Abstract
Observer-driven pattern recognition is the standard for interpretation of medical images. To achieve global parity in interpretation, semi-quantitative scoring systems have been developed based on observer assessments; these are widely used in scoring coronary artery disease, the arthritides and neurological conditions and for indicating the likelihood of malignancy. However, in an era of machine learning and artificial intelligence, it is increasingly desirable that we extract quantitative biomarkers from medical images that inform on disease detection, characterisation, monitoring and assessment of response to treatment. Quantitation has the potential to provide objective decision-support tools in the management pathway of patients. Despite this, the quantitative potential of imaging remains under-exploited because of variability of the measurement, lack of harmonised systems for data acquisition and analysis, and crucially, a paucity of evidence on how such quantitation potentially affects clinical decision-making and patient outcome. This article reviews the current evidence for the use of semi-quantitative and quantitative biomarkers in clinical settings at various stages of the disease pathway including diagnosis, staging and prognosis, as well as predicting and detecting treatment response. It critically appraises current practice and sets out recommendations for using imaging objectively to drive patient management decisions.
Keywords: Imaging biomarkers, Clinical decision making, Quantitation, Standardisation
Key points
Biomarkers derived from medical images inform on disease detection, characterisation and treatment response.
Quantitative imaging biomarkers have potential to provide objective decision-support tools in the management pathway of patients.
Measurement variability needs to be understood and systems for data acquisition and analysis harmonised before using quantitative imaging measurements to drive clinical decisions.
Introduction
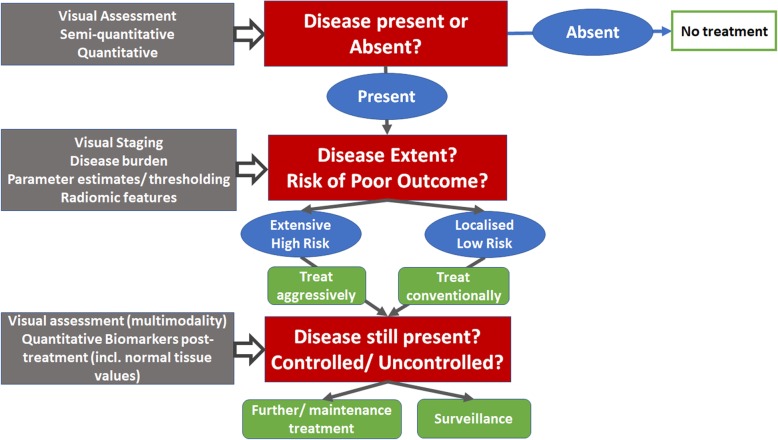
Interpretation of medical images relies on visual assessment. Accumulated and learnt knowledge of anatomical and physiological variations determines recognition of appearances that are within “normal limits” and allows a pathological change in appearances outside these limits to be identified. Observer-driven pattern recognition dominates the way that imaging data are used in routine clinical practice (Fig. 1). A semi-quantitative approach to image analysis has been advocated in various scenarios. These use observer-based categorical scoring systems to classify images according to the presence or absence of certain features. Examples used widely in healthcare for clinical decision-making include reporting and data systems (RADS) [1, 2]. Increasingly, however, advancement in standardisation efforts, applications of analysis techniques to extract quantitative information and machine and deep learning techniques are transforming how medical images may be exploited.
Fig. 1.
Schematic of questions requiring decisions (red boxes), imaging assessments (grey boxes), the results of the imaging assessments (blue ovals) and the management decisions they potentially influence (green boxes)
In some clinical scenarios, automated quantitation may be more objective and accurate than manual assessment; thresholds can be applied above or below which a disease state is recognised and subsequent changes interpreted as clinically relevant [3]. Unlike biomaterials, images potentially can be transferred worldwide easily, cheaply and quickly for biomarker extraction in an automated, reproducible and blinded manner. Nevertheless, despite the substantial advantages of quantitation, very few quantitative imaging biomarkers are used in clinical decision-making due to several obstacles. Harmonisation of data acquisition and analysis is non-trivial. Lack of international standards without routine quality assurance (QA) and quality control (QC) processes results in poorly validated quantitative biomarkers that are subject to errors in interpretation [4–6]. This has profound implications for diagnosis (correct interpretation of the presence of the disease state) [7] and treatment decision-making (based on interpretation of response vs non-response) [8] and reduces the validity of combination biomarkers derived from hybrid (multi-modality) imaging systems. The imaging community needs to engage in delivering high-quality data for quantification and adoption of machine learning to ultimately exploit quantitative imaging information for clinical decision-making [9]. This manuscript describes the current evidence and future recommendations for using semi-quantitative or quantitative imaging biomarkers as decision-support tools in clinical trials and ultimately in routine clinical practice.
Validated imaging biomarkers currently used to support clinical decision-making
The need for absolute quantitation (versus semi-quantitative assessment) in decision-making should be clearly established. Absolute quantitation is demanding and resource intensive because hardware and software differences across centres and instrumentation and their evolution impact the quality of quantified data. Rigorous on-going QA and QC are essential to support the validity and clinically acceptable repeatability of the measurement, and efforts are on-going within RSNA and the ESR and other academic societies. Critically also, definitive thresholds to confidently separate normal from pathological tissues based on absolute quantitative metrics often do not have wide applicability or acceptance.
Semi-quantitative scoring systems
Semi-quantitative readouts of scores based on an observer-recognition process are widely used because visual interpretation often has proven adequate and is linked to outcome. For example, MRI scoring systems for grading hypoxic-ischaemic injury in neonates using a combination of T1-weighted (T1W) imaging, T2-weighted (T2W) imaging and diffusion-weighted imaging (DWI) have shown that higher post-natal grades were associated with poorer neuro-developmental outcome [10]. In cervical spondylosis, grading of high T2-weighted (T2W) signal within the spinal cord has been related variably to disease severity and outcome [11, 12]. In common diseases such as osteoarthritis, where follow-up scans to assess progression are vital in treatment decision-making, such scoring approaches also are useful [13]; web-based knowledge transfer tools using the developed scoring systems indicate good agreement between readers with both radiological and clinical background specialisms in interpreting the T2W MRI data [14]. Similar analyses have been extensively applied in diseases such as multiple sclerosis [15] and even to delineate the rectal wall from adjacent fibrosis [16]. In cancer imaging, 18FDG PET/CT studies use the Deauville scale (liver and mediastinum uptake as reference) as the standard for response assessment in lymphoma [17]. Semi-quantitative scoring systems also form the basis of the breast imaging (BI)-RADS and prostate imaging (PI)-RADS systems in breast and prostate cancer respectively. Their wide adoption has led to spawning of similar classification scores for liver imaging (LI)-RADS [18–20], thyroid imaging (TI)-RADS [20] and bladder (vesicle imaging, VI)-RADS [21] tumours. Multiparametric MRI scores are also used for detection of recurrent gynaecological malignancy [22] and grading of renal cancer [23]. Manual assessment of lung nodule diameter and volume doubling time have reached a wide acceptance in the decision-making of incidental detection, screening [24] and prediction of response [25]. These parameters might be substituted or improved by artificial intelligence in the near future [26].
Quantitative measures of size/volume
The simplest quantitative measure used routinely is size. Size is linked to outcome in both non-malignant and malignant disease [27]. Ventricular size on echocardiography is robust and incorporated into large multicentre trials [28, 29] and into routine clinical care. Left ventricular ejection fraction (LVEF) is routinely extracted from both ultrasound and MRI measurements. In inflammatory diseases such as rheumatoid arthritis, where bone erosions are a central feature, assessment of the volume of disease on high-resolution CT provides a surrogate marker of disease severity [30] and is associated with the degree of physical impairment and mortality [31, 32]. Yet these methods remain to be implemented in a clinical setting because intensive segmentation and post-processing resources are required. In cancer studies, unidimensional measurements (RECIST1.0 and 1.1) [27] are used for response because of the perceived robustness and simplicity of the measurement, although reproducibility is variable [33], resulting in uncertainty [34]. Although numerous studies have linked disease volume to outcome over decades of research [35–38], volume is not routinely documented in clinical reports because of the need for segmentation of irregularly shaped tumours. Volume is indicative of prognosis and response, for example in cervix cancer where evidence is strong [39]. In other cancer types, such as lung, metabolic active tumour volume on PET has a profound link to survival [40, 41]. Metabolic active tumour volume also has proven to be a prognostic factor in several lymphoma studies [42] and is being explored as a biomarker for response to treatment [43–45]. The availability of automated volume segmentation at the point of reporting is essential for routine adoption.
Extractable quantitative imaging biomarkers with potential to support clinical decision-making
Quantitative imaging biomarkers that characterise tissue features (e.g. calcium, fat and iron deposition, cellularity, perfusion, hypoxia, diffusion, necrosis, metabolism, lung airspace density, fibrosis) can provide information that characterises a disease state and reflects histopathology. Multiple quantitative features can be incorporated into algorithms for recognising disease and its change over time (both natural course and in response to therapy). This involves an informatics style approach with data built from atlases derived from validated cases. Curation of anatomical databases annotated according to disease presence, phenotype and grade can then be used with the clinical data to build predictive models that act as decision-support tools. This has been proposed for brain data [46] but requires a collection of good quality validated data sets, carefully archived and curated. Harnessing the quantitative information contained in images with rigorous processes for acquisition and analysis, together with deep-learning algorithms such as has been demonstrated for brain ageing [47] and treatment response [48], will provide a valuable decision-support framework.
Ultrasound
Quantitation in ultrasound imaging has derived parameters related to cardiac output (left ventricular ejection fraction), tissue stiffness (elastography) and vascular perfusion (contrast-enhanced ultrasound) where parameters are related to blood flow. Ultrasound elastography is an emerging field; it has been shown to differentiate liver fibrosis [49], benign and malignant breast and prostate masses and invasive and intraductal breast cancers [50, 51]. It also has been explored for quantifying muscle stiffness in Parkinson’s disease [52], where low interobserver variation and significant differences in Young’s modulus between mildly symptomatic and healthy control limbs make it a useful assessment tool. Furthermore, it has shown acceptable inter-frame coefficient of variation for identifying unstable coronary plaques [53]. Blood flow quantified by power Doppler has potential as a bedside test for intramuscular blood flow in the muscular dystrophies [54]. Quantified parameters peak intensity (PI), mean transit time (MTT) and time to peak (TTP) are available from contrast-enhanced ultrasound, but rarely used because of competing studies with CT and MRI that also capture morphology.
CT
CT biomarkers are dependent on a single biophysical parameter, differential absorption of X-rays due to differences in tissue density, either on unenhanced scans or following administration of iodine-based contrast agent, which increases X-ray absorption in highly perfused tissues. Other developments have utilised tissue density as a parameter in multicentre trials for quantification of emphysema (COPDGene and SPIROMICS) [55–57] and interstitial pulmonary fibrosis (IPF-NET) [58] and for assessment of obstructive (reversible) airways disease [59, 60]. The studies have made use of various open source and bespoke research software tools, but generally, these imaging-based biomarkers have been used to guide treatment [61, 62] and demonstrated direct correlation with outcomes and functional parameters [63]. Drawbacks include poor standardisation of imaging protocols (voltage, slice thickness, respiration, I.V. contrast, kernel size) and post-processing software [64], although many of these issues have been resolved using phantom quality assurance and specified imaging procedures for every CT system used in these studies [65, 66]. Standardisation of instrumentation would simplify comparability between centres and enable long-term data acquisition consistency even after scanner updates [66]. In cardiac imaging, tissue density biomarkers using coronary artery calcium scoring have been extensively applied in large studies evaluating cardiac risk [67] and luminal size on coronary angiography used in outcome studies [68, 69]. Dual-energy CT quantifies iodine concentration directly and is being investigated for characterising pulmonary nodules and pleural tumours [70, 71].
MR including multiparametric data
MRI is more versatile than US and CT because it can be manipulated to derive a number of parameters based on multiple intrinsic properties of tissue (including T1- and T2 relaxation times, proton density, diffusion, water-fat fraction) and how these are altered in the presence of other macromolecules (e.g. proteins giving rising to magnetisation transfer and chemical exchange transfer effects) and externally administered contrast agents (Gadolinium chelates). Perfusion metrics have also been derived with arterial spin labelling, which does not require externally administered agents [72]. The apparent diffusion coefficient (ADC) is the most widely used metric in oncology for disease detection [73, 74], prognosis [75] and response evaluation [76, 77]. Post-processing methods to derive absolute quantitation are extensively debated [78, 79], but the technique is robust with good reproducibility in multicentre, multivendor trials across tumour types [80]. Refinements to model intravascular incoherent motion (IVIM) and diffusion kurtosis are currently research tools. In cardiovascular MRI, there is a growing interest in quantifying T1 relaxation time, rather than just relying on its effect on image contrast; when combined with the use of contrast agents, T1 mapping allows investigation of interstitial remodeling in ischaemic and non-ischaemic heart disease [81]. T1 values are useful to distinguish inflammatory processes in the heart [82], multiple sclerosis in the central nervous system [83], iron and fat content in the liver [84, 85] and adrenal [86], which correlates with fibrosis scores on histology [87]. Multiparametric MRI biomarkers (T1 and proton density fat fraction) achieve a > 90% AUC for differentiating patients with significant liver fibrosis and steatosis on histology [88] and are being supplemented by measurements of tissue stiffness (MR elastography) where a measurement repeatability coefficient of 22% has been demonstrated in a metaanalysis [89]. Chemical exchange saturation transfer (CEST) MRI interrogates endogenous biomolecules with amide, amine and hydroxyl groups; exogenous CEST agents such as glucose provide quantitative imaging biomarkers of metabolism and perfusion. Quantitative CEST imaging shows promise in assessing cerebral ischaemia [90], lymphedema [91], osteoarthritis [92] and metabolism/pH of solid tumours [93]. However, the small signal requires higher field strength acquisition and substantial post-processing.
Positron emission tomography (PET)-SUV metrics
Quantitation of 18FDG PET/CT studies is mainly performed by standardised uptake values (SUVs), although other metrics such as metabolic active tumour volume (MATV) and total lesion glycolysis are being introduced in studies and the clinic [94, 95]. The most frequently used metric to assess the intensity of FDG accumulation in cancer lesions is, however, still the maximum SUV. SUV represents the tumour tracer uptake normalised for injected activity per kilogram body weight. SUV and any of the other PET quantitative metrics are affected by technical (calibration of systems, synchronisation of clocks and accurate assessment of injected 18FDG activity), physical (procedure, methods and settings used for image acquisition, image reconstruction and quantitative image analysis) and physiological factors (FDG kinetics and patient biology/physiology) [96]. To mitigate these factors, guidelines have been developed in order to standardise imaging procedures [96, 97] and to harmonise PET/CT system performance at a European level [97, 98]. Newer targeted PET agents are only assessed qualitatively on their distribution (Table 1).
Table 1.
Imaging biomarkers for disease detection (semi-quantitative and quantitative) with examples of current evidence for their use that would support decision-making
| Disease detection | |||||||
|---|---|---|---|---|---|---|---|
| Biomarker | SemiQ/Q | Disease | Question answered | Utility of biomarker | Data from | Potential decision for | |
| Non-malignant disease |
LVEF-US LVEF-MRI |
Q |
Cardiac function |
Cardiac output Cardiac output |
ICC US 0.72, single centre sensitivity 69% [29] ICC MRI 0.86,correlation of MRI and cineventriculography 0.72 [99] |
Single centre US |
Inotropes Inotropes |
| Renal volume-US, CT, MRI | Q | Renal failure | Mass of parenchyma |
ICC on US 0.64–0.86 [101] Correlation of US with CT 0.76–0.8 [102] Interobserver reproducibility on MRI 87–88% [103] |
Single centre | Renal replacement, safety and toxicity of other pharmaceuticals | |
| Young’s modulus on elastography-US | Q |
Thyroid [104], breast [50] and prostate cancer [51] Parkinson’s disease |
Tumour presence Muscle stiffness |
Thyroid sensitivity 80%, specificity 95% [104] Breast AUC 0.898 for conventional US, 0.932 for shear wave elastography, and 0.982 for combined data [105] Prostate sensitivity 0.84, spec 0.84 [51] |
Thyroid, breast: single centre Prostate meta-analysis |
Treatment with surgery/radiotherapy/chemotherapy | |
| Lung tissue density | Q | Emphysema [106, 107] and fibrosis [58] | Airways obstruction, interstitial lung disease present |
Emphysema (density assessment) influences BODE (body mass index, airflow obstruction, dyspnea and exercise capacity) index. Odds ratio of interstitial lung abnormalities for reduced lung capacity 2.3 |
Multicentre Single centre |
Surgery, valve and drug treatment | |
| Fibrosis and ground-glass index on CT lung | SQ | Idiopathic lung fibrosis | Development of inflammation and fibrosis | Mortality predicted by pulmonary vascular volume (HR 1.23 (1.08–1.40), p = 0.001) and honeycombing (HR 1.18 (1.06–1.32), p = 0.002) [108] | Single centre | Drug treatment | |
| ADC/pCT | SQ | Ischaemic stroke | Presence of salvageable tissue versus infarct core | Measure of infarct core/penumbra used for patient stratification for research [109] | Planned multicentre | Treatment | |
| Malignant disease | Lung RADS, PanCan, NCCN criteria [110, 111] | SQ | Lung nodules | Risk of malignancy | AUC for malignancy 0.81–0.87 [110] | Multicentre | Time period of follow-up or surgery |
| CT blood flow, perfusion, permeability metrics | Q |
Malignant neck lymph nodes Hepatocellular cancer |
Tumour presence |
Sensitivity 0.73, specificity 0.70 [112] AUC 0.75, sensitivity 0.79, specificity 0.75 [113] |
Single centre Single centre |
Staging and management (surgery, radiotherapy or chemotherapy) | |
|
BI-RADS [114] PI-RADS [115] LI-RADS [116] |
SQ | Cancer | Risk of malignancy |
PPV: BI-RADS0 14.1 %, BI-RADS4 39.1 % and BI-RADS5 92.9 % PI-RADS2 pooled sensitivity 0.85, pooled specificity 0.71 Pooled sensitivity for malignancy 0.93 |
Dutch breast cancer screening programme Meta-analysis Systematic review |
Staging and management stratification (surgery, radiotherapy, chemotherapy, combination) | |
| ADC | Q |
Cancer [117] Liver lesions [118] Prostate cancer [119] |
Tumour presence |
Liver AUC 0.82–0.95 Prostate AUC 0.84 |
Single centre Single centre |
Staging and management stratification (surgery, radiotherapy, chemotherapy, combination) | |
| Dynamic contrast enhanced metrics (Ktrans, Kep, blood flow, Ve) | Q |
Liver tumour Recurrent glioblastoma |
Hepatocellular cancer AUC 0.85, sensitivity 0.85, specificity 0.81 [113] Brain- KtransAccuracy 86% [120] |
Single centre Single centre |
Further treatment | ||
| 18 FDG SUV | Q |
Cancer Sarcoma [121] Lung cancer [105] |
Tumour presence |
Sarcoma—sensitivity 0.91, specificity 0.85, accuracy 0.88 Lung—sensitivity 0.68 to 0.95 depending on histology |
Meta-analysis Meta-analysis |
Staging and management stratification (surgery, radiotherapy, chemotherapy, combination) | |
|
Targeted radionuclides [68]Ga DOTATOC and [68]Ga DOTATATE [124, 125] [68]Ga PSMA [4] |
Non-Q | Cancer | Tumour presence |
Sensitivity 97% and specificity 92% for octreotide [126] Sensitivity 100% and specificity 100% for PSMA [127] |
Single centre Single centre |
Validation remains difficult because of biopsying multiple positive sites. | |
Biomarkers used visually in the clinic are given in italics, and those that are used quantitatively are in bold
Abbreviations: ADC apparent diffusion coefficient, APT amide proton transfer, AUC area under curve, BI-RADS breast imaging reporting and data systems, CBV cerebral blood volume, CoV coefficient of variation, CR complete response, CT computerised tomography, DCE dynamic contrast enhanced, DFS disease-free survival, DOTATOC DOTA octreotitide, DOTATATE DOTA octreotate, DSC dynamic susceptibility contrast, ECG electro cardiogram, FDG fluorodeoxyglucose, FLT fluoro thymidine, HR hazard ratio, HU Hounsfield unit, ICC intraclass correlation, IQR interquartile range, LVEF left ventricular ejection fraction, MRF magnetic resonance fingerprinting, MRI magnetic resonance imaging, MTR magnetisation transfer ratio, NCCN National Comprehensive Cancer Network, OS overall survival, pCT perfusion computerised tomography, PERCIST positron emission tomography response criteria in solid tumours, PD progressive disease, PFS progression-free survival, PPV positive predictive value, PI-RADS prostate imaging reporting and data systems, PR partial response, PSMA prostate-specific membrane antigen, RECIL response evaluation in lymphoma, RECIST response evaluation criteria in solid tumours, ROC receiver operating characteristic, SD stable disease, SUV standardised uptake value, SWE shear wave elastography, US ultrasound
Radiomic signature biomarkers
Radiomics describes the extraction and analysis of quantitative features from radiological images. The assumption is that radiomic features reflect pathophysiological processes expressed by other “omics”, such as genomics, transcriptomics, metabolomics and proteomics [128]. Hundreds to thousands of radiomic features (mathematical descriptors of texture, heterogeneity or shape) can be extracted from a region or volume of interest (ROI/VOI), derived manually or semi-automatically by a human operator, or automatically by a computer algorithm. The radiomic “signature” (summary of all features) is expected to be specific for a given patient, patient group, tissue or disease [129, 130]: it depends on the type of imaging data (CT, MRI, PET) and is influenced by image acquisition parameters (e.g. resolution, reconstruction algorithm, repetition/echo times for MRI), hardware (e.g. scanner model, coils), VOI/ROI segmentation [131] and image artifacts.
Unlike biopsies, radiomic analyses, although not tissue specific, capture heterogeneity across the entire volume [132], potentially making them more indicative of therapy response, resistance and survival. They may be therefore better suited to decision support in terms of treatment selection and risk stratification. Current radiomics research in X-ray mammography [133] and cross-sectional imaging (lung, head and neck, prostate, GI tract, brain) has shown promising results [134], leading to extrapolation in non-malignant disease. Image quality optimisation and standardisation of data acquisition are mandatory for widespread application. At present, individual research groups derive differing versions of a similar signature and there is a tendency to change the signature from study to study. Since radiomic signatures are typically multi-dimensional data, they are an ideal input for advanced machine learning techniques, such as artificial neural networks, especially when big multicentric datasets are available. Early reports from multicentre trials indicate that reproducibility of feature selection is good when extracted from CT [135] as well as MRI [136] data.
Selecting and translating appropriate imaging biomarkers to support clinical decision-making
Automated quantitative assessments rather than scoring systems are easier to incorporate into artificial intelligence systems. For this, threshold values need to be established and a probability function of the likelihood of disease vs. no disease derived from the absolute quantitation (e.g. bone density measurements) [137]. Alternatively, ratios of values to adjacent healthy tissue can be used to recognise disease. Similarly, for prognostic information, thresholds established from large databases will define action limits for altering management based on the likelihood of a good or poor outcome predicted by imaging data. This will enable the clinical community to move towards using imaging as a “virtual biopsy”. The current evidence for use of quantitative imaging biomarkers for diagnostic and prognostic purposes is given in Tables 1 and 2 respectively.
Table 2.
Imaging biomarkers for disease characterisation (semi-quantitative and quantitative) with examples of current evidence for their use that would support decision-making
| Biomarker | SemiQ/Q | Disease | Question answered | Utility of biomarker | Data from | Potential decision for | |
|---|---|---|---|---|---|---|---|
| Non-malignant disease | Young’s modulus | Q | Coronary plaques [53] | Risk of rupture | Reproducibility CoV 22% vessel wall, 19% in plaque. AUC for focal neurology Youngs modulus + degree = 0.78 | Single centre | Stenting, coronary bypass surgery |
| Plaque density, vessel luminal diameter | Q | Coronary artery stenosis | Risk of plaque rupture; risk of significant cardiac ischaemia, infarction, death |
No luminal narrowing but with coronary artery calcium (CAC) score > 0 had a 5-year mortality HR 1.8 compared with those whose CACS = 0. No luminal narrowing but CAC ≥ 100 had mortality risks similar to individuals with non-obstructive coronary artery disease [138] CT angiography significantly better at predicting events than stress echo/ECG [68] Coronary death/non-fatal myocardial infarction was lower in patients with stable angina receiving CT angiography than in the standard-care group (HR = 0.59) [69] |
Multicentre Multicentre Multicentre |
Statins, stenting, coronary bypass surgery | |
| 18F-Na | SQ |
Aortic valve disease Coronary plaque [139] Acute events from abdominal aortic aneurysm |
Valve stenosis present Likelihood of plaque rupture Likelihood of aneurysm rupture |
Reproducibility NaF uptake 10% [140] Baseline 18F-NaF uptake correlated closely with the change in calcium score at 1 year [141] 18F-NaF uptake (maximum tissue-to-background ratio 1·90 [IQR 1.61–2.17]) associated with ruptured plaques and those with high-risk features [142] Aneurysms in the highest tertile of 18F-NaF uptake expanded 2.5× more rapidly than those in the lowest tertile and were 3× more likely to rupture [143] |
Single Multicentre |
Coronary stenting, aneurysm stenting | |
| MTR | Q | Multiple sclerosis | Disease progression |
MTR significantly correlates with T2 lesion volume [144] Grey matter MTR histogram peak height and average lesion MTR percentage change after 12 months independent predictors of disability worsening at 8 years [145] Change in brain MTR specificity 76.9% and PPV 59.1% for Expanded Disability Status Scale score deterioration [146] |
Multicentre Single centre Single centre |
Timing of therapeutic intervention | |
| Malignant disease | 18 FDG-SUV | Q |
Cancer Oesophageal cancer |
Good or poor prognosis tumour in terms of PFS and OS |
Wide variation between individuals and tumours [147] Oesophageal cancer HR 1.86 for OS, 2.52 for DFS [148] |
Meta-analysis | Neoadjuvant or adjuvant therapy or treatment modality combinations |
| 18FLT-SUV | Q | Cancer | High proliferative activity present | Sizeable overlap in values with normal proliferating tissues [75] | Review of data from single centre studies | Neoadjuvant or adjuvant therapy or treatment modality combinations | |
|
ADC MRF (ADC, T1 and T2) |
Q Q Q |
Cancer, correlates with tumour grade | Risk of recurrence or metastasis |
Area under ROC, sensitivity and specificity of nADCmean for G3 intrahepatic cholangiocarcinoma versus G1+G2 were 0.71, 89.5% and 55.5% [149] “Unfavourable” ADC in cervix cancer predictive of disease-free survival (HR 1.55) [150] ADC and T2 together give AUC of 0.83 for separating high- or intermediate-grade from low-grade prostate cancer [151] |
Single centre Meta-analysis Single centre |
Need of biopsy or other invasive diagnosis Neoadjuvant or adjuvant therapy Decision for radical treatment or active surveillance |
|
| DSC-MRI | SQ (rCBV) | Brain cancer | Grading glioma | AUC = 0.77 for discriminating glioma grades II and III [152] | Meta-analysis | Type and time of intervention/treatment | |
| APT | Q | Glioma | Proliferation | APT correlates with tumour grade and Ki67 index [153] | Single centre | Therapeutic strategies | |
|
DCE-CT parameters Blood flow, permeability |
Q |
Rectal cancer Lung cancer |
Blood flow 75% accuracy for detecting rectal tumours with lymph node metastases [154] CT permeability predicted survival independent of treatment in lung cancer [155] |
Single centre Single centre |
Surgical dissection, adjuvant radiotherapy Adjuvant therapy |
||
| DCE-MRI parameters | Q |
Cervix cancer Endometrial cancer Rectal cancer Breast cancer |
Risk of recurrence or metastasis, survival |
Tumour volume with increasing signal is a strong independent prognostic factor for DFS and OS in cervical cancer [156] Low tumour blood flow and low rate constant for contrast agent intravasation (kep) associated with high-risk histological subtype in endometrial cancer [157] Ktrans, Kep and Ve significantly higher in rectal cancers with distant metastasis [158] Ktrans, iAUCqualitative and ADC predict low-risk breast tumors (AUC of combined parameters 0.78) |
Single centre Single centre Single centre Single centre |
Neoadjuvant, adjuvant or multimodality treatment strategies | |
| Radiomic signature [159] | Q | Multiple tumour types [160, 161] | Tumour with good or poor prognosis |
Data endpoints, feature selection techniques and classifiers were significant factors in affecting predictive accuracy in lung cancer [162] Radiomic signature (24 selected features) is significantly associated with LN status in colorectal cancer [163] |
Single centre Single centre |
Neoadjuvant or adjuvant treatment, immunotherapy Lymph node dissection, adjuvant treatment |
Biomarkers used visually in the clinic are given in italics, and those that are used quantitatively are in bold
Abbreviations: ADC apparent diffusion coefficient, APT amide proton transfer, AUC area under curve, BI-RADS breast imaging reporting and data systems, CBV cerebral blood volume, CoV coefficient of variation, CR complete response, CT computerised tomography, DCE dynamic contrast enhanced, DFS disease-free survival, DOTATOC DOTA octreotitide, DOTATATE DOTA octreotate, DSC dynamic susceptibility contrast, ECG electro cardiogram, FDG fluorodeoxyglucose, FLT fluoro thymidine, HR hazard ratio, HU Hounsfield unit, ICC intraclass correlation, IQR interquartile range, LVEF left ventricular ejection fraction, MRF magnetic resonance fingerprinting, MRI magnetic resonance imaging, MTR magnetisation transfer ratio, NCCN National Comprehensive Cancer Network, OS overall survival, pCT perfusion computerised tomography, PERCIST positron emission tomography response criteria in solid tumours, PD progressive disease, PFS progression-free survival, PPV positive predictive value, PI-RADS prostate imaging reporting and data systems, PR partial response, PSMA prostate-specific membrane antigen, RECIL response evaluation in lymphoma, RECIST response evaluation criteria in solid tumours, ROC receiver operating characteristic, SD stable disease, SUV standardised uptake value, SWE shear wave elastography, US ultrasound
For assessing treatment response (Table 3), the key element in biomarker selection relates to the type of treatment and expected pathological response. For non-targeted therapies, tissue necrosis to cytotoxic agents is expected, so biomarkers that read-out on increased free water (CT Hounsfield units) or reduced cell density (ADC) are most useful. With specific targeted agents (e.g. antiangiogenics), specific biomarker read-outs (perfusion metrics by US, CT or MRI) are more appropriate [185]. Both non-targeted and targeted agents shut down tumour metabolism, so that in glycolytic tumours, FDG metrics are exquisitely sensitive [186]. Distortion and changes following surgery, or changes in the adjacent normal tissue following radiotherapy [122], reduce quantitative differences between irradiated non-malignant and residual malignant tissue, so must be taken into account [187]. In multicentre trials, it is also crucial to establish the repeatability of the quantitative biomarker across multiple sites and vendor platforms for response interpretation [4].
Table 3.
Imaging biomarkers for disease response assessment (semi-quantitative and quantitative) with examples of current evidence for their use that would support decision-making
| Biomarker | SemiQ/Q | Disease | Question answered | Utility of biomarker | Data from | Potential decision for | |
|---|---|---|---|---|---|---|---|
| Non-malignant disease | Volumetric high resolution CT density (quantitative interstitial lung disease, QILD) | Q | Scleroderma | Response to cyclophosphamide | 24-month changes in QILD scores in the whole lung correlated significantly 24-month changes in forced vital capacity (ρ = − 0.37), diffusing capacity (ρ = − 0.22) and breathlessness (ρ = − 0.26) [164] | Single centre | Continue, change or stop treatment |
| Left Ventricular ejection fraction LVEF | Q |
Pulmonary hypertension Myocardial ischaemia/infarction |
Right and left cardiac sufficiency Improvement in cardiac function |
Increases in 6-min walk distance were significant correlated with change in right ventricular ejection fraction and left ventricular end-diastolic volume [165] Monitoring cardiac function [166] |
Multicentre Multicentre |
Continue, change or stop treatment | |
| Malignant disease | RECIST/morphological volume | Q | Cancer | Response | Current guidelines for response assessment [167] | Multicentre | Continue, change or stop treatment |
| PERCIST/metabolic volume [168] | Q | Cancer | Response | Current guidelines for response assessment | Multicentre | Continue, change or stop treatment | |
| Scoring systems for disease burden | SQ |
Multiple sclerosis Rheumatoid arthritis |
Reduction in disease burden |
Effects on MRI lesions over 6–9 months predict the effects on relapses at 12–24 months) [169] International consensus on scoring system [170] |
Meta-analysis Review |
Continue, change or stop therapy | |
| DSC-MRI | SQ (rCBV) | Brain cancer | Differentiation of treatment effects and tumour progression | In 2 meta-analyses MRI had high pooled sensitivities and specificities: 87% (95% CI, 0.82–0.91) to 90% (95% CI, 0.85-0.94) sensitivity and 86% (95% CI, 0.77–0.91) to 88% (95% CI, 0.83-0.92) specificity [171, 172] | Meta-analysis | Decision to treat | |
| 18F FDG-SUVmax [173] | Q | Multiple cancer types | Response to therapy |
Rectal cancer-pooled sensitivity, 73%; pooled specificity, 77%; pooled AUC, 0.83 [174] Intratreatment low SUVmax (persistent low or decrease of 18F-FDG uptake) predictive of loco-regional control in head and neck cancer [175] |
Meta-analysis Meta-analysis |
Continue, change or stop therapy | |
| Deauville or RECIL score on 18 F-FDG-PET | SQ | Lymphoma | CR, PR, SD or PD [176] | Assessment of tumour burden in lymphoma clinical trials can use the sum of longest diameters of a maximum of three target lesions [177] | Multicentre | Continue, change or stop therapy | |
|
Targeted agents HER2 PSMA |
SQ |
Breast cancer [178] Prostate cancer [179] |
Reduction in tumour cells expressing these antigens |
Tumour receptor specific Effects of treatment on receptor expression |
Single centre studies, review | Continue, change or stop therapy | |
| ADC [117] |
SQ Q |
Rectal cancer Breast cancer |
Response to neoadjuvant chemotherapy Response to neoadjuvant chemotherapy |
Additional value in both the prediction and detection of (complete) response to therapy compared with conventional sequences alone [180] After 12 weeks of therapy, change in ADC predicts complete pathologic response to neoadjuvant chemotherapy (AUC = 0.61, p = 0.013) [181] |
Review Multicentre |
Continue, change or stop therapy, proceed to surgery | |
| CT perfusion/blood flow | Q | Oesophageal cancer | Response to chemoradiotherapy | Multivariate analysis identified blood flow as a significant independent predictor of response [182] | Single centre | Further treatment | |
| DCE-MR parameters | Q | Multiple cancer types | Response to therapy | Particular benefit in assessing therapy response to antiangiogenic agents [183] | Review | Change therapeutic strategy | |
| CT density HU | Q | Gastrointestinal stromal tumours | Response to chemotherapy | Decrease in tumour density of > 15% on CT had a sensitivity of 97% and a specificity of 100% in identifying PET responders versus 52% and 100% by RECIST [184] | Continue, change or stop therapy |
Biomarkers used visually in the clinic are given in italics, and those that are used quantitatively are in bold
Abbreviations: ADC apparent diffusion coefficient, APT amide proton transfer, AUC area under curve, BI-RADS breast imaging reporting and data systems, CBV cerebral blood volume, CoV coefficient of variation, CR complete response, CT computerised tomography, DCE dynamic contrast enhanced, DFS disease-free survival, DOTATOC DOTA octreotitide, DOTATATE DOTA octreotate, DSC dynamic susceptibility contrast, ECG electro cardiogram, FDG fluorodeoxyglucose, FLT fluoro thymidine, HR hazard ratio, HU Hounsfield unit, ICC intraclass correlation, IQR interquartile range, LVEF left ventricular ejection fraction, MRF magnetic resonance fingerprinting, MRI magnetic resonance imaging, MTR magnetisation transfer ratio, NCCN National Comprehensive Cancer Network, OS overall survival, pCT perfusion computerised tomography, PERCIST positron emission tomography response criteria in solid tumours, PD progressive disease, PFS progression-free survival, PPV positive predictive value, PI-RADS prostate imaging reporting and data systems, PR partial response, PSMA prostate-specific membrane antigen, RECIL response evaluation in lymphoma, RECIST response evaluation criteria in solid tumours, ROC receiver operating characteristic, SD stable disease, SUV standardised uptake value, SWE shear wave elastography, US ultrasound
Advancing new quantitative imaging biomarkers as decision-support tools to clinical practice
To become clinically useful, biomarkers must be rigorously evaluated for their technical performance, reproducibility, biological and clinical validity, and cost-effectiveness [6]. Table 4 gives current recommendations for use of quantitative biomarkers as decision support tools.
Table 4.
Recommendations for the use of quantitative imaging biomarkers as decision-support tools
| Recommendation | Current evidence | Action needed |
|---|---|---|
| Consider need for quantitation in relation to the decision being made | Semi-quantitative imaging biomarkers are successfully used in many clinical pathways. |
• Classification systems retain a subjective element that could benefit from standardisation and refinement. • Development of automated and thresholding would enable more quantitative assessments |
| Use validated IB methodology for semi-quantitative and quantitative measures | Many single and multicentre trials validating quantitative imaging biomarkers with clinical outcome now exist. |
• Harmonisation of methodology • Standardised reporting systems |
| Establish evidence on the use of quantitation by inclusion into clinical trials | Clinical trials are usually planned by non-imagers. Integration of imaging biomarkers into trials is dependent on what is available routinely to non-imagers in the clinic, rather than exploiting an imaging technique to its optimal potential. |
• Inventory of imaging biomarkers accessible through a web-based portal would inform the inclusion and utilisation of imaging biomarkers within trials (The European Imaging Biomarkers Alliance initiative). • Certified biomarkers conforming to set standards (Quantitative Imaging Biomarkers Alliance initiative) |
| Validate against pathology or clinical outcomes to make imaging a “virtual biopsy” |
Several major databanks hold imaging and clinical or pathology data • CaBIG (USA) • UK MRC Biobank (UK) • German National Cohort Study (Germany) |
• Large data collection for validation of imaging and pathology • Curation in imaging biobanks |
| Select appropriate quality assured quantitative IB | Trials with embedded QA/QC procedures have indicated good reproducibility of quantitative imaging biomarkers (e.g. EU iMi QuIC:ConCePT project) | • Ensure curation and archiving of longitudinal imaging data with outcomes within trials |
| Open-source interchange kernel | Low comparability between image-derived biomarkers if hardware and software of different manufacturers are used. | • Harmonisation of image acquisition and post-processing over manufacturers |
Technical validation establishes whether a biomarker can be derived reliably in different institutions (comparability) and on widely available platforms. Provision must be made if specialist hardware or software is required, or if a key tracer or contrast agent is not licensed for clinical use. Reproducibility, a mandatory requirement, is very rarely demonstrated in practice [188] because inclusion of a repeat baseline study is resource and time intensive for both patients and researchers. Multicentre technical validation using standardised protocols may occur after initial biological validation (evidence that known perturbations in biology alter the imaging biomarker signal in a way that supports the measurement characteristics assigned to the biomarker). Subsequent clinical validation, showing that the same relationships are observed in patients, may then occur in parallel to multicentre technical validation.
Once a biomarker is shown to have acceptable technical, biological and clinical validation, a decision must be made to qualify the biomarker for a specific purpose or use. Increasingly, the role of imaging in the context of other non-imaging biomarkers needs to be considered as part of a multiparametric healthcare assessment. For example, circulating biomarkers such as circulating tumour DNA are often more specific at detecting disease but do not localise or stage tumours. The integration of imaging biomarkers with tissue and liquid biomarkers is likely to replace many traditional and more simplistic approaches to decision-support systems that are used currently.
The cost-effectiveness of a biomarker is increasingly important in financially restricted healthcare systems where value-based care is increasingly considered [189]. However, the information may be derived from scans done as part of the patients’ clinical work-up. Nevertheless, additional imaging/image processing is expensive compared to liquid- and tissue-based biomarkers. Costs can be off-set against the cost saving from the unnecessary use of expensive but ineffective novel and targeted drugs. Health economic assessment is therefore an important part of translating a new biomarker into routine clinical practice. In an era of artificial intelligence, where radiologists are faced with an ever-increasing volume of digital data, it makes sense to increase our efforts at utilising validated, quantified imaging biomarkers as key elements in supporting management decisions for patients.
Acknowledgements
This paper was reviewed and endorsed by the ESR Executive Council in March 2019.
Abbreviations
- ADC
Apparent diffusion coefficient
- APT
Amide proton transfer
- AUC
Area under curve
- CBV
Cerebral blood volume
- CEST
Chemical exchange saturation transfer
- CoV
Coefficient of variation
- CR
Complete response
- CT
Computerised tomography
- DCE
Dynamic contrast enhanced
- DFS
Disease-free survival
- DOTATOC
DOTA octreotitide
- DOTATATE
DOTA-octreotate
- DSC
Dynamic susceptibility contrast
- DWI
Diffusion-weighted imaging
- ECG
Electrocardiogram
- ESR
European Society of Radiology
- FDG
Fluorodeoxyglucose
- FLT
Fluorothymidine
- HR
Hazard ratio
- HU
Hounsfield unit
- ICC
Intraclass correlation
- IPF
Interstitial pulmonary fibrosis
- IQR
Interquartile range
- LVEF
Left ventricular ejection fraction
- MATV
Metabolic active tumour volume
- MRF
Magnetic resonance fingerprinting
- MRI
Magnetic resonance imaging
- MTR
Magnetisation transfer ratio
- MTT
Mean transit time
- NCCN
National Comprehensive Cancer Network
- OS
Overall survival
- pCT
Perfusion computerised tomography
- PERCIST
Positron emission tomography response criteria in solid tumours
- PD
Progressive disease
- PFS
Progression free survival
- PPV
Positive predictive value
- PI
Peak intensity
- PR
Partial response
- PSMA
Prostate specific membrane antigen
- QA
Quality assurance
- QC
Quality control
- RADS
Reporting and data systems (BI, breast imaging; LI, liver imaging; PI, prostate imaging; TI, thyroid imaging; VI, vesicle imaging)
- RECIL
Response evaluation in lymphoma
- RECIST
Response evaluation criteria in solid tumours
- ROC
Receiver operating characteristic
- ROI
Region of interest
- RSNA
Radiological Society of North America
- SD
Stable disease
- SUV
Standardised uptake value
- SWE
Shear wave elastography
- TTP
Time to peak
- US
Ultrasound
- VOI
Voxel of interest
Authors’ contributions
All authors have contributed to the conception of the work, have drafted the work and have approved the submitted final version of the manuscript.
Authors’ information
All authors are either past or current members of the European Biomarkers Alliance subcommittee.
Funding
None declared for this work.
Availability of data and materials
Not applicable
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
*The European Imaging Biomarkers ALLiance (EIBALL) is a subcommittee of the ESR Research Committee. Its mission is to facilitate imaging biomarker development and standardisation and promote their use in clinical trials and in clinical practice by collaboration with specialist societies, international standards agencies and trials organisations.
https://www.myesr.org/research/esr-research-committee#paragraph_grid_5924
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mercado CL. BI-RADS update. Radiol Clin North Am. 2014;52:481–487. doi: 10.1016/j.rcl.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Barentsz JO, Weinreb JC, Verma S, et al. Synopsis of the PI-RADS v2 guidelines for multiparametric prostate magnetic resonance imaging and recommendations for use. Eur Urol. 2016;69:41–49. doi: 10.1016/j.eururo.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hosny Ahmed, Parmar Chintan, Quackenbush John, Schwartz Lawrence H., Aerts Hugo J. W. L. Artificial intelligence in radiology. Nature Reviews Cancer. 2018;18(8):500–510. doi: 10.1038/s41568-018-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zacho HD, Nielsen JB, Afshar-Oromieh A, et al. Prospective comparison of (68)Ga-PSMA PET/CT, (18)F-sodium fluoride PET/CT and diffusion weighted-MRI at for the detection of bone metastases in biochemically recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45:1884–1897. doi: 10.1007/s00259-018-4058-4. [DOI] [PubMed] [Google Scholar]
- 5.Boellaard R, Delgado-Bolton R, Oyen WJ, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–354. doi: 10.1007/s00259-014-2961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Connor JP, Aboagye EO, Adams JE, et al. Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol. 2017;14:169–186. doi: 10.1038/nrclinonc.2016.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhuang Mingzan, García David Vállez, Kramer Gerbrand M., Frings Virginie, Smit E.F., Dierckx Rudi, Hoekstra Otto S., Boellaard Ronald. Variability and Repeatability of Quantitative Uptake Metrics in 18F-FDG PET/CT of Non–Small Cell Lung Cancer: Impact of Segmentation Method, Uptake Interval, and Reconstruction Protocol. Journal of Nuclear Medicine. 2018;60(5):600–607. doi: 10.2967/jnumed.118.216028. [DOI] [PubMed] [Google Scholar]
- 8.Barrington SF, Kirkwood AA, Franceschetto A, et al. PET-CT for staging and early response: results from the Response-Adapted Therapy in Advanced Hodgkin Lymphoma study. Blood. 2016;127:1531–1538. doi: 10.1182/blood-2015-11-679407. [DOI] [PubMed] [Google Scholar]
- 9.Hosny Ahmed, Parmar Chintan, Quackenbush John, Schwartz Lawrence H., Aerts Hugo J. W. L. Artificial intelligence in radiology. Nature Reviews Cancer. 2018;18(8):500–510. doi: 10.1038/s41568-018-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trivedi SB, Vesoulis ZA, Rao R, et al. A validated clinical MRI injury scoring system in neonatal hypoxic-ischemic encephalopathy. Pediatric radiology. 2017;47:1491–1499. doi: 10.1007/s00247-017-3893-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Machino Masaaki, Ando Kei, Kobayashi Kazuyoshi, Ito Kenyu, Tsushima Mikito, Morozumi Masayoshi, Tanaka Satoshi, Ota Kyotaro, Ito Keigo, Kato Fumihiko, Ishiguro Naoki, Imagama Shiro. Alterations in Intramedullary T2-weighted Increased Signal Intensity following Laminoplasty in Cervical Spondylotic Myelopathy Patients. SPINE. 2018;43(22):1595–1601. doi: 10.1097/BRS.0000000000002674. [DOI] [PubMed] [Google Scholar]
- 12.Chen CJ, Lyu RK, Lee ST, Wong YC, Wang LJ. Intramedullary high signal intensity on T2-weighted MR images in cervical spondylotic myelopathy: prediction of prognosis with type of intensity. Radiology. 2001;221:789–794. doi: 10.1148/radiol.2213010365. [DOI] [PubMed] [Google Scholar]
- 13.Khanna D, Ranganath VK, Fitzgerald J, et al. Increased radiographic damage scores at the onset of seropositive rheumatoid arthritis in older patients are associated with osteoarthritis of the hands, but not with more rapid progression of damage. Arthritis Rheum. 2005;52:2284–2292. doi: 10.1002/art.21221. [DOI] [PubMed] [Google Scholar]
- 14.Jaremko Jacob L., Azmat Omar, Lambert Robert G.W., Bird Paul, Haugen Ida K., Jans Lennart, Weber Ulrich, Winn Naomi, Zubler Veronika, Maksymowych Walter P. Validation of a Knowledge Transfer Tool According to the OMERACT Filter: Does Web-based Real-time Iterative Calibration Enhance the Evaluation of Bone Marrow Lesions in Hip Osteoarthritis? The Journal of Rheumatology. 2017;44(11):1713–1717. doi: 10.3899/jrheum.161101. [DOI] [PubMed] [Google Scholar]
- 15.Molyneux PD, Miller DH, Filippi M, et al. Visual analysis of serial T2-weighted MRI in multiple sclerosis: intra- and interobserver reproducibility. Neuroradiology. 1999;41:882–888. doi: 10.1007/s002340050860. [DOI] [PubMed] [Google Scholar]
- 16.Stollfuss JC, Becker K, Sendler A, et al. Rectal carcinoma: high-spatial-resolution MR imaging and T2 quantification in rectal cancer specimens. Radiology. 2006;241:132–141. doi: 10.1148/radiol.2411050942. [DOI] [PubMed] [Google Scholar]
- 17.Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32:3048–3058. doi: 10.1200/JCO.2013.53.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chernyak Victoria, Fowler Kathryn J., Kamaya Aya, Kielar Ania Z., Elsayes Khaled M., Bashir Mustafa R., Kono Yuko, Do Richard K., Mitchell Donald G., Singal Amit G., Tang An, Sirlin Claude B. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology. 2018;289(3):816–830. doi: 10.1148/radiol.2018181494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elsayes Khaled M., Hooker Jonathan C., Agrons Michelle M., Kielar Ania Z., Tang An, Fowler Kathryn J., Chernyak Victoria, Bashir Mustafa R., Kono Yuko, Do Richard K., Mitchell Donald G., Kamaya Aya, Hecht Elizabeth M., Sirlin Claude B. 2017 Version of LI-RADS for CT and MR Imaging: An Update. RadioGraphics. 2017;37(7):1994–2017. doi: 10.1148/rg.2017170098. [DOI] [PubMed] [Google Scholar]
- 20.Tessler FN, Middleton WD, Grant EG et al (2017) ACR thyroid imaging, reporting and data system (TI-RADS): white paper of the ACR TI-RADS committee. J Am Coll Radiol. 14:587–595 [DOI] [PubMed]
- 21.Panebianco V, Narumi Y, Altun E, et al. Multiparametric Magnetic Resonance Imaging for Bladder Cancer: Development of VI-RADS (Vesical Imaging-Reporting And Data System) Eur Urol. 2018;74:294–306. doi: 10.1016/j.eururo.2018.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitajima K, Tanaka U, Ueno Y, et al. Role of diffusion weighted imaging and contrast-enhanced MRI in the evaluation of intrapelvic recurrence of gynecological malignant tumour. PLoS One. 2015;10:e0117411. doi: 10.1371/journal.pone.0117411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cornelis F, Tricaud E, Lasserre AS, et al. Multiparametric magnetic resonance imaging for the differentiation of low and high grade clear cell renal carcinoma. Eur Radiol. 2015;25:24–31. doi: 10.1007/s00330-014-3380-x. [DOI] [PubMed] [Google Scholar]
- 24.Martin MD, Kanne JP, Broderick LS, Kazerooni EA, Meyer CA. Lung-RADS: pushing the limits. Radiographics. 2017;37:1975–1993. doi: 10.1148/rg.2017170051. [DOI] [PubMed] [Google Scholar]
- 25.Sabra Mona M., Sherman Eric J., Tuttle R. Michael. Tumor volume doubling time of pulmonary metastases predicts overall survival and can guide the initiation of multikinase inhibitor therapy in patients with metastatic, follicular cell-derived thyroid carcinoma. Cancer. 2017;123(15):2955–2964. doi: 10.1002/cncr.30690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kadir T, Gleeson F. Lung cancer prediction using machine learning and advanced imaging techniques. Transl Lung Cancer Res. 2018;7:304–312. doi: 10.21037/tlcr.2018.05.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., Dancey J., Arbuck S., Gwyther S., Mooney M., Rubinstein L., Shankar L., Dodd L., Kaplan R., Lacombe D., Verweij J. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) European Journal of Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 28.Yao GH, Zhang M, Yin LX, et al. Doppler Echocardiographic Measurements in Normal Chinese Adults (EMINCA): a prospective, nationwide, and multicentre study. Eur Heart J Cardiovasc Imaging. 2016;17:512–522. doi: 10.1093/ehjci/jev330. [DOI] [PubMed] [Google Scholar]
- 29.Elgendy A, Seppelt IM, Lane AS. Comparison of continous-wave Doppler ultrasound monitor and echocardiography to assess cardiac output in intensive care patients. Crit Care Resusc. 2017;19:222–229. [PubMed] [Google Scholar]
- 30.Figueiredo CP, Kleyer A, Simon D, et al. Methods for segmentation of rheumatoid arthritis bone erosions in high-resolution peripheral quantitative computed tomography (HR-pQCT) Semin Arthritis Rheum. 2018;47:611–618. doi: 10.1016/j.semarthrit.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 31.Welsing PM, van Gestel AM, Swinkels HL, Kiemeney LA, van Riel PL. The relationship between disease activity, joint destruction, and functional capacity over the course of rheumatoid arthritis. Arthritis Rheum. 2001;44:2009–2017. doi: 10.1002/1529-0131(200109)44:9<2009::AID-ART349>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 32.Ødegård Sigrid, Landewé Robert, van der Heijde Désirée, Kvien Tore K., Mowinckel Petter, Uhlig Till. Association of early radiographic damage with impaired physical function in rheumatoid arthritis: A ten-year, longitudinal observational study in 238 patients. Arthritis & Rheumatism. 2005;54(1):68–75. doi: 10.1002/art.21548. [DOI] [PubMed] [Google Scholar]
- 33.Marcus CD, Ladam-Marcus V, Cucu C, Bouche O, Lucas L, Hoeffel C. Imaging techniques to evaluate the response to treatment in oncology: current standards and perspectives. Crit Rev Oncol Hematol. 2009;72:217–238. doi: 10.1016/j.critrevonc.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Levine ZH, Pintar AL, Hagedorn JG, Fenimore CP, Heussel CP. Uncertainties in RECIST as a measure of volume for lung nodules and liver tumours. Med Phys. 2012;39:2628–2637. doi: 10.1118/1.3701791. [DOI] [PubMed] [Google Scholar]
- 35.Hawnaur JM, Johnson RJ, Buckley CH, Tindall V, Isherwood I. Staging, volume estimation and assessment of nodal status in carcinoma of the cervix: comparison of magnetic resonance imaging with surgical findings. Clin Radiol. 1994;49:443–452. doi: 10.1016/S0009-9260(05)81738-6. [DOI] [PubMed] [Google Scholar]
- 36.Soutter WP, Hanoch J, D'Arcy T, Dina R, McIndoe GA, DeSouza NM. Pretreatment tumour volume measurement on high-resolution magnetic resonance imaging as a predictor of survival in cervical cancer. BJOG. 2004;111:741–747. doi: 10.1111/j.1471-0528.2004.00172.x. [DOI] [PubMed] [Google Scholar]
- 37.Jiang Y, You K, Qiu X, et al. Tumour volume predicts local recurrence in early rectal cancer treated with radical resection: a retrospective observational study of 270 patients. Int J Surg. 2018;49:68–73. doi: 10.1016/j.ijsu.2017.11.052. [DOI] [PubMed] [Google Scholar]
- 38.Tayyab M, Razack A, Sharma A, Gunn J, Hartley JE. Correlation of rectal tumour volumes with oncological outcomes for low rectal cancers: does tumour size matter? Surg Today. 2015;45:826–833. doi: 10.1007/s00595-014-1068-0. [DOI] [PubMed] [Google Scholar]
- 39.Wagenaar HC, Trimbos JB, Postema S, et al. Tumour diameter and volume assessed by magnetic resonance imaging in the prediction of outcome for invasive cervical cancer. Gynecol Oncol. 2001;82:474–482. doi: 10.1006/gyno.2001.6267. [DOI] [PubMed] [Google Scholar]
- 40.Lee JW, Lee SM, Yun M, Cho A. Prognostic value of volumetric parameters on staging and posttreatment FDG PET/CT in patients with stage IV non-small cell lung cancer. Clin Nucl Med. 2016;41:347–353. doi: 10.1097/RLU.0000000000001126. [DOI] [PubMed] [Google Scholar]
- 41.Kurtipek E, Cayci M, Duzgun N, et al. (18)F-FDG PET/CT mean SUV and metabolic tumour volume for mean survival time in non-small cell lung cancer. Clin Nucl Med. 2015;40:459–463. doi: 10.1097/RLU.0000000000000740. [DOI] [PubMed] [Google Scholar]
- 42.Meignan Michel, Cottereau Anne Ségolène, Versari Annibale, Chartier Loïc, Dupuis Jehan, Boussetta Sami, Grassi Ilaria, Casasnovas René-Olivier, Haioun Corinne, Tilly Hervé, Tarantino Vittoria, Dubreuil Julien, Federico Massimo, Salles Gilles, Luminari Stefano, Trotman Judith. Baseline Metabolic Tumor Volume Predicts Outcome in High–Tumor-Burden Follicular Lymphoma: A Pooled Analysis of Three Multicenter Studies. Journal of Clinical Oncology. 2016;34(30):3618–3626. doi: 10.1200/JCO.2016.66.9440. [DOI] [PubMed] [Google Scholar]
- 43.Meignan M, Itti E, Gallamini A, Younes A. FDG PET/CT imaging as a biomarker in lymphoma. Eur J Nucl Med Mol Imaging. 2015;42:623–633. doi: 10.1007/s00259-014-2973-6. [DOI] [PubMed] [Google Scholar]
- 44.Kanoun S, Tal I, Berriolo-Riedinger A, et al. Influence of software tool and methodological aspects of total metabolic tumour volume calculation on baseline [18F]FDG PET to predict survival in Hodgkin lymphoma. PLoS One. 2015;10:e0140830. doi: 10.1371/journal.pone.0140830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kostakoglu L, Chauvie S. Metabolic tumour volume metrics in lymphoma. Semin Nucl Med. 2018;48:50–66. doi: 10.1053/j.semnuclmed.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 46.Mori S, Oishi K, Faria AV, Miller MI. Atlas-based neuroinformatics via MRI: harnessing information from past clinical cases and quantitative image analysis for patient care. Annu Rev Biomed Eng. 2013;15:71–92. doi: 10.1146/annurev-bioeng-071812-152335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cole JH, Poudel RPK, Tsagkrasoulis D, et al. Predicting brain age with deep learning from raw imaging data results in a reliable and heritable biomarker. Neuroimage. 2017;163:115–124. doi: 10.1016/j.neuroimage.2017.07.059. [DOI] [PubMed] [Google Scholar]
- 48.Xu Yiwen, Hosny Ahmed, Zeleznik Roman, Parmar Chintan, Coroller Thibaud, Franco Idalid, Mak Raymond H., Aerts Hugo J.W.L. Deep Learning Predicts Lung Cancer Treatment Response from Serial Medical Imaging. Clinical Cancer Research. 2019;25(11):3266–3275. doi: 10.1158/1078-0432.CCR-18-2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferraioli G, Wong VW, Castera L, et al. Liver ultrasound elastography: an update to the world federation for ultrasound in medicine and biology guidelines and recommendations. Ultrasound Med Biol. 2018;44:2419–2440. doi: 10.1016/j.ultrasmedbio.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 50.Lee SH, Chung J, Choi HY, et al. Evaluation of screening US-detected breast masses by combined use of elastography and color doppler US with B-Mode US in women with dense breasts: a multicenter prospective study. Radiology. 2017;285:660–669. doi: 10.1148/radiol.2017162424. [DOI] [PubMed] [Google Scholar]
- 51.Woo S, Suh CH, Kim SY, Cho JY, Kim SH. Shear-wave elastography for detection of prostate cancer: a systematic review and diagnostic meta-analysis. AJR Am J Roentgenol. 2017;209:806–814. doi: 10.2214/AJR.17.18056. [DOI] [PubMed] [Google Scholar]
- 52.Du LJ, He W, Cheng LG, Li S, Pan YS, Gao J. Ultrasound shear wave elastography in assessment of muscle stiffness in patients with Parkinson’s disease: a primary observation. Clin Imaging. 2016;40:1075–1080. doi: 10.1016/j.clinimag.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 53.Ramnarine KV, Garrard JW, Kanber B, Nduwayo S, Hartshorne TC, Robinson TG. Shear wave elastography imaging of carotid plaques: feasible, reproducible and of clinical potential. Cardiovasc Ultrasound. 2014;12:49. doi: 10.1186/1476-7120-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dori A, Abbasi H, Zaidman CM. Intramuscular blood flow quantification with power doppler ultrasonography. Muscle Nerve. 2016;54:872–878. doi: 10.1002/mus.25108. [DOI] [PubMed] [Google Scholar]
- 55.Regan Elizabeth A., Hokanson John E., Murphy James R., Make Barry, Lynch David A., Beaty Terri H., Curran-Everett Douglas, Silverman Edwin K., Crapo James D. Genetic Epidemiology of COPD (COPDGene) Study Design. COPD: Journal of Chronic Obstructive Pulmonary Disease. 2010;7(1):32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sieren JP, Newell JD, Jr, Barr RG, et al. SPIROMICS protocol for multicenter quantitative computed tomography to phenotype the lungs. Am J Respir Crit Care Med. 2016;194:794–806. doi: 10.1164/rccm.201506-1208PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keene JD, Jacobson S, Kechris K, et al. Biomarkers predictive of exacerbations in the SPIROMICS and COPDGene cohorts. Am J Respir Crit Care Med. 2017;195:473–481. doi: 10.1164/rccm.201607-1330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andrade J, Schwarz M, Collard HR, et al. The Idiopathic Pulmonary Fibrosis Clinical Research Network (IPFnet): diagnostic and adjudication processes. Chest. 2015;148:1034–1042. doi: 10.1378/chest.14-2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Washko GR, Diaz AA, Kim V, et al. Computed tomographic measures of airway morphology in smokers and never-smoking normals. J Appl Physiol (1985). 2014;116:668–673. doi: 10.1152/japplphysiol.00004.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jarjour NN, Erzurum SC, Bleecker ER, et al. Severe asthma: lessons learned from the National Heart, Lung, and Blood Institute Severe Asthma Research Program. Am J Respir Crit Care Med. 2012;185:356–362. doi: 10.1164/rccm.201107-1317PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schuhmann M, Raffy P, Yin Y, et al. Computed tomography predictors of response to endobronchial valve lung reduction treatment. Comparison with Chartis. Am J Respir Crit Care Med. 2015;191:767–774. doi: 10.1164/rccm.201407-1205OC. [DOI] [PubMed] [Google Scholar]
- 62.Van Der Molen MC, Klooster K, Hartman JE, Slebos DJ. Lung volume reduction with endobronchial valves in patients with emphysema. Expert Rev Med Devices. 2018;15:847–857. doi: 10.1080/17434440.2018.1538780. [DOI] [PubMed] [Google Scholar]
- 63.Salisbury ML, Lynch DA, van Beek EJ, et al. Idiopathic pulmonary fibrosis: the association between the adaptive multiple features method and fibrosis outcomes. Am J Respir Crit Care Med. 2017;195:921–929. doi: 10.1164/rccm.201607-1385OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goyal M, Menon BK, Derdeyn CP. Perfusion imaging in acute ischaemic stroke: let us improve the science before changing clinical practice. Radiology. 2013;266:16–21. doi: 10.1148/radiol.12112134. [DOI] [PubMed] [Google Scholar]
- 65.Guo J, Wang C, Chan KS, et al. A controlled statistical study to assess measurement variability as a function of test object position and configuration for automated surveillance in a multicenter longitudinal COPD study (SPIROMICS) Med Phys. 2016;43:2598. doi: 10.1118/1.4947303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rodriguez A, Ranallo FN, Judy PF, Fain SB. The effects of iterative reconstruction and kernel selection on quantitative computed tomography measures of lung density. Med Phys. 2017;44:2267–2280. doi: 10.1002/mp.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Al-Mallah MH. Coronary artery calcium scoring: do we need more prognostic data prior to adoption in clinical practice? JACC Cardiovasc Imaging. 2018;11:1807–1809. doi: 10.1016/j.jcmg.2017.11.041. [DOI] [PubMed] [Google Scholar]
- 68.Hoffmann U, Ferencik M, Udelson JE, et al. Prognostic value of noninvasive cardiovascular testing in patients with stable chest pain: insights from the PROMISE trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) Circulation. 2017;135:2320–2332. doi: 10.1161/CIRCULATIONAHA.116.024360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Newby DE, Adamson PD, Berry C, et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. 2018;379:924–933. doi: 10.1056/NEJMoa1805971. [DOI] [PubMed] [Google Scholar]
- 70.Altenbernd J, Wetter A, Umutlu L, et al. Dual-energy computed tomography for evaluation of pulmonary nodules with emphasis on metastatic lesions. Acta Radiol. 2016;57:437–443. doi: 10.1177/0284185115582060. [DOI] [PubMed] [Google Scholar]
- 71.Lennartz Simon, Le Blanc Markus, Zopfs David, Große Hokamp Nils, Abdullayev Nuran, Laukamp Kai Roman, Haneder Stefan, Borggrefe Jan, Maintz David, Persigehl Thorsten. Dual-Energy CT–derived Iodine Maps: Use in Assessing Pleural Carcinomatosis. Radiology. 2019;290(3):796–804. doi: 10.1148/radiol.2018181567. [DOI] [PubMed] [Google Scholar]
- 72.Barker P, Golay X, Zaharchuk G (2013) Clinical perfusion MRI: techniques and applications. Cambridge University Press.
- 73.Bittencourt LK, de Hollanda ES, de Oliveira RV. Multiparametric MR imaging for detection and locoregional staging of prostate cancer. Top Magn Reson Imaging. 2016;25:109–117. doi: 10.1097/RMR.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 74.Lopci Egesta, Franzese Ciro, Grimaldi Marco, Zucali Paolo Andrea, Navarria Pierina, Simonelli Matteo, Bello Lorenzo, Scorsetti Marta, Chiti Arturo. Imaging biomarkers in primary brain tumours. European Journal of Nuclear Medicine and Molecular Imaging. 2014;42(4):597–612. doi: 10.1007/s00259-014-2971-8. [DOI] [PubMed] [Google Scholar]
- 75.Bollineni VR, Kramer G, Liu Y, Melidis C, deSouza NM. A literature review of the association between diffusion-weighted MRI derived apparent diffusion coefficient and tumour aggressiveness in pelvic cancer. Cancer Treat Rev. 2015;41:496–502. doi: 10.1016/j.ctrv.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 76.Galban CJ, Hoff BA, Chenevert TL, Ross BD (2017) Diffusion MRI in early cancer therapeutic response assessment. NMR Biomed. 30 [DOI] [PMC free article] [PubMed]
- 77.Shukla‐Dave Amita, Obuchowski Nancy A., Chenevert Thomas L., Jambawalikar Sachin, Schwartz Lawrence H., Malyarenko Dariya, Huang Wei, Noworolski Susan M., Young Robert J., Shiroishi Mark S., Kim Harrison, Coolens Catherine, Laue Hendrik, Chung Caroline, Rosen Mark, Boss Michael, Jackson Edward F. Quantitative imaging biomarkers alliance (QIBA) recommendations for improved precision of DWI and DCE‐MRI derived biomarkers in multicenter oncology trials. Journal of Magnetic Resonance Imaging. 2018;49(7):e101–e121. doi: 10.1002/jmri.26518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zeng Q, Shi F, Zhang J, Ling C, Dong F, Jiang B. A modified tri-exponential model for multi-b-value diffusion-weighted imaging: a method to detect the strictly diffusion-limited compartment in brain. Front Neurosci. 2018;12:102. doi: 10.3389/fnins.2018.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Langkilde F, Kobus T, Fedorov A, et al. Evaluation of fitting models for prostate tissue characterization using extended-range b-factor diffusion-weighted imaging. Magn Reson Med. 2018;79:2346–2358. doi: 10.1002/mrm.26831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Winfield JM, Tunariu N, Rata M, et al. Extracranial soft-tissue tumours: repeatability of apparent diffusion coefficient estimates from diffusion-weighted MR imaging. Radiology. 2017;284:88–99. doi: 10.1148/radiol.2017161965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taylor AJ, Salerno M, Dharmakumar R, Jerosch-Herold M. T1 mapping: basic techniques and clinical applications. JACC Cardiovasc Imaging. 2016;9:67–81. doi: 10.1016/j.jcmg.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 82.Toussaint Marcel, Gilles Raymond J., Azzabou Noura, Marty Benjamin, Vignaud Alexandre, Greiser Andreas, Carlier Pierre G. Characterization of Benign Myocarditis Using Quantitative Delayed-Enhancement Imaging Based on Molli T1 Mapping. Medicine. 2015;94(43):e1868. doi: 10.1097/MD.0000000000001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jurcoane A, Wagner M, Schmidt C, et al. Within-lesion differences in quantitative MRI parameters predict contrast enhancement in multiple sclerosis. J Magn Reson Imaging. 2013;38:1454–1461. doi: 10.1002/jmri.24107. [DOI] [PubMed] [Google Scholar]
- 84.Katsube T, Okada M, Kumano S, et al. Estimation of liver function using T1 mapping on Gd-EOB-DTPA-enhanced magnetic resonance imaging. Invest Radiol. 2011;46:277–283. doi: 10.1097/RLI.0b013e318200f67d. [DOI] [PubMed] [Google Scholar]
- 85.Mozes FE, Tunnicliffe EM, Moolla A et al (2018) Mapping tissue water T1 in the liver using the MOLLI T1 method in the presence of fat, iron and B0 inhomogeneity. NMR Biomed e4030 [DOI] [PMC free article] [PubMed]
- 86.Adam SZ, Nikolaidis P, Horowitz JM, et al. Chemical shift MR imaging of the adrenal gland: principles, pitfalls, and applications. Radiographics. 2016;36:414–432. doi: 10.1148/rg.2016150139. [DOI] [PubMed] [Google Scholar]
- 87.Yang L, Ding Y, Rao S, et al. Staging liver fibrosis in chronic hepatitis B with T1 relaxation time index on gadoxetic acid-enhanced MRI: comparison with aspartate aminotransferase-to-platelet ratio index and FIB-4. J Magn Reson Imaging. 2017;45:1186–1194. doi: 10.1002/jmri.25440. [DOI] [PubMed] [Google Scholar]
- 88.McDonald N, Eddowes PJ (2018) Multiparametric magnetic resonance imaging for quantitation of liver disease: a two-centre cross-sectional observational study. Sci Rep 8:9189 [DOI] [PMC free article] [PubMed]
- 89.Serai SD, Obuchowski NA, Venkatesh SK, et al. Repeatability of MR elastography of liver: a meta-analysis. Radiology. 2017;285:92–100. doi: 10.1148/radiol.2017161398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tietze A, Blicher J, Mikkelsen IK, et al. Assessment of ischemic penumbra in patients with hyperacute stroke using amide proton transfer (APT) chemical exchange saturation transfer (CEST) MRI. NMR Biomed. 2014;27:163–174. doi: 10.1002/nbm.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Donahue MJ, Donahue PC, Rane S, et al. Assessment of lymphatic impairment and interstitial protein accumulation in patients with breast cancer treatment-related lymphedema using CEST MRI. Magn Reson Med. 2016;75:345–355. doi: 10.1002/mrm.25649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Krishnamoorthy G, Nanga RPR, Bagga P, Hariharan H, Reddy R. High quality three-dimensional gagCEST imaging of in vivo human knee cartilage at 7 Tesla. Magn Reson Med. 2017;77:1866–1873. doi: 10.1002/mrm.26265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lindeman LR, Randtke EA, High RA, Jones KM, Howison CM, Pagel MD. A comparison of exogenous and endogenous CEST MRI methods for evaluating in vivo pH. Magn Reson Med. 2018;79:2766–2772. doi: 10.1002/mrm.26924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.David S, Visvikis D, Roux C, Hatt M. Multi-observation PET image analysis for patient follow-up quantitation and therapy assessment. Phys Med Biol. 2011;56:5771–5788. doi: 10.1088/0031-9155/56/18/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McDonald JE, Kessler MM, Gardner MW, et al. Assessment of total lesion glycolysis by (18)F FDG PET/CT significantly improves prognostic value of GEP and ISS in myeloma. Clin Cancer Res. 2017;23:1981–1987. doi: 10.1158/1078-0432.CCR-16-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boellaard R. Standards for PET image acquisition and quantitative data analysis. J Nucl Med. 2009;50(Suppl 1):11s–20s. doi: 10.2967/jnumed.108.057182. [DOI] [PubMed] [Google Scholar]
- 97.Boellaard R, O'Doherty MJ, Weber WA, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2010;37:181–200. doi: 10.1007/s00259-009-1297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kaalep Andres, Sera Terez, Rijnsdorp Sjoerd, Yaqub Maqsood, Talsma Anne, Lodge Martin A., Boellaard Ronald. Feasibility of state of the art PET/CT systems performance harmonisation. European Journal of Nuclear Medicine and Molecular Imaging. 2018;45(8):1344–1361. doi: 10.1007/s00259-018-3977-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hoffmann R, von Bardeleben S, ten Cate F, et al. Assessment of systolic left ventricular function: a multi-centre comparison of cineventriculography, cardiac magnetic resonance imaging, unenhanced and contrast-enhanced echocardiography. Eur Heart J. 2005;26:607–616. doi: 10.1093/eurheartj/ehi083. [DOI] [PubMed] [Google Scholar]
- 100.Donal E, Delgado V, Magne J, et al. Rational and design of EuroCRT: an international observational study on multi-modality imaging and cardiac resynchronization therapy. Eur Heart J Cardiovasc Imaging. 2017;18:1120–1127. doi: 10.1093/ehjci/jex021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.de Amorim Paiva CC, de Mello Junior CF, Guimaraes Filho HA, et al. Reproducibility of renal volume measurement in adults using 3-dimensional sonography. J Ultrasound Med. 2014;33:431–435. doi: 10.7863/ultra.33.3.431. [DOI] [PubMed] [Google Scholar]
- 102.Janki S1, Kimenai HJAN, Dijkshoorn ML, Looman CWN, Dwarkasing RS, IJzermans JNM (2018) Validation of ultrasonographic kidney volume measurements: a reliable imaging modality. Exp Clin Transplant 16:16–22 [DOI] [PubMed]
- 103.Di Leo G, Di Terlizzi F, Flor N, Morganti A, Sardanelli F. Measurement of renal volume using respiratory-gated MRI in subjects without known kidney disease: intraobserver, interobserver, and interstudy reproducibility. Eur J Radiol. 2011;80:e212–e216. doi: 10.1016/j.ejrad.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 104.Veyrieres JB, Albarel F, Lombard JV, et al. A threshold value in Shear Wave elastography to rule out malignant thyroid nodules: a reality? Eur J Radiol. 2012;81:3965–3972. doi: 10.1016/j.ejrad.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 105.Chang CY, Chang SJ, Chang SC, Yuan MK. The value of positron emission tomography in early detection of lung cancer in high-risk population: a systematic review. Clin Respir J. 2013;7:1–6. doi: 10.1111/j.1752-699X.2012.00290.x. [DOI] [PubMed] [Google Scholar]
- 106.Martinez CH, Chen YH, Westgate PM, et al. Relationship between quantitative CT metrics and health status and BODE in chronic obstructive pulmonary disease. Thorax. 2012;67:399–406. doi: 10.1136/thoraxjnl-2011-201185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lynch DA, Moore CM, Wilson C, et al. CT-based visual classification of emphysema: association with mortality in the COPDGene study. Radiology. 2018;288:859–866. doi: 10.1148/radiol.2018172294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jacob J, Bartholmai BJ, Rajagopalan S et al (2017) Mortality prediction in idiopathic pulmonary fibrosis: evaluation of computer-based CT analysis with conventional severity measures. Eur Respir J. 49 [DOI] [PubMed]
- 109.Jovin TG, Saver JL, Ribo M, et al. Diffusion-weighted imaging or computerized tomography perfusion assessment with clinical mismatch in the triage of wake up and late presenting strokes undergoing neurointervention with Trevo (DAWN) trial methods. Int J Stroke. 2017;12:641–652. doi: 10.1177/1747493017710341. [DOI] [PubMed] [Google Scholar]
- 110.van Riel SJ, Ciompi F, Jacobs C, et al. Malignancy risk estimation of screen-detected nodules at baseline CT: comparison of the PanCan model, Lung-RADS and NCCN guidelines. Eur Radiol. 2017;27:4019–4029. doi: 10.1007/s00330-017-4767-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Heuvelmans MA, Walter JE, Vliegenthart R, et al. Disagreement of diameter and volume measurements for pulmonary nodule size estimation in CT lung cancer screening. Thorax. 2018;73:779–781. doi: 10.1136/thoraxjnl-2017-210770. [DOI] [PubMed] [Google Scholar]
- 112.Matoba M, Tsuji H, Shimode Y, Nagata H, Tonami H. Diagnostic performance of adaptive 4D volume perfusion CT for detecting metastatic cervical lymph nodes in head and neck squamous cell carcinoma. AJR Am J Roentgenology. 2018;211:1106–1111. doi: 10.2214/AJR.17.19241. [DOI] [PubMed] [Google Scholar]
- 113.Zhang D, Xu A. Application of dual-source CT perfusion imaging and MRI for the diagnosis of primary liver cancer. Oncol Lett. 2017;14:5753–5758. doi: 10.3892/ol.2017.6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Timmers JM, van Doorne-Nagtegaal HJ, Zonderland HM, et al. The Breast Imaging Reporting and Data System (BI-RADS) in the Dutch breast cancer screening programme: its role as an assessment and stratification tool. Eur Radiol. 2012;22:1717–1723. doi: 10.1007/s00330-012-2409-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang L, Tang M, Chen S, Lei X, Zhang X, Huan Y. A meta-analysis of use of Prostate Imaging Reporting and Data System Version 2 (PI-RADS V2) with multiparametric MR imaging for the detection of prostate cancer. Eur Radiol. 2017;27:5204–5214. doi: 10.1007/s00330-017-4843-7. [DOI] [PubMed] [Google Scholar]
- 116.van der Pol CB, Lim CS, Sirlin CB, et al. Accuracy of the liver imaging reporting and data system in computed tomography and magnetic resonance image analysis of hepatocellular carcinoma or overall malignancy-a systematic review. Gastroenterology. 2019;156:976–986. doi: 10.1053/j.gastro.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 117.deSouza NM, Winfield JM, Waterton JC, et al. Implementing diffusion-weighted MRI for body imaging in prospective multicentre trials: current considerations and future perspectives. Eur Radiol. 2018;28:1118–1131. doi: 10.1007/s00330-017-4972-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hu Yao, Tang Hao, Li Haojie, Li Anqin, Li Jiali, Hu Daoyu, Li Zhen, Kamel Ihab R. Assessment of different mathematical models for diffusion-weighted imaging as quantitative biomarkers for differentiating benign from malignant solid hepatic lesions. Cancer Medicine. 2018;7(7):3501–3509. doi: 10.1002/cam4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bao J, Wang X, Hu C, Hou J, Dong F, Guo L. Differentiation of prostate cancer lesions in the transition zone by diffusion-weighted MRI. Eur J Radiol Open. 2017;4:123–128. doi: 10.1016/j.ejro.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nael K, Bauer AH, Hormigo A, et al. Multiparametric MRI for differentiation of radiation necrosis from recurrent tumour in patients with treated glioblastoma. AJR Am J Roentgenol. 2018;210:18–23. doi: 10.2214/AJR.17.18003. [DOI] [PubMed] [Google Scholar]
- 121.Bastiaannet E, Groen H, Jager PL, et al. The value of FDG-PET in the detection, grading and response to therapy of soft tissue and bone sarcomas; a systematic review and meta-analysis. Cancer Treat Rev. 2004;30:83–101. doi: 10.1016/j.ctrv.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 122.van Dijk LV, Brouwer CL, van der Laan HP, et al. Geometric image biomarker changes of the parotid gland are associated with late xerostomia. Int J Radiat Oncol Biol Phys. 2017;99:1101–1110. doi: 10.1016/j.ijrobp.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 123.Lu SJ, Gnanasegaran G, Buscombe J, Navalkissoor S. Single photon emission computed tomography/computed tomography in the evaluation of neuroendocrine tumours: a review of the literature. Nucl Med Commun. 2013;34:98–107. doi: 10.1097/MNM.0b013e32835bd59d. [DOI] [PubMed] [Google Scholar]
- 124.Ambrosini V, Campana D, Tomassetti P, Fanti S. 68Ga-labelled peptides for diagnosis of gastroenteropancreatic NET. Eur J Nucl Med Mol Imaging. 2012;39(Suppl 1):S52–S60. doi: 10.1007/s00259-011-1989-4. [DOI] [PubMed] [Google Scholar]
- 125.Maxwell JE, Howe JR. Imaging in neuroendocrine tumours: an update for the clinician. Int J Endocr Oncol. 2015;2:159–168. doi: 10.2217/ije.14.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gabriel M, Decristoforo C, Kendler D, et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumours: comparison with somatostatin receptor scintigraphy and CT. J Nuclear Med. 2007;48:508–518. doi: 10.2967/jnumed.106.035667. [DOI] [PubMed] [Google Scholar]
- 127.Park Sonya Youngju, Zacharias Claudia, Harrison Caitlyn, Fan Richard E., Kunder Christian, Hatami Negin, Giesel Frederik, Ghanouni Pejman, Daniel Bruce, Loening Andreas M., Sonn Geoffrey A., Iagaru Andrei. Gallium 68 PSMA-11 PET/MR Imaging in Patients with Intermediate- or High-Risk Prostate Cancer. Radiology. 2018;288(2):495–505. doi: 10.1148/radiol.2018172232. [DOI] [PubMed] [Google Scholar]
- 128.Lambin Philippe, Rios-Velazquez Emmanuel, Leijenaar Ralph, Carvalho Sara, van Stiphout Ruud G.P.M., Granton Patrick, Zegers Catharina M.L., Gillies Robert, Boellard Ronald, Dekker André, Aerts Hugo J.W.L. Radiomics: Extracting more information from medical images using advanced feature analysis. European Journal of Cancer. 2012;48(4):441–446. doi: 10.1016/j.ejca.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278:563–577. doi: 10.1148/radiol.2015151169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Aerts HJ, Velazquez ER, Leijenaar RT, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006. doi: 10.1038/ncomms5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yip SS, Aerts HJ. Applications and limitations of radiomics. Phys Med Biol. 2016;61:R150–R166. doi: 10.1088/0031-9155/61/13/R150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.O'Connor JP, Rose CJ, Waterton JC, Carano RA, Parker GJ, Jackson A. Imaging intratumor heterogeneity: role in therapy response, resistance, and clinical outcome. Clin Cancer Res. 2015;21:249–257. doi: 10.1158/1078-0432.CCR-14-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Drukker K, Giger ML, Joe BN (2018) Combined benefit of quantitative threecompartment breast image analysis and mammography radiomics in the classification of breast masses in a clinical data set Radiology. 290:621–628 [DOI] [PMC free article] [PubMed]
- 134.Lu Miaolong, Zhan Xianquan. The crucial role of multiomic approach in cancer research and clinically relevant outcomes. EPMA Journal. 2018;9(1):77–102. doi: 10.1007/s13167-018-0128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhao B, Tan Y, Tsai WY, et al. Reproducibility of radiomics for deciphering tumour phenotype with imaging. Sci Rep. 2016;6:23428. doi: 10.1038/srep23428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Peerlings J, Woodruff H, Winfield J et al (2019) Stability of radiomics features in apparent diffusion coefficient maps from a multi-centre test-retest trial. Sci Rep 9(1):4800 [DOI] [PMC free article] [PubMed]
- 137.Kanis JA, Harvey NC, Cooper C, Johansson H, Oden A, McCloskey EV. A systematic review of intervention thresholds based on FRAX : a report prepared for the National Osteoporosis Guideline Group and the International Osteoporosis Foundation. Arch Osteoporos. 2016;11:25. doi: 10.1007/s11657-016-0278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cho I, Al’Aref SJ, Berger A, et al. Prognostic value of coronary computed tomographic angiography findings in asymptomatic individuals: a 6-year follow-up from the prospective multicentre international CONFIRM study. Eur Heart J. 2018;39:934–941. doi: 10.1093/eurheartj/ehx774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dweck MR, Chow MW, Joshi NV, et al. Coronary arterial 18F-sodium fluoride uptake: a novel marker of plaque biology. J Am Coll Cardiol. 2012;59:1539–1548. doi: 10.1016/j.jacc.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 140.Pawade TA, Cartlidge TR, Jenkins WS et al (2016) Optimization and reproducibility of aortic valve 18F-fluoride positron emission tomography in patients with aortic stenosis. Circ Cardiovasc imaging. 9 [DOI] [PMC free article] [PubMed]
- 141.Dweck MR, Jenkins WS, Vesey AT, et al. 18F-sodium fluoride uptake is a marker of active calcification and disease progression in patients with aortic stenosis. Circ Cardiovasc Imaging. 2014;7:371–378. doi: 10.1161/CIRCIMAGING.113.001508. [DOI] [PubMed] [Google Scholar]
- 142.Joshi NV, Vesey AT, Williams MC, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet. 2014;383:705–713. doi: 10.1016/S0140-6736(13)61754-7. [DOI] [PubMed] [Google Scholar]
- 143.Forsythe RO, Dweck MR, McBride OMB, et al. 18F-sodium fluoride uptake in abdominal aortic aneurysms: the SoFIA3 study. J Am Coll Cardiol. 2018;71:513–523. doi: 10.1016/j.jacc.2017.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mesaros S, Rocca M, Sormani M, et al. Bimonthly assessment of magnetisation transfer magnetic resonance imaging parameters in multiple sclerosis: a 14-month, multicentre, follow-up study. Mult Scler. 2010;16:325–331. doi: 10.1177/1352458509358713. [DOI] [PubMed] [Google Scholar]
- 145.Agosta F, Rovaris M, Pagani E, Sormani MP, Comi G, Filippi M. Magnetisation transfer MRI metrics predict the accumulation of disability 8 years later in patients with multiple sclerosis. Brain. 2006;129:2620–2627. doi: 10.1093/brain/awl208. [DOI] [PubMed] [Google Scholar]
- 146.Rovaris M, Agosta F, Sormani MP, et al. Conventional and magnetisation transfer MRI predictors of clinical multiple sclerosis evolution: a medium-term follow-up study. Brain. 2003;126:2323–2332. doi: 10.1093/brain/awg232. [DOI] [PubMed] [Google Scholar]
- 147.Deantonio L, Caroli A, Puta E, et al. Does baseline [18F] FDG-PET/CT correlate with tumour staging, response after neoadjuvant chemoradiotherapy, and prognosis in patients with rectal cancer? Radiat Oncol. 2018;13:211. doi: 10.1186/s13014-018-1154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pan L, Gu P, Huang G, Xue H, Wu S. Prognostic significance of SUV on PET/CT in patients with esophageal cancer: a systematic review and meta-analysis. European journal of gastroenterology & hepatology. 2009;21:1008–1015. doi: 10.1097/MEG.0b013e328323d6fa. [DOI] [PubMed] [Google Scholar]
- 149.Lewis S, Besa C, Wagner M, et al. Prediction of the histopathologic findings of intrahepatic cholangiocarcinoma: qualitative and quantitative assessment of diffusion-weighted imaging. Eur Radiol. 2018;28:2047–2057. doi: 10.1007/s00330-017-5156-6. [DOI] [PubMed] [Google Scholar]
- 150.Wang YT, Li YC, Yin LL, Pu H. Can diffusion-weighted magnetic resonance imaging predict survival in patients with cervical cancer? A meta-analysis. Eur J Radiol. 2016;85:2174–2181. doi: 10.1016/j.ejrad.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 151.Yu AC, Badve C, Ponsky LE, et al. Development of a combined MR fingerprinting and diffusion examination for prostate cancer. Radiology. 2017;283:729–738. doi: 10.1148/radiol.2017161599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Delgado Anna F., Delgado Alberto F. Discrimination between Glioma Grades II and III Using Dynamic Susceptibility Perfusion MRI: A Meta-Analysis. American Journal of Neuroradiology. 2017;38(7):1348–1355. doi: 10.3174/ajnr.A5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Su C., Liu C., Zhao L., Jiang J., Zhang J., Li S., Zhu W., Wang J. Amide Proton Transfer Imaging Allows Detection of Glioma Grades and Tumor Proliferation: Comparison with Ki-67 Expression and Proton MR Spectroscopy Imaging. American Journal of Neuroradiology. 2017;38(9):1702–1709. doi: 10.3174/ajnr.A5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hayano K, Shuto K, Koda K, Yanagawa N, Okazumi S, Matsubara H. Quantitative measurement of blood flow using perfusion CT for assessing clinicopathologic features and prognosis in patients with rectal cancer. Dis Colon Rectum. 2009;52:1624–1629. doi: 10.1007/DCR.0b013e3181afbd79. [DOI] [PubMed] [Google Scholar]
- 155.Win T, Miles KA, Janes SM, et al. Tumour heterogeneity and permeability as measured on the CT component of PET/CT predict survival in patients with non-small cell lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:3591–3599. doi: 10.1158/1078-0432.CCR-12-1307. [DOI] [PubMed] [Google Scholar]
- 156.Lund KV, Simonsen TG, Kristensen GB, Rofstad EK. Pretreatment late-phase DCE-MRI predicts outcome in locally advanced cervix cancer. Acta Oncol. 2017;56:675–681. doi: 10.1080/0284186X.2017.1294762. [DOI] [PubMed] [Google Scholar]
- 157.Fasmer Kristine E, Bjørnerud Atle, Ytre-Hauge Sigmund, Grüner Renate, Tangen Ingvild L, Werner Henrica MJ, Bjørge Line, Salvesen Øyvind O, Trovik Jone, Krakstad Camilla, Haldorsen Ingfrid S. Preoperative quantitative dynamic contrast-enhanced MRI and diffusion-weighted imaging predict aggressive disease in endometrial cancer. Acta Radiologica. 2017;59(8):1010–1017. doi: 10.1177/0284185117740932. [DOI] [PubMed] [Google Scholar]
- 158.Yu J, Xu Q, Huang DY, et al. Prognostic aspects of dynamic contrast-enhanced magnetic resonance imaging in synchronous distant metastatic rectal cancer. Eur Radiol. 2017;27:1840–1847. doi: 10.1007/s00330-016-4532-y. [DOI] [PubMed] [Google Scholar]
- 159.Wilson R, Devaraj A. Radiomics of pulmonary nodules and lung cancer. Transl Lung Cancer Res. 2017;6:86–91. doi: 10.21037/tlcr.2017.01.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lee JW, Lee SM. Radiomics in oncological PET/CT: clinical applications. Nucl Med Mol Imaging. 2018;52:170–189. doi: 10.1007/s13139-017-0500-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kumar V, Gu Y, Basu S, et al. Radiomics: the process and the challenges. Magn Reson imaging. 2012;30:1234–1248. doi: 10.1016/j.mri.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang Y, Oikonomou A, Wong A, Haider MA, Khalvati F. Radiomics-based prognosis analysis for non-small cell lung cancer. Sci Rep. 2017;7:46349. doi: 10.1038/srep46349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Huang Yan-qi, Liang Chang-hong, He Lan, Tian Jie, Liang Cui-shan, Chen Xin, Ma Ze-lan, Liu Zai-yi. Development and Validation of a Radiomics Nomogram for Preoperative Prediction of Lymph Node Metastasis in Colorectal Cancer. Journal of Clinical Oncology. 2016;34(18):2157–2164. doi: 10.1200/JCO.2015.65.9128. [DOI] [PubMed] [Google Scholar]
- 164.Goldin JG, Kim GHJ, Tseng CH, et al. Longitudinal changes in quantitative interstitial lung disease on computed tomography after immunosuppression in the Scleroderma Lung Study II. Ann Am Thorac Soc. 2018;15:1286–1295. doi: 10.1513/AnnalsATS.201802-079OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Peacock AJ, Crawley S, McLure L, et al. Changes in right ventricular function measured by cardiac magnetic resonance imaging in patients receiving pulmonary arterial hypertension-targeted therapy: the EURO-MR study. Circ Cardiovasc Imaging. 2014;7:107–114. doi: 10.1161/CIRCIMAGING.113.000629. [DOI] [PubMed] [Google Scholar]
- 166.Steinhoff Gustav, Nesteruk Julia, Wolfien Markus, Kundt Günther, Börgermann Jochen, David Robert, Garbade Jens, Große Jana, Haverich Axel, Hennig Holger, Kaminski Alexander, Lotz Joachim, Mohr Friedrich-Wilhelm, Müller Paula, Oostendorp Robert, Ruch Ulrike, Sarikouch Samir, Skorska Anna, Stamm Christof, Tiedemann Gudrun, Wagner Florian Mathias, Wolkenhauer Olaf. Cardiac Function Improvement and Bone Marrow Response –. EBioMedicine. 2017;22:208–224. doi: 10.1016/j.ebiom.2017.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schwartz Lawrence H., Seymour Lesley, Litière Saskia, Ford Robert, Gwyther Stephen, Mandrekar Sumithra, Shankar Lalitha, Bogaerts Jan, Chen Alice, Dancey Janet, Hayes Wendy, Hodi F. Stephen, Hoekstra Otto S., Huang Erich P., Lin Nancy, Liu Yan, Therasse Patrick, Wolchok Jedd D., de Vries Elisabeth. RECIST 1.1 – Standardisation and disease-specific adaptations: Perspectives from the RECIST Working Group. European Journal of Cancer. 2016;62:138–145. doi: 10.1016/j.ejca.2016.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumours. J Nucl Med. 2009;50(Suppl 1):122s–150s. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sormani MP, De Stefano N. Defining and scoring response to IFN-beta in multiple sclerosis. Nat Rev Neurol. 2013;9:504–512. doi: 10.1038/nrneurol.2013.146. [DOI] [PubMed] [Google Scholar]
- 170.Østergaard Mikkel, Bird Paul, Gandjbakhch Frédérique, Eshed Iris, Haugen Ida K., Haavardsholm Espen A., Lillegraven Siri, Foltz Violaine, Glinatsi Daniel, Peterfy Charles, Ejbjerg Bo, Bøyesen Pernille, Mease Philip J., Hermann Kay-Geert, Emery Paul, Genant Harry K., Conaghan Philip G. The OMERACT MRI in Arthritis Working Group — Update on Status and Future Research Priorities. The Journal of Rheumatology. 2015;42(12):2470–2472. doi: 10.3899/jrheum.141248. [DOI] [PubMed] [Google Scholar]
- 171.Patel P, Baradaran H, Delgado D, et al. MR perfusion-weighted imaging in the evaluation of high-grade gliomas after treatment: a systematic review and meta-analysis. Neuro Oncol. 2017;19:118–127. doi: 10.1093/neuonc/now148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.van Dijken BRJ, van Laar PJ, Holtman GA, van der Hoorn A. Diagnostic accuracy of magnetic resonance imaging techniques for treatment response evaluation in patients with high-grade glioma, a systematic review and meta-analysis. Eur Radiol. 2017;27:4129–4144. doi: 10.1007/s00330-017-4789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Aide N, Lasnon C, Veit-Haibach P, Sera T, Sattler B, Boellaard R. EANM/EARL harmonization strategies in PET quantification: from daily practice to multicentre oncological studies. Eur J Nucl Med Mol Imaging. 2017;44:17–31. doi: 10.1007/s00259-017-3740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Maffione AM, Marzola MC, Capirci C, Colletti PM, Rubello D. Value of 18F-FDG PET for predicting response to neoadjuvant therapy in rectal cancer: systematic review and meta-analysis. AJR Am J Roentgenology. 2015;204:1261–1268. doi: 10.2214/AJR.14.13210. [DOI] [PubMed] [Google Scholar]
- 175.Martens RM, Noij DP, Ali M, et al. Functional imaging early during (chemo)radiotherapy for response prediction in head and neck squamous cell carcinoma; a systematic review. Oral Oncol. 2019;88:75–83. doi: 10.1016/j.oraloncology.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 176.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Younes A, Hilden P, Coiffier B, et al. International Working Group consensus response evaluation criteria in lymphoma (RECIL 2017) Ann Oncol. 2017;28:1436–1447. doi: 10.1093/annonc/mdx097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dalm Simone, Verzijlbergen John, De Jong Marion. Review: Receptor Targeted Nuclear Imaging of Breast Cancer. International Journal of Molecular Sciences. 2017;18(2):260. doi: 10.3390/ijms18020260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bakht Martin K., Oh So Won, Youn Hyewon, Cheon Gi Jeong, Kwak Cheol, Kang Keon Wook. Influence of Androgen Deprivation Therapy on the Uptake of PSMA-Targeted Agents: Emerging Opportunities and Challenges. Nuclear Medicine and Molecular Imaging. 2016;51(3):202–211. doi: 10.1007/s13139-016-0439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hotker AM, Tarlinton L, Mazaheri Y, et al. Multiparametric MRI in the assessment of response of rectal cancer to neoadjuvant chemoradiotherapy: a comparison of morphological, volumetric and functional MRI parameters. Eur Radiol. 2016;26:4303–4312. doi: 10.1007/s00330-016-4283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Partridge Savannah C., Zhang Zheng, Newitt David C., Gibbs Jessica E., Chenevert Thomas L., Rosen Mark A., Bolan Patrick J., Marques Helga S., Romanoff Justin, Cimino Lisa, Joe Bonnie N., Umphrey Heidi R., Ojeda-Fournier Haydee, Dogan Basak, Oh Karen, Abe Hiroyuki, Drukteinis Jennifer S., Esserman Laura J., Hylton Nola M. Diffusion-weighted MRI Findings Predict Pathologic Response in Neoadjuvant Treatment of Breast Cancer: The ACRIN 6698 Multicenter Trial. Radiology. 2018;289(3):618–627. doi: 10.1148/radiol.2018180273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hayano K, Okazumi S, Shuto K, et al. Perfusion CT can predict the response to chemoradiation therapy and survival in esophageal squamous cell carcinoma: initial clinical results. Oncol Rep. 2007;18:901–908. [PubMed] [Google Scholar]
- 183.O'Connor JP, Jackson A, Parker GJ, Roberts C, Jayson GC. Dynamic contrast-enhanced MRI in clinical trials of antivascular therapies. Nat Rev Clin Oncol. 2012;9:167–177. doi: 10.1038/nrclinonc.2012.2. [DOI] [PubMed] [Google Scholar]
- 184.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumour treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 185.El Alaoui-Lasmaili K, Faivre B. Antiangiogenic therapy: markers of response, “normalization” and resistance. Crit Rev Oncol Hematol. 2018;128:118–129. doi: 10.1016/j.critrevonc.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 186.Sheikhbahaei S, Mena E, Yanamadala A, et al. The value of FDG PET/CT in treatment response assessment, follow-up, and surveillance of lung cancer. AJR Am J Roentgenol. 2017;208:420–433. doi: 10.2214/AJR.16.16532. [DOI] [PubMed] [Google Scholar]
- 187.Goense L, van Rossum PS, Reitsma JB, et al. Diagnostic performance of 18F-FDG PET and PET/CT for the detection of recurrent esophageal cancer after treatment with curative intent: a systematic review and meta-analysis. J Nucl Med. 2015;56:995–1002. doi: 10.2967/jnumed.115.155580. [DOI] [PubMed] [Google Scholar]
- 188.Sullivan DC, Obuchowski NA, Kessler LG, et al. Metrology standards for quantitative imaging biomarkers. Radiology. 2015;277:813–825. doi: 10.1148/radiol.2015142202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Waterton John C., Pylkkanen Liisa. Qualification of imaging biomarkers for oncology drug development. European Journal of Cancer. 2012;48(4):409–415. doi: 10.1016/j.ejca.2011.11.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable