Summary
Tomato chlorosis virus (ToCV) causes an important disease that primarily affects tomato, although it has been found infecting other economically important vegetable crops and a wide range of wild plants. First described in Florida (USA) and associated with a ‘yellow leaf disorder’ in the mid‐1990s, ToCV has been found in 35 countries and territories to date, constituting a paradigmatic example of an emergent plant pathogen. ToCV is transmitted semipersistently by whiteflies (Hemiptera: Aleyrodidae) belonging to the genera Bemisia and Trialeurodes. Whitefly transmission is highly efficient and cases of 100% infection are frequently observed in the field. To date, no resistant or tolerant tomato plants are commercially available and the control of the disease relies primarily on the control of the insect vector.
Taxonomy
Tomato chlorosis virus is one of the 14 accepted species in the genus Crinivirus, one of the four genera in the family Closteroviridae of plant viruses.
Virion and genome properties
The genome of ToCV is composed of two molecules of single‐stranded positive‐sense RNA, named RNA1 and RNA2, separately encapsidated in long, flexuous, rod‐like virions. As has been shown for other closterovirids, ToCV virions are believed to have a bipolar structure. RNA1 contains four open reading frames (ORFs) encoding proteins associated with virus replication and suppression of gene silencing, whereas RNA2 contains nine ORFs encoding proteins putatively involved in encapsidation, cell‐to‐cell movement, gene silencing suppression and whitefly transmission.
Host range
In addition to tomato, ToCV has been found to infect 84 dicot plant species belonging to 25 botanical families, including economically important crops.
Transmission
Like all species within the genus Crinivirus, ToCV is semipersistently transmitted by whiteflies, being one of only two criniviruses transmitted by members of the genera Bemisia and Trialeurodes.
Disease symptoms
Tomato ‘yellow leaf disorder’ syndrome includes interveinal yellowing and thickening of leaves. Symptoms first develop on lower leaves and then advance towards the upper part of the plant. Bronzing and necrosis of the older leaves are accompanied by a decline in vigour and reduction in fruit yield. In other hosts the most common symptoms include interveinal chlorosis and mild yellowing on older leaves.
Control
Control of the disease caused by ToCV is based on the use of healthy seedlings for transplanting, limiting accessibility of alternate host plants that can serve as virus reservoirs and the spraying of insecticides for vector control. Although several wild tomato species have been shown to contain genotypes resistant to ToCV, there are no commercially available resistant or tolerant tomato varieties to date.
Keywords: Closteroviridae, criniviruses, emergent diseases, tomato, tomato chlorosis virus, whiteflies
Discovery and Emergence of ToCV
The family Closteroviridae of plant viruses includes the genera Ampelovirus, Closterovirus, Crinivirus, Velarivirus, and a few unassigned species. The genus Crinivirus includes the 14 whitefly‐transmitted members of the family (ICTV, 2018). Most individual criniviruses cause significant diseases in important crops including common bean, beet, cucurbits, lettuce, potato and tomato, whereas some of them are asymptomatic and only cause disease when found in mixed infections with other viruses (Tzanetakis et al., 2013).
Tomato chlorosis virus (ToCV), a crinivirus, was first identified in the mid‐1990s from greenhouse‐grown tomato plants showing a ‘yellow leaf disorder’ syndrome in north‐central Florida, USA. This syndrome had been observed since 1989 but was initially attributed to physiological or nutritional disorders (Wisler et al., 1998a, 1998b). Symptoms observed in ToCV‐infected tomato plants include chlorotic areas that evolve into interveinal bright yellowing, beginning on lower leaves and gradually progressing to the upper part of the plant (Fig. 1). In the initial stage of the infection, chlorotic areas are frequently polygonal in shape, which is limited by main veins. In advanced stages, interveinal yellow areas can develop reddish‐brown necrotic flecks. Lower leaves present as rolled, thickened and brittle, being crispy to the touch. Although no obvious symptoms are produced on fruits, significant yield reduction occurs due to a loss of photosynthetic area. Symptomatic plants are less vigorous, showing yield losses as a result of reduced fruit growth and delayed ripening. Symptoms of ToCV on tomato are indistinguishable from those induced by a related crinivirus, tomato infectious chlorosis virus (TICV) (Duffus et al., 1996). In other hosts, the most common symptoms caused by ToCV include interveinal chlorosis or mild yellowing on older leaves.
Figure 1.
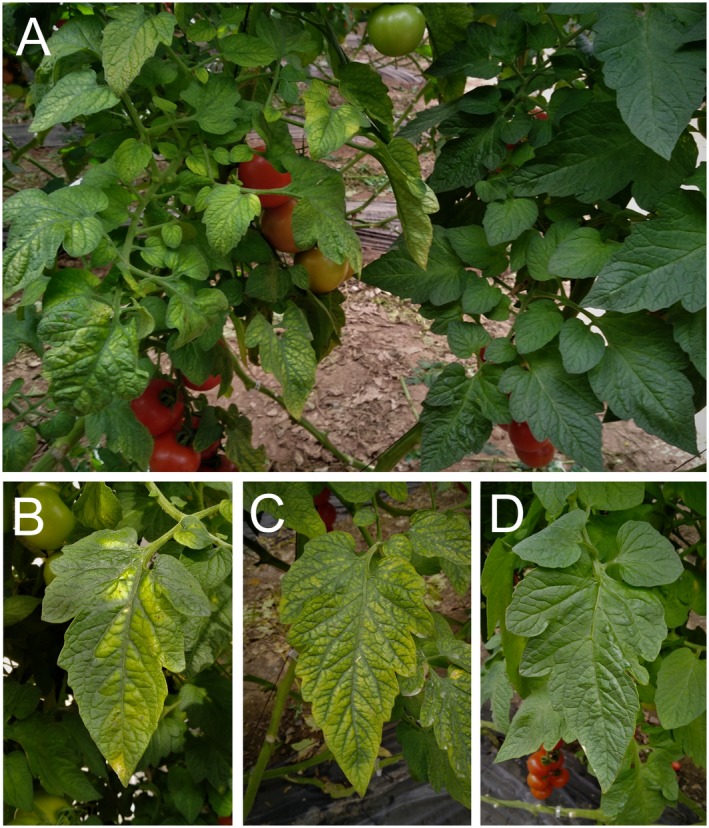
Symptoms of tomato chlorosis virus infection on naturally infected tomato plants. (A) Commercial plant showing generalized yellowing (left) close to a healthy plant (right). Individual leaflets showing polygonal chlorotic areas (B) or interveinal bright yellowing (C) are shown alongside an asymptomatic leaflet (D).
Soon after tomato yellow leaf disorder was recognized as being caused by a new crinivirus in the USA, similar symptoms were detected in Spain (Navas‐Castillo et al., 2000). These symptoms were observed as early as 1997 and its association with ToCV infection was confirmed for the first time in Europe. Since then, the virus has been detected infecting tomato in many areas around the world. Altogether, ToCV has been found in 35 countries and territories in the Americas, Europe, Asia and Africa (Table 1, Fig. 2). During the 22 years since the presence of ToCV in the USA was confirmed, a linear progression of the disease has been observed throughout the world. This expansion is still evident; overall, a mean of 1.5 new countries or territories have been invaded per year (Fig. 2). Confirmation of viral presence in new areas could be dependent on a greater research effort on ToCV detection and identification. However, it is evident that recognition of new symptoms in a given area by farmers, who used to be the main actors in initial warnings given to scientists, has been occurring in new countries in recent years. In this sense, ToCV constitutes a paradigmatic example of an emergent plant pathogen that will surely invade new areas in the near future. It is worth noting the recent detection of ToCV in northern Europe, specifically in the Netherlands and the UK (EPPO, 2018a, 2018b). The climatic conditions in these countries were not thought to be conducive to the transmission of the virus, and its spread here was not foreseen. Cultivation of tomato under greenhouses, together with the fact that ToCV can be transmitted by Bemisia tabaci and Trialeurodes vaporariorum, the latter less dependent on warm climates than B. tabaci, means that the infections could spread to new areas. Climate change could aggravate this situation as the geographical range, phenology, density and activity of the vectors could be altered (Canto et al., 2009). Another worrisome situation is related to the recent report of ToCV in Nigeria (Mohammed et al., 2018), where the disease has been observed simultaneously in seven states of the country. Expansion of the virus to other tropical countries in sub‐Saharan Africa will alter the scenario in this huge area, whose agriculture is already seriously threatened by other insect‐borne viruses.
Table 1.
Dates and references for first reports of tomato chlorosis virus in the countries and territories showed in Fig. 2
| Year* | Country/territory | Reference |
|---|---|---|
| 1996 | United States | Wisler et al. (1998b) |
| 1997 | Spain | Navas‐Castillo et al. (2000) |
| 1998 | Portugal | Louro et al. (2000) |
| 1998 | Taiwan | Tsai et al. (2004) |
| 2000 | Italy | Accotto et al. (2001) |
| 2000 | Morocco | Hanafi (2002) |
| 2000 | South Africa | Jones (2001) |
| 2001 | Canary Islands (ES) | Font et al. (2003) |
| 2001 | Greece | Dovas et al. (2002) |
| 2001 | Puerto Rico (US) | Wintermantel et al. (2001) |
| 2002 | France | Dalmon et al. (2005) |
| 2003 | Israel | Segev et al. (2004) |
| 2004 | Cyprus | Papayiannis et al. (2006) |
| 2004 | Réunion (FR) | Delatte et al. (2006) |
| 2005 | Mayotte (FR) | Massé et al. (2008) |
| 2005 | Mexico | Alvarez‐Ruiz et al. (2007) |
| 2006 | Brazil | Barbosa et al. (2008) |
| 2006 | Cuba | Martínez‐Zubiaur et al. (2008) |
| 2006 | Lebanon | Abou‐Jawdah et al. (2006) |
| 2007 | Costa Rica | Castro et al. (2009) |
| 2007 | Mauritius | Lett et al. (2009) |
| 2008 | Japan | Hirota et al. (2010) |
| 2008 | Turkey | Çevik and Erkiş (2008) |
| 2008 | Indonesia | Suastika et al. (2011) |
| 2010 | Hungary | Bese et al. (2011) |
| 2011 | Sudan | Fiallo‐Olivé et al. (2011) |
| 2012 | China | Zhao et al. (2013) |
| 2012 | Uruguay | Arruabarrena et al. (2014) |
| 2013 | Korea | Lee et al. (2018) |
| 2014 | Tunisia | Mnari‐Hattab et al. (2014) |
| 2014 | Saudi Arabia | Al‐Saleh et al. (2014) |
| 2014 | Jordan | Salem et al. (2015) |
| 2017 | Nigeria | Mohammed et al. (2018) |
| 2017 | Netherlands | EPPO (2018a) |
| 2018 | United Kingdom | EPPO (2018b) |
ES, Spain; US, United States; FR, France.
Whenever it could be determined from the literature, dates correspond to collection of samples that were confirmed to be infected.
Figure 2.
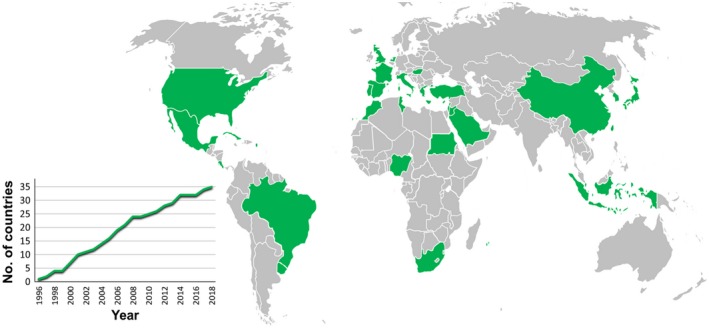
Geographical distribution of tomato chlorosis virus (ToCV). A complete list of countries and territories where the virus has been reported (in green) and references for first reports are shown in Table 1. The inset graph shows the evolution through time of the number of countries and territories where ToCV has been found to date.
In addition to tomato, ToCV has been found infecting a high number of diverse plant species. Overall, 85 dicot plant species belonging to 25 botanical families have been described as natural hosts for the virus (Table 2). These hosts include economically important vegetable (cowpea, eggplant, lettuce, potato, pumpkin, sweet pepper and tomato) and ornamental crops, as well as weeds and other wild plants. For their global importance, sweet pepper and potato crops can be highlighted in addition to tomato. ToCV infections on sweet pepper have been reported from Spain (Lozano et al., 2004), Brazil (Barbosa et al., 2010), Costa Rica (Vargas et al., 2011), Tunisia (Gharsallah et al., 2015) and Saudi Arabia (Shakeel et al., 2017). The effect of the disease on sweet pepper has been revealed by controlled experiments using B. tabaci as a vector (Fortes et al., 2012). These experiments showed that varieties of the three basic types of pepper grown in the Mediterranean basin (Italian, California and Lamuyo) are efficiently infected by the virus. Also, in addition to confirming the development of the symptoms observed in the field (stunting growth of the plant, curling, interveinal yellowing and abnormal elongation in leaves), an important decrease of marketable fruit yield was observed due to reduction in fruit number and size (Fig. 3). Potato plants have been also shown to be naturally infected by ToCV in Spain (Fortes and Navas‐Castillo, 2008, 2012), Brazil (Freitas et al., 2012) and Tunisia (Gharsallah et al., 2015). Transmission experiments have shown the presence of ToCV in potato tubers from infected plants, which subsequently produced infected plants themselves, and that this species served as virus source for tomato infection via B. tabaci transmission (Fortes and Navas‐Castillo, 2012). Although the above experiments did not show any symptoms in the ToCV‐infected potato cv. Safrane plants, plants of cv. Ágata reported as being infected by ToCV in Brazil showed leaf roll and interveinal chlorosis symptoms on older leaves (Freitas et al., 2012).
Table 2.
Natural hosts reported for tomato chlorosis virus.
| Family | Plant species* | Reference |
|---|---|---|
| Aizoaceae | Heliotropium lasiocarpum | Shakeel et al. (2017) |
| Amaranthaceae | Alternanthera philoxeroides | Tang et al. (2017) |
| Amaranthus retroflexus | Orfanidou et al. (2014) | |
| Amaranthus viridis | Shakeel et al. (2017) | |
| Apocynaceae | Calotropis procera | Shakeel et al. (2017) |
| Araliaceae | Aralia nudicaulis | Shakeel et al. (2017) |
| Asteraceae | Cirsium arvense | Orfanidou et al. (2014) |
| Conyza canadensis | Kil et al. (2015b) | |
| Conyza sp. | Orfanidou et al. (2014) | |
| Erigeron annuus | Kil et al. (2015a) | |
| Lactuca saligna | Shakeel et al. (2017) | |
| Lactuca sativa (lettuce) | Orfanidou et al. (2014) | |
| Lactuca serriola | Shakeel et al. (2017) | |
| Sonchus asper | Kil et al. (2015b) | |
| Sonchus olereaceus | Shakeel et al. (2017) | |
| Youngia japonica | Kil et al. (2015b) | |
| Boraginaceae | Trigonotis peduncularis | Kil et al. (2015b) |
| Brassicaceae | Brassica sp. † | Solórzano‐Morales et al. (2011) |
| Cardamine flexuosa | Kil et al. (2015b) | |
| Eruca vesicaria (garden rocket) | Boiteux et al. (2016) | |
| Raphanus raphanistrum (radish) | Boiteux et al. (2016) | |
| Caryophyllaceae | Cerastium glomeratum | Kil et al. (2015b) |
| Stellaria media | Kil et al. (2015b) | |
| Chenopodiaceae | Chenopodium album | Orfanidou et al. (2014) |
| Chenopodium murale | Shakeel et al. (2017) | |
| Chenopodium opulifolium | Shakeel et al. (2017) | |
| Compositae | Zinnia elegans (zinnia) | Tsai et al. (2004) |
| Convolvulaceae | Convolvulus arvensis | Orfanidou et al. (2014) |
| Ipomoea cholulensis | Kil et al. (2015b) | |
| Ipomoea hederaceae | Kil et al. (2015b) | |
| Cucurbitaceae | Cucurbita moschata (pumpkin) | Solórzano‐Morales et al. (2011) |
| Fabaceae | Vicia angustifolia | Kil et al. (2015b) |
| Vicia tetrasperma | Kil et al. (2015b) | |
| Vigna unguiculata (cowpea) | Wang et al. (2018a) | |
| Fumariaceae | Fumaria officinalis | Orfanidou et al. (2014) |
| Malvaceae | Abelmoschus esculentus (okra) | Shakeel et al. (2017) |
| Abutilon theophrasti | Orfanidou et al. (2014) | |
| Malva parviflora | Shakeel et al. (2017) | |
| Malva sylvestris | Orfanidou et al. (2014) | |
| Mazaceae | Mazus pumilus | Kil et al. (2015b) |
| Oxalidaceae | Oxalis pes‐caprae | Orfanidou et al. (2014) |
| Phytolacaceae | Phytolacca americana | Kil et al. (2015b) |
| Phytolacca icosandra | Solórzano‐Morales et al. (2011) | |
| Plantaginaceae | Plantago major | Solórzano‐Morales et al. (2011) |
| Veronica hederifolia | Orfanidou et al. (2014) | |
| Portulacaceae | Portulaca oleracea (purslane) ‡ | Orfanidou et al. (2014) |
| Primulaceae | Anagalis foemina | Orfanidou et al. (2014) |
| Rubiaceae | Galium aparine | Orfanidou et al. (2014) |
| Rutaceae | Ruta chalepensis | Solórzano‐Morales et al. (2011) |
| Solanaceae | Capsicum annuum (sweet pepper) | Lozano et al. (2004) |
| Datura stramonium | Alvarez‐Ruiz et al. (2007) | |
| Nicandra physaloides | Souza et al. (2019) | |
| Nicotiana tabacum (tobacco) | Fiallo‐Olivé et al. (2014) | |
| Physalis angulata | Fonseca et al. (2013) | |
| Physalis ixocarpa | Trenado et al. (2007) | |
| Physalis peruviana | Trenado et al. (2007) | |
| Solanum aethiopicum (scarlet eggplant) | Fonseca et al. (2016) | |
| Solanum americanum | Arruabarrena et al. (2015) | |
| Solanum arcanum | García‐Cano et al. (2010) | |
| Solanum chilense | García‐Cano et al. (2010) | |
| Solanum chmielewskii | García‐Cano et al. (2010) | |
| Solanum corneliomulleri | García‐Cano et al. (2010) | |
| Solanum elaeagnifolium | Gharsallah et al. (2015) | |
| Solanum galapagense | García‐Cano et al. (2010) | |
| Solanum habrochaites | García‐Cano et al. (2010) | |
| Solanum huaylasense | García‐Cano et al. (2010) | |
| Solanum jamaicense | Boiteux et al. (2018) | |
| Solanum lycopersicum (tomato) | Wisler et al. (1998b) | |
| Solanum mammosum | Boiteux et al. (2018) | |
| Solanum melongena (eggplant) | Zhou et al. (2015) | |
| Solanum neorickii | García‐Cano et al. (2010) | |
| Solanum nigrescens | Alvarez‐Ruiz et al. (2007) | |
| Solanum nigrum | Font et al. (2004) | |
| Solanum paniculatum | Boiteux et al. (2018) | |
| Solanum pennellii | García‐Cano et al. (2010) | |
| Solanum peruvianum | García‐Cano et al. (2010) | |
| Solanum pimpinellifolium | García‐Cano et al. (2010) | |
| Solanum scuticum | Boiteux et al. (2018) | |
| Solanum sessiliflorum | Boiteux et al. (2018) | |
| Solanum sisymbriifolium | Arruabarrena et al. (2015) | |
| Solanum stramoniifolium | Boiteux et al. (2018) | |
| Solanum subinerme | Boiteux et al. (2018) | |
| Solanum tuberosum (potato) | Fortes and Navas‐Castillo (2008) | |
| Solanum velleum | Boiteux et al. (2018) | |
| Zygophyllaceae | Tribulus terrestris | Shakeel et al. (2017) |
For cultivated hosts (highlighted in bold), the common name is given in parentheses after the scientific name.
The infected Brassica plants found in Costa Rica, unidentified to the species level, were reported as weeds.
Although purslane is grown in many areas, the infected samples from Greece were reported as weeds.
Figure 3.

Effect of tomato chlorosis virus infection on pepper (cv. Pescara) plants inoculated using viruliferous Bemisia tabaci and maintained under controlled conditions. (A) Plant growth reduction in an infected plant (right) in comparison to a mock‐inoculated plant (left). (B) Reduction in size of fruits from infected plants (right) in comparison to those from mock‐inoculated plants (left). Reproduced from Fortes et al. (2012).
Genome Organization and Diversity
To date, 17 complete ToCV genome sequences have been published, one each from the USA (Wintermantel et al., 2005), Spain (Lozano et al., 2006b, 2007), Greece (Kataya et al., 2008), Brazil (Albuquerque et al., 2013) and Taiwan (Kang et al., 2018), two from China (Zhao et al., 2014a, 2014b) and ten from Korea (Lee et al., 2018).
The ToCV genome has the typical organization of bipartite criniviruses, with two molecules of linear, positive‐sense, single‐stranded RNA, namely RNA1 (8593–8596 nt) and RNA2 (8242–8247 nt) (Lozano et al., 2006b, 2007; Wintermantel et al., 2005) (Fig. 4). RNA1 contains four open reading frames (ORF 1a/1b to ORF3) potentially encoding proteins associated with virus replication and the suppression of gene silencing. RNA2 contains nine ORFs (ORF4 to ORF12) possibly encoding proteins putatively involved in virus encapsidation, cell‐to‐cell movement, membrane association, whitefly transmission and the suppression of gene silencing. Both RNAs, encapsidated in separated flexuous rod particles of approximately 800–850 nm in length (Liu et al., 2000), are needed for infectivity (Orílio et al., 2014). Although there is no direct evidence, it is supposed that ToCV virions have the rattlesnake structure described for the crinivirus lettuce infectious yellows virus (LIYV), composed of a long body and a short tail (Tian et al., 1999). This feature is common to all members of the family Closteroviridae (Agranovsky et al., 1995).
Figure 4.
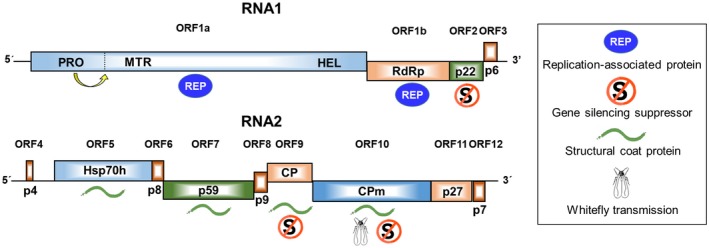
Schematic representation of the genomic structure of tomato chlorosis virus RNA1 and RNA2. Boxes represent open reading frames (ORF) with the putative protein products indicated inside. The inset shows the symbols used to represent the (putative) functions of proteins: replication‐associated proteins, gene silencing suppresors, structural coat proteins and whitefly transmission. CP, coat protein; CPm, minor coat protein; HSP70h, heat shock protein 70 homologue; HEL, helicase; MTR, methyl transferase; PRO, proteinase, RdRp, RNA‐dependent RNA polymerase.
ORF 1a encodes a 221 kDa multifunctional protein typical of members of the family Closteroviridae (Karasev, 2000), containing protease, methyltransferase and helicase domains (Wintermantel et al., 2005). ORF 1b expresses a 59 kDa RNA‐dependent RNA polymerase (RdRp) likely through a +1 ribosomal frameshift at the end of ORF 1a, as happens in other members of the family Closteroviridae, although no specific structures that favour the slippage have been identified (Wintermantel et al., 2005). ORF 2 encodes p22, a protein with little similarity to other proteins in searched databases, although an ORF of similar size and location is present in the genome of other criniviruses. The p22 protein has been shown to be an efficient and strong RNA silencing suppressor by the classical Agrobacterium co‐infiltration assay on Nicotiana benthamiana plants, in which it has an extremely long‐lasting local activity (Cañizares et al., 2008). It has been suggested that the suppression mechanism of p22 is mediated by its preferential binding to long dsRNAs, preventing them from being cleaved (Landeo‐Ríos et al., 2016). ORF 3 encodes a putative protein of 6 kDa (p6) with a transmembrane domain similar to other 3ʹ‐end proteins of criniviruses.
ORF 4 encodes a putative 4 kDa protein (p4) with a hydrophobic domain, suggesting it functions as a transmembrane protein. Similarly sized hydrophobic proteins are putatively encoded in this location in most other criniviruses, although comparison of the amino acid sequences does not reveal obvious similarity for these proteins, with the possible exception of sweet potato chlorotic stunt virus (Kreuze et al., 2002). ORF 5 encodes a heat shock protein 70 homologue (HSP70h) that likely associates with virion tails and is involved in their assembly, along with cell‐to‐cell movement based on similarity with the closteroviruses beet yellows virus (BYV) and citrus tristeza virus (Alzhanova et al., 2001; Napuli et al., 2000; Peremyslov et al., 1999; Satyanarayana et al., 2000). ORF 6 encodes a putative 8 kDa protein (p8) with similarity to 6 kDa proteins encoded by beet pseudoyellows virus and strawberry pallidosis‐associated virus (Tzanetakis and Martin, 2004; Tzanetakis et al., 2005). ORF 7 encodes a 59 kDa protein (p59) that also associates with virion tails and is likely involved in cell‐to‐cell movement (Alzhanova et al., 2001; Satyanarayana et al., 2000). ORF 8 encodes a putative 9 kDa protein (p9) for which no function or similarity has been identified. ORFs 9 and 10 encode 29 kDa and 76 kDa major and minor coat proteins (CP and CPm), respectively. As is typical of all members of the family Closteroviridae, the CP encapsidates most of the virion particle, while CPm is associated with the virion tail (Agranovsky et al., 1995). Studies on LIYV have suggested that CPm may also be involved in virus transmission by the whitefly vectors (Chen et al., 2011; Stewart et al., 2010). CP and CPm may also be involved in cell‐to‐cell movement based on studies conducted with BYV (Alzhanova et al., 2000, 2001). ToCV CP and CPm have also been shown to have silencing suppression activity (Cañizares et al., 2008). ORF 11 encodes a 27 kDa protein (p27) which seems unique to criniviruses. ORF 12 encodes a putative 7 kDa protein (p7) unique to ToCV among criniviruses characterized to date. This putative protein contains a transmembrane domain, which suggests it may be a functional protein, although no similarity has been found to any other protein in the searched databases.
ToCV populations have a heterogeneous and complex genetic structure similar to those described for animal and plant RNA viral quasispecies. This was shown by analysing the intraisolate genetic structure of 755 molecular clones distributed in four genomic regions within the RNA‐dependent RNA polymerase (RNA1) and HSP70h, CP and CPm (RNA2) ORFs of ToCV isolates from Spain (Lozano et al., 2009). Also, it has been shown that the structure of the ToCV populations clearly differ depending on the RNA segment considered, being more complex for RNA1 (encoding replication‐associated proteins) than for RNA2 (encoding encapsidation, systemic‐movement and insect transmission‐relevant proteins) (Lozano et al., 2009; our unpublished results). This observation supports the idea that, in multicomponent RNA viruses, function can generate profound differences in the genetic structures of the different genomic segments.
A few studies have shown a low genetic diversity of ToCV when analysing partial sequences (HSP70h, CP and CPm genes) within a country (Barbosa et al., 2013; Orfanidou et al., 2014). However, when all available full‐length genome sequences have been compared, three major clusters can be recognized (Lee et al., 2018).
Availability of infectious clones
Orílio et al. (2014) constructed full‐length cDNA infectious clones for both RNA1 and RNA2 of a Spanish ToCV isolate, AT80/99. In vitro transcripts of the RNA1 clone, under the control of SP6 RNA promoter, were able to replicate in N. benthamiana mesophyll protoplasts. On the other hand, in vivo transcripts of the RNA1 and RNA2 clones, under the control of 35S CaMV promoter, resulted in infection on N. benthamiana plants after agroinoculation. As has been reported for other criniviruses, infection could not be achieved directly on the natural host of the virus, tomato. However, grafting of agroinfected N. benthamiana stem scions onto tomato plants allowed systemic infection of tomato plants, which then showed the characteristic yellowing symptoms caused by ToCV. The viruses generated in tomato were shown to be whitefly transmitted, both by B. tabaci Mediterranean (MED, formerly Q biotype) and Middle East‐Asia Minor 1 (MEAM1, formerly B biotype), thus proving the functional integrity of the virus progeny generated from the infectious clones. Full‐length cDNA clones under a 35S CaMV promoter have been also obtained for a Chinese ToCV isolate, whose biological activity was demonstrated through agroinoculation of N. benthamiana plants (Zhao et al., 2016). A recent paper has reported that the latter clones resulted infectious in tomato plants, although details on the inoculation method used or the efficiency of infection were not given (Shi et al., 2018).
Epidemiology
Criniviruses emerged as a major problem for world agriculture at the end of the twentieth century with the establishment of some of their whitefly vectors in temperate climates. ToCV is one of two criniviruses that are transmitted by whiteflies of the genera Bemisia and Trialeurodes. These include several species of the B. tabaci complex [MED, MEAM1 and New World (NW, formerly A biotype)], T. vaporariorum and T. abutilonea (Navas‐Castillo et al., 2000; Wisler et al., 1998b). The high number of natural plant hosts (see Table 2) and being readily transmitted by several whitefly species have contributed to successful emergence of ToCV worldwide (see Table 1 and Fig. 2). In Spain, for example, ToCV prevalence in tomato crops is reported to be very high, frequently at levels of 50–100% (Fortes et al., 2012; Lozano et al., 2006a; Velasco et al., 2008). Field investigations conducted in Brazil on tomato have shown that the main dispersal mechanism of the disease caused by ToCV is primary spread, with epidemics being caused by successive influxes of viruliferous whiteflies (Macedo et al., 2019).
The presence of wild and cultivated (e.g. sweet pepper and potato) alternative hosts for ToCV (Table 2) adds a new dimension to the complexity of its epidemiology. Although there are no in‐depth studies regarding the epidemiological consequences of wild host presence or the coincidence of tomato with sweet pepper, potato or other susceptible crops in the field, some insights can be gained from laboratory experiments. Thus, both choice and non‐choice transmission experiments using B. tabaci MEAM1 as a vector and tomato or potato plants as source of inoculum have shown that ToCV transmission rates follow the order tomato > potato > sweet pepper (Mituti et al., 2018). These studies also showed that tomato is a better source of inoculum than potato.
Whitefly transmission
ToCV is transmitted semipersistently and, based on similarity with LIYV (Chen et al., 2011), is believed to be foregut‐borne. Although it is commonly accepted that ToCV is a phloem‐limited virus, only recently has it been shown using the electrical penetration graph technique that transmission occurs primarily after salivation activity in the phloem sieve elements (waveform E1) (Maluta et al., 2017). Unexpectedly, this investigation suggests that a very low rate of infection (below 2%) could also occur before salivation during stylet puncture in mesophyll, companion or parenchyma cells, without ruling out brief stylet punctures in phloem sieve elements. Similar results have been observed with lettuce chlorosis virus, another crinivirus, with a low rate of transmission before whiteflies performed waveform E1 (Johnson et al., 2002).
The transmission efficiency differs among the whitefly species, following the order B. tabaci MED > B. tabaci MEAM1 ≈ T. abutilonea > B. tabaci NW > T. vaporariorum (Shi et al., 2018; Wintermantel and Wisler, 2006). The difference in transmission between MED and MEAM1, the most invasive species in the B. tabaci complex, has been associated to differences in virus acquisition and accumulation rate (Shi et al., 2018).
Virus–plant–whitefly interactions
Recently, a number of papers have been published describing the interactions between ToCV, tomato plants and whitefly vectors. The parameters most commonly studied include preference and performance of the insects on infected and healthy plants. The results of these studies are apparently contradictory, even in cases where they were carried out with the same whitefly species, suggesting differences in the experimental conditions. Thus, two studies with B. tabaci MEAM1 reached different conclusions regarding the preference of adults for virus‐infected tomato leaves in comparison to healthy leaves; the preference for virus‐infected plants was shown only for non‐viruliferous adults by Shi et al. (2018) while Fereres et al. (2016) found preference for both viruliferous and non‐viruliferous adults. Also, studies on performance of whiteflies on ToCV‐infected plants, as determined by fecundity and other reproductive parameters, resulted in contrasting conclusions. Thus, according to Shi et al. (2018), B. tabaci MED adults performed better on infected plants while according to Li et al. (2018), they performed worst. On the other hand, B. tabaci MEAM1 adults performed similarly on infected and healthy plants according to Shi et al. (2018) and Maluta et al. (2019), while they performed worst on infected plants according to Watanabe et al. (2018). Although the effects of plant pathogens on the behaviour and biological performance of their vectors have been shown for different host–pathogen–vector combinations, from the available studies carried out with ToCV it is clear that additional research is needed to correctly understand the interactions between the virus, the plant host and whiteflies, and their further epidemiological implications.
Mixed infections and synergism
Mixed virus infections are frequently found in wild and cultivated plants. In some cases, the interaction between two or more viruses results in a synergistic interaction. Synergism has a facilitative effect on one or all of the viral partners and can be manifested by an increase in virus replication, symptomatology, cellular tropism, within host movement and transmission rate (reviewed by Syller, 2012).
In the case of ToCV in tomato, mixed infections with other viruses have been described to be frequent in several countries. The viruses involved in mixed infections with ToCV include tomato yellow leaf curl virus (a monopartite Old World begomovirus transmitted by B. tabaci) (Martínez‐Zubiaur et al., 2008), tomato severe rugose virus (a bipartite New World begomovirus transmitted by B. tabaci) (Macedo et al., 2014), pepino mosaic virus (a potexvirus transmitted by mechanical contact) (Davino et al., 2008) and tomato torrado virus (a torradovirus transmitted by B. tabaci and T. vaporariorum) (Gómez et al., 2010). The above papers describing mixed infections with begomoviruses reported that the symptoms observed in plants infected with both are more severe than those induced by the begomovirus alone. Although these observations are suggestive of a synergistic interaction with a facilitative effect of ToCV on the begomovirus, to our knowledge there is no study available in which this phenomenon has been examined in depth.
On the other hand, the interaction between ToCV and tomato spotted wilt virus (TSWV) (an orthotospovirus transmitted by thrips) has been studied under experimental conditions (García‐Cano et al., 2006). A synergistic reaction was observed in mixed infections in tomato plants susceptible to both viruses when doubly infected with ToCV and TSWV, resulting in the rapid death of plants. Also, a pronounced enhancement of ToCV accumulation, but not of TSWV, was observed in mixed infections. Synergism was also observed in tomato plants carrying the Sw‐5 resistance gene, which are resistant to TSWV. Pre‐infection with ToCV resulted in susceptibility to TSWV, whereas co‐inoculations did not, suggesting that a threshold level or a time lapse is needed for ToCV to interfere or down‐regulate the defence response in the TSWV‐resistant plants.
Control Strategies
As ToCV cannot be transmitted from plant to plant in the absence of whitefly vectors, suppression of vector populations can keep virus dispersion to a minimum. However, chemical control of the vector, widely used by tomato growers in many areas, has not proven to be effective for control of the disease caused by ToCV. This is especially true for open‐field cultivation. A few simple crop management strategies have been evaluated for controlling the disease damage based on limiting the access of the whitefly vectors to the plants. Thus, Velasco et al. (2008) compared the prevalence of tomato yellowing caused by ToCV in south‐eastern Spain (Murcia Region) where different greenhouse or nethouse covers are commonly used. These covers include five categories ranging from nethouses with 6 × 6 threads per centimetre (equivalent to open‐air cultivation with the only purpose of avoiding wind damage) to polycarbonate plastic greenhouses with 10 × 20 threads per centimetre window screens. Only when the higher quality covers are used was a significant reduction of the disease observed, as determined by the area under the disease progress curve. Similarly, experiments were carried out in east China (Shandong Province) by Wang et al. (2018b) to compare the influence of different structures and covers on ToCV prevalence. In this case four types of greenhouse were assayed, and it was found that adding nets to the greenhouse ventilation windows reduced the level of ToCV infection. These two reports show that reducing entry of whiteflies to the greenhouses by using simple containment structures results in an efficient protection of the crop from ToCV infection.
Diagnostics
Availability of specific and sensitive diagnostic methods is a key factor for designing control strategies against plant pathogens. In the case of ToCV, both serological and molecular diagnostic methodologies have been developed. For serological detection of ToCV, a polyclonal antiserum produced using viral capsid protein expressed in Escherichia coli was proposed for routine diagnosis in double antibody sandwich enzyme‐linked immunosorbent assay (DAS‐ELISA) but about 5% of false negatives were reported to occur (Jacquemond et al., 2009). Polyclonal antibodies are now available to be used in DAS‐ELISA from a number of commercial sources [e.g. the German Collection of Microorganisms and Cell Cultures (DSMZ) and the company Loewe].
Several reverse transcription‐polymerase chain reaction (RT‐PCR) protocols have been developed to allow rapid and specific detection of ToCV. These include a multiplex RT‐PCR assay which allows the simultaneous detection of ToCV and TICV by amplifying part of the HSP70h gene of both criniviruses followed by a multiplex nested PCR amplification (Dovas et al., 2002). A real‐time TaqMan RT‐PCR assay has been also developed for the multiplex detection of ToCV and TICV in plants and vector whiteflies, with a higher sensitivity than the conventional RT‐PCR test (Papayiannis et al., 2011).
Molecular hybridization of petiole cross‐section tissue printing has been used for ToCV detection in plants by using a digoxigenin‐labelled RNA probe obtained from the ToCV CP gene (Fortes et al., 2012; Trenado et al., 2007). This probe has proved to be useful for diagnosis of ToCV in epidemiological studies and for evaluation of genetic resistance in wild tomato germplasm (García‐Cano et al., 2010; Gómez et al., 2010).
In recent years, several reverse transcription loop‐mediated isothermal amplification (RT‐LAMP) protocols have been also developed to detect ToCV in plants and whiteflies (Karwitha et al., 2016; Kil et al., 2015a; Zhao et al., 2015).
Search for genetic resistance
To date, there is no commercial tomato cultivar reported to be resistant or tolerant to ToCV. However, experiments carried out in Spain, Brazil and Uruguay have identified several wild tomato species (Solanum section Lycopersicon) that contain genotypes reacting with mild symptoms and/or low viral titres to the natural or experimental inoculation with ToCV (García‐Cano et al., 2010; González‐Arcos et al., 2018; Mansilla‐Córdova et al., 2018). The most promising materials include genotypes of S. chmielewskii, S. habrochaites and S. lycopersicum × S. peruvianum. Also, an inbred line of tomato, LT05, has shown high tolerance to ToCV (González‐Arcos et al., 2018).
Conclusions and Prospects
In summary, ToCV can be considered a bona fide emergent plant virus that is still expanding its geographical and host ranges by means of its insect vectors. In addition, human‐assisted movement of plant materials, planting monocultures or exotic crops, and introducing other agricultural practices may enhance virus spread. Getting a more precise knowledge of the mechanisms involved in its transmission by whiteflies, ongoing worldwide emergence, and disease development will guide future research efforts with the final goal of developing effective control measures. Some of the key research issues on which emphasis should be placed are listed below:
The real molecular and biological diversity of ToCV should be revealed by sequencing and generation of infectious clones of isolates from plant hosts other than tomato.
The molecular mechanisms underlying symptom development in plants infected by ToCV, an issue only barely studied (e.g. Seo et al., 2018), should receive further attention in order to understand how the disease initiates and progresses in the plant after horizontal transmission by whiteflies.
Almost nothing is known about the molecular determinants of whitefly transmissibility of ToCV, especially on the insect side, and this can be extended to other criniviruses and semipersistently transmitted plant viruses in general. Identification of virus receptors in the whitefly will be a key step to clarify the mechanism of transmission and to design strategies to disrupt disease spread.
Establishing correlations between climatic variables and distribution of whiteflies of the B. tabaci complex [especially those shown to efficiently transmit ToCV: MED (formerly Q biotype), MEAM1 (formerly B biotype) and NW (formerly A biotype)], T. vaporariorum and T. abutilonea will help to predict areas where ToCV is likely to emerge. This takes on special importance from the perspective of climate change.
The few known cases of synergisms in which ToCV is involved are insufficiently understood and should be further investigated, as should novel interactions that occur when the virus comes into contact with new viruses following its ongoing emergence.
The development of resistant or tolerant commercial tomato (and other crop) varieties is urgently needed to complement basic and general control measures to reduce whitefly numbers and virus inoculum.
Acknowledgements
ToCV research in the Navas‐Castillo group has been funded by the Ministerio de Economía, Industria y Competitividad (Spain) and the European Regional Development Fund through grants AGL2013‐48913‐C2‐1‐R and AGL2016‐75819‐C2‐2‐R. The authors thank Jesús Ariza for allowing them to take photographs of the ToCV‐infected tomato plants.
References
- Abou‐Jawdah, Y. , El Mohtar, C. , Atamian, H. and Sobh, H. (2006) First report of Tomato chlorosis virus in Lebanon. Plant Dis. 90, 378. [DOI] [PubMed] [Google Scholar]
- Accotto, G.P. , Vaira, A.M. , Vecchiati, M. , Finetti Sialer, M.M. , Gallitelle, D. and Davino, M. (2001) First report of Tomato chlorosis virus in Italy. Plant Dis. 85, 1208. [DOI] [PubMed] [Google Scholar]
- Agranovsky, A.A. , Lesemann, D.E. , Maiss, E. , Hull, R. and Atabekov, J.G. (1995) ‘Rattlesnake’ structure of a filamentous plant RNA virus built of two capsid proteins. Proc. Natl. Acad. Sci. USA. 92, 2470–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque, L.C. , Villanueva, F. , Resende, R.O. , Navas‐Castillo, J. , Barbosa, J.C. and Inoue‐Nagata, A.K. (2013) Molecular characterization reveals Brazilian Tomato chlorosis virus to be closely related to a Greek isolate. Trop. Plant Pathol. 38, 332–336. [Google Scholar]
- Al‐Saleh, M.A. , Al‐Shahwan, I.M. , Shakeel, M.T. , Amer, M.A. , Orfanidou, C.G. and Katis, N.I. (2014) First report of Tomato chlorosis virus (ToCV) in tomato crops in Saudi Arabia. Plant Dis. 98, 1590. [DOI] [PubMed] [Google Scholar]
- Alvarez‐Ruiz, P. , Jimenez, C.G. , Leyva‐López, N.E. and Méndez‐Lozano, J. (2007) First report of Tomato chlorosis virus infecting tomato crops in Sinaloa, Mexico. Plant Pathol. 56, 1043. [Google Scholar]
- Alzhanova, V.V. , Hagiwara, Y. , Peremyslov, V.V. and Dolja, V.V. (2000) Genetic analysis of the cell‐to‐cell movement of beet yellows closterovirus. Virology, 268, 192–200. [DOI] [PubMed] [Google Scholar]
- Alzhanova, V.V. , Napuli, A.J. , Creamer, R. and Dolja, V.V. (2001) Cell‐to‐cell movement and assembly of a plant closterovirus: roles for the capsid proteins and HSP70 homolog. EMBO J. 20, 6997–7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruabarrena, A. , Rubio, L. , González‐Arcos, M. , Maeso, D. , Fonseca, M.E.N. and Boiteux, L.S. (2014) First report of Tomato chlorosis virus infecting tomato crops in Uruguay. Plant Dis. 98, 1445. [DOI] [PubMed] [Google Scholar]
- Arruabarrena, A. , Rubio, L. , González‐Arcos, M. , Sánchez‐Campos, S. , Fonseca, M.E.N. and Boiteux, L.S. (2015) First report of Solanum sisymbriifolium and S. americanum as natural weed hosts of Tomato chlorosis virus (Genus Crinivirus) in South America. Plant Dis. 99, 895. [Google Scholar]
- Barbosa, J.C. , Teixeira, A.P.M. , Moreira, A.G. , Camargo, L.E.A. , Bergamin Filho, A. , Kitajima, E.W. and Rezende, J.A.M. (2008) First report of Tomato chlorosis virus infecting tomato crops in Brazil. Plant Dis. 92, 1709. [DOI] [PubMed] [Google Scholar]
- Barbosa, J.C. , Teixeira, L.D.D. and Rezende, J.A.M. (2010) First report on the susceptibility of sweet pepper crops to Tomato chlorosis virus in Brazil. Plant Dis. 94, 374. [DOI] [PubMed] [Google Scholar]
- Barbosa, J.C. , Rezende, J.A.M. and Filho, A.B. (2013) Low genetic diversity suggests a single introduction and recent spread of Tomato chlorosis virus in Brazil. J. Phytopathol. 161, 884–886. [Google Scholar]
- Bese, G. , Bóka, K. , Krizbai, L. and Takács, A. (2011) First report of Tomato chlorosis virus in tomato from Hungary. Plant Dis. 95, 363. [DOI] [PubMed] [Google Scholar]
- Boiteux, L.S. , Fonseca, M.E.N. , Reis, A. , Costa, A.F. , Fontes, M.G. and González‐Arcos, M. (2016) Wild radish (Raphanus spp.) and garden rocket (Eruca sativa) as new brassicaceae hosts of Tomato chlorosis virus in South America. Plant Dis. 100, 1027. [Google Scholar]
- Boiteux, L.S. , Lima, M.F. , Fonseca, M.E.N. , Mendonça, J.L. , Costa, A.F. , Silva‐Filho, J.G. , Fontes, M.G. and González‐Arcos, M. (2018) Identification of eight solanum (subgenus Leptostemonum) species a novel natural hosts of Tomato chlorosis virus in Brazil. Plant Dis. 102, 1673. [Google Scholar]
- Cañizares, M.C. , Navas‐Castillo, J. and Moriones, E. (2008) Multiple suppressors of RNA silencing encoded by both genomic RNAs of the crinivirus, Tomato chlorosis virus . Virology, 379, 168–174. [DOI] [PubMed] [Google Scholar]
- Canto, T. , Aranda, M.A. and Fereres, A. (2009) Climate change effects on physiology and population processes of hosts and vectors that influence the spread of hemipteran‐borne plant viruses. Glob. Chang. Biol. 15, 1884–1894. [Google Scholar]
- Castro, R.M. , Hernandez, E. , Mora, F. , Ramirez, P. and Hammond, R.W. (2009) First report of Tomato chlorosis virus in tomato in Costa Rica. Plant Dis. 93, 970. [DOI] [PubMed] [Google Scholar]
- Çevik, B. and Erkiş, G. (2008) First report of Tomato chlorosis virus in Turkey. Plant Pathol. 57, 767. [Google Scholar]
- Chen, A.Y. , Walker, G.P. , Carter, D. and Ng, J.C. (2011) A virus capsid component mediates virion retention and transmission by its insect vector. Proc. Natl. Acad. Sci. USA. 108, 16777–16782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmon, A. , Bouyer, S. , Cailly, M. , Girard, M. , Lecoq, H. , Debiez, C. and Jacquemond, M. (2005) First report of Tomato chlorosis virus and Tomato infectious chlorosis virus in tomato crops in France. Plant Dis. 89, 1243. [DOI] [PubMed] [Google Scholar]
- Davino, S. , Davino, M. , Bellardi, M.G. and Agosteo, G.E. (2008) Pepino mosaic virus and Tomato chlorosis virus causing mixed infection in protected tomato crops in Sicily. Phytopathol. Mediterr. 47, 35–41. [Google Scholar]
- Delatte, H. , Naze, F. , Cottineau, J.S. , Lefeuvre, P. , Hostachy, B. , Reynaud, B. and Lett, J.‐M. (2006) Occurrence of Tomato chlorosis virus on tomato in Réunion Island. Plant Pathol. 55, 289. [Google Scholar]
- Dovas, C.I. , Katis, N.I. and Avgelis, A.D. (2002) Multiplex detection of criniviruses associated with epidemics of a yellowing disease of tomato in Greece. Plant Dis. 86, 1345–1349. [DOI] [PubMed] [Google Scholar]
- Duffus, J.E. , Liu, H.‐Y. and Wisler, G.C. (1996) Tomato infectious chlorosis virus – a new clostero‐like virus transmitted by Trialeurodes vaporariorum . Eur. J. Plant Pathol. 102, 219–226. [Google Scholar]
- EPPO (2018a) First report of Tomato chlorosis virus in the Netherlands. EPPO Rep. Serv. 2018, 37. [Google Scholar]
- EPPO (2018b) First report of Tomato chlorosis virus in the United Kingdom. EPPO Rep. Serv. 2018, 219. [Google Scholar]
- Fereres, A. , Peñaflor, M.F.G.V. , Favaro, C.F. , Azevedo, K.E.X. , Landi, C.H. , Maluta, N.K.P. , Bento, J.M.S. and Lopes, J.R.S. (2016) Tomato infection by whitefly‐transmitted circulative and non‐circulative viruses induce contrasting changes in plant volatiles and vector behaviour. Viruses, 8, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiallo‐Olivé, E. , Hamed, A.A. , Moriones, E. and Navas‐Castillo, J. (2011) First report of Tomato chlorosis virus infecting tomato in Sudan. Plant Dis. 95, 1592. [DOI] [PubMed] [Google Scholar]
- Fiallo‐Olivé, E. , Espino, A.I. , Botella‐Guillén, M. , Gómez‐González, E. , Reyes‐Carlos, J.A. and Navas‐Castillo, J. (2014) Tobacco: a new natural host of Tomato chlorosis virus in Spain. Plant Dis. 98, 1162. [DOI] [PubMed] [Google Scholar]
- Fonseca, M.E.N. , Boiteux, L.S. , Abreu, H. , Nogueira, I. and Pereira‐Carvalho, R.C. (2013) Physalis angulata: a new natural host of Tomato chlorosis virus in Brazil. Plant Dis. 97, 692. [DOI] [PubMed] [Google Scholar]
- Fonseca, M.E.N. , Boiteux, L.S. , Lima, M.F. , Mendonça, J.L. , Costa, A.F. , Fontes, M.G. , Costa, H. and González‐Arcos, M. (2016) First report of Tomato chlorosis virus infecting eggplant and scarlet eggplant in Brazil. Plant Dis. 100, 867–868. [Google Scholar]
- Font, M.I. , Vaira, A.M. , Accotto, G.P. , Lacasa, A. , Serra, J. , Gomila, J. , Juárez, M. , Espino, A.I. and Jordá, M.C. (2003) Amarilleos en los cultivos de tomate asociados a Tomato chlorosis virus (ToCV) y Tomato infectious chlorosis virus (TICV) en España. Bol. San. Veg. Plagas, 29, 109–121. [Google Scholar]
- Font, M.I., Juárez, M., Martínez, O. and Jordá, C. (2004) Current status and newly discovered natural hosts of Tomato infectious chlorosis virus and Tomato chlorosis virus in Spain. Plant Dis. 88, 82. [DOI] [PubMed] [Google Scholar]
- Fortes, I.M. and Navas‐Castillo, J. (2008) Potato (Solanum tuberosum) is an experimental and natural host of Tomato chlorosis virus In: 3rd Conference of the International Working Group on Legume and Vegetable Viruses, p. 56. Ljubljana: IWGLVV. [Google Scholar]
- Fortes, I.M. and Navas‐Castillo, J. (2012) Potato, an experimental and natural host of the crinivirus Tomato chlorosis virus . Eur. J. Plant Pathol. 134, 81–86. [Google Scholar]
- Fortes, I.M. , Moriones, E. and Navas‐Castillo, J. (2012) Tomato chlorosis virus in pepper: prevalence in commercial crops in southeastern Spain and symptomatology under experimental conditions. Plant Pathol. 61, 994–1001. [Google Scholar]
- Freitas, D.M.S. , Nardin, I. , Shimoyama, N. , Souza‐Dias, J.A.C. and Rezende, J.A.M. (2012) First report of Tomato chlorosis virus in potato in Brazil. Plant Dis. 96, 593. [DOI] [PubMed] [Google Scholar]
- García‐Cano, E. , Resende, R.O. , Fernández‐Muñoz, R. and Moriones, E. (2006) Synergistic interaction between Tomato chlorosis virus and Tomato spotted wilt virus results in breakdown of resistance in tomato. Phytopathology, 96, 1263–1269. [DOI] [PubMed] [Google Scholar]
- García‐Cano, E. , Navas‐Castillo, J. , Moriones, E. and Fernández‐Muñoz, R. (2010) Resistance to Tomato chlorosis virus in wild tomato species that impair virus accumulation and disease symptom expression. Phytopathology, 100, 582–592. [DOI] [PubMed] [Google Scholar]
- Gharsallah, C. , Halima, A.B. , Fakhfakh, H. and Gorsane, F. (2015) Insights into the genetic diversity and the phylogenetic analysis of Tunisian isolates of Tomato chlorosis virus . Phytoparasitica, 43, 87–96. [Google Scholar]
- Gómez, P. , Sempere, R.N. , Amari, K. , Gómez‐Aix, C. and Aranda, M.A. (2010) Epidemics of Tomato torrado virus, Pepino mosaic virus and Tomato chlorosis virus in tomato crops: do mixed infections contribute to torrado disease epidemiology? Ann. Appl. Biol. 156, 401–410. [Google Scholar]
- González‐Arcos, M. , de Noronha Fonseca, M.E. , Arruabarrena, A. , Lima, M.F. , Michereff‐Filho, M. , Moriones, E. , Fernández‐Muñoz, R. and Boiteaux, L.S. (2018) Identification of genetic sources with attenuated Tomato chlorosis virus‐induced symptoms in Solanum (section Lycopersicon) germplasm. Euphytica, 214, 178. [Google Scholar]
- Hanafi, A. (2002) Invasive species. A real challenge to IPM in the Mediterranean region? EWSN Newsletter, 13, 4. [Google Scholar]
- Hirota, T. , Natsuaki, T. , Murai, T. , Nishigawa, H. , Niibori, K. , Goto, K. , Hartono, S. , Suastika, G. and Okuda, S. (2010) Yellowing disease of tomato caused by Tomato chlorosis virus newly recognized in Japan. J. Gen. Plant Pathol. 76, 168–171. [Google Scholar]
- ICTV (2018) Virus Taxonomy: 2018 Release. Available at https://talk.ictvonline.org/taxonomy/ (accessed on Mar 31, 2019). [Google Scholar]
- Jacquemond, M. , Verdin, E. , Dalmon, A. , Guilbaud, L. and Gognalons, P. (2009) Serological and molecular detection of Tomato chlorosis virus and Tomato infectious chlorosis virus in tomato. Plant Pathol. 58, 210–220. [Google Scholar]
- Johnson, D.D. , Walker, G.P. and Creamer, R. (2002) Stylet penetration behavior resulting in inoculation of a semipersistently transmitted closterovirus by the whitefly Bemisia argentifolii . Entomol. Exp. Appl. 102, 115–123. [Google Scholar]
- Jones, D.R. (2001) Pest risk analysis of Tomato chlorosis virus. York: CSL. [Google Scholar]
- Kang, Y.‐C. , Wang, Y.‐C. , Hsia, C.‐M. , Tsai, W.‐S. , Huang, L.‐H. , Yeh, S.‐D. and Chen, T.‐C. (2018) Molecular characterization and detection of a genetically distinct Tomato chlorosis virus strain in Taiwan. Plant Dis. 102, 600–607. [DOI] [PubMed] [Google Scholar]
- Karasev, A.V. (2000) Genetic diversity and evolution of closteroviruses. Annu. Rev. Phytopathol. 38, 293–324. [DOI] [PubMed] [Google Scholar]
- Karwitha, M. , Feng, Z.K. , Shen, Y. , Essendi, W. , Zhang, W.N. , Li, J.Y. and Tao, X.R. (2016) Rapid detection of Tomato chlorosis virus from infected plant and whitefly by one‐step reverse transcription loop‐mediated isothermal amplification. J. Phytopathol. 164, 255–263. [Google Scholar]
- Kataya, A.R.A. , Stavridou, E. , Farhan, K. and Livieratos, I.C. (2008) Nucleotide sequence analysis and detection of a Greek isolate of Tomato chlorosis virus . Plant Pathol. 57, 819–824. [Google Scholar]
- Kil, E.‐J. , Kim, S. , Lee, Y.‐J. , Kang, E.‐H. , Lee, M. , Cho, S.‐H. , Kim, M.‐K. , Lee, K.‐Y. , Heo, N.‐Y. , Choi, H.‐S. , Kwon, S.‐T. and Lee, S. (2015a) Advanced loop‐mediated isothermal amplification method for sensitive and specific detection of Tomato chlorosis virus using a uracil DNA glycosylase to control carry‐over contamination. J. Virol. Methods, 213, 68–74. [DOI] [PubMed] [Google Scholar]
- Kil, E.‐J. , Lee, J.‐J. , Cho, S. , Auh, C.‐K. , Kim, D. , Lee, K.‐Y. , Kim, M.‐K. , Choi, H.‐S. , Kim, C.‐S. and Lee, S. (2015b) Identification of natural weed hosts of Tomato chlorosis virus in Korea by RT‐PCR with root tissues. Eur. J. Plant Pathol. 142, 419–426. [Google Scholar]
- Kreuze, J.F. , Savenkov, E.I. and Valkonen, J.P. (2002) Complete genome sequence and analyses of the subgenomic RNAs of Sweet potato chlorotic stunt virus reveal several new features for the genus Crinivirus . J. Virol. 76, 9260–9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landeo‐Ríos, Y. , Navas‐Castillo, J. , Moriones, E. and Cañizares, M.C. (2016) The p22 RNA silencing suppressor of the crinivirus Tomato chlorosis virus preferentially binds long dsRNAs preventing them from cleavage. Virology, 488, 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y.‐J. , Kil, E.‐J. , Kwak, H.‐R. , Kim, M. , Seo, J.‐K. , Lee, S. and Choi, H.‐S. (2018) Phylogenetic characterization of Tomato chlorosis virus population in Korea: evidence of reassortment between isolates from different origins. Plant Pathol. J. 34, 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett, J.‐M. , Hoareau, M. , Reynaud, B. , Saison, A. , Hostachy, B. , Lobin, K. and Benimadhu, S.P. (2009) First report of Tomato chlorosis virus in tomato on Mauritius Island. Plant Dis. 93, 111. [DOI] [PubMed] [Google Scholar]
- Li, J. , Ding, T.B. , Chi, H. and Chu, D. (2018) Effects of Tomato chlorosis virus on the performance of its key vector, Bemisia tabaci, in China. J. Appl. Entomol. 142, 296–304. [Google Scholar]
- Liu, H.‐Y. , Wisler, G.C. and Duffus, J.E. (2000) Particle length of whitefly‐transmitted criniviruses. Plant Dis. 84, 803–805. [DOI] [PubMed] [Google Scholar]
- Louro, D. , Accotto, G.P. and Vaira, A.M. (2000) Occurrence and diagnosis of Tomato chlorosis virus in Portugal. Eur. J. Plant Pathol. 106, 589–592. [Google Scholar]
- Lozano, G. , Moriones, E. and Navas‐Castillo, J. (2004) First report of sweet pepper (Capsicum annuum) as a natural host plant for Tomato chlorosis virus . Plant Dis. 88, 224. [DOI] [PubMed] [Google Scholar]
- Lozano, G. , Fortes, I.M. , García‐Cano, E. , Fernández‐Muñoz, R. , Moriones, E. and Navas‐Castillo, J. (2006a) El virus del amarilleo del tomate (Tomato chlorosis virus, ToCV): una amenaza más para los cultivos protegidos de tomate y pimiento. Agríc. Vergel. 293, 263–268. [Google Scholar]
- Lozano, G. , Moriones, E. and Navas‐Castillo, J. (2006b) Complete nucleotide sequence of the RNA2 of the crinivirus tomato chlorosis virus. Arch. Virol. 151, 581–587. [DOI] [PubMed] [Google Scholar]
- Lozano, G. , Moriones, E. and Navas‐Castillo, J. (2007) Complete sequence of the RNA1 of a European isolate of tomato chlorosis virus. Arch. Virol. 152, 839–841. [DOI] [PubMed] [Google Scholar]
- Lozano, G. , Grande‐Pérez, A. and Navas‐Castillo, J. (2009) Populations of genomic RNAs devoted to the replication or spread of a bipartite plant virus differ in genetic structure. J. Virol. 83, 12973–12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo, M.A. , Barreto, S.S. , Hallwass, M. and Inoue‐Nagata, A.K. (2014) High incidence of Tomato chlorosis virus alone and in mixed infection with begomoviruses in two tomato fields in the Federal District and Goiás state, Brazil. Trop. Plant Pathol. 39, 449–452. [Google Scholar]
- Macedo, M.A. , Inoue‐Nagata, A.K. , Silva, T.N.Z. , Freitas, D.M.S. , Rezende, J.A.M. , Michereff Filho, M. , Nascimento, A.R. , Lourenção, A.L. and Bergamin Filho, A. (2019) Temporal and spatial progress of the diseases caused by the crinivirus Tomato chlorosis virus and the begomovirus Tomato severe rugose virus in tomatoes in Brazil. Plant Pathol. 68, 72–84. [Google Scholar]
- Maluta, N.K.P. , Garzo, E. , Moreno, A. , Navas‐Castillo, J. , Fiallo‐Olivé, E. , Lopes, J.R.S. and Fereres, A. (2017) Stylet penetration activities of the whitefly Bemisia tabaci associated with inoculation of the crinivirus Tomato chlorosis virus . J. Gen. Virol. 98, 1515–1520. [DOI] [PubMed] [Google Scholar]
- Maluta, N. , Fereres, A. and Lopes, J.R.S. (2019) Plant‐mediated indirect effects of two viruses with different transmission modes on Bemisia tabaci feeding behavior and fitness. J. Pest Sci. 92, 405–416. [Google Scholar]
- Mansilla‐Córdova, P.J. , Bampi, D. , Rondinel‐Mendoza, N.V. , Melo, P.C.T. , Lourenção, A.L. and Rezende, J.A.M. (2018) Screening tomato genotypes for resistance and tolerance to Tomato chlorosis virus . Plant Pathol. 67, 1231–1237. [Google Scholar]
- Martínez‐Zubiaur, Y. , Fiallo‐Olivé, E. , Carrillo‐Tripp, J. and Rivera‐Bustamante, R. (2008) First report of Tomato chlorosis virus infecting tomato single and mixed infections with Tomato yellow leaf curl virus in Cuba. Plant Dis. 92, 836. [DOI] [PubMed] [Google Scholar]
- Massé, D. , Lefeuvre, P. , Delatte, H. , Abdoul Karime, A.L. , Hostachy, B. , Reynaud, B. and Lett, J.‐M. (2008) Tomato chlorosis virus: first report in Mayotte Island. Plant Pathol. 57, 388. [Google Scholar]
- Mituti, T. , Molina, J.P.E. and Rezende, J.A.M. (2018) Bioassays on the role of tomato, potato and sweet pepper as sources of Tomato chlorosis virus transmitted by Bemisia tabaci MEAM1. Eur. J. Plant Pathol. 152, 613–619. [Google Scholar]
- Mnari‐Hattab, M. , Zammouri, S. , Salleh, W. , Hdider, C. and Hajlaoui, M.R. (2014) First report of severe yellowing outbreaks on tomato in Tunisia associated with Tomato chlorosis virus infection. New Dis. Rep. 30, 3. [Google Scholar]
- Mohammed, I.U. , Yakub, A.M. , Yusuf, I. , Muhammad, A. , Navas‐Castillo, J. and Fiallo‐Olivé, E. (2018) First report of Tomato chlorosis virus infecting tomato in Nigeria. Plant Dis. 102, 257. [DOI] [PubMed] [Google Scholar]
- Napuli, A.J. , Falk, B.W. and Dolja, V.V. (2000) Interaction between HSP70‐homolog and filamentous virions of the Beet yellows virus . Virology, 274, 232–239. [DOI] [PubMed] [Google Scholar]
- Navas‐Castillo, J. , Camero, R. , Bueno, M. and Moriones, E. (2000) Severe yellowing outbreaks in tomato in Spain associated with infections of Tomato chlorosis virus . Plant Dis. 84, 835–837. [DOI] [PubMed] [Google Scholar]
- Orfanidou, C.G. , Dimitriou, C. , Papayiannis, L.C. , Maliogka, V.I. and Katis, N.I. (2014) Epidemiology and genetic diversity of criniviruses associated with tomato yellows disease in Greece. Virus Res. 186, 120–129. [DOI] [PubMed] [Google Scholar]
- Orílio, A.F. , Fortes, I.M. and Navas‐Castillo, J. (2014) Infectious cDNA clones of the crinivirus Tomato chlorosis virus are competent for systemic plant infection and whitefly‐transmission. Virology, 464, 365–374. [DOI] [PubMed] [Google Scholar]
- Papayiannis, L.C. , Ioannou, N. , Dovas, C.I. , Maliogka, V.I. and Katis, N.I. (2006) First report of Tomato chlorosis virus on tomato crops in Cyprus. Plant Pathol. 55, 567. [Google Scholar]
- Papayiannis, L.C. , Harkou, I.S. , Markou, Y.M. , Demetriou, C.N. and Katis, N.I. (2011) Rapid discrimination of Tomato chlorosis virus, Tomato infectious chlorosis virus and co‐amplification of plant internal control using real‐time RT‐PCR. J. Virol. Methods. 176, 53–59. [DOI] [PubMed] [Google Scholar]
- Peremyslov, V.V. , Hagiwara, Y. and Dolja, V.V. (1999) HSP70 homolog functions in cell‐to‐cell movement of a plant virus. Proc. Natl. Acad. Sci. USA. 96, 14771–14776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem, N.M. , Mansour, A.N. , Abdeen, A.O. , Araj, S. and Khrfan, W.I. (2015) First report of Tomato chlorosis virus infecting tomato crops in Jordan. Plant Dis. 99, 1286. [Google Scholar]
- Satyanarayana, T. , Gowda, S. , Mawassi, M. , Albiach‐Marti, M.R. , Ayllon, M.A. , Robertson, C. , Garnsey, S.M. and Dawson, W.O. (2000) Closterovirus encoded HSP70 homolog and P61 in addition to both coat proteins function in efficient virion assembly. Virology, 278, 253–265. [DOI] [PubMed] [Google Scholar]
- Segev, L. , Wintermantel, W.M. , Polston, J.E. and Lapidot, M. (2004) First report of Tomato chlorosis virus in Israel. Plant Dis. 88, 1160. [DOI] [PubMed] [Google Scholar]
- Seo, J.‐K. , Kim, M.‐K. , Kwak, H.‐R. , Choi, H.‐S. , Nam, M. , Choe, J. , Choi, B. , Han, S.‐J. , Kang, J.‐H. and Jung, C. (2018) Molecular dissection of distinct symptoms induced by tomato chlorosis virus and tomato yellow leaf curl virus based on comparative transcriptome analysis. Virology, 516, 1–20. [DOI] [PubMed] [Google Scholar]
- Shakeel, M.T. , Al‐Saleh, M.A. , Amer, M.A. , Al‐Shahwan, I.M. , Umar, M. , Dimou, N. , Orfanidou, C.G. , Zakri, A.M. and Katis, N.I. (2017) Molecular characterization and natural host range of Tomato chlorosis virus in Saudi Arabia. J. Plant Pathol. 99, 415–421. [Google Scholar]
- Shi, X. , Tang, X. , Zhang, X. , Zhang, D. , Li, F. , Yan, F. , Zhang, Y. , Zhou, X. and Liu, Y. (2018) Transmission efficiency, preference and behavior of Bemisia tabaci MEAM1 and MED under the influence of Tomato chlorosis virus . Front. Plant Sci. 8, 2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solórzano‐Morales, A. , Barboza, N. , Hernández, E. , Mora‐Umaña, F. , Ramírez, P. and Hammond, R.W. (2011) Newly discovered natural hosts of Tomato chlorosis virus in Costa Rica. Plant Dis. 95, 497. [DOI] [PubMed] [Google Scholar]
- Souza, T.A. , Macedo, M.A. and Inoue‐Nagata, A. (2019) Natural infection of apple‐of‐Peru (Nicandra physaloides) with Tomato chlorosis virus in Brazil. Plant Dis. 103, 593. [Google Scholar]
- Stewart, L.R. , Medina, V. , Tian, T. , Turina, M. , Falk, B.W. and Ng, J.C. (2010) A mutation in the Lettuce infectious yellows virus minor coat protein disrupts whitefly transmission but not in planta systemic movement. J. Virol. 84, 12165–12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suastika, G. , Hartono, S. , Nishigawa, H. and Natsuaki, T. (2011) Yellowing disease outbreaks in tomato in Indonesia associated with infection of Tomato chlorosis virus and Tomato infectious chlorosis virus . J. ISSAAS. 17, 233. [Google Scholar]
- Syller, J. (2012) Facilitative and antagonistic interactions between plant viruses in mixed infections. Mol. Plant Pathol. 13, 204–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, X. , Shi, X. , Zhang, D. , Li, F. , Yan, F. , Zhang, Y. , Liu, Y. and Zhou, X. (2017) Detection and epidemic dynamic of ToCV and CCYV with Bemisia tabaci and weed in Hainan of China. Virology J. 14, 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, T. , Rubio, L. , Yeh, H.H. , Crawford, B. and Falk, B.W. (1999) Lettuce infectious yellows virus: in vitro acquisition analysis using partially purified virions and the whitefly Bemisia tabaci . J. Gen. Virol. 80, 1111–1117. [DOI] [PubMed] [Google Scholar]
- Trenado, H.P. , Fortes, I.M. , Louro, D. and Navas‐Castillo, J. (2007) Physalis ixocarpa and P. peruviana, new natural hosts of Tomato chlorosis virus . Eur. J. Plant Pathol. 118, 193–196. [Google Scholar]
- Tsai, W.S. , Shih, S.L. , Green, S.K. , Hanson, P. and Liu, H.Y. (2004) First report of the occurrence of Tomato chlorosis virus and Tomato infectious chlorosis virus in Taiwan. Plant Dis. 88, 311. [DOI] [PubMed] [Google Scholar]
- Tzanetakis, I.E. and Martin, R.R. (2004) Complete nucleotide sequence of a strawberry isolate of Beet pseudoyellows virus . Virus Genes, 28, 239–246. [DOI] [PubMed] [Google Scholar]
- Tzanetakis, I.E. , Reed, J. and Martin, R.R. (2005) Nucleotide sequence, genome organization and phylogenetic analysis of Strawberry pallidosis associated virus, a new member of the genus Crinivirus . Arch. Virol. 150, 273–286. [DOI] [PubMed] [Google Scholar]
- Tzanetakis, I.E. , Martin, R.R. and Wintermantel, W.M. (2013) Epidemiology of criniviruses: an emerging problem in world agriculture. Front. Microbiol. 119, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas, J.A. , Hernández, E. , Barboza, N. , Mora, F. and Ramírez, P. (2011) First report of Tomato chlorosis virus infecting sweet pepper in Costa Rica. Plant Dis. 95, 1482. [DOI] [PubMed] [Google Scholar]
- Velasco, L. , Simón, B. , Janssen, D. and Cenis, J.L. (2008) Incidences and progression of tomato chlorosis virus disease and tomato yellow leaf curl virus disease in tomato under different greenhouse covers in southeast Spain. Ann. Appl. Biol. 153, 335–344. [Google Scholar]
- Wang, X.Y. , Feng, J. , Zang, L.Y. , Yan, Y.L. , Yang, Y.Y. and Zhu, X.P. (2018a) Natural occurrence of Tomato chlorosis virus in cowpea (Vigna unguiculata) in China. Plant Dis. 102, 254. [Google Scholar]
- Wang, F. , Liu, J. , Dong, Y. , Chen, P. , Zhu, X. , Liu, Y. and Ma, J. (2018b) Insect‐proof netting technique: Effective control of Bemisia tabaci and Tomato chlorosis virus (ToCV) in protected cultivations in China. Chil. J. Agric. Res. 78, 48–58. [Google Scholar]
- Watanabe, L.F.M. , Bello, V.H. , De Marchi, B.R. , Sartori, M.M.P. , Pavan, M.A. and Krause‐Sakate, R. (2018) Performance of Bemisia tabaci MEAM1 and Trialeurodes vaporariorum on Tomato chlorosis virus (ToCV) infected plants. J. App. Entomol. 142, 1008–1015. [Google Scholar]
- Wintermantel, W.M. and Wisler, G.C. (2006) Vector specificity, host range, and genetic diversity of Tomato chlorosis virus . Plant Dis. 90, 814–819. [DOI] [PubMed] [Google Scholar]
- Wintermantel, W.M. , Polston, J.E. , Escudero, J. and Paoli, E.R. (2001) First report of Tomato chlorosis virus in Puerto Rico. Plant Dis. 85, 228. [DOI] [PubMed] [Google Scholar]
- Wintermantel, W.M. , Wisler, G.C. , Anchieta, A.G. , Liu, H.‐Y. , Karasev, A.V. and Tzanetakis, I.E. (2005) The complete nucleotide sequence and genome organization of tomato chlorosis virus. Arch. Virol. 150, 2287–2298. [DOI] [PubMed] [Google Scholar]
- Wisler, G.C. , Duffus, J.E. , Liu, H.‐Y. and Li, R.H. (1998a) Ecology and epidemiology of whitefly‐transmitted closteroviruses. Plant Dis. 82, 270–280. [DOI] [PubMed] [Google Scholar]
- Wisler, G.C. , Li, R.H. , Liu, H.‐Y. , Lowry, D.S. and Duffus, J.E. (1998b) Tomato chlorosis virus: A new whitefly‐transmitted, phloem limited, bipartite closterovirus of tomato. Phytopathology, 88, 402–409. [DOI] [PubMed] [Google Scholar]
- Zhao, R.N. , Wang, R. , Wang, N. , Fan, Z.F. , Zhou, T. , Shi, Y.C. and Chai, M. (2013) First report of Tomato chlorosis virus in China. Plant Dis. 97, 1123. [DOI] [PubMed] [Google Scholar]
- Zhao, L.M. , Li, G. , Gao, Y. , Liu, Y.J. , Sun, G.Z. and Zhu, X.P. (2014a) Molecular detection and complete genome sequences of Tomato chlorosis virus isolates from infectious outbreaks in China. J. Phytopathol. 162, 627–634. [Google Scholar]
- Zhao, R. , Wang, N. , Wang, R. , Chen, H. , Shi, Y. , Fan, Z. and Zhou, T. (2014b) Characterization and full genome sequence analysis of a Chinese isolate of tomato chlorosis virus. Acta Virol. 58, 92–94. [DOI] [PubMed] [Google Scholar]
- Zhao, L.M. , Li, G. , Gao, Y. , Zhu, Y.R. , Liu, J. and Zhu, X.P. (2015) Reverse transcription loop‐mediated isothermal amplification assay for detecting tomato chlorosis virus. J. Virol. Methods, 213, 93–97. [DOI] [PubMed] [Google Scholar]
- Zhao, R. , Wang, N. , Liu, S. , Ling, K.S. , Fan, Z. and Zhou, T. (2016) P22 of tomato chlorosis virus, a ribonucleic acid silencing suppressor, is naturally expressed in the infected plant. Acta Virol. 60, 423–425. [DOI] [PubMed] [Google Scholar]
- Zhou, Y. , Yan, J.Y. , Qiao, G.H. , Liu, M. , Zhang, W. and Li, X.H. (2015) First report of Tomato chlorosis virus infecting eggplant (Solanum melongena) in China. Plant Dis. 99, 1657. [Google Scholar]


