Summary
Receptor‐like kinases (RLKs) play a prominent role in the interaction between plants and extracellular pathogens. Intriguingly, in the past few years several studies have demonstrated that a number of RLKs influence plant susceptibility to viruses and, in some cases, interact with viral proteins. In this review, we will summarize and discuss recent advances suggesting a direct role for RLKs in plant–virus interactions.
Keywords: BAK1, BAM1, BKK1, CLV1, NIK1, receptor‐like kinase, virus
Introduction
Receptor kinases are proteins localized at the cell surface, formed by an extracellular domain (ECD), a transmembrane domain (TMD), and an intracellular kinase domain (KD). In plants, these proteins are usually known as receptor‐like kinases (RLKs) and play an essential role in a multitude of cellular processes, from developmental control to the response to environmental stimuli (Breiden and Simon, 2016; Ye et al., 2017; He et al., 2018). In comparison with other organisms, plants have evolved a notably large family of RLKs, including more than 600 members in Arabidopsis and more than 1000 members in rice (Gish and Clark, 2011). The ECDs of plant RLKs are considerably diverse, and may include extensin‐like, lectin‐like, epidermal‐growth‐factor‐like (including cysteine‐rich), lysine motif, and leucine‐rich repeat (LRR) domains, among others (Gish and Clark, 2011). RLK ECDs may homo‐ or hetero‐multimerize in order to perceive endogenous or exogenous ligands, including peptides, steroids, oligosaccharides, polysaccharides, and lipopolisaccharides, and transduce these signals to the cell interior (Breiden and Simon, 2016; Ye et al., 2017; He et al., 2018; Smakowska‐Luzan et al., 2018), which often results in the initiation of specific intracellular signalling events.
RLKs play a prominent role in plant–pathogen interactions (Tang et al., 2017). Some RLKs have been shown to act as pattern‐recognition receptors (PRRs), mediating the perception of molecular patterns of plant or microbial origin, usually termed damage‐associated molecular patterns (DAMPs) or pathogen‐associated molecular patterns (PAMPs), respectively (Zipfel, 2014). These molecular patterns are usually recognized as danger signals by plant cells, leading to the activation of immune responses (Macho and Zipfel, 2014) and the subsequent development of pattern‐triggered immunity (PTI). Other RLKs have been found to interact with PRRs, providing a scaffold for their association with other proteins or regulating the activation of signalling (Tang et al., 2017; Smakowska‐Luzan et al., 2018). Additionally, non‐PRR RLKs may also contribute to the plant response to pathogen perception by regulating molecular cross‐talks or developmental rearrangements associated with immunity (Tang et al., 2017).
While the importance of RLKs, especially PRRs, in plant defence against bacteria, fungi, oomycetes, and even insects is widely accepted, their contribution to plant–virus interactions is currently under debate. In principle, a potential role of RLKs in the perception of invading viruses seems counterintuitive, since these proteins relay signals from the extracellular space, while viruses are intracellular pathogens; consistently, RNA interference (RNAi) is considered the main antiviral defence mechanism (Yang and Li, 2018). However, it cannot be excluded that the viral infection could result in the release to the apoplast of tell‐tale molecules, either derived from the virus itself (proteins or nucleic acids) or as a result of cellular damage or the activation of plant defence mechanisms (DAMPs) (Fig. 1). Additionally, RLKs involved in the regulation of developmental processes and hormonal responses might have an indirect impact on the viral infection, hence contributing to the final outcome of the interaction between virus and host; due to space limitations, RLKs with no known role in plant defence and not described as targeted by viruses will not be discussed in this review (Fig. 1). Interestingly, recent works have unveiled that a number of RLKs indeed influence plant susceptibility to viruses, in some cases being direct targets of viral proteins (Korner et al., 2013; Zorzatto et al., 2015; Gouveia et al., 2016; Niehl et al., 2016; Calil and Fontes, 2017; Carluccio et al., 2018; Li et al., 2018; Rosas‐Diaz et al., 2018); some of these RLKs are well‐known players in plant immunity, while others may exert novel functions in antiviral defence (Fig. 2).
Figure 1.
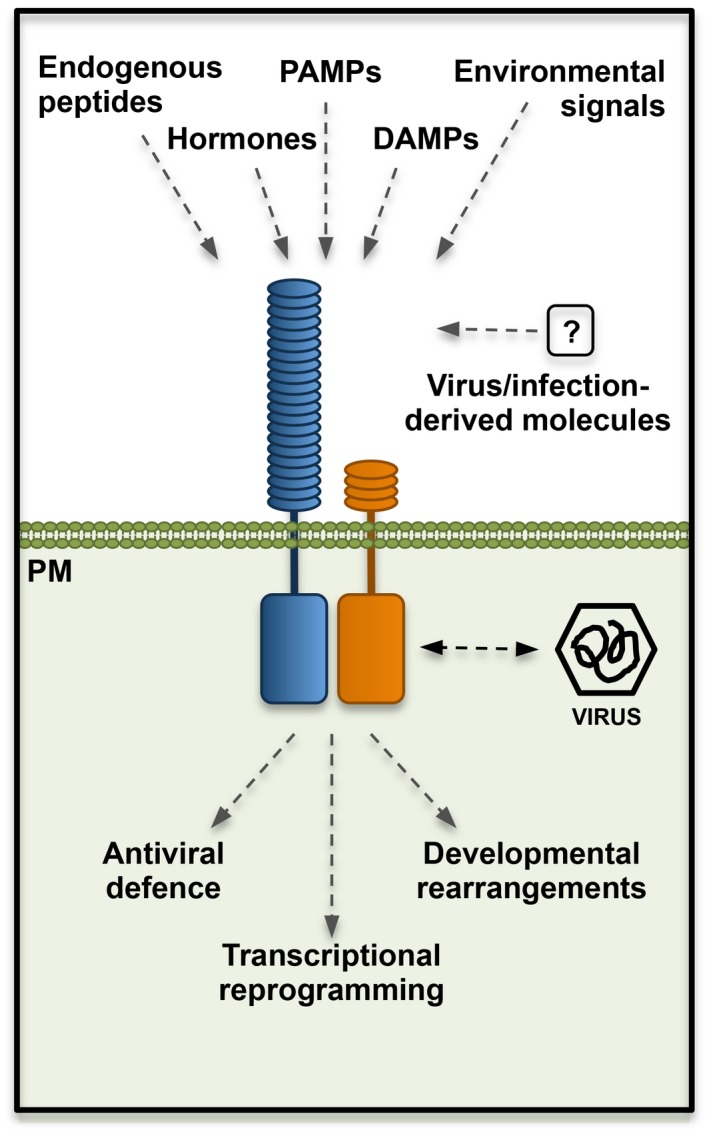
Extracellular signals potentially perceived by RLKs and potential outcomes with an impact on plant–virus interactions. These signals may include not only molecules from plant or pathogen origin, but also environmental cues. PM, plasma membrane.
Figure 2.
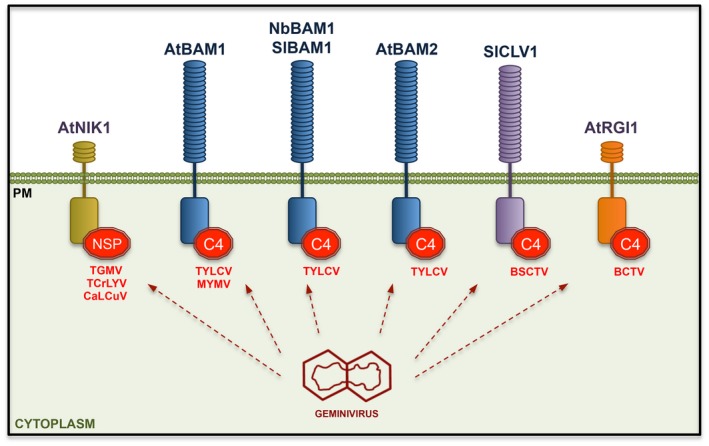
RLKs are targets of viral proteins. Different proteins from plant viruses interact with RLKs, including the specific plant and virus species employed in the corresponding studies. TGMV, Tomato golden mosaic virus (TGMV); TCrLYV, Tomato crinkle leaf yellow virus (TCrLYV); CaLCuV, Cabbage leaf curl virus; TYLCV, Tomato yellow leaf curl virus; BSCTV, Beet severe curly top virus; BCTV, Beet curly top virus.
Potential role of RLK‐mediated PAMP‐ or DAMP‐triggered immunity in antiviral defence in plants
Somatic embryogenesis receptor kinases (SERKs) are a small group of LRR RLKs that act as co‐receptors for multiple receptor kinase complexes, including those formed by most LRR‐containing PRRs identified to date, playing a major role in the activation of PTI triggered by PAMPs or DAMPs (Ma et al., 2016). SERK3/BRI1‐ASSOCIATED KINASE 1 (BAK1) is possibly the member of this family making the largest contribution to PTI (Ma et al., 2016). Importantly, a role for BAK1 in antiviral defence was demonstrated by Yang et al. (2010) and, later, by Korner et al. (2013), who showed that a bak1 Arabidopsis mutant displayed increased susceptibility to three unrelated RNA viruses, namely Oilseed rape mosaic virus (ORMV) (genus Tobamovirus), Tobacco mosaic virus (TMV) (genus Tobamovirus), and Turnip crinkle virus (TCV) (genus Carmovirus); mutation in the closest BAK1 homologue, SERK4/BKK1, also results in enhanced TCV accumulation (Yang et al., 2010). Interestingly, infection by these viruses induces the expression of the DAMP propeptide‐encoding ProPEP2 and ProPEP3, which in turn produce the immune peptide Pep1, as well as the expression of the genes encoding the Pep1 receptors, PEPR1/2, which use BAK1 as co‐receptor (Schulze et al., 2010; Postel et al., 2010). However, lack of PEPR1/2 did not affect viral accumulation, suggesting that it is the contribution of BAK1 to a signalling pathway other than PEPR1‐mediated PTI what increases viral resistance. Another interesting finding is that crude TCV viral extracts, but not purified virions, induce hallmark PTI readouts (MAPK activation, ethylene production, and inhibition of root growth) in a BAK1‐dependent manner (Korner et al., 2013), which hints at the existence of an infection‐derived molecule perceived by a BAK1‐containing receptor complex (Fig. 1). This is further supported by the observation that viral infections often result in the induction of PTI‐related genes (Whitham et al., 2003; Love et al., 2005; Carr et al., 2010; Hanssen et al., 2011; Nicaise and Candresse, 2017), and by the discovery of at least two viral proteins, the capsid protein (CP) from Plum pox virus (PPV) (genus Potyvirus) and the movement protein (MP) of Cucumber mosaic virus (CMV) (genus Cucumovirus), with PTI‐suppressing activity (Nicaise and Candresse, 2017; Kong et al., 2018). That suppressing PTI could be beneficial for an invading virus is not inconceivable, since the activation of PTI results in multiple outputs associated with plant defence, some of which may have a negative effect on the viral infection: at least in some cases, accumulation of reactive oxygen species (ROS) can have a deleterious effect on the viral invasion (Wu et al., 2017), and the activation of salicylic acid (SA) signalling has proven effective against a plethora of different plant viruses (reviewed in Alazem and Lin (2015)). Additionally, and since most plant viruses are transmitted by insect vectors, indirect effects on viral propagation should also be considered.
More challenging perhaps is to imagine what kind of viral molecule could act as a PAMP, given that a general feature of PAMPs, according to the accepted definition, is their high degree of conservation due to strong evolutionary constraints, while viral proteins mutate at a high speed – a property that underlies the limited durability of any sequence‐based antiviral resistance strategy. In this scenario, it seems more likely that if a viral PAMP exists, this is a conserved structure that can be recognized as non‐self, rather than a specific protein/peptide/nucleic acid sequence (Fig. 1). Alternatively, it could be virus‐induced DAMPs which activate PTI‐like responses, likely through their perception by PRR complexes that involve BAK1.
Interestingly, double‐stranded RNA (dsRNA) (or the dsRNA analog poly(I:C)) has been shown to trigger PTI‐like responses in both Arabidopsis and Nicotiana benthamiana (Niehl et al., 2016). Since dsRNA is generated during viral infections (as the viral genome itself, replicative intermediates, or as the result of the activation of antiviral gene silencing), but is not abundant in the plant cell in uninfected conditions, dsRNA would be a good candidate to be recognized as a signal of the viral invasion and be considered a viral PAMP. Nevertheless, perception of dsRNA/poly(I:C) depends on SERK1, and not on BAK1 or BKK1, which suggests that the previously observed involvement of the latter in antiviral defence relies on a different pathway (Yang et al., 2010; Korner et al., 2013; Niehl et al., 2016); whether SERK1 has a general antiviral role remains to be determined. Additionally, whether perception of dsRNA is intracellular or extracellular, and, in the latter case, how the virus‐derived molecules would reach the apoplast in sufficient amounts to be detected, is still unclear.
Taken together, and although fragmentary, previous work suggests that plant viruses might be both targets and suppressors of PTI or PTI‐associated responses. However, at present, alternative options cannot be excluded. For example, BAK1 contributes to several other RLK‐mediated signalling pathways (Ma et al., 2016) and has been shown to regulate jasmonic acid (JA) content in Nicotiana attenuata (Yang et al., 2011); this or a similar, yet unknown, role of BAK1 on other, non‐PTI‐related pathways may underlie its effect on the viral infection. The viral susceptibility phenotype of mutants in other PTI components is inconsistent (Korner et al., 2013; Nicaise and Candresse, 2017), which may nevertheless reflect different virus invasion strategies. A more systematic, comprehensive analysis, including RNA and DNA viruses belonging to different families, will be required to gain a global overview of the potential interplay between plant viruses and PTI responses.
Receptor‐like kinases targeted by viral proteins
In recent years, perhaps unexpectedly, several RLKs have been identified as direct targets of viral proteins (Fig. 2), and this number is likely to increase in the near future. In some cases, the biological function of the targeted RLK in plant–virus interactions has been unveiled, while in others it remains elusive, but whether these proteins are acting as receptors of extracellular cues during the viral infection is an open question common to all of them.
The first RLKs to be identified as interactors of a viral protein were Arabidopsis NSP‐INTERACTING KINASE 1, 2, and 3 (AtNIK1‐3), which are bound and inhibited by the nuclear shuttle protein (NSP) of bipartite geminiviruses (Tomato golden mosaic virus (TGMV), Tomato crinkle leaf yellow virus (TCrLYV), and Cabbage leaf curl virus (CaLCuV)) (Fontes et al., 2004). Interestingly, plasma membrane‐localized AtNIK1 initiates a novel antiviral response in plants, mediated by the ribosomal protein RPL10a, which results in the translational shutdown of viral genes and is suppressed by the viral NSP (Carvalho et al., 2008; Rocha et al., 2008; Zorzatto et al., 2015). Still to be elucidated are the mechanisms employed by monopartite geminiviruses, which lack an NSP, as well as viruses belonging to other families, to interfere with this signalling cascade. Of note, AtNIK1 interacts with AtBAK1 (Smakowska‐Luzan et al., 2018), raising the possibility that the previously observed contribution of AtBAK1 to antiviral immunity relies on this pathway and not PTI. However, considering that both AtNIK1 and AtBAK1 belong to the same subfamily of LRR‐RLKs (subfamily II) in Arabidopsis, and share a similar structural organization (Shiu and Bleecker, 2001), a role for NIK1 as a co‐receptor or signalling partner for other RLK(s) should not be ruled out.
Another geminiviral protein, C4/AC4, has been recently shown to bind RLKs in the CLAVATA 1 (CLV1) clade (reviewed in Zeng et al. (2018)). C4 from Tomato yellow leaf curl virus (TYLCV) and Mungbean yellow mosaic virus (MYMV) interacts with Arabidopsis BARELY ANY MERISTEM 1 (AtBAM1) and, at least in the case of TYLCV, its close homologue AtBAM2 (Carluccio et al., 2018; Rosas‐Diaz et al., 2018). C4 from TYLCV also interacts with the BAM1 orthologs from tomato and N. benthamiana (Rosas‐Diaz et al., 2018). The interaction between C4 and BAM1/2 is particularly strong at plasmodesmata (PD) and, strikingly, BAM1/2 seem to be required for the cell‐to‐cell spread of RNA interference (RNAi) from the vasculature, a function suppressed by C4 (Carluccio et al., 2018; Rosas‐Diaz et al., 2018). Since RNAi is considered a key antiviral mechanism in plants, it is tempting to speculate that BAM1/2 or other components involved in the spread of RNAi through the PD will be convergently targeted by proteins encoded by diverse viruses, something that remains to be seen. However, it is worth noting that, at present, an alternative function of BAM1/2 on the viral infection, e.g. in viral movement through the PD, cannot be excluded.
The C4 protein from Beet severe curly top virus (BSCTV), on the other hand, can interact with SlCLV1, and this has been suggested to potentially underpin symptom development (Li et al., 2018); however, the functional relevance of this interaction is still poorly understood. Interestingly, C4 from yet another geminivirus, Beet curly top virus (BCTV), was previously found to bind the Arabidopsis RLK RGF INSENSITIVE 1 (AtRGI1) (Piroux et al., 2007), although this interaction was not characterized further. Taken together, these results suggest that geminivirus C4 might target an array of phylogenetically related RLKs (Zeng et al., 2018), although the number and significance of these intriguing interactions would need to be globally addressed.
Outlook
A growing body of data generated in the past few years seems to point at a potential role of RLKs in plant–virus interactions, which may occur at different levels: RLKs may mediate antiviral PTI‐like or alternative defence responses, activate developmental pathways re‐wired by the viral infection, or facilitate the cell‐to‐cell movement of antiviral signals or the virus itself, among other things. Nevertheless, the results currently available in the literature are rather fragmentary and getting a global overview of how different RLKs contribute to the viral infection will necessarily require a more comprehensive and integrative approach, in which viruses belonging to different families, with diverse replication and transmission strategies, are tested and compared. Additionally, and since fast evolution makes viral proteins excellent probes for plant functions, the identification and study of RLKs targeted by viruses might help uncover new roles of RLKs with an impact on the viral invasion of the plant – a potentiality that has already materialized in the novel functions uncovered for NIK1 and BAM1/2.
Acknowledgements
Work in APM’s laboratory is funded by the Shanghai Center for Plant Stress Biology (Chinese Academy of Sciences, CAS) and the Chinese 1000 Talents Program; work in RL‐D’s laboratory is funded by the Shanghai Center for Plant Stress Biology (CAS), the 100 Talent Program from CAS, and the National Natural Science Foundation of China (NSFC) (Grant 31671994). We would like to thank Jose Rufian Plaza for critical reading of the manuscript and useful suggestions. We apologize to colleagues whose work could not be cited due to space limitation.
Contributor Information
Alberto P. Macho, Email: alberto.macho@sibs.ac.cn.
Rosa Lozano‐Duran, Email: lozano-duran@sibs.ac.cn.
References
- Alazem, M. and Lin, N.S. (2015) Roles of plant hormones in the regulation of host–virus interactions. Mol. Plant Pathol. 16, 529–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiden, M. and Simon, R. (2016) Q&A: How does peptide signaling direct plant development? BMC Biol. 14, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calil, I.P. and Fontes, E.P.B. (2017) Plant immunity against viruses: antiviral immune receptors in focus. Ann. Bot. 119, 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carluccio, A.V. , Prigigallo, M.I. , Rosas‐Diaz, T. , Lozano‐Duran, R. and Stavolone, L. (2018) S‐acylation mediates mungbean yellow mosaic virus AC4 localization to the plasma membrane and in turns gene silencing suppression. PLoS Pathog. 14, e1007207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr, J.P. , Lewsey, M.G. and Palukaitis, P. (2010) Signaling in induced resistance. Adv. Virus Res. 76, 57–121. [DOI] [PubMed] [Google Scholar]
- Carvalho, C.M. , Santos, A.A. , Pires, S.R. , Rocha, C.S. , Saraiva, D.I. , Machado, J.P. , Mattos, E.C. , Fietto, L.G. and Fontes, E.P. (2008) Regulated nuclear trafficking of rpL10A mediated by NIK1 represents a defense strategy of plant cells against virus. PLoS Pathog. 4, e1000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes, E.P. , Santos, A.A. , Luz, D.F. , Waclawovsky, A.J. and Chory, J. (2004) The geminivirus nuclear shuttle protein is a virulence factor that suppresses transmembrane receptor kinase activity. Genes Dev. 18, 2545–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gish, L.A. and Clark, S.E. (2011) The RLK/Pelle family of kinases. Plant J. 66, 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia, B.C. , Calil, I.P. , Machado, J.P. , Santos, A.A. and Fontes, E.P. (2016) Immune receptors and co‐receptors in antiviral innate immunity in plants. Front Microbiol. 7, 2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen, I.M. , van Esse, H.P. , Ballester, A.R. , Hogewoning, S.W. , Parra, N.O. , Paeleman, A. , Lievens, B. , Bovy, A.G. and Thomma, B.P. (2011) Differential tomato transcriptomic responses induced by pepino mosaic virus isolates with differential aggressiveness. Plant Physiol. 156, 301–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Y. , Zhou, J. , Shan, L. and Meng, X. (2018) Plant cell surface receptor‐mediated signaling ‐ a common theme amid diversity. J. Cell Sci. 131, jcs209353. 10.1242/jcs.209353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, J. , Wei, M. , Li, G. , Lei, R. , Qiu, Y. , Wang, C. , Li, Z.H. and Zhu, S. (2018) The cucumber mosaic virus movement protein suppresses PAMP‐triggered immune responses in Arabidopsis and tobacco. Biochem. Biophys. Res. Commun. 498, 395–401. [DOI] [PubMed] [Google Scholar]
- Korner, C.J. , Klauser, D. , Niehl, A. , Dominguez‐Ferreras, A. , Chinchilla, D. , Boller, T. , Heinlein, M. and Hann, D.R. (2013) The immunity regulator BAK1 contributes to resistance against diverse RNA viruses. Mol. Plant Microbe Interact, 26, 1271–1280. [DOI] [PubMed] [Google Scholar]
- Li, H. , Zeng, R. , Chen, Z. , Liu, X. , Cao, Z. , Xie, Q. , Yang, C. and Lai, J. (2018) S‐acylation of a geminivirus C4 protein is essential for regulating the CLAVATA pathway in symptom determination. J. Exp. Bot. 69, 4459–4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love, A.J. , Yun, B.W. , Laval, V. , Loake, G.J. and Milner, J.J. (2005) Cauliflower mosaic virus, a compatible pathogen of Arabidopsis, engages three distinct defense‐signaling pathways and activates rapid systemic generation of reactive oxygen species. Plant Physiol. 139, 935–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, X. , Xu, G. , He, P. and Shan, L. (2016) SERKing coreceptors for receptors. Trends Plant Sci., 21, 1017–1033. [DOI] [PubMed] [Google Scholar]
- Macho, A.P. and Zipfel, C. (2014) Plant PRRs and the activation of innate immune signaling. Mol. Cell, 54, 263–272. [DOI] [PubMed] [Google Scholar]
- Nicaise, V. and Candresse, T. (2017) Plum pox virus capsid protein suppresses plant pathogen‐associated molecular pattern (PAMP)‐triggered immunity. Mol. Plant Pathol. 18, 878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehl, A. , Wyrsch, I. , Boller, T. and Heinlein, M. (2016) Double‐stranded RNAs induce a pattern‐triggered immune signaling pathway in plants. New Phytol. 211, 1008–1019. [DOI] [PubMed] [Google Scholar]
- Piroux, N. , Saunders, K. , Page, A. and Stanley, J. (2007) Geminivirus pathogenicity protein C4 interacts with Arabidopsis thaliana shaggy‐related protein kinase AtSKeta, a component of the brassinosteroid signalling pathway. Virology, 362, 428–440. [DOI] [PubMed] [Google Scholar]
- Postel, S. , Küfner, I. , Beuter, C. , Mazzotta, S. , Schwedt, A. , Borlotti, A. , Halter, T. , Kemmerling, B. and Nürnberger, T. (2010) The multifunctional leucine‐rich repeat receptor kinase BAK1 is implicated in Arabidopsis development and immunity. Eur. J. Cell Biol. 89 169–174. [DOI] [PubMed] [Google Scholar]
- Rocha, C.S. , Santos, A.A. , Machado, J.P. and Fontes, E.P. (2008) The ribosomal protein L10/QM‐like protein is a component of the NIK‐mediated antiviral signaling. Virology, 380, 165–169. [DOI] [PubMed] [Google Scholar]
- Rosas‐Diaz, T. , Zhang, D. , Fan, P. , Wang, L. , Ding, X. , Jiang, Y. , Jimenez‐Gongora, T. , Medina‐Puche, L. , Zhao, X. , Feng, Z. , Zhang, G. , Liu, X. , Bejarano, E.R. , Tan, L. , Zhang, H. , Zhu, J.K. , Xing, W. , Faulkner, C. , Nagawa, S. and Lozano‐Duran, R. (2018) A virus‐targeted plant receptor‐like kinase promotes cell‐to‐cell spread of RNAi. Proc. Natl. Acad. Sci. USA, 115, 1388–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze, B. , Mentzel, T. , Jehle, A.K. , Mueller, K. , Beeler, S. , Boller, T. , Felix, G. and Chinchilla, D. (2010) Rapid heteromerization and phosphorylation of ligand‐activated plant transmembrane receptors and their associated kinase BAK1. J. Biol. Chem. 285, 9444–9451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu, S.H. and Bleecker, A.B. (2001) Receptor‐like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc. Natl. Acad. Sci. USA, 98, 10763–10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smakowska‐Luzan, E. , Mott, G.A. , Parys, K. , Stegmann, M. , Howton, T.C. , Layeghifard, M. , Neuhold, J. , Lehner, A. , Kong, J. , Grunwald, K. , Weinberger, N. , Satbhai, S.B. , Mayer, D. , Busch, W. , Madalinski, M. , Stolt‐Bergner, P. , Provart, N.J. , Mukhtar, M.S. , Zipfel, C. , Desveaux, D. , Guttman, D.S. and Belkhadir, Y. (2018) An extracellular network of Arabidopsis leucine‐rich repeat receptor kinases. Nature, 553, 342–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, D. , Wang, G. and Zhou, J.M. (2017) Receptor kinases in plant‐pathogen interactions: more than pattern recognition. Plant Cell, 29, 618–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham, S.A. , Quan, S. , Chang, H.S. , Cooper, B. , Estes, B. , Zhu, T. , Wang, X. and Hou, Y.M. (2003) Diverse RNA viruses elicit the expression of common sets of genes in susceptible Arabidopsis thaliana plants. Plant J. 33, 271–283. [DOI] [PubMed] [Google Scholar]
- Wu, J. , Yang, R. , Yang, Z. , Yao, S. , Zhao, S. , Wang, Y. , Li, P. , Song, X. , Jin, L. , Zhou, T. , Lan, Y. , Xie, L. , Zhou, X. , Chu, C. , Qi, Y. , Cao, X. and Li, Y. (2017) ROS accumulation and antiviral defence control by microRNA528 in rice. Nat. Plants, 3, 16203. [DOI] [PubMed] [Google Scholar]
- Yang, D.H. , Hettenhausen, C. , Baldwin, I.T. and Wu, J. (2011) BAK1 regulates the accumulation of jasmonic acid and the levels of trypsin proteinase inhibitors in Nicotiana attenuata's responses to herbivory. J. Exp. Bot. 62, 641–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H. , Gou, X. , He, K. , Xi, D. , Du, J. , Lin, H. and Li, J. (2010) BAK1 and BKK1 in Arabidopsis thaliana confer reduced susceptibility to turnip crinkle virus. Eur. J. Plant Pathol. 127, 149–156. [Google Scholar]
- Yang, Z. and Li, Y. (2018) Dissection of RNAi‐based antiviral immunity in plants. Curr. Opin. Virol. 32, 88–99. [DOI] [PubMed] [Google Scholar]
- Ye, Y. , Ding, Y. , Jiang, Q. , Wang, F. , Sun, J. and Zhu, C. (2017) The role of receptor‐like protein kinases (RLKs) in abiotic stress response in plants. Plant Cell Rep. 36, 235–242. [DOI] [PubMed] [Google Scholar]
- Zeng, R. , Liu, X. , Yang, C. and Lai, J. (2018) Geminivirus C4: interplaying with receptor‐like kinases. Trends Plant Sci. 23, 1044–1046. [DOI] [PubMed] [Google Scholar]
- Zipfel, C. (2014) Plant pattern‐recognition receptors. Trends Immunol. 35, 345–351. [DOI] [PubMed] [Google Scholar]
- Zorzatto, C. , Machado, J.P. , Lopes, K.V. , Nascimento, K.J. , Pereira, W.A. , Brustolini, O.J. , Reis, P.A. , Calil, I.P. , Deguchi, M. , Sachetto‐Martins, G. , Gouveia, B.C. , Loriato, V.A. , Silva, M.A. , Silva, F.F. , Santos, A.A. , Chory, J. and Fontes, E.P. (2015) NIK1‐mediated translation suppression functions as a plant antiviral immunity mechanism. Nature, 520, 679–682. [DOI] [PMC free article] [PubMed] [Google Scholar]


