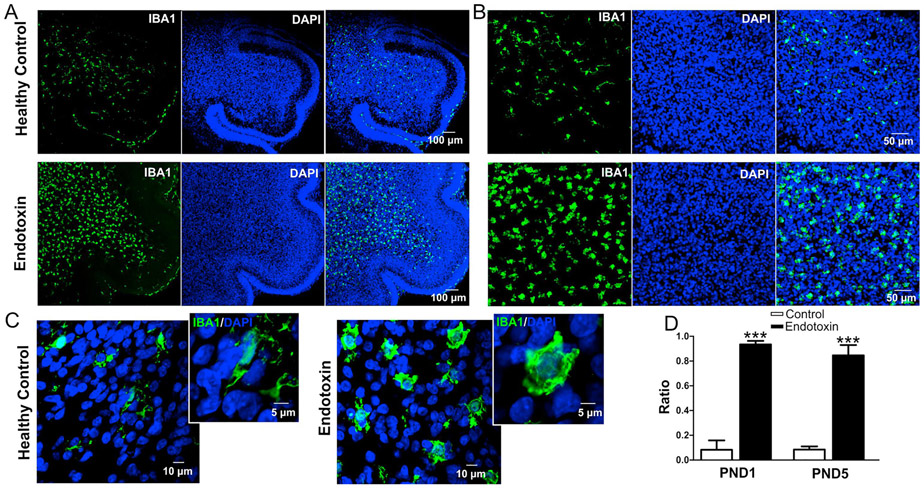
An analysis of microglial morphology in the cerebellar white matter tracts showed that microglia in the endotoxin group had an amoeboid soma and retracted and thickened processes indicative of microglial activation. In the healthy control group, the microglia had normal soma size and ramified processes, indicative of ‘resting’ or ‘normal’ microglia (Figure 1A-C). The ratio of activated to total (activated + resting) microglia was significantly increased in the endotoxin treated kits, compared with healthy control at PND1 (control: n=5, from 3 litters; endotoxin: n=5, from 4 litters, p < 0.0001) and PND 5 (control: n=5, from 3 litters; endotoxin: n=5, from 4 litters, p<0.0001) (Figure 1D).
Figure 1.
Microglial activation in endotoxin group kits compared with age-matched healthy controls. A-C, Representative images of microglial distribution and morphology in cerebellar white matter in healthy control and endotoxin kits at PND1 (A), 20x magnification (B), and 63x magnification (C). D, The ratio of activated to total microglia in healthy control and endotoxin kits at PND1 and PND5. ***p<0.001.
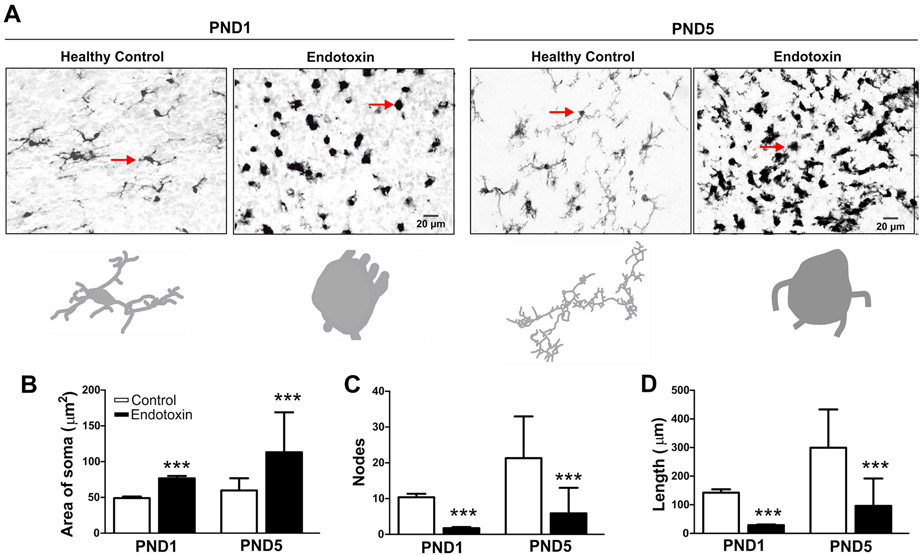
We further analyzed microglial morphology using Neurolucida and Neurolucida Explorer software. At both PND1 and PND5, the size of the soma was significantly increased (Figure 2A, B) while the nodes and length of the microglial processes were significant decreased in the endotoxin treated versus control kits (control group n=5, from 3 litters; endotoxin group: n=5, from 4 litters) (Figure 2C, D).
Figure 2.
Microglial morphology in healthy controls and endotoxin kits. Brain slices were stained with IBA1 antibody (microglial marker) and images (40x) were randomly acquired from the white matter areas from healthy controls and endotoxin kits. A, Representative images of microglia in healthy control and endotoxin kits at PND1 and PND5. The arrows indicate the microglia that corresponding to the Neurolucida tracing images. B-D, Microglial morphological analysis. The microglia in the endotoxin kits had significantly increased area of the soma (B), significant decreased node (C) and length (D) of the processes, compared to the healthy control kits at PND1 and PND5. ***p<0.001.
3.2. Maternal inflammation differentially regulates mRNA expression of pro- and anti-inflammatory cytokines
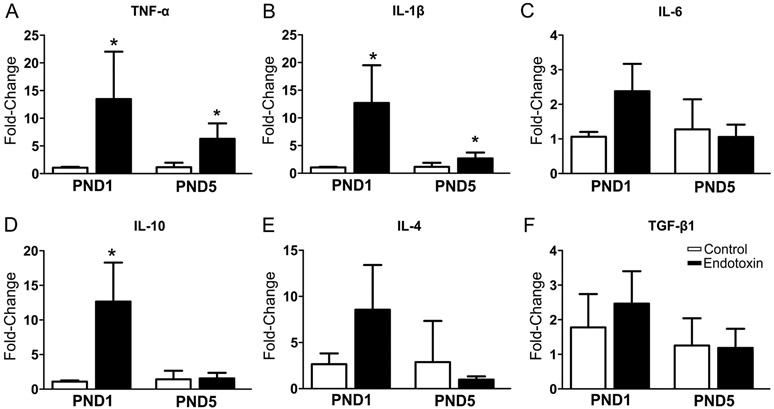
We compared the mRNA expressions of pro- and anti-inflammatory cytokines in endotoxin and age-matched healthy control kits at PND 1 (control group: n=9, from 6 litters; endotoxin group: n=9, from 7 litters) and PND5 (control group: n=6, from 4 litters; endotoxin group: n=6, from 4 litters). We found that mRNA expression of pro-inflammatory cytokines TNF-α and IL-1β increased significantly in endotoxin treated kits at both PND1 and PND5 (Fig. 3A, B); while mRNA expression of the anti-inflammatory cytokine IL-10 was significantly increased at PND1, but not at PND5 (Fig. 3D). There was no statistical difference between the two groups in the mRNA expression of IL-6, IL-4 and TGF-β1 (Figure 3C, E, F).
Figure 3.
mRNA expression of pro- and anti-inflammatory cytokines in the whole cerebella. There is a significant increase in pro-inflammatory cytokines TNF-α (A), IL-1β (B) in the endotoxin kits at PND1 and PND5 when compared to controls. The anti-inflammatory cytokines IL-10 (D) significantly increased only at PND1. There was no significant change in mRNA expression of IL-6 (C), IL-4 (E) and TGF-β1 (F). *p<0.05.
3.3. Maternal inflammation leads to impaired Purkinje cell development
Qualitatively, the morphology of the Purkinje cell layer differed between control and endotoxin treated kits at PND1 and PND5. Purkinje cells were more clustered and at times overlapped with one another in the endotoxin kits and had fewer dendrites than healthy controls at PND1 (Figure 4A). At PND5, Purkinje cells in endotoxin kits continued to have less developed dendritic processes than healthy controls, which had well-defined dendritic structures (Figure 4B). We measured the mean maximal length of dendrites and calbindin staining intensity from control (n=4, from 4 litters) and endotoxin kits (n=4, from 3 litters) at PND5. We found that the mean maximal length of dendrites (Figure 4C) and calbindin staining intensity (Figure 4D) significantly decreased in endotoxin kits when compared to healthy controls. Due to the overlapping of Purkinje cells in endotoxin treated kits at PND1 and PND5, the soma size and linear density of Purkinje cells were not quantitatively analyzed at these two time points. At PND60, quantitative analysis demonstrated a significant decrease both in soma size and linear density of Purkinje cells in endotoxin treated kits compared with healthy controls (p<0.001) (Figure 4E, F, G).
3.4. Maternal inflammation leads to impaired cerebellar function
Rabbits from healthy control and endotoxin groups that were 4-6 weeks of age received eye-blink training sessions (90 CS-US trials per day) for 5 consecutive days. A probe session (30 trials with only CS) was carried out on the sixth day. Cerebellar learning, indicated by the percentage of conditioned responses, was calculated and averaged. We found that during the 5-day training period, the endotoxin treated kits showed fewer conditioned responses at day 3, 4 and 5 than control kits. Moreover, during the probe trial on day 6 (24 hour after the last training session), the percentage of conditioned responses significantly decreased in the endotoxin treated kits, compared with healthy controls (Figure 5), indicating that cerebellar learning was impaired.
4. Discussion
Microglial activation has been implicated in a variety of neurological diseases, including autism and ADHD (Suzuki, Sugihara et al. 2013). A clinical study in adult males patients with autism that used positron emission tomography to assess microglial activation showed increased microglial activation throughout the brain, with the most substantial increase occurring in the cerebellum (Suzuki, Sugihara et al. 2013). Microglia play a crucial role in the pruning and development of the Purkinje cell layer in the postnatal period with precise programmed cell death and engulfment of dying Purkinje neurons during development (Marin-Teva, Dusart et al. 2004). Any perturbation or change in microglial function that can disrupt this precise developmental regulation can lead to impaired development of the Purkinje cell layer. Global hypoxia and hypoxic-ischemic induced injury to the forebrain was shown to result in defects in myelination in the cerebellum along with injury to granular cells and Purkinje cells, with both microglia and astrocytes being implicated in the pathogenesis (Biran, Heine et al. 2011). Another study evaluating the effects of focal cerebral ischemia demonstrated increased necrosis and apoptosis in the cerebellum that may involve changes in regulation of programmed cell death in granular and Purkinje cells (Peng, Feng et al. 2005).
Cerebellar injury and hypoplasia have been noted in premature infants and infants with HIE (Tam, Ferriero et al. 2009; Volpe 2009; Kidokoro, Neil et al. 2013; Traudt, McPherson et al. 2014; Klein, Lemmon et al. 2016). A reduction in cerebellar volume has been associated with insults such as intraventricular hemorrhage and prematurity, and correlates with neurologic impairments at later time points (Shah, Anderson et al. 2006; Tam, Rosenbluth et al. 2011; Morita, Morimoto et al. 2015; Jeong, Shim et al. 2016). In one study 66% of preterm infants with isolated cerebellar hemorrhage developed neurologic abnormalities ranging from motor dysfunction to learning disorders (Limperopoulos, Bassan et al. 2007). Cerebellar volume loss has been noted in premature infants even in the absence of focal cerebellar injury (Allin, Matsumoto et al. 2001). However it has not always been clear whether the cerebellar volume reduction is due to, or independent of, supratentorial injury (Srinivasan, Allsop et al. 2006; Tam, Ferriero et al. 2009). Pre-clinical models of chorioamnionitis using maternal LPS administration of LPS induced fetal inflammation have demonstrated the presence of microgliosis in the cerebellar white matter and injury to granule cells in neonatal sheep, and increased presence of pro-inflammatory cytokines in the cerebellar white matter in rhesus monkeys (Dean, Farrag et al. 2009; Strackx, Sparnaaij et al. 2015; Schmidt, Kannan et al. 2016; McDougall, Hale et al. 2017). Several preclinical models of other forms of neonatal brain injury including IVH and HIE have demonstrated abnormalities in the cerebellum ranging from injury and/or disorganization of the Purkinje cell layer to gliosis and abnormal development of the granule cells (Biran, Verney et al. 2012; Campanille, Saraceno et al. 2015). The mechanisms for the cerebellar hypoplasia are not clearly elucidated. Some of the proposed mechanisms include injury from hemoglobin degradation products, decreased circulating IGF1 seen in preterm rabbits (Sveinsdottir, Lansberg et al. 2017), gliosis, inflammation (McDougall, Hale et al. 2017) and alterations in sonic hedgehog signaling in Purkinje cells (Nguyen, Sabeur et al. 2018).
In this study we evaluated morphological changes, cytokine regulation, and functional impairment of cerebellum in a maternal inflammation induced rabbit model of neonatal brain injury. We found that intrauterine endotoxin exposure resulted in intense microglial activation in the white matter tracts of the cerebellum in newborn kits, which lasted for more than 7 days post-injury. This was evidenced by the significant increase in the ratio of activated microglia to total microglia, the morphology changes (amoeboid soma and retracted processes) of the microglia present in the cerebellar white matter tracts, and upregulation of pro-inflammatory cytokines. We have previously shown similar microglial activation in the periventricular region (PVR) in the cerebrum with persistence of microglial activation and elevated pro-inflammatory cytokines at PND5 (Zhang, Jyoti et al. 2018). In this study we used a lower dose of endotoxin to ensure long term survival of the rabbit kits for evaluating cerebellar function. However, even with a lower dose we demonstrate gliosis, and an increase in inflammatory cytokines that rapidly decreases by PND5. This alteration in microglial function and transient inflammation at a vulnerable period in cerebellar development may be responsible for the impairment in cerebellar function and abnormality in Purkinje cell layer structure seen in the older rabbits at 1-2 months of age.
We further evaluated the impact of maternal inflammation on the cerebellar function and Purkinje cell morphology at young adulthood. We found that Purkinje cells in kits exposed to intrauterine inflammation had smaller soma and decreased densities compared with healthy controls at two months of age. In a rat model of perinatal asphyxia, a disarrayed Purkinje cell layer with a higher number of abnormal calbindin-stained Purkinje cells were reported (Campanille, Saraceno et al. 2015), which may indicate a loss in proper migration (Laure-Kamionowska and Maslinska 2011). The reduction in calbindin protein expression was also reported in hypoxic guinea pig fetus at term, which could result in activation of Ca2+ -mediated pathways (Katsetos, Spandou et al. 2001). In our model the changes in the Purkinje cell morphology could be due to the alteration of calbindin protein expression, which needs to be further determined. Moreover, endotoxin treated kits showed cerebellar function impairment, indicated by a significantly decreased percentage of conditioned responses during the eye-blink test. Evidence indicates a strong link between cerebral white matter lesions and cerebellar underdevelopment (Argyropoulou, Xydis et al. 2003; Shah, Anderson et al. 2006). Studies show that microglia play an important role in cerebellum development (Perez-Pouchoulen, VanRyzin et al. 2015) and promote the death of developing Purkinje cells (Marin-Teva, Dusart et al. 2004). In our study, the decreased Purkinje cell density may be results from the intense and prolonged pre-inflammatory microglial activation resulting in loss or impaired development of Purkinje cells.
Eyeblink classical conditioning has been used to study the involvement of the cerebellum in motor learning. In eyeblink conditioning, a conditioned stimulus precedes and co-terminates with an unconditioned stimulus. Initially, the conditioned stimulus cannot elicit a reliable eye blink, but the unconditioned stimulus can elicit a reliable eye blink. An eye blink to the conditioned stimulus can be developed over the course of a few hundred paired conditioned stimulus-unconditioned stimulus trials, which is called the conditioned response. We found in our rabbit model that it took longer to acquire the conditioned response and that conditioned responses were fewer than in controls. These results are consistent with those of a recent study in patients born prematurely, who when tested as adults exhibited slower learning rates and fewer conditioned responses than age-matched healthy young adults who were born at term.. Despite these functional changes, there were no MRI detectable cerebellar lesions, cerebellar hypoplasia or white matter injury (Tran, Huening et al. 2017). Purkinje cell activity plays an important role in conditioned response execution (Berthier and Moore 1986; Katz and Steinmetz 1997; Green and Steinmetz 2005). The changes in the morphology and density of Purkinje cells in endotoxin kits might be responsible for the functional impairment of cerebellum. However, it is possible that extra-cerebellar pathology may also contribute to this learning deficit.
5. Limitations of the study
In this study we have demonstrated gross structural changes in the Purkinje cell layer. However, detailed analysis of single cell dendritic branching patterns and functional assessment by evaluating the electrophysiological properties of the Purkinje cells will help in better defining the abnormalities and injury to these cells. Future studies will focus on these aspects to better define the alterations in the Purkinje cell layer following a perinatal insult. Other limitations are that, the endotoxin animals that survive up to 1 month of age inherently have a milder injury, which may under-represent the extent of injury to the cerebellum in this model. Moreover, sex differences are not adequately explored here due to the smaller sample sizes used. These will be addressed in future studies.
6. Conclusions
This study indicates that maternal inflammation leads to intense cerebellar microglial activation in neonatal rabbit kits and abnormal development of Purkinje cells along with impairment in cerebellar learning that is detectable at 4-6 weeks of age. Thus, therapeutic interventions targeting neuroinflammation may attenuate cerebellar injury and improve cerebellar learning.
Highlights.
Maternal inflammation induces microglial activation in cerebellum of neonatal rabbits
Maternal endotoxin-exposure induced cerebellar inflammation in the neonates
Intrauterine endotoxin exposure results in Purkinje cell abnormality in young adult rabbits
Intrauterine inflammation impairs cerebellar function in rabbits at 1 month of age
Acknowledgment
This research was supported by National Institute of Health under NICHD R01HD068562 and NINDS R01NS093416 (S. Kannan).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allin M, Matsumoto H, et al. (2001). "Cognitive and motor function and the size of the cerebellum in adolescents born very pre-term." Brain 124(Pt 1): 60–66. [DOI] [PubMed] [Google Scholar]
- Argyropoulou MI, Xydis V, et al. (2003). "MRI measurements of the pons and cerebellum in children born preterm; associations with the severity of periventricular leukomalacia and perinatal risk factors." Neuroradiology 45(10): 730–734. [DOI] [PubMed] [Google Scholar]
- Attwell PJ, Ivarsson M, et al. (2002). "Cerebellar mechanisms in eyeblink conditioning." Ann N Y Acad Sci 978: 79–92. [DOI] [PubMed] [Google Scholar]
- Balakrishnan B, Dai H, et al. (2013). "Maternal endotoxin exposure results in abnormal neuronal architecture in the newborn rabbit." Dev Neurosci 35(5): 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear JJ and Wu YW (2016). "Maternal Infections During Pregnancy and Cerebral Palsy in the Child." Pediatr Neurol 57: 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthier NE and Moore JW (1986). "Cerebellar Purkinje cell activity related to the classically conditioned nictitating membrane response." Exp Brain Res 63(2): 341–350. [DOI] [PubMed] [Google Scholar]
- Biran V, Heine VM, et al. (2011). "Cerebellar abnormalities following hypoxia alone compared to hypoxic-ischemic forebrain injury in the developing rat brain." Neurobiol Dis 41(1): 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biran V, Verney C, et al. (2012). "Perinatal cerebellar injury in human and animal models." Neurol Res Int 2012: 858929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracha V, Zbarska S, et al. (2009). "The cerebellum and eye-blink conditioning: learning versus network performance hypotheses." Neuroscience 162(3): 787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KL and Woodruff-Pak DS (2012). "Eyeblink conditioning in the developing rabbit." Dev Psychobiol 54(4): 423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanille V, Saraceno GE, et al. (2015). "Long lasting cerebellar alterations after perinatal asphyxia in rats." Brain Res Bull 116: 57–66. [DOI] [PubMed] [Google Scholar]
- Christian KM and Thompson RF (2003). "Neural substrates of eyeblink conditioning: acquisition and retention." Learn Mem 10(6): 427–455. [DOI] [PubMed] [Google Scholar]
- Dean JM, Farrag D, et al. (2009). "Cerebellar white matter injury following systemic endotoxemia in preterm fetal sheep." Neuroscience 160(3): 606–615. [DOI] [PubMed] [Google Scholar]
- Gelman A and Hill J (2007). Data analysis using regression and multilevel/hierarchical models. Cambridge ; New York, Cambridge University Press. [Google Scholar]
- Green JT and Steinmetz JE (2005). "Purkinje cell activity in the cerebellar anterior lobe after rabbit eyeblink conditioning." Learn Mem 12(3): 260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong HJ, Shim SY, et al. (2016). "Cerebellar Development in Preterm Infants at Term-Equivalent Age Is Impaired after Low-Grade Intraventricular Hemorrhage." J Pediatr 175: 86–92 e82. [DOI] [PubMed] [Google Scholar]
- Kannan S, Dai H, et al. (2012). "Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model." Sci Transl Med 4(130): 130ra146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan S, Saadani-Makki F, et al. (2011). "Decreased cortical serotonin in neonatal rabbits exposed to endotoxin in utero." J Cereb Blood Flow Metab 31(2): 738–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan S, Saadani-Makki F, et al. (2007). "Microglial activation in perinatal rabbit brain induced by intrauterine inflammation: detection with 11C-(R)-PK11195 and small-animal PET." J Nucl Med 48(6): 946–954. [DOI] [PubMed] [Google Scholar]
- Katsetos CD, Spandou E, et al. (2001). "Acute hypoxia-induced alterations of calbindin-D28k immunoreactivity in cerebellar Purkinje cells of the guinea pig fetus at term." J Neuropathol Exp Neurol 60(5): 470–482. [DOI] [PubMed] [Google Scholar]
- Katz DB and Steinmetz JE (1997). "Single-unit evidence for eye-blink conditioning in cerebellar cortex is altered, but not eliminated, by interpositus nucleus lesions." Learn Mem 4(1): 88–104. [DOI] [PubMed] [Google Scholar]
- Kidokoro H, Neil JJ, et al. (2013). "New MR imaging assessment tool to define brain abnormalities in very preterm infants at term." AJNR Am J Neuroradiol 34(11): 2208–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JL, Lemmon ME, et al. (2016). "Clinical and neuroimaging features as diagnostic guides in neonatal neurology diseases with cerebellar involvement." Cerebellum Ataxias 3: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulak P, Maciorkowska E, et al. (2016). "Volumetric Magnetic Resonance Imaging Study of Brain and Cerebellum in Children with Cerebral Palsy." Biomed Res Int 2016: 5961928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laure-Kamionowska M and Maslinska D (2011). "Cerebellar cortical neurons misplaced in the white matter due to disturbed migration during development of human brain." Folia Neuropathol 49(4): 282–294. [PubMed] [Google Scholar]
- Limperopoulos C, Bassan H, et al. (2007). "Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors?” Pediatrics 120(3): 584–593. [DOI] [PubMed] [Google Scholar]
- Louis ED, Babij R, et al. (2013). "Quantification of cerebellar hemispheric purkinje cell linear density: 32 ET cases versus 16 controls." Mov Disord 28(13): 1854–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenner MJ, Blumberg SJ, et al. (2016). "Prevalence of cerebral palsy and intellectual disability among children identified in two U.S. National Surveys, 2011-2013." Ann Epidemiol 26(3): 222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Teva JL, Dusart I, et al. (2004). "Microglia promote the death of developing Purkinje cells." Neuron 41(4): 535–547. [DOI] [PubMed] [Google Scholar]
- McDougall ARA, Hale N, et al. (2017). "Erythropoietin Protects Against Lipopolysaccharide-Induced Microgliosis and Abnormal Granule Cell Development in the Ovine Fetal Cerebellum." Front Cell Neurosci 11: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T, Morimoto M, et al. (2015). "Low-grade intraventricular hemorrhage disrupts cerebellar white matter in preterm infants: evidence from diffusion tensor imaging." Neuroradiology 57(5): 507–514. [DOI] [PubMed] [Google Scholar]
- Nguyen V, Sabeur K, et al. (2018). "Sonic Hedgehog Agonist Protects Against Complex Neonatal Cerebellar Injury." Cerebellum 17(2): 213–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea TM, Allred EN, et al. (2009). "The ELGAN study of the brain and related disorders in extremely low gestational age newborns." Early Hum Dev 85(11): 719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Nores WL, et al. (2006). "Learning-induced plasticity in deep cerebellar nucleus." J Neurosci 26(49): 12656–12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng JH, Feng Y, et al. (2005). "Apoptosis and necrosis in developing cerebellum and brainstem induced after focal cerebral hypoxic-ischemic injury." Brain Res Dev Brain Res 156(1): 87–92. [DOI] [PubMed] [Google Scholar]
- Perez-Pouchoulen M, VanRyzin JW, et al. (2015). "Morphological and Phagocytic Profile of Microglia in the Developing Rat Cerebellum." eNeuro 2(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadani-Makki F, Kannan S, et al. (2008). "Intrauterine administration of endotoxin leads to motor deficits in a rabbit model: a link between prenatal infection and cerebral palsy." Am J Obstet Gynecol 199(6): 651 e651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AF, Kannan PS, et al. (2016). "Intra-amniotic LPS causes acute neuroinflammation in preterm rhesus macaques." J Neuroinflammation 13(1): 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah DK, Anderson PJ, et al. (2006). "Reduction in cerebellar volumes in preterm infants: relationship to white matter injury and neurodevelopment at two years of age." Pediatr Res 60(1): 97–102. [DOI] [PubMed] [Google Scholar]
- Srinivasan L, Allsop J, et al. (2006). "Smaller cerebellar volumes in very preterm infants at term-equivalent age are associated with the presence of supratentorial lesions." AJNR Am J Neuroradiol 27(3): 573–579. [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ (2012). "The cerebellum and cognition: evidence from functional imaging studies." Cerebellum 11(2): 352–365. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ and Schmahmann JD (2010). "Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing." Cortex 46(7): 831–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strackx E, Sparnaaij MA, et al. (2015). "Lipopolysaccharide-induced chorioamnionitis causes acute inflammatory changes in the ovine central nervous system." CNS Neurol Disord Drug Targets 14(1): 77–84. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Sugihara G, et al. (2013). "Microglial activation in young adults with autism spectrum disorder." JAMA Psychiatry 70(1): 49–58. [DOI] [PubMed] [Google Scholar]
- Sveinsdottir K, Lansberg JK, et al. (2017). "Impaired Cerebellar Maturation, Growth Restriction, and Circulating Insulin-Like Growth Factor 1 in Preterm Rabbit Pups." Dev Neurosci 39(6): 487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam EW, Ferriero DM, et al. (2009). "Cerebellar development in the preterm neonate: effect of supratentorial brain injury." Pediatr Res 66(1): 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam EW, Rosenbluth G, et al. (2011). "Cerebellar hemorrhage on magnetic resonance imaging in preterm newborns associated with abnormal neurologic outcome." J Pediatr 158(2): 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AR, Lacadie C, et al. (2017). "Fine Motor Skill Mediates Visual Memory Ability with Microstructural Neuro-correlates in Cerebellar Peduncles in Prematurely Born Adolescents." Cereb Cortex 27(1): 322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RF (1986). "The neurobiology of learning and memory." Science 233(4767): 941–947. [DOI] [PubMed] [Google Scholar]
- Tran L, Huening BM, et al. (2017). "Cerebellar-dependent associative learning is impaired in very preterm born children and young adults." Sci Rep 7(1): 18028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traudt CM, McPherson RJ, et al. (2014). "Systemic glycerol decreases neonatal rabbit brain and cerebellar growth independent of intraventricular hemorrhage." Pediatr Res 75(3): 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis KE, Leitner Y, et al. (2015). "Cerebellar white matter pathways are associated with reading skills in children and adolescents." Hum Brain Mapp 36(4): 1536–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe JJ (2009). "Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important." J Child Neurol 24(9): 1085–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Zhang Z, et al. (2017). "Maternal Inflammation Results in Altered Tryptophan Metabolism in Rabbit Placenta and Fetal Brain." Dev Neurosci 39(5): 399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Bassam B, et al. (2016). "Maternal inflammation leads to impaired glutamate homeostasis and up-regulation of glutamate carboxypeptidase II in activated microglia in the fetal/newborn rabbit brain." Neurobiol Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Jyoti A, et al. (2018). "Trajectory of inflammatory and microglial activation markers in the postnatal rabbit brain following intrauterine endotoxin exposure." Neurobiol Dis 111:153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]