Abstract
Background and objectives
Haemoglobin content is the well accepted indicator for anaemia assessment. The high prevalence of anaemia, maternal health care issues and adverse delivery outcome in Jharkhand, we investigated whether delivering women with anaemia would present a modifiable risk of preterm (PTB) and low birth weight (LBW).
Methods
A facility-based cross-sectional study involving pregnant women, with screening for pregnancy endpoints and haemoglobin assay, were conducted. Anaemia was classified according to World Health Organization's definition of anaemia in pregnancy. Confounding variables were adjusted in a logistic model. The adjusted odds ratios (AORs) with 95% confidence intervals (CIs) were used for analyzing the association among maternal anaemia, PTB and LBW.
Results
We observed a high prevalence of anaemia (78.45%) in delivering women, whereas high prevalence of preterm birth (34.75%) and LBW (32.81%) in delivering women overall. In the adjusted analysis, overall anaemia in pregnancy was strongly associated with preterm birth (OR, 3.42; 95% CI, 1.98–5.88; P ≤ .0001) as compared to LBW (OR, 1.12; 95% CI, 0.65–1.61; P = .0003). The risk of PTB and LBW were dependent on the stratification of the anaemia group, as the strongest association was observed in severe (OR, 4.86) followed by mild (OR, 3.66) and moderate (OR, 3.18) anaemia in PTB; whereas risk of LBW was found in severe (OR, 2.5) followed by moderate (OR, 1.11) and mild (OR, 0.57) anaemia. The risk of PTB and LBW across six pregnancy haemoglobin groups were compared, haemoglobin of 10–10.9 g/dl (OR, 1.25) and ≤ 8 g/dl (OR, 1.03) have shown association with PTB and LBW, respectively. However, high haemoglobin concentration was not associated with either PTB or LBW.
Conclusions
Anaemia in delivering women was associated with an elevated risk of PTB and LBW and the risk increased with the severity of anaemia in pregnant women.
Keywords: Maternal anaemia, Preterm birth, Low birth weight, Pregnancy, Jharkhand
1. Introduction
Anaemia is the most prominent hematological manifestation during gestation and is a global health problem affecting nearly half of all pregnant women, primarily with a low socioeconomic status worldwide, including the state of Jharkhand, India [1,2]. Burden of anaemia in pregnant women in developed countries like Australia, United States, United Kingdom and Germany ranges from 9% to 51% [[3], [4], [5]],. These measures contrast with those of developing countries like Ghana, Sudan Nepal, Bangladesh, Pakistan and India, where the burden of anaemia ranges from 44% to 81% [2,[6], [7], [8], [9], [10]]. In India the burden among states ranges from 40% to 72% [2,11]. The prevalence of anaemia during pregnancy in Jharkhand is just over 71% [12]; which is among the highest reported for Indian states. Our previously published data indicates much higher prevalence i.e. 86% and 72%, respectively in pregnant and delivering women [2,12] in the state. Anaemia during gestation and in delivering women is a global problem for mothers and babies resulting in deaths of approximately 115,000 mothers and 590,000 perinatal babies annually [13,14]. Anaemia in gestation and delivery influence the risk of hemorrhage, labor complications, aberrant delivery [15] and also escalate the risk of infection to both mother and developing embryo as result of perturbed immunity and disparity in hormonal orchestration [[16], [17], [18], [19]]. The gestational complications, maternal mortality, low birth weight and adverse birth outcome are among the prominent consequences of anaemia in pregnancy in most developing countries, particularly in south-east Asia [20,21]. Despite the advancing knowledge in obstetric technology, achievements in maternal and child health (MCH) related issues, MCH and nutritional awareness programs over the past decade [[22], [23], [24]]; anaemia remains enigmatic for pregnancy and delivering women in low- and middle-income countries including India [3,25]. An estimated 15 million babies are born preterm and 20 million LBW infants born every year and its prevalence is global public health problem, particularly in developing countries, this number is still rising despite of extensive integrated efforts and improved maternal and child health care interventions across the globe [26,27]. Anaemia is long being considered as an indicator of both poor nutrition and impaired health for women in general and particularly more adversely affecting the reproducing women and increases the risk of maternal and neonatal adverse outcomes. The adversity and impact of maternal anaemia is vulnerability to preterm birth and low birth weight babies in addition to various other gestational complications, such as the risk of miscarriages, stillbirths, intra-uterine growth retardation and risk of postpartum hemorrhage [28]. These adversities are the consequences of altered physiological equilibrium and accumulative gynaecological and obstetric factors.
Maternal anaemia during pregnancy and delivery has been previously linked with risk of poor birth outcomes, such as preterm births, low birth weight, lower haemoglobin concentration and maternal mortality [29,30]. Conflicting observations were reported in regard to cause of poor birth outcome and low birth weight due to maternal anaemia. Some authors have found that anaemia in pregnancy and in delivering women is associated with preterm birth [31], low birth weight [32] and maternal mortality [33]; whereas others have documented increased risk of preterm and low birth weight [30]. Unfortunately, India is major contributor of maternal health issues and neonatal complications despite the advancing knowledge in obstetric technology, various existing public health and medical interventions and decades of research into prematurity. In this country, the incidence of preterm deliveries/birth (PTB) and low birth weight continues to contribute significantly to the burden on maternal and child health (MCH) care [30,[34], [35], [36]]. In view of magnitude, allied morbidity and mortality, the problem of adverse birth outcomes including PTB needs both attention and intervention if India is to improve its maternal and child health record.
Maternal early and culminating pregnancy haemoglobin concentration has rarely been explored as a risk of poor birth outcomes. This study examined whether delivering women with anaemia and/or without anaemia would be at higher risk of preterm birth and low birth weight (LBW), in view of startling status of anaemia 69% and 71% in males and females respectively, higher than in almost all other states in India [12,37]. The identification of risk factors is advantageous for predicting preterm birth because it allows for the initiation of risk-specific treatment for women at-risk. Identifying these risk factors may provide insights into a better understanding of the mechanisms leading to preterm birth.
The prevalence of maternal health-associated factors, such as susceptibility to infectious diseases, poor nutritional status, and hematological perturbation in women's reproductive age, magnitude of adverse delivery, including preterm birth and lacking intent for both prioritization for pre-natal investigations and its incorporation in routine data collection on maternal health status at regional level, prompted us to investigate the actual prevalence of preterm delivery and low birth weight babies vis-à-vis impact of maternal haemoglobin concentration in resources poor settings in tertiary health care facility from endemic population of semi-urban Hazaribag, district of Jharkhand. Additionally, to the best of our information this acquisition of data on preterm, low birth weight and investigation on maternal health care will be the first systematic report of its kind from the region of high priority and risk i.e. Jharkhand, a region not only relatively neglected and under investigated but comprehensively overlooked. The very initiative and investigative work, itself points the significance of this work in view of supporting the concept of healthy mothers give births to healthy babies, is an outmost aspect of reproductive and family health care programme. Further, this work aims to address and provide an evidence-based platform for decision and policy maker to conceptualize and re-orient a much needed region specific strategies for assessment, diagnosis, intervention, prevention and treatment. The present outcomes promises to provide broad spectrum and multi-disciplinary insights to address woman-centred public health issues at the community level in developing and low-income countries; where poverty, resources poor emergency obstetrical care, illiteracy and multiple pregnancies take their toll on mother's health.
2. Methods
2.1. Ethics statement and subject consent
The Institutional Ethics Committee (IEC) of the Vinoba Bhave University, Hazaribag, Jharkhand approved the project and consent proposal (memo no. VBU/R/2481′A'/2015 dated 08-07-2015). The project followed human ethical guidelines of the Medical Ethics Committee, Ministry of Health, Government of India; for all aspects of the project, including collection of human blood samples after obtaining written consent from the study participants under proposed protocols activities. This study did not involve any minors. Thus, signed and written approval was given by an adult subject herself. All study participants were included only after informed consent.
2.2. Study population
A cross-sectional investigation was conducted in the antenatal care units (ANC) and delivery units (DU), of Sadar hospital in the Hazaribag district of Jharkhand, India. Pregnant women aged ≥18 years presenting themselves at the ANC for routine care and at DU were screened and enrolled. Recruitment and enrolment took place from September 2016 to December 2017.
2.3. Study enrolments
In DU, 870 pregnant/delivering women were referred and screened. Of these 539 were willing to understand our study protocol, out of which 515 consented and agreed to peripheral sampling, while 24 refused to participate in the study. The 515 enrolled subjects were interviewed by trained technical staff with data collection focused on socio-demographic and anthropometric characteristics, upon pregnancy screening report and obstetric complications during pregnancy, birth outcome i.e. pre-term, post-term and term delivery and mode of delivery i.e. normal, caesarean delivery and still birth.
2.4. Determination of gestation
To assess the gestational age, we adopted the simplest method i.e. symphysis-pubis fundal height (SFH) measurement (also known as palpation of uterine height measurement), the most widely used method globally, especially in resource poor settings [38]. Assessments were performed by nurses followed by a gynaecologist. However, ultrasounds were used for confirmation of age, in case of any ambiguity or doubt in positioning of foetus and estimation of gestational ages.
2.5. Study definitions for birth outcomes and maternal variables
Birth at <37 weeks of gestation was defined as preterm birth and prevalence of preterm across the cohort further subcategorized into preterm (24–36 weeks), early preterm (24–33 weeks) and late preterm (34–36 weeks). Low birth weight (LBW) infants were those that weighed <2500 g at birth. Maternal height and weight were taken at the first visit to ANC and DU. Based on this information the body mass index (BMI) were calculated as weight (kg) divided by the squared height (meters); a low BMI was defined as a BMI < 22.0 kg/m2. We classified women into 2 groups (anaemia (Hb < 12 g/dl) and no anaemia (Hb ≥ 12 g/dl)), 4 groups (mild-moderate-to-severe, mild anaemia (Hb10–11.8 g/dl), moderate anaemia (Hb of 8–9.9 g/l), severe anaemia (Hb of <8 g/dl) and normal haemoglobin i.e. no anaemia (Hb ≥ 12 g/dl)) and 6 groups by intervals of 8 g/dl from severe anaemia (Hb < 8 g/dl) to the highest Hb group (≥12 g/dl) by pregnancy Hb concentration. Hb categorization, particularly the stratification of anaemia, was based on the WHO guidelines [39].
2.6. Haemoglobin concentration
Haemoglobin (Hb) levels were recorded at the first ANC and DU visit. The concentration of haemoglobin was determined in peripheral blood samples using a portable HemoCue haemoglobinometer (HemoCue AB, Ängelholm, Sweden) following manufacturer's instructions. The concentration of Hb was recorded on the study questionnaire. The concentration of Hb was also estimated following acid haematin method [40] in laboratory, double-checked by the laboratory technician.
2.7. Data management and statistical analysis
All clinical, demographic and anthropometric information were carefully checked for correctness and data with missing information/parameters were removed before analysis. Data were entered in MS-Excel and analyses were performed using SPSS v.16 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism version 5.0 (GraphPad Software, Inc., CA, USA). Maternal age, body mass index and Hb concentration were expressed as mean ± SD. The number and rate (%), and the rate's exact binomial 95% confidence interval (CI) were calculated for preterm birth and LBW across the Hb groups. An independent samples t-test was applied to compare the differences in means, and the χ2 test was performed to examine differences in proportions. An unconditional logistic regression model was made to calculate odds ratios (ORs) and 95% CIs. The ORs were adjusted for mother's age at delivery, body mass index, parity, and education level. Tests for linear trends across the Hb groups were also conducted with the Hb group as an ordinal variable. The P-value was calculated with two-sided tests and a statistical significance level of 0.05 was applied.
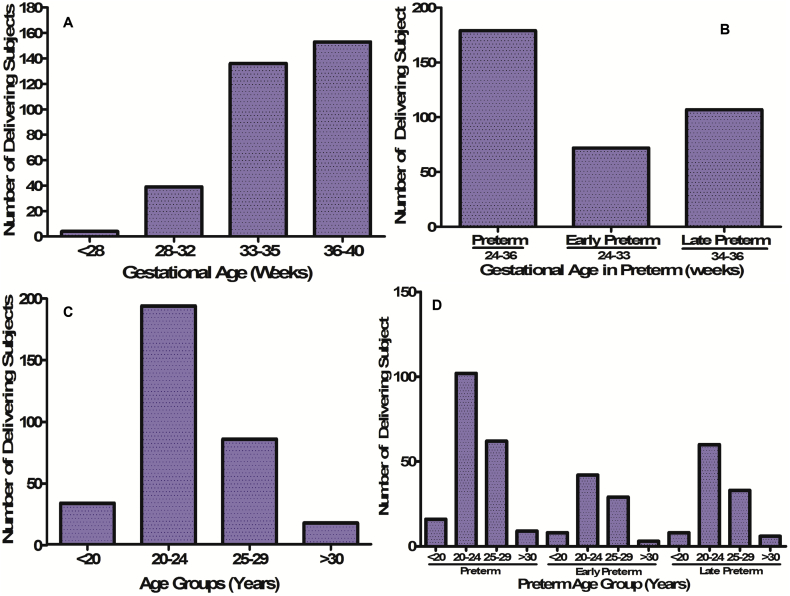
3. Results
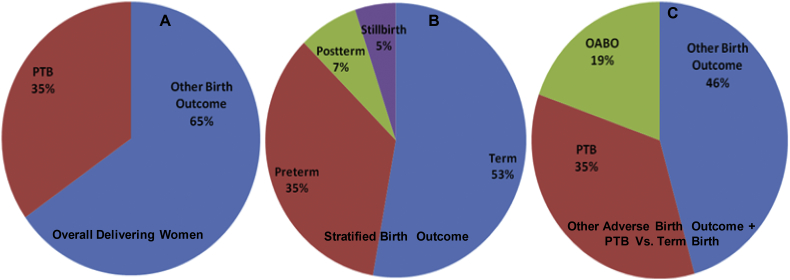
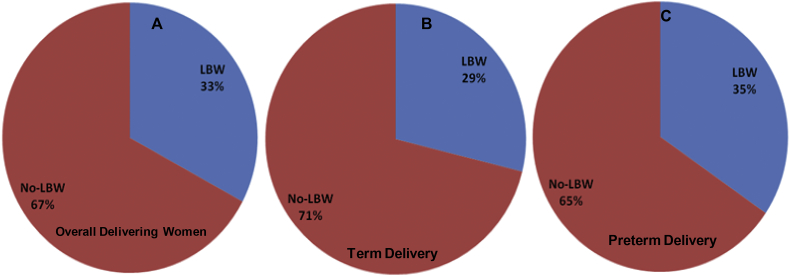
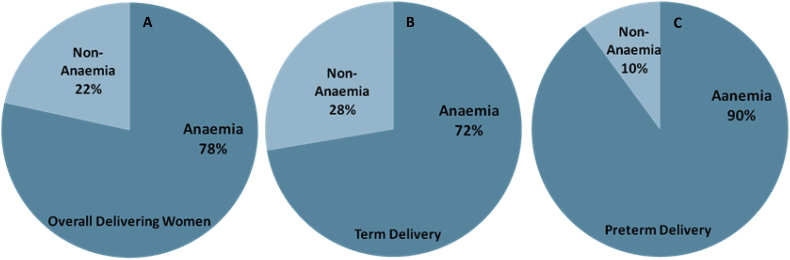
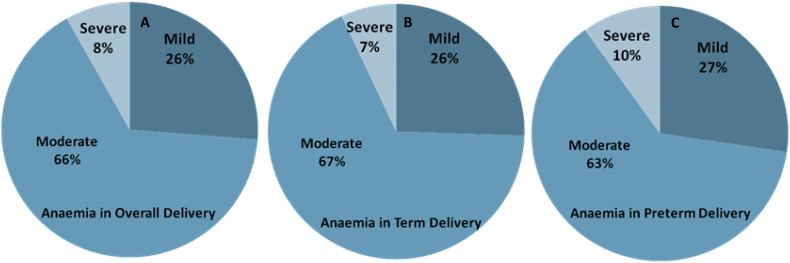
The maternal and gestational mean age of the population at delivery was 23.2 ± 3.5 years and 33.3 ± 1.5 weeks, respectively. The mean maternal and gestational age of the preterm group at delivery was 21.7 ± 2.3 years and 32.2 ± 1.2 weeks, respectively. Further, stratified maternal and gestational age groups of all the studied subjects are given (Fig. 1). The mean body mass index and mean Hb concentration was 21.5 ± 3.2 kg/m2 and 10.6 ± 5.3 g/dl at the time of health examinations undertaken before pregnancy. Detailed documented maternal characteristics of anaemic women as compared to non-anaemic women at the DU are given (Table 1). We investigated the prevalence and burden of preterm delivery in both overall delivering women and in stratified birth groups. We found 35% preterm birth and 65% other category of birth outcome overall among delivering women (Fig. 2A). In the stratified birth and in comparison of preterm versus term birth group, we observed 35% and 33% preterm delivery, respectively (Fig. 2B and 2C). Similarly, we observed 33% LBW in overall delivery, but 29% in term delivery compared with 35% LBW in preterm deliveries (Fig. 3A–C). The overall prevalence of anaemia was 78.4% in delivering women; whereas 72% women delivering at term were anaemic, compared with 90% in preterm delivery (Fig. 4A–C). Further, we have evaluated the classified anaemia i.e. mild, moderate and severe anaemia and observed the prevalence of stratified anaemia in all the three groups of delivering women (Fig. 5A–C). We found 10% severe anaemia in preterm delivery as compared to 7% and 8% in term and overall delivery groups, respectively. Additionally, it remarkable that, overall, there was a very high prevalence of anaemia (90%) in women delivering preterm (35%) with low birth weight babies (35%) in the present investigation. Precisely, all preterm births were found with low birth babies among overall delivery in the present investigation.
Fig. 1.
Clinically stratified gestational and maternal ages. (A) Gestational age group of all delivering women. (B) Gestational ages of stratified preterm birth group. (C) Maternal age group of all delivery unit attendee at the time of delivery. (D) Maternal age group of all preterm subjects at the time of delivery.
Table 1.
Maternal Characteristics by anaemia in delivering women at delivery unit, Sadar hospital, Hazaribag, Jharkhand.
| Characteristics | Levels | Anaemia |
No anaemia |
P-valuesb |
|---|---|---|---|---|
| N, (%) | N, (%) | |||
| Maternal age (Years) | ||||
| ˂20 | 43(10.7) | 17(15.3) | 0.009 | |
| 20–24 | 207(51.2) | 57(51.3) | ||
| 25–29 | 102(25.2) | 14(12.6) | ||
| ≥30 | 52(12.8) | 23(20.7) | ||
| Parity | ||||
| Primigravid | 303(75) | 72(64.8) | 0.006 | |
| Secundigravid | 86(21.3) | 27(24.3) | ||
| Multigravida | 15(3.7) | 12(10.8) | ||
| Gestational age at delivery (weeks) | ||||
| ˂28 | 8(1.9) | 5(4.5) | 0.004 | |
| 28–32 | 47(11.6) | 13(11.7) | ||
| 33–35 | 136(33.6) | 19(17.1) | ||
| ≥36 | 213(52.7) | 74(66.6) | ||
| Body mass index (Kg/m2) | ||||
| <21 | 14(3.4) | 7(6.3) | 0.02 | |
| 21–24.9 | 56(13.8) | 13(11.7) | ||
| 25–29.9 | 190(47.1) | 37(33.3) | ||
| 30–34-9 | 104(25.7) | 43(38.7) | ||
| ≥35 | 40(9.9) | 11(9.9) | ||
| Educational status | ||||
| No formal schooling | 137(33.9) | 53(47.7) | 0.004 | |
| Schooling at any level | 213(52.7) | 39(35.1) | ||
| Graduation and above | 54(13.3) | 19(17.1) | ||
Defined as 3 or more pregnancies.
chi2-test.
Fig. 2.
Burden of Premature birth and other birth outcome. (A) Prevalence of preterm birth (PTB) in delivering women. (B) Stratified birth outcomes in delivering women. (C) Comparison of PTB and other adverse birth outcome (OABO) with term delivery in investigated population of Hazaribag, district of Jharkhand.
Fig. 3.
Prevalence of low birth weight (LBW) as compared to no-LBW baby. (A) Low birth weight baby (LBW) in delivering women. (B) LBW baby in term delivering women. (C) LBW baby in preterm delivering women in investigated population of Hazaribag, district of Jharkhand.
Fig. 4.
Prevalence of maternal Anaemia as compared to non-anemic (A) Maternal Anaemia in delivering women. (B) Maternal Anaemia in term delivering women. (C) Maternal Anaemia in preterm delivering women in investigated population of Hazaribag, district of Jharkhand.
Fig. 5.
Prevalence of classified Anaemia according to WHO guidelines (A) Stratified Anaemia in delivering women. (B) Stratified Anaemia in term delivering women. (C) Stratified Anaemia in preterm delivering women in investigated population of Hazaribag, district of Jharkhand.
Interestingly, we have observed higher mean haemoglobin concentration in term delivery as compared to preterm delivery in case of sever and moderate anaemia; whereas significantly lower haemoglobin concentration were observed in overall delivering women as compared to term delivery (Fig. 6).
Fig. 6.
Concentration of Haemoglobin in mild, moderate and severe Anaemia in (A) Overall delivering women. (B) Term delivering women. (C) Preterm delivering women (D) Comparison between preterm and term delivery in case of sever and moderate Anaemia in investigated population of Hazaribag, district of Jharkhand.
The preterm birth and LBW proportion was highest in women with moderate-to-severe anaemia when the subjects were classified into 4 groups. The risk of preterm birth and LBW progressively increased with the severity of pregnancy anaemia among 6 groups of maternal Hb (Table 2).
Table 2.
Birth outcomes by maternal pregnancy anaemia and haemoglobin groups.
| Pregnancy Haemoglobin Groups(g/dl) | Number of women | Preterm birth |
Low birth weight |
||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | 95% CI | P | N | % | 95% CI | P | ||
| 2 Groups | |||||||||
| Anaemic | 404 | 161 | 39.8 | 1.9–5.8 | 0.0006 | 133 | 32.9 | 0.6–1.6 | 0.9 |
| Non-Anaemic | 111 | 18 | 16.2 | 0.1–0.5 | 36 | 32.4 | 0.6–1.5 | ||
| 4 Groups | |||||||||
| Mild | 106 | 44 | 41.5 | 1.8–6.4 | 0.006 | 23 | 21.6 | 0.3–1.1 | 0.03 |
| Moderate | 265 | 101 | 38.1 | 1.7–5.1 | 92 | 34.7 | 0.6–1.7 | ||
| Severe | 33 | 16 | 48.4 | 2.1–11.3 | 18 | 54.5 | 1.1–5.5 | ||
| Non-Anaemic | 111 | 18 | 16.2 | 0.1–0.5 | 36 | 32.4 | 0.6–1.5 | ||
| 6 Groups | |||||||||
| <8 | 49 | 16 | 32.6 | 1.1–5.4 | 0.9 | 18 | 36.7 | 0.6–2.4 | 0.8 |
| 8–8.9 | 132 | 44 | 33.3 | 1.3–4.8 | 43 | 32.5 | 0.6–1.7 | ||
| 9–9.9 | 164 | 57 | 34.7 | 1.5–5.1 | 57 | 34.7 | 0.7–1.8 | ||
| 10–10.9 | 112 | 44 | 39.2 | 1.7–6.2 | 32 | 28.5 | 0.4–1.5 | ||
| 11–11.9 | 50 | 17 | 34 | 1.2–5.7 | 18 | 36 | 0.2–2.3 | ||
| ≥12 | 8 | 1 | 12.5 | 0.1–6.3 | 1 | 12.5 | 0.1–2.5 | ||
In logistic regression analysis, anaemic women had a strongly associated (OR, 3.4) and statistically significant (P = .0001) risk of PTB than non-anaemic women; whereas anaemic women had relatively weakly associated (OR, 1.1) and statistically significant (P = .0003) risk of LBW. Although, association with risk of preterm birth progressively increased with the severity of maternal anaemia during pregnancy and/or in delivering women; with a highest risk of preterm birth (OR, 4.8) was observed in severely anaemic group of women. Contrary to the risk of PTB, the association with risk of low birth weight (OR, 2.5) was observed mainly in the severely anaemic group of women (Table 3). The investigated population had very strong, progressive and inclined risk of preterm birth irrespective of the status of anaemia stratification and lower haemoglobin content (Table 3 and Fig. 6).
Table 3.
Adjusted odds ratios for birth outcome and low birth weight by maternal pregnancy anaemia and haemoglobin groups.
| Pregnancy Haemoglobin Groups(g/dl) | Preterm birth |
Low birth weight |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| 2 Groups | ||||||
| Anaemic | 3.42 | 1.98–5.88 | 0.0001 | 1.12 | 0.65–1.61 | 0.0003 |
| Non-Anaemic | Reference | Reference | ||||
| 4 Groups | ||||||
| Mild | 3.66 | 1.94–6.92 | 0.0001 | 0.57 | 0.31–1.06 | 0.0003 |
| Moderate | 3.18 | 1.81–5.58 | 0.0001 | 1.11 | 0.69–1.77 | 0.0003 |
| Severe | 4.86 | 2.08–11.36 | 0.0001 | 2.5 | 1.13–5.52 | 0.0003 |
| Non-Anaemic | Reference | Reference | ||||
| 6 Groups | ||||||
| <8 | 0.94 | 0.41–2.17 | 0.02 | 1.03 | 0.45–2.34 | 0.05 |
| 8–8.9 | 0.97 | 0.48–1.93 | 0.02 | 0.85 | 0.43–1.69 | 0.05 |
| 9–9.9 | 1.03 | 0.53–2.01 | 0.02 | 0.94 | 0.48–1.83 | 0.05 |
| 10–10.9 | 1.25 | 0.62–2.52 | 0.02 | 0.71 | 0.35–1.44 | 0.05 |
| 11–11.9 | Reference | Reference | ||||
| ≥12 | 0.27 | 0.03–2.44 | 0.02 | 0.25 | 0.02–2.23 | 0.05 |
4. Discussion
Anaemia is the most prominent hematological manifestation of pregnancy, delivery and heralded cause of gestational complications and adverse birth outcomes. In the present investigation, we observed stunningly high, that is, over 78% anaemia in pregnant and delivering women. Our observation of very high prevalence of anaemia in reproducing women population from Jharkhand is closely in accordance with the findings for the state of Tripura and Assam and other north-eastern states in India [41] and a comparatively higher prevalence of anaemia as compared with other states [11,[42], [43], [44], [45], [46]]. Our finding of high prevalence of anaemia among reproducing women is also in line with observations from developing and African countries [[47], [48], [49], [50], [51], [52]]. Upon conception and with the progression of gestation, the reproductive physiology of women is marked by the higher demand of haemoglobin [53] due to increased requirement of iron for the growing foetus and placenta. The increased demand of fresh haemoglobin, the inability of women to produce it endogenously due to poor iron supplementation in their diet and depleted reserve iron due to previous recurrent menstrual loss potentially causes maternal anaemia during pregnancy in a high proportion of women from developing countries. Persistent depletion and causative conditions of anaemia, significantly contributes to the gestational complications and adverse birth outcomes. Physiologically, anaemia is marked by the condition of deficiency of haemoglobin content in healthy red blood cells [54,55]; attributed either to one unlikely cause of enigmatic deficiency of haemoglobin or mostly caused by a complex array of factors ranging from endogenous biological orchestrating components including ion-elemental regulations [56] to various exogenous supplementary, dietary and socio-economic factors [57]. Based on direct evidence from our previous observation of high prevalence of anaemia in reproducing women [2] and present observation also endorsing the distinctively high prevalence (over 78%); it will be pertinent to peruse and discuss the various plausible reasons with coordinated underlying factors for such unfortunate but sizable burden.
Interpretation of observed high incidence of anaemia in this region should be viewed and correlated; also in the ambit of dominant and indigenous non-biological perspectives, before considering biological plausibility as integrated factors for continued and persistent burden. Those predominant non-biological factors like, geographical landscape i.e. region under investigation, native inhabitants with sizable under nourished and mainstream aloof tribe population, educational background, social taboos and critically limited availability and accessibility of resources poor public health facilities in general are consolidated and indispensable contributory elements to the magnitude of anaemia. This accumulative component of high prevalence of anaemia also differentiates as well as signifies our study from both across the globe and within Indian states [2,12,37,58,59].
Further, in case of Jharkhand, women health issues, health care and prevention strategies tend to magnify. Associated morbidity also amplifies due to difficult geographical location of the state and the administrative landscape hinders effective intervention and implementation of medical support. Dietary deficiency, hematological dysfunction and iron regulation are conventionally acknowledged and plausible biological causative cascade for genesis of anaemia [20,60,61]. Additionally, various parasitic and other infections (TORCH (Toxoplasma, Rubella, Cytomegalovirus and Herpes) pathogens) including opportunistic urinary tract infections (UTI) during gestation are also potential contributing factors of maternal anaemia [[62], [63], [64], [65], [66], [67]]. The conception, pregnancy and delivery are marked by the finely tuned and regulated immunological phenomena of indigenous inflammation. The very proinflammatory micro environment have shown causative association in both, inflammation-induced anaemia [21,43] through a mechanism, which down regulates the iron adsorption and erythropoiesis [68] and in infection-induced inflammation mediated anaemia during pregnancy and delivery. During an early pregnancy, a physiologically indispensible and activated mechanism works in such a coordinated fashion that up regulates the blood and plasma volumes [69,70]. The very precisely orchestrated phenomena of increased blood volume observed by Dieckmann and others that the total amount of circulatory haemoglobin at term pregnancy was increased about 13% and total erythrocyte volume increased by 20% [71] leading to relative decrease in haemoglobin concentration and absolute increase in amount during pregnancy. This uneven increase in plasma to total blood volume during pregnancy makes the interpretation of haemoglobin estimation more complex and thus warrants the need of repetitive and time dependent measurement during various stages of pregnancy for precise assessment and management of anaemia in gestation and delivering women.
However, in the present investigation, we have neither experimentally elucidated the causative mechanism for anaemia nor was the basic objective to understand the reason of high prevalence in pregnancy and/or delivery from this region. Rather, our objective was to investigate the actual burden of anaemia in pregnant and delivering women, various birth outcome including preterm and low birth weight, and its associated risk of preterm and low birth weight babies with maternal anaemia. We observed overall 34.5% and 32.8%, preterm and low birth weight babies, respectively. In the case of anaemia in delivering women, we found 39.8% and 32.9% preterm and low birth weight babies, respectively (Table 2). Further, upon stratification of anaemia; highest, 48.4% followed by 41.5% and 38.1% of preterm birth was observed in severe, mild and moderate anaemia, respectively; whereas, highest 54.5% followed by 34.7% and 21.6% of low birth weight were observed in sever, moderate and mild anaemia, respectively. It is quite interesting to note that highest prevalence of both preterm and low birth weight was observed in severe anaemia in the investigated region and population; overall and stratified prevalence is once again alarmingly high as compared to other states across the India as well higher than the national average [[44], [45], [46],72] anaemia and subsequent impact on birth outcome may be attributed to the low socio-economic factor in general, and particularly the literacy and education contributes significantly to the burden, as also observed in our present investigation (Table 1). We observed that anaemic women are less educated and/or having any level of formal education than non-anaemic women in this study, and maternal educational level was adjusted for regression analysis. We speculate that women with secondary and/or higher education are more aware about personal care and hygiene by virtue of education as compared to their counterparts; and have lesser risk of being anaemic [73,74]. This also highlights the necessity of multisectorial integrated efforts to combat the anaemia and other maternal health problems, as education and empowerment of women and children are beyond the purview of the health sector. The magnitude of anaemia is variably pandemic in nature but precisely afflicting the underprivileged community in developing countries including India and its aetiology is interwoven and multifactorial. Aetiological factors include nutritional deficiencies and parasitic infection with a huge burden on women of child bearing age and children in general and particularly during pregnancy [49,75]. However, prevalence and severity of accumulated factors to anaemia in pregnancy varies by geographical location, socio-economic status, dietary practices, awareness and wellness index including the incidence of infectious diseases [76]. Varied concentrations of haemoglobin and stratified anaemia during pregnancy have implication in the genesis of preterm and low birth weight babies [77,78] and we have also observed the significant impact of low haemoglobin concentration on preterm and LBW in the present investigation (Table 2). Possibly geographical diversity, low socio-economic status of the population at large, due to a sizable rural and semi-urban society, limited and poor health resources and services utilization, overall health and nutritional status of mothers during pregnancy could be the reasons for vulnerability and exposure to gestational complications leading to preterm and low birth weight in this region.
Our observation of significantly high prevalence of anaemia in general and its association with preterm birth and low birth weight babies suggests quite high-risk factors for adverse birth outcome in general and particularly for PTB and LBW in all delivering women. This indicated the alarming situation for community reproductive healthcare management across the investigated region, where poverty, illiteracy, geographical diversity, socio-economic disparities and multiple pregnancies take their toll on mother's health. Our findings regarding preterm and LBW associated with anaemia are critically alarming and emerge out as the most threatening health scenario in this region despite the high priority of maternal and child health programs. The reasons can be attributed to several factors, ranging from young maternal age [79], parity [80], severity and duration of anaemia [60], stages of gestation [81,82], term specific haemoglobin concentration [[83], [84], [85]], substantial inter and intra regional differences in basic health amenities [20,86,87] and poor nutritional intake [88,89] including hidden elemental deficiencies [90,91] leading to critical BMI [[92], [93], [94]]; which are the direct and contributory causative factors in several adverse effects including low birth weight and preterm delivery.
Our result of anaemia in early pregnancy support the observation made by Zhang et al. [95] in their finding that diagnosis of anaemia in early stage of pregnancy is likely to be a stronger associating factor for adverse birth outcome than anaemia diagnosed later in gestation. Specifically, anaemia found in first trimester was associated with modest increased risk of all preterm births. Additionally, our observation of the high prevalence of moderate and mild anaemia (41.5% and 38.1% respectively) and its association with preterm and low birth weight are in accordance with the observation of Zhang et al. [95]. Further, spontaneous and medically predicted preterm in non-anaemic delivering women was not associated with anaemia as also described by Zhang et al. [95]. These findings emphasise that the strong assortment in the risk profile for preterm birth depends on heterogeneity of clinical subtypes, as well as by exposure window, i.e. trimester in pregnancy when anaemia was diagnosed [96]. Interestingly, anaemia before pregnancy is also strongly associated with risk of adverse birth outcome including preterm births and LBW [60,97]. These findings are distinctive, affirmative and conclusive in terms of providing staggering estimate, associated risk factor for reproductive health and on adverse delivery outcome. However, the present investigation have some limitations, that we could not access the impact of infection vis-à-vis adverse birth outcome, impact evaluation of nutritional components vis-à-vis information on iron supplementation during gestation and post-delivery comparative evaluation of anaemia components for region specific better understanding of the burden on pregnancy and effects on birth outcomes.
Our investigations and findings on the prevalence and association of maternal anaemia in preterm and LBW babies, poses challenges to current implementation and impact of various Family Welfare and MCH care programme and raised the necessity for critical assessment of existing programme, policies, and ongoing intervention strategies; as well as to reorient the region specific policies and intensify the prenatal-cum-maternal health care management, if deplorable reproductive health and its associated indicators in Jharkhand, has to be improved. Further, our observation also suggests that community awareness, strengthening of rural and remote health facility for marginalized population and integrated immediate and long term maternal health management; need to be prioritized for the improvement of overall maternal health indicators and particularly, reduction in the magnitude of maternal anaemia from investigated region.
5. Conclusion
Our results suggest that maternal anaemia in pregnant and delivering women has an unacceptably high prevalence and severe anaemia was high. Overall anaemia and its severity significantly influences preterm and low birth deliveries and all the stratified anaemia is strongly associated with preterm birth, whereas severity of anaemia is strongly associated with low birth weight in the investigated population. In view of conclusive observation, evaluation of haemoglobin in all the trimesters of pregnancy and, particularly in early pregnancy is fairly independent and promising predictor of anaemia and any form of anaemia in pregnancy is potential risk factor for preterm and LBW. Both this anaemia mediated prevalence of, PTB and LBW in delivering women from investigated region of Jharkhand are alarmingly much higher than the previous and recent national average; is indicative of serious health issue afflicting women and children across Jharkhand and emphasizes the need of early, active diagnosis and integrated investigation in order to curb the menace of public and community health importance.
Ethics statement and subject consent
All human blood samples used in this study were collected after obtaining written consent from the study participants under protocols activities approved by the Institutional Ethics Committee (IEC) of the Vinoba Bhave University, Hazaribag, Jharkhand and human ethical guidelines as reflected in the guidelines of the Medical Ethics Committee, Ministry of Health, Govt. of India. Present study does not involve any minor/children. Thus, signed and written approval was given by adult subject herself. All study participants were included only after informed consent. The study protocol and consent proposal is approved from IEC, VBU having memo no. VBU/R/2481′A'/2015 dated 08-07-2015.
Consent for publication
All authors read and approved the final version for publication consideration.
Availability of supporting data
All data available with the submitted manuscript.
Competing interests
The authors have declared that no competing interests exist.
Funding
This research was supported by Dr. D. S. Kothari's postdoctoral grant under UGC, Govt. of India (letter no. F.4-2/2006 (BSR)/13-690/2012 (BSR) dated 25th May 2012)) and ISID-Small grant, USA. Fall-2012 to Mohammad Sohail and also supported by Emeritus Grant Letter No.F.66/ 2017-18/EMERITUS-2017-18-GEN-11523/(SAII) dated 07-April, 2017 to Mohammad Raziuddin. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions statement
Conceived and designed the experiments: MS, AKS. MR. Performed the experiments: SK, PKIG, SA, SA, KPS. Analyzed the data: MS, KPS, SA, MR, NG. Designed and implemented clinical studies and collected samples: SK, PKIG, SA, MS Contributed reagents/materials/analysis tools: SN, PKM, BKG, AKS, MR, NG. Wrote the paper: MS, KPS, MR.
Acknowledgments
The active support from Mr. Arjun Prasad and Mrs. Devyanti Devi at Sadar Hospital, Hazaribag in clinical sample collection is gratefully acknowledged. We thank all the OPD physicians at Sadar Hospital, Hazaribag for their expertise and dedication in providing health care to the community particularly, to the poor and underprivileged. We also wish to acknowledge honourable Vice-Chancellor of Vinoba Bhave University and Civil Surgeon at Sadar Hospital Hazaribag for support and kind assistance for the work.
Contributor Information
Mohammad Raziuddin, Email: mrazi.vbu@gmail.com.
Mohammad Sohail, Email: soh.khan@hotmail.com, soh.khan@vbu.ac.in.
References
- 1.Lee A.I., Okam M.M. Anemia in pregnancy. Hematol. Oncol. Clin. North Am. 2011;25:241–259. doi: 10.1016/j.hoc.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Sohail M., Shakeel S., Kumari S., Bharti A., Zahid F., Anwar S., Singh K.P., Islam M., Sharma A.K., Lata S., Ali V., Adak T., Das P., Raziuddin M. Prevalence of malaria infection and risk factors associated with anaemia among pregnant women in Semiurban Community of Hazaribag, Jharkhand, India. Biomed. Res. Int. 2015;2015:740512. doi: 10.1155/2015/740512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevens G.A., Finucane M.M., De-Regil L.M., Paciorek C.J., Flaxman S.R., Branca F., Pena-Rosas J.P., Bhutta Z.A., Ezzati M. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob. Health. 2013;1:e16–e25. doi: 10.1016/S2214-109X(13)70001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rukuni R., Knight M., Murphy M.F., Roberts D., Stanworth S.J. Screening for iron deficiency and iron deficiency anaemia in pregnancy: a structured review and gap analysis against UK national screening criteria. BMC Pregnancy Childbirth. 2015;15:269. doi: 10.1186/s12884-015-0679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO . World Health Organization; 2016. Global Health Observatory Data Repository/ World Health Statistics. [Google Scholar]
- 6.Anlaakuu P., Anto F. Anaemia in pregnancy and associated factors: a cross sectional study of antenatal attendants at the Sunyani Municipal Hospital, Ghana. BMC Res. Notes. 2017;10:402. doi: 10.1186/s13104-017-2742-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdelgadir M.A., Khalid A.R., Ashmaig A.L., Ibrahim A.R., Ahmed A.A., Adam I. Epidemiology of anaemia among pregnant women in Geizera, Central Sudan. J. Obstet. Gynaecol. 2012;32:42–44. doi: 10.3109/01443615.2011.617849. [DOI] [PubMed] [Google Scholar]
- 8.Bondevik G.T., Lie R.T., Ulstein M., Kvale G. Seasonal variation in risk of anemia among pregnant Nepali women. Int. J. Gynaecol. Obstet. 2000;69:215–222. doi: 10.1016/s0020-7292(00)00206-x. [DOI] [PubMed] [Google Scholar]
- 9.Chowdhury H.A., Ahmed K.R., Jebunessa F., Akter J., Hossain S., Shahjahan M. Factors associated with maternal anaemia among pregnant women in Dhaka city. BMC Womens Health. 2015;15:77. doi: 10.1186/s12905-015-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ansari N.B., Badruddin S.H., Karmaliani R., Harish H., Jehan I., Pasha O., Moss N., McClure E.M., Goldenberg R.L. Anemia prevalence and risk factors in pregnant women in an urban area of Pakistan. Food Nutr. Bull. 2008;29:132–139. doi: 10.1177/156482650802900207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suryanarayana R., Chandrappa M., Santhuram A.N., Prathima S., Sheela S.R. Prospective study on prevalence of anemia of pregnant women and its outcome: a community based study. J. Family Med. Prim. Care. 2017;6:739–743. doi: 10.4103/jfmpc.jfmpc_33_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.N.F.H.S. (NFHS-4) IIPS; Jharkhand, Mumbai: 2017. International Institute for Population Sciences, India, 2015–16. [Google Scholar]
- 13.Salhan S., Tripathi V., Singh R., Gaikwad H.S. Evaluation of hematological parameters in partial exchange and packed cell transfusion in treatment of severe anemia in pregnancy. Anemia. 2012;2012:608658. doi: 10.1155/2012/608658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feleke B.E., Feleke T.E. Pregnant mothers are more anemic than lactating mothers, a comparative cross-sectional study, Bahir Dar, Ethiopia. BMC Hematol. 2018;18:2. doi: 10.1186/s12878-018-0096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Figueiredo A.C.M.G., Gomes-Filho I.S., Silva R.B., Pereira P.P.S., Mata F., Lyrio A.O., Souza E.S., Cruz S.S., Pereira M.G. Maternal anemia and low birth weight: a systematic review and meta-analysis. Nutrients. 2018;10 doi: 10.3390/nu10050601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muhangi L., Woodburn P., Omara M., Omoding N., Kizito D., Mpairwe H., Nabulime J., Ameke C., Morison L.A., Elliott A.M. Associations between mild-to-moderate anaemia in pregnancy and helminth, malaria and HIV infection in Entebbe, Uganda. Trans. R. Soc. Trop. Med. Hyg. 2007;101:899–907. doi: 10.1016/j.trstmh.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gangopadhyay R., Karoshi M., Keith L. Anemia and pregnancy: a link to maternal chronic diseases. Int. J. Gynaecol. Obstet. 2011;115(Suppl. 1):S11–S15. doi: 10.1016/S0020-7292(11)60005-2. [DOI] [PubMed] [Google Scholar]
- 18.Robinson D.P., Klein S.L. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm. Behav. 2012;62:263–271. doi: 10.1016/j.yhbeh.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abu-Ouf N.M., Jan M.M. The impact of maternal iron deficiency and iron deficiency anemia on child’s health. Saudi Med. J. 2015;36:146–149. doi: 10.15537/smj.2015.2.10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahman M.M., Abe S.K., Rahman M.S., Kanda M., Narita S., Bilano V., Ota E., Gilmour S., Shibuya K. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: systematic review and meta-analysis. Am. J. Clin. Nutr. 2016;103:495–504. doi: 10.3945/ajcn.115.107896. [DOI] [PubMed] [Google Scholar]
- 21.Wirth J.P., Woodruff B.A., Engle-Stone R., Namaste S.M., Temple V.J., Petry N., Macdonald B., Suchdev P.S., Rohner F., Aaron G.J. Predictors of anemia in women of reproductive age: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr. 2017;106:416S–427S. doi: 10.3945/ajcn.116.143073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chowdhury A.M., Bhuiya A., Chowdhury M.E., Rasheed S., Hussain Z., Chen L.C. The Bangladesh paradox: exceptional health achievement despite economic poverty. Lancet. 2013;382:1734–1745. doi: 10.1016/S0140-6736(13)62148-0. [DOI] [PubMed] [Google Scholar]
- 23.Mangla M., Singla D. Gestational Gigantomastia: a systematic review of case reports. J. Midlife Health. 2017;8:40–44. doi: 10.4103/jmh.JMH_92_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tandon R., Brandl B., Baryshnikova N., Landshammer A., Steenpass L., Keminer O., Pless O., Muller F.J. Generation of two human isogenic iPSC lines from fetal dermal fibroblasts. Stem Cell Res. 2018;33:120–124. doi: 10.1016/j.scr.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Branca F., Mahy L., Mustafa T.S. The lack of progress in reducing anaemia among women: the inconvenient truth. Bull. World Health Organ. 2014;92:231. doi: 10.2471/BLT.14.137810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iams J.D., Romero R., Culhane J.F., Goldenberg R.L. Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. The Lancet. 2008;371 doi: 10.1016/S0140-6736(08)60108-7. [DOI] [PubMed] [Google Scholar]
- 27.Chawanpaiboon S., Vogel J.P., Moller A.B., Lumbiganon P., Petzold M., Hogan D., Landoulsi S., Jampathong N., Kongwattanakul K., Laopaiboon M., Lewis C., Rattanakanokchai S., Teng D.N., Thinkhamrop J., Watananirun K., Zhang J., Zhou W., Gulmezoglu A.M. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob. Health. 2018;7:e37–e46. doi: 10.1016/S2214-109X(18)30451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Figueiredo A., Gomes-Filho I.S., Silva R.B., Pereira P.P.S., Mata F., Lyrio A.O., Souza E.S., Cruz S.S., Pereira M.G. Maternal anemia and low birth weight: a systematic review and meta-analysis. Nutrients. 2018;10 doi: 10.3390/nu10050601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norman J.E., Morris C., Chalmers J. The effect of changing patterns of obstetric care in Scotland (1980–2004) on rates of preterm birth and its neonatal consequences: perinatal database study. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beck S., Wojdyla D., Say L., Betran A.P., Merialdi M., Requejo J.H., Rubens C., Menon R., Van Look P.F. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull. World Health Organ. 2010;88:31–38. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slattery M.M., Morrison J.J. Preterm delivery. Lancet. 2002;360:1489–1497. doi: 10.1016/S0140-6736(02)11476-0. [DOI] [PubMed] [Google Scholar]
- 32.McCormick M.C. The contribution of low birth weight to infant mortality and childhood morbidity. N. Engl. J. Med. 1985;312:82–90. doi: 10.1056/NEJM198501103120204. [DOI] [PubMed] [Google Scholar]
- 33.Little M.P., Brocard P., Elliott P., Steer P.J. Hemoglobin concentration in pregnancy and perinatal mortality: a London-based cohort study. Am. J. Obstet. Gynecol. 2005;193:220–226. doi: 10.1016/j.ajog.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 34.WHO . World Health Organisation; Geneva: 2012. Essential Interventions, Commodities and Guidlines for Reproductive, Maternal, Newborn and Child Health. [Google Scholar]
- 35.Froen J.F., Myhre S.L., Frost M.J., Chou D., Mehl G., Say L., Cheng S., Fjeldheim I., Friberg I.K., French S., Jani J.V., Kaye J., Lewis J., Lunde A., Morkrid K., Nankabirwa V., Nyanchoka L., Stone H., Venkateswaran M., Wojcieszek A.M., Temmerman M., Flenady V.J. eRegistries: electronic registries for maternal and child health. BMC Pregnancy Childbirth. 2016;16:11. doi: 10.1186/s12884-016-0801-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molla Y.B., Rawlins B., Makanga P.T., Cunningham M., Avila J.E., Ruktanonchai C.W., Singh K., Alford S., Thompson M., Dwivedi V., Moran A.C., Matthews Z. Geographic information system for improving maternal and newborn health: recommendations for policy and programs. BMC Pregnancy Childbirth. 2017;17:26. doi: 10.1186/s12884-016-1199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.N.F.H.S. (NFHS-3) IIPS; Jharkhand, Mumbai: 2007. International Institute for Population Sciences, India, 2005–06. [Google Scholar]
- 38.Indira R., Oumachigui A., Narayan K.A., Rajaram P., Ramalingam G. Symphysis-fundal height measurement--a reliable parameter for assessment of fetal growth. Int. J. Gynaecol. Obstet. 1990;33:1–5. doi: 10.1016/0020-7292(90)90646-3. [DOI] [PubMed] [Google Scholar]
- 39.WHO Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitam. Mineral Nutr. Inf. Syst. 2015;11(1) [Google Scholar]
- 40.Sahli H. 5th ed. 1909. Klinische Untersuchungsmethoden. Leipsic and Vienns. 845. [Google Scholar]
- 41.Gogoi M., Prusty R.M. Maternal anaemia, pregnancy complications and birth outcome: evidences from North-East India. J. North East India Stud. 2013;3:74–85. [Google Scholar]
- 42.Kumar K.J., Asha N., Murthy D.S., Sujatha M., Manjunath V. Maternal anemia in various trimesters and its effect on newborn weight and maturity: an observational study. Int. J. Prev. Med. 2013;4:193–199. [PMC free article] [PubMed] [Google Scholar]
- 43.Finkelstein J.L., Layden A.J., Stover P.J. Vitamin B-12 and perinatal health. Adv. Nutr. 2015;6:552–563. doi: 10.3945/an.115.008201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bora R., Sable C., Wolfson J., Boro K., Rao R. Prevalence of anemia in pregnant women and its effect on neonatal outcomes in Northeast India. J. Matern. Fetal Neonatal Med. 2014;27:887–891. doi: 10.3109/14767058.2013.845161. [DOI] [PubMed] [Google Scholar]
- 45.Nair M., Choudhury M.K., Choudhury S.S., Kakoty S.D., Sarma U.C., Webster P., Knight M. Association between maternal anaemia and pregnancy outcomes: a cohort study in Assam, India. BMJ Glob. Health. 2016;1 doi: 10.1136/bmjgh-2015-000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srilatha J. Prevalence of anemia in pregnant mothers and their outcome: a study in a semi urban area. Int. J. Reprod. Contracept. Obstet. Gynecol. 2017;6:4886–4889. [Google Scholar]
- 47.Osungbade K.O., Oladunjoye A.O. Preventive treatments of iron deficiency anaemia in pregnancy: a review of their effectiveness and implications for health system strengthening. J. Pregnancy. 2012;2012:454601. doi: 10.1155/2012/454601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taner C.E., Ekin A., Solmaz U., Gezer C., Cetin B., Kelesoglu M., Erpala M.B., Ozeren M. Prevalence and risk factors of anemia among pregnant women attending a high-volume tertiary care center for delivery. J. Turk. Ger. Gynecol. Assoc. 2015;16:231–236. doi: 10.5152/jtgga.2015.15071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asrie F. Prevalence of anemia and its associated factors among pregnant women receiving antenatal care at Aymiba Health Center, Northwest Ethiopia. J. Blood Med. 2017;8:35–40. doi: 10.2147/JBM.S134932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ebuy Y., Alemayehu M., Mitiku M., Goba G.K. Determinants of severe anemia among laboring mothers in Mekelle city public hospitals, Tigray region, Ethiopia. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Esike O.C., Anozie B.C., Onoh C.R., Sunday U.C., Nwokpor O.S., Umeora O.U.J. The prevalence of anemia in pregnancy at booking in Abakaliki, Nigeria. Trop. J. Obstet. Gynaecol. 2016;33:332–336. [Google Scholar]
- 52.Rahmati S., Delpishe A., Azami M., Hafezi Ahmadi M.R., Sayehmiri K. Maternal Anemia during pregnancy and infant low birth weight: a systematic review and meta-analysis. Int. J. Reprod. Biomed. (Yazd) 2017;15:125–134. [PMC free article] [PubMed] [Google Scholar]
- 53.Siu A.L. Screening for iron deficiency anemia and iron supplementation in pregnant women to improve maternal health and birth outcomes: U.S. preventive services task force recommendation statement. Ann. Intern. Med. 2015;163:529–536. doi: 10.7326/M15-1707. [DOI] [PubMed] [Google Scholar]
- 54.Basu S.M. Anaemia and pregnancy. In: Gupta S., editor. Obstetric Anaesthesia. 1st ed. Arya Publications; Delhi: 2004. pp. 433–456. [Google Scholar]
- 55.Rutter T.W., Tremper K.K. The physiology of oxygen transport and red cell transfusion. In: Thomas E.J., Healy K.P.R., editors. Wylie and Churchill-Davidson’s Anesthesia. 7th ed. Arnold; London: 2003. pp. 167–183. [Google Scholar]
- 56.Wallace D.F. The regulation of iron absorption and homeostasis. Clin. Biochem. Rev. 2016;37:51–62. [PMC free article] [PubMed] [Google Scholar]
- 57.Derso T., Abera Z., Tariku A. Magnitude and associated factors of anemia among pregnant women in Dera District: a cross-sectional study in Northwest Ethiopia. BMC Res. Notes. 2017;10:359. doi: 10.1186/s13104-017-2690-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumar S., Shen J., Zolnik B., Sadrieh N., Burgess D.J. Optimization and dissolution performance of spray-dried naproxen nano-crystals. Int. J. Pharm. 2015;486:159–166. doi: 10.1016/j.ijpharm.2015.03.047. [DOI] [PubMed] [Google Scholar]
- 59.Maiti S., Unisha S., Agarwal P.K. Health care and health among tribal women in Jharkhand: a situational analysis. Stud. Tribes Tribals. 2005;3:37–46. [Google Scholar]
- 60.Yi S.W., Han Y.J., Ohrr H. Anemia before pregnancy and risk of preterm birth, low birth weight and small-for-gestational-age birth in Korean women. Eur. J. Clin. Nutr. 2013;67:337–342. doi: 10.1038/ejcn.2013.12. [DOI] [PubMed] [Google Scholar]
- 61.Hisano M., Suzuki R., Sago H., Murashima A., Yamaguchi K. Vitamin B6 deficiency and anemia in pregnancy. Eur. J. Clin. Nutr. 2010;64:221–223. doi: 10.1038/ejcn.2009.125. [DOI] [PubMed] [Google Scholar]
- 62.Viana M.B. Anemia and infection: a complex relationship. Rev. Bras. Hematol. Hemoter. 2011;33:90–92. doi: 10.5581/1516-8484.20110024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kwaan H.C. Infection and anemia. Infect. Disord. Drug Targets. 2011;11:40–44. doi: 10.2174/187152611794407791. [DOI] [PubMed] [Google Scholar]
- 64.Dotters-Katz S.K., Grotegut C.A., Heine R.P. The effects of anemia on pregnancy outcome in patients with pyelonephritis. Infect. Dis. Obstet. Gynecol. 2013;2013:780960. doi: 10.1155/2013/780960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McClure E.M., Meshnick S.R., Mungai P., Malhotra I., King C.L., Goldenberg R.L., Hudgens M.G., Siega-Riz A.M., Dent A.E. The association of parasitic infections in pregnancy and maternal and fetal anemia: a cohort study in coastal Kenya. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0002724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tay S.C., Nani E.A., Walana W. Parasitic infections and maternal anaemia among expectant mothers in the Dangme East District of Ghana. BMC Res. Notes. 2017;10:3. doi: 10.1186/s13104-016-2327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waldorf K.M.A., McAdams R.M. Influence of infection during pregnancy on fetal development. Reproduction. 2013;5:2–20. doi: 10.1530/REP-13-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thurnham D.I. Macular zeaxanthins and lutein -- a review of dietary sources and bioavailability and some relationships with macular pigment optical density and age-related macular disease. Nutr. Res. Rev. 2007;20:163–179. doi: 10.1017/S0954422407842235. [DOI] [PubMed] [Google Scholar]
- 69.Dieckmann W.J., Wegner C.K. The blood in normal pregnancy: I. Blood and plasma volumes. Arch. Int. Med. 1934;53 [Google Scholar]
- 70.Thomson K.J., McGregor M., Hirshemer A., Dayton Ohio, G J.G. Studies on the circulation in pregnancy. Am. J. Obs. Gyn. 1938;36:48–59. [Google Scholar]
- 71.Dieckmann W.J. Kimpton; London: 1952. The Toxaemias of Pregnancy. [Google Scholar]
- 72.Ahankari A.S., Myles P.R., Dixit J.V., Tata L.J., Fogarty A.W. Risk factors for maternal anaemia and low birth weight in pregnant women living in rural India: a prospective cohort study. Public Health. 2017;151:63–73. doi: 10.1016/j.puhe.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 73.Melku M., Addis Z., Alem M., Enawgaw B. Prevalence and predictors of maternal anemia during pregnancy in Gondar, Northwest Ethiopia: an Institutional based cross-sectional study. Anemia. 2014;2014:108593. doi: 10.1155/2014/108593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stephen G., Mgongo M., Hussein Hashim T., Katanga J., Stray-Pedersen B., Msuya S.E. Anaemia in pregnancy: prevalence, risk factors, and adverse perinatal outcomes in Northern Tanzania. Anemia. 2018;2018:1846280. doi: 10.1155/2018/1846280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vivek R.G., Halappanavar A.B., Vivek P.R., Halki S.B. Prevalence of anemia and its epidemiological. Determinants Pregnant Women. 2012;5:216–223. [Google Scholar]
- 76.Balarajan Y., Ramakrishnan U., Ozaltin E., Shankar A.H., Subramanian S.V. Anaemia in low-income and middle-income countries. Lancet. 2011;378:2123–2135. doi: 10.1016/S0140-6736(10)62304-5. [DOI] [PubMed] [Google Scholar]
- 77.Steer P.J. Maternal hemoglobin concentration and birth weight. Am. J. Clin. Nutr. 2000;71 doi: 10.1093/ajcn/71.5.1285s. 1285S–7S. [DOI] [PubMed] [Google Scholar]
- 78.Blankson M.L., Goldenberg R.L., Cutter G., Cliver S.P. The relationship between maternal hematocrit and pregnancy outcome: black-white differences. J. Natl. Med. Assoc. 1993;85:130–134. [PMC free article] [PubMed] [Google Scholar]
- 79.Muhihi A., Sudfeld C.R., Smith E.R., Noor R.A., Mshamu S., Briegleb C., Bakari M., Masanja H., Fawzi W., Chan G.J. Risk factors for small-for-gestational-age and preterm births among 19,269 Tanzanian newborns. BMC Pregnancy Childbirth. 2016;16:110. doi: 10.1186/s12884-016-0900-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kozuki N., Lee A.C., Silveira M.F., Sania A., Vogel J.P., Adair L., Barros F., Caulfield L.E., Christian P., Fawzi W., Humphrey J., Huybregts L., Mongkolchati A., Ntozini R., Osrin D., Roberfroid D., Tielsch J., Vaidya A., Black R.E., Katz J. The associations of parity and maternal age with small-for-gestational-age, preterm, and neonatal and infant mortality: a meta-analysis. BMC Public Health. 2013;13(Suppl. 3):S2. doi: 10.1186/1471-2458-13-S3-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang G., Bacelis J., Lengyel C., Teramo K., Hallman M., Helgeland O., Johansson S., Myhre R., Sengpiel V., Njolstad P.R., Jacobsson B., Muglia L. Assessing the causal relationship of maternal height on birth size and gestational age at birth: a mendelian randomization analysis. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raju T.N. Moderately preterm, late preterm and early term infants: research needs. Clin. Perinatol. 2013;40:791–797. doi: 10.1016/j.clp.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Banerjee J., Asamoah F.K., Singhvi D., Kwan A.W., Morris J.K., Aladangady N. Haemoglobin level at birth is associated with short term outcomes and mortality in preterm infants. BMC Med. 2015;13:16. doi: 10.1186/s12916-014-0247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Savajols E., Burguet A., Grimaldi M., Godoy F., Sagot P., Semama D.S. Maternal haemoglobin and short-term neonatal outcome in preterm neonates. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lu Z.M., Goldenberg R.L., Cliver S.P., Cutter G., Blankson M. The relationship between maternal hematocrit and pregnancy outcome. Obstet. Gynecol. 1991;77:190–194. doi: 10.1097/00006250-199102000-00005. [DOI] [PubMed] [Google Scholar]
- 86.Bhutta Z.A., Black R.E. Global maternal, newborn, and child health--so near and yet so far. N. Engl. J. Med. 2013;369:2226–2235. doi: 10.1056/NEJMra1111853. [DOI] [PubMed] [Google Scholar]
- 87.Newnham J.P., Dickinson J.E., Hart R.J., Pennell C.E., Arrese C.A., Keelan J.A. Strategies to prevent preterm birth. Front. Immunol. 2014;5:584. doi: 10.3389/fimmu.2014.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kumar R.K., Singhal A., Vaidya U., Banerjee S., Anwar F., Rao S. Optimizing nutrition in preterm low birth weight infants-consensus summary. Front. Nutr. 2017;4:20. doi: 10.3389/fnut.2017.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Y., Zhou H., Perkins A., Wang Y., Sun J. Maternal dietary nutrient intake and its association with preterm birth: a case-control study in Beijing, China. Nutrients. 2017;9 [Google Scholar]
- 90.Gernand A.D., Paul R.R., Ullah B., Taher M.A., Witter F.R., Wu L., Labrique A.B., West K.P., Jr., Christian P. A home calendar and recall method of last menstrual period for estimating gestational age in rural Bangladesh: a validation study. J. Health Popul. Nutr. 2016;35:34. doi: 10.1186/s41043-016-0072-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Terrin G., Berni Canani R., Di Chiara M., Pietravalle A., Aleandri V., Conte F., De Curtis M. Zinc in early life: a key element in the fetus and preterm neonate. Nutrients. 2015;7:10427–10446. doi: 10.3390/nu7125542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vinturache A., McKeating A., Daly N., Sheehan S., Turner M. Maternal body mass index and the prevalence of spontaneous and elective preterm deliveries in an Irish obstetric population: a retrospective cohort study. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-015258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kosa J.L., Guendelman S., Pearl M., Graham S., Abrams B., Kharrazi M. The association between pre-pregnancy BMI and preterm delivery in a diverse southern California population of working women. Matern. Child Health J. 2011;15:772–781. doi: 10.1007/s10995-010-0633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Haider B.A., Olofin I., Wang M., Spiegelman D., Ezzati M., Fawzi W.W. Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. 2013;346:f3443. doi: 10.1136/bmj.f3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Q., Ananth C.V., Rhoads G.G., Li Z. The impact of maternal anemia on perinatal mortality: a population-based, prospective cohort study in China. Ann. Epidemiol. 2009;19:793–799. doi: 10.1016/j.annepidem.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 96.Ren A., Wang J., Ye R.W., Li S., Liu J.M., Li Z. Low first-trimester hemoglobin and low birth weight, preterm birth and small for gestational age newborns. Int. J. Gynaecol. Obstet. 2007;98:124–128. doi: 10.1016/j.ijgo.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 97.Klebanoff M.A. Paternal and maternal birthweights and the risk of infant preterm birth. Am. J. Obstet. Gynecol. 2008;198:58. doi: 10.1016/j.ajog.2007.06.013. e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data available with the submitted manuscript.