Abstract
Introduction
The central regulatory system that generates biological rhythms is regulated by circadian clock genes expressed by cells in the suprachiasmatic nucleus. Signals from this system are converted to adrenocortical hormones through the sympathetic nervous system and transmitted to peripheral organs. Another system releases glucocorticoids (GCs) in response to stress through the HPA-axis. Here we investigated the second messenger GC, which is shared by these systems and influences the expression of circadian clock genes of cells of the musculoskeletal system and in viable bone tissue.
Methods
We used mouse-derived cell lines, which differentiate into osteoblasts (MC3T3-E1, C2C12, and 10T1/2) as well as primary cultures of mouse osteoblasts to determine the expression levels of circadian clock genes that respond to GC. Mice (mPer2m/m) with an inactivating mutation in the period circadian clock 2 gene (Per2) exhibit marked dysrhythmia. Here we compared the bone morphologies of mPer2m/m mice with those of wild-type (WT) mice.
Results
The expression of major circadian clock genes was detected in each cell line, and their responsiveness to GC was confirmed. We focused on Per2, a negative regulator of the circadian clock and found that a Per2-loss-of-function mutation increased the proliferative capacity of osteoblasts. Treatment of mutant mice with slow-release GC and bisphosphonate affected the maturation of bone tissue, which reflects a tendency to retard calcification.
Conclusion
Our investigations of the mechanisms that regulate circadian rhythm function in tissues of the musculoskeletal system that respond to the stress hormone GC, reveal that Per2 is required for the maturation of bone tissue. Thus, the influences of the systems that control circadian rhythms and the responses to stress by regenerating tissue used for regenerative medicine must be considered and studied in greater detail.
Keywords: Circadian rhythm, Glucocorticoids, Period circadian clock 2 gene, Second messenger
Abbreviations: CRH, corticotropin-releasing hormone; ACTH, adrenocorticotropic hormone; HPA-axis, hypothalamic-pituitary-adrenal-axis; ASPS, advanced sleep phase syndrome; BMSCs, bone marrow stem cells; BV/TV, bone volume/tissue volume; OV/TV, Osteoid volume/Tissue volume; OV/BV, Osteoid volume/ Bone volume; OS/BS, Osteoid surface/ Bone surface; Tb.Th, Trabecular thickness; ES/BS, Eroded surface/ Bone surface; MS/OS, Mineralizing surface/Osteoid surface; G.P.Th, growth plate thickness
Highlights
-
•
Circadian clock genes expressed in musculoskeletal cells respond to GCs.
-
•
Per2, a negative regulatory gene, influences the proliferation of osteoblasts.
-
•
Circadian rhythms and GCs affect bone maturation and may control regenerating tissues.
1. Introduction
The 2017 Nobel Prize in Physiology or Medicine was awarded to researchers who identified genes responsible for controlling the circadian rhythm and their mechanisms of action (https://www.nobelprize.org/prizes/medicine/2017/summary/). The central control system generating circadian rhythm resides in the suprachiasmatic nucleus (SCN) and is regulated by factors expressed by circadian clock genes through negative feedback mechanisms [1], [2]. The master regulators of the circadian clock system CLOCK and ARNTL/BMAL1 form a heterodimer and bind to an enhancer sequence called the E box in circadian clock genes such as Per and Cry to promote their transcription. Proteins encoded by Per (PER1, 2, and 3) and Cry (CRY1 and 2) form a complex that suppresses transcriptional activation by the CLOCK–ARNTL complex to generate the core loop of a circadian rhythm [1], [2].
Circadian clock genes are intimately associated with sleep. For example, a mutation in Per2 causes a sleep disorder, advanced sleep phase syndrome (ASPS), which is inherited and caused by a missense mutation (S662G) in PER2, which alters the circadian period [3]. Further, Per2 function is associated with other pathologies. For example, Per2 is involved in suppressing tumors through other genes that affect cell proliferation and death such as cyclin-dependent kinase 1, cyclin A1, transformed mouse 3T3 cell double minute 2, and growth arrest and DNA-damage-inducible 45 alpha [4]. Therefore, disruption of the circadian clock machinery may increase the risk of developing cancer [4], [5]. Further, Per2 regulates time-of-day-dependent variation in the passive cutaneous anaphylactic reaction of mice by controlling the rhythmic secretion of glucocorticoids (GCs) from the adrenal glands, gating the responses to GCs of mast cells at certain times of day, or both [6]. These findings suggest that the circadian clock system is intimately involved in allergic diseases such as asthma, allergic rhinitis, and chronic urticaria through its effects on GC levels.
Adrenocortical hormones are secreted in a rhythmic manner. The amplitudes of their levels undergo circadian variations during intervals of approximately 24 h [7]. The phase of the rhythm in humans peaks upon waking, while the phase in mice is nocturnal [8]. The circadian signal emitted by the SCN is regulated through preganglionic sympathetic neurons in the spinal cord [9]. GCs subsequently produced by the adrenal cortex serve as messengers to transmit the clock signal to peripheral cells. Thus, the signaling pathway through which the activity of the SCN-sympathetic nerves, which function as the central control system of rhythm, is altered to produce GCs, and the rhythmic input signal of GCs is transmitted to cells distributed throughout the body [5]. In contrast, GCs produced by the adrenal cortex play a central role in host defenses in response to stress, and together with mineral corticoids and androgens, mediate the regulation of electrolyte levels, blood pressure, and sugar metabolism [10]. The secretion of GCs under stress is controlled by the so-called hypothalamic pituitary adrenal (HPA) axis, whose components are located in the hypothalamus, which releases corticotropin-releasing hormone (CRH), which in turn binds to a receptor that mediates the release of adrenocorticotrophic hormone (ACTH) from adenohypophysial cells [11]. ACTH facilitates the secretion of GCs (cortisol in humans and corticosteroids in rodents) from the adrenal cortex, independent of SCN-sympathetic nerves [11].
Evidence indicates that GCs act directly and indirectly in bone tissue to cause osteoporosis [reviewed in 12]. However, we are aware of only a few studies [13], [14], [15], [18] on the interactions of bone with circadian clock genes. Moreover, the expression of clock genes in osteoblasts is regulated by the sympathetic nervous system and the hormone leptin, which regulates bone remodeling, a homeostatic function that maintains a constant bone mass [13]. In homozygous Arntl/Bmal1-deficient (Arntl−/−) mice, osteoclast differentiation is increased by downregulating the expression of osteoprotegerin [14]. Further, osteoclast-specific Arntl/Bmal1-knockout mice have increased bone mass because of diminished osteoclast differentiation [15], and ARNTL/BMAL1-deficient osteoblasts have a higher ability compared with wild-type (WT) mice to support osteoclastogenesis by inducing the synthesis of RANKL, which is an endogenous osteoclast-activating factor secreted mainly by osteoblasts and activated T cells [16], [17]. These results suggest that bone resorption and mass are control by a complex mechanism employed by the osteoblastic Clock system [18]. These studies of the association of ARNTL/BMAL1 with bone metabolism include insufficient data about the influence of PER2 on bone tissues. Here we used Per2-mutant mice (mPer2m/m) to determine how GCs, the second messengers that regulate rhythmic and stress responses, influence the expression of circadian clock genes of cells that regulate bone homeostasis, and assessed the effects of PER2 on bone tissue.
2. Methods
2.1. Cell culture
The mouse preosteoblastic cell line MC3T3-E1, the premyoblastic cell line C2C12, and the fibroblast-like cell line 10T1/2 were cultured in α-modified Minimum Essential Medium (α-MEM) (WAKO, Osaka, Japan) with 10% fetal bovine serum (FBS) (BioWest, Nuaillé, France); DMEM with 10% FBS, Basal medium Eagle (BME) with 10% FBS; RPMI 1640 with 10% FBS; and α-MEM with 10% FBS and 50 μg/ml M-CSF, respectively. The growth- and conditioned media were changed every three days. All cultures were maintained at 37 °C in humidified air in an atmosphere containing 5% CO2 and were passaged every 7 days. Dr. T. Ogasawara provide the MC3T3-E1 and 10T1/2 cell lines were obtained from the RIKEN BRC-Cell bank (Tsukuba, Japan).
2.2. Mice
Animal experiments were conducted according to the ARRIVE Guidelines for Reporting Animal Research [19] as previously described [20]. Adult female (15 weeks old) Per2 mutant mice (mPer2m/m) [21] with an ICR background and the same strain of wild-type mice were housed in a temperature-controlled (24 ± 1 °C) room under a 12-h light:12-h dark cycle. The mPer2m/m mice carry an in-frame deletion in the sequence encoding the PER-ARNT-SIM–B (PAS) domain and are deficient in mPer2-mediated transcriptional regulation [21]. These mice were provided by S. Shibata. Primary murine osteoblasts (pOBs) were isolated from the skulls of newborn mice. Neonatal mouse skulls were dissected free of adherent soft tissue, washed in PBS, and sequentially digested with 0.2% dispase and 0.1% collagenase. The experiments were conducted according to the institutional ethical guidelines for research involving animals. The Institutional Review Board of Saitama Medical University approved this study.
2.3. RT-PCR analysis of clock gene expression
Confluent cultures were incubated in the presence of 0–103 nM dexamethasone (Dex) for 0–8 days. Total RNA (2 μg) was extracted from cells using ISOGEN (Nippon Gene, Tokyo, Japan) and subjected to RT-PCR using a SuperScript One-Step RT-PCR System with a Platinum Taq Kit (Invitrogen, California, USA) according to the manufacturer's instructions. Gene-specific primer pairs are shown in Table 1. For RT-PCR analysis, cDNA synthesis was performed for 30 min at 45 °C, and the products were denatured for 2 min at 94 °C. PCR amplification was performed as follows: 26 cycles of denaturation for 60 s at 94 °C with primer annealing for 90 s at 48 °C (Arntl/Bmal1), 50 °C (Per1, Per3, Cry1, and Cry2) and 55 °C (Per2, Clock); and extension for 180 s at 72 °C. Gapdh mRNA was used as a loading control. Expression of mRNA was detected using a semiquantitative RT-PCR assay. The PCR conditions were determined so that the band intensity was a linear function of the number of cycles. Bands were quantified using a LAS-3000mini luminescent image analyzer (Fuji Film, Japan), and the intensities were normalized to those of Gapdh.
Table 1.
RT-PCR primers.
| Genes | Upstream (5′-3′) | Downstream (5′-3′) |
|---|---|---|
| Per1 | ccatggacatgtctact | agaggaccaggggacat |
| Per2 | ctacctggtcaaggtgcaagag | ggtttgaatcttgccactgg |
| Per3 | tcctgatggtaagacattccag | gcgtgaacaatcacactcactt |
| Cry1 | cgtctgtttgtgattcgggg | attcacgccacaggagttgc |
| Cry2 | agaaggtgaagaggaacagcac | tagatgtatcgagaggggaagc |
| Arntl/Bmal1 | tcaagaatgcaagggaggcc | aacaggtagaggcgaagtcc |
| Clock | ctatgcttcctggtaacgcg | gcctattattggtggtgccc |
| Gapdh | tgaaggtcggtgtgaacggatttggc | tgaaggtcggtgtgaacggatttggc |
2.4. Cell proliferation
Cells (4 × 103 cells per well) were added to the wells of 96-well plates. To assess the effects of Dex on pOBs derived from WT and mPer2m/m mice, the cells were incubated in growth medium with 100 nM Dex in the presence or absence of 100 μM zoledronic acid (ZA). Concentrations were determined the approximate value required to express clock genes by the dilution method using concentrations between 1 and 1000 (DEX: nM, ZA: μM). The sample cells were quantified using a Cell Titer 96 Aqueous One Solution Cell Proliferation Assay (Promega, USA), according to the manufacturer's instructions. Briefly, 20 μl of MTS was added to 100 μl of culture medium. After incubation for 2 h at 37 °C, the absorbance was measured at 490 nm using a Model 680 XR plate reader (BIO RAD, USA). The measurements represent the mean of at least three independent experiments (six replicates per data point).
2.5. Experiments using mice
Slow-release pellets (Innovative Research of America, Sarasota, FL) containing prednisolone (PSL) (15 mg/21 day) were implanted into the lateral side of the neck according to the manufacturer's protocol. Bisphosphonate (BP), in the form of ZA, was provided by Novartis Pharma AG (Basel, Switzerland). We treated WT and mPer2m/m mice with once-weekly subcutaneous injections of 100 μg/kg ZA. PSL was released from pellets implanted into the mice for 21 days, and the weekly injections of ZA continued for 21-days. ZA inhibits bone resorption by inducing osteoclasts to undergo apoptosis and was therefore used to assess osteogenic capacity.
2.6. Microfocus X-ray computed tomography (μ-CT)
Microfocus X-ray computed tomography (μCT) (ELE SCAN; Nittetsu Elex Co. Ltd., Tokyo, Japan), performed according to the manufacturer's protocol, was used to obtain cross-sectional views of the secondary spongiosa in the distal tibial metaphysis, approximately 0.28 mm distal to the growth plate. μCT settings were as follows: energy, 67.0 kV; current, 100 μA; and slice thickness, 13.09 μm (n = 4 mice per group). Three-dimensional (3D) digital images were reconstructed using above software.
2.7. Bone histomorphometry
Bone specimens were fixed, left undecalcified, and embedded in a volume of ethanol (70–80%), which was 25-times the volume of the sample, as previously described [20]. Calcein (16 mg/kg) (Dojindo, Kumamoto, Japan) was injected intraperitoneally 4 days and 1 day before the mice were killed. The dose for subcutaneous administration was 0.1 mL/10 g body weight. A computer and digitizer tablet (Histometry RT Camera; System Supply Co., Ltd., Nagano, Japan) were used for histomorphometric analysis, which was conducted by the Niigata Bone Science Institute (Niigata, Japan). The nomenclature, symbols, and units follow the recommendations of the Nomenclature Committee of the American Society for Bone and Mineral Research [22]. The perimeter, trabecular thickness (Tb.Th, mcm) is structural indices of cancellous bone tissue. We calculated the bone formation parameters as follows: bone volume (BV/TV, %), osteoid volume (OV/TV, %; OV/BV, %), and osteoid surface (OS/BS, %) were calculated. The bone resorption parameters were as follows: the amount of eroded surface (ES/BS, %) calculated as a percentage of the total bone surface. Mineralizing surface (MS/OS, %) is kinetic indices of cancellous bone tissue. We calculated the dynamic histomorphometric parameters of the growing long bone metaphysis, the growth plate thickness (G.P.Th, μm).
2.8. Statistical analysis
Results are expressed as the mean ± SEM of three replicates, comparisons were performed using one-way analysis of variance or the Student t test, and p < 0.05 indicates a significant difference.
3. Results
3.1. Effect of Dex on the expression of circadian clock genes in vitro
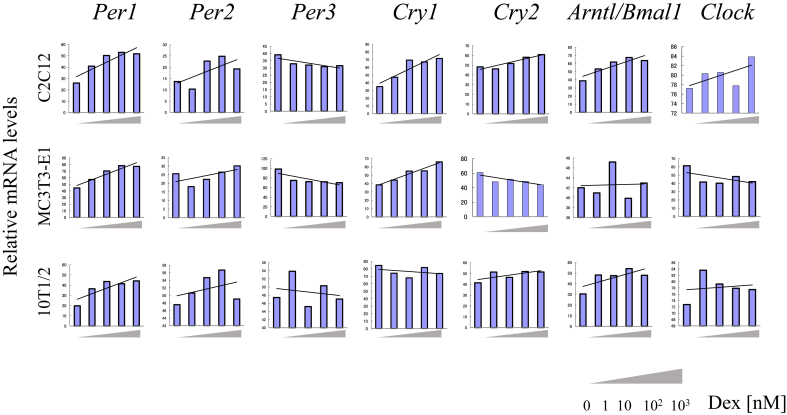
The relevant phenotypes of the cell lines analyzed for their transcriptional responses to Dex are as follows: The osteoblast-like cell line MC3T3-E1 differentiates into osteoblasts [23]. The C2C12 cell line exhibits a myoblast-like phenotype [24]. The 10T1/2 cell line exhibits fibroblast-like characteristics [25]. Generally, the levels of Per1, Per2, and Cry1 mRNAs increased as a function increasing Dex concentrations in MC3T3-E1 and C2C12 cells, whereas the levels of Per3 mRNA decreased. The levels of Cry2, Arnt/Bmal1, and Clock mRNAs did not change in a manner that demonstrated a convincing effect of Dex concentrations. In undifferentiated 10T1/2 cells, the levels of mRNAs encoding circadian clock genes varied, except those of Per1 and Per2 (Fig. 1).
Fig. 1.
Analysis of the expression of circadian clock genes in mouse cell lines treated with dexamethasone (Dex). A. RT-PCR analysis of mRNAs encoding Clock proteins expressed by the C2C12, MC3T3-E1, and 10T1/2 cell lines. Cultures were treated with 0–103 nM Dex.
3.2. Expression of circadian clock genes in pOBs of WT and mPer2m/m mice
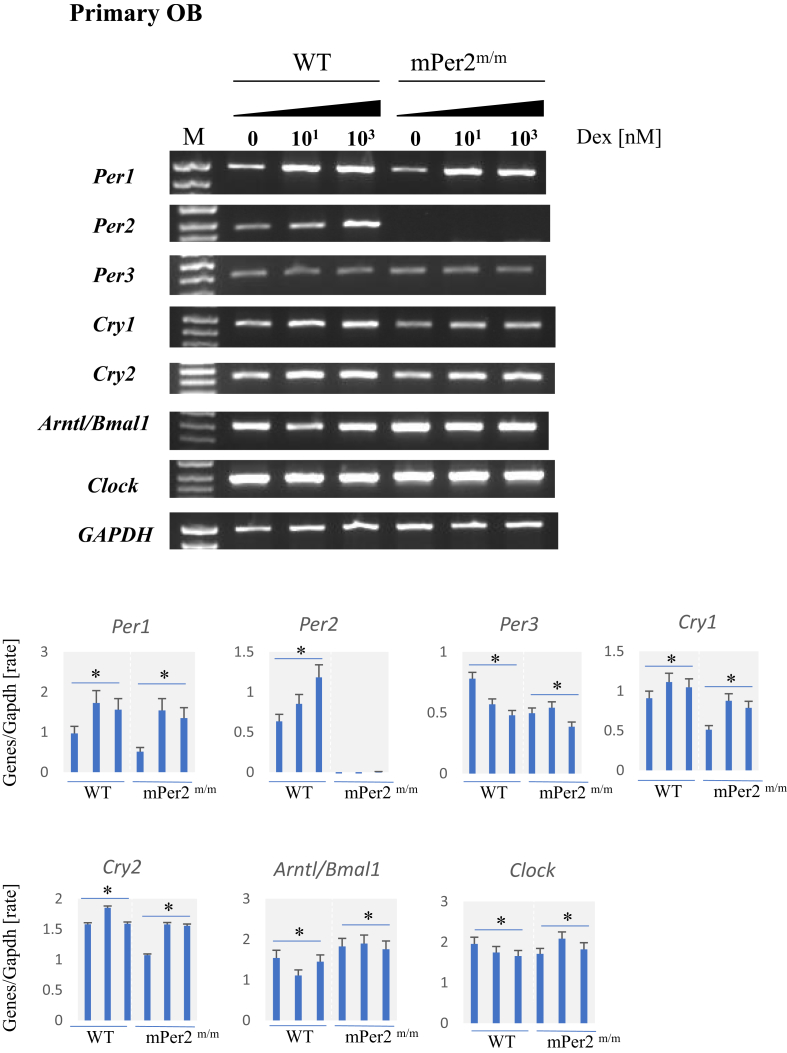
In the primary culture of osteoblasts from WT and mPer2m/m mice, the levels of Per1, Per2(only in those of WT) and Cry1 mRNAs generally increased, according to the increase in concentrations of Dex, whereas the levels of Per3 mRNA decreased. These data are consistent with those of the cell lines (Section 2.2). The quantification using the luminescent image analyzer also showed that the expression patterns of circadian clock genes in mPer2m/m mice were almost similar to those of WT mice (Fig. 2).
Fig. 2.
Analysis of the expression of circadian clock genes in primary cultures of osteoblasts of wild-type (WT) and mPer2m/m mice treated with Dex. Top panel. Agarose gel electrophoresis of Clock gene expression in primary osteoblast (pOB) cultures treated with different concentrations of Dex. Bottom panel. Quantitation of mRNA levels normalized to those of Gapdh mRNA (*p < 0.05).
3.3. Osteoblast proliferation and inhibition by Dex and ZA
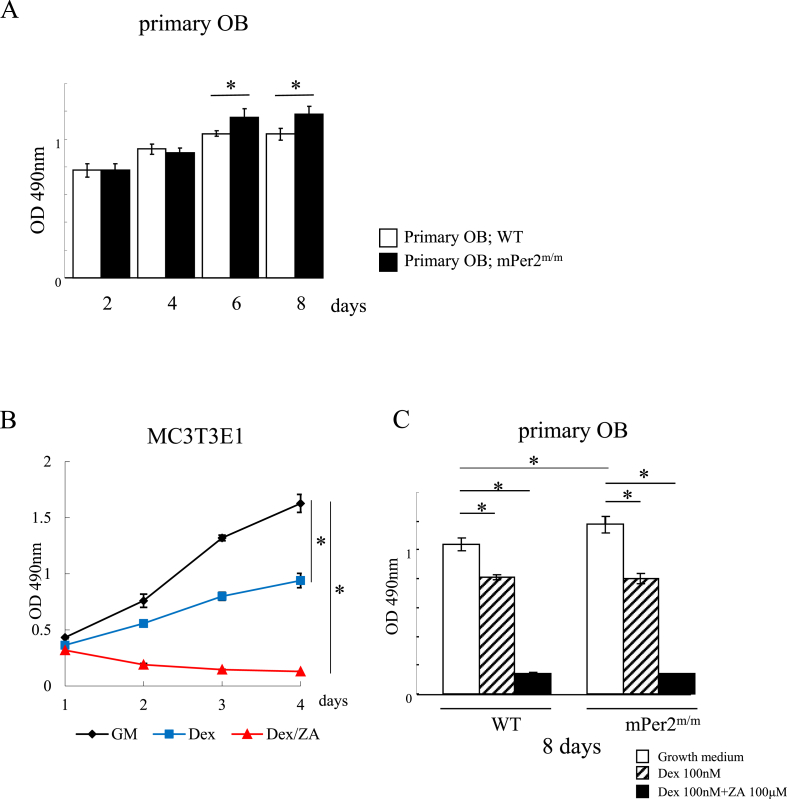
The proliferation of pOBs isolated from WT and mPer2m/m mice differed, starting on day 6 (Fig. 3A). The proliferative capacity of pOBs derived from mPer2m/m mice was significantly higher. Fig. 3B shows that the proliferation of MC3T3-E1 cells was significantly inhibited (approximately 2-fold) by the administration of 100 nM Dex. Further addition of 100 μM ZA completely inhibited proliferation. The proliferative capacity of pOBs from WT and mPer2m/m mice was also inhibited by approximately 25% by Dex and completely inhibited when mice were administered Dex combined with ZA, consistent with the data for MC3T3-E1 cells (Fig. 3C).
Fig. 3.
Analysis of the proliferation of osteoblast. A. Proliferation of pOBs of wild-type (WT) and mPer2m/m mice (*p < 0.05). B. Proliferation of MC3T3-E1 cells in the presence and absence of 100 nM each of Dex and zoledronic acid (ZA). Growth medium without supplements (black line), with Dex (blue line), and Dex plus ZA (red line). C. Proliferation of pOBs of WT and mPer2m/m mice after 8 days in culture in the absence or presence (100 nM each) of Dex, ZA, and Dex plus ZA (*p < 0.05).
3.4. μ-CT morphometric analysis and bone histomorphometry
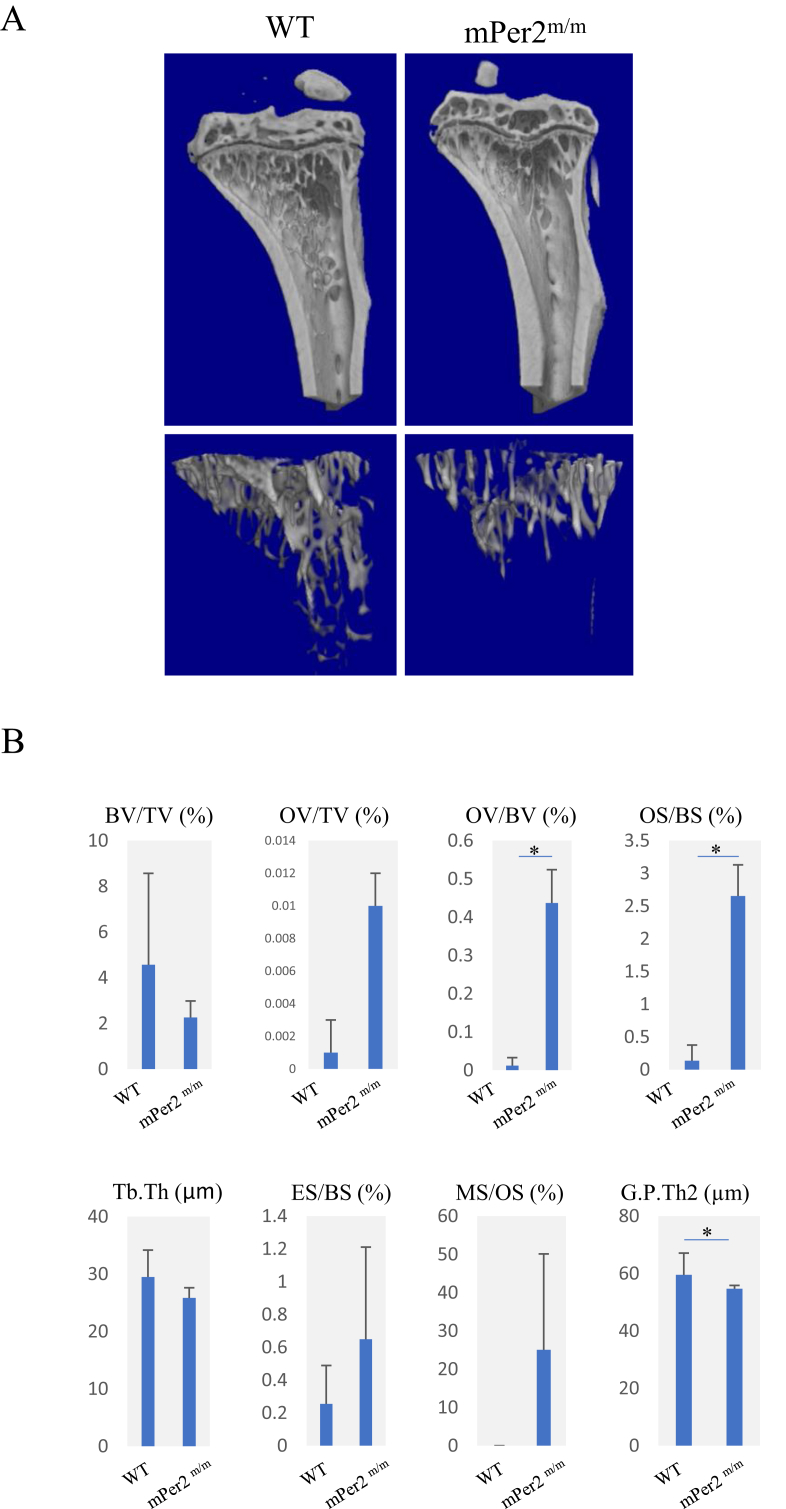
To evaluate osteogenic capacity, we treated the mice with PSL and ZA, which inhibits osteoblast proliferation (Fig. 3C). Representative 3-D images of bone structures in the tibiae of WT and mPer2m/m mice treated with PSL and ZA are shown in Fig. 4A. Compared with the trabeculae of WT mice treated with both ZA and PSL, those in PSL/ZA-treated mPer2m/m mice were smaller (Fig. 4A, lower panels). Bone morphometry analysis (Fig. 4B) showed that although PSL/ZA-treated mPer2m/m mice had tended to decrease BV/TV than compared with PSL/ZA-treated WT, the OV/BV and OS/BS values of PSL/ZA-treated mPer2m/m mice seemed higher compared with those of WT, suggesting that PSL/ZA-treated mPer2m/m mice showed a tendency to retard calcification.
Fig. 4.
Morphometric analysis of the fibulae of WT and mPer2m/m treated with ZA. ZA was administered daily for 21 days to WT and mPer2m/m mice injected with slow-release pellets containing prednisolone (PSL). A. μ-CT 3D images of fibulae. Left: sagittal view (upper panel) and axial view (lower panel) of WT mice demonstrating the usual cortical bone and trabeculae. Right: sagittal view (upper panel) and axial view (lower panel) of mPer2m/m mice revealing more thicker cortical bone and smaller trabeculae than those of WT. B. Morphometric analysis of the fibulae of WT and mPer2m/m mice treated with ZA (*p < 0.05).
4. Discussion
Increases in the levels of stress hormones, particularly in people whose cortisol response to the administration of CRH is increased, suffer from sleep abnormalities, which are worsened in association with the exacerbation of stress symptoms [10]. These findings suggest that the normal response to stress, which is controlled by the HPA-axis, is required for normal sleep. The presence of abnormal activity rhythms in mPer2-deficient mice demonstrate that the GC rhythm is absent. Further, diurnal feeding rhythm is undetectable in mPer2-deficient mice [26]. Inadequate sleep leads to increased appetite, obesity [24], and increased mortality [25], demonstrating the deep relationship between sleep disorders and stress, which significantly affects the body, although stress is not addressed by these studies. However, the responses to restraint stress, hypoglycemia, and ACTH injection are intact in mPer2 deficient mice [26]. Therefore, Per2 may not significantly influence the regulation of the activity of the HPA-axis, at least in rodents. In contrast, the Per1 promoter region harbors a GC response element (GRE), and the transcription of Per1 is controlled by GC in hepatic cells (HepG2) [27]. Further, GC signaling resets the circadian cycle in peripheral tissues as described in above [11]. In our present study of osteogenic cells, we show that mPer2 responses to GC were similar to those of mPer1. The GRE-like region may therefore exist in Per2.
The regulation of cartilage differentiation is mediated by direct stimulation of mPer1 expression by PTH through a protein kinase A-CREB-dependent mechanism in chondrocytes, which regulates the subsequent Arntl/Clock-dependent synthesis of the extracellular matrix [28], [29]. Further, circadian clock genes are expressed by chondrocytes to regulate the synthesis of the extracellular matrix in cartilage [28], and the promoter activity of the Indian hedgehog gene (Ihh) is regulated by PER1 and ARNTL/BMAL1, which are coordinately expressed in the growth plate [29]. A deficit in ARNTL/BMAL1 expression leads to the disruption of the rhythmic expression profiles of Per1 and Ihh in the growth plate [29]. IHH controls the differentiation and proliferation of growth plate chondrocytes [30]. These findings indicate that endochondral ossification may be regulated by the Clock gene product PER1 expressed in chondrocytes during postnatal skeletogenesis through a mechanism that regulates the rhythmic expression of Ihh [29]. We found that the concomitant use of BP and GC changes the concentration of RANKL in the bloodstream and that the epiphyseal growth plates widen in the femurs of mice administered BP because of the expansion of hypertrophic chondrocyte layers [20], [31]. Here we show that in the absence of Per2 expression, the characteristic widening of growth plates was suppressed and the trabeculae decreased further (Fig. 4), suggesting their involvement in bone growth related to endochondral ossification.
The loss of Per2 expression causes abnormalities of erythrocytes and impairs oxygen transport, which significantly shortens the life span of erythrocytes [32]. Therefore, PER2 function in the bone marrow may influence the regulation of life span of circulating erythrocytes. Recently, Zhou et al. (2018) reported that osteogenic differentiation of bone marrow stem cells (BMSCs) in vitro is enhanced when RNA interference techniques are used to inhibit the expression of Arntl/Bmal1 or Per2, indicating that ARNTL/BMAL1 and PER2 negatively regulate the osteogenic potential of BMSCs and may have a synergistic effect on the osteogenic differentiation of BMSCs [33]. We show here that the proliferation of osteoblast derived from mPer2m/m mice was enhanced compared with those of WT mice, suggesting that an absence of PER2 shortens the cell cycle and the biological clock. Increasing calcification in untreated mPer2m/m mice was repressed by treatment with PSL, thus inhibiting the generation of viable, mature bone that frames the trabecula in a cancellous bone. Therefore, these findings suggest that Per2 regulates bone homeostasis to contribute to the stabilization of osseous tissue. Future molecular genetic studies should focus on revealing the complex mechanisms that interact to regulate the expression of circadian clock genes in vivo.
5. Conclusions
The current study demonstrates that bone metabolism is influenced by Per2. To advance regenerative medicine, it is important to deepen our understanding of the biology of sleep, circadian rhythms, and tissue repair [34].
Acknowledgments
We thank Professor Shigenobu Shibata at Waseda University for providing Per2 knockout mice, and Dr. Naoko Hori for assistance. This work was supported by JSPS KAKENHI Grant Number JP17791488.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Jin X., Shearman L.P., Weaver D.R., Zylka M.J., de Vries G.J., Reppert S.M. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell. 1999;96(1):57–68. doi: 10.1016/s0092-8674(00)80959-9. [DOI] [PubMed] [Google Scholar]
- 2.Shearman L.P., Sriram S., Weaver D.R., Maywood E.S., Chaves I., Zheng B. Interacting molecular loops in the mammalian circadian clock. Science (New York, NY) 2000;288(5468):1013–1019. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- 3.Toh K.L., Jones C.R., He Y., Eide E.J., Hinz W.A., Virshup D.M. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science (New York, NY) 2001;291(5506):1040–1043. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- 4.Fu L., Pelicano H., Liu J., Huang P., Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111(1):41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- 5.Katamune C., Koyanagi S., Shiromizu S., Matsunaga N., Shimba S., Shibata S. Different roles of negative and positive components of the circadian clock in oncogene-induced neoplastic transformation. J Biol Chem. 2016;291(20):10541–10550. doi: 10.1074/jbc.M115.706481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamura Y., Harama D., Shimokawa N., Hara M., Suzuki R., Tahara Y. Circadian clock gene Period2 regulates a time-of-day-dependent variation in cutaneous anaphylactic reaction. J Allergy Clin Immunol. 2011;127(4):1038–1045. doi: 10.1016/j.jaci.2011.02.006. e1-3. [DOI] [PubMed] [Google Scholar]
- 7.Tronche F., Kellendonk C., Reichardt H.M., Schutz G. Genetic dissection of glucocorticoid receptor function in mice. Curr Opin Genet Dev. 1998;8(5):532–538. doi: 10.1016/s0959-437x(98)80007-5. [DOI] [PubMed] [Google Scholar]
- 8.Keenan D.M., Roelfsema F., Veldhuis J.D. Endogenous ACTH concentration-dependent drive of pulsatile cortisol secretion in the human. Am J Physiol Endocrinol Metab. 2004;287(4):E652–E661. doi: 10.1152/ajpendo.00167.2004. [DOI] [PubMed] [Google Scholar]
- 9.Ishida A., Mutoh T., Ueyama T., Bando H., Masubuchi S., Nakahara D. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metabol. 2005;2(5):297–307. doi: 10.1016/j.cmet.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Hori H., Teraishi T., Sasayama D., Ozeki Y., Matsuo J., Kawamoto Y. Poor sleep is associated with exaggerated cortisol response to the combined dexamethasone/CRH test in a non-clinical population. J Psychiatr Res. 2011;45(9):1257–1263. doi: 10.1016/j.jpsychires.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Balsalobre A., Brown S.A., Marcacci L., Tronche F., Kellendonk C., Reichardt H.M. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science (New York, NY) 2000;289(5488):2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 12.Suarez-Bregua P., Guerreiro P.M., Rotllant J. Stress, glucocorticoids and bone: a Review from mammals and fish. Front Endocrinol. 2018;9:526. doi: 10.3389/fendo.2018.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu L., Patel M.S., Bradley A., Wagner E.F., Karsenty G. The molecular clock mediates leptin-regulated bone formation. Cell. 2005;122(5):803–815. doi: 10.1016/j.cell.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 14.Zhou X., Yu R., Long Y., Zhao J., Yu S., Tang Q. BMAL1 deficiency promotes skeletal mandibular hypoplasia via OPG downregulation. Cell Prolif. 2018;51(5) doi: 10.1111/cpr.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu C., Ochi H., Fukuda T., Sato S., Sunamura S., Takarada T. Circadian clock regulates bone resorption in mice. J Bone Miner Res. 2016;31(7):1344–1355. doi: 10.1002/jbmr.2803. [DOI] [PubMed] [Google Scholar]
- 16.Fuller K., Wong B., Fox S., Choi Y., Chambers T.J. TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts. J Exp Med. 1998;188(5):997–1001. doi: 10.1084/jem.188.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuzaki K., Udagawa N., Takahashi N., Yamaguchi K., Yasuda H., Shima N. Osteoclast differentiation factor (ODF) induces osteoclast-like cell formation in human peripheral blood mononuclear cell cultures. Biochem Biophys Res Commun. 1998;246(1):199–204. doi: 10.1006/bbrc.1998.8586. [DOI] [PubMed] [Google Scholar]
- 18.Takarada T., Xu C., Ochi H., Nakazato R., Yamada D., Nakamura S. Bone resorption is regulated by circadian clock in osteoblasts. J Bone Miner Res. 2017;32(4):872–881. doi: 10.1002/jbmr.3053. [DOI] [PubMed] [Google Scholar]
- 19.Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6) doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abe T., Sato T., Kokabu S., Hori N., Shimamura Y., Sato T. Zoledronic acid increases the circulating soluble RANKL level in mice, with a further increase in lymphocyte-derived soluble RANKL in zoledronic acid- and glucocorticoid-treated mice stimulated with bacterial lipopolysaccharide. Cytokine. 2016;83:1–7. doi: 10.1016/j.cyto.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Zheng B., Larkin D.W., Albrecht U., Sun Z.S., Sage M., Eichele G. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400(6740):169–173. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]
- 22.Dempster D.W., Compston J.E., Drezner M.K., Glorieux F.H., Kanis J.A., Malluche H. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28(1):2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sudo H., Kodama H.A., Amagai Y., Yamamoto S., Kasai S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol. 1983;96(1):191–198. doi: 10.1083/jcb.96.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yaffe D., Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270(5639):725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- 25.Reznikoff C.A., Brankow D.W., Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973;33(12):3231–3238. [PubMed] [Google Scholar]
- 26.Yang S., Liu A., Weidenhammer A., Cooksey R.C., McClain D., Kim M.K. The role of mPer2 clock gene in glucocorticoid and feeding rhythms. Endocrinology. 2009;150(5):2153–2160. doi: 10.1210/en.2008-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koyanagi S., Okazawa S., Kuramoto Y., Ushijima K., Shimeno H., Soeda S. Chronic treatment with prednisolone represses the circadian oscillation of clock gene expression in mouse peripheral tissues. Mol Endocrinol. 2006;20(3):573–583. doi: 10.1210/me.2005-0165. [DOI] [PubMed] [Google Scholar]
- 28.Hinoi E., Ueshima T., Hojo H., Iemata M., Takarada T., Yoneda Y. Up-regulation of per mRNA expression by parathyroid hormone through a protein kinase A-CREB-dependent mechanism in chondrocytes. J Biol Chem. 2006;281(33):23632–23642. doi: 10.1074/jbc.M512362200. [DOI] [PubMed] [Google Scholar]
- 29.Takarada T., Kodama A., Hotta S., Mieda M., Shimba S., Hinoi E. Clock genes influence gene expression in growth plate and endochondral ossification in mice. J Biol Chem. 2012;287(43):36081–36095. doi: 10.1074/jbc.M112.408963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohba S. Hedgehog signaling in endochondral ossification. J Dev Biol. 2016;4(2) doi: 10.3390/jdb4020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hori N., Abe T., Sato T., Kokabu S., Shimamura Y., Sato T. Data in support of the bone analysis of NOD-SCID mice treated with zoledronic acid and prednisolone. Data Brief. 2016;7:1486–1490. doi: 10.1016/j.dib.2016.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun Q., Zhao Y., Yang Y., Yang X., Li M., Xu X. Loss of the clock protein PER2 shortens the erythrocyte life span in mice. J Biol Chem. 2017;292(30):12679–12690. doi: 10.1074/jbc.M117.783985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhuo H., Wang Y., Zhao Q. The interaction between Bmal1 and Per2 in mouse BMSC osteogenic differentiation. Stem Cell Int. 2018;2018:3407821. doi: 10.1155/2018/3407821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swanson C.M., Kohrt W.M., Buxton O.M., Everson C.A., Wright K.P., Jr., Orwoll E.S. The importance of the circadian system & sleep for bone health. Metabolism. 2018;84:28–43. doi: 10.1016/j.metabol.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]