Abstract
Objectives
Chronic obstructive pulmonary disease (COPD) is a fatal disease that shortens one's life expectancy and reduces the quality of life of patients. The current known treatments for COPD can only act to alleviate the symptoms. Recently, stem cells have demonstrated efficacy in various medical areas. The aim of this study was to investigate the possibility of using human Wharton's jelly-derived mesenchymal stem cells (MSC)s for lung recovery in a COPD mouse model.
Methods
Human Wharton's jelly was obtained during natural delivery or caesarean section from healthy women. Wharton's jelly-derived MSC was confirmed with expression of CD14, CD34, CD45, CD73, CD90, and CD105 using flow cytometry. Mice model (C57BL/6) of COPD were induced by injecting 10 μL elastase into the trachea and they were divided into three treatment groups (sham, vehicle, stem cell). The sham group was not induced COPD, nor provided any treatment; the vehicle group comprised of COPD-induced mice treated with normal saline; the stem cell group comprised of COPD-induced mice treated with Wharton's jelly-derived MSCs. The vehicle and mesenchymal stem cells (5 × 104 cells) were injected in tail vein 7 days following COPD induction. Mice were euthanized 7 days after vehicle and stem cell injection, and pathologic findings were confirmed. Mean Linear Intercept (MLI) was measured after emphysema-induced alveoli were identified.
Results
Cell surface markers were positive for CD105, CD90, and CD73 and negative for CD45, CD34, and CD14. Pathological tests showed that COPD-induced mice had significantly increased emphysema volume as compared with that in the sham group. The degree of emphysema in the stem cell group was reduced based on pathologic findings. The mean MLI of the sham group was measured as 38.85 ± 6.45. The mean MLI of the vehicle and stem cell groups were 163.05 ± 48.94 and 123.59 ± 30.53, respectively, and there was a statistically significant difference between the two groups (p = 0.008).
Conclusions
Though the number of mice in the experiment was not large, human Wharton's jelly-derived MSCs showed pulmonary regenerative effects in the COPD mouse model. Although we cannot confirm the effects of Wharton's jelly-derived MSCs in COPD through this experiment, it can be used as a basis for a larger clinical experiment.
Keywords: Alveolar size, Cell regeneration, Elastase, Emphysema, Mean linear intercept, Mouse model
1. Introduction
Chronic obstructive pulmonary disease (COPD), which is associated with high mortality, is characterized by the irreversible destruction of lung parenchyma due to chronic inflammation, resulting in a deterioration of pulmonary function. Currently known treatments include smoking cessation and oxygenation in patients with low blood oxygen saturation, but these treatments do not cure the disease [1]. Bronchodilators and steroids may be used, but they cannot prevent disease progression and only help alleviate some symptoms.
In Korea, according to the National Health Insurance Corporation, about 230,000 to 250,000 patients have been diagnosed with COPD [2]. In addition, the average annual hospitalization per patient, which is an indicator of disease severity, is 27 days, showing a trend toward high incidence. The annual average cost of medical treatment is 4.7 times higher than the average medical cost per capita. Stem cell therapy has been performed in various areas and there have been attempts to apply cell regeneration to COPD.
Weiss et al. performed a placebo-controlled, randomized clinical trial at six centres using allogeneic mesenchymal stem cells (MSCs — Prochymal; Osiris Therapeutics Inc, Columbia, MD, USA) [3]. In their study, MSCs did not show clinical efficacy for COPD, but systemic inflammation levels were reduced. Since then, there has been no progress in clinical trials of COPD using stem cells. Therefore, we investigated the efficacy of Wharton's jelly-derived MSCs, a new source of allogeneic stem cells, in COPD, rather than the previously used bone marrow-derived or adipose-derived stem cells.
2. Materials and methods
2.1. Human umbilical cord
Umbilical cord tissues obtained through normal delivery or caesarean section from healthy mothers at Daegu Catholic University Hospital were collected. Those who had a history of malignant neoplasms, had received immunosuppressive agents, chemotherapy, or radiotherapy, had participated in other clinical trials within the last 30 days, had previously received stem cell therapy, possessed cognitive limitations that prevented them from understanding the experiment, and those with infectious diseases were excluded from the donation.
Informed consents including agreement to study human derivatives were received by all donors. This study was approved by the institutional ethics committee/review board of our institution (CR-16-162).
2.2. Mesenchymal stem cell extraction and characterization
The umbilical cord tissue was trimmed and treated with Collagenase I. Then, it was cultured in Dulbecco's modified Eagle's medium supplemented with 10% foetal bovine serum (FBS), 1% penicillin and streptomycin, plasmocin, platelet-derived growth factor, and fibroblast growth factor at 37 °C in a 5% CO2 incubator. We confirmed the expression of CD14, CD34, CD45, CD73, CD90, and CD105 using flow cytometry to determine whether the cultured cells had the phenotype specified by the international standard of MSC [4]. MSC extraction for flow cytometry and animal model injection were performed in passage 6.
2.3. Mouse COPD model and MSC injection
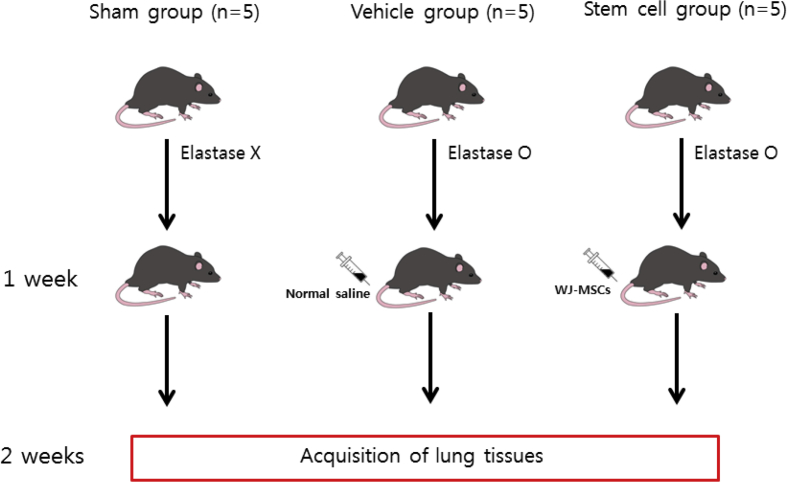
A total of 15 male C57BL/6 mice (KOATECH, Gyeonggi-do, Korea) were obtained and divided into three groups to perform the experiment (5 mice of each group): a stem cell group, a vehicle group, and a sham group (Fig. 1). A COPD model was induced in the stem cell group and the vehicle group with elastase (30 ??l) and the sham group was administered normal saline instead of elastase. Elastase/normal saline was injected into the bronchus through an endotracheal tube. Seven days after COPD modelling, MSCs (5 × 104 cells) were administered through the tail vein along with 200??l of phosphate buffered saline to the stem cell group and 200??l of normal saline was administered through the tail vein to the vehicle group. The sham group did not undergo any further treatment. After another week, mice were euthanized, and their lungs were stained using haematoxylin and eosin (H & E) and examined histopathologically.
Fig. 1.
Schematic overview of the experiment.
2.4. Quantification of MLI
Emphysema-induced alveoli were identified under the microscope and their mean linear intercept (MLI) was measured as an indicator of the degree of COPD. Quantification of MLI was performed as previously described [5]. Briefly, digital images of the accessory lobe section (20X magnification) stained with hematoxylin and eosin were obtained using a bright field microscope. To quantify the MLI, 3 non-overlapping views (1000 μm x 1000 μm) were selected randomly from the appropriate area without arteries, veins, main airway, and alveolar ducts. Using the ruler tool, a grid with 10 uniformly distributed vertical lines and 10 uniformly distributed horizontal lines of defined length (1000 μm) was placed in the selected viewing area. Each line was spaced 100 μm apart. A one-slice value was defined as the straight-line length between two adjacent alveolar epithelia. All slice values were measured along the 1000 μm-long line. All intercept values between the 10 and 1000 vertices were then quantified for each grid. The MLI was considered as the average length of the pieces from a total of 3 grids analysed for the three sections prepared for each of the accessory lobes.
All cell and animal experiments were conducted at the Daegu Gyeongbuk Medical Innovation Foundation (DGMIF) and were approved by the Institutional Animal Care and Use Committee of the Experimental Animal Center of the DGMIF based on the Animal Protection Act.
2.5. Statistical analysis
MLI, which was expressed as continuous data, was subjected to statistical analysis. The means ± standard deviation was calculated. Data were compared using the Kruskal–Wallis test to verify the difference between the three groups and Mann–Whitney U test was used for post hoc analysis. Values of P < 0.05 were considered statistically significant and Bonferroni's method was applied. All statistical analyses were performed using the IBM SPSS software version 25.
3. Results
3.1. Donated umbilical cord and identification of cultured MSCs
Umbilical cord tissues obtained from through normal delivery or caesarean section from 10 healthy mothers between the ages of 33 and 40. The tissues from umbilical cords were about 5–10 g each in quantity. A total of 6 cell lines were obtained, and one cell line was randomly selected and used for the experiment.
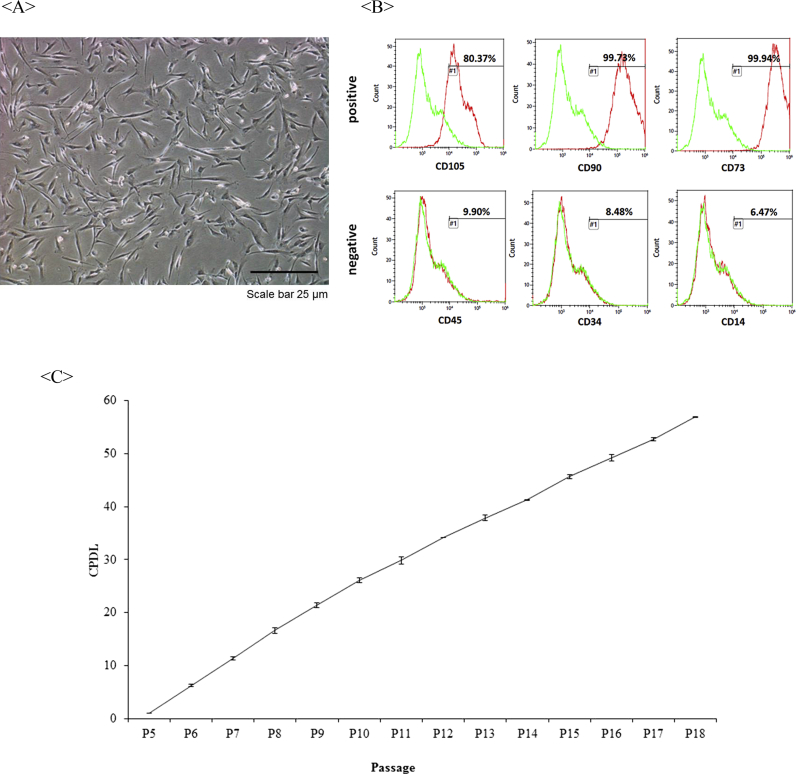
Cell surface markers administered in passage 6 were positive for CD105, CD90, and CD73 and negative for CD45, CD34, and CD14 (Fig. 2).
Fig. 2.
Stem cell characterization. A. Image of human Wharton's jelly-derived MSCs under a bright field microscope at passage 6. B. Cell surface markers in Wharton's jelly-derived MSCs using FACS. Wharton's jelly-derived MSCs were positive for CD105, CD90, and CD73 markers and negative for CD45, CD34, and CD14 markers. C. Growth curve of Wharton's jelly-derived MSCs expressed in CPDL. MSC: mesenchymal stem cell. FACS: fluorescence activated cell sorting. CPDL: cell population doubling level.
3.2. Verification of the regenerative effect of Wharton's jelly-derived MSC
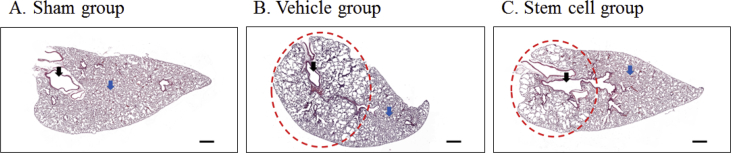
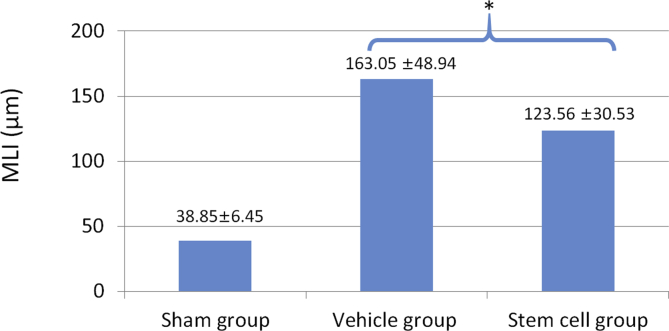
The mouse COPD model was induced satisfactorily using elastase (Fig. 3). Microscopically, we could confirm that the alveolar size of the vehicle group was larger than that of the sham group, and that the COPD-inducing effect was alleviated in the stem cell group. MLI was measured to identify the degree of COPD induction and the ability to recover from COPD by the administration of stem cells. MLI was the largest in the vehicle group (COPD + saline) with an average of 163.05 ± 48.94 μm; the average was 123.56 ± 30.53 μm in the stem cell group (COPD + MSCs) and 38.85 ± 6.45 μm in the sham group (Table 1). The MLI of the three groups were significantly different (P = 0.002). In post-hoc analysis, there was a statistically significant difference in MLI between the COPD-induced group (stem cell + vehicle) and the sham group, and between the stem cell group and the vehicle group (P = 0.008, Fig. 4).
Fig. 3.
Representative histopathological findings for each group. Black arrow: Bronchiole, Blue arrow: Alveolus, Red circle: Alveolar emphysema. Scale bar = 500 ㎛.
Table 1.
Mean Linear Intercept values measured in each group.
| Group | Mean ± SD | Group | Mean ± SD | Group | Mean ± SD |
|---|---|---|---|---|---|
| Sham | 34.48 ± 6.36 | Vehicle | 134.01 ± 39.49 | Stem cell | 125.88 ± 40.17 |
| 38.30 ± 7.75 | 143.93 ± 30.20 | 122.39 ± 31.21 | |||
| 40.73 ± 4.63 | 154.58 ± 22.08 | 131.48 ± 32.65 | |||
| 36.68 ± 5.35 | 214.15 ± 65.78 | 116.55 ± 23.17 | |||
| 44.07 ± 4.08 | 168.60 ± 38.40 | 121.49 ± 12.37 | |||
| Average | 38.85 ± 6.45 | Average | 163.05 ± 48.94 | Average | 123.56 ± 30.53 |
Fig. 4.
Mean Linear Intercept of each group. * Difference between the stem cell group and the vehicle group; P = 0.008 using the Mann–Whitney U test. MLI: Mean Linear Intercept.
4. Discussion
Previous experiments have demonstrated the effectiveness of Wharton's jelly-derived MSC in lung injury animal models [6], [7]. However, these experiments used an acute lung injury or lung fibrosis model, not the COPD model, and the efficacy was verified by studying the cytokines rather than pathologically. In the present experiment, we demonstrate pathologically the effect of Wharton's jelly-derived MSC in an animal model of COPD.
The MLI method was introduced by Dunnill in 1964 and has commonly been used for a quantitative evaluation of histological analyses of lung tissue [8]. The degree to which COPD has progressed can be measured indirectly through MLI values. Comparing the results of our study with those of Cruz et al. and Tibboel et al., the mean MLI value of COPD-induced mice in our study is very suggestive of well-developed emphysema [9], [10]. Through our experiments, we conducted preliminary experiments to make an appropriate COPD model. Elastase was injected into mice through the oral or intra-tracheal route, and we found that injecting it through the intra-tracheal route was a more reliable method of making a COPD model. For inducing COPD, the use of elastase has an advantage over other methods, which is why elastase was chosen for our experiments. Moreover, the degree of severity of COPD can be achieved by adjusting the amount of elastase [11].
There are various methods for injecting stem cells depending on the target organ. In the case of lungs, direct injection into the bronchi and intravenous injection are possible. In our experiment, intravenous injection was performed. In fact, when targeting other organs with intravenous infusion, the lungs present an obstacle [12], [13]. Most of the stem cells injected into the vein are trapped in the lungs, which is advantageous for treating lung disease because it ensures a relatively easy injection route.
In this study, stem cells showed an effect on COPD in the animal experiments; however, there is no basis for this in the human body. Only one paper has shown that when treated with stem cells, systemic inflammation was reduced in COPD patients [3]. Since then, efforts to treat COPD using MSCs have continued, and the application of stem cell therapy in COPD patients has been achieved [14]. Several variables are involved in stem cell treatment, including the source of the stem cells, timing of the treatment, number of stem cells injected, method of injection, and choice of the patient population. In fact, several clinical trials have been conducted using various evaluating parameters and cells from various sources [15].
The reason for using Wharton's jelly in our study among different kinds of stem cell sources is simple. In a clinical situation, the jelly is easy to obtain, and it is possible to obtain a large number of cells continuously. Compared with other MSCs, Wharton's jelly-derived MSCs have been reported to have a superior angiogenic and regenerative effect [16], [17]. The anti-inflammatory and tissue repair properties of Wharton's jelly-derived MSCs have not yet been examined as extensively as those of BM-MSCs or AT-MSCs [18]. However, in some reports, the mechanism underlying these properties have been well explained. Wharton's jelly-derived MSCs are known to regulate inflammatory cells through multiple cytokines, growth factors, and their receptors, such as interleukin-6, epidermal growth factor, transforming growth factor-alpha, and insulin-like growth factor-1 [19], [20], [21], [22].
The safety of intravenous injection of umbilical cord-derived MSC has also been demonstrated in several studies in terms of allogeneic cell transplantation [23], [24], [25], [26], [27], [28], [29].
One limitation of our experiments is that we could not identify the mechanism underlying the effect of Wharton's jelly-derived MSCs in the COPD mouse model through various cytokine assays. The lack of a study on whether pulmonary function has improved is also a matter of concern. In addition, experiments to determine whether injected stem cells reside in the tissues of the subject may help to clarify the effect of Wharton's jelly-derived MSCs, and will be performed in a future study. Additional experiments will be performed in the near future to determine the mechanism underlying the effect of Wharton's jelly-derived MSCs in the COPD mouse model.
5. Conclusions
Although the number of mice used in the experiment was not high, human Wharton's jelly-derived MSCs showed pulmonary regenerative effects in the COPD mouse model. Although we cannot confirm the effects of Wharton's jelly-derived MSCs in COPD through this experiment, the results can be used as a basis for a larger clinical experiment. We also need to define evaluation methods for pulmonary function recovery and develop optimal protocols for cell infusion in terms of stem cell concentration, infusion timing, and method of administration.
Funding
This work was supported by the grant of Research Institute of Medical Science, Catholic University of Daegu (2017).
Conflicts of interest
No potential conflict of interest was reported by the authors.
Acknowledgment
The authors thank researchers at Daegu Gyeongbuk Medical Innovation Foundation (DGMIF) for assistance with laboratory work.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Sin D.D., McAlister F.A., Man S.P., Anthonisen N.R. Contemporary management of chronic obstructive pulmonary disease: scientific review. Jama. 2003;290:2301–2312. doi: 10.1001/jama.290.17.2301. [DOI] [PubMed] [Google Scholar]
- 2.National Health Insurance Service Analysis of COPD treatment status in Korea. http://www.nhis.or.kr/bbs7/boards/B0039/23784 [cited May, 2017]. Available from:
- 3.Weiss D.J., Casaburi R., Flannery R., LeRoux-Williams M., Tashkin D.P. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest. 2013;143:1590–1598. doi: 10.1378/chest.12-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F.C., Krause D.S. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 5.Liu Z., Fu S., Tang N. A standardized method for measuring internal lung surface area via mouse pneumonectomy and prosthesis implantation. J Vis Exp. 2017;125 doi: 10.3791/56114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moodley Y., Atienza D., Manuelpillai U., Samuel C.S., Tchongue J., Ilancheran S. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am J Pathol. 2009;175:303–313. doi: 10.2353/ajpath.2009.080629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J., Li D., Liu X., Tang S., Wei F. Human umbilical cord mesenchymal stem cells reduce systemic inflammation and attenuate LPS-induced acute lung injury in rats. J Inflamm. 2012;9:33. doi: 10.1186/1476-9255-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunnill M.S. Evaluation of a simple method of sampling the lung for quantitative histological analysis. Thorax. 1964;19:443. doi: 10.1136/thx.19.5.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruz F.F., Antunes M.A., Abreu S.C., Fujisaki L.C., Silva J.D., Xisto D.G. Protective effects of bone marrow mononuclear cell therapy on lung and heart in an elastase-induced emphysema model. Respir Physiol Neurobiol. 2012;182:26–36. doi: 10.1016/j.resp.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Tibboel J., Keijzer R., Reiss I., de Jongste J.C., Post M. Intravenous and intratracheal mesenchymal stromal cell injection in a mouse model of pulmonary emphysema. COPD. 2014;11:310–318. doi: 10.3109/15412555.2013.854322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghorani V., Boskabady M.H., Khazdair M.R., Kianmeher M. Experimental animal models for COPD: a methodological review. Tob Induc Dis. 2017;15:25. doi: 10.1186/s12971-017-0130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer U.M., Harting M.T., Jimenez F., Monzon-Posadas W.O., Xue H., Savitz S.I. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18:683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schrepfer S., Deuse T., Reichenspurner H., Fischbein M.P., Robbins R.C., Pelletier M.P. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39:573–576. doi: 10.1016/j.transproceed.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 14.ClinicalTrials.Gov. Available from: https://clinicaltrials.gov (accessed on 8 August 2019).
- 15.Cheng S.L., Lin C.H., Yao C.L. Mesenchymal stem cell administration in patients with chronic obstructive pulmonary disease: state of the science. Stem cells Int. 2017;2017:1–14. doi: 10.1155/2017/8916570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nartprayut K., Kheolamai P., Manochantr S., Chayosumrit M., Issaragrisil S., Supokawej A. Cardiomyocyte differentiation of perinatally-derived mesenchymal stem cells. Mol Med Rep. 2013;7:1465–1469. doi: 10.3892/mmr.2013.1356. [DOI] [PubMed] [Google Scholar]
- 17.Yannarelli G., Dayan V., Pacienza N., Lee C.J., Medin J., Keating A. Human umbilical cord perivascular cells exhibit enhanced cardiomyocyte reprogramming and cardiac function after experimental acute myocardial infarction. Cell Transplant. 2013;22:1651–1666. doi: 10.3727/096368912X657675. [DOI] [PubMed] [Google Scholar]
- 18.Janczewski A., Wojtkiewicz J., Malinowska E., Doboszyńska A. Can youthful mesenchymal stem cells from Wharton's jelly bring a breath of fresh air for COPD? Int J Mol Sci. 2017;18:2449. doi: 10.3390/ijms18112449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munir H., Luu N.T., Clarke L.S., Nash G.B., McGettrick H.M. Comparative ability of mesenchymal stromal cells from different tissues to limit neutrophil recruitment to inflamed endothelium. PLoS One. 2016;11 doi: 10.1371/journal.pone.0155161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao C.V., Li X., Toth P., Lei Z.M. Expression of epidermal growth factor, transforming growth factor-α, and their common receptor genes in human umbilical cords. J Clin Endocrinol Metab. 1995;80:1012–1020. doi: 10.1210/jcem.80.3.7883816. [DOI] [PubMed] [Google Scholar]
- 21.Palka J., Bankowski E., Jaworski S. An accumulation of IGF-I and IGF-binding proteins in human umbilical cord. Mol Cell Biochem. 2000;206:133–139. doi: 10.1023/a:1007005610960. [DOI] [PubMed] [Google Scholar]
- 22.Anzalone R., lo Iacono M., Corrao S., Magno F., Loria T., Cappello F. New emerging potentials for human Wharton's jelly mesenchymal stem cells: immunological features and hepatocyte-like differentiative capacity. Stem Cells Dev. 2010;19:423–438. doi: 10.1089/scd.2009.0299. [DOI] [PubMed] [Google Scholar]
- 23.Liang J., Zhang H., Hua B., Wang H., Wang J., Han Z. Allogeneic mesenchymal stem cells transplantation in treatment of multiple sclerosis. Mult Scler J. 2009;15:644–646. doi: 10.1177/1352458509104590. [DOI] [PubMed] [Google Scholar]
- 24.Wu K.H., Tsai C., Wu H.P., Sieber M., Peng C.T., Chao Y.H. Human application of ex vivo expanded umbilical cord-derived mesenchymal stem cells: enhance hematopoiesis after cord blood transplantation. Cell Transplant. 2013;22:2041–2051. doi: 10.3727/096368912X663533. [DOI] [PubMed] [Google Scholar]
- 25.Wu K.H., Sheu J.N., Wu H.P., Tsai C., Sieber M., Peng C.T. Cotransplantation of umbilical cord–derived mesenchymal stem cells promote hematopoietic engraftment in cord blood transplantation: a pilot study. Transplantation. 2013;95:773–777. doi: 10.1097/TP.0b013e31827a93dd. [DOI] [PubMed] [Google Scholar]
- 26.Hu J., Yu X., Wang Z., Wang F., Wang L., Gao H. Long term effects of the implantation of Wharton's jelly-derived mesenchymal stem cells from the umbilical cord for newly-onset type 1 diabetes mellitus. Endocr J. 2013;60:347–357. doi: 10.1507/endocrj.ej12-0343. [DOI] [PubMed] [Google Scholar]
- 27.Ma L., Zhou Z., Zhang D., Yang S., Wang J., Xue F. Immunosuppressive function of mesenchymal stem cells from human umbilical cord matrix in immune thrombocytopenia patients. Thromb Haemost. 2012;107:937. doi: 10.1160/TH11-08-0596. [DOI] [PubMed] [Google Scholar]
- 28.Shi M., Zhang Z., Xu R., Lin H., Fu J., Zou Z. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med. 2012;1:725–731. doi: 10.5966/sctm.2012-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z., Lin H., Shi M., Xu R., Fu J., Lv J. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol. 2012;27:112–120. doi: 10.1111/j.1440-1746.2011.07024.x. [DOI] [PubMed] [Google Scholar]