Abstract
Myopia is very prevalent worldwide, especially among Asian populations. Orthokeratology is a proven intervention to reduce myopia progression. The current study investigated association between baseline corneal biomechanics and orthokeratology responses, and changes of corneal biomechanics from long-term orthokeratology. We fitted 59 adult subjects having myopia between −4.00D to −5.00D with overnight orthokeratology. Corneal biomechanics was measured through dynamic bidirectional corneal applanation (in terms of corneal hysteresis, CH and corneal resistance factor, CRF) and corneal indentation (in terms of corneal stiffness, S and tangent modulus, E). Subjects with poor orthokeratology responses had lower E (mean 0.474 MPa) than subjects with good orthokeratology responses (mean 0.536 MPa). Successful orthokeratology for 6 months resulted in reducing CH (reduced by 5.8%) and CRF (reduced by 8.7%). Corneal stiffness was stable, but E showed an increasing trend. Among subjects with successful orthokeratology, a higher baseline S resulted in greater myopia reduction (Pearson correlation coefficient, r = 0.381, p = 0.02).
Subject terms: Epidemiology, Outcomes research
Introduction
The increase in myopia has reached epidemic proportions in Asia. Holden et al.1 estimated that by 2050, more than 5.6 billion people worldwide would be affected by myopia. Recent meta-analysis reports have confirmed that orthokeratology is an effective intervention to slow the progression of myopia2–5. In orthokeratology, myopia reduction occurs through alteration of the anterior corneal shape6,7, whereas myopia control has been hypothesized to be due to myopic defocus at the peripheral retina following changes in the anterior corneal shape8–10. The retina is capable of responding to different types of optical defocus to drive eye growth11,12.
Altering the physical dimensions of the cornea may affect corneal biomechanics. After the launch of the Ocular Response Analyzer (ORA; Reichert Inc., USA) over a decade ago to clinically measure corneal biomechanics, numerous studies have confirmed changes in these measurements after orthokeratology13–16. Corneal hysteresis (CH), which is a measure of the viscoelastic properties of the cornea, is derived from the inward and outward intraocular pressures measured using the ORA. The corneal resistance factor (CRF) indicates overall corneal resistance. CH and/or CRF have been reported to be reduced after orthokeratology. However, few studies have investigated the association between orthokeratology responses and baseline corneal biomechanics. For example, Gonzalez-Meijome et al.16 reported that eyes with lower CH had relatively faster responses following orthokeratology, but their study involved lens wearing for only 3 hours.
Corneal indentation is an alternative method for measuring corneal biomechanics17,18. The corneal tangent modulus can be measured at both the central and peripheral corneas19,20 and is a stable parameter throughout the course of a day21. Our group previously studied variation in the tangent modulus after short-term orthokeratology22. Although short-term overnight orthokeratology did not change the tangent modulus, a higher baseline tangent modulus had greater corneal flattening along the flattest meridian. The current study incorporated both the ORA and corneal indentation to investigate the association between baseline corneal biomechanics and long-term orthokeratology responses and changes in corneal biomechanics following long-term orthokeratology. This study had two objectives: to monitor changes in corneal biomechanics from long-term successful orthokeratology and to determine which baseline parameters in corneal biomechanics were significantly associated with successful orthokeratology.
Results
Fifty-nine subjects were eligible and fitted with orthokeratology lenses, of whom 37 completed the 6-month wearing period. These subjects included 7 males and 30 females with ages ranging from 18 to 30 years. Table 1 summarizes the reasons the 22 subjects dropped out of the study. Among these 22 subjects, 14 had poor myopia reduction from orthokeratology with a significant amount of residual refractive errors. Among the remaining 8 subjects, seven withdrew from the study within the first week of orthokeratology. Lens fitting was optimal, and we attempted to modify the lenses. Unfortunately, these 7 subjects did not wait for new lenses to arrive from overseas and discontinued the study. One subject had significant corneal staining at the 3-month follow-up visit. We advised the subject to stop wearing the lenses until complete corneal health recovery, but she decided to discontinue this study. A comparison of the baseline ocular parameters of the 37 subjects (37 eyes) who completed the study and the 14 subjects (14 eyes, randomly selected) who dropped out due to lack of an orthokeratology response or significant residual refractive error revealed that these two groups shared similar CH, CRF, and asphericity along the steepest (Steep-Q) and flattest meridians (Flat-Q) (Table 2). The eyes of the subjects who dropped out had significantly flatter keratometry results along the steepest and flattest meridians; in addition, they had similar corneal stiffness, but the normalized corneal tangent modulus was significantly lower.
Table 1.
Summary of dropout cases.
| Main reason of dropout | Number of cases |
|---|---|
| Non-responsive | 2 |
| Significant residual refractive error | 12 (residual sphere was −1.90D ± 0.76D in 24 eyes) |
| Poor lens centration | 2 |
| Significant corneal staining | 3 |
| Lens discomfort | 1 |
| Personal reason | 2 |
Table 2.
Mean ± standard deviation, or median (interquartile range) of ocular parameters in the completed and dropout groups.
| Parameters | Completed subjects (37 eyes from 37 subjects) | Dropout subjects (14 eyes from 14 subjects) | Comparison between two groups |
|---|---|---|---|
| Steep-K (D) | 44.03 ± 1.74 | 43.15 ± 1.14 | t = −2.118, p = 0.041 |
| Flat-K (D) | 42.91 ± 1.58 | 42.02 ± 1.13 | t = −2.227, p = 0.033 |
| Steep-Q | −0.184 ± 0.148 | −0.115 ± 0.133 | t = 1.602, p = 0.121 |
| Flat-Q | −0.341 ± 0.098 | −0.323 ± 0.106 | t = 0.554, p = 0.585 |
| CH (mmHg) | 10.40 (1.50) | 10.78 (1.49) | p = 0.441* |
| CRF (mmHg) | 9.95 ± 1.30 | 10.31 ± 1.26 | t = 0.919, p = 0.367 |
| S (N/mm) | 0.061 ± 0.007 | 0.058 ± 0.008 | t = −0.947, p = 0.353 |
| EN (MPa) | 0.536 ± 0.118 | 0.474 ± 0.085 | t = −3.073, p = 0.046 |
Significant difference was bold.
Steep-K: keratometry along the steepest meridian; Flat-K: keratometry along the flattest meridian; Steep-Q: asphericity along the steepest meridian; Flat-Q: asphericity along the flattest meridian; CH: corneal hysteresis; CRF: corneal resistance factor; S: corneal stiffness; EN: normalized corneal tangent modulus.
Comparisons using unpaired t-tests, except CH using Mann-Whitney test*.
Table 3 shows variations in different ocular parameters throughout the study period. The refractive sphere declined rapidly within the first month and continued to reduce thereafter, albeit more slowly (RMANOVA: F = 403.4, p < 0.001). Refractive astigmatism was relatively stable initially but increased slightly at the first month and at the end of the study (RMANOVA: F = 3.910, p = 0.002). Compared with that of the baseline, a mean increase in astigmatism of 0.24 ± 0.36D was observed. The spherical equivalent (SEQ, refractive sphere plus half refractive astigmatism) was also significantly reduced throughout the study period (RMANOVA: F = 380.2, p < 0.001). The subjects achieved good and stable uncorrected visual acuity after wearing the lenses during the first week (Friedman: p < 0.001). Both the steepest and flattest corneal curvatures flattened significantly after one night and one week of orthokeratology, respectively. CCT had a small thinning effect and stabilized one week after orthokeratology (Friedman: p < 0.001).
Table 3.
Ocular parameters throughout the study period. Results are expressed in mean ± standard deviation, or median (interquartile range).
| Ocular parameter | Baseline | 1 night | 1 week | 1 month | 3 months | 6 months | Statistics |
|---|---|---|---|---|---|---|---|
| Sphere (D) | −4.44 ± 0.35 | −2.66 ± 0.76* | −0.87 ± 0.67* | −0.32 ± 0.60* | −0.24 ± 0.65* | 0.11 ± 0.57* |
RMANOVA F = 403.4, p < 0.001 |
| Astigmatism (D) | −0.50 ± 0.33 | −0.62 ± 0.38 | −0.55 ± 0.36 | −0.70 ± 0.45* | −0.66 ± 0.36 | −0.74 ± 0.34* |
RMANOVA F = 3.910, p = 0.002 |
| SEQ (D) | −4.69 ± 0.32 | −2.97 ± 0.81* | −1.15 ± 0.76* | −0.67 ± 0.68* | −0.56 ± 0.65* | −0.26 ± 0.64* |
RMANOVA F = 380.2, p < 0.001 |
| BCVA (logMAR) | −0.10 (0.10) | −0.10 (0.10) | −0.10 (0.10) | −0.10 (0.10) | −0.10 (0.10) | −0.08 (0.10) | Friedman, p = 0.110 |
| UCVA (logMAR) | 1.02 (0.16) | 0.70 (0.36) | 0.10 (0.14)* | 0.02 (0.18)* | 0.01 (0.14)* | 0.00 (0.16)* | Friedman, p < 0.001 |
| Steep-K (D) | 44.03 ± 1.74 | 43.40 ± 1.68* | 42.44 ± 1.68* | 42.05 ± 1.67* | 42.08 ± 1.66* | 42.09 ± 1.66* |
RMANOVA F = 140.0, p < 0.001 |
| Flat-K (D) | 42.87 (2.23) | 42.17 (1.99) | 41.48 (2.07)* | 40.95 (2.23)* | 41.14 (2.17)* | 40.92 (1.93)* | Friedman, p < 0.001 |
| CCT (µm) | 545.3 (42.7) | 546.0 (43.3) | 537.7 (43.7)* | 535.0 (49.0)* | 538.3 (47.7)* | 538.0 (44.3)* | Friedman, p < 0.001 |
| CH (mmHg) | 10.53 ± 1.19 | 10.22 ± 1.17* | 10.00 ± 1.26* | 9.99 ± 1.12* | 9.91 ± 1.22* | 9.92 ± 1.25* |
RMANOVA F = 9.320, p < 0.001 |
| CRF (mmHg) | 9.90 (1.80) | 9.67 (1.77) | 9.30 (1.70)* | 9.10 (1.87)* | 8.97 (1.60)* | 8.90 (1.47)* | Friedman, p < 0.001 |
| S (N/mm) | 0.060 (0.008) | 0.060 (0.010) | 0.059 (0.007) | 0.057 (0.006) | 0.059 (0.007) | 0.057 (0.005) | Friedman, p = 0.182 |
| EN (MPa) | 0.536 ± 0.118 | 0.539 ± 0.129 | 0.562 ± 0.093 | 0.578 ± 0.116* | 0.579 ± 0.097* | 0.571 ± 0.106 |
RMANOVA F = 3.263, p = 0.008 |
Significant difference was bold.
RMANOVA: repeated measures analysis of variance.
*post-hoc test (Dunnett’s method) showing significant difference with baseline.
SEQ: spherical equivalent; BCVA: best corrected visual acuity; UCVA: uncorrected visual acuity; Steep-K: keratometry along the steepest meridian; Flat-K: keratometry along the flattest meridian; CCT: central corneal thickness; CH: corneal hysteresis; CRF: corneal resistance factor; S: corneal stiffness; EN: normalized corneal tangent modulus.
Both CH and CRF decreased throughout the study. Compared with those at the baseline, the CRF at 6 months was decreased by 8.7% (−0.87 ± 0.76 mmHg), and CH was decreased by 5.8% (−0.61 ± 0.76 mmHg). Corneal stiffness did not change significantly, but the normalized tangent modulus showed an increasing trend (Table 3), this measure was significantly higher than the baseline after one month and three months of orthokeratology.
Only the baseline corneal stiffness demonstrated a significant correlation with sphere reduction after 6 months of orthokeratology (r = 0.381, p = 0.020). A linear regression model revealed how the myopia reduction was related to the baseline corneal stiffness (Fig. 1). The baseline tangent modulus was positively correlated with myopia reduction, but this correlation did not reach statistical significance (r = 0.311, p = 0.061). Correlations between other ocular parameters (namely CH, CRF, steepest and flattest keratometry, steepest and flattest asphericity) and myopia reduction were even weaker (Pearson’s correlation coefficient or Spearman’s rho ranged between 0.045 and 0.188).
Figure 1.
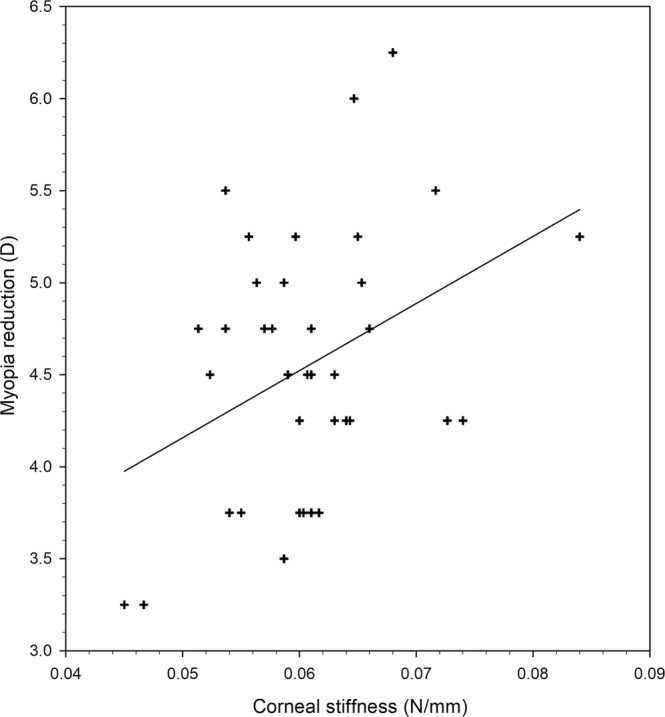
Linear regression analysis between myopia reduction at the 6th month of orthokeratology and baseline corneal stiffness. Pearson correlation coefficient (r) is 0.381, p = 0.02. Regression equation is y = 36.466x + 2.334.
We calculated the post hoc power using G-power version 3.1.9.2 (Franz Faul, Universität Kiel, Germany). Based on the 5.8% decrease in CH after 6 months of orthokeratology, the current study had over 99% power to detect changes in corneal biomechanics at an alpha value of 5%.
Discussion
Orthokeratology is an effective technique to temporarily reduce myopia23 and even reduce myopia progression24–26. This technique alters the corneal dimensions by flattening the anterior curvature6,27, reducing the central thickness, and increasing the mid-peripheral thickness28. After orthokeratology, the corneal epithelium is reduced29,30, but the corneal stroma thickens31,32. These changes in the morphological and histological properties of the cornea may alter its biomechanical properties33. The current study applied both a dynamic bidirectional corneal applanation technology (i.e. the ORA) and corneal indentation to monitor changes in corneal biomechanics through the 6-month orthokeratology period. The corneal stiffness was stable, whereas the tangent modulus exhibited a significant increasing trend (Table 3). Previous studies that have used the ORA have reported changes in CH or the CRF14–16,34.
Following initiation of orthokeratology, our subjects showed a significant reduction in the spherical component during the first month, and good uncorrected visual acuity was achieved in the first week (Table 3). These findings are consistent with those of previous studies that have used the same type of lens35. By contrast, the astigmatic component increased slightly. Orthokeratology usually cannot provide a significant reduction in refractive astigmatism. Even for studies using toric orthokeratology lenses, the purpose was mainly to improve lens centration rather than reducing astigmatism totally36–38.
We previously reported a reduction in the CRF with a relatively stable CH after one session of overnight orthokeratology15,22. Orthokeratology for longer periods exerts different effects on CH and the CRF. Chen et al.33 reported that both CH and the CRF declined significantly after one week of orthokeratology and that the reduction in the CRF was greater. This result was affirmed by Yeh et al.13, who monitored CH and the CRF for one month. The reduction in the CRF was greater (7.4%) than that in CH (2.8%). Moreover, Nieto-Bona et al.14 reported a similar reduction in CH and the CRF (6%) after one month of orthokeratology. Fewer studies have monitored CH and CRF throughout the course of long-term orthokeratology. One study in China found a significant initial drop in both CH and the CRF, but these parameters returned to the pretreatment levels after 3 months of orthokeratology34. The current study found significant reductions in both CH and the CRF throughout the 6-month orthokeratology period, with a greater reduction in the CRF. As shown in Table 3, the CH reduction plateaued after one month of orthokeratology whereas the CRF exhibited a sustained decreasing trend. Since the corneal stroma constitutes 90% of the entire corneal thickness, CH and the CRF are mainly associated with the corneal stromal thickness39. Supporting this hypothesis, Chen et al.33 reported significant associations of changes in CH and the CRF with changes in the stromal thickness.
Studying corneal biomechanics using the ORA has certain drawbacks because of the wide inter-subject variation in CH and the CRF13. Corneal indentation is an alternative corneal biomechanics measurement method in vivo19. Corneal stiffness is defined as the force required to indent a cornea to a particular depth (1 mm in our device). This biomechanical property depends on the physical properties of the cornea, including its thickness and radius. Tangent modulus is an intrinsic mechanical property of a tissue. Hon et al.40 used both the ORA and corneal indentation to compare the corneal biomechanics of low and high myopes. Although high myopes exhibited lower mean CH than low myopes, as documented in the literature, the two groups demonstrated nearly the same CH spread due to wide inter-subject variation. Furthermore, high myopes had not only a lower mean tangent modulus but also a narrow spread of the tangent modulus.
One novel finding of the current study is that long-term orthokeratology increases the tangent modulus while the corneal stiffness remains stable. This result implies that even though the geometric properties (i.e. thickness and radius) of the cornea change, some physical properties (e.g. stiffness) do not. However, orthokeratology altered the intrinsic material properties of the cornea. A thorough evaluation of the sub-corneal layers is required, because these layers have different elastic properties41,42. For example, Zhong et al.43 found that the basal cell and stromal keratocyte densities declined after 5 years of orthokeratology. Nieto-Bona et al.44 obtained similar findings for the basal cell and anterior stromal keratocyte densities after 6 and 12 months of orthokeratology. In addition, they reported thinning of Bowman’s membrane during the same period and speculated that imaging artefacts due to epithelial compression might affect this measurement. Among the anterior sub-corneal layers, Bowman’s membrane has the highest elastic modulus, followed by the stroma among the other anterior sub-corneal layers41,42. Confocal microscopy can be incorporated to measure Bowman’s membrane and the stromal thickness in future studies. Owens et al.45 compared rabbit eyes after two weeks of overnight orthokeratology with untreated eyes and did not find a significant difference in the stromal collagen fibril diameter. In vivo measurement of corneal collagen fibres may further enrich our understanding of corneal biomechanics46,47.
One objective of our study was to evaluate whether baseline corneal biomechanics had significant associations with the orthokeratology success (i.e. myopia reduction). Myopia reduction was used as an indicator of the orthokeratology success because it was more than flattening of the corneal curvatures48–50. Furthermore, myopes seek orthokeratology for myopia reduction. In the 59 subjects fitted with orthokeratology lenses, 14 did not have a good orthokeratology response and thus dropped out of this study. When we compared the ocular parameters between the subjects who completed the 6-month orthokeratology (37 eyes of 37 subjects) and those who dropped out (14 eyes of 14 subjects), the dropout group had a flatter baseline corneal curvature and a significantly lower tangent modulus (Table 2). In myopic orthokeratology, the anterior corneal curvature is flattened6,27; thus, myopia reduction will be limited if the baseline corneal curvature is flat and spherical51,52. The dropout group had a flatter corneal curvature, but their corneal asphericity was similar to that of the completed group. The dropout could also be related to the lenses (Menicon Z Night contact lenses) used in the current study. Santodomingo-Rubido et al.50 used the same lens type and reported that only 68% of children could achieve an optimum lens fit from the first contact lens. Moreover, 32% of their subjects required a total of 35 adjustments to obtain an optimum lens fit. Chan et al.35 reported that myopia reduction only reached 57% and 81% after wearing these lenses for one night and one week, respectively. The prolonged trial period due to many lens modifications discouraged our adult subjects from continuing the study. Our lenses had fenestrations for better corneal health that could further reduce the rate of myopia reduction53. Among the measured corneal biomechanics, only the tangent modulus differed between the two groups; the baseline tangent modulus of the group that completed the study was 0.536 MPa, which was comparable with that of healthy subjects40, Hon et al.40 reported a mean tangent modulus of 0.57 MPa in low myopes (mean SER of −1.37D) and 0.47 MPa in high myopes (mean SER of −9.07D). The mean tangent modulus of our dropout group (0.474 MPa) was low in myopes with myopia between −4.00D and −5.00D. Therefore, incorporating an evaluation of corneal biomechanics may help screen good orthokeratology responders. However, orthokeratology practitioners should refer to both corneal curvatures and biomechanics (Table 2). Vinciguerra et al.54 suggested integrating biomechanical evaluation with tomographic and topographic analyses for diagnosis of subclinical keratoconus.
We performed a correlation analysis for the group who completed the study, but except for corneal stiffness, no other baseline corneal parameter demonstrated a significant association with myopia reduction. Gonzalez-Meijome et al.16 was the first study to investigate whether orthokeratology responses were correlated with corneal biomechanics. They proposed that corneas with a low CH or CRF might respond faster to myopic orthokeratology. They used corneal curvatures as the response of interest, whereas we used myopia reduction in this study, because orthokeratology resulted in more myopia reduction than flattening of the corneal curvature48–50. The difference in the results could be due to different wearing conditions: our subjects wore the lenses during sleep, whereas their subjects wore the lenses for only 3 hours and under an open-eye condition16. Furthermore, we set stringent inclusion criteria related to age and the myopia range to account for confounding factors that could affect corneal biomechanics. We concluded that neither the baseline CH nor CRF had a significant association with myopia reduction during long-term orthokeratology.
In the current study, corneal stiffness was defined as the force required to induce unit corneal displacement. This measure is influenced by the corneal dimensions, including its curvature and thickness. Corneas with a higher baseline stiffness appear to lead to greater myopia reduction (Fig. 1). However, we should interpret this result with caution. Our data points were very scattered, and higher corneal stiffness only accounted for 14.5% (r = 0.381) of the cases with greater myopia reduction. Therefore, other factors should contribute to the higher myopia reduction in these eyes. Neither central corneal curvatures nor asphericity had significant correlations with myopia reduction. Consistent with our observation, Chan et al.55 previously reported that corneal asphericity could not predict myopia reduction after overnight orthokeratology.
Other factors could affect the performance of overnight orthokeratology. Younger patients, such as children, were more likely to have adverse effects from overnight lens wear56. The incorporation of fenestrations in our orthokeratology lenses could reduce lens binding53. Additionally, the experience of the practitioner is crucial in orthokeratology57. Our frequent aftercare visit schedule, which involved an experienced orthokeratology practitioner, and conduct of the study at a university clinic ensured a high standard of performance58.
The current study monitored corneal biomechanics in long-term orthokeratology using two technologies: dynamic bidirectional corneal applanation and corneal indentation. The strength of this study lies in its stringent inclusion criteria regarding baseline myopia and age, because corneal biomechanics differ with age and refractive groups. However, our study has several limitations. Corneal biomechanics is age-dependent59–62. Our subjects were all adults, although orthokeratology usually is offered to children for myopia control. Answering the question of whether our findings apply to children requires further research. Corneal responses could vary based on the orthokeratology lens design63. Further studies can include confocal microscopy to evaluate histological changes. We concede that no single parameter can adequately describe all corneal biomechanical properties. CH represents the viscoelastic property of the cornea62; thus, evaluating corneal deformation and deflection through dynamic Scheimpflug imaging is another promising technology to study corneal biomechanics in vivo64,65.
To conclude, intrinsic corneal tissue properties together with corneal curvature contribute to successful overnight orthokeratology. A cornea with a flat corneal curvature together with an unusually low tangent modulus may not respond well to myopic orthokeratology. In successful overnight orthokeratology, the baseline corneal stiffness has a weak but significant association with myopia reduction.
Data collection
Subjects
In total, 275 subjects were screened for eligibility to participate in this study. The inclusion criteria were an age between 18 and 30 years, myopia between −4.00D and −5.00D in sphere power, and with-the-rule astigmatism within 1.50D. In addition, differences in refractive errors for both the sphere and astigmatic components, of the left and right eyes were required to be within 1.00D. These stringent criteria eliminated confounding factors in corneal biomechanics, such as age61,66 and myopia39,40. The exclusion criteria were long-term contact lens use or a history of ocular diseases. In addition, those with a best-corrected visual acuity of less than 0.10 logMAR in each eye measured using the Early Treatment Diabetic Retinopathy Study chart (Prevision Vision, La Salle, IL) under normal room lighting conditions were excluded. All procedures were performed in accordance with the ethical standards of the institution and the 1964 Declaration of Helsinki. Ethics clearance was obtained from the institutional review board of The Hong Kong Polytechnic University. Informed consent was obtained from all participants included in the study. This study was registered at ClinicalTrials.gov (NCT02719535, registered March 25, 2016) and in the University of Hong Kong HKU Clinical Trials Register (HKUCTR-1957, registered Feb 1, 2016).
Methods
The following baseline data were collected: non-cycloplegic manifest refraction, ocular biometry through partial coherence interferometry (Zeiss IOLMaster; Zeiss Humphrey, Dublin, CA), corneal topography (Medmont E300, Medmont Pty Ltd., Vermont, VIC, Australia), corneal thickness through swept-source optical coherence tomography (Casia SS-1000, Tomey, Nagoya, Japan), and corneal biomechanics using an ORA. CH, CRF, and corneal-compensated intraocular pressure (IOPcc) were measured using the ORA. Three acquisitions were obtained, each with a waveform score of at least 6.067. The CRF is a measure of the overall resistance of the cornea. The IOPcc is a measure of intraocular pressure (IOP) and is less affected by corneal parameters, such as the central corneal thickness (CCT)68. After all non-contact procedures were completed, the cornea was anaesthetized using one drop of 0.4% benoxinate. The IOP was measured through Goldmann applanation tonometry, followed by corneal indentation. The corneal stiffness and tangent modulus measurements were described in detail in the literature19. Briefly, first, the indenter is brought into contact with the central cornea. Then, the preload is stabilized (as confirmed by an audible sound), and the indenter is moved forwards and backwards at 12 mm/s to indent the cornea by 1 mm. Each indentation is completed in less than 0.25 seconds. Finally, the corneal stiffness is read from the indentation device. In this study, the average stiffness from three measurements was used to calculate the tangent modulus using the central corneal radius and CCT. Because the corneal tangent modulus varies with the IOP17, the tangent modulus was normalized to the mean IOP in normal eyes (15.5 mmHg) using the IOPcc40.
Lenses used
Menicon Z Night contact lenses (NKL Contactlenzen, Netherlands) made of super-high gas permeable material (Dk: 163 × 10−11) were used. The back optic zone radius of the lenses ranged from 7.20 to 9.50 mm in 0.05 mm steps. The lens diameters were either 10.20 mm or 10.60 mm. Three fenestrations were located in the reverse curve 120°apart to enhance tear exchange. The lenses were fitted according to the manufacturer’s instructions using a computer programme (Easy Fit Software, Menicon Co Ltd., Nagoya, Japan). This computer-assisted lens fitting method has a very high first-fit success rate for myopic orthokeratology in low to moderate myopes35. Lens fitting requires ocular information, such as corneal topography and non-cycloplegic manifest refraction. The required lenses were ordered, and a trial fitting was arranged.
Wearing schedule
After a successful trial fitting, a delivery visit was arranged. Subjects were asked to return to the University Optometry Clinic for regular follow-up per the following schedule: after the first overnight wear and after 1 week, 1 month, 3 months, and 6 months of lens wear. Each visit was completed within two hours of waking in the morning and removing the lenses. Lens parameters were modified when necessary, especially in case of poor lens centration or significant residual refractive error.
At each follow-up visit, the corneal topography, thickness, and biomechanics were measured using both the ORA and corneal indentation in addition to the subjective refraction and ocular biometry. Both the habitual uncorrected visual acuity (UCVA) and best-corrected visual acuity (BCVA) were measured.
Statistical analysis
Data of one eye of each subject who completed the 6-month orthokeratology were analyzed. Only one eye per subject was included to avoid inter-eye correlation that could influence the analysis results. We compared the refractive sphere, refractive astigmatism, and BCVA between the two eyes at the 6-month visit. No significant difference was found between the two eyes (refractive sphere, p = 0.657; refractive astigmatism, p = 0.364; BCVA, p = 0.390). Since we used myopia reduction rather than corneal flattening to represent orthokeratology success48–50, the eye with the residual sphere closer to plano at the 6-month visit was selected. If the residual sphere was the same in both eyes, then the eye with less residual astigmatism was selected. For subjects with the same residual refractive errors in both eyes, the right eye was selected. Some previous studies also selected a “better” eye rather than using a random order to select an eye for data analysis69–71. Normality was checked using the Shapiro-Wilks test. Repeated-measure analysis of variance (RMANOVA) or the Friedman test was used to compare changes in ocular parameters throughout the study (i.e. from baseline to the end of the 6-month orthokeratology period). When a significant difference was found, a post hoc test (Dunnett’s method) was used to compare results from the different follow-up visits with the baseline data. Baseline ocular parameters that were significantly correlated (Pearson or Spearman) with myopia reduction were identified. A linear regression model was applied when a significant correlation was found. Reduction of the sphere at the 6-month visit was used as the dependent outcome response, and the various baseline ocular parameters were treated as independent predictors. All data analyses and graphical presentations were completed using SigmaPlot 13 (Systat Software, Inc.).
Acknowledgements
This study is supported by a General Research Fund (RGC 15101415/PolyU B-Q46G) of the Research Grants Council of the Hong Kong SAR Government. We would also like to thank the support from Abbott Medical Optics Asia Ltd, and Menicon Co. Ltd. on contact lens solutions.
Author Contributions
A.K.C. Lam and Y. Hon designed the clinical study. Y. Hon conducted data collection. A.K.C. Lam analyzed data. S.Y.Y. Leung, S.H. Lu, J. Chong and D.C.C. Lam designed the corneal indentation device and provided technical support. A.K.C. Lam wrote the manuscript. A.K.C. Lam and D.C.C. Lam reviewed the manuscript.
Competing Interests
The corneal indentation device is patented by The Hong Kong University of Science and Technology. The authors declare no other competing financial and non-financial interests in the study.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Holden BA, et al. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology. 2016;123:1036–1042. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Si JK, et al. Orthokeratology for myopia control: a meta-analysis. Optometry and vision science: official publication of the American Academy of Optometry. 2015;92:252–257. doi: 10.1097/OPX.0000000000000505. [DOI] [PubMed] [Google Scholar]
- 3.Sun Y, et al. Orthokeratology to control myopia progression: a meta-analysis. PloS one. 2015;10:e0124535. doi: 10.1371/journal.pone.0124535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li SM, et al. Efficacy, Safety and Acceptability of Orthokeratology on Slowing Axial Elongation in Myopic Children by Meta-Analysis. Current eye research. 2016;41:600–608. doi: 10.3109/02713683.2015.1050743. [DOI] [PubMed] [Google Scholar]
- 5.Cooper J, Tkatchenko AV. A Review of Current Concepts of the Etiology and Treatment of Myopia. Eye & contact lens. 2018;44:231–247. doi: 10.1097/ICL.0000000000000499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen D, Lam AK, Cho P. Posterior corneal curvature change and recovery after 6 months of overnight orthokeratology treatment. Ophthalmic & physiological optics: the journal of the British College of Ophthalmic Opticians (Optometrists) 2010;30:274–280. doi: 10.1111/j.1475-1313.2010.00710.x. [DOI] [PubMed] [Google Scholar]
- 7.Yoon JH, Swarbrick HA. Posterior corneal shape changes in myopic overnight orthokeratology. Optometry and vision science: official publication of the American Academy of Optometry. 2013;90:196–204. doi: 10.1097/OPX.0b013e31828121eb. [DOI] [PubMed] [Google Scholar]
- 8.Queiros A, Gonzalez-Meijome JM, Jorge J, Villa-Collar C, Gutierrez AR. Peripheral refraction in myopic patients after orthokeratology. Optometry and vision science: official publication of the American Academy of Optometry. 2010;87:323–329. doi: 10.1097/OPX.0b013e3181d951f7. [DOI] [PubMed] [Google Scholar]
- 9.Kang P, Swarbrick H. Peripheral refraction in myopic children wearing orthokeratology and gas-permeable lenses. Optometry and vision science: official publication of the American Academy of Optometry. 2011;88:476–482. doi: 10.1097/OPX.0b013e31820f16fb. [DOI] [PubMed] [Google Scholar]
- 10.Gardner DJ, Walline JJ, Mutti DO. Choroidal Thickness and Peripheral Myopic Defocus during Orthokeratology. Optometry and vision science: official publication of the American Academy of Optometry. 2015;92:579–588. doi: 10.1097/OPX.0000000000000573. [DOI] [PubMed] [Google Scholar]
- 11.Tse DY, To CH. Graded competing regional myopic and hyperopic defocus produce summated emmetropization set points in chick. Investigative ophthalmology & visual science. 2011;52:8056–8062. doi: 10.1167/iovs.10-5207. [DOI] [PubMed] [Google Scholar]
- 12.McFadden SA, et al. Integration of defocus by dual power Fresnel lenses inhibits myopia in the mammalian eye. Investigative ophthalmology & visual science. 2014;55:908–917. doi: 10.1167/iovs.13-11724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeh TN, et al. Short-term effects of overnight orthokeratology on corneal epithelial permeability and biomechanical properties. Investigative ophthalmology & visual science. 2013;54:3902–3911. doi: 10.1167/iovs.13-11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.González-Mesa A, Nieto-Bona A, Villa-Collar C, Lorente-Velazquez A. Biomechanical properties in corneal refractive therapy during adaptation period and after treatment interruption. J Optom. 2012;5:164–170. doi: 10.1016/j.optom.2012.06.006. [DOI] [Google Scholar]
- 15.Chen D, Lam AK, Cho P. A pilot study on the corneal biomechanical changes in short-term orthokeratology. Ophthalmic & physiological optics: the journal of the British College of Ophthalmic Opticians (Optometrists) 2009;29:464–471. doi: 10.1111/j.1475-1313.2008.00625.x. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Meijome JM, Villa-Collar C, Queiros A, Jorge J, Parafita MA. Pilot study on the influence of corneal biomechanical properties over the short term in response to corneal refractive therapy for myopia. Cornea. 2008;27:421–426. doi: 10.1097/ICO.0b013e318164e49d. [DOI] [PubMed] [Google Scholar]
- 17.Ko MW, Leung LK, Lam DC, Leung CK. Characterization of corneal tangent modulus in vivo. Acta ophthalmologica. 2013;91:e263–269. doi: 10.1111/aos.12066. [DOI] [PubMed] [Google Scholar]
- 18.Ko MW, Leung LK, Lam DC. Comparative study of corneal tangent elastic modulus measurement using corneal indentation device. Medical engineering & physics. 2014;36:1115–1121. doi: 10.1016/j.medengphy.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Lam AK, Hon Y, Leung LK, Lam DC. Repeatability of a novel corneal indentation device for corneal biomechanical measurement. Ophthalmic & physiological optics: the journal of the British College of Ophthalmic Opticians (Optometrists) 2015;35:455–461. doi: 10.1111/opo.12219. [DOI] [PubMed] [Google Scholar]
- 20.Hon Y, Chen GZ, Lu SH, Lam DC, Lam AK. In vivo measurement of regional corneal tangent modulus. Scientific reports. 2017;7:14974. doi: 10.1038/s41598-017-14750-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hon Y, et al. Diurnal Variation of Corneal Tangent Modulus in Normal Chinese. Cornea. 2016;35:1600–1604. doi: 10.1097/ICO.0000000000000968. [DOI] [PubMed] [Google Scholar]
- 22.Lam AK, et al. Influence of Short-Term Orthokeratology to Corneal Tangent Modulus: A Randomized Study. Current eye research. 2018;43:474–481. doi: 10.1080/02713683.2017.1418895. [DOI] [PubMed] [Google Scholar]
- 23.Sorbara L, Fonn D, Simpson T, Lu F, Kort R. Reduction of myopia from corneal refractive therapy. Optometry and vision science: official publication of the American Academy of Optometry. 2005;82:512–518. doi: 10.1097/01.opx.0000166772.68413.0e. [DOI] [PubMed] [Google Scholar]
- 24.Walline JJ, Jones LA, Sinnott LT. Corneal reshaping and myopia progression. The British journal of ophthalmology. 2009;93:1181–1185. doi: 10.1136/bjo.2008.151365. [DOI] [PubMed] [Google Scholar]
- 25.Cho P, Cheung SW. Retardation of myopia in Orthokeratology (ROMIO) study: a 2-year randomized clinical trial. Investigative ophthalmology & visual science. 2012;53:7077–7085. doi: 10.1167/iovs.12-10565. [DOI] [PubMed] [Google Scholar]
- 26.Hiraoka T, Kakita T, Okamoto F, Takahashi H, Oshika T. Long-term effect of overnight orthokeratology on axial length elongation in childhood myopia: a 5-year follow-up study. Investigative ophthalmology & visual science. 2012;53:3913–3919. doi: 10.1167/iovs.11-8453. [DOI] [PubMed] [Google Scholar]
- 27.Tsukiyama J, Miyamoto Y, Higaki S, Fukuda M, Shimomura Y. Changes in the anterior and posterior radii of the corneal curvature and anterior chamber depth by orthokeratology. Eye & contact lens. 2008;34:17–20. doi: 10.1097/ICL.0b013e3180515299. [DOI] [PubMed] [Google Scholar]
- 28.Alharbi A, Swarbrick HA. The effects of overnight orthokeratology lens wear on corneal thickness. Investigative ophthalmology & visual science. 2003;44:2518–2523. doi: 10.1167/iovs.02-0680. [DOI] [PubMed] [Google Scholar]
- 29.Choo, J., Caroline, P. & Harlin, D. How does the cornea change under corneal reshaping contact lenses? Eye & contact lens30, 211–213, discussion 218 (2004). [DOI] [PubMed]
- 30.Wang J, et al. Topographical thickness of the epithelium and total cornea after overnight wear of reverse-geometry rigid contact lenses for myopia reduction. Investigative ophthalmology & visual science. 2003;44:4742–4746. doi: 10.1167/iovs.03-0239. [DOI] [PubMed] [Google Scholar]
- 31.Haque S, Fonn D, Simpson T, Jones L. Corneal refractive therapy with different lens materials, part 1: corneal, stromal, and epithelial thickness changes. Optometry and vision science: official publication of the American Academy of Optometry. 2007;84:343–348. doi: 10.1097/OPX.0b013e318042af1d. [DOI] [PubMed] [Google Scholar]
- 32.Reinstein DZ, Gobbe M, Archer TJ, Couch D, Bloom B. Epithelial, stromal, and corneal pachymetry changes during orthokeratology. Optometry and vision science: official publication of the American Academy of Optometry. 2009;86:E1006–1014. doi: 10.1097/OPX.0b013e3181b18219. [DOI] [PubMed] [Google Scholar]
- 33.Chen R, et al. The relationship between corneal biomechanics and anterior segment parameters in the early stage of orthokeratology: A pilot study. Medicine. 2017;96:e6907. doi: 10.1097/MD.0000000000006907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mao XJ, Huang CC, Chen L, Lu F. A study on the effect of the corneal biomechanical properties undergoing overnight orthokeratology. Zhonghua yan ke za zhi Chinese journal of ophthalmology. 2010;46:209–213. [PubMed] [Google Scholar]
- 35.Chan KY, Cheung SW, Cho P. Clinical performance of an orthokeratology lens fitted with the aid of a computer software in Chinese children. Contact lens & anterior eye: the journal of the British Contact Lens Association. 2012;35:180–184. doi: 10.1016/j.clae.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Chen CC, Cheung SW, Cho P. Toric orthokeratology for highly astigmatic children. Optometry and vision science: official publication of the American Academy of Optometry. 2012;89:849–855. doi: 10.1097/OPX.0b013e318257c20f. [DOI] [PubMed] [Google Scholar]
- 37.Lyu B, Hwang KY, Kim SY, Kim SY, Na KS. Effectiveness of Toric Orthokeratology in the Treatment of Patients with Combined Myopia and Astigmatism. Korean journal of ophthalmology: KJO. 2016;30:434–442. doi: 10.3341/kjo.2016.30.6.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen C, Cheung SW, Cho P. Myopia control using toric orthokeratology (TO-SEE study) Investigative ophthalmology & visual science. 2013;54:6510–6517. doi: 10.1167/iovs.13-12527. [DOI] [PubMed] [Google Scholar]
- 39.Wong YZ, Lam AK. The roles of cornea and axial length in corneal hysteresis among emmetropes and high myopes: a pilot study. Current eye research. 2015;40:282–289. doi: 10.3109/02713683.2014.922193. [DOI] [PubMed] [Google Scholar]
- 40.Hon Y, Chen GZ, Lu SH, Lam DC, Lam AK. High myopes have lower normalised corneal tangent moduli (less ‘stiff’ corneas) than low myopes. Ophthalmic & physiological optics: the journal of the British College of Ophthalmic Opticians (Optometrists) 2017;37:42–50. doi: 10.1111/opo.12335. [DOI] [PubMed] [Google Scholar]
- 41.Last JA, Thomasy SM, Croasdale CR, Russell P, Murphy CJ. Micron (Oxford, England: 1993) 2012. Compliance profile of the human cornea as measured by atomic force microscopy; pp. 1293–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomasy SM, et al. Elastic modulus and collagen organization of the rabbit cornea: epithelium to endothelium. Acta biomaterialia. 2014;10:785–791. doi: 10.1016/j.actbio.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhong X, et al. Differences between overnight and long-term wear of orthokeratology contact lenses in corneal contour, thickness, and cell density. Cornea. 2009;28:271–279. doi: 10.1097/ICO.0b013e318186e620. [DOI] [PubMed] [Google Scholar]
- 44.Nieto-Bona A, Gonzalez-Mesa A, Nieto-Bona MP, Villa-Collar C, Lorente-Velazquez A. Long-term changes in corneal morphology induced by overnight orthokeratology. Current eye research. 2011;36:895–904. doi: 10.3109/02713683.2011.593723. [DOI] [PubMed] [Google Scholar]
- 45.Owens, H., Hayes, S. & Meek, K. M. Stromal response to orthokeratology: an x-ray diffraction study. Acta Ophthalmologica Scandinavica85 (2007).
- 46.Winkler M, et al. Nonlinear optical macroscopic assessment of 3-D corneal collagen organization and axial biomechanics. Investigative ophthalmology & visual science. 2011;52:8818–8827. doi: 10.1167/iovs.11-8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dias JM, Ziebarth NM. Anterior and posterior corneal stroma elasticity assessed using nanoindentation. Experimental eye research. 2013;115:41–46. doi: 10.1016/j.exer.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swarbrick HA, Wong G, O’Leary DJ. Corneal response to orthokeratology. Optometry and vision science: official publication of the American Academy of Optometry. 1998;75:791–799. doi: 10.1097/00006324-199811000-00019. [DOI] [PubMed] [Google Scholar]
- 49.Cho P, Cheung SW, Edwards M. The longitudinal orthokeratology research in children (LORIC) in Hong Kong: a pilot study on refractive changes and myopic control. Current eye research. 2005;30:71–80. doi: 10.1080/02713680590907256. [DOI] [PubMed] [Google Scholar]
- 50.Santodomingo-Rubido J, Villa-Collar C, Gilmartin B, Gutierrez-Ortega R, Sugimoto K. Long-term Efficacy of Orthokeratology Contact Lens Wear in Controlling the Progression of Childhood Myopia. Current eye research. 2017;42:713–720. doi: 10.1080/02713683.2016.1221979. [DOI] [PubMed] [Google Scholar]
- 51.Joe JJ, Marsden HJ, Edrington TB. The relationship between corneal eccentricity and improvement in visual acuity with orthokeratology. Journal of the American Optometric Association. 1996;67:87–97. [PubMed] [Google Scholar]
- 52.Lui WO, Edwards MH. Orthokeratology in low myopia. Part 1: efficacy and predictability. Contact lens & anterior eye: the journal of the British Contact Lens Association. 2000;23:77–89. doi: 10.1016/S1367-0484(00)80016-8. [DOI] [PubMed] [Google Scholar]
- 53.Cho P, Chan B, Cheung SW, Mountford J. Do fenestrations affect the performance of orthokeratology lenses? Optometry and vision science: official publication of the American Academy of Optometry. 2012;89:401–410. doi: 10.1097/OPX.0b013e31824cb743. [DOI] [PubMed] [Google Scholar]
- 54.Vinciguerra R, Ambrosio R, Jr., Roberts CJ, Azzolini C, Vinciguerra P. Biomechanical Characterization of Subclinical Keratoconus Without Topographic or Tomographic Abnormalities. Journal of refractive surgery (Thorofare, N.J.: 1995) 2017;33:399–407. doi: 10.3928/1081597X-20170213-01. [DOI] [PubMed] [Google Scholar]
- 55.Chan B, Cho P, Mountford J. Relationship between corneal topographical changes and subjective myopic reduction in overnight orthokeratology: a retrospective study. Clinical & experimental optometry. 2010;93:237–242. doi: 10.1111/j.1444-0938.2010.00489.x. [DOI] [PubMed] [Google Scholar]
- 56.Li W, Sun X, Wang Z, Zhang Y. A survey of contact lens-related complications in a tertiary hospital in China. Contact lens & anterior eye: the journal of the British Contact Lens Association. 2018;41:201–204. doi: 10.1016/j.clae.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 57.Cho P, Cheung SW, Edwards MH. Practice of orthokeratology by a group of contact lens practitioners in Hong Kong–Part 1. General overview. Clinical & experimental optometry. 2002;85:365–371. doi: 10.1111/j.1444-0938.2002.tb02387.x. [DOI] [PubMed] [Google Scholar]
- 58.Chan B, Cho P, Cheung SW. Orthokeratology practice in children in a university clinic in Hong Kong. Clinical & experimental optometry. 2008;91:453–460. doi: 10.1111/j.1444-0938.2008.00259.x. [DOI] [PubMed] [Google Scholar]
- 59.Elsheikh A, Geraghty B, Rama P, Campanelli M, Meek KM. Characterization of age-related variation in corneal biomechanical properties. Journal of the Royal Society, Interface. 2010;7:1475–1485. doi: 10.1098/rsif.2010.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Knox Cartwright NE, Tyrer JR, Marshall J. Age-related differences in the elasticity of the human cornea. Investigative ophthalmology & visual science. 2011;52:4324–4329. doi: 10.1167/iovs.09-4798. [DOI] [PubMed] [Google Scholar]
- 61.Terai N, Raiskup F, Haustein M, Pillunat LE, Spoerl E. Identification of biomechanical properties of the cornea: the ocular response analyzer. Current eye research. 2012;37:553–562. doi: 10.3109/02713683.2012.669007. [DOI] [PubMed] [Google Scholar]
- 62.Garcia-Porta N, et al. Corneal biomechanical properties in different ocular conditions and new measurement techniques. ISRN ophthalmology. 2014;2014:724546. doi: 10.1155/2014/724546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marcotte-Collard, R., Simard, P. & Michaud, L. Analysis of Two Orthokeratology Lens Designs and Comparison of Their Optical Effects on the Cornea. Eye & contact lens (2018). [DOI] [PubMed]
- 64.Roberts CJ, et al. Introduction of Two Novel Stiffness Parameters and Interpretation of Air Puff-Induced Biomechanical Deformation Parameters With a Dynamic Scheimpflug Analyzer. Journal of refractive surgery (Thorofare, N.J.: 1995) 2017;33:266–273. doi: 10.3928/1081597X-20161221-03. [DOI] [PubMed] [Google Scholar]
- 65.Vinciguerra R, et al. In Vivo Early Corneal Biomechanical Changes After Corneal Cross-linking in Patients With Progressive Keratoconus. Journal of refractive surgery (Thorofare, N.J.: 1995) 2017;33:840–846. doi: 10.3928/1081597X-20170922-02. [DOI] [PubMed] [Google Scholar]
- 66.Elsheikh A, Wang D, Pye D. Determination of the modulus of elasticity of the human cornea. Journal of refractive surgery (Thorofare, N.J.: 1995) 2007;23:808–818. doi: 10.3928/1081-597X-20071001-11. [DOI] [PubMed] [Google Scholar]
- 67.Mandalos A, et al. Inter-examiner reproducibility of Ocular Response Analyzer using the waveform score quality index in healthy subjects. Journal of glaucoma. 2013;22:152–155. doi: 10.1097/IJG.0b013e318227e63e1. [DOI] [PubMed] [Google Scholar]
- 68.Medeiros FA, Weinreb RN. Evaluation of the influence of corneal biomechanical properties on intraocular pressure measurements using the ocular response analyzer. Journal of glaucoma. 2006;15:364–370. doi: 10.1097/01.ijg.0000212268.42606.97. [DOI] [PubMed] [Google Scholar]
- 69.Manjunath V, Taha M, Fujimoto JG, Duker JS. Choroidal thickness in normal eyes measured using Cirrus HD optical coherence tomography. American journal of ophthalmology. 2010;150:325–329.e321. doi: 10.1016/j.ajo.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Branchini L, et al. Reproducibility of choroidal thickness measurements across three spectral domain optical coherence tomography systems. Ophthalmology. 2012;119:119–123. doi: 10.1016/j.ophtha.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miki, A. et al. Dynamic Scheimpflug Ocular Biomechanical Parameters in Healthy and Medically Controlled Glaucoma Eyes. Journal of glaucoma (2019). [DOI] [PubMed]


