Abstract
After 15 years of research into Alzheimer's disease (AD) therapeutics, including billions of US dollars provided by federal agencies, pharmaceutical companies, and private foundations, there are still no meaningful therapies that can delay the onset or slow the progression of AD. An understanding of the proteolytic processing of amyloid precursor protein (APP) and the hypothesis that pathogenic mechanisms in familial and sporadic forms of AD are very similar led to the assumption that pharmacological inhibition of secretases or immunological approaches to clear amyloid depositions in the brain would have been the core to drug discovery strategies and successful therapies. However, there are other understudied approaches including targeting genes, gene networks, and metabolic pathways outside the proteolytic processing of APP. The advancement of newly developed sequencing technologies and mass spectrometry, as well as the availability of animal models expressing human apolipoprotein E isoforms, has been critical in rationalizing additional AD therapeutics. The purpose of this review is to present one of those approaches, based on the role of ligand‐activated nuclear liver X and retinoid X receptors in the brain. This therapeutic approach was initially proposed utilizing in vitro models 15 years ago and has since been examined in numerous studies using AD‐like mouse models.
Linked Articles
This article is part of a themed section on Therapeutics for Dementia and Alzheimer's Disease: New Directions for Precision Medicine. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.18/issuetoc
Abbreviations
- AD
Alzheimer's disease
- APOE
apolipoprotein E
- APP
amyloid precursor protein
- Aβ
amyloid β
- BBB
blood–brain barrier
- CAs
cholestenoic acids
- LOAD
late‐onset Alzheimer's disease
- LXR
liver X receptors
- NR
nuclear receptor
- PS1
presenilin 1
- RXR
retinoid X receptors.
1. INTRODUCTION
Alzheimer's disease (AD) is a neurodegenerative disorder, manifested by memory deficits, profound behavioural and cognitive impairment, and characteristic brain pathology—senile plaques, containing amyloid β (Aβ) and intracellular neurofibrillary tangles, composed of hyperphosphorylated Tau. A third of older adults who are clinically asymptomatic may possess pathophysiological features of AD, including structural changes in the brain up to 20 years prior to observable symptoms. An estimated 5.7 million Americans are diagnosed with AD and this number is expected to triple by 2050 (Alzheimer's_Association, 2018).
There are two forms of AD: the first is familial, autosomal dominant disease, caused by rare mutations of the gene driving the expression of amyloid precursor protein (APP), or genes coding for presenilin 1 (PS1) or presenilin 2. Presenilins are part of the γ‐secretase complex responsible for normal and physiological cleavage of APP in its transmembrane region. The amyloidogenic pathway of APP proteolytic processing results in secretion of Aβ peptides. The cases with familial autosomal dominant form of AD are only less than 3–5% of all AD cases, and almost 30 years after the cloning of APP, we have a fairly good understanding of why and how the disease develops and progresses. Our understanding, however, about the pathogenic mechanisms of the second form of AD, which is sporadic and late in onset (LOAD), is still limited. While specific gene mutations for LOAD have not been identified, so far the inheritance of ε4 allele of apolipoprotein E (APOE) is the strongest genetic risk factor for LOAD (Corder et al., 1993). However, the molecular and cellular mechanisms behind the risk conferred by APOEε4 and APOE4 protein remain elusive. The risk is dose‐dependent such that one copy of APOE4 confers 2–3 times the risk for LOAD, while two copies of APOE4 confer 12 times the risk for LOAD, lowers the age of onset, and is associated with a much more aggressive course of pathology (see also Kanekiyo, Xu, & Bu, 2014). It is important to underline that the cognitive deficits and pathology of the familial and LOAD at autopsy are indistinguishable.
The main function of APOE, primarily secreted by astrocytes in the CNS, is the transport of brain cholesterol and phospholipids. Based on a number of studies, it has been established that the lipidation status of APOE significantly impacts amyloid deposition (DeMattos et al., 2004; Fagan et al., 2004; Fryer et al., 2003; Hirsch‐Reinshagen et al., 2004; Hirsch‐Reinshagen et al., 2005; Jiang et al., 2008; Wahrle et al., 2004; Wahrle et al., 2005; Wellington et al., 2002). The lipidation of APOE is a complex biochemical process at the cell surface membrane and a prerequisite for its normal function in the brain. In AD, two contrasting roles for APOE were proposed that depend on its lipidation status: (a) a role as a pathological chaperone affecting Aβ aggregation (LaDu et al., 1994; LaDu et al., 1995) and (b) a role in Aβ clearance through the blood–brain barrier (BBB) and Aβ degradation by astrocytes and microglia (Koistinaho et al., 2004; Wyss‐Coray et al., 2003). For both processes, it is thought that internalization of APOE‐Aβ complexes is mediated through receptor binding—a process that poorly recognizes non‐lipidated APOE (Ruiz et al., 2005). In support of this hypothesis, studies have shown that Aβ binding to lipid‐poor APOE facilitates Aβ aggregation, while binding to lipidated APOE has an inhibitory effect (LaDu et al., 1994; LaDu et al., 1995). Utilizing multiple sample preparations and model systems, Verghese et al. (2013) found that only a small percentage of soluble Aβ directly interacts with APOE. The physiological relevance of this small population of APOE‐Aβ complexes is not known or how in the long term this could impact the overall progression of amyloid pathology. Importantly, they still observed that APOE isoforms and lipidation states influence the clearance of Aβ by astrocytes, in a mouse model assessed by in vivo microdialysis. They propose this modulation of Aβ clearance by APOE is through their interactions with cellular receptors, such as LDL receptor‐related protein 1, transporters, or membrane surfaces. Further work is needed to determine the extent of these interactions and possible mechanisms by which APOE modulates Aβ clearance that can then be therapeutically targeted.
The well‐understood proteolytic processing of APP, the importance of β‐ and γ‐secretases in this process (Selkoe, 2001), and the similarities in amyloid deposition and Tau pathology between familial AD and LOAD led to the assumption that the inhibition of APP processing and clearance of Aβ species and plaques in the brain of LOAD patients would have been the core of drug discovery strategies and successful therapies (Michaelson, 2014). This assumption has yet to produce any meaningful therapies and, at this time, there are no therapies that delay the age of onset or diminish the progression of AD. There must be other AD therapeutic approaches, including the targeting of genes, gene networks, and metabolic pathways outside APP proteolytic processing—that at the time were understudied and, thus, initially regarded not worth pursuing. The advancement of newly developed sequencing technologies and mass spectrometry, as well as the availability of animal models expressing human APOE isoforms, has been critical in rationalizing additional directions in AD therapeutics. The purpose of this review is to present one of those approaches, based on a better understanding of the role of ligand‐activated nuclear liver X and retinoid X receptors (LXR/RXR) in the brain. This therapeutic approach was suggested and demonstrated in vivo more than 10 years ago (Koldamova et al., 2005) and has been supported by numerous in vitro and in vivo studies.
2. NUCLEAR RECEPTORS
Members of the nuclear receptor (NR) superfamily are ligand‐activated transcription factors characterized by the unique property of directly binding to DNA response elements. They control the expression of specific genes, thereby regulating multiple developmental, homeostatic, and metabolic processes (Evans & Mangelsdorf, 2014; Olivares, Moreno‐Ramos, & Haider, 2015; Whitney et al., 2002). The NR superfamily are represented by 48 members in human, 49 in mouse, and more than 270 genes in Caenorhabditis elegans. The NR superfamily share a common structural organization, with most containing six domains: two regulatory transactivation domains—one at the N‐terminus, AF‐1, and another at the C‐terminus, AF‐2, separated by a DNA‐binding domain, a domain involved in dimerization, and a ligand‐binding domain. The C‐terminal region downstream from the AF‐2, called F‐domain, is highly variable and exists only in some of the receptor subfamilies (Figure 1). A unified nomenclature of the NR was proposed and accepted in 1999 and is based on the phylogenetic tree connecting 65 of the known NR genes (Nuclear Receptors Nomenclature Committee, 1999). The nomenclature separates the NR into subfamilies, groups, and numbered genes within each of the groups and allows the incorporation of any number of new members of the corresponding subfamily (Benoit et al., 2006; Gustafsson, 2016; Olivares et al., 2015). Clustering of mammalian NRs into four major classes is still widely accepted and is based on the dimerization status and DNA‐binding properties of each receptor using its trivial name. Thus, the NR superfamily can be divided into subtypes: type I NRs, also called steroid receptors, which bind as homodimers to a palindromic DNA response element sequence of two half sites. Steroid hormone ligand binding to type I NRs causes the dissociation of heat shock proteins, homodimerization, cytosolic translocation into the nucleus, and binding to hormone response elements within the promoter of a target gene. In contrast, type II nonsteroidal NRs, including PPARα, β, and γ; RXRα, β, and γ; retinoic acid receptor (RARα, β, and γ); and LXRα and β, bind as RXR heterodimers to RXR direct repeats. They are retained in the nucleus regardless of the ligand‐binding status and in the absence of ligands are tightly bound to a corepressor complex (NCoR or SMRT and HDAC3). Ligand binding to type II NRs results in a functional transcription factor, dissociation of the corepressor proteins, chromatin remodelling, and transcriptional activation (Robinson‐Rechavi, Garcia, & Laudet, 2003). Furthermore, it was postulated that ligand binding to PPARs and LXRs induces an allosteric change in the receptor that results in SUMOylation response. This SUMOylated PPAR or LXR monomer stabilizes repressive nuclear complexes on the promoters of inflammatory genes (reviewed in Glass & Saijo, 2010). Although there is evidence in in vitro macrophages that supports this model (Ghisletti et al., 2009), the requirement for receptor SUMOylation for the repressive actions of PPARs and LXRs on inflammation needs validation in animal models. This could be an important pathway to further explore given the chronic inflammatory state observed in the brains of AD patients. Class III receptors bind as homodimers to direct repeats. An important member of this class are RXRs. Class IV receptors bind as monomers to a single response element sequence half site and their ligands are still unknown (Mangelsdorf et al., 1995; Mangelsdorf & Evans, 1995; Olivares et al., 2015). Despite the fact that most NRs show widespread expression in the brain, including high expression of members of the PPAR, RXR, and LXR, we are only beginning to understand their roles in development, behaviour, neurological and psychiatric disorders, and neurodegenerative disease (Gofflot et al., 2007).
Figure 1.
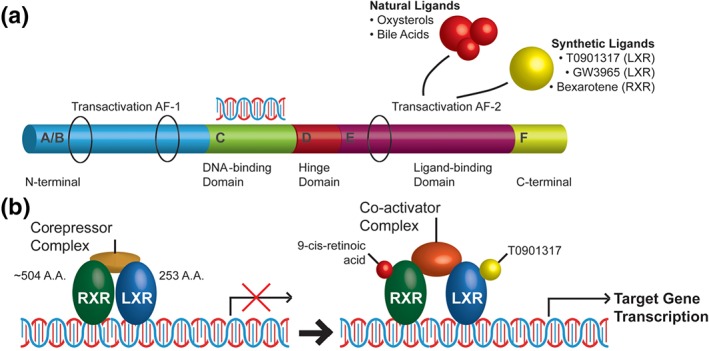
Molecular structure and ligand activation of nuclear receptors. (a) Nuclear receptors share a common structural organization with six domains (A through F): two regulatory transactivation domains, one at the N‐terminus, AF‐1, and another—AF‐2, at the C‐terminus. AF‐1 and AF‐2 are separated by DNA‐binding domain, a hinge—domain involved in dimerization, and a ligand‐binding domain; the C‐terminal, F‐region downstream from AF‐2, is highly variable and exists only in some of the receptor subfamilies. (b) LXR/RXR heterodimers in the absence of a ligand are tightly bound to a corepressor complex and a response element, usually upstream of the target gene. Ligand binding results in conformational change of the heterodimer, dissociation of the corepressor complex, recruitment of co‐activators, chromatin remodelling, and transcriptional activation
3. LIVER X RECEPTORS
3.1. LXR in the brain—Role in development and lipid homeostasis
LXRs—LXRα and LXRβ—are key transcriptional regulators of cholesterol metabolism, lipogenesis, and inflammation, acting as sterol sensors protecting cells from cholesterol burden by stimulating the first step of reverse cholesterol transport—cholesterol efflux and lipidation of apolipoproteins at the cell surface (Baranowski, 2008). The two LXR isoforms show unique expression patterns: the highest expression of LXRα is detected in the liver, kidney, lung, and adipose tissue, while LXRβ is ubiquitously expressed, and in the brain and spinal cord, its expression is 3–5 times higher than the expression of LXRα (Bookout et al., 2006; Gofflot et al., 2007). LXRs form permissive heterodimers with RXR, so that they may be activated by either LXR or RXR ligands, and simultaneous binding of both agonists usually elicits a stronger response (Figure 1). Their endogenous agonists include multiple oxidized cholesterol derivatives, oxysterols—24(S)‐hydroxycholesterol, 22(R)‐hydroxycholesterol 24(S), 25‐epoxycholesterol, and 27‐hydroxycholesterol. Cytochrome P450 enzyme—cholesterol 24‐hydroxylase (CYP46A1)—is the enzyme responsible for the generation of the most abundant endogenous agonist in the brain (McMillin & DeMorrow, 2016; Mertens, Kalsbeek, Soeters, & Eggink, 2017; Russell, 2003). An important group of endogenous LXR ligands in the brain are cholestenoic acids (CAs), derived as intermediates in the brain cholesterol metabolism. The biosynthetic enzymes that generate CAs in the mammalian CNS—CYP39A1, CYP27A1, CYP7B1, and HSD3B7—are expressed in the brain (McMillin & DeMorrow, 2016; Mertens et al., 2017; Ogundare et al., 2010; Russell, 2003; Theofilopoulos et al., 2014). Deficiencies of CYP7B1 and CYP27A1, characterized by mutations, result in hereditary neurological diseases—hereditary spastic paresis type 5 and cerebrotendinous xanthomatosis, which are clinically well defined in human patients (Arnoldi et al., 2012; Björkhem, 2013). CAs generated in the liver and intestine circulate in peripheral blood and penetrate the BBB, but since their levels in the brain are very low, a bile acid synthesis pathway in the brain has been proposed (McMillin & DeMorrow, 2016).
LXRs have many essential roles in brain development and homeostasis. LXRβ knockout mice display adult onset motor neuron degeneration with impaired motor coordination. This phenotype was associated with axonal dystrophy, astrogliosis, and intracellular accumulation of lipids (Andersson, Gustafsson, Warner, & Gustafsson, 2005). Animals lacking both LXRα and β have a variety of abnormalities in the brain including closing of the lateral ventricles, which are lined with cells containing lipid droplets, enlarged vasculature, loss of neurons, astrogliosis, and myelin disorganization (Wang et al., 2002). The brain is the most cholesterol‐rich organ, with cholesterol vital for brain development and normal adult function. Cholesterol synthesis and metabolism in the brain is largely separated from that of peripheral tissue by the BBB, which is impermeable to lipoproteins, preventing blood cholesterol from reaching the brain parenchyma. During development, astrocytes and neurons produce abundant amounts of cholesterol; however, as the brain matures, neurons down‐regulate many of the genes important for cholesterol synthesis, leaving adult neurons reliant on cholesterol synthesized mainly by astrocytes to maintain membrane plasticity and cellular function (Baranowski, 2008). The neurons still have an important role in cholesterol homeostasis by metabolizing cholesterol to 24(S)‐hydroxycholesterol. This metabolite is freely released from neurons and can cross the BBB allowing for removal of excess cholesterol from the brain.
In the brain, cholesterol is transported between astrocytes and neurons or oligodendrocytes as a component of HDL‐like particles with APOE secreted mostly by astrocytes as the major apolipoprotein component in the CNS in a process similar to reverse cholesterol transport: the difference is that cholesterol transported by HDL‐like particles does not reach the liver. Lipoproteins produced and released by astrocytes are discoidal in shape, and along with APOE, they contain phospholipids and cholesterol but lack the core lipids (cholesterol esters or triglycerides), which are present in peripheral HDL. Cholesterol and phospholipids transport in the brain depends on ABCA1, a member of the ATP‐binding cassette family of transporters, which facilitates the efflux of cellular cholesterol and phospholipids through the cell membrane onto extracellular lipid‐poor APOE to form pre‐HDL‐like particles (Lee & Parks, 2005; Van Eck, Pennings, Hoekstra, Out, & Van Berkel, 2005). The initial lipidation of APOE continues until formation of mature HDL‐like particles at the cell membrane—a process facilitated by ABCA1 and ABCG1. LXRs directly regulate the transcription of ABCA1, ABCG1, and the entire APOE gene cluster, thus the overall expression of APOE and its lipidation state. A better understanding of brain lipid metabolism has led to the assumption that the pharmacological activation of NRs—LXR/RXR—in the brain using nonsteroidal synthetic ligands may have therapeutic application.
3.2. AD‐associated therapeutic effects of ligand‐activated LXRs
Seminal articles first demonstrated that LXR activation decreased the amyloidogenic processing of APP and Aβ secretion (Burns et al., 2006; Koldamova et al., 2005; Lefterov et al., 2007; Riddell et al., 2007; Sun, Yao, Kim, & Tall, 2003). These studies demonstrated that LXR activation increased Abca1 expression and importantly reduced Aβ secretion (Sun et al., 2003). This was validated in a mouse AD model, where a short treatment with LXR agonist, T0901317, in 3‐month old predepositing APP23 mice significant increased APOE and ABCA1 levels along with a related reduction in the levels of brain soluble Aβ40 and of Aβ42 (Koldamova et al., 2005). Similarly, Burns et al. (2006) demonstrated a reduction in Aβ levels both in vitro and in vivo follow treatment with T0901317, which was dependent on intact ABCA1 function. Later studies utilizing chronic LXR agonist, GW3965 or T0901317, treatment showed significantly reduced levels of insoluble Aβ (Jiang et al., 2008; Lefterov et al., 2007). The role of LXRs in amyloid deposition was validated by experiments utilizing LXRα and LXRβ knockout mice. Zelcer et al. (2007) demonstrated that endogenous LXR signalling impacts the development of AD‐related pathology. Lastly, it was shown that T0901317 treatment decreased hippocampal Aβ42 levels in Tg2576 transgenic mice and importantly reversed the contextual memory deficit observed in these mice (Riddell et al., 2007).
3.2.1. Aβ clearance, APOE, and ABCA1
The modulation of APP processing, Aβ production and clearance, and reversal of memory deficits by LXR ligands has inspired a whole research direction exploring the potential of LXR agonists to diminish AD pathogenesis. This has resulted in a number of articles exploring the potential of LXR agonists to improve cognitive performance, increase the clearance of Aβ, and diminish senile plaque levels. In the past 10 years, we and others using a number of different in vivo models and behavioural paradigms have validated the initial findings that LXR agonists are able to reverse the cognitive impairments in AD model mice (Carter et al., 2017; Donkin et al., 2010; Fitz et al., 2010; Fitz et al., 2014; Vanmierlo et al., 2011; Wesson et al., 2011). The ability of LXR agonists to reverse cognitive deficits in a mouse AD model appears to be reliant on intact ABCA1 (Carter et al., 2017; Donkin et al., 2010; Fitz et al., 2010; Sandoval‐Hernandez et al., 2016). Donkin et al. (2010) presented that an 8‐week treatment of a high GW3965 dose during the early stages of amyloid deposition could diminish the levels of senile plaques in an APP/PS1 mouse model, which was dependent on functioning Abca1. It is hypothesized that LXR agonists improve memory in AD rodent models through increasing the levels and lipidation states of APOE that consequently increases Aβ clearance across the BBB. For example, employing microdialysis of freely moving mice, we have shown that a short 15‐day treatment with a LXR ligand significantly reduced Aβ levels in the interstitial fluid of predepositing APP23 mice (Fitz et al., 2014), suggesting increased clearance of soluble forms of Aβ. In APP23 mice, 7 weeks of T0901317 significantly reduced the Thioflavin S‐positive plaque area in the brain (Terwel et al., 2011). Cognitive performance was restored; however, the levels of plaques were unchanged following T0901317 treatment in APP/PS1 mice that express human APOE3 or APOE4 and are haplodeficient in Abca1 (Carter et al., 2017). Again suggesting the importance of Abca1 in the mechanism of action of LXR agonists. Interestingly, there was a significant reduction in soluble oligomeric Aβ levels in APOE4 expressing mice following this T0901317 treatment. Similarly, Vanmierlo et al. (2011) observed a reversal of hippocampal‐dependent memory deficits but no change in plaque load following T0901317 treatment in APPSLxPS1 mice. Furthermore, we have shown that the AD phenotype was exacerbated in APP23 mice fed a high‐fat/high‐cholesterol diet for 4 months. Again, T0901317 treatment reversed the memory deficits and this was correlated with a reduction in amyloid plaque load, insoluble Aβ, and soluble Aβ oligomers (Fitz et al., 2014). We hypothesize that clearance of soluble Aβ is an important response to the increased levels and lipidation of APOE. Fully lipidated APOE could also impact Aβ metabolism by decreasing Aβ aggregation, maintaining Aβ in a soluble state, and facilitating its clearance across the BBB or by glial cells.
3.2.2. Other mechanisms
In addition to increasing Aβ clearance via BBB, LXR agonists could stimulate its enzymatic degradation, and its phagocytosis by microglia (Jiang et al., 2008; Zelcer et al., 2007). Interestingly, primary microglia exposed to medium from APOE knockout astrocytes treated with T0901317 did not impact microglial phagocytosis of Aβ (Terwel et al., 2011). Furthermore, PGF2α, a major metabolite of arachidonic acid involved in the regulation of chronic and acute inflammation, was shown to effectively antagonize the activation of LXR by T0901317, reducing Aβ clearance by microglia (Zhuang et al., 2013). This suggests that the increased APOE levels and lipidation following treatment with T0901317 can impact the Aβ phagocytic capacity of microglia and clearance rate of Aβ. Likewise, in APP23 mice with substantial plaque deposition and presence of plaque associated microglia, long‐term treatment (7 weeks) with T0901317 strongly decreased the burden of Aβ (insoluble Aβ and plaques), accompanied by a significant increase in the levels of brain ABCA1 and APOE (Terwel et al., 2011). However, the cellular accumulation of Aβ in pericytes was unchanged following LXR activation (Saint‐Pol et al., 2012), suggesting cell specific changes following LXR agonist treatment, possibly due to receptor expression.
LXR agonist treatments have other positive effects on the brain, which could modulate the overall AD pathology. In agreement with previous findings, chronic T0901317 treatment decreased Aβ levels and reversed memory loss. This effect was accompanied by a decrease in the number of GFAP‐positive, activated astrocytes and an increase in the number of cholinergic neurons in the medial septum of APP‐expressing mice (Cui et al., 2012). In a primary neuronal culture, pretreatment with GW3965 was shown to prevent the significant oligomeric Aβ‐induced reduction in the density of mature dendritic spines, synaptic contact number, and expression of pre‐ (VGlut1, SYT1) and post‐synaptic (SHANK2, NMDA) protein markers (Baez‐Becerra, Filipello, Sandoval‐Hernandez, Arboleda, & Arboleda, 2018). Twenty‐four‐month‐old 3xTg‐AD mice treated for 6 days with GW3965 demonstrated improvements in Morris water maze performance and this was accompanied by decreased DNA methylation associated mainly with neurogenesis‐associated genes (HMGB3 and RBBP7) and synapse‐related genes (SYP, SYN1, and DLG3; Sandoval‐Hernandez, Hernandez, et al., 2016). We identified an enrichment of genes associated with GO categories “Microtubule Based Process” and “Synapse Organization and Biosynthesis” in the brain of APP/PS1 mice expressing APOE4 and Abca1 haplodeficient treated with T0901317. Members of the β‐protocadherin family, which are essential in establishing functional synapses, were also up‐regulated in these APOE4 mice (Carter et al., 2017). These studies illustrate the ability of LXR agonism to impact synaptic function and integrity and furthermore alleviate the accelerated synaptopathy observed in AD and characteristic of APOE4 expression. There is accumulating evidence indicating that synaptic dysfunctions are among the earliest pathogenic events that are correlated with learning and memory losses in AD; the impact of LXR on synaptopathy is a critical direction for future research. We postulate that the increased lipidation of APOE can affect the phenotype through an increased supply of cholesterol and phospholipids to neurons—“trophic effect.”
An LXR agonist also exhibited significant beneficial effects on the disruption of the brain neurovascular unit in a mouse AD model. In very old (24 months) 3xTg‐AD mice, short‐term GW3965 treatment was able to significantly decrease astrogliosis and partially restore the hippocampal microvascular morphology by decreasing tortuosity and increasing its length (Sandoval‐Hernandez, Restrepo, Cardona‐Gomez, & Arboleda, 2016). This restoration in the microvasculature could have a significant impact on neuronal function and hippocampal‐dependent learning and memory, which is greatly impacted by AD pathology.
4. RETINOID X RECEPTORS
4.1. RXR expression in the brain and ligand activation
The mechanism of action of RXR agonists is quite complex given the ability of RXR to both homodimerize and heterodimerize with all Class II nonsteroidal NRs, including LXR (Mangelsdorf et al., 1995; Mangelsdorf & Evans, 1995). Since the detailed molecular mechanisms of NR activation are out of the scope of this review, the reader is directed to several fundamental publications (Bookout et al., 2006; Evans & Mangelsdorf, 2014; Mangelsdorf et al., 1995; Olivares et al., 2015; Robinson‐Rechavi et al., 2003). In the brain, RXRs partner mainly with LXRs, PPARs, and RARs. Three different isoforms of RXR encoded by separate genes have been identified: RXRα, RXRβ, and RXRγ, with at least one of these isoforms expressed in every cell of the body. There are two endogenous ligands that can activate RXR at very low concentrations: 9‐cis‐retinoic acid (RA) and 9‐cis‐13,14‐dihydroretinoic acid (Ruhl et al., 2015). A number of genes have been suggested as responsive to ligand‐activated RAR/RXR including cytokines (Mey, 2001) and cytokine receptors (Mey, 2006). It has also been shown that RXR agonists can increase APOE production and lipidation by modulating the expression of ABCA1 (Zhao et al., 2014). In addition, RXR/PPAR heterodimers are important in lipid metabolism, cellular proliferation, and inflammatory responses (Villapol, 2018).
4.2. RXR activation—Therapeutic effect in mouse AD model
The potential of ligand‐activated RXR as AD therapies is supported by (a) transcriptional regulation of ABCA1, ABCG1, and APOE gene and protein expression and the overall control of APOE lipidation status (Zhao et al., 2014); (b) the importance of RXR homodimers in RA‐mediated signalling, neuronal plasticity, and memory (Ruhl et al., 2015); and (c) the anti‐inflammatory properties of PPAR/RXR activating drugs and anti‐inflammatory effects of LXR/RXR in vitro and in vivo (Villapol, 2018). Cramer et al. first reported that a short 3‐day treatment with bexarotene, a selective RXR agonist approved by the FDA for treatment of cutaneous T cell lymphoma, enhanced the clearance of soluble Aβ within hours as assessed by in vivo microdialysis and correlated to increased APOE levels in predepositing APP/PS1 mice. A 7‐day treatment with bexarotene reduced the area of Thioflavin S‐positive plaques by over 50% and reversed cognition, social, and olfactory deficits in middle age and old APP/PS1 mice (Cramer et al., 2012). The original report was followed by four publications, which tried to replicate the positive results of bexarotene on AD pathology observed in the original article (Fitz, Cronican, Lefterov, & Koldamova, 2013; Price et al., 2013; Tesseur et al., 2013; Veeraraghavalu et al., 2013). We wanted to test the efficacy of bexarotene in relationship to human APOE3 and APOE4 and found that 15 days of bexarotene treatment in APP/PS1 mice expressing human APOE3 or APOE4 reversed the memory impairments to the levels of their non‐APP controls. We also observed significantly decreased levels of interstitial fluid Aβ assessed by in vivo microdialysis and cortical A11 positive oligomers (Figure 2). We were unable, however, to confirm bexarotene's effect on amyloid plaques (Fitz et al., 2013). Likewise, Veeraraghavalu et al. demonstrated the ability of bexarotene to reduce soluble Aβ40 levels in the same APP/PS1 mouse model, but treatment had no impact on plaque burden in three mouse strains that exhibit Aβ pathology (APP/PS1, 5xFAD, and APPPS1‐21). Price et al. and Tesseur et al. demonstrated RXR target enhancement but no change in amyloid burden following a similar bexarotene treatment as that used by Cramer, in mouse (APP/PS1) and beagle models respectively (Price et al., 2013; Tesseur et al., 2013). It was noted that the Price, Tesseur, and Veeraraghavalu studies used different formulations of bexarotene, which potentially alter its pharmacokinetics and bioavailability. Landreth stressed that the therapeutic potential of bexarotene was its ability to clear soluble Aβ and improve cognitive performance (Landreth et al., 2013). In agreement, other studies have shown that RXR agonists are able to increase Aβ clearance across the BBB (Bachmeier, Beaulieu‐Abdelahad, Crawford, Mullan, & Paris, 2013) and diminish the levels of interstitial Aβ (Ulrich et al., 2013) with both processes reliant on APOE expression or levels. In mice expressing endogenous APP and human APOE4, bexarotene treatment reversed APOE4‐driven accumulation of Aβ42 and hyperphosphorylated Tau in hippocampal neurons and APOE4‐induced decrease in presynaptic markers (VGluT1). In addition, and importantly, bexarotene alleviated lipidation deficiency of APOE4 and cognitive impairments in these APOE4 expressing mice (Boehm‐Cagan & Michaelson, 2014). From the studies discussed above, it appears that the effects of bexarotene require functional ABCA1. Using APP/PS1 mice with global deletion of Abca1, Corona et al. demonstrated that bexarotene treatment did not diminish the levels of soluble Aβ and had no effect on cognitive impairments (Corona, Kodoma, Casali, & Landreth, 2016). In the same study, however, the effect of bexarotene on the microglial inflammatory profiles (IL1‐β, IL‐6, TNFα, TGFβ, CCL2, and ATF3) was retained, confirming that not all of its effects are mediated through Abca1 transcriptional up‐regulation. Tai et al. demonstrated that bexarotene increased the level of APOE4 lipoprotein association/lipidation and APOE4/Aβ complex levels, which decreased oligomeric Aβ levels and improved synaptic viability in 5xFAD mice with APOE4 targeted replacement (Tai et al., 2014). They postulate that RXR agonists could address a loss of function associated with APOE4 that is exacerbated by Aβ. However, they did observe hepatomegaly even after a short‐term treatment, a potentially negative systemic side effect also observed following LXR agonist treatments. It should also be noted that an increased level of APOE in response to short‐term bexarotene treatment does not always translate to cognitive improvement, as other groups have shown using different rodent models (Balducci et al., 2015; LaClair et al., 2013; O'Hare et al., 2016).
Figure 2.
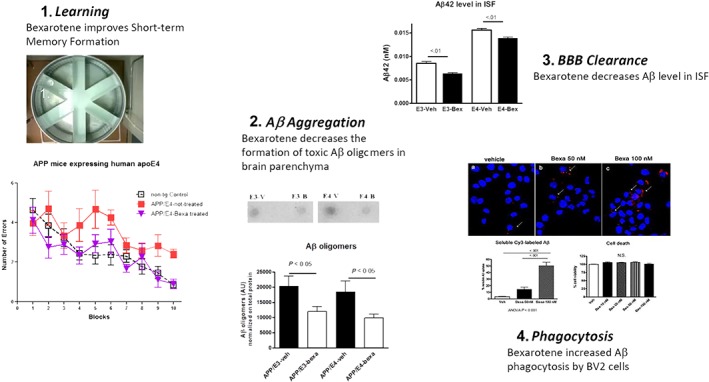
Therapeutic potential of bexarotene to ameliorate pathological phenotype of Alzheimer's disease mouse model. (1) Treatment with bexarotene (7 days) was shown to reverse cognitive decline, social, and olfactory deficits in APP/PS1 mice as well as short‐term memory deficits exhibited by APOE4 expressing mice (modified from Fitz et al., 2013). (2) Bexarotene application decreases the formation of toxic Aβ species in brain parenchyma, which have been associated with diminished cognitive performance (Fitz et al., 2013). (3) Using in vivo microdialysis groups have shown that bexarotene significantly decreases the amount of soluble Aβ in the interstitial fluid, suggesting an increased clearance rate of Aβ. This has been highlighted as one of the possible beneficial effects of bexarotene treatment on AD pathogenesis (Fitz et al., 2013). (4) The increased clearance rate of Aβ could be due to accelerated microglial phagocytosis of Aβ following treatment with bexarotene in culture (Hilyte‐labelled Aβ is phagocytosed after bexarotene treatment; Lefterov et al., 2015)
Recently, a bexarotene derivative, OAB‐14, was shown to significantly alleviate cognitive deficits in APP/PS1 mice and rapidly clear soluble Aβ and amyloid plaques by promoting microglia phagocytosis. OAB‐14 significantly increased the expression of ABCA1 and the lipidation of APOE while attenuating synaptic degeneration, neuronal loss, and neuroinflammation in APP/PS1 mice (Yuan et al., 2019). The potential mechanisms of action are also considered to be due to the ability of bexarotene to directly impact amyloid pathology: (a) altering its clearance across the BBB (Kuntz et al., 2015), (b) bexarotene can weakly bind to Aβ preventing self‐assembly (Huy et al., 2017), or (c) bexarotene binds APP inhibiting the intramembrane cleavage by γ‐secretase to release Aβ (Kamp et al., 2018). The strongest support of the assumption that the synthetic RXR ligand, bexarotene, has a therapeutic effect in AD‐like animal models, however, is the increased levels and ABCA1‐mediated lipidation of APOE following treatment.
4.2.1. Other mechanisms
RXR agonists also have the potential to modulate other AD‐associated pathologies resulting in a positive outcome following treatment. We observed in vitro the ability of bexarotene to increase the proliferation of neural progenitors and neuronal differentiation and stimulated neurite outgrowth. This effect was validated in a mouse model, demonstrating that following bexarotene treatment, both APOE3 and APOE4 mice had an increased number of neural progenitors in the dentate gyrus. Furthermore, bexarotene significantly improved the compromised dendritic structure in the hippocampus of APOE4 mice (Mounier et al., 2015). RNA‐Seq data showed an enrichment of Gene Ontology categories related to neuronal differentiation, neurite growth, and neuritogenesis in APOE4 mice treated with bexarotene. Interestingly, RNA‐Seq data demonstrated that bexarotene treatment affected Notch1 signalling known to be important in cell fate decisions in uncommitted proliferating cells and differentiation of immature neurons. Additional genes associated with changes in the Notch1 pathway following bexarotene treatment include Dlk1, nerve growth factor receptor, and EGF receptor (Mounier et al., 2015). Most studies have demonstrated that adult neurogenesis, particularly in the dentate gyrus, improves behavioural deficits in rodents (reviewed in Lepousez, Nissant, & Lledo, 2015). In this regard, it is important to emphasize that bexarotene treatment attenuated neuronal loss in the subiculum and cortex in 5xFAD mice, which correlated with significant increases in the levels of post‐synaptic marker PSD95 and the presynaptic marker synaptophysin (Mariani et al., 2017). Tachibana et al. presented the ability of bexarotene to restore the levels of post‐synaptic proteins (PSD95, GluR1, and NR1) which were observed to be diminished with age and important in synaptic plasticity (Tachibana et al., 2016). RXR agonists were also shown to have therapeutic potential in reversing the age‐associated decreases in myelin debris removal and remyelination (Natrajan et al., 2015). These studies demonstrate the ability of bexarotene‐activated RXRs to promote neuronal function, which could significantly improve cognitive deficits. It will be important for further research to determine the mechanisms of neuroprotection of bexarotene given the number of pathways that have been reported to be altered in response to ligand‐mediated RXR activation.
4.2.2. Expression of immune receptors and phagocytosis
A recent study showed that administration of agonists for LXR or RXR, GW3965 and bexarotene, increases the expression of the phagocytic receptors such as Axl, MerTK, and Trem2 in plaque‐associated macrophages with reduced plaque burden in mouse model of AD (Savage et al., 2015). TREM2 is an innate immune receptor expressed on myeloid cells including microglia and modulates microglial function—migration, survival as well as Aβ clearance (Zhao et al., 2018; Zheng et al., 2017). In an earlier study using RNA‐seq, we demonstrated that bexarotene treatment of APP/PS1 mice increased the expression of the genes involved in phagocytosis including Trem2, Tyrobp, and Apoe (Nam et al., 2016). Subsequent studies showed that an interaction of TREM2 with APOE facilitates Aβ clearance by microglia (Yeh, Wang, Tom, Gonzalez, & Sheng, 2016), while an AD‐associated R47H variant of TREM2 has an impaired APOE‐binding affinity (Atagi et al., 2015). Finally, genome‐wide changes in histone modifications and transcriptomic profiles in the brain of APP transgenic mice support the idea that ligand activation of LXR/RXR modulates the expression of genes involved in immune and inflammatory pathways and is tightly linked to the expression and function of phagocytic receptors in microglia (Nam et al., 2016).
5. FUTURE DIRECTIONS
Studies since 2003 highlight the therapeutic potential of LXR agonists to ameliorate pathological hallmarks of AD—cognitive deficits and Aβ deposition. Targeting early pathological changes with an LXR agonist and delaying AD onset even modestly will clearly have a major public health and economic impact. While many studies have shown the therapeutic potential of LXR agonists in AD, their move into clinical trials have been limited due to their documented off target effects. RXR and LXR synthetic agonists have been coupled with an undesirable increase in serum and hepatic triglyceride levels, probably through LXRα‐dependent pathways in the liver, leading to hepatic steatosis (Grefhorst et al., 2002; Tai et al., 2014). There is an increased need for developing second generation LXR and RXR agonists, which would affect AD pathogenesis without the negative peripheral side effects (reviewed in Komati et al., 2017). This could be achieved through development of highly selective LXRβ agonists or improved permeability across the BBB. An example of this potential is a recently developed LXRβ selective agonist, which increased levels of ABCA1 and soluble APOE with diminished levels of soluble Aβ. Mice treated with this LXRβ selective agonist failed to exhibit the increased liver fat content observed with nonselective LXR agonist (Stachel et al., 2016).
5.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Cidlowski et al., 2017; Alexander, Fabbro et al., 2017; Alexander, Kelly et al., 2017).
ACKNOWLEDGEMENTS
This research was funded by the Alzheimer's Association (AARF‐16‐443213 KN and AARG‐590509 NFF), NIA (K01AG044490 NFF, R01AG057565 IL & RK, R56AG057565 IL & RK and RF1AG056371 IL & RK) and NIEHS (R01ES024233 IL & RK).
Fitz NF, Nam KN, Koldamova R, Lefterov I. Therapeutic targeting of nuclear receptors, liver X and retinoid X receptors, for Alzheimer's disease. Br J Pharmacol. 2019;176:3599–3610. 10.1111/bph.14668
All authors participated in literature review and composing this review.
Contributor Information
Nicholas F. Fitz, Email: nffitz@pitt.edu.
Iliya Lefterov, Email: iliyal@pitt.edu.
REFERENCES
- Alexander, S. P. , Cidlowski, J. A. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The concise guide to PHARMACOLOGY 2017/18: Nuclear hormone receptors. British Journal of Pharmacology, 174(Suppl 1), S208–S224. 10.1111/bph.13880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174(S1), S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators (2017). THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Transporters. British Journal of Pharmacology, 174(S1), S360–S446. 10.1111/bph.13883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer's_Association (2018). 2018 Alzheimer's disease facts and figures (vol 14, pg 367, 2018). Alzheimers Dement, 14, 701–701. [Google Scholar]
- Andersson, S. , Gustafsson, N. , Warner, M. , & Gustafsson, J. A. (2005). Inactivation of liver X receptor β leads to adult‐onset motor neuron degeneration in male mice. Proceedings of the National Academy of Sciences of the United States of America, 102, 3857–3862. 10.1073/pnas.0500634102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoldi, A. , Crimella, C. , Tenderini, E. , Martinuzzi, A. , D'Angelo, M. G. , Musumeci, O. , … Bassi, M. T. (2012). Clinical phenotype variability in patients with hereditary spastic paraplegia type 5 associated with CYP7B1 mutations. Clinical Genetics, 81, 150–157. 10.1111/j.1399-0004.2011.01624.x [DOI] [PubMed] [Google Scholar]
- Atagi, Y. , Liu, C. C. , Painter, M. M. , Chen, X. F. , Verbeeck, C. , Zheng, H. , … Bu, G. (2015). Apolipoprotein E is a ligand for triggering receptor expressed on myeloid cells 2 (TREM2). The Journal of Biological Chemistry, 290, 26043–26050. 10.1074/jbc.M115.679043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmeier, C. , Beaulieu‐Abdelahad, D. , Crawford, F. , Mullan, M. , & Paris, D. (2013). Stimulation of the retinoid X receptor facilitates β‐amyloid clearance across the blood‐brain barrier. Journal of Molecular Neuroscience: MN, 49, 270–276. 10.1007/s12031-012-9866-6 [DOI] [PubMed] [Google Scholar]
- Baez‐Becerra, C. , Filipello, F. , Sandoval‐Hernandez, A. , Arboleda, H. , & Arboleda, G. (2018). Liver X receptor agonist GW3965 regulates synaptic function upon amyloid β exposure in hippocampal neurons. Neurotoxicity Research, 33, 569–579. 10.1007/s12640-017-9845-3 [DOI] [PubMed] [Google Scholar]
- Balducci, C. , Paladini, A. , Micotti, E. , Tolomeo, D. , La Vitola, P. , Grigoli, E. , … Forloni, G. (2015). The continuing failure of bexarotene in Alzheimer's disease mice. Journal of Alzheimer's Disease: JAD, 46, 471–482. 10.3233/JAD-150029 [DOI] [PubMed] [Google Scholar]
- Baranowski, M. (2008). Biological role of liver X receptors. Journal of Physiology and Pharmacology: An Official Journal of the Polish Physiological Society, 59(Suppl 7), 31–55. [PubMed] [Google Scholar]
- Benoit, G. , Cooney, A. , Giguere, V. , Ingraham, H. , Lazar, M. , Muscat, G. , … Laudet, V. (2006). International union of pharmacology. LXVI. Orphan nuclear receptors. Pharmacological Reviews, 58, 798–836. 10.1124/pr.58.4.10 [DOI] [PubMed] [Google Scholar]
- Björkhem, I. (2013). Cerebrotendinous xanthomatosis. Current Opinion in Lipidology, 24, 283–287. 10.1097/MOL.0b013e328362df13 [DOI] [PubMed] [Google Scholar]
- Boehm‐Cagan, A. , & Michaelson, D. M. (2014). Reversal of apoE4‐driven brain pathology and behavioral deficits by bexarotene. The Journal of Neuroscience, 34, 7293–7301. 10.1523/JNEUROSCI.5198-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookout, A. L. , Jeong, Y. , Downes, M. , Yu, R. T. , Evans, R. M. , & Mangelsdorf, D. J. (2006). Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell, 126, 789–799. 10.1016/j.cell.2006.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns, M. P. , Vardanian, L. , Pajoohesh‐Ganji, A. , Wang, L. , Cooper, M. , Harris, D. C. , … Rebeck, G. W. (2006). The effects of ABCA1 on cholesterol efflux and Aβ levels in vitro and in vivo. Journal of Neurochemistry, 98, 792–800. 10.1111/j.1471-4159.2006.03925.x [DOI] [PubMed] [Google Scholar]
- Carter, A. Y. , Letronne, F. , Fitz, N. F. , Mounier, A. , Wolfe, C. M. , Nam, K. N. , … Koldamova, R. (2017). Liver X receptor agonist treatment significantly affects phenotype and transcriptome of APOE3 and APOE4 Abca1 haplo‐deficient mice. PLoS ONE, 12, e0172161 10.1371/journal.pone.0172161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder, E. H. , Saunders, A. M. , Strittmatter, W. J. , Schmechel, D. E. , Gaskell, P. C. , Small, G. W. , … Pericak‐Vance, M. (1993). Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science, 261, 921–923. 10.1126/science.8346443 [DOI] [PubMed] [Google Scholar]
- Corona, A. W. , Kodoma, N. , Casali, B. T. , & Landreth, G. E. (2016). ABCA1 is necessary for bexarotene‐mediated clearance of soluble amyloid β from the hippocampus of APP/PS1 mice. Journal of Neuroimmune Pharmacology: The Official Journal of the Society on NeuroImmune Pharmacology, 11, 61–72. 10.1007/s11481-015-9627-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer, P. E. , Cirrito, J. R. , Wesson, D. W. , Lee, C. Y. , Karlo, J. C. , Zinn, A. E. , … Landreth, G. E. (2012). ApoE‐directed therapeutics rapidly clear β‐amyloid and reverse deficits in AD mouse models. Science, 335, 1503–1506. 10.1126/science.1217697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, W. , Sun, Y. , Wang, Z. , Xu, C. , Peng, Y. , & Li, R. (2012). Liver X receptor activation attenuates inflammatory response and protects cholinergic neurons in APP/PS1 transgenic mice. Neuroscience, 210, 200–210. 10.1016/j.neuroscience.2012.02.047 [DOI] [PubMed] [Google Scholar]
- DeMattos, R. B. , Cirrito, J. R. , Parsadanian, M. , May, P. C. , O'Dell, M. A. , Taylor, J. W. , … Holtzman, D. M. (2004). ApoE and clusterin cooperatively suppress Aβ levels and deposition: Evidence that ApoE regulates extracellular Aβ metabolism in vivo. Neuron, 41, 193–202. 10.1016/S0896-6273(03)00850-X [DOI] [PubMed] [Google Scholar]
- Donkin, J. J. , Stukas, S. , Hirsch‐Reinshagen, V. , Namjoshi, D. , Wilkinson, A. , May, S. , … Wellington, C. L. (2010). ATP‐binding cassette transporter A1 mediates the beneficial effects of the liver X receptor agonist GW3965 on object recognition memory and amyloid burden in amyloid precursor protein/presenilin 1 mice. The Journal of Biological Chemistry, 285, 34144–34154. 10.1074/jbc.M110.108100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, R. M. , & Mangelsdorf, D. J. (2014). Nuclear receptors, RXR, and the big bang. Cell, 157, 255–266. 10.1016/j.cell.2014.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan, A. M. , Christopher, E. , Taylor, J. W. , Parsadanian, M. , Spinner, M. , Watson, M. , … Holtzman, D. M. (2004). ApoAI deficiency results in marked reductions in plasma cholesterol but no alterations in amyloid‐β pathology in a mouse model of Alzheimer's disease‐like cerebral amyloidosis. The American Journal of Pathology, 165, 1413–1422. 10.1016/S0002-9440(10)63399-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz, N. F. , Castranio, E. L. , Carter, A. Y. , Kodali, R. , Lefterov, I. , & Koldamova, R. (2014). Improvement of memory deficits and amyloid‐β clearance in aged APP23 mice treated with a combination of anti‐amyloid‐β antibody and LXR agonist. Journal of Alzheimer's Disease: JAD, 41, 535–549. 10.3233/JAD-132789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz, N. F. , Cronican, A. , Pham, T. , Fogg, A. , Fauq, A. H. , Chapman, R. , … Koldamova, R. (2010). Liver X receptor agonist treatment ameliorates amyloid pathology and memory deficits caused by high‐fat diet in APP23 mice. The Journal of Neuroscience, 30, 6862–6872. 10.1523/JNEUROSCI.1051-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz, N. F. , Cronican, A. A. , Lefterov, I. , & Koldamova, R. (2013). Comment on “ApoE‐directed therapeutics rapidly clear β‐amyloid and reverse deficits in AD mouse models”. Science, 340, 924–c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer, J. D. , Taylor, J. W. , DeMattos, R. B. , Bales, K. R. , Paul, S. M. , Parsadanian, M. , & Holtzman, D. M. (2003). Apolipoprotein E markedly facilitates age‐dependent cerebral amyloid angiopathy and spontaneous hemorrhage in amyloid precursor protein transgenic mice. The Journal of Neuroscience, 23, 7889–7896. 10.1523/JNEUROSCI.23-21-07889.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisletti, S. , Huang, W. , Jepsen, K. , Benner, C. , Hardiman, G. , Rosenfeld, M. G. , & Glass, C. K. (2009). Cooperative NCoR/SMRT interactions establish a corepressor‐based strategy for integration of inflammatory and anti‐inflammatory signaling pathways. Genes & Development, 23, 681–693. 10.1101/gad.1773109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass, C. K. , & Saijo, K. (2010). Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nature Reviews Immunology, 10, 365–376. 10.1038/nri2748 [DOI] [PubMed] [Google Scholar]
- Gofflot, F. , Chartoire, N. , Vasseur, L. , Heikkinen, S. , Dembele, D. , Le Merrer, J. , & Auwerx, J. (2007). Systematic gene expression mapping clusters nuclear receptors according to their function in the brain. Cell, 131, 405–418. 10.1016/j.cell.2007.09.012 [DOI] [PubMed] [Google Scholar]
- Grefhorst, A. , Elzinga, B. M. , Voshol, P. J. , Plosch, T. , Kok, T. , Bloks, V. W. , … Kuipers, F. (2002). Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride‐rich very low density lipoprotein particles. The Journal of Biological Chemistry, 277, 34182–34190. 10.1074/jbc.M204887200 [DOI] [PubMed] [Google Scholar]
- Gustafsson, J.‐A. (2016). Historical overview of nuclear receptors. The Journal of Steroid Biochemistry and Molecular Biology, 157, 3–6. 10.1016/j.jsbmb.2015.03.004 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch‐Reinshagen, V. , Maia, L. F. , Burgess, B. L. , Blain, J. F. , Naus, K. E. , McIsaac, S. A. , … Wellington, C. L. (2005). The absence of ABCA1 decreases soluble ApoE levels but does not diminish amyloid deposition in two murine models of Alzheimer disease. The Journal of Biological Chemistry, 280, 43243–43256. 10.1074/jbc.M508781200 [DOI] [PubMed] [Google Scholar]
- Hirsch‐Reinshagen, V. , Zhou, S. , Burgess, B. L. , Bernier, L. , McIsaac, S. A. , Chan, J. Y. , … Wellington, C. L. (2004). Deficiency of ABCA1 impairs apolipoprotein E metabolism in brain. The Journal of Biological Chemistry, 279, 41197–41207. 10.1074/jbc.M407962200 [DOI] [PubMed] [Google Scholar]
- Huy, P. D. Q. , Thai, N. Q. , Bednarikova, Z. , Phuc, L. H. , Linh, H. Q. , Gazova, Z. , & Li, M. S. (2017). Bexarotene does not clear amyloid β plaques but delays fibril growth: Molecular mechanisms. ACS Chemical Neuroscience, 8, 1960–1969. 10.1021/acschemneuro.7b00107 [DOI] [PubMed] [Google Scholar]
- Jiang, Q. , Lee, C. Y. , Mandrekar, S. , Wilkinson, B. , Cramer, P. , Zelcer, N. , … Landreth, G. E. (2008). ApoE promotes the proteolytic degradation of Aβ. Neuron, 58, 681–693. 10.1016/j.neuron.2008.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp, F. , Scheidt, H. A. , Winkler, E. , Basset, G. , Heinel, H. , Hutchison, J. M. , … Huster, D. (2018). Bexarotene binds to the amyloid precursor protein transmembrane domain, alters its α‐helical conformation, and inhibits γ‐secretase nonselectively in liposomes. ACS Chemical Neuroscience, 9, 1702–1713. 10.1021/acschemneuro.8b00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekiyo, T. , Xu, H. , & Bu, G. (2014). ApoE and Aβ in Alzheimer's disease: Accidental encounters or partners? Neuron, 81, 740–754. 10.1016/j.neuron.2014.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koistinaho, M. , Lin, S. , Wu, X. , Esterman, M. , Koger, D. , Hanson, J. , … Paul, S. M. (2004). Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid‐β peptides. Nature Medicine, 10, 719–726. 10.1038/nm1058 [DOI] [PubMed] [Google Scholar]
- Koldamova, R. P. , Lefterov, I. M. , Staufenbiel, M. , Wolfe, D. , Huang, S. , Glorioso, J. C. , … Lazo, J. S. (2005). The liver X receptor ligand T0901317 decreases amyloid β production in vitro and in a mouse model of Alzheimer's disease. The Journal of Biological Chemistry, 280, 4079–4088. 10.1074/jbc.M411420200 [DOI] [PubMed] [Google Scholar]
- Komati, R. , Spadoni, D. , Zheng, S. , Sridhar, J. , Riley, K. E. , & Wang, G. (2017). Ligands of therapeutic utility for the liver X receptors. Molecules, 22 10.3390/molecules22010088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz, M. , Candela, P. , Saint‐Pol, J. , Lamartiniere, Y. , Boucau, M. C. , Sevin, E. , … Gosselet, F. (2015). Bexarotene promotes cholesterol efflux and restricts apical‐to‐basolateral transport of amyloid‐β peptides in an in vitro model of the human blood‐brain barrier. Journal of Alzheimer's Disease: JAD, 48, 849–862. 10.3233/JAD-150469 [DOI] [PubMed] [Google Scholar]
- LaClair, K. D. , Manaye, K. F. , Lee, D. L. , Allard, J. S. , Savonenko, A. V. , Troncoso, J. C. , & Wong, P. C. (2013). Treatment with bexarotene, a compound that increases apolipoprotein‐E, provides no cognitive benefit in mutant APP/PS1 mice. Molecular Neurodegeneration, 8, 18 10.1186/1750-1326-8-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaDu, M. J. , Falduto, M. T. , Manelli, A. M. , Reardon, C. A. , Getz, G. S. , & Frail, D. E. (1994). Isoform‐specific binding of apolipoprotein E to β‐amyloid. The Journal of Biological Chemistry, 269, 23403–23406. [PubMed] [Google Scholar]
- LaDu, M. J. , Pederson, T. M. , Frail, D. E. , Reardon, C. A. , Getz, G. S. , & Falduto, M. T. (1995). Purification of apolipoprotein E attenuates isoform‐specific binding to β‐amyloid. The Journal of Biological Chemistry, 270, 9039–9042. 10.1074/jbc.270.16.9039 [DOI] [PubMed] [Google Scholar]
- Landreth, G. E. , Cramer, P. E. , Lakner, M. M. , Cirrito, J. R. , Wesson, D. W. , Brunden, K. R. , & Wilson, D. A. (2013). Response to comments on “ApoE‐directed therapeutics rapidly clear β‐amyloid and reverse deficits in AD mouse models”. Science, 340, 924–g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. Y. , & Parks, J. S. (2005). ATP‐binding cassette transporter AI and its role in HDL formation. Current Opinion in Lipidology, 16, 19–25. 10.1097/00041433-200502000-00005 [DOI] [PubMed] [Google Scholar]
- Lefterov, I. , Bookout, A. , Wang, Z. , Staufenbiel, M. , Mangelsdorf, D. , & Koldamova, R. (2007). Expression profiling in APP23 mouse brain: Inhibition of Aβ amyloidosis and inflammation in response to LXR agonist treatment. Molecular Neurodegeneration, 2, 20 10.1186/1750-1326-2-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefterov, I. , Schug, J. , Mounier, A. , Nam, K. N. , Fitz, N. F. , & Koldamova, R. (2015). RNA‐sequencing reveals transcriptional up‐regulation of Trem2 in response to bexarotene treatment. Neurobiology of Disease, 82, 132–140. 10.1016/j.nbd.2015.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepousez, G. , Nissant, A. , & Lledo, P. M. (2015). Adult neurogenesis and the future of the rejuvenating brain circuits. Neuron, 86, 387–401. 10.1016/j.neuron.2015.01.002 [DOI] [PubMed] [Google Scholar]
- Mangelsdorf, D. J. , & Evans, R. M. (1995). The RXR heterodimers and orphan receptors. Cell, 83, 841–850. 10.1016/0092-8674(95)90200-7 [DOI] [PubMed] [Google Scholar]
- Mangelsdorf, D. J. , Thummel, C. , Beato, M. , Herrlich, P. , Schütz, G. , Umesono, K. , … Evans, R. M. (1995). The nuclear receptor superfamily: The second decade. Cell, 83, 835–839. 10.1016/0092-8674(95)90199-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani, M. M. , Malm, T. , Lamb, R. , Jay, T. R. , Neilson, L. , Casali, B. , … Landreth, G. E. (2017). Neuronally‐directed effects of RXR activation in a mouse model of Alzheimer's disease. Scientific Reports, 7, 42270 10.1038/srep42270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillin, M. , & DeMorrow, S. (2016). Effects of bile acids on neurological function and disease. The FASEB Journal, 30, 3658–3668. 10.1096/fj.201600275R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens, K. L. , Kalsbeek, A. , Soeters, M. R. , & Eggink, H. M. (2017). Bile acid signaling pathways from the enterohepatic circulation to the central nervous system. Frontiers in Neuroscience, 11, 617 10.3389/fnins.2017.00617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mey, J. (2001). Retinoic acid as a regulator of cytokine signaling after nerve injury. Zeitschrift Fur Naturforschung C, Journal of Biosciences, 56, 163–176. 10.1515/znc-2001-3-401 [DOI] [PubMed] [Google Scholar]
- Mey, J. (2006). New therapeutic target for CNS injury? The role of retinoic acid signaling after nerve lesions. Journal of Neurobiology, 66, 757–779. 10.1002/neu.20238 [DOI] [PubMed] [Google Scholar]
- Michaelson, D. M. (2014). APOE ε4: The most prevalent yet understudied risk factor for Alzheimer's disease. Alzheimer's & Dementia: The Journal of the Alzheimer's Association, 10, 861–868. 10.1016/j.jalz.2014.06.015 [DOI] [PubMed] [Google Scholar]
- Mounier, A. , Georgiev, D. , Nam, K. N. , Fitz, N. F. , Castranio, E. L. , Wolfe, C. M. , … Koldamova, R. (2015). Bexarotene‐activated retinoid X receptors regulate neuronal differentiation and dendritic complexity. The Journal of Neuroscience, 35, 11862–11876. 10.1523/JNEUROSCI.1001-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam, K. N. , Mounier, A. , Fitz, N. F. , Wolfe, C. , Schug, J. , Lefterov, I. , & Koldamova, R. (2016). RXR controlled regulatory networks identified in mouse brain counteract deleterious effects of Aβ oligomers. Scientific Reports, 6, 24048 10.1038/srep24048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natrajan, M. S. , de la Fuente, A. G. , Crawford, A. H. , Linehan, E. , Nunez, V. , Johnson, K. R. , … Franklin, R. J. (2015). Retinoid X receptor activation reverses age‐related deficiencies in myelin debris phagocytosis and remyelination. Brain: A Journal of Neurology, 138, 3581–3597. 10.1093/brain/awv289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuclear Receptors Nomenclature Committee (1999). A unified nomenclature system for the nuclear receptor superfamily. Cell, 97, 161–163. [DOI] [PubMed] [Google Scholar]
- Ogundare, M. , Theofilopoulos, S. , Lockhart, A. , Hall, L. J. , Arenas, E. , Sjövall, J. , … Griffiths, W. J. (2010). Cerebrospinal fluid steroidomics: Are bioactive bile acids present in brain? The Journal of Biological Chemistry, 285, 4666–4679. 10.1074/jbc.M109.086678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare, E. , Jeggo, R. , Kim, E. M. , Barbour, B. , Walczak, J. S. , Palmer, P. , … Hobson, P. (2016). Lack of support for bexarotene as a treatment for Alzheimer's disease. Neuropharmacology, 100, 124–130. 10.1016/j.neuropharm.2015.04.020 [DOI] [PubMed] [Google Scholar]
- Olivares, A. M. , Moreno‐Ramos, O. A. , & Haider, N. B. (2015). Role of nuclear receptors in central nervous system development and associated diseases. Journal of Experimental Neuroscience, 9, 93–121. 10.4137/JEN.S25480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, A. R. , Xu, G. , Siemienski, Z. B. , Smithson, L. A. , Borchelt, D. R. , Golde, T. E. , & Felsenstein, K. M. (2013). Comment on “ApoE‐directed therapeutics rapidly clear β‐amyloid and reverse deficits in AD mouse models”. Science, 340, 924–d. [DOI] [PubMed] [Google Scholar]
- Riddell, D. R. , Zhou, H. , Comery, T. A. , Kouranova, E. , Lo, C. F. , Warwick, H. K. , … Jacobsen, J. S. (2007). The LXR agonist TO901317 selectively lowers hippocampal Aβ42 and improves memory in the Tg2576 mouse model of Alzheimer's disease. Molecular and Cellular Neurosciences, 34, 621–628. 10.1016/j.mcn.2007.01.011 [DOI] [PubMed] [Google Scholar]
- Robinson‐Rechavi, M. , Garcia, H. , & Laudet, V. (2003). The nuclear receptor superfamily. Journal of Cell Science, 116, 585–586. 10.1242/jcs.00247 [DOI] [PubMed] [Google Scholar]
- Ruhl, R. , Krzyzosiak, A. , Niewiadomska‐Cimicka, A. , Rochel, N. , Szeles, L. , Vaz, B. , … Krężel, W. (2015). 9‐cis‐13,14‐dihydroretinoic acid is an endogenous retinoid acting as RXR ligand in mice. PLoS Genetics, 11, e1005213 10.1371/journal.pgen.1005213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz, J. , Kouiavskaia, D. , Migliorini, M. , Robinson, S. , Saenko, E. L. , Gorlatova, N. , … Strickland, D. K. (2005). The apoE isoform binding properties of the VLDL receptor reveal marked differences from LRP and the LDL receptor. Journal of Lipid Research, 46, 1721–1731. 10.1194/jlr.M500114-JLR200 [DOI] [PubMed] [Google Scholar]
- Russell, D. W. (2003). The enzymes, regulation, and genetics of bile acid synthesis. Annual Review of Biochemistry, 72, 137–174. 10.1146/annurev.biochem.72.121801.161712 [DOI] [PubMed] [Google Scholar]
- Saint‐Pol, J. , Vandenhaute, E. , Boucau, M. C. , Candela, P. , Dehouck, L. , Cecchelli, R. , … Gosselet, F. (2012). Brain pericytes ABCA1 expression mediates cholesterol efflux but not cellular amyloid‐β peptide accumulation. Journal of Alzheimer's Disease: JAD, 30, 489–503. 10.3233/JAD-2012-112090 [DOI] [PubMed] [Google Scholar]
- Sandoval‐Hernandez, A. G. , Hernandez, H. G. , Restrepo, A. , Munoz, J. I. , Bayon, G. F. , Fernandez, A. F. , … Arboleda, G. H. (2016). Liver X receptor agonist modifies the DNA methylation profile of synapse and neurogenesis‐related genes in the triple transgenic mouse model of Alzheimer's disease. Journal of Molecular Neuroscience: MN, 58, 243–253. 10.1007/s12031-015-0665-8 [DOI] [PubMed] [Google Scholar]
- Sandoval‐Hernandez, A. G. , Restrepo, A. , Cardona‐Gomez, G. P. , & Arboleda, G. (2016). LXR activation protects hippocampal microvasculature in very old triple transgenic mouse model of Alzheimer's disease. Neuroscience Letters, 621, 15–21. 10.1016/j.neulet.2016.04.007 [DOI] [PubMed] [Google Scholar]
- Savage, J. C. , Jay, T. , Goduni, E. , Quigley, C. , Mariani, M. M. , Malm, T. , … Landreth, G. E. (2015). Nuclear receptors license phagocytosis by trem2+ myeloid cells in mouse models of Alzheimer's disease. The Journal of Neuroscience, 35, 6532–6543. 10.1523/JNEUROSCI.4586-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe, D. J. (2001). Presenilin, Notch, and the genesis and treatment of Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America, 98, 11039–11041. 10.1073/pnas.211352598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachel, S. J. , Zerbinatti, C. , Rudd, M. T. , Cosden, M. , Suon, S. , Nanda, K. K. , … Renger, J. (2016). Identification and in vivo evaluation of liver X receptor β‐selective agonists for the potential treatment of Alzheimer's disease. Journal of Medicinal Chemistry, 59, 3489–3498. 10.1021/acs.jmedchem.6b00176 [DOI] [PubMed] [Google Scholar]
- Sun, Y. , Yao, J. , Kim, T. W. , & Tall, A. R. (2003). Expression of liver X receptor target genes decreases cellular amyloid β peptide secretion. The Journal of Biological Chemistry, 278, 27688–27694. 10.1074/jbc.M300760200 [DOI] [PubMed] [Google Scholar]
- Tachibana, M. , Shinohara, M. , Yamazaki, Y. , Liu, C. C. , Rogers, J. , Bu, G. , & Kanekiyo, T. (2016). Rescuing effects of RXR agonist bexarotene on aging‐related synapse loss depend on neuronal LRP1. Experimental Neurology, 277, 1–9. 10.1016/j.expneurol.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai, L. M. , Koster, K. P. , Luo, J. , Lee, S. H. , Wang, Y. T. , Collins, N. C. , … LaDu, M. J. (2014). Amyloid‐β pathology and APOE genotype modulate retinoid X receptor agonist activity in vivo. The Journal of Biological Chemistry, 289, 30538–30555. 10.1074/jbc.M114.600833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwel, D. , Steffensen, K. R. , Verghese, P. B. , Kummer, M. P. , Gustafsson, J. A. , Holtzman, D. M. , & Heneka, M. T. (2011). Critical role of astroglial apolipoprotein E and liver X receptor‐α expression for microglial Aβ phagocytosis. The Journal of Neuroscience, 31, 7049–7059. 10.1523/JNEUROSCI.6546-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesseur, I. , Lo, A. C. , Roberfroid, A. , Dietvorst, S. , Van Broeck, B. , Borgers, M. , … De Strooper, B. (2013). Comment on “ApoE‐directed therapeutics rapidly clear β‐amyloid and reverse deficits in AD mouse models”. Science, 340, 924–e. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos, S. , Griffiths, W. J. , Crick, P. J. , Yang, S. , Meljon, A. , Ogundare, M. , … Wang, Y. (2014). Cholestenoic acids regulate motor neuron survival via liver X receptors. The Journal of Clinical Investigation, 124, 4829–4842. 10.1172/JCI68506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich, J. D. , Burchett, J. M. , Restivo, J. L. , Schuler, D. R. , Verghese, P. B. , Mahan, T. E. , … Holtzman, D. M. (2013). In vivo measurement of apolipoprotein E from the brain interstitial fluid using microdialysis. Molecular Neurodegeneration, 8, 13 10.1186/1750-1326-8-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eck, M. , Pennings, M. , Hoekstra, M. , Out, R. , & Van Berkel, T. J. (2005). Scavenger receptor BI and ATP‐binding cassette transporter A1 in reverse cholesterol transport and atherosclerosis. Current Opinion in Lipidology, 16, 307–315. 10.1097/01.mol.0000169351.28019.04 [DOI] [PubMed] [Google Scholar]
- Vanmierlo, T. , Rutten, K. , Dederen, J. , Bloks, V. W. , van Vark‐van der Zee, L. C. , Kuipers, F. , … Mulder, M. (2011). Liver X receptor activation restores memory in aged AD mice without reducing amyloid. Neurobiology of Aging, 32, 1262–1272. 10.1016/j.neurobiolaging.2009.07.005 [DOI] [PubMed] [Google Scholar]
- Veeraraghavalu, K. , Zhang, C. , Miller, S. , Hefendehl, J. K. , Rajapaksha, T. W. , Ulrich, J. , … Sisodia, S. S. (2013). Comment on “ApoE‐directed therapeutics rapidly clear β‐amyloid and reverse deficits in AD mouse models”. Science, 340, 924–f. [DOI] [PubMed] [Google Scholar]
- Verghese, P. B. , Castellano, J. M. , Garai, K. , Wang, Y. , Jiang, H. , Shah, A. , … Holtzman, D. M. (2013). ApoE influences amyloid‐β (Aβ) clearance despite minimal apoE/Aβ association in physiological conditions. Proceedings of the National Academy of Sciences of the United States of America, 110, E1807–E1816. 10.1073/pnas.1220484110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villapol, S. (2018). Roles of peroxisome proliferator‐activated receptor γ on brain and peripheral inflammation. Cellular and Molecular Neurobiology, 38, 121–132. 10.1007/s10571-017-0554-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahrle, S. E. , Jiang, H. , Parsadanian, M. , Hartman, R. E. , Bales, K. R. , Paul, S. M. , & Holtzman, D. M. (2005). Deletion of Abca1 increases Aβ deposition in the PDAPP transgenic mouse model of Alzheimer disease. The Journal of Biological Chemistry, 280, 43236–43242. 10.1074/jbc.M508780200 [DOI] [PubMed] [Google Scholar]
- Wahrle, S. E. , Jiang, H. , Parsadanian, M. , Legleiter, J. , Han, X. , Fryer, J. D. , … Holtzman, D. M. (2004). ABCA1 is required for normal central nervous system ApoE levels and for lipidation of astrocyte‐secreted apoE. The Journal of Biological Chemistry, 279, 40987–40993. 10.1074/jbc.M407963200 [DOI] [PubMed] [Google Scholar]
- Wang, L. , Schuster, G. U. , Hultenby, K. , Zhang, Q. , Andersson, S. , & Gustafsson, J. A. (2002). Liver X receptors in the central nervous system: From lipid homeostasis to neuronal degeneration. Proceedings of the National Academy of Sciences of the United States of America, 99, 13878–13883. 10.1073/pnas.172510899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellington, C. L. , Walker, E. K. , Suarez, A. , Kwok, A. , Bissada, N. , Singaraja, R. , … Hayden, M. R. (2002). ABCA1 mRNA and protein distribution patterns predict multiple different roles and levels of regulation. Laboratory Investigation, 82, 273–283. 10.1038/labinvest.3780421 [DOI] [PubMed] [Google Scholar]
- Wesson, D. W. , Borkowski, A. H. , Landreth, G. E. , Nixon, R. A. , Levy, E. , & Wilson, D. A. (2011). Sensory network dysfunction, behavioral impairments, and their reversibility in an Alzheimer's β‐amyloidosis mouse model. The Journal of Neuroscience, 31, 15962–15971. 10.1523/JNEUROSCI.2085-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney, K. D. , Watson, M. A. , Collins, J. L. , Benson, W. G. , Stone, T. M. , Numerick, M. J. , … Kliewer, S. A. (2002). Regulation of cholesterol homeostasis by the liver X receptors in the central nervous system. Molecular Endocrinology, 16, 1378–1385. 10.1210/mend.16.6.0835 [DOI] [PubMed] [Google Scholar]
- Wyss‐Coray, T. , Loike, J. D. , Brionne, T. C. , Lu, E. , Anankov, R. , Yan, F. , … Husemann, J. (2003). Adult mouse astrocytes degrade amyloid‐β in vitro and in situ. Nature Medicine, 9, 453–457. 10.1038/nm838 [DOI] [PubMed] [Google Scholar]
- Yeh, F. L. , Wang, Y. , Tom, I. , Gonzalez, L. C. , & Sheng, M. (2016). TREM2 binds to apolipoproteins, including APOE and CLU/APOJ, and thereby facilitates uptake of amyloid‐β by microglia. Neuron, 91, 328–340. 10.1016/j.neuron.2016.06.015 [DOI] [PubMed] [Google Scholar]
- Yuan, C. , Guo, X. , Zhou, Q. , Du, F. , Jiang, W. , Zhou, X. , … Zou, L. (2019). OAB‐14, a bexarotene derivative, improves Alzheimer's disease‐related pathologies and cognitive impairments by increasing β‐amyloid clearance in APP/PS1 mice. Biochimica et Biophysica Acta Molecular Basis of Disease, 1865, 161–180. 10.1016/j.bbadis.2018.10.028 [DOI] [PubMed] [Google Scholar]
- Zelcer, N. , Khanlou, N. , Clare, R. , Jiang, Q. , Reed‐Geaghan, E. G. , Landreth, G. E. , … Tontonoz, P. (2007). Attenuation of neuroinflammation and Alzheimer's disease pathology by liver x receptors. Proceedings of the National Academy of Sciences of the United States of America, 104, 10601–10606. 10.1073/pnas.0701096104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, J. , Fu, Y. , Liu, C. C. , Shinohara, M. , Nielsen, H. M. , Dong, Q. , … Bu, G. (2014). Retinoic acid isomers facilitate apolipoprotein E production and lipidation in astrocytes through the retinoid X receptor/retinoic acid receptor pathway. The Journal of Biological Chemistry, 289, 11282–11292. 10.1074/jbc.M113.526095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. , Wu, X. , Li, X. , Jiang, L. L. , Gui, X. , Liu, Y. , … Xu, H. (2018). TREM2 is a receptor for β‐amyloid that mediates microglial function. Neuron, 97, 1023–1031.e1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, H. , Jia, L. , Liu, C. C. , Rong, Z. , Zhong, L. , Yang, L. , … Bu, G. (2017). TREM2 promotes microglial survival by activating Wnt/β‐catenin pathway. The Journal of Neuroscience, 37, 1772–1784. 10.1523/JNEUROSCI.2459-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang, J. , Zhang, H. , Zhou, R. , Chen, L. , Chen, J. , & Shen, X. (2013). Regulation of prostaglandin F2α against β amyloid clearance and its inflammation induction through LXR/RXR heterodimer antagonism in microglia. Prostaglandins & Other Lipid Mediators, 106, 45–52. 10.1016/j.prostaglandins.2013.09.002 [DOI] [PubMed] [Google Scholar]


