Abstract
Organ shortage represents one of the major limitations to the development of kidney transplantation. To increase the donor pool and to answer the ever increasing kidney request, physicians are recurring to marginal kidneys as kidneys from older donors, from hypertensive or diabetic donors and from non-heart beating donors. These kidneys are known to have frequently a worse outcome in the recipients. To date major problem is to evaluate such kidneys in order to use or to discard them before transplantation. The use of such kidneys create other relevant question as whether to use them as single or dual transplant and to allocate them fairly according transplant programs. The pre-transplant histological evaluation, the clinical evaluation of the donor or both the criteria joined has been used and according the time each criterion prevailed over the others. Aim of this review has been to examine the advantages and the drawbacks of any criterion and how they have changed with time. To date any criterion has several limitations and several authors have argued for the development of new guidelines in the field of the kidney evaluation for transplantation. Several authors argue that the use of omic technologies should improve the organ evaluation and studies are ongoing to evaluate these technologies either in the donor urine or in the biopsies taken before transplantation.
Keywords: Kidney evaluation, Pre-transplant biopsies, Kidney donor evaluation, Kidney risk profile index, Omic technologies, Deceased donor score, Donor risk score
Core tip: With the extension of donor pool to high risk donors, the kidney pre-transplant evaluation became mandatory. Different criteria have been used, each of them with advantages and limitations. Probably the use of pre-transplant kidney biopsies in those kidneys coming from donors with the highest profile index seem to give the better results. These could be improved applying omic technologies either to donor urine or to pre-transplant biopsies. However the application of omic technologies is time consuming and not everywhere applicable. Several studies on these technologies are to date ongoing, but their results are yet not known.
INTRODUCTION
To date, organ shortage represents one of the major limitations to the development of kidney transplantation.
To increase the donor pool many transplant programs accept kidneys from the so-called extended criteria donors (ECDs)[1,2]. Kidneys from the ECD pool are known to have worse outcomes in recipients with a higher rate of delayed graft function (DGF), primary non function (PNF), and reduced function of the allograft and reduced graft survival[3]. The main challenge is to evaluate such kidneys before transplantation either for a better and fair allocation or for discarding the kidney in the case of a very poor evaluation of the offered kidney.
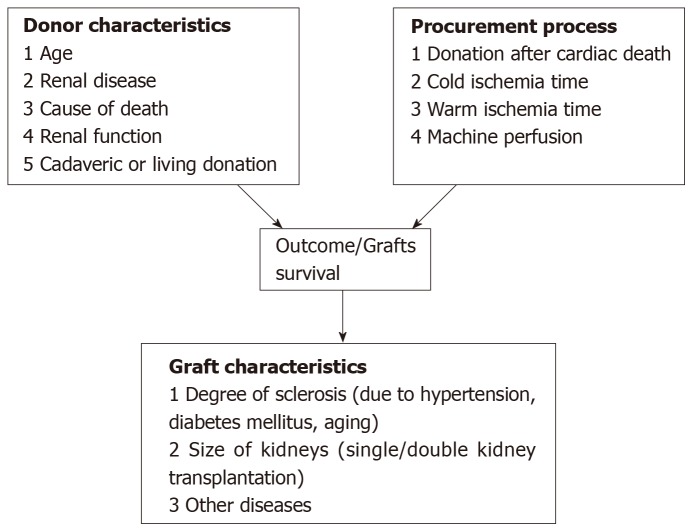
Several factors related to the donors are known to influence the post-transplant outcomes. Figure 1 identifies which donor, procurement and graft characteristics principally influence the outcomes. They may be divided into clinical and histological factors and factors related to the donor and related to the offered kidney and to the procurement management.
Figure 1.
Main donor, procurement and graft related factors influencing the post-transplant outcomes.
Historically, the evaluation of the kidneys from ECDs has been made histologically by the so-called zero-time biopsy[4], by clinical evaluation of the donor by different kidney allocation scores or by a combination of histological and clinical parameters.
Additionally, it should be highlighted that the need of a clear evaluation of the “so called” marginal donors became a must with the increased use of such kidneys. With time the experience documented that several kidneys from ECD pool performed well, while other kidneys labeled as standard criteria donors (SCD) did not perform well. Hence, the opportunity of a safe evaluation also for SCD. De facto the recent kidney donor risk index (KDRI) automatically offers the evaluation for any kidney.
The aim of this review is to describe the aforementioned evaluation criteria of ECD kidneys and to describe how they have changed with time.
SELECTION CRITERIA OF THE ARTICLES INCLUDED IN THIS REVIEW AND THEIR DRAWBACKS
The criteria to evaluate the kidneys have been histological, clinical and mixed histological-clinical. We have searched for all the papers concerning these points. The main studies concerning the most important scoring systems are shown on Table 1. With the exception of the two single centre studies as Maryland Aggregate Pathology Index (MAPI) and the Irish nomogram, all the studies considered included a large number of patients with the limitation to be retrospective in the attempt to validate the original findings. Clearly, in this review are also included articles documenting the drawbacks of the different scoring systems and these articles may include a limited number of patients. Similarly, the studies evaluating the omics on the renal biopsies or on the donor urine have a limited number of patients.
Table 1.
Descriptive table of selected clinical scoring system
| Score | Authors | Variables included in risk score | Score grades | Outcome |
| Expanded criteria donor | Port et al[58], 2002 | Donor age | SCD | Relative risk of graft failure compared to SCD |
| Cerebrovascular accident as cause of death | ECD | RR>1.7 | ||
| Serum creatinine> 1.5mg/dL | ||||
| History of hypertension | ||||
| Deceased donor score | Nyberg et al[65], 2003 | Age | 5-year graft survival | |
| History of hypertension | A (0-9 points) | Grade A 82% | ||
| Creatinine clearance | B (10-19 points) | Grade B 79% | ||
| HLA mismatch | C (20-29 points) | Grade C 72% | ||
| Cause of death | D (30-39 points) | Grade D 65% | ||
| Donor risk score (DRS) | Schold et al[67], 2005 | Donor risk factors | 5-year graft survival | |
| Race | I | Grade I 76.7% | ||
| Age | II | Grade II 73.6% | ||
| History of hypertension | III | Grade III 66.3% | ||
| History of diabetes | IV | Grade IV54.8% | ||
| Cause of death | V | Grade V 47.6% | ||
| History of hypertension | ||||
| History of diabetes | ||||
| Cause of death | ||||
| HLA-Dr mismatch | ||||
| CMV mismatch | ||||
| Cold ischemia time | ||||
| DGF nomogram | Irish et al[70], 2003 | Donor risk factors | Continuous point score | Delayed graft function |
| Age | ||||
| Serum creatinine | ||||
| History of hypertension | ||||
| Cause of death | ||||
| Donor after cardiac death | ||||
| Recipient risk factors | ||||
| Peak PRA | ||||
| Race | ||||
| Gender | ||||
| History of diabetes mellitus | ||||
| Previous transplant | ||||
| Pretransplant dialysis | ||||
| Pretransplant transfusions | ||||
| Combined transplantation | ||||
| HLA mismatch | ||||
| Cold ischemia time | ||||
| KDRI | Rao et al[71], 2009 | Donor risk factors | KDRI quintile | 5-year graft survival |
| Age | 0.45-0.79 | 82% | ||
| Race | 0.80-0.96 | 79% | ||
| Height | 0.97-1.15 | NA | ||
| Weight | 1.16-1.45 | NA | ||
| History of hypertension | >1.45 | 63% | ||
| History of diabetes | ||||
| Cause of death | ||||
| Serum creatinine | ||||
| Hepatitis C | ||||
| Donation after cardiac death | ||||
| HLA-B mismatch | ||||
| HLA-DR mismatch | ||||
| Cold ischemia time | ||||
| Double or en bloc transplant | ||||
| Donor-only KDRI | OPTN[72], 2014 | Donor risk factors | 5-year graft survival | |
| Age | <0.6 | 80% | ||
| Race | 0.61-0.79 | 78% | ||
| Height | 0.80-0.99 | 74% | ||
| Weight | 1.00-1.19 | 66% | ||
| History of hypertension | 1.20-1.59 | 59% | ||
| History of diabetes | 1.60-1.99 | 52% | ||
| Cause of death | >1.99 | 44% | ||
| Serum creatinine | ||||
| Hepatitis C | ||||
| Donation after cardiac death |
ECD: Expanded criteria donor; KDRI: Kidney donor risk index; OPTN: The Organ Procurement and Transplantation Network; SCD: Standard criteria donor.
HISTOLOGICAL EVALUATION OF DONOR KIDNEYS
By 1999, Karpinski et al[5] considering that kidneys from high risk donors had worse outcomes in the recipient after transplantation tried to establish which donor or kidney variables were most relevant to these poor outcomes. For high donor risk, they considered donation after cardiac death donors, donors over 55 years of age, donors with a history of hypertension or diabetes, and donors with abnormal kidney anatomy or abnormal renal function[6]. The study found that a low calculated creatinine clearance (CrCl) and donor kidney pathology were the main predictors of worse outcomes
In particular, the donor renal pathology was scored 0-3 in each of four distinct aspects: Glomerulosclerosis, interstitial fibrosis, tubular atrophy and vascular disease (Table 2). Previous studies have documented the relevance of pre-implantation histological findings on recipient outcomes[7-9]. None of these studies had been concordant, and the study of Karpinski et al[5]may be considered a pioneering study documenting the relevance of the pathology score over the transplant outcomes.
Table 2.
Histological score according Karpinski
| Histological score | |
| Glomerular score | 0 = no globally sclerosed glomeruli |
| 1 = < 20% global glomerulosclerosis | |
| 2 = 20-50% global glomerulosclerosis | |
| 3 = > 50% global glomerulosclerosis | |
| Tubular score | 0 = absent |
| 1 = < 20% of tubules affected | |
| 2 = 20-50% of tubules affected | |
| 3 = > 50% of tubules affected | |
| Interstitial score | 0 = absent |
| 1 = < 20% of cortical parenchyma replaced by fibrous connective tissue | |
| 2 = 20-50% of cortical parenchyma replaced by fibrous connective tissue | |
| 3 = > 50% of cortical parenchyma replaced by fibrous connective tissue | |
| Vascular score | 0 = absent |
| 1 = increased wall thickness but to a degree that is less than the diameter of the lumen | |
| 2 = wall thickness that is equal or slightly greater than the diameter of the lumen | |
| 3 = wall thickness that far exceeds the diameter of the lumen, with extreme narrowing |
Since the study of Karpinski et al[5], several studies have documented the relevance of the pathology score of donor kidneys over the outcomes, while other studies did not find a similar usefulness of the pathology score.
One of the most important studies in favor of the pathology score has been the study of Remuzzi et al[10]. According to this study, the pathology score allows transplant kidneys with a score up to 3 to be used as single kidneys, while kidneys with a score from 4 to 6 are better allocated as dual transplants and kidneys with a score of 7 or higher should be discarded.
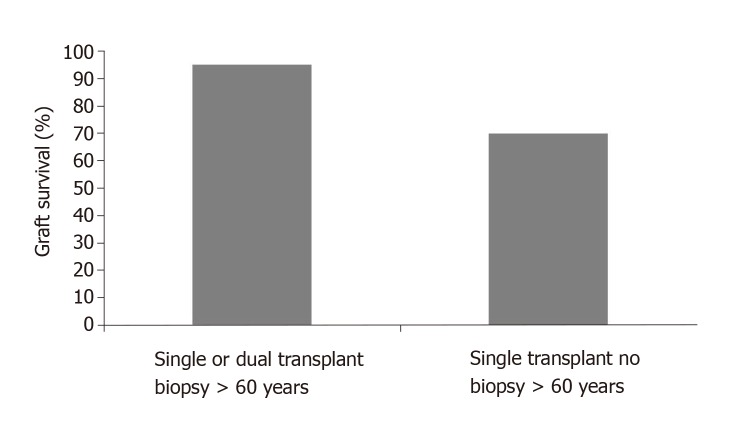
Additionally, the study documents the importance of the pre-transplant renal biopsy for donors over 60 years when comparing the renal outcomes with and without biopsy (Figure 2).
Figure 2.
36 month graft survival for donors over 60 years according pre-transplant biopsy.
In a different study, Mancilla et al[11] suggested the utility of zero-time biopsy in the case of living donor kidneys, particularly for donors with borderline renal function or with a history of familial renal disorders[12,13]. In a study from Kayler et al[14] a correlation of histological findings on pre-implantation biopsy with kidney graft survival was also found but was restricted to vascular lesions, while glomerulo-sclerosis and low-grade interstitial fibrosis did not have statistical significance.
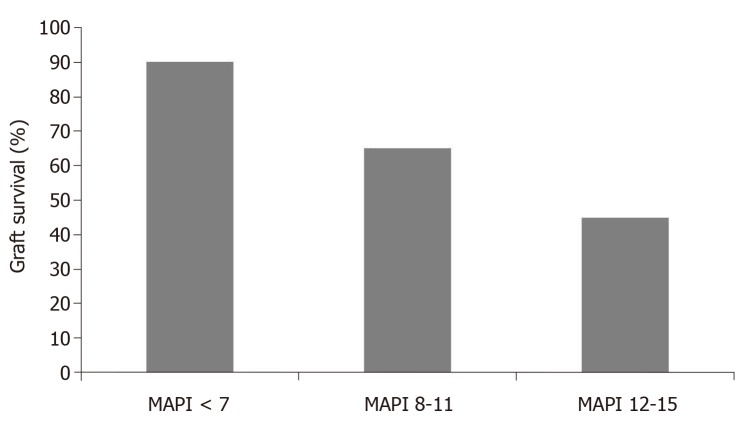
Based on 371 pre-transplant biopsies and correlating the findings with post-transplant outcomes, Munivenkatappa et al[15] developed the MAPI. In the study, glomerulosclerosis, glomerular size and periglomerular fibrosis in addition to vascular pathology and arteriolar hyalinosis were considered in developing the MAPI score (Table 3). The authors found that the five-year actuarial graft survival rate was related to the MAPI scoring (Figure 3) and that the MAPI score at the multivariate analysis correlated with the risk of graft failure better than any other clinical parameter (Table 4). This study suddenly received several comments, which brought up several unanswered questions about the relevance of pre-transplant biopsies in predicting post-transplant outcomes. Many of these questions were raised by Nickeleit[16].
Table 3.
Maryland Aggregate Pathology Index scoring system for pre-transplant kidney biopsies
| HR (95%CI) | P value |
MAPI points |
||
| Absent | Present | |||
| Arteriolar hyalinosis | 3.93 (2.02-7.64) | <0.0001 | 0 | 4 |
| PGF (any) | 4.09 (1.65-10.14) | 0.002 | 0 | 3 |
| Scar (any) | 2.58 (1.24-5.38) | 0.01 | 0 | 3 |
| GS > 15% | 1.87 (1.17-2.99) | 0.009 | 0 | 2 |
| WLR interlobular arteries > 0.5 | 2.05 (1.21-3.47) | 0.008 | 0 | 2 |
MAPI: Maryland Aggregate Pathology Index; WLR: Wall to lumen ratio; CI: Confidence interval.
Figure 3.
Five years graft survival for the study population according low, intermediate and high Maryland Aggregate Pathology Index score ranges. MAPI: Maryland Aggregate Pathology Index.
Table 4.
Cox Multivariate analysis showing association of Maryland Aggregate Pathology Index score and clinical parameters to risk of graft failure
| HR (95%CI) | P value | |
| MAPI | 1.21 (1.05-1.40) | 0.008 |
| Donor age | 1.03 (1.00-1.07) | 0.096 |
| Cold ischemia (h) | 3.66 (0.77-17.40) | 0.102 |
| Donor history of hypertension | 1.62 (0.67-3.97) | 0.287 |
| Donor terminal creatinine> 1.5 mg/dL | 1.34 (0.43-4.18) | 0.611 |
| CVA as cause of donor death | 0.98 (0.35-2.73) | 0.973 |
CVA: Cerebrovascular accident; MAPI: Maryland Aggregate Pathology Index.
One point that is not clarified is whether wedge specimens or needle biopsies should be used. This issue is well described in a further paper[17] that considers wedge biopsies to be safer and superior to core biopsies in finding significant findings.
Another point is whether frozen or paraffinized sections should be used, even if the original MAPI score found paraffinized sections to be more reliable.
Additionally, it should be better defined when zero-time biopsies should be taken: before or after reperfusion. Biopsy time is relevant in detecting the complement activation that is predictive of early antibody mediated rejection[18].
An important point, not well considered by the MAPI score is how the lesions should be scored and whether the Banff criterion is appropriate[19]. This point is relevant for comparing zero-time biopsies with subsequent post-transplant biopsies. Nickeleit[16]’s conclusions were that much remains to be determined about zero-time biopsies and that consensus guidelines remain to be defined.
Recommendations on these points have been given by two German workshops and described by Pisarski et al[20] in 2016. The German recommendations advocate a detailed assessment of the findings and do not agree with the recommendations of the Interpretation Biopsy Banff Working Group[21], whose approach is adopted for a general pathologist, without specific training in the field.
The issue of an expert pathologist was addressed in 2012 in a study of the pre-implantation biopsies in the Organ Procurement Organization (OPOS) that found a lack of concordance among OPOS pathologists[22]. The lack of a correlation between the findings of on-call pathologists and the lack of association between their findings and the transplant outcomes is highlighted by two papers[23,24] that advocate for specific training in renal pathology to optimize the histological evaluation of donor kidneys. It could also be argued that a renal pathologist “per se” could not be expert enough in evaluating such biopsies. Probably a specific training should be the best solution.
By 2011, Mueller et al[25], reviewing several studies on histopathology-based variables at zero-time biopsies, highlighted the limitations due to sampling errors, confounding clinical variables, and inter-observer variability[26,27] and advocated for a validated approach for the analysis of pathology findings. In particular, they advocate for the use of omic technologies such as proteomics, transcriptomics and metabolomics that could have the potential to improve the significance of the histological findings. Table 5 highlights the principal studies that were conducted until 2011[28,39].
Table 5.
Studies on molecular markers measured in 0-h biopsies (up to 2011)
| Ref. | Pats | f/u | Findings/timing of biopsy-technology |
| Hoffmann et al[28], 2002 | 24 | 1 h | IRI injury ass w increased adhesion, chemotaxis, apoptosis, monocyte recruitment/activation transcripts.Post-reperfusion/RT-PCR |
| Hauser et al[29],2004 | 36 | 1 | Increased Communication, apoptosis, inflammation |
| Kainzet al[30],2004 | 10 | 1 | DD kidneys distinctly different transcripts in the TI but not in the G compartment compared to LD. End of CIT/microarrays |
| Avihingsanon et al[31],2005 | 75 | 6 | 15 selected genes associated with outcomes, included DGF, REJ and 6 mofunction. Post-reperfusion/RT-PCR |
| Kainzet al[32],2007 | 31 | 12 | Increased immunity, signal transduction, oxidative stress response associated with lower 1-year function |
| Park et al[33],2007 | 15 | 12 | Increased inflammation and immune response at 1-year in uncomplicated grafts |
| Mas et al[34],2008 | 33 | 3 | Increased immunity, inflammation and apoptosis genes associated with DGF. End of CIT/microarrays |
| Mueller et al[35],2008 | 87 | 12 | Increased acute phase, complement, chemochines and reduced metabolism, transporters in DD versus LD, transcriptome identifies risk for DGF better than clinical ± histological markers. Post-reperfusion/ microarrays |
| Perco et al[36], 2009 | 82 | 12 | Increasedimmunity/defense, communication, apoptosis in damaged kidneys, CADI score + clinic explained 14%, 3 biomarkers 28% of 1-year creatinine variability. End of CIT/ microarrays |
| Naesens et al[37], 2009 | 28 | 36 | Complement genes differ between LD and DD and are associated with early and late function. End of CIT and post-transplant/ microarrays |
| Bodonyi-Kovacs et al[38], 2010 | 75 | 48 | Pre-selected genes associated with 2-year graft function. Post-reperfusion/ RT-PCR |
| Cravedi et al[39], 2010 | 49 | 12 | LDvs DD differ by inflammation, donor age and ITGB2 prognostic for 1-year function. Post-reperfusion/RT-PCR |
f/u:Follow up in months; IRI:Ischemia-reperfusion injury; DD:Deceased donor; LD:Living donor; IGF:Immediate graft function; DGF:Delayed graft function; REJ:Rejection; CIT: Cold ischemia time; TI:Tubulointerstitial; G:Glomerular.
A study from Krol et al[40], documented that the apoptosis of tubular epithelial cells in pre-implantation biopsies is related to DGF. Their findings were confirmed by another study [41]that found a relationship between high BAX/BCL2 expression in pre-implantation biopsies and DGF, confirming that apoptosis-related gene expression levels are predictors of DGF.
A recent study[42] confirmed that zero-time biopsies in ECDs showed a significant increase in the transcripts of MCP-1, RANTES, TGF beta and IL 10, documenting a higher gene expression of inflammatory cytokines in ECDs that could predict the post-transplant outcome.
In recent years, several studies, often retrospective, and several reviews and meta-analyses did not confirm the utility of zero-time biopsy in allocating or discarding ECD kidneys. Wang et al[43] reviewed 47 studies published between 1994 and 2014, where each study included pre-transplant biopsies format least 50 donors and compared the histological findings with post-transplant outcomes. Overall, 15 scoring systems were proposed by the studies, but none were able to correlate with post-transplant outcomes.
Naesens[44] reviewed the problems and the utility of zero-time biopsy and highlighted that the major problems were the wedge vs core needle biopsy[45,46]; frozen vs paraffin-embedded tissue[47,48]; pathologist’s experience[23,24]; different composite histological scoring such as the Pirani score[49], Chronic Allograft Damage Index (CADI)[50], and Donor Score[23]; and the lack of utilizing hard clinical end-points in evaluating graft and recipient outcomes. The author concluded that zero-time biopsies are not useful for assigning or discarding kidneys or improving dual kidney transplantation programs. The author recognizes that the molecular phenotype in pre-transplant biopsies could be useful in donor selection and in peri-transplant management even if the time required could make such a procedure difficult[51-54].
Two recent Italian studies on the utility of pre-implantation biopsy in allocating ECD kidneys[4,55] concluded that histological evaluation was not superior to donor clinical evaluation in allocating ECD kidneys either as a single kidney or as a dual kidney transplant. The authors concluded that, according to their experience, the histological score poorly evaluates the donor kidney quality. Accordingly, the use of histological criteria to assign as single or dual kidneys does not seem to offer advantages over the evaluation made on clinical basis.
A Banff Pre-implantation Biopsy Working Group has been established to develop guidelines for the interpretation of pre-implantation renal biopsies[56]. The last working group meeting stated that to date, histological parameters are poorly correlated with post-transplant outcomes and that remain significant limitations in understanding the role of pre-implantation biopsies.
Recently, Carpenter et al[57] from Columbia University examined their experience and compared procurement biopsies with reperfusion paraffin-embedded biopsies and with post-transplant biopsies. All the findings were then correlated with allograft failures and patient deaths. No agreement has been found between frozen procurement biopsies and paraffin-embedded biopsies, and frozen procurement biopsies were poorly correlated with post-transplant biopsies and the hard end-point considered.
COMBINED CLINICAL AND HISTOLOGICAL EVALUATION OF DONOR KIDNEYS
A different approach to evaluating ECD kidneys has been to combine histological findings with clinical donor-related parameters. The latter have been identified since the publication of the study by Port et al[58]. In a study in 2001, Verran et al[59] found that the combination of abnormal biopsy findings with donor age and donor cardiovascular disease and hypertension was associated with poor outcomes.
In an Italian study[60], donor kidneys were assigned with good results according to donor renal function [estimated glomerular filtration rate (eGFR) under or over 50 mL/min] and the previously mentioned Karpinski score.
The largest study that evaluated the predictive value of clinical and histological findings taken together was conducted by Anglicheauet al[61]. The authors, evaluating 313 kidney transplants from donors aged >50 years, developed the so-called Anglicheau score. The best predictive parameters were a history of hypertension in the donor, serum creatinine levels under or over 1.5 mg/dL and glomerulosclerosis less than or over 10%. These parameters in the multivariate analysis significantly correlated with renal function at 1 year post-transplantation.
A different study[62] recognizes the utility of zero-time biopsy, but, as none of the histological variables and scores provided a good prediction of post-transplant outcomes, the histological findings need to be integrated with all the known donor-related clinical parameters.
Finally, a very recent Spanish study[63] highlights the utility of evaluating the pre-transplant donor biopsies in the donor with the highest kidney donor profile index (KDPI) that is based on several deceased donor variables.
CLINICAL EVALUATION OF DONOR KIDNEYS
In an attempt to improve the evaluation of the donor kidneys, principally in the US, where the donor kidney evaluation is strictly connected with their discard or their allocation to different recipients according to national programs, several clinical donor quality scoring systems have been performed.
The first one was the characterization and a better definition of ECDs. According to the report of the Kidney Working Group[1], kidneys belonging to the ECD were kidneys with a relative risk of graft failure of 1.7 with respect to standard kidneys. These kidneys are characterized by a donor age older than 59 years with two of the following characteristics: cerebrovascular accident as cause of death, history of hypertension or creatinine over 1.5 mg/dL[2].
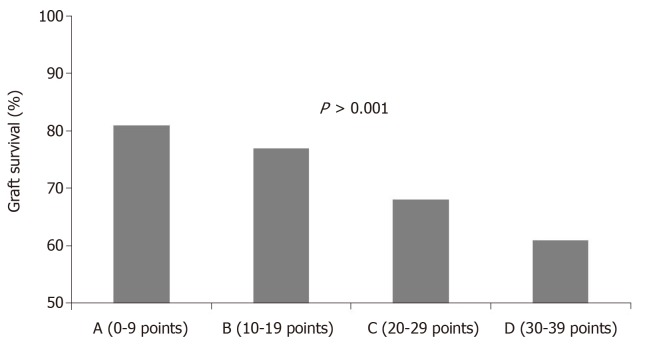
Nyberg et al[64] evaluated 241 consecutive cadaveric renal transplants and gave a score based on recognized clinical factors responsible for DGF. These factors were age, cause of death, history of hypertension, diabetes mellitus, creatinine clearance and presence in the donor of renal artery stenosis. A scoring system was developed from these seven donor variables, allowing stratification of cadaver kidneys into four classes (grades A, B, C, D). Univariate and multivariate analyses were performed, and a significant decline in early renal function was observed with an increase in the score. Additionally, the multivariate analysis had a better prognostic value with respect to each single variable considered in the univariate analysis.
Later, Nyberg et al[65], in an attempt to validate his scoring system, applied the analysis to a wider population, including 34324 transplant patients from the UNOS registry in the period between 1994 and 1999. This study allowed us to evaluate the feasibility of the score on a larger follow-up. The study allowed the recognition of five clinical variables as predictive of a poorer outcome [age, cause of death, history of hypertension, creatinine clearance and human leukocyte antigen (HLA) mismatch]. This score was called the Deceased Donor Score or Nyberg score and was able to predict renal function at 12 mo and graft survival at 6 years (Figure 4).
Figure 4.
Grade of deceased donor kidney score significantly influenced graft survival at 6 years after transplantation.
A further study by the same author[66] also confirmed these data for kidneys receiving machine reperfusion.
To further improve clinical factors able to evaluate kidney status and to predict outcomes after transplantation, Schold et al[67] studied different clinical variables that were applied to transplants included in the National Scientific Transplant Registry from 1996 to 2002.
The variables were age, race, and history of hypertension, diabetes mellitus, and cause of death, cold ischemia time, HLA mismatch, and immunological status and CMV status. This was called the Donor Risk Score and allowed for the calculation of the multivariate estimates for graft loss by donor grade (Figure 5).
Figure 5.
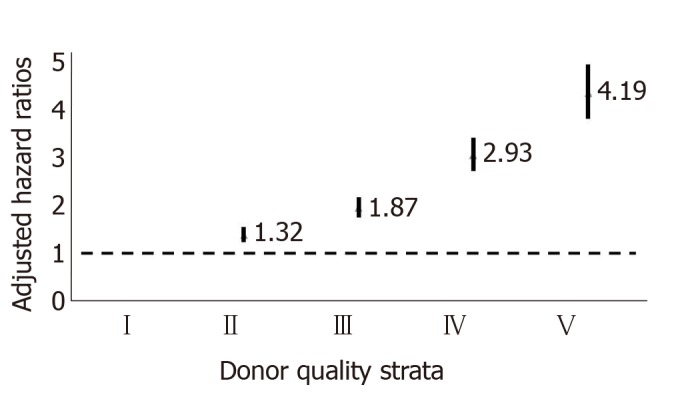
Multivariate estimates for graft loss by donor grade (Hazard ratio expressed as mean +/- confidence interval.
A further study[68] compared the different clinical risk scores and documented that the Donor Risk Score was better associated with subsequent allograft function.
ECD-KDRI-KDPI
As already mentioned, by 2002, in an attempt to improve the utilization of marginal deceased donor kidneys, the concept of ECD vs SCD was introduced[1,2]. With time this dichotomy (SCD/ECD) demonstrated several drawbacks. Indeed, the experience documented that several kidneys labeled as ECD performed well, while other kidneys labeled as SCD did not perform well[69]. To improve these limitations other different scoring systems have been attempted. The donor score of Nyberg and the donor risk score of Schold have been described. Additionally, Irish et al[70] applied a nomogram aimed at predicting the risk of DGF based on 16 donor and recipient risk factors. Moore et al[68] documented that Schold’s donor risk score is the scoring system that best predicts graft outcomes, but the need still remains for a simple and validated system that applies to the entire donor population viewed as a continuum and not in a dichotomous fashion.
In 2009, Rao et al[71]analyzed 69440 deceased donor adult transplants registered in the Scientific Registry of Transplant Recipients (SRTR) and proposed a new continuous KDRI for deceased donor kidneys combining donor and transplant variables.
Rao’s KDRI included 14 donor and transplant factors, each associated with shorter graft survival. Table 6 shows the mentioned risk factors.
Table 6.
Donor and transplant factors and corresponding hazard ratios for graft failure
| Hazard ratio | 95%CI | P value | |
| Donor parameter | |||
| Age | 1.013 | 1.011-1.015 | < 0.0001 |
| Afro American race | 1.20 | 1.13-1.27 | < 0.0001 |
| Serum creatinine | 1.25 | 1.17-1.23 | < 0.0001 |
| Hypertensive | 1.13 | 1.08-1.19 | < 0.0001 |
| Diabetic | 1.14 | 1.04-1.24 | 0.0040 |
| Cause of Death | 1.09 | 1.04-1.14 | 0.0002 |
| Height | 0.96 | 0.94-0.97 | < 0.0001 |
| Weight | 0.98 | 0.97-0.99 | 0.0003 |
| Donation after cardiac death | 1.14 | 1.02-1.28 | 0.0246 |
| HCV positive | 1.27 | 1.13-1.43 | < 0.0001 |
| Transplant parameter | |||
| HLA-DR mismatch | 0.88 | 0.84-0.92 | < 0.0001 |
| Cold ischemia time | 1.005 | 1.003-1.008 | < 0.0001 |
| En bloc transplant | 0.70 | 0.57-0.84 | 0.0002 |
| Double kidney transplant | 0.86 | 0.75-1.00 | 0.0494 |
HLA:Human leukocyte antigen; HCV:Hepatitis C virus.
The KDRI is a continuous spectrum for any kind of donor (ECD and SCD) and allows for dividing the donor population into quintiles based on their KDRI. By the end of 2014, the KDRI was implemented by the OPTN[72]. Indeed, as some transplant factors are not known at the time of transplant, the donor-only KDRI based on 10 donor factors has been implemented.
All the mentioned donor scoring systems are shown in Table 1[73]. Woodside et al[74] examined the SRTR data from 2002 to 2010, and applying the KDRI, they found that kidneys belonging to the same KDRI quintile had similar outcomes independently of their belonging to ECD or SCD. However, ECD kidneys had a higher discard rate.
The use of the KDRI was further validated by several studies. Jun et al[75] examined the use of the KDRI in donors with acute kidney injury (AKI) and found a good correlation between KDRI quintiles and graft outcomes.
A different study[76] documented that the KDRI was a good prognostic tool for graft outcomes in deceased donor kidney transplantation with a short cold ischemia time. In this study, the KDRI correlated with renal function at 1 year, and a high KDRI was associated with a high risk of graft failure.
Recently, a Spanish study validated the usefulness of the KDRI in a European population[77]. The study evaluated 144 renal transplants. All kidneys transplanted were evaluated by the KDRI and biopsied. The aims of the study were to verify the concordance between the KDRI and the histological findings and to validate the prognostic value of the KDRI for transplant outcomes. The study concluded that there was a poor concordance between the KDRI and histological score and that the KDRI had a good prognostic value.
Strictly connected with the KDRI is the KDPI. The KDPI represents the relative risk of graft failure in the case of a particular deceased donor compared to a reference donor. The KDPI was introduced in 2014 in the US[78] and is derived by ranking the KDRI on a scale of 0-100% with reference to a donor cohort in the OPTN. It is useful and is represented by a number that helps in deciding the allocation of a specific organ[79].The KDRI and KDPI are strictly related.
These scoring systems have advantages over the ECD system because they represent a continuum, are based on 10 donor factors and represent a measure of donor quality.
Limitations of the KDRI and KDPI are represented by the fact that they do not include all of the donors’ factors that could impact the graft outcome. Additionally, the KDPI is a measure of the donor and is not specific for each kidney taken individually.
The KDPI is useful for introducing the concept of the so-called longevity matching. The concept consists of allocating kidneys with a higher KDPI to patients on dialysis with a lower life expectancy. A retrospective study[80] documented those patients older than 50 years or with a long waiting list time who were transplanted with kidneys with a high KDPI had a better survival than similar patients remaining on dialysis. This is particularly evident for patients older than 70 years[81]. Notwithstanding, a German study[82]reporting the experience of transplanting kidneys with a high KDPI observed that poor kidney quality, even when matching donors and recipients is the main factor responsible for poor outcomes. Several studies have evaluated the utility of the KDPI even outside of the US.
In a retrospective study, Lehner et al[83] evaluated the utility of the KDPI in almost 1000 European kidney transplants. The study found rather good outcomes in the case of donors with a very high KDPI. A Spanish study[84] evaluated the KDPI score on 389 transplants. The study documented that only the KDPI correlated with the risk of graft failure. This study also documented the utility of the KDPI measure in a cohort of European patients.
To further improve the KDPI, a retrospective study[85] was conducted in the US. The study evaluated the KDPI in adult transplant recipients in the OPTN/UNOS database from 2000 to 2015. This study, while validating the usefulness of the KDPI, found that terminal serum creatinine of the donor (one of the components of the KDPI) is not a useful variable.
Another European study[86] analyzed 1,305 kidney transplants. The study retrospectively applied the KDPI in 889 deceased donors and the living donor kidney profile index (LKDPI) in 416 living donors using the LKDPI realized by a US study for living donation[87].The European study was able to validate both the KDPI and LDKPI.
A major concern is what to do with donor kidneys with very a high KDPI (>80%).
In the US, the discard rate of these kidneys is approximately 50%. However, the allocation of kidneys with a KDPI higher than 80% in patients older than 60 years results in a lower patient mortality compared to patients who remain on the waiting list[88]. Indeed, several kidneys with a KDPI higher than 80% are viable. A recent study[89] evaluated the 1-year eGFR and graft failure for kidneys transplanted with a KDPI higher than 80%. The discard of such kidneys had been decided with the help of a pre-Tx kidney biopsy, renal resistance and kidney injury biomarker levels. The 1-year eGFR was low but satisfying. The authors request the use of new biological tools for a proper evaluation of these kidneys.
An Italian multicenter study tried to reduce the discard rate of kidneys with a KDPI higher than 80% using pre-transplant kidney biopsy for these kidneys[90]. The discard rate was reduced from 50% to 15%-37% according to the KDPI. The 1-year eGFR was lower for these marginal kidneys, but the graft survival was similar to that of standard kidneys. The study highlighted the utility of pre-transplant biopsy for kidneys with a very high KDPI.
Finally, a recently raised relevant question is whether the KDPI may be universally applied in allocating marginal kidneys or whether it is UNOS specific. A recent study from Ruggenenti et al[91] documented the allocation and good graft survival of 37 renal transplants with donors with a KDPI between 96% and 100% after a pre-transplant biopsy. These kidneys should have been discarded according to the UNOS criteria[92]. Similar findings have come from a previous study by Ekser et al[93]. The 5-year graft survival was 91%, and the mean KDPI was 97%. More than 80% of these kidneys should have been discarded according to the UNOS[94].
The question of UNOS specificity of the KDPI is examined in a recent study by Ruggenenti et al[95]. According to the author, the difference in ethnicity may only partially explain the different results and the different discard rates of UNOS and several European studies[96]. The author highlights the usefulness of pre-transplant biopsy for kidneys of donors with a very high KDPI.
In conclusion, the KDRI/KDPI represents an easy scoring system that could facilitate the decision to discard organs or allocate them in the best way.
According to several studies, the KDPI may also be applicable to European patients, even though this point is to date debated.
Based on the KDPI, the UNOS is implementing new allocation systems such as “longevity matching”. Each candidate willing to participate in the “longevity matching” will receive an “estimated post-transplant survival score” (EPTS) and will receive a graft according to the matching KDPI/EPTS.
The allocation of kidneys with the highest KDPI is debated. Often, these kidneys are discarded[97], but the use of pre-transplant biopsy may allow allocation of many of these kidneys, thus reducing the discard rate[98].
MACHINE PERFUSION AND PERFUSATE BIOMARKERS
Hypothermic machine perfusion is increasingly used in deceased donor kidney transplantation, but the question still remains on how efficient are MP in assessing the quality of an organ?
One study evaluating the reasons for discarding 12536 ECD kidneys found that 15% of perfused kidneys were discarded partly based on high renovascular resistance (RR)[99]. In a prospective study by Jochmans et al[100] RR values of 302 MP kidneys were evaluated. The study conclusions were that RR as a standalone quality assessment tool cannot be used to predict the graft outcomes.
More recently, Parikh et al[101]in a prospective observational cohort study examined the association between pump parameters and graft outcomes. They found an association between 1 h perfusate flow and DGF but with a border line value.
In conclusion, according the currently available data, there is a weak correlation between perfusion parameters and graft outcomes and additional studies are needed.
FUTURE PERSPECTIVES AND EMERGING TECHNOLOGIES
All the scoring systems, either histological or clinical, need to be improved with the help of new tools. Indeed, several cited studies advocate for newest approach in the evaluation of donor kidneys. Nickeleit[16] stated that new consensus guidelines remain to be defined on zero-time biopsies. Mueller et al[25] highlighting the confounding variables, advocate for the use of omic technologies in the evaluation of kidney biopsies. This point is also highlighted by the Banff Pre-Implantation Biopsy Working Group[56]. The usefulness of biomarkers in the evaluation of donor kidneys has also been highlighted by another recent study[90].
There are a number of emerging technologies to examine an organ at molecular level ranging from proteomics to metabolomics to transcription studies.
The most important study on proteomics is the study of Reese et al[102] who examined the association between four different biomarkers and the post-transplant renal function. All the urine injury biomarkers strongly associated with donor AKI, but resulted of limited value in predicting DGF or early graft function
By using transcription analysis, Scian et al[103] validated a set of three genes (CCL5, CXCR4 and ITGB2) that was up regulated in kidneys with a low eGFR post-transplantation.
Gustafson et al[104] still by transcription analysis found a set of 13 genes (Table 7) associated with allograft loss at two or three years after transplantation.
Table 7.
Genes included in the study
| ID | Symbol | Gene description | CADI-12 correlation | P value |
| 3954887 | CHCHD 10 | Coiled-coil-helix-coiled-coil-helix domain containing 10 | 0.404 | 2.85 x 10-5 |
| 4019160 | KLHL 13 | Kelch-like family member 13 (Drosophila) | 0.369 | 1.49 x 10-4 |
| 3326826 | FJX1 | Four jointed box 1 (Drosophila) | 0.367 | 1.60 x 10-4 |
| 3120343 | MET | Met proto-oncogene (hepatocyte growth factor receptor) | 0.352 | 3.01 x 10-4 |
| 2864449 | SERUNC5 | Seine incorporator 5 | 0.318 | 0.0012 |
| 2567583 | RNF149 | Ring finger protein 149 | 0.280 | 0.0046 |
| 2879105 | SPRY4 | Sprout homolog 4 (Drosophila) | 0.270 | 0.0062 |
| 3776504 | TG1F1 | TGFB-induced factor homeobox 1 | 0.244 | 0.0140 |
| 2898441 | KAAG1 | Kidney associated antigen 1 | 0.240 | 0.0154 |
| 3361971 | ST5 | Suppression of tumorigenity 5 | 0.232 | 0.0197 |
| 2459352 | WNT9A | Wingless-type MMTV integration site family member 9A | 0.212 | 0.0332 |
| 3021696 | ASB15 | Ankrin repeat and SOCS box-containing 15 | -0.263 | 0.0079 |
| 3193339 | RXRA | Retinoid X receptor alpha | -0.300 | 0.0023 |
CADI-12: Chronic allograft damage index at 12 mo.
By metabolomics studies, Guy et al[105] found in the perfusate of the hypothermic machine significant lower levels of gluconate, glucose, inosine and leucine in kidneys with DGF.
Finally, a novel technique able to recondition the kidney and to restore normal function prior to transplantation is the ex vivo normothermic perfusion. Phase I studies in ECD documented its safety and feasibility in clinical practice[106].
Some studies are ongoing, but their results are to date unknown.
An important study aims to evaluate the relevance of molecular biomarkers of aging in the blood of donors. This study (Senesce Test) has been completed, but no results are available yet (NCT02335333)[107]. Another NIH study coordinated by Yale University is testing biomarkers characteristic of renal injury in the urine of the donor and in the perfusion media (NCT01848249)[108].
The PREDICTION study aims to evaluate the improvement in viability of marginal kidneys treated by pulsatile perfusion[109].
CONCLUSION
The increase in the demand of kidneys for transplantation may only be satisfied with the increase in the use of marginal donors as kidneys from aged donors or with the use of donation after cardiac death donors.
Such kidneys need to be carefully evaluated either to be discarded or for a fair allocation.
The histological evaluation met several drawbacks as the time of the biopsy (pre or post reperfusion, the type of biopsy (wedge versus core biopsy), the pathologist involved in the evaluation (pathologist on-call or trained pathologist in this field).
Additionally, the difficulty of obtaining adequate histological analysis from pre implantation biopsies and the risk/benefit considerations to prolong cold ischemia time waiting for chronic histological abnormalities that often show poor correlation with clinical outcomes represents the most relevant drawback. All these drawbacks led to give more importance to the clinical evaluation of the donor. The KDRI/KDPI is an easily applicable scoring system, but this system also has its drawbacks especially in the evaluation of donors with the highest KDPI.
In the US, the use of KDPI led to a very high discard rate of the marginal donor kidneys, while other studies documented that several of these kidneys might be usefully transplanted.
Overall, is not easy to establish how many centers have taken part to the different scoring system as many of them are retrospective studies.
The elaboration of the Port scoring of standard criteria donors versus expanded criteria donors has been done comparing retrospectively 24756 SCD versus 4312 ECD from almost all the UNOS centers.
The MAPI has been done in a single center considering 371 transplants.
The Nyberg deceased donor score was made in three steps. In a first step 241 transplants were enrolled in two centers. Then in the attempt to give more strength to the scoring system, this was evaluated retrospectively on 34324 UNOS kidney transplants and in a third phase on 48952 UNOS kidney transplants.
The Donor risk score of Schold was evaluated retrospectively on 45850 data from SRTR.
The DGF nomogram of Irish was evaluated in a single center in UK on 217 prospective transplant patients.
Finally the KDRI of Rao was retrospectively evaluated on 69440 patients from SRTR. Subsequently the scoring was evaluated prospectively in different countries.
A hope for the future seems to come from the use of biomarkers. However, to date the use of urine biomarkers offers discordant results and does not provide sufficient power to be used in the kidney evaluation.
According recent studies, the use of pre-implantation biopsy has been shown to have its major utility in the evaluation of kidneys with a very high KDPI.
A very recent study from Moeckli et al[110] helps in clarifying what’s new in the current and emerging techniques of kidney evaluation. In particular the study concerns the use of omics and states that the most promising is transcriptome profile, also according the already cited studies.
Waiting for the advent of omics it seems that the best strategy in evaluating kidneys for transplantation is the clinical one. In the case of a very high KDRI pretransplant biopsy may be useful in allocating or not the kidneys
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Manuscript source: Invited manuscript
Peer-review started: March 8, 2019
First decision: April 16, 2019
Article in press: July 30, 2019
Specialty type: Transplantation
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Akbulut S, Boteon YL, Cantarovich F, Hibberd AD, Hilmi I, Gonzalez F, Sureshkumar K S-Editor: Wang JL L-Editor: Filipodia E-Editor: Qi LL
Contributor Information
Maurizio Salvadori, Department of Transplantation Renal Unit, Careggi University Hospital, viale Pieraccini 18, Florence 50139, Italy. maurizio.salvadori1@gmail.com.
Aris Tsalouchos, Nephrology and Dialysis Unit, Saints Cosmas and Damian Hospital, Via Cesare Battisti, Pescia (PT) 2-51017, Italy.
References
- 1.Rosengard BR, Feng S, Alfrey EJ, Zaroff JG, Emond JC, Henry ML, Garrity ER, Roberts JP, Wynn JJ, Metzger RA, Freeman RB, Port FK, Merion RM, Love RB, Busuttil RW, Delmonico FL. Report of the Crystal City meeting to maximize the use of organs recovered from the cadaver donor. Am J Transplant. 2002;2:701–711. doi: 10.1034/j.1600-6143.2002.20804.x. [DOI] [PubMed] [Google Scholar]
- 2.Metzger RA, Delmonico FL, Feng S, Port FK, Wynn JJ, Merion RM. Expanded criteria donors for kidney transplantation. Am J Transplant. 2003;3 Suppl 4:114–125. doi: 10.1034/j.1600-6143.3.s4.11.x. [DOI] [PubMed] [Google Scholar]
- 3.van Ittersum FJ, Hemke AC, Dekker FW, Hilbrands LB, Christiaans MH, Roodnat JI, Hoitsma AJ, van Diepen M. Increased risk of graft failure and mortality in Dutch recipients receiving an expanded criteria donor kidney transplant. Transpl Int. 2017;30:14–28. doi: 10.1111/tri.12863. [DOI] [PubMed] [Google Scholar]
- 4.Casati C, Colombo VG, Perrino M, Rossetti OM, Querques M, Giacomoni A, Binaggia A, Colussi G. Renal Transplants from Older Deceased Donors: Use of Preimplantation Biopsy and Differential Allocation to Dual or Single Kidney Transplant according to Histological Score Has No Advantages over Allocation to Single Kidney Transplant by Simple Clinical Indication. J Transplant. 2018;2018:4141756. doi: 10.1155/2018/4141756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karpinski J, Lajoie G, Cattran D, Fenton S, Zaltzman J, Cardella C, Cole E. Outcome of kidney transplantation from high-risk donors is determined by both structure and function. Transplantation. 1999;67:1162–1167. doi: 10.1097/00007890-199904270-00013. [DOI] [PubMed] [Google Scholar]
- 6.Alexander JW. High-risk donors: diabetics, the elderly, and others. Transplant Proc. 1992;24:2221–2222. [PubMed] [Google Scholar]
- 7.Wang HJ, Kjellstrand CM, Cockfield SM, Solez K. On the influence of sample size on the prognostic accuracy and reproducibility of renal transplant biopsy. Nephrol Dial Transplant. 1998;13:165–172. doi: 10.1093/ndt/13.1.165. [DOI] [PubMed] [Google Scholar]
- 8.Leunissen KM, Bosman FT, Nieman FH, Kootstra G, Vromen MA, Noordzij TC, van Hooff JP. Amplification of the nephrotoxic effect of cyclosporine by preexistent chronic histological lesions in the kidney. Transplantation. 1989;48:590–593. [PubMed] [Google Scholar]
- 9.Johnson LB, Kuo PC, Schweitzer EJ, Ratner LE, Klassen DK, Hoehn-Saric EW, dela Torre A, Weir MR, Strange J, Bartlett ST. Double renal allografts successfully increase utilization of kidneys from older donors within a single organ procurement organization. Transplantation. 1996;62:1581–1583. doi: 10.1097/00007890-199612150-00009. [DOI] [PubMed] [Google Scholar]
- 10.Remuzzi G, Cravedi P, Perna A, Dimitrov BD, Turturro M, Locatelli G, Rigotti P, Baldan N, Beatini M, Valente U, Scalamogna M, Ruggenenti P Dual Kidney Transplant Group. Long-term outcome of renal transplantation from older donors. N Engl J Med. 2006;354:343–352. doi: 10.1056/NEJMoa052891. [DOI] [PubMed] [Google Scholar]
- 11.Mancilla E, Avila-Casado C, Uribe-Uribe N, Morales-Buenrostro LE, Rodríguez F, Vilatoba M, Gabilondo B, Aburto S, Rodríguez RM, Magaña S, Magaña F, Alberú J. Time-zero renal biopsy in living kidney transplantation: a valuable opportunity to correlate predonation clinical data with histological abnormalities. Transplantation. 2008;86:1684–1688. doi: 10.1097/TP.0b013e3181906150. [DOI] [PubMed] [Google Scholar]
- 12.Pham PC, Wilkinson AH, Pham PT. Evaluation of the potential living kidney donor. Am J Kidney Dis. 2007;50:1043–1051. doi: 10.1053/j.ajkd.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 13.Davis CL. Evaluation of the living kidney donor: current perspectives. Am J Kidney Dis. 2004;43:508–530. doi: 10.1053/j.ajkd.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 14.Kayler LK, Mohanka R, Basu A, Shapiro R, Randhawa PS. Correlation of histologic findings on preimplant biopsy with kidney graft survival. Transpl Int. 2008;21:892–898. doi: 10.1111/j.1432-2277.2008.00681.x. [DOI] [PubMed] [Google Scholar]
- 15.Munivenkatappa RB, Schweitzer EJ, Papadimitriou JC, Drachenberg CB, Thom KA, Perencevich EN, Haririan A, Rasetto F, Cooper M, Campos L, Barth RN, Bartlett ST, Philosophe B. The Maryland aggregate pathology index: a deceased donor kidney biopsy scoring system for predicting graft failure. Am J Transplant. 2008;8:2316–2324. doi: 10.1111/j.1600-6143.2008.02370.x. [DOI] [PubMed] [Google Scholar]
- 16.Nickeleit V. Pathology: donor biopsy evaluation at time of renal grafting. Nat Rev Nephrol. 2009;5:249–251. doi: 10.1038/nrneph.2009.50. [DOI] [PubMed] [Google Scholar]
- 17.Yong ZZ, Kipgen D, Aitken EL, Khan KH, Kingsmore DB. Wedge Versus Core Biopsy at Time Zero: Which Provides Better Predictive Value for Delayed Graft Function With the Remuzzi Histological Scoring System? Transplant Proc. 2015;47:1605–1609. doi: 10.1016/j.transproceed.2015.03.050. [DOI] [PubMed] [Google Scholar]
- 18.Haas M, Ratner LE, Montgomery RA. C4d staining of perioperative renal transplant biopsies. Transplantation. 2002;74:711–717. doi: 10.1097/00007890-200209150-00021. [DOI] [PubMed] [Google Scholar]
- 19.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Yamaguchi Y. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 20.Pisarski P, Schleicher C, Hauser I, Becker JU. German recommendations for pretransplantation donor kidney biopsies. Langenbecks Arch Surg. 2016;401:133–140. doi: 10.1007/s00423-016-1384-5. [DOI] [PubMed] [Google Scholar]
- 21.Mengel M, Sis B, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Cendales L, Demetris AJ, Drachenberg CB, Farver CF, Rodriguez ER, Wallace WD, Glotz D Banff meeting report writing committee. Banff 2011 Meeting report: new concepts in antibody-mediated rejection. Am J Transplant. 2012;12:563–570. doi: 10.1111/j.1600-6143.2011.03926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh P, Farber JL, Doria C, Francos GC, Gulati R, Ramirez CB, Maley WR, Frank AM. Peritransplant kidney biopsies: comparison of pathologic interpretations and practice patterns of organ procurement organizations. Clin Transplant. 2012;26:E191–E199. doi: 10.1111/j.1399-0012.2011.01584.x. [DOI] [PubMed] [Google Scholar]
- 23.Azancot MA, Moreso F, Salcedo M, Cantarell C, Perello M, Torres IB, Montero A, Trilla E, Sellarés J, Morote J, Seron D. The reproducibility and predictive value on outcome of renal biopsies from expanded criteria donors. Kidney Int. 2014;85:1161–1168. doi: 10.1038/ki.2013.461. [DOI] [PubMed] [Google Scholar]
- 24.Freedman BI, Divers J, High KP. The authors reply: Kidney Int. 2014;85:1242–1243. doi: 10.1038/ki.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mueller TF, Solez K, Mas V. Assessment of kidney organ quality and prediction of outcome at time of transplantation. Semin Immunopathol. 2011;33:185–199. doi: 10.1007/s00281-011-0248-x. [DOI] [PubMed] [Google Scholar]
- 26.El-Husseini A, Sabry A, Zahran A, Shoker A. Can donor implantation renal biopsy predict long-term renal allograft outcome? Am J Nephrol. 2007;27:144–151. doi: 10.1159/000099944. [DOI] [PubMed] [Google Scholar]
- 27.Randhawa P. Role of donor kidney biopsies in renal transplantation. Transplantation. 2001;71:1361–1365. doi: 10.1097/00007890-200105270-00001. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann SC, Kampen RL, Amur S, Sharaf MA, Kleiner DE, Hunter K, John Swanson S, Hale DA, Mannon RB, Blair PJ, Kirk AD. Molecular and immunohistochemical characterization of the onset and resolution of human renal allograft ischemia-reperfusion injury. Transplantation. 2002;74:916–923. doi: 10.1097/00007890-200210150-00003. [DOI] [PubMed] [Google Scholar]
- 29.Hauser P, Schwarz C, Mitterbauer C, Regele HM, Mühlbacher F, Mayer G, Perco P, Mayer B, Meyer TW, Oberbauer R. Genome-wide gene-expression patterns of donor kidney biopsies distinguish primary allograft function. Lab Invest. 2004;84:353–361. doi: 10.1038/labinvest.3700037. [DOI] [PubMed] [Google Scholar]
- 30.Kainz A, Mitterbauer C, Hauser P, Schwarz C, Regele HM, Berlakovich G, Mayer G, Perco P, Mayer B, Meyer TW, Oberbauer R. Alterations in gene expression in cadaveric vs. live donor kidneys suggest impaired tubular counterbalance of oxidative stress at implantation. Am J Transplant. 2004;4:1595–1604. doi: 10.1111/j.1600-6143.2004.00554.x. [DOI] [PubMed] [Google Scholar]
- 31.Avihingsanon Y, Ma N, Pavlakis M, Chon WJ, Uknis ME, Monaco AP, Ferran C, Stillman I, Schachter AD, Mottley C, Zheng XX, Strom TB. On the intraoperative molecular status of renal allografts after vascular reperfusion and clinical outcomes. J Am Soc Nephrol. 2005;16:1542–1548. doi: 10.1681/ASN.2005020210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kainz A, Perco P, Mayer B, Soleiman A, Steininger R, Mayer G, Mitterbauer C, Schwarz C, Meyer TW, Oberbauer R. Gene-expression profiles and age of donor kidney biopsies obtained before transplantation distinguish medium term graft function. Transplantation. 2007;83:1048–1054. doi: 10.1097/01.tp.0000259960.56786.ec. [DOI] [PubMed] [Google Scholar]
- 33.Park W, Griffin M, Grande JP, Cosio F, Stegall MD. Molecular evidence of injury and inflammation in normal and fibrotic renal allografts one year posttransplant. Transplantation. 2007;83:1466–1476. doi: 10.1097/01.tp.0000265501.33362.d3. [DOI] [PubMed] [Google Scholar]
- 34.Mas VR, Archer KJ, Yanek K, Dumur CI, Capparuccini MI, Mangino MJ, King A, Gibney EM, Fisher R, Posner M, Maluf D. Gene expression patterns in deceased donor kidneys developing delayed graft function after kidney transplantation. Transplantation. 2008;85:626–635. doi: 10.1097/TP.0b013e318165491f. [DOI] [PubMed] [Google Scholar]
- 35.Mueller TF, Reeve J, Jhangri GS, Mengel M, Jacaj Z, Cairo L, Obeidat M, Todd G, Moore R, Famulski KS, Cruz J, Wishart D, Meng C, Sis B, Solez K, Kaplan B, Halloran PF. The transcriptome of the implant biopsy identifies donor kidneys at increased risk of delayed graft function. Am J Transplant. 2008;8:78–85. doi: 10.1111/j.1600-6143.2007.02032.x. [DOI] [PubMed] [Google Scholar]
- 36.Perco P, Kainz A, Wilflingseder J, Soleiman A, Mayer B, Oberbauer R. Histogenomics: association of gene expression patterns with histological parameters in kidney biopsies. Transplantation. 2009;87:290–295. doi: 10.1097/TP.0b013e318191b4c0. [DOI] [PubMed] [Google Scholar]
- 37.Naesens M, Li L, Ying L, Sansanwal P, Sigdel TK, Hsieh SC, Kambham N, Lerut E, Salvatierra O, Butte AJ, Sarwal MM. Expression of complement components differs between kidney allografts from living and deceased donors. J Am Soc Nephrol. 2009;20:1839–1851. doi: 10.1681/ASN.2008111145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bodonyi-Kovacs G, Putheti P, Marino M, Avihingsanon Y, Uknis ME, Monaco AP, Strom TB, Pavlakis M. Gene expression profiling of the donor kidney at the time of transplantation predicts clinical outcomes 2 years after transplantation. Hum Immunol. 2010;71:451–455. doi: 10.1016/j.humimm.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cravedi P, Maggiore U, Mannon RB. Low-density array PCR analysis of reperfusion biopsies: an adjunct to histological analysis. Nephrol Dial Transplant. 2010;25:4077–4086. doi: 10.1093/ndt/gfq297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krol R, Chudek J, Karkoszka H, Ziaja J, Kolonko A, Pawlicki J, Kajor M, Wiecek A, Cierpka L. Apoptosis of tubular epithelial cells in preimplantation biopsies of kidney grafts with immediate, slow and delayed function. Ann Transplant. 2011;16:17–22. doi: 10.12659/aot.881990. [DOI] [PubMed] [Google Scholar]
- 41.Goncalves-Primo A, Mourão TB, Andrade-Oliveira V, Campos EF, Medina-Pestana JO, Tedesco-Silva H, Gerbase-DeLima M. Investigation of apoptosis-related gene expression levels in preimplantation biopsies as predictors of delayed kidney graft function. Transplantation. 2014;97:1260–1265. doi: 10.1097/01.TP.0000442579.12285.e8. [DOI] [PubMed] [Google Scholar]
- 42.Mazeti-Felicio CM, Caldas HC, Fernandes-Charpiot IMM, Dezotti CZ, Baptista MASF, Abbud-Filho M. Preimplantation Kidney Biopsies of Extended Criteria Donors Have a Heavier Inflammatory Burden Than Kidneys From Standard Criteria Donors. Transplant Direct. 2017;3:e180. doi: 10.1097/TXD.0000000000000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang CJ, Wetmore JB, Crary GS, Kasiske BL. The Donor Kidney Biopsy and Its Implications in Predicting Graft Outcomes: A Systematic Review. Am J Transplant. 2015;15:1903–1914. doi: 10.1111/ajt.13213. [DOI] [PubMed] [Google Scholar]
- 44.Naesens M. Zero-Time Renal Transplant Biopsies: A Comprehensive Review. Transplantation. 2016;100:1425–1439. doi: 10.1097/TP.0000000000001018. [DOI] [PubMed] [Google Scholar]
- 45.Haas M, Segev DL, Racusen LC, Bagnasco SM, Melancon JK, Tan M, Kraus ES, Rabb H, Ugarte RM, Burdick JF, Montgomery RA. Arteriosclerosis in kidneys from healthy live donors: comparison of wedge and needle core perioperative biopsies. Arch Pathol Lab Med. 2008;132:37–42. doi: 10.5858/2008-132-37-AIKFHL. [DOI] [PubMed] [Google Scholar]
- 46.Muruve NA, Steinbecker KM, Luger AM. Are wedge biopsies of cadaveric kidneys obtained at procurement reliable? Transplantation. 2000;69:2384–2388. doi: 10.1097/00007890-200006150-00029. [DOI] [PubMed] [Google Scholar]
- 47.De Vusser K, Lerut E, Kuypers D, Vanrenterghem Y, Jochmans I, Monbaliu D, Pirenne J, Naesens M. The predictive value of kidney allograft baseline biopsies for long-term graft survival. J Am Soc Nephrol. 2013;24:1913–1923. doi: 10.1681/ASN.2012111081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goumenos DS, Kalliakmani P, Tsamandas AC, Maroulis I, Savidaki E, Fokaefs E, Papachristou E, Karavias D, Vlachojannis JG. The prognostic value of frozen section preimplantation graft biopsy in the outcome of renal transplantation. Ren Fail. 2010;32:434–439. doi: 10.3109/08860221003658241. [DOI] [PubMed] [Google Scholar]
- 49.Remuzzi G, Grinyò J, Ruggenenti P, Beatini M, Cole EH, Milford EL, Brenner BM. Early experience with dual kidney transplantation in adults using expanded donor criteria. Double Kidney Transplant Group (DKG) J Am Soc Nephrol. 1999;10:2591–2598. doi: 10.1681/ASN.V10122591. [DOI] [PubMed] [Google Scholar]
- 50.Ortiz F, Paavonen T, Törnroth T, Koskinen P, Finne P, Salmela K, Kyllönen L, Grönhagen-Riska C, Honkanen E. Predictors of renal allograft histologic damage progression. J Am Soc Nephrol. 2005;16:817–824. doi: 10.1681/ASN.2004060475. [DOI] [PubMed] [Google Scholar]
- 51.Naesens M, Sarwal MM. Molecular diagnostics in transplantation. Nat Rev Nephrol. 2010;6:614–628. doi: 10.1038/nrneph.2010.113. [DOI] [PubMed] [Google Scholar]
- 52.Kamińska D, Kościelska-Kasprzak K, Drulis-Fajdasz D, Hałoń A, Polak W, Chudoba P, Jańczak D, Mazanowska O, Patrzałek D, Klinger M. Kidney ischemic injury genes expressed after donor brain death are predictive for the outcome of kidney transplantation. Transplant Proc. 2011;43:2891–2894. doi: 10.1016/j.transproceed.2011.08.062. [DOI] [PubMed] [Google Scholar]
- 53.lznerowicz A, Chudoba P, Kamińska D, Kościelska-Kasprzak K, Drulis-Fajdasz D, Hałoń A, Janczak D, Boratyńska M, Klinger M, Patrzałek D, Polak WG. Duration of brain death and cold ischemia time, but not warm ischemia time, increases expression of genes associated with apoptosis in transplanted kidneys from deceased donors. Transplant Proc. 2011;43:2887–2890. doi: 10.1016/j.transproceed.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 54.Mitterbauer C, Schwarz C, Hauser P, Steininger R, Regele HM, Rosenkranz A, Oberbauer R. Impaired tubulointerstitial expression of endothelin-1 and nitric oxide isoforms in donor kidney biopsies with postischemic acute renal failure. Transplantation. 2003;76:715–720. doi: 10.1097/01.TP.0000082820.13813.19. [DOI] [PubMed] [Google Scholar]
- 55.Colussi G, Casati C, Colombo VG, Camozzi MLP, Salerno FR. Renal transplants from older deceased donors: Is pre-implantation biopsy useful? A monocentric observational clinical study. World J Transplant. 2018;8:110–121. doi: 10.5500/wjt.v8.i4.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liapis H, Gaut JP, Klein C, Bagnasco S, Kraus E, Farris AB, 3rd, Honsova E, Perkowska-Ptasinska A, David D, Goldberg J, Smith M, Mengel M, Haas M, Seshan S, Pegas KL, Horwedel T, Paliwa Y, Gao X, Landsittel D, Randhawa P Banff Working Group. Banff Histopathological Consensus Criteria for Preimplantation Kidney Biopsies. Am J Transplant. 2017;17:140–150. doi: 10.1111/ajt.13929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carpenter D, Husain SA, Brennan C, Batal I, Hall IE, Santoriello D, Rosen R, Crew RJ, Campenot E, Dube GK, Radhakrishnan J, Stokes MB, Sandoval PR, D'Agati V, Cohen DJ, Ratner LE, Markowitz G, Mohan S. Procurement Biopsies in the Evaluation of Deceased Donor Kidneys. Clin J Am Soc Nephrol. 2018;13:1876–1885. doi: 10.2215/CJN.04150418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Port FK, Bragg-Gresham JL, Metzger RA, Dykstra DM, Gillespie BW, Young EW, Delmonico FL, Wynn JJ, Merion RM, Wolfe RA, Held PJ. Donor characteristics associated with reduced graft survival: an approach to expanding the pool of kidney donors. Transplantation. 2002;74:1281–1286. doi: 10.1097/00007890-200211150-00014. [DOI] [PubMed] [Google Scholar]
- 59.Verran DJ, deLeon C, Chui AK, Chapman JR. Factors in older cadaveric organ donors impacting on renal allograft outcome. Clin Transplant. 2001;15:1–5. doi: 10.1034/j.1399-0012.2001.150101.x. [DOI] [PubMed] [Google Scholar]
- 60.Wright DL, Kemp TL. The dual-task methodology and assessing the attentional demands of ambulation with walking devices. Phys Ther. 1992;72:306–12; discussion 313-5. doi: 10.1093/ptj/72.4.306. [DOI] [PubMed] [Google Scholar]
- 61.Anglicheau D, Loupy A, Lefaucheur C, Pessione F, Létourneau I, Côté I, Gaha K, Noël LH, Patey N, Droz D, Martinez F, Zuber J, Glotz D, Thervet E, Legendre C. A simple clinico-histopathological composite scoring system is highly predictive of graft outcomes in marginal donors. Am J Transplant. 2008;8:2325–2334. doi: 10.1111/j.1600-6143.2008.02394.x. [DOI] [PubMed] [Google Scholar]
- 62.Hopfer H, Kemény É. Assessment of donor biopsies. Curr Opin Organ Transplant. 2013;18:306–312. doi: 10.1097/MOT.0b013e3283607a6e. [DOI] [PubMed] [Google Scholar]
- 63.Sánchez-Escuredo A, Sagasta A, Revuelta I, Rodas LM, Paredes D, Musquera M, Diekmann F, Campistol JM, Solé M, Oppenheimer F. Histopathological evaluation of pretransplant donor biopsies in expanded criteria donors with high kidney donor profile index: a retrospective observational cohort study. Transpl Int. 2017;30:975–986. doi: 10.1111/tri.12966. [DOI] [PubMed] [Google Scholar]
- 64.Nyberg SL, Matas AJ, Rogers M, Harmsen WS, Velosa JA, Larson TS, Prieto M, Ishitani MB, Sterioff S, Stegall MD. Donor scoring system for cadaveric renal transplantation. Am J Transplant. 2001;1:162–170. [PubMed] [Google Scholar]
- 65.Nyberg SL, Matas AJ, Kremers WK, Thostenson JD, Larson TS, Prieto M, Ishitani MB, Sterioff S, Stegall MD. Improved scoring system to assess adult donors for cadaver renal transplantation. Am J Transplant. 2003;3:715–721. doi: 10.1034/j.1600-6143.2003.00111.x. [DOI] [PubMed] [Google Scholar]
- 66.Nyberg SL, Baskin-Bey ES, Kremers W, Prieto M, Henry ML, Stegall MD. Improving the prediction of donor kidney quality: deceased donor score and resistive indices. Transplantation. 2005;80:925–929. doi: 10.1097/01.tp.0000173798.04043.af. [DOI] [PubMed] [Google Scholar]
- 67.Schold JD, Kaplan B, Baliga RS, Meier-Kriesche HU. The broad spectrum of quality in deceased donor kidneys. Am J Transplant. 2005;5:757–765. doi: 10.1111/j.1600-6143.2005.00770.x. [DOI] [PubMed] [Google Scholar]
- 68.Moore J, Ramakrishna S, Tan K, Cockwell P, Eardley K, Little MA, Rylance P, Shivakumar K, Suresh V, Tomlinson K, Ready A, Borrows R. Identification of the optimal donor quality scoring system and measure of early renal function in kidney transplantation. Transplantation. 2009;87:578–586. doi: 10.1097/TP.0b013e3181949e71. [DOI] [PubMed] [Google Scholar]
- 69.Friedewald JJ. Utilization and outcomes of marginal kidneys--using Kidney Donor Risk Index to move beyond the current labels. Am J Transplant. 2012;12:1971–1972. doi: 10.1111/j.1600-6143.2012.04149.x. [DOI] [PubMed] [Google Scholar]
- 70.Irish WD, McCollum DA, Tesi RJ, Owen AB, Brennan DC, Bailly JE, Schnitzler MA. Nomogram for predicting the likelihood of delayed graft function in adult cadaveric renal transplant recipients. J Am Soc Nephrol. 2003;14:2967–2974. doi: 10.1097/01.asn.0000093254.31868.85. [DOI] [PubMed] [Google Scholar]
- 71.Rao PS, Schaubel DE, Guidinger MK, Andreoni KA, Wolfe RA, Merion RM, Port FK, Sung RS. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009;88:231–236. doi: 10.1097/TP.0b013e3181ac620b. [DOI] [PubMed] [Google Scholar]
- 72.OPTN. Organ Procurement and Transplantation Network. Available from: http://optn.transplant.hrsa.gov//converge/resorces/allocationcalculators.asp?index=81isSubmit=trueextra=true#bottom.
- 73.Wang ZG. Adherence to standardization and integrity in translational medicine research. Chin J Traumatol. 2014;17:311–312. [PubMed] [Google Scholar]
- 74.Woodside KJ, Merion RM, Leichtman AB, de los Santos R, Arrington CJ, Rao PS, Sung RS. Utilization of kidneys with similar kidney donor risk index values from standard versus expanded criteria donors. Am J Transplant. 2012;12:2106–2114. doi: 10.1111/j.1600-6143.2012.04146.x. [DOI] [PubMed] [Google Scholar]
- 75.Jun H, Jung CW, Lim S, Kim MG. Kidney Donor Risk Index as the Predictor for the Short-term Clinical Outcomes After Kidney Transplant From Deceased Donor With Acute Kidney Injury. Transplant Proc. 2017;49:88–91. doi: 10.1016/j.transproceed.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 76.Han M, Jeong JC, Koo TY, Jeon HJ, Kwon HY, Kim YJ, Ryu HJ, Ahn C, Yang J. Kidney donor risk index is a good prognostic tool for graft outcomes in deceased donor kidney transplantation with short, cold ischemic time. Clin Transplant. 2014;28:337–344. doi: 10.1111/ctr.12318. [DOI] [PubMed] [Google Scholar]
- 77.Del Moral Martín RMG, Retamero Díaz JA, Cava Molina M, Cobacho Tornel BM, Bravo Soto J, Osuna Ortega A, O'Valle Ravassa F. Validation of KDRI/KDPI for the selection of expanded criteria kidney donors. Nefrologia. 2018;38:297–303. doi: 10.1016/j.nefro.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 78.Leichtman AB, McCullough KP, Wolfe RA. Improving the allocation system for deceased-donor kidneys. N Engl J Med. 2011;364:1287–1289. doi: 10.1056/NEJMp1102728. [DOI] [PubMed] [Google Scholar]
- 79.Smith JM, Biggins SW, Haselby DG, Kim WR, Wedd J, Lamb K, Thompson B, Segev DL, Gustafson S, Kandaswamy R, Stock PG, Matas AJ, Samana CJ, Sleeman EF, Stewart D, Harper A, Edwards E, Snyder JJ, Kasiske BL, Israni AK. Kidney, pancreas and liver allocation and distribution in the United States. Am J Transplant. 2012;12:3191–3212. doi: 10.1111/j.1600-6143.2012.04259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Massie AB, Luo X, Chow EK, Alejo JL, Desai NM, Segev DL. Survival benefit of primary deceased donor transplantation with high-KDPI kidneys. Am J Transplant. 2014;14:2310–2316. doi: 10.1111/ajt.12830. [DOI] [PubMed] [Google Scholar]
- 81.Hernandez RA, Malek SK, Milford EL, Finlayson SR, Tullius SG. The combined risk of donor quality and recipient age: higher-quality kidneys may not always improve patient and graft survival. Transplantation. 2014;98:1069–1076. doi: 10.1097/TP.0000000000000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tittelbach-Helmrich D, Thurow C, Arwinski S, Schleicher C, Hopt UT, Bausch D, Drognitz O, Pisarski P. Poor organ quality and donor-recipient age mismatch rather than poor donation rates account for the decrease in deceased kidney transplantation rates in a Germany Transplant Center. Transpl Int. 2015;28:191–198. doi: 10.1111/tri.12478. [DOI] [PubMed] [Google Scholar]
- 83.Lehner LJ, Kleinsteuber A, Halleck F, Khadzhynov D, Schrezenmeier E, Duerr M, Eckardt KU, Budde K, Staeck O. Assessment of the Kidney Donor Profile Index in a European cohort. Nephrol Dial Transplant. 2018;33:1465–1472. doi: 10.1093/ndt/gfy030. [DOI] [PubMed] [Google Scholar]
- 84.Arias-Cabrales C, Pérez-Sáez MJ, Redondo-Pachón D, Buxeda A, Burballa C, Bermejo S, Sierra A, Mir M, Burón A, Zapatero A, Crespo M, Pascual J. Usefulness of the KDPI in Spain: A comparison with donor age and definition of standard/expanded criteria donor. Nefrologia. 2018;38:503–513. doi: 10.1016/j.nefro.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 85.Chopra B, Sureshkumar KK. Limitation of Terminal Serum Creatinine as a Kidney Donor Profile Index Variable in Predicting Long-Term Kidney Transplant Outcomes. Transplant Proc. 2018;50:1272–1275. doi: 10.1016/j.transproceed.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 86.Rehse G, Halleck F, Khadzhynov D, Lehner LJ, Kleinsteuber A, Staeck A, Duerr M, Budde K, Staeck O. Validation of the Living Kidney Donor Profile Index in a European cohort and comparison of long-term outcomes with US results. Nephrol Dial Transplant. 2019;34:1063–1070. doi: 10.1093/ndt/gfy118. [DOI] [PubMed] [Google Scholar]
- 87.Massie AB, Leanza J, Fahmy LM, Chow EK, Desai NM, Luo X, King EA, Bowring MG, Segev DL. A Risk Index for Living Donor Kidney Transplantation. Am J Transplant. 2016;16:2077–2084. doi: 10.1111/ajt.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jay CL, Washburn K, Dean PG, Helmick RA, Pugh JA, Stegall MD. Survival Benefit in Older Patients Associated With Earlier Transplant With High KDPI Kidneys. Transplantation. 2017;101:867–872. doi: 10.1097/TP.0000000000001405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Doshi MD, Reese PP, Hall IE, Schröppel B, Ficek J, Formica RN, Weng FL, Hasz RD, Thiessen-Philbrook H, Parikh CR. Utility of Applying Quality Assessment Tools for Kidneys With KDPI ≥80. Transplantation. 2017;101:1125–1133. doi: 10.1097/TP.0000000000001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gandolfini I, Buzio C, Zanelli P, Palmisano A, Cremaschi E, Vaglio A, Piotti G, Melfa L, La Manna G, Feliciangeli G, Cappuccilli M, Scolari MP, Capelli I, Panicali L, Baraldi O, Stefoni S, Buscaroli A, Ridolfi L, D'Errico A, Cappelli G, Bonucchi D, Rubbiani E, Albertazzi A, Mehrotra A, Cravedi P, Maggiore U. The Kidney Donor Profile Index (KDPI) of marginal donors allocated by standardized pretransplant donor biopsy assessment: distribution and association with graft outcomes. Am J Transplant. 2014;14:2515–2525. doi: 10.1111/ajt.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ruggenenti P, Silvestre C, Boschiero L, Rota G, Furian L, Perna A, Rossini G, Remuzzi G, Rigotti P. Long-term outcome of renal transplantation from octogenarian donors: A multicenter controlled study. Am J Transplant. 2017;17:3159–3171. doi: 10.1111/ajt.14459. [DOI] [PubMed] [Google Scholar]
- 92.Bae S, Massie AB, Luo X, Anjum S, Desai NM, Segev DL. Changes in Discard Rate After the Introduction of the Kidney Donor Profile Index (KDPI) Am J Transplant. 2016;16:2202–2207. doi: 10.1111/ajt.13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ekser B, Furian L, Broggiato A, Silvestre C, Pierobon ES, Baldan N, Rigotti P. Technical aspects of unilateral dual kidney transplantation from expanded criteria donors: experience of 100 patients. Am J Transplant. 2010;10:2000–2007. doi: 10.1111/j.1600-6143.2010.03188.x. [DOI] [PubMed] [Google Scholar]
- 94.Ekser B, Powelson JA, Fridell JA, Goggins WC, Taber TE. Is the kidney donor profile index (KDPI) universal or UNOS-specific? Am J Transplant. 2018;18:1031–1032. doi: 10.1111/ajt.14538. [DOI] [PubMed] [Google Scholar]
- 95.Ruggenenti P, Remuzzi G. Invited letter in response to: "Is the kidney donor profile index (KDPI) universal or UNOS-specific?". Am J Transplant. 2018;18:1033–1034. doi: 10.1111/ajt.14652. [DOI] [PubMed] [Google Scholar]
- 96.Rege A, Irish B, Castleberry A, Vikraman D, Sanoff S, Ravindra K, Collins B, Sudan D. Trends in Usage and Outcomes for Expanded Criteria Donor Kidney Transplantation in the United States Characterized by Kidney Donor Profile Index. Cureus. 2016;8:e887. doi: 10.7759/cureus.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tice BG, Datta M, Mousseau J, Aliaga L, Altinok O, Barrios Sazo MG, Betancourt M, Bodek A, Bravar A, Brooks WK, Budd H, Bustamante MJ, Butkevich A, Martinez Caicedo DA, Castromonte CM, Christy ME, Chvojka J, da Motta H, Devan J, Dytman SA, Díaz GA, Eberly B, Felix J, Fields L, Fiorentini GA, Gago AM, Gallagher H, Gran R, Harris DA, Higuera A, Hurtado K, Jerkins M, Kafka T, Kordosky M, Kulagin SA, Le T, Maggi G, Maher E, Manly S, Mann WA, Marshall CM, Martin Mari C, McFarland KS, McGivern CL, McGowan AM, Miller J, Mislivec A, Morfín JG, Muhlbeier T, Naples D, Nelson JK, Norrick A, Osta J, Palomino JL, Paolone V, Park J, Patrick CE, Perdue GN, Rakotondravohitra L, Ransome RD, Ray H, Ren L, Rodrigues PA, Savage DG, Schellman H, Schmitz DW, Simon C, Snider FD, Solano Salinas CJ, Tagg N, Valencia E, Velásquez JP, Walton T, Wolcott J, Zavala G, Zhang D, Ziemer BP MINERvA Collaboration. Measurement of ratios of νμ charged-current cross sections on C, Fe, and Pb to CH at neutrino energies 2-20 GeV. Phys Rev Lett. 2014;112:231801. doi: 10.1103/PhysRevLett.112.231801. [DOI] [PubMed] [Google Scholar]
- 98.Philipse E, Lee APK, Bracke B, Hartman V, Chapelle T, Roeyen G, de Greef K, Ysebaert DK, van Beeumen G, Couttenye MM, Van Craenenbroeck AH, Hellemans R, Bosmans JL, Abramowicz D. Does Kidney Donor Risk Index implementation lead to the transplantation of more and higher-quality donor kidneys? Nephrol Dial Transplant. 2017;32:1934–1938. doi: 10.1093/ndt/gfx257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sung RS, Christensen LL, Leichtman AB, Greenstein SM, Distant DA, Wynn JJ, Stegall MD, Delmonico FL, Port FK. Determinants of discard of expanded criteria donor kidneys: impact of biopsy and machine perfusion. Am J Transplant. 2008;8:783–792. doi: 10.1111/j.1600-6143.2008.02157.x. [DOI] [PubMed] [Google Scholar]
- 100.Jochmans I, Moers C, Smits JM, Leuvenink HG, Treckmann J, Paul A, Rahmel A, Squifflet JP, van Heurn E, Monbaliu D, Ploeg RJ, Pirenne J. The prognostic value of renal resistance during hypothermic machine perfusion of deceased donor kidneys. Am J Transplant. 2011;11:2214–2220. doi: 10.1111/j.1600-6143.2011.03685.x. [DOI] [PubMed] [Google Scholar]
- 101.Parikh CR, Hall IE, Bhangoo RS, Ficek J, Abt PL, Thiessen-Philbrook H, Lin H, Bimali M, Murray PT, Rao V, Schröppel B, Doshi MD, Weng FL, Reese PP. Associations of Perfusate Biomarkers and Pump Parameters With Delayed Graft Function and Deceased Donor Kidney Allograft Function. Am J Transplant. 2016;16:1526–1539. doi: 10.1111/ajt.13655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reese PP, Hall IE, Weng FL, Schröppel B, Doshi MD, Hasz RD, Thiessen-Philbrook H, Ficek J, Rao V, Murray P, Lin H, Parikh CR. Associations between Deceased-Donor Urine Injury Biomarkers and Kidney Transplant Outcomes. J Am Soc Nephrol. 2016;27:1534–1543. doi: 10.1681/ASN.2015040345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Scian MJ, Maluf DG, Archer KJ, Turner SD, Suh JL, David KG, King AL, Posner MP, Brayman KL, Mas VR. Identification of biomarkers to assess organ quality and predict posttransplantation outcomes. Transplantation. 2012;94:851–858. doi: 10.1097/TP.0b013e318263702b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gustafson E, Asif S, Kozarcanin H, Elgue G, Meurling S, Ekdahl KN, Nilsson B. Control of IBMIR Induced by Fresh and Cryopreserved Hepatocytes by Low Molecular Weight Dextran Sulfate Versus Heparin. Cell Transplant. 2017;26:71–81. doi: 10.3727/096368916X692609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guy AJ, Nath J, Cobbold M, Ludwig C, Tennant DA, Inston NG, Ready AR. Metabolomic analysis of perfusate during hypothermic machine perfusion of human cadaveric kidneys. Transplantation. 2015;99:754–759. doi: 10.1097/TP.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 106.Hosgood SA, Saeb-Parsy K, Wilson C, Callaghan C, Collett D, Nicholson ML. Protocol of a randomised controlled, open-label trial of ex vivo normothermic perfusion versus static cold storage in donation after circulatory death renal transplantation. BMJ Open. 2017;7:e012237. doi: 10.1136/bmjopen-2016-012237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.GerberD. Biomarkers of Aging as Predictors of Kidney Transplant Function. [accessed 2019 Mar 1]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT02335333 ClinicalTrials.gov Identifier: NCT02335333. [Google Scholar]
- 108.Parikh CR. Deceased Donor Biomarkers and Recipient Outcomes (DDS). [accessed 2019 Mar 1]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT01848249 ClinicalTrials.gov Identifier: NCT01848249.
- 109.Cravedi P, Remuzzi G, Rota G, De Pascale S, La Canna F, Piccolo G, Rossini G, Vesconi S. Pulsed Perfusion for Marginal Kidneys (PREDICTION). [accessed 2019 Mar 1]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT02055950 ClinicalTrials.gov Identifier: NCT02055950.
- 110.Moeckli B, Sun P, Lazeyras F, Morel P, Moll S, Pascual M, Bühler LH. Evaluation of donor kidneys prior to transplantation: an update of current and emerging methods. Transpl Int. 2019;32:459–469. doi: 10.1111/tri.13430. [DOI] [PubMed] [Google Scholar]