Many microorganisms, including the plant pathogen Pseudomonas syringae, produce amphiphilic compounds known as biosurfactants. While biosurfactants are known to disperse hydrophobic compounds and to reduce water tension, they have other properties that can benefit the cells that produce them. Leaf-colonizing bacteria experience frequent water stress, since liquid water is present only transiently on or in leaf sites that they colonize. The demonstration that syringafactin, a biosurfactant produced by P. syringae, is sufficiently hygroscopic to increase water availability to cells, thus relieving water stress, reveals that P. syringae can modify its local habitat both on leaf surfaces and in the leaf apoplast. Such habitat modification may be a common role for biosurfactants produced by other bacterial species that colonize habitats (such as soil) that are not always water saturated.
KEYWORDS: biosensors, biosurfactants, epiphytes, hygroscopicity
ABSTRACT
The epiphytic bacterium Pseudomonas syringae strain B728a produces the biosurfactant syringafactin, which is hygroscopic. The water-absorbing potential of syringafactin is high. Syringafactin attracts 250% of its weight in water at high relative humidities but is less hygroscopic at lower relative humidities. This finding suggests that the benefit of syringafactin to the producing cells is strongly context dependent. The contribution of syringafactin to the water availability around cells on different matrices was assessed by examining the water stress exhibited by biosensor strains expressing gfp via the water-stress-activated proU promoter. Wild-type cells exhibited significantly less green fluorescent protein (GFP) fluorescence than a syringafactin-deficient strain on dry filters in atmospheres of high water saturation, as well as on leaf surfaces, indicating greater water availability. When infiltrated into the leaf apoplast, wild-type cells also subsequently exhibited less GFP fluorescence than the syringafactin-deficient strain. These results suggest that the apoplast is a dry but humid environment and that, just as on dry but humid leaf surfaces, syringafactin increases liquid water availability and reduces the water stress experienced by P. syringae.
IMPORTANCE Many microorganisms, including the plant pathogen Pseudomonas syringae, produce amphiphilic compounds known as biosurfactants. While biosurfactants are known to disperse hydrophobic compounds and to reduce water tension, they have other properties that can benefit the cells that produce them. Leaf-colonizing bacteria experience frequent water stress, since liquid water is present only transiently on or in leaf sites that they colonize. The demonstration that syringafactin, a biosurfactant produced by P. syringae, is sufficiently hygroscopic to increase water availability to cells, thus relieving water stress, reveals that P. syringae can modify its local habitat both on leaf surfaces and in the leaf apoplast. Such habitat modification may be a common role for biosurfactants produced by other bacterial species that colonize habitats (such as soil) that are not always water saturated.
INTRODUCTION
While leaf surfaces support large numbers of bacteria, leaves are considered a relatively harsh environment for bacterial colonization. Leaves are frequently dry environmental habitats that are also subject to high UV fluxes, as well as fluctuations in temperature and humidity (1–5). Water stress is considered one of the major factors limiting bacterial survival on leaves (6, 7). As in many environments, the frequent lack of water on leaves is expected to limit the ability of epiphytes like Pseudomonas syringae to colonize such surfaces. However, P. syringae successfully colonizes and survives on the surfaces of leaves, often subsequently causing disease in the host plant after it enters the leaf interior (4). The leaf apoplast is composed largely of air-filled voids between parenchymal cells, which facilitate gas exchange for photosynthesis (4, 8). Although the apoplast has been suggested to be a dry environment (6), the air in the apoplast would have high water saturation, given that it would be in equilibrium with the water found in plant cells; this would result in it being a humid but dry environment. Transcriptomic analysis of P. syringae cells recovered from both epiphytic and endophytic sites revealed high levels of expression of genes involved in tolerance to water stress (9). This finding supports the idea that water limitation is experienced by this species both in the interior and on the exterior of leaves. However, the traits that enable P. syringae to grow and to survive on and in dry leaves are poorly understood.
While leaves are frequently dry, the relative humidity (RH) of air near the leaf surface is expected to often differ substantially from that surrounding the leaf. Because of friction with the leaf surface, air movement is rapidly inhibited as it crosses the leaf, creating a thin layer of still air, known as the laminar boundary layer, that surrounds the leaf. The thickness of this layer is inversely proportional to wind speed but usually is less than about 10 mm (10). Much of the water vapor that exits the leaf via its stomata is apparently retained within the laminar boundary layer (2, 3, 6, 11–16). Thus, the air surrounding the leaf surface can exhibit a much higher RH than the atmosphere away from the leaf (10, 17). While the high RH expected in the boundary layer would reduce the rate of evaporation of water from bacterial cells on the leaf surface, it would not be expected to eliminate the osmotic or matric water stresses that cells would experience when unbound liquid water is not present.
Biosurfactants are amphiphilic compounds produced by various microorganisms (18). Most studies of biosurfactants have described their ability to disperse hydrophobic compounds, often enabling their consumption by the biosurfactant-producing bacteria (18, 19). Most biosurfactants can also reduce water tension, thereby enabling the dispersal of water across hydrophobic surfaces such as leaves (3, 20). This trait might be beneficial to epiphytic bacteria. Burch et al. (2) recently reported that certain biosurfactants, such as syringafactin, a biosurfactant produced by P. syringae, also have the underappreciated characteristic of being hygroscopic. Syringafactin is a lipopeptide containing eight amino acids linked to an acyl tail, making it amphipathic (21). The peptide head of this molecule contains several hydroxyl groups capable of hydrogen bonding with water. This structure suggested that syringafactin could interact with and absorb water. Burch et al. (2) verified the hygroscopic nature of syringafactin by showing that, after being desiccated, syringafactin could be rewetted when exposed to a water-saturated atmosphere. Syringafactin production appears to be beneficial to P. syringae on leaf surfaces. When a wild-type P. syringae strain and a syfA mutant strain deficient in syringafactin production were coinoculated onto bean leaves in a field experiment, the wild-type strain maintained greater population sizes on plants than did the syfA mutant strain (2). This suggested that the wild-type strain was more tolerant of water stress experienced during fluctuating environmental conditions in the field.
The goal of this study was to test the hypothesis that the contributions of syringafactin to the epiphytic fitness of P. syringae are due to its ability to absorb water vapor from the air and to retain water once cells have been wetted, thereby maintaining a more hydrated state in the vicinity of cells producing this compound. This would reduce the water stress experienced by cells on plants in the absence of liquid water. Such a role would require syringafactin to bind abundant water under the conditions that cells would experience on leaf surfaces. While the RH experienced by bacteria on the surface of plants is expected to be higher than that in the air surrounding the plant, the actual RH and its temporal variability on leaves are unknown. Furthermore, although syringafactin was shown to bind abundant water in a water-saturated environment (2), it is not clear whether it can do so under conditions experienced by cells on a plant. By determining both the RH-dependent water-binding capabilities of syringafactin and the apparent water status of bacteria on leaves, it should be possible both to test the hypothesis described above and to provide insight regarding the water environment experienced by cells on leaves. Such information is needed to determine the contexts in which syringafactin would benefit the producing cells. To measure the water status of cells, we utilize a whole-cell bacterial biosensor described by Axtell and Beattie (1), which assesses the expression of proU, a gene contributing to production of the compatible solute proline, by linking it to a green fluorescent protein (GFP) reporter gene. Cells harboring this reporter gene construct exhibit GFP fluorescence that is directly proportional to the level of either matric or osmotic stress that they experience (1). In this study, we assessed the water stress experienced by both the wild-type P. syringae B728a strain and a syfA mutant strain deficient in syringafactin production, on both the surface and interior of plants, by quantifying the fluorescence of individual bacterial cells using epifluorescence microscopy. As described below, our results strongly suggest that syringafactin production by P. syringae can reduce the water stress that cells experience both on the leaf surface and in the leaf apoplast. This work thus reveals an important and previously unrecognized role for microbial biosurfactants in the varied environments that such epiphytes colonize.
RESULTS
Syringafactin is very hygroscopic only at high relative humidities.
Since the hypothesized ecological role of syringafactin depends on its ability to interact with water, we examined the conditions under which syringafactin would absorb water. Purified dehydrated syringafactin was exposed to different controlled RH conditions maintained by suspension over different saturated salt solutions (Fig. 1). The weight of the syringafactin was determined both before exposure and after 3 days of exposure to a given RH. Although the water-absorbing potential of syringafactin generally increased with increasing RH, syringafactin absorbed less than its own weight in water at most levels of atmospheric water saturation. Importantly, its water-binding capacity increased dramatically at RHs greater than about 97%, and it absorbed 250% of its weight in water in fully water-saturated air (Fig. 1). This finding indicates that the high water-binding capability of syringafactin under conditions with high levels of atmospheric water saturation maximizes its potential ecological value under such conditions. In contrast to the high water-binding capability of syringafactin in water-saturated atmospheres, levan and alginate, polysaccharides that are produced abundantly by P. syringae under certain conditions, absorb only 35% and 36% of their weight, respectively, under these conditions (data not shown).
FIG 1.
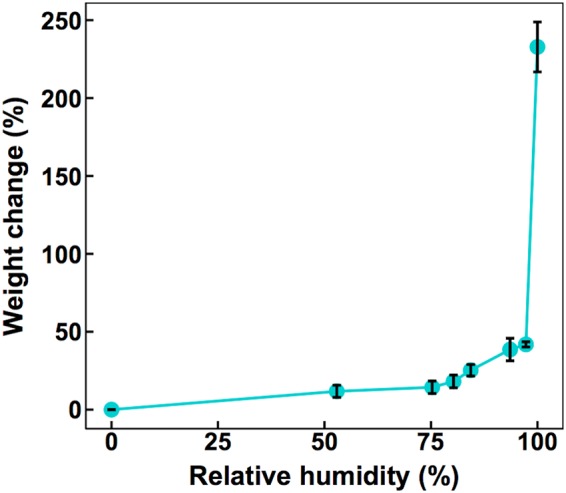
Water binding by syringafactin. Weight gain due to water absorption is expressed as a proportion of the initial weight of dehydrated syringafactin, which was exposed for 3 days to atmospheres containing the RH shown on the abscissa. The vertical bars represent the standard error of the mean percent weight change.
Syringafactin contributes to water availability for cells on filters.
To determine whether syringafactin made enough water available to bacterial cells to alleviate water stress, we compared the water stress levels exhibited by P. syringae cells differing in syringafactin production when immobilized on membrane filters. Since they would lack the humid laminar boundary layer of leaves, filters were used as a more direct means to determine the conditions under which, and the degree to which, the water status of cells would be modulated by the presence of syringafactin. Cells of the wild-type strain and the syfA mutant strain harboring the proU-gfp reporter gene construct, grown on low-salt minimal medium in which they would be expected to experience the same low level of water stress, did not differ in their expression of GFP fluorescence before application to filters (see Fig. S1 in the supplemental material). The wild-type strain and a syfA mutant P. syringae strain were then grown on filters placed on agar plates for 8 h before the filters were transferred to chambers maintaining 52% RH or 100% RH. Filters were incubated in the chambers for 4 h and then immersed in a low-salt-containing minimal nutrient medium for 2 h to resuscitate cells and to enable the translation of GFP resulting from transcription of the reporter gene. As a positive control, 2.5 mg of exogenous syringafactin was added to cells of the syfA mutant strain after they had grown on the filter. Wild-type cells exhibited significantly less GFP fluorescence than did the syfA mutant when incubated at 52% RH (Fig. 2A). The GFP fluorescence of the syfA mutant strain exposed to exogenous syringafactin was similar to that of the wild-type strain (Fig. 2A). Similarly, at 100% RH, the GFP fluorescence exhibited by the syfA mutant strain to which exogenous syringafactin had been applied was significantly less than that exhibited by this strain in the absence of added syringafactin (Fig. 2B). Furthermore, the GFP fluorescence of the syfA mutant was still higher than that of the wild-type strain when both were incubated at 100% RH (Fig. 2B). The finding that both the wild-type strain alone and the syfA mutant strain with applied syringafactin exhibited similarly lower levels of GFP fluorescence than the syfA mutant strain itself at 100% RH supports our hypothesis that not only is syringafactin capable of making water more available to cells under high RH conditions but also wild-type cells produce sufficient amounts of syringafactin to confer this phenotype. Surprisingly, this trend was also seen at 52% RH, suggesting that the lesser amounts of water retained by syringafactin at this lower RH were still enough to reduce somewhat the water stress that producing cells experienced.
FIG 2.
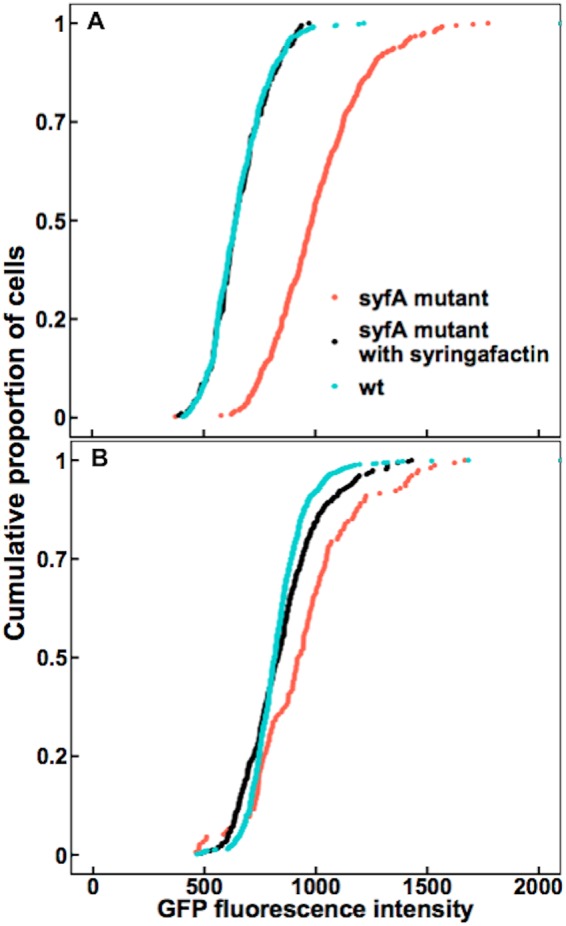
Syringafactin reduces water stress experienced by P. syringae on filters. Lower water stress is experienced by wild-type P. syringae cells than by syfA mutant cells on filters at 52% RH and 100% RH. A wild-type (wt) strain (blue) and a syfA mutant strain (red) harboring a proU-gfp reporter, as well as the syfA mutant strain with added syringafactin (black), were incubated on membrane filters on minimal medium plates for 8 h before the filters were transferred to glass slides placed in chambers maintained at either 52% RH (A) or 100% RH (B). Single-cell fluorescence was quantified by microscopy. (A) After exposure to 52% RH, the median fluorescence of wild-type cells was 643 (n = 449), while that of syfA mutant cells was 984 (n = 366) and that of syfA mutant cells with added syringafactin was 643 (n = 192). Significance between sample distributions was tested using the Wilcoxon rank-sum test (wild-type strain versus syfA mutant strain, P < 2.2 × 10−16, W = 153,460; wild-type strain versus syfA mutant strain with syringafactin, P = 0.70, W = 43,693; syfA mutant strain versus syfA mutant strain with syringafactin, P < 2.2 × 10−16, W = 65,276). (B) After exposure to 100% RH, the median fluorescence of wild-type cells was 815 (n = 767), while that of syfA mutant cells was 924 (n = 155) and that of syfA mutant cells with syringafactin was 831 (n = 308). Significance between sample distributions was tested using the Wilcoxon rank-sum test (wild-type strain versus syfA mutant strain, P = 7.71 × 10−11, W = 39,768; wild-type strain versus syfA mutant strain with syringafactin, P = 0.36, W = 113,890; syfA mutant strain versus syfA mutant strain with syringafactin, P = 2.78 × 10−6, W = 30,238).
Syringafactin improves water availability for cells on leaf surfaces, irrespective of the dryness of air away from the leaf.
Given that syringafactin could make water more available to cells on abiotic surfaces, we determined the extent of water stress experienced by bacterial cells on leaves exposed to various environmental conditions, and we asked whether cells could ameliorate this stress by producing syringafactin. We hypothesized that, at high RH, the wild-type cells would exhibit lower GFP fluorescence than the syfA mutant cells, due to the attraction of water by syringafactin production. To test this, wild-type and syfA mutant strains harboring the proU-gfp reporter gene fusion were sprayed onto the leaves of bean plants, which were then immediately placed in a 100% RH chamber for 2 days to enable bacterial growth and production of any extracellular products. The sprayed leaves initially were covered with many small droplets of bacterial suspension; after 2 days, however, most of the leaf was free of any droplets. Only a few water droplets persisted on the leaves, suggesting that the water had evaporated from the leaves to condense on the chamber walls and any residual water was redistributed to produce large dry areas on the leaf surface. When examined 2 days after inoculation, wild-type cells exhibited less GFP expression than did the syfA mutant cells (Fig. 3A). Presumably, many of the cells on these leaves were localized at sites on the leaf that were devoid of liquid water and thus they experienced water stress, although the RH on the leaves must have been near 100%.
FIG 3.
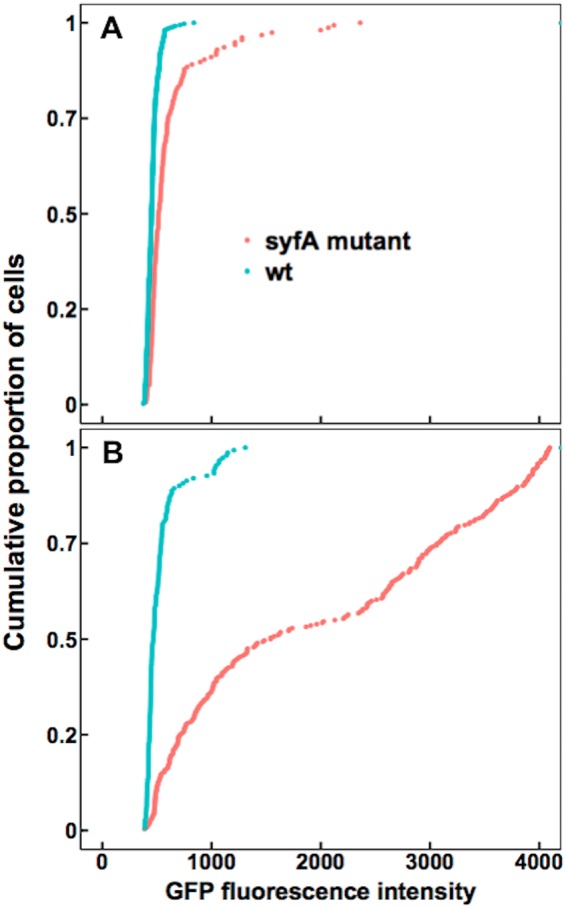
Syringafactin-producing strains of P. syringae experience less water stress than syfA mutants on humid leaves and after transient exposure to low RH on plants. GFP fluorescence exhibited by wild-type (wt) P. syringae strain B728a (blue) or a syfA mutant strain (red) harboring a proU-gfp reporter recovered from the leaves of plants incubated at 100% RH for 2 days (A) and from the leaves of plants incubated at 100% RH for 2 days, followed by drying at 50% RH for 20 min before reexposure to 100% RH for 2 h (B), was assessed in separate experiments. The median fluorescence of wild-type cells on continuously humid leaves was 450 (n = 371), while that of syfA mutant cells was 516 (n = 156). The median fluorescence of the wild-type cells on leaves that were transiently dried was 467 (n = 150), while that of the syfA mutant cells was 1,541 (n = 282). Significance between sample distributions was tested using the Wilcoxon rank-sum test (wild-type strain versus syfA mutant strain in panel A, W = 45,442, P < 2.2 × 10−16; wild-type strain versus syfA mutant strain in panel B, W = 38,207, P < 2.2 × 10−16).
To determine the environmental contexts in which syringafactin could be produced and under which it could confer protection against water stress, we exposed P. syringae cells to various drying conditions on leaves. Leaves were exposed sequentially to wet conditions under which bacterial cells could multiply on leaves, followed by dry conditions under which multiplication would cease. Although a difference in GFP fluorescence was observed between the wild-type and syfA mutant strains harboring the proU-gfp reporter gene when cells were applied to dry plants that remained dry throughout the experiments (Fig. S2), it is noteworthy that this difference seemed to be caused by only about 20% of the cell population, rather than a majority. Indeed, in a subsequent experiment, most of the cells died quickly after immediate drying on surfaces (Fig. S3). This phenomenon has been seen previously on leaves (7), and these cells would not have been capable of expressing the reporter gene. Therefore, we assessed the water stress experienced by bacteria exposed to dry conditions following moist incubation conditions after inoculation of bacteria onto the plant, which would have allowed their colonization and any habitat modification to occur. Under one such condition, inoculated bean plants were immediately incubated in a 100% RH chamber for 2 days before leaf surfaces were dried by exposure of the plants to 50% RH for 20 min and then were incubated at 100% RH for 2 hours (Fig. 3B). Such plants would have experienced liquid water on leaf surfaces only in the initial 2-day incubation period, and cells would have subsequently found themselves on dry leaves exposed to varying RH conditions. We presume that the apparent transcription of the proU-gfp reporter gene, as evidenced by the GFP fluorescence output, would have reflected the conditions experienced by the cells during the final 2-h period of dry but humid incubation. Under those conditions, it was evident that, while the wild-type strain exhibited the same relatively low GFP fluorescence (indicative of low water stress) as it had on leaves continually exposed to high RH conditions, the syfA mutant strain apparently experienced considerable water stress, as indicated by its high GFP fluorescence (Fig. 3B). The GFP fluorescence of most syfA mutant cells was much higher than that of the wild-type strain, which suggested that they experienced more water stress than the wild-type strain (Fig. 3B). These results suggest that syringafactin production by the wild-type strain could sequester sufficient water to prevent water stress when cells exposed to desiccating conditions were subsequently exposed to a water-saturated environment.
Wild-type cells experience less water stress than syfA mutant cells when exposed to fluctuating RH conditions at less than full atmospheric water saturation.
Given that plants frequently experience conditions of less than full atmospheric water saturation (100% RH) under field conditions (3), we examined the potential for syringafactin to modulate the water availability for cells on the surface of leaves under these conditions. Bean leaves sprayed with either the wild-type strain or the syfA mutant strain harboring the proU-gfp reporter gene fusion were immediately incubated at 100% RH for 2 days to enable bacterial growth and metabolism. The plants were then exposed to 50% RH for 1 h, to allow liquid water to evaporate from the leaf, before being incubated at 97% RH for 2 days. As was seen when such dried colonized leaves were subsequently exposed to 100% RH, the GFP fluorescence of the syfA mutant strain was significantly higher than that of the wild-type strain (Fig. 4), indicating that the mutant strain exhibited a higher degree of water stress than the wild-type strain. Given that the water-binding capability of syringafactin at 97% RH is much lower than that in a fully water-saturated atmosphere, it seems likely that the RH experienced by cells on plants incubated at 97% RH was actually much higher because of the modulation of the air in the laminar boundary layer surrounding the leaves by water vapor released by plant transpiration. This would enable syringafactin to bind water and thus to hydrate the cells of the syringafactin-producing strain.
FIG 4.
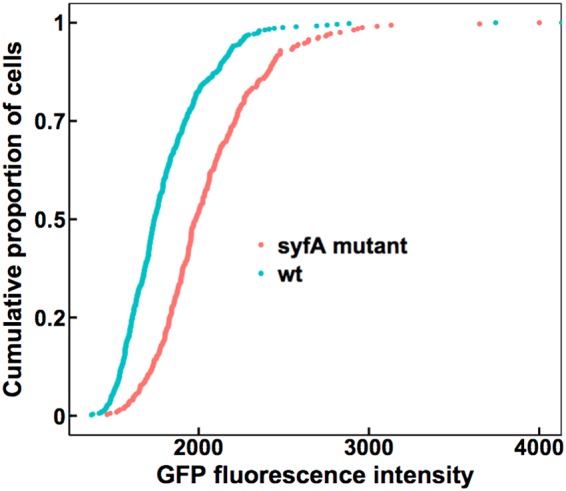
Wild-type cells experience less water stress than syfA mutant cells when exposed to 97% RH on leaf surfaces. GFP fluorescence exhibited by wild-type (wt) P. syringae strain B728a (blue) or a syfA mutant strain (red) harboring a proU-gfp reporter recovered from the leaves of plants incubated at 100% RH for 2 days, followed by drying at 50% RH for 20 min before reexposure to 97% RH for 2 days, was assessed. The median fluorescence of the wild-type cells was 1,741 (n = 489), while that of the syfA mutant cells was 1,984 (n = 324). Single-cell fluorescence was quantified by microscopy. Significance between sample distributions was tested using the Wilcoxon rank-sum test (wild-type strain versus syfA mutant strain, W = 119,610, P < 2.2 × 10−16).
Syringafactin helps make water available to bacteria in the leaf apoplast.
After colonizing the leaf surface, P. syringae cells can enter the leaf apoplast through stomata, where they can grow to sufficient numbers to eventually cause disease (4). Given that the apoplast is often dry, at least initially after bacterial entry, we determined whether syringafactin production could reduce water stress in this habitat, just as it apparently does on the leaf surface. Wild-type cells and syfA mutant cells harboring the proU-gfp reporter gene construct were infiltrated into bean leaves under a vacuum. Before the cells were infiltrated into the leaves, aliquots of cells taken from the liquid culture used as the inoculum were assessed for GFP fluorescence to determine whether the two strains differed in apparent water availability at time zero. While the levels of GFP fluorescence exhibited by the two strains were initially quite similar (Fig. 5A), cells of the syfA mutant strain exhibited higher GFP fluorescence than did cells of the wild-type strain when assessed 24 h after inoculation, indicating that cells of the mutant strain were beginning to experience somewhat more water stress than cells of the wild-type strain (Fig. 5B). By 48 h after inoculation, the levels of GFP fluorescence of the two strains in the apoplast were much higher than those after 24 h, indicating that the intercellular spaces had become even drier during the infection process (Fig. 5C). Importantly, by 48 h after incubation, syfA mutant cells exhibited much higher GFP fluorescence than did the wild-type cells, indicating that they were experiencing greater water stress than the wild-type strain (Fig. 5C). Thus, syringafactin production by the wild-type strain had ameliorated the water stress that it otherwise would have experienced.
FIG 5.
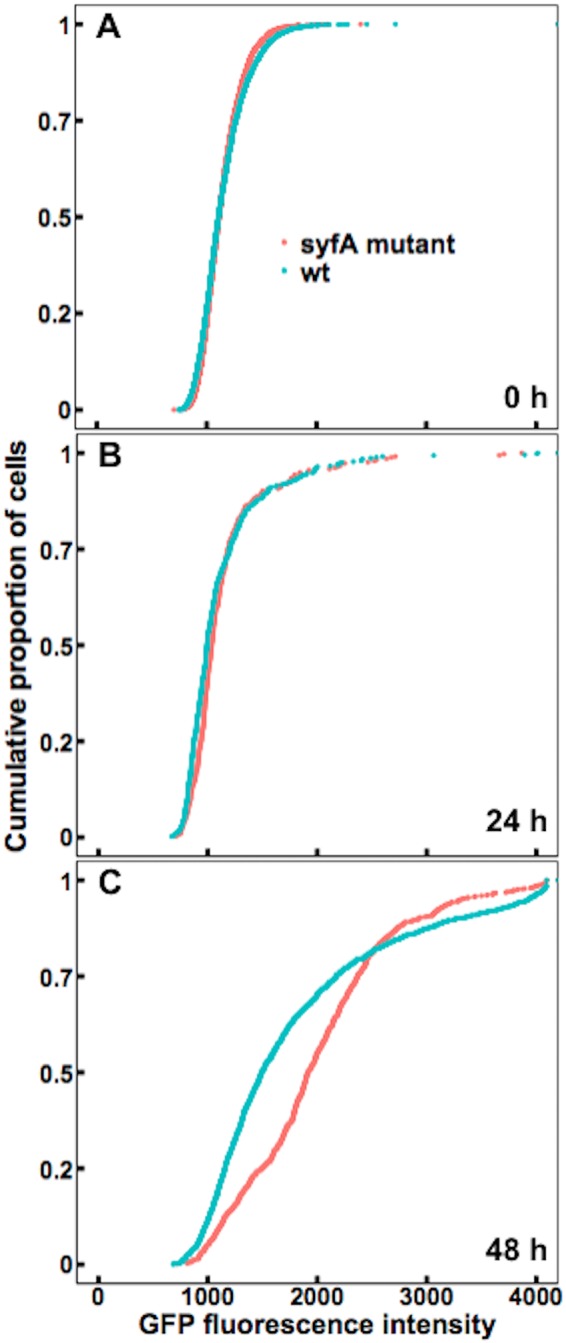
Wild-type P. syringae cells in the leaf apoplast experience less water stress than syfA mutant cells. GFP fluorescence exhibited by wild-type (wt) strain B728a (blue) or a syfA mutant strain (red) harboring a proU-gfp reporter, recovered from the interior of plants 0 h (A), 24 h (B), or 48 h (C) after cells were vacuum infiltrated into the apoplasts of bean leaves, was assessed. (A) At 0 h, the median fluorescence of the wild-type cells was 1,107 (n = 4,023), while that of the syfA mutant cells was 1,112 (n = 4,000). (B) After 24 h, the median fluorescence of the wild-type cells was 992 (n = 447), while that of the syfA mutant cells was 1,035 (n = 336). (C) After 48 h, the median fluorescence of the wild-type cells was 1,492 (n = 1,528), while that of the syfA mutant cells was 1,914 (n = 908). Single-cell fluorescence was quantified by microscopy. Significance between sample distributions was tested using the two-sample t test (A) (wild-type strain versus syfA mutant strain, degrees of freedom = 8,021, P = 0.97), the Wilcoxon rank-sum test (B) (wild-type strain versus syfA mutant strain, W = 83,004, P = 0.01), or the Wilcoxon rank-sum test (C) (wild-type strain versus syfA mutant strain, W = 856,960, P < 2.2 × 10−16).
DISCUSSION
This study determines whether syringafactin plays an important role in the epiphytic and endophytic growth of P. syringae. It is reasonable to assume that free liquid water is required for bacterial growth in and on leaves, since it would be required to mobilize soluble nutrients (8). However, leaf surfaces are dry much of the time and cells must survive such conditions in order to grow during the brief periods when water might become available. Thus, life on a leaf surface is probably stressful, since it is a dry environment that is only transiently wet (1, 2, 5, 6, 22).
We found that the hygroscopic biosurfactant syringafactin produced by P. syringae B728a reduced the water stress experienced by this bacterium both on dry leaves and in the apoplast, by attracting water vapor from the atmosphere and also perhaps by retaining water after cells were wetted. Syringafactin is quite hygroscopic under the high RH conditions expected in both the apoplast and the humid laminar boundary layer immediately above the leaf surface. Although syringafactin can bind substantial water only in air that is nearly fully saturated with water vapor (Fig. 1), these conditions are consistent with models of the abiotic conditions that prevail on leaves. Such models predict that the water content of air immediately surrounding the leaf, known as the laminar boundary layer, can differ greatly from that of air further away from the leaf (10, 11, 15, 23), since the laminar boundary layer traps water vapor exiting the leaf via stomata. Thus, a very humid microenvironment is proposed to surround even dry leaves (2, 3, 6, 11–16). While syringafactin absorbs substantial amounts of water only at RH greater than 97%, cells that exist within the laminar boundary layer (<10 mm) apparently often experience such high RH (10). Even when plants are exposed to a relatively dry environment (97% RH), the reduced water stress exhibited by the wild-type strain, compared to the syfA mutant strain, on such dry leaves (Fig. 4) can be attributed to water made available by binding to the syringafactin produced by the wild-type strain. Thus, even though the water-binding capability of syringafactin is strongly dependent on the RH of the atmosphere, cells in and on plants apparently reside in a sufficiently humid atmosphere for them to benefit from water binding by syringafactin.
This study also supports predictions that the physical characteristics of leaves lead to great spatial heterogeneity in the microhabitats that cells experience. For example, the syfA mutant strain harboring the proU-gfp reporter gene exhibited greater GFP fluorescence than the wild-type strain even on plants that had been maintained in a water-saturated environment after they had been sprayed with bacterial suspensions (Fig. 3A). These results suggest that many cells of this mutant strain experienced lesser amounts of free water than did cells of the wild-type strain. The observation that water limitations might occur in such a scenario supports and advances previous models of the movement and distribution of water on leaves. For instance, rather than being evenly distributed across the leaf surface, water is thought to be more prevalent at the base of trichomes or at leaf veins or cracks in the cuticle (6, 7, 24). Nutrients are also apparently very spatially variable in their abundance (25), but their coincidence with sites where water is most likely to be retained is unknown. While there was apparently very slow removal of water from the leaf surface as a whole even at 100% RH in our study, the remaining water also seemed to have been redistributed as in other studies, collecting in a few sites on the leaf (6, 7, 24). This was quite apparent in this study, since leaves that were sprayed with cells initially harbored many small droplets but the leaves contained only a few apparent water droplets after 2 days in a water-saturated environment. Most of the leaf surface was apparently devoid of water, and none of the leaves harbored films of water. These observations, along with our results, support the hypothesis that the hydrophobic leaf surface is very poorly wettable, and cells migrating to most areas of the leaf would experience a relatively dry environment due to the localized retention of water, even under relatively humid conditions, and would often encounter a complete lack of water (1–3).
The hypothesis that syringafactin production benefited cells by making water more available, even in leaves exposed to relatively low RH, was supported by the results observed both in cells on continuously wet and humid leaves (Fig. 3A) and when leaf surfaces were dried before being placed again under humid conditions (Fig. 5). In both cases, the wild-type strain presumably could have made syringafactin under the moist conditions initially present on the leaves after inoculation. As expected, a much higher level of GFP expression was seen for the syfA mutant strain when plants were exposed to 50% RH than when plants were in a continually water-saturated environment. Given that a quantitative relationship between GFP expression of cells harboring such a reporter gene construct and the level of either matric or osmotic stress to which cells were exposed has been observed (1), it seems clear that many of the cells of the syfA mutant strain experienced lower water availability on these drier leaves than did those on the plants maintained under humid conditions. Importantly, a much larger difference in GFP fluorescence exhibited by the wild-type and syfA mutant strains was observed when cells were inoculated on plants exposed to low-humidity conditions after their growth on the plants (Fig. 5). We presume that any liquid water would have been removed from both wild-type and syfA mutant strains in such a strong drying event, but apparently only the wild-type strain could become rehydrated or would have locally retained water due to its production of the hygroscopic syringafactin. Additional support for this model was also provided by studies in which plants were incubated at 97% RH after colonization. Under those conditions, the wild-type strain still exhibited less GFP fluorescence than did the syfA mutant strain, indicating that it was wetter (Fig. 4). These results suggest that, after being produced during periods when cells are metabolically active on leaves, syringafactin benefits cells during their subsequent, and probably inevitable, exposure to periods of low atmospheric RH by maintaining the rehydration of cells during periods of high RH. Alternatively, syringafactin could suppress the dehydration of cells by retaining free water around the cells after leaves are periodically wetted. As discussed above, the RH of the air at the leaf surface is probably often above 97% for plants incubated in a chamber at such humidity. In this setting, the wild-type cells would be expected to be more highly hydrated than mutants lacking the ability to produce syringafactin. Such a scenario is supported by the results of Burch et al. (2), who observed that, although the ability of syringafactin to wet an abiotic surface was lost at 50% RH, the syringafactin was able to rehydrate and to rewet the surface when the filter was reexposed to 100% RH. This finding suggests that, once cells have grown on moist leaves and produced syringafactin, they subsequently benefit from its production by being able to absorb water and make it more available during fluctuating atmospheric moisture conditions.
Features of syringafactin suggest that it might influence the environment of cells only very locally. While it is highly hygroscopic, syringafactin is apparently not readily dispersed across the leaf surface, since over 70% of purified syringafactin was bound to the waxy cuticle of the leaf after topical application (2). This observation suggests that syringafactin largely remains in the local environment of the bacterium that secreted it. This hypothesis was further supported by the observation that the syfA mutant strain inoculated onto bean plants did not maintain epiphytic population sizes as large as those of the wild-type strain, irrespective of whether it was inoculated alone on leaves or coinoculated with the wild-type strain (2). This finding suggests that the syfA mutant strain did not share in any benefits of syringafactin production by the wild-type strain. Therefore, syringafactin seems to largely affect only the local environment of the cell that produces it, rather than serving as a “public good.” Given that bacterial cells on the leaf surface may need only a small localized quantity of water to avoid water stress (26), the production of a nondiffusible hygroscopic material such as syringafactin might be an economical way for cells to modify their local water environment. In fact, it has been shown that hygroscopic salts on the leaf surface can readily absorb water even at relatively low ambient RH (26), which suggests that the same phenomenon can occur for syringafactin. While both levan and alginate produced by P. syringae on leaves would also be expected to remain in the vicinity of the producing cells, it is noteworthy that the amounts of water absorbed by these polymers in humid atmospheres were much smaller (more than 7-fold smaller, on a weight basis) than that absorbed by syringafactin. While there has been some suggestion that alginate might benefit producing cells by binding water (3), it appears that the contributions of syringafactin to such a process exceed those of such polymers unless they are produced in much greater amounts on or in plants.
These studies of the water available to bacteria colonizing the bean apoplast provided great insights into the important role of syringafactin in this habitat and the nature of the plant apoplast itself. The leaf apoplast was described previously as “a large, air-filled intercellular space” (4). The amount of free water available in the apoplast is still largely unknown (6), but it has been suggested that the apoplast is in fact a dry environment, especially when stomata are open (27). Indeed, studies using a proU-inaZ reporter gene indicated that liquid water is apparently largely absent from the apoplast (28). Those earlier studies, however, did not provide any insight regarding the RH in the apoplast. It could be speculated that the water within plant cells would be in close equilibrium with water vapor in the apoplast, given the relatively little ventilation that would be expected from diffusion through the stomata. It would be in such a setting that one might expect syringafactin to effectively contribute to the fitness of P. syringae, since its ability to bind water is much higher in atmospheres that are nearly saturated with water vapor. Therefore, it was important to note that, in the apoplast, the wild-type strain experienced less water stress than did the syfA mutant at 24 h, and especially at 48 h, after infiltration (Fig. 5B and C). Given that P. syringae strain B728a is a pathogen of the bean variety used in the study, it was somewhat surprising to find that at least some degree of water limitation was experienced by some cells of both the wild-type and syfA mutant strains only 24 h after inoculation (Fig. 5B).
A recent study showed that certain effectors, such as HopM1, in pathogens such as P. syringae pv. tomato strain DC3000 mediate the release of water from the plant into the apoplast (8). Indeed, at least transient water soaking is a typical symptom of the infection of many plants by pathogenic bacteria. It is thought that the release of water makes apoplastic nutrients more available to bacteria within this habitat and that, since nutrient limitation probably limits bacterial population sizes in the apoplast, water availability is a determinant of the success of a pathogen. In such a setting, it was somewhat surprising that a portion of cells of the wild-type P. syringae B728a strain saw lower water availability 24 h after inoculation than in broth medium itself (Fig. 5A and B). It is possible that effector-mediated water release in beans is transient. However, it is noteworthy that the apoplast became even drier between 24 h and 48 h postinoculation (Fig. 5C). While examining incompatible interactions of plant-pathogenic bacteria and host plants, Freeman and Beattie (29) found that by 24 h postinoculation, plants can actively withhold water from bacterial pathogens that enter the leaf apoplast. This suggests that plant responses to the presence of a compatible pathogen such as strain B728a might be delayed and that such water withholding would occur only later during the interaction. The finding that cells of the wild-type strain, and particularly the syfA mutant strain, exhibited substantial water stress 48 h after inoculation is consistent with such a model. Earlier work has also shown that hosts (such as beans) that are compatible with P. syringae produce defensive phytoalexins ≥2 days after the infection process is initiated (30). Furthermore, the growth of strain B728a in beans slows with time and typically ceases by 2 days after inoculation (31). This is consistent with a model of decreasing water availability during the infection process. In such a setting, alleviation of water stress by syringafactin production would benefit P. syringae.
The demonstration that syringafactin helps provide water to P. syringae in natural habitats provides support for an important new role for microbial biosurfactants. It seems likely that at least some of the many microorganisms that produce biosurfactants (19, 32) could similarly benefit. This might be particularly true of those that live in non-water-saturated environments, such as soil, that experience periodic water stress. By better understanding the roles of various biosurfactants produced by bacteria, we can gain more insight into the behavior of biosurfactant-producing microbes and the contribution of such compounds to plant-microbe interactions. We should also gain more insight into the interactions between bacteria and the leaf surface. Since biosurfactant producers occur on edible plants, such as lettuce (2), they may influence the behavior of human pathogens, such as Salmonella, which can coexist with and benefit from interactions with other epiphytic bacteria (33). Hence, a better understanding of biosurfactant production and the use of biosurfactant-producing bacteria as biocontrol agents may help to mitigate both human and plant pathogens, thereby improving both human health and crop yield (32).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Pseudomonas syringae B728a strains were grown either on King’s B medium (KB) plates containing 1.5% technical agar or on half-strength 1/2-21 C medium plates (1, 34, 35). Antibiotics were used at the following concentrations: spectinomycin, 100 μg/ml; kanamycin, 50 μg/ml; and tetracycline, 15 μg/ml.
Syringafactin extraction.
Syringafactin was extracted using a protocol described by Burch et al. (2), which was modified from a protocol described by Berti et al. (21). P. syringae B728a strains were grown on agar plates for 3 days. Cells were then suspended in water and centrifuged at room temperature at 5,000 × g for 10 min. The centrifuge was a Beckman J2-21M high-speed centrifuge with a JA-20 fixed-angle rotor holding eight 50-ml tubes. The supernatant was mixed with ethyl acetate (1.5:1) in a separatory flask. The organic fraction was retained while the aqueous fraction was discarded. The organic fraction was reduced to dryness in a rotary evaporator (Büchi). The remaining powder was resuspended in methanol and dried completely in a SpeedVac concentrator (Savant).
Measuring water absorption by syringafactin.
Dried purified syringafactin was added to preweighed 1.5-ml microcentrifuge tubes, which were weighed on an analytical balance (Fisher Scientific) and then placed, with the cap open, in a sealed chamber (magenta box) containing a given saturated salt solution to maintain constant RH in the air in the chamber (36). Salts that were used were magnesium nitrate, sodium chloride, ammonium sulfate, potassium chloride, potassium nitrate, and potassium sulfate, which maintained RHs of 52%, 75%, 80%, 84%, 93%, and 97%, respectively. Open tubes were left in the chamber for 3 days before being taken out, rapidly sealed, and reweighed.
Transformation of P. syringae B728a strains.
Wild-type P. syringae strain B728a and a syfA mutant strain (31) were both transformed with plasmid pPProGreen carrying a fusion of proU with a promoterless gfp reporter gene (1), using previously described methods (37).
Preparation of cultured cells for in vitro GFP measurements.
Wild-type and syfA mutant strains harboring the proU-gfp reporter gene were harvested with a loop from half-strength 1/2-21 C agar plates that had been grown for 1 day at 20°C and were suspended in 1 ml of half-strength 1/2-21 C broth to an adjusted concentration of 108 cells/ml. In addition, in some studies, syfA mutant cells were suspended to a concentration of 108 cells/ml in 500 μl of half-strength 1/2-21 C broth containing 2.5 mg of purified syringafactin. Ten-microliter drops of each treatment were then pipetted onto 0.4-μm-pore-size Isopore filters, which were placed on the surface of half-strength 1/2-21 C agar plates. All filters were left on the plates for 8 h at room temperature. After 8 h, the filters were transferred to glass slides in chambers containing saturated solutions of magnesium nitrate or water, which maintained the atmosphere within the container at 52% RH or 100% RH, respectively. After 4 h, the filters were placed in 1.5-ml microcentrifuge tubes containing 1 ml of half-strength 1/2-21 C broth to ensure that the cells had the opportunity to resuscitate from water stress and to translate the gfp reporter gene. The fluorescence of P. syringae B728a cells harboring the proU-gfp reporter was at a very low background level in both low-salt minimal medium and water, indicating that the resuscitation step itself did not induce GFP fluorescence.
After 2 h, the tubes containing the filters were vortex mixed for 20 s to remove cells, and a 5-μl aliquot of each solution was then pipetted onto a glass slide and left to dry for 20 min. Coverslips were applied to the slides using Aqua PolyMount (product no. 18606; Polysciences). Slides were then examined with an epifluorescence microscope, as described below. It should be noted that the experiments at 52% RH were performed on a different day than the experiments at 100% RH. Thus, the GFP fluorescence intensities in these experiments cannot be directly compared.
Cell viability assays over time on filters were performed in a similar manner except that filters were exposed to 52% RH, as described above, for various periods of time before the filters were removed. Appropriate serial dilutions of cells washed from filters as described above were plated onto a KB plate. All plates were incubated at 20°C, and colonies were enumerated after 2 days.
Preparation of epiphytic cells for GFP measurements.
Wild-type and syfA mutant strains were harvested with a loop from half-strength 1/2-21 C agar plates that had been grown for 24 h at 20°C and were suspended in 50-ml volumes of water to an adjusted concentration of 106 cells/ml. Cells were then sprayed onto the leaves of 2-week-old bean plants (Phaseolus vulgaris cv. Bush Blue Lake 274). Four to six seedlings were grown in each pot. All plants were incubated in sealed plastic tents or in large sealed plastic tubs under various RH conditions, which were maintained with saturated salt solutions as described above. Plants were sprayed to wetness and then immediately placed in a sealed plastic tent at 100% RH or in a sealed plastic tub maintained at 97% RH, which was then placed in a plastic tent maintaining 100% RH. Plants were kept in the chambers for 2 days to ensure equilibrium. When leaves were to be dried between chamber transfers, plants were placed at room RH for 20 min to 1 h until water droplets on the leaves had evaporated. Primary leaves were excised from plants (three leaves per replicate) and were immersed in 150 ml of 5 mM KPO4 buffer (pH 7.0) in a beaker. Each beaker was placed in a sonicator (Branson 5510MT) for 10 min to remove cells from the leaf surface. Buffer containing the released cells was filtered through a 0.4-μm-pore-size Isopore filter to capture and to immobilize the cells. Filters were attached to glass slides and coverslips with Aqua PolyMount. Slides were then examined with an epifluorescence microscope.
Preparation of apoplastic samples.
Wild-type and syfA mutant strains were harvested with a loop from half-strength 1/2-21 C agar plate cultures that had been grown for 1 day at 20°C. Each strain was suspended in 1 liter of water to a concentration of 106 cells/ml. Cells were vacuum infiltrated into the leaves of 2-week-old plants (Phaseolus vulgaris cv. Bush Blue Lake 274, with four to six seedlings per pot), as in other studies (9, 30). All plants were stored on the bench at room RH (∼50% RH). At time zero, 5 μl of each inoculum was pipetted onto a glass slide and left to dry for 20 min. Coverslips were applied to the slides using Aqua PolyMount. Slides were then used for examination of cells under the epifluorescence microscope. At 24 h and 48 h, primary leaves were excised from plants (three leaves per replicate) and cut into strips before being immersed in 45 ml of 10 mM KPO4 contained in Falcon 50-ml conical tubes. Each tube was sonicated for 10 min; after sonication, each tube was vortex mixed for 20 s. The cell suspensions were then decanted from the tubes into 50-ml centrifuge tubes and centrifuged at room temperature at 7,000 rpm (4,720 × g) for 10 min. The centrifuge was a Fisherbrand accuSpin Micro 17 with a 24 × 1.5/2.0 ml rotor. The supernatant was discarded, and the remaining pellets were resuspended in 10-μl volumes of 10 mM KPO4. Five microliters of each suspension was then pipetted onto a glass slide and left to dry for 20 min. Coverslips were applied to the slides using Aqua PolyMount. Slides were then examined with an epifluorescence microscope.
Quantification of GFP fluorescence.
An M2 AxioImager was used for all microscopic analyses. A GFP filter set was used to view cells in all experiments, at ×100 magnification, and all images were taken in black and white with a 12-bit Retiga camera. Bitplane Imaris image processing and manipulation software was used to quantify the average GFP fluorescence exhibited by each individual cell in digital images. The location of each individual cell was automatically assigned to the cells in a given image, and plant debris and bacterial cellular aggregates were identified visually and deselected. For each object (individual bacterial cell) identified, the program calculated the mean pixel intensity. Unless otherwise noted, experiments were performed on different days and images were captured with different exposure times and illumination intensities. Differences in GFP fluorescence intensity can be directly compared only within a given experiment illustrated in a given figure.
Statistical analysis.
The software environment R (38) was used to perform the Wilcoxon rank-sum test (39), a nonparametric test of the null hypothesis that it is equally likely that a randomly selected value from one sample will be lesser or greater than a randomly selected value from another sample. The test is appropriate for comparing the distributions of data that are not normally distributed. The mean pixel intensities for all cells in a given sample were combined and ordered. Ranks were assigned starting with the smallest observation and summed in order to determine the W statistic. Medians were also reported for data that were not normally distributed. R was also used to perform the two-sample t test (40) to determine whether two population means were equal. The test calculates the test statistic, t, and compares it with the distribution of possible values for t to determine a P value. The two-sample t test (two sided) was performed on data that were normally distributed. For normally distributed data, means were reported instead of medians. Statistical significance was determined at a P value of ≤0.05 except when multiple comparisons were made (Fig. 2). To account for the false-discovery rate, the Bonferroni correction (41) was used, which involved dividing the original P value of 0.05 by the number of comparisons made; this revealed that each comparison was significant at a P value of ≤0.017.
Supplementary Material
ACKNOWLEDGMENTS
We thank Steven Ruzin and Denise Schichnes at the College of Natural Resource Biological Imaging Facility. We also thank Ellen Simms and Fan Dong for their assistance in statistical analysis and Helen Kurkjian for her assistance in statistical analysis and R.
This work was supported by the National Science Foundation Louis Stokes Alliances for Minority Participation Bridge to the Doctorate Fellowship, the Chancellor’s Fellowship for Graduate Study, and the William Carroll Smith Fellowship.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01014-19.
REFERENCES
- 1.Axtell CA, Beattie GA. 2002. Construction and characterization of a proU-gfp transcriptional fusion that measures water availability in a microbial habitat. Appl Environ Microbiol 68:4604–4612. doi: 10.1128/AEM.68.9.4604-4612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burch AY, Zeisler V, Yokota K, Schreiber L, Lindow SE. 2014. The hygroscopic biosurfactant syringafactin produced by Pseudomonas syringae enhances fitness on leaf surfaces during fluctuating humidity. Environ Microbiol 16:2086–2098. doi: 10.1111/1462-2920.12437. [DOI] [PubMed] [Google Scholar]
- 3.Lindow SE, Brandl MT. 2003. Microbiology of the phyllosphere. Appl Environ Microbiol 69:1875–1883. doi: 10.1128/aem.69.4.1875-1883.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melotto M, Underwood WR, He SY. 2008. Role of stomata in plant innate immunity and foliar bacterial diseases. Annu Rev Phytopathol 46:101–122. doi: 10.1146/annurev.phyto.121107.104959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Remus-Emsermann MNP, Schlechter RO. 2018. Phyllosphere microbiology: at the interface between microbial individuals and the plant host. New Phytol 218:1327–1333. doi: 10.1111/nph.15054. [DOI] [PubMed] [Google Scholar]
- 6.Beattie GA. 2011. Water relations in the interaction of foliar bacterial pathogens with plants. Annu Rev Phytopathol 49:533–555. doi: 10.1146/annurev-phyto-073009-114436. [DOI] [PubMed] [Google Scholar]
- 7.Monier J-M, Lindow SE. 2003. Differential survival of solitary and aggregated bacterial cells promotes aggregate formation on leaf surfaces. Proc Natl Acad Sci U S A 100:15977–15982. doi: 10.1073/pnas.2436560100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xin XF, Nomura K, Aung K, Velasquez AC, Yao J, Boutrot F, Chang JH, Zipfel C, He SY. 2016. Bacteria establish an aqueous living space in plants crucial for virulence. Nature 539:524–529. doi: 10.1038/nature20166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu X, Lund SP, Scott RA, Greenwald JW, Records AH, Nettleton D, Lindow SE, Gross DC, Beattie GA. 2013. Transcriptional responses of Pseudomonas syringae to growth in epiphytic versus apoplastic leaf sites. Proc Natl Acad Sci U S A 110:e425–e434. doi: 10.1073/pnas.1221892110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferro DN, Southwick EE. 1984. Microclimates of small arthropods: estimating humidity within the leaf boundary layer. Environ Entomol 13:926–929. doi: 10.1093/ee/13.4.926. [DOI] [Google Scholar]
- 11.Drake BG, Raschke K, Salisbury FB. 1970. Temperature and transpiration resistances of Xanthium leaves as affected by air temperature, humidity, and wind speed. Plant Physiol 46:324–330. doi: 10.1104/pp.46.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin TA, Hinckley TM, Meinzer FC, Sprugel DG. 1999. Boundary layer conductance, leaf temperature and transpiration of Abies amabilis branches. Tree Physiol 19:435–443. doi: 10.1093/treephys/19.7.435. [DOI] [PubMed] [Google Scholar]
- 13.Parlange JY, Waggoner PE, Heichel GH. 1971. Boundary layer resistance and temperature distribution on still and flapping leaves. I. Theory and laboratory experiments. Plant Physiol 48:437–442. doi: 10.1104/pp.48.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parlange JY, Waggoner PE. 1972. Boundary layer resistance and temperature distribution on still and flapping leaves. II. Field experiments. Plant Physiol 50:60–63. doi: 10.1104/pp.50.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuepp PH. 1993. Tansley review no. 59: leaf boundary layers. New Phytol 125:477–507. doi: 10.1111/j.1469-8137.1993.tb03898.x. [DOI] [PubMed] [Google Scholar]
- 16.Waggoner PE. 1965. Microclimate and plant disease. Annu Rev Phytopathol 3:103–126. doi: 10.1146/annurev.py.03.090165.000535. [DOI] [Google Scholar]
- 17.Longrée K. 1939. The effect of temperature and relative humidity on the powdery mildew of roses. Cornell University, Ithaca, NY. [Google Scholar]
- 18.Burch AY, Browne PJ, Dunlap CA, Price NP, Lindow SE. 2011. Comparison of biosurfactant detection methods reveals hydrophobic surfactants and contact-regulated production. Environ Microbiol 13:2681–2691. doi: 10.1111/j.1462-2920.2011.02534.x. [DOI] [PubMed] [Google Scholar]
- 19.Ron EZ, Rosenberg E. 2001. Natural roles of biosurfactants. Environ Microbiol 3:229–236. doi: 10.1046/j.1462-2920.2001.00190.x. [DOI] [PubMed] [Google Scholar]
- 20.Bunster L, Fokkema NJ, Schippers B. 1989. Effect of surface-active Pseudomonas spp. on leaf wettability. Appl Environ Microbiol 55:1340–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berti AD, Greve NJ, Christensen QH, Thomas MG. 2007. Identification of a biosynthetic gene cluster and the six associated lipopeptides involved in swarming motility of Pseudomonas syringae pv. tomato DC3000. J Bacteriol 189:6312–6323. doi: 10.1128/JB.00725-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreiber L. 1996. Wetting of the upper needle surface of Abies grandis: influence of pH, wax chemistry and epiphyllic microflora on contact angles. Plant Cell Environ 19:455–463. doi: 10.1111/j.1365-3040.1996.tb00337.x. [DOI] [Google Scholar]
- 23.Yarwood CE, Hazen WE. 1944. The relative humidity at leaf surfaces. Am J Bot 31:129–135. doi: 10.2307/2437635. [DOI] [Google Scholar]
- 24.Gnanamanickam SS, Immanuel JE. 2006. Epiphytic bacteria, their ecology and functions, p 131–153. In Gnanamanickam SS. (ed), Plant-associated bacteria. Springer, Dordrecht, Netherlands. [Google Scholar]
- 25.Leveau JH, Lindow SE. 2001. Appetite of an epiphyte: quantitative monitoring of bacterial sugar consumption in the phyllosphere. Proc Natl Acad Sci U S A 98:3446–3453. doi: 10.1073/pnas.061629598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burkhardt J, Hunsche M. 2013. “Breath figures” on leaf surfaces: formation and effects of microscopic leaf wetness. Front Plant Sci 4:422. doi: 10.3389/fpls.2013.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang D, Tian C, Yin K, Wang W, Qiu J-L. 2019. Postinvasive bacterial resistance conferred by open stomata in rice. Mol Plant Microbe Interact 32:255–266. doi: 10.1094/MPMI-06-18-0162-R. [DOI] [PubMed] [Google Scholar]
- 28.Wright CA, Beattie GA. 2004. Pseudomonas syringae pv. tomato cells encounter inhibitory levels of water stress during the hypersensitive response of Arabidopsis thaliana. Proc Natl Acad Sci U S A 101:3269–3274. doi: 10.1073/pnas.0400461101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freeman BC, Beattie GA. 2009. Bacterial growth restriction during host resistance to Pseudomonas syringae is associated with leaf water loss and localized cessation of vascular activity in Arabidopsis thaliana. Mol Plant Microbe Interact 22:857–867. doi: 10.1094/MPMI-22-7-0857. [DOI] [PubMed] [Google Scholar]
- 30.Lyon FM, Wood R. 1975. Production of phaseollin, coumestrol and related compounds in bean leaves inoculated with Pseudomonas spp. Physiol Plant Pathol 6:117–124. doi: 10.1016/0048-4059(75)90039-9. [DOI] [Google Scholar]
- 31.Wilson M, Hirano SS, Lindow SE. 1999. Location and survival of leaf-associated bacteria in relation to pathogenicity and potential for growth within the leaf. Appl Environ Microbiol 65:1435–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burch AY, Shimada BK, Mullin SWA, Dunlap CA, Bowman MJ, Lindow SE. 2012. Pseudomonas syringae coordinates production of a motility-enabling surfactant with flagellar assembly. J Bacteriol 194:1287–1298. doi: 10.1128/JB.06058-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poza-Carrion C, Suslow T, Lindow S. 2013. Resident bacteria on leaves enhance survival of immigrant cells of Salmonella enterica. Phytopathology 103:341–351. doi: 10.1094/PHYTO-09-12-0221-FI. [DOI] [PubMed] [Google Scholar]
- 34.Halverson LJ, Firestone MK. 2000. Differential effects of permeating and nonpermeating solutes on the fatty acid composition of Pseudomonas putida. Appl Environ Microbiol 66:2414–2421. doi: 10.1128/aem.66.6.2414-2421.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.King EO, Ward MK, Raney DE. 1954. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med 44:301–307. [PubMed] [Google Scholar]
- 36.Jarrett DG. 1995. Constant temperature and humidity chamber for standard resistors, p 501–506. In The impact of metrology on global trade: proceedings of the 1995 National Conference of Standards Laboratories workshop and symposium. National Conference of Standards Laboratories, Boulder, CO. [Google Scholar]
- 37.Burch AY, Finkel OM, Cho JK, Belkin S, Lindow SE. 2013. Diverse microhabitats experienced by Halomonas variabilis on salt-secreting leaves. Appl Environ Microbiol 79:845–852. doi: 10.1128/AEM.02791-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.R Core Team. 2013. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: https://www.R-project.org. [Google Scholar]
- 39.Wilcoxon F. 1945. Individual comparisons by ranking methods. Biometrics 1:80–83. doi: 10.2307/3001968. [DOI] [Google Scholar]
- 40.Student 1908. The probable error of a mean. Biometrika 6:1–25. doi: 10.1093/biomet/6.1.1. [DOI] [Google Scholar]
- 41.Bonferroni CE. 1936. Teoria statistica delle classi e calcolo delle probabilità. Pubbl R Ist Super Sci Econ Commer Fir 8:3–62. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


