Abstract
We report about a case of a compassionate off-label use of the anti-interleukin-5-agent mepolizumab in a ventilated patient with life-threatening asthma attack in eosinophilic asthma. The patient suffered from severe eosinophilic asthma and was transmitted to our hospital with an asthma attack and a life-threatening respiratory state under ventilation. Since high dose steroids had not yielded a sufficient respiratory improvement mepolizumab was administered subcutaneously. After administration of mepolizumab respiratory state and ventilation parameter improved significantly. Two days after administration the patient was weaned could be extubated 8 days later and recovered completely from the asthma attack.
The presented clinical case is suggestive of future clinical trials or registry studies to evaluate potential clinical benefits of anti-interleukin-5 treatment in patients with severe exacerbations of eosinophilic asthma.
Keywords: Anti-IL-5-therapy, Asthma, Eosinophilia, Exacerbation, Intensive care medicine
1. Introduction
Asthma is a heterogeneous chronic inflammatory airway disease exhibiting a broad range of severity from mild symptoms to a very severe, potentially life-threatening condition. Severe forms occur in approximately 10% of all asthma patients [[1], [2], [3]]. Eosinophils play a central role in the pathogenesis and regulation of allergic and eosinophilic asthma. Since interleukin-5 (IL-5) plays a critical role in eosinophil differentiation, maturation, recruitment and activation in tissues, IL-5 antagonization has been introduced as a therapeutic target. Therefore, monoclonal antibodies directed against IL-5 or its receptor have been developed and demonstrated impressive efficacy in patients with severe eosinophilic asthma [[4], [5], [6]].
In the present case, we report on a compassionate use of mepolizumab in a patient with life-threatening asthma attack since high dose steroids had not yielded a sufficient respiratory improvement.
2. Case presentation
A 43-year-old woman was admitted to our intensive care unit (ICU) subsequent to initiation of invasive ventilation because of a Glasgow Coma Scale below eight points following intoxication with unknown doses of tricyclic antidepressants (TCA), quetiapine and non-steroidal anti-inflammatory drugs (ibuprofen) due to suicidal intentions. The patient had an established diagnosis of mixed-type bronchial asthma with relative blood eosinophils up to a maximum of 16% as assessed during previous examinations. Signs of chronic rhinosinusitis with polyps were not present. In the past, up to two asthma exacerbations per year had occurred but ICU admission had never been required before. The current asthma-attack was probably aggravated by the intoxication with non-steroidal anti-inflammatory drugs (ibuprofen). During the previous hospital stay two months ago 30 mg of oral prednisolone were given daily, subsequently tapered down and completely terminated six weeks before the current admission. In addition, inhalational therapy had been changed at that point of time replacing budesonide (200 μg twice daily) with a fixed-dose combination of budesonide and formoterol (320/9 μg twice daily) and tiotropium (18 μg twice daily).
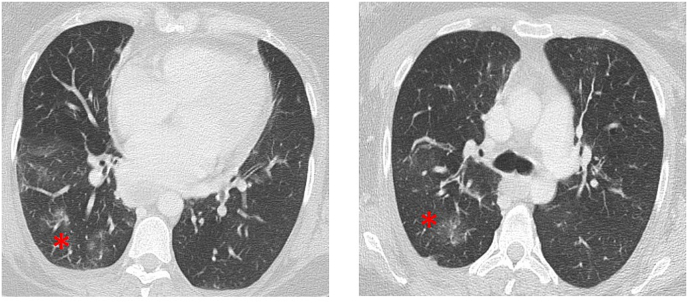
In the current ICU stay, electrocardiographic findings as well as serum B-type natriuretic peptide were normal as were inflammatory markers such as leucocyte counts, C-reactive protein and procalcitonin were within the normal interval (4.6 mg/l and smaller than 0.1 μg/l, respectively). At time of admission, relative blood eosinophils were two percent (see Fig. 2, corresponding to 180 per μl absolute count). The initial blood gas analysis during Biphasic Positive Airway Pressure-(BIPAP)-ventilation after intubation (settings: inspiratory pressure (Pi) 30 mmHg, expiratory airway pressure (PEEP) 10 mmHg, respiratory rate 26/min, inspiratory to expiratory time 1:2.6, and inspiratory oxygen concentration of 80%) showed a respiratory acidosis in arterial blood gas analysis: pH 7.117, carbon dioxide partial pressure (pCO2) 91 mmHg (see Fig. 3), oxygen partial pressure 202 mmHg, oxygen saturation 94%, bicarbonate 26 mmol/l, base excess 1.8, hemoglobin 7.8 g/l. Computed tomography of the chest revealed bilateral small interstitial infiltrates (Fig. 1) while showing no signs of severe pneumonia.
Fig. 2.
Course of blood eosinophiles (given in percent relative to total leucocyte count) in weeks where time 0 corresponds to the event of intubation. Medication is noted in the graph at the time when administered. od: once daily, qid: four times a day.
Fig. 3.
Development of respiratory state with the parameters Carbon dioxide partial pressure (pCO2), inspiratory pressure of invasive ventilation (Pi) and pH-value over the course of time where time 0 corresponds to event of intubation. Medication is noted in the graph at the time when administered. qid: four times a day.
Fig. 1.
Representative Computed Tomography Scan of the lung after admission to the Intensive Care Unit. Interstitial infiltrates are indicated by asterisks.
Due to these infiltrates and an increased temperature up to 38° degrees of Celsius we initiated antibiotic treatment with sultamicillin plus sulbactam which was replaced by meropenem after four days of persistent fever and increased inflammatory serum markers (initial C-reactive Protein 4.6 mg/l increased up to 118 mg/l, initial White Blood cell Count 6.27 G/l increased up to 21 G/l).
BIPAP ventilation with Pi pressures up to 30 mmHg and various PEEP levels did not improve the respiratory situation. pCO2 increased up to 100 mmHg accompanied by an acidosis of pH 7.12 (Fig. 3). Due to the critical situation several applications of prednisolone within seven days (50 mg given four times per day, see Fig. 2) were applied in addition to inhalation of epinephrine, salbutamol/ipratropiumbromide every 15–30 minutes. During the first two days, intravenous reproterol as well as magnesium were added. In addition, the regional Poison Control Center was contacted for information how to treat intoxications with the involved substances. The advice given was to continue ICU treatment and to wait and see while no specific detoxification procedures were recommended.
Because of the critical ventilatory state during the severe long-lasting status asthmaticus and the previous elevated relative blood eosinophils up to 16%, we finally decided to administer 100 mg mepolizumab subcutaneously (s.c.) in terms of a compassionate off-label use. The course of ventilatory parameters and eosinophils related to clinical course and the applied therapy regimen are also displayed in Fig. 2, Fig. 3.
As had been expected, relative eosinophils dropped significantly after administering mepolizumab (Fig. 2). Regarding the clinical course, the respiratory situation improved considerably after two days with lower Pi pressures, an improved decarboxylation, pH normalization (see Fig. 3), and a decrease of the intrinsic PEEP to eight mmHg. The patient was weaned and could be extubated eight days later and recovered completely.
3. Discussion and conclusion
In the last decade, several asthma phenotypes were identified which differ in their underlying pathogenesis. Of particular clinical relevance is the allergic and eosinophilic type. Both occur with a high prevalence and could be targeted by new innovative treatment strategies. Disease characteristics comprising of late onset and severity, blood eosinophilia count, and frequent exacerbations requiring systemic corticosteroids support the diagnosis of eosinophilic asthma in the present patient.
IL-5 is a potent eosinophil cytokine; it is responsible for growth and differentiation in the bone marrow, survival and mobilization, as well as emigration from bone marrow to the blood. It is therefore not surprising that in patients with severe eosinophilic asthma, targeting IL-5 by specific monoclonal antibodies is associated with a marked decrease in blood and lung eosinophilia, the number of exacerbations and clinical improvement. The monoclonal antibody mepolizumab binds and inactivates IL-5 and reduces exacerbations of asthma patients [6,7], in particular in addition to glucocorticosteroid therapy. Cytokine-based therapies could be the key to spare glucocorticoid-based treatments in severe asthma [8]. It is indicated as add-on treatment for severe refractory eosinophilic asthma in adult patients (>18 years). In late 2015, the U. S. Food and Drug Administration and European Medicines Agency approved mepolizumab for use in patients at the dose of 100 mg s.c. every four weeks [9].
During ventilation consequent to a suicide attempt our patient developed a severe asthma exacerbation which led to difficult conditions of ventilation, leading to therapy-refractory hypercapnia. Two to four days after mepolizumab administration clinical symptoms showed a significant improvement accompanied by a noticeably better ventilator situation which might be caused by reduced eosinophilic inflammation. The steroid administration could be responsible for reduced levels of inflammation but it did not result in a clinical improvement after 7 days, especially in ventilation.
As far as we know, this is the first report to describe s.c. mepolizumab administration during exacerbation of asthma under ICU-conditions. In a different published case, mepolizumab was also administered in a patient with allergic asthma with recurring severe acute asthma exacerbations during such an exacerbation episode but not during ICU conditions [10]. In that case, the patient was treated with omalizumab s.c. repeatedly and, additionally, with a thermoplastic approach which did not show any clinical meaningful results, however. The authors describe that the use of mepolizumab during another exacerbation led to a prompt clinical improvement and facilitated hospital discharge of the patient. Yet another clinical case reported the use of mepolizumab in allergic bronchopulmonary aspergillosis complicated with nontuberculous mycobacterial infection [11]. The authors reported that tapering of oral glucocorticosteroids after administering of mepolizumab was possible while blood eosinophils, need for inhaled budesonide/formoterol and IgE and exacerbation rate characterized by asthmatic symptoms all decreased.
Due to the pharmacokinetic and pharmacodynamic characteristics significant effects of anti-IL-5 therapy are expected after four weeks [12,13]. But maximum plasma concentration is reached after four to eight days after s.c. administration to healthy subjects or patients with asthma.
In principle, a short-term response to mepolizumab treatment could be explained by different mechanisms. Firstly, it can be argued that the response to prednisolone treatment was prolonged by the severity of asthma exacerbation. In this scenario, mepolizumab was added to the treatment regimen shortly before the condition of the patient would have improved anyhow and therefore had no relevant additional therapeutic benefit. Secondly, again given the severity of the underlying disease, the effects of systemic corticosteroids may have not been pronounced enough and only the additional impact of mepolizumab helped the patient. Finally, mepolizumab may influence eosinophil functions that are not responsive to glucocorticosteroids. This assumption is supported by clinical experience in patients with severe eosinophilic asthma treated with oral corticosteroids who not uncommonly report impressive symptomatic benefits upon initiation of mepolizumab treatment.
In these patients, local maturation rather than systemic recruitment of mature cells might contribute to persistent airway eosinophilia. Group 2 innate lymphoid cells are a major source of IL-5 and can facilitate eosinophilic inflammatory responses in the absence of CD4(+) lymphocytes, thereby promoting the persistence of airway eosinophilia through uncontrolled localized production of IL-5 despite high-dose corticosteroid therapy [14].
A combined effect of prednisolone and mepolizumab is further supported by findings confirming that patients with severe eosinophilic asthma have an exaggerated eosinophilopoeitic process in their airways. Treatment with mepolizumab significantly attenuated systemic differentiation of eosinophils but did not suppress local airway eosinophil differentiation to mature cells [15]. Targeting IL-5-driven eosinophil differentiation locally within the lung may be of relevance in order to achieve optimal control of airway eosinophilia and asthma.
In conclusion, mepolizumab is an attractive therapeutic agent in eosinophilic asthma, especially if patients are already treated with glucocorticoids. The present clinical case is suggestive of future clinical trials or registry studies to evaluate potential clinical benefits of anti-IL-5 treatment in patients with severe eosinophilic asthma exacerbations.
Conflicts of interest
Timm Greulich Speaker's Fees: AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, CSL Behring, Grifols, GSK, Mundipharma, Novartis, Advisory Boards: Astra Zeneca, Berlin-Chemie, Boehringer-Ingelheim, CSL-Behring, Grifols, GSK, Novartis, Grant (AATD-Lab): Grifols, Rembert Koczulla Speaker's fees and Advisory boards: Chiesi, Grifols, Astra Zeneca, Boehringer Ingelheim, Mundipharma, Teva, Novartis, GSK, Berlin Chemie, Orion Pharma, Manuel J Richter has received support from United Therapeutics and Bayer Pharma AG, and speaker fees from Actelion, Mundipharma, Roche, and United Therapeutics/OMT, Claus Vogelmeier reports personal fees from Boehringer Ingelheim, Chiesi, Menarini, Mundipharma, Novartis, Teva and grants and personal fees from AstraZeneca, GlaxoSmithKline, Grifols, Roland Buhl reports personal fees from AstraZeneca, Chiesi, Cipla and Teva, and grants and personal fees from Boehringer Ingelheim, GlaxoSmithKline, Novartis, and Roche, Stefan Kuhnert reports lecture fees from AstraZeneca and Elpen Pharma, and lecture fees and grants from Novartis, Marco Idzko reports lecture and adboard fees from AstraZeneca, Berlin Chemie, Boehringer Ingelheim, Grifols, GSK, Mundipharma, Novartis, Roche and Teva, Andreas Hoffmann has received support from CSL Behring.
Funding
This manuscript did not entail any funding sources outside of our institution.
Authors' contributions
All authors contributed equally to this case report. All authors read and approved the final manuscript.
Consent for publication
Consent for publication has been obtained from the involved patient.
References
- 1.Cisternas M.G., Blanc P.D., Yen I.H., Katz P.P., Earnest G., Eisner M.D., Shiboski S., Yelin E.H. A comprehensive study of the direct and indirect costs of adult asthma. J. Allergy Clin. Immunol. 2003;111:1212–1218. doi: 10.1067/mai.2003.1449. [DOI] [PubMed] [Google Scholar]
- 2.Custovic A., Johnston S.L., Pavord I., Gaga M., Fabbri L., Bel E.H., Le Souëf P., Lötvall J., Demoly P., Akdis C.A., Ryan D., Mäkelä M.J., Martinez F., Holloway J.W., Saglani S., O'Byrne P., Papi A., Sergejeva S., Magnan A., Del Giacco S., Kalayci O., Hamelmann E., Papadopoulos N.G. EAACI position statement on asthma exacerbations and severe asthma. Allergy. 2013;68:1520–1531. doi: 10.1111/all.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Omachi T.A., Iribarren C., Sarkar U., Tolstykh I., Yelin E.H., Blanc P.D., Eisner M.D., Katz P.P. Risk factors for death in adults with severe asthma. Ann. Allergy Asthma Immunol. 2008;101:130–136. doi: 10.1016/S1081-1206(10)60200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bel E.H., Wenzel S.E., Thompson P.J., Prazma C.M., Keene O.N., Yancey S.W., Ortega H.G., Pavord I.D. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N. Engl. J. Med. 2014;371:1189–1197. doi: 10.1056/NEJMoa1403291. [DOI] [PubMed] [Google Scholar]
- 5.Ortega H.G., Liu M.C., Pavord I.D., Brusselle G.G., FitzGerald J.M., Chetta A., Humbert M., Katz L.E., Keene O.N., Yancey S.W., Chanez P. Mepolizumab treatment in patients with severe eosinophilic asthma. N. Engl. J. Med. 2014;371:1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 6.Pavord I.D., Korn S., Howarth P., Bleecker E.R., Buhl R., Keene O.N., Ortega H., Chanez P. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. The Lancet. 2012;380:651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 7.Haldar P., Brightling C.E., Hargadon B., Gupta S., Monteiro W., Sousa A., Marshall R.P., Bradding P., Green R.H., Wardlaw A.J., Pavord I.D. Mepolizumab and exacerbations of refractory eosinophilic asthma. N. Engl. J. Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung K.F., Wenzel S.E., Brozek J.L., Bush A., Castro M., Sterk P.J., Adcock I.M., Bateman E.D., Bel E.H., Bleecker E.R., Boulet L.-P., Brightling C., Chanez P., Dahlen S.-E., Djukanovic R., Frey U., Gaga M., Gibson P., Hamid Q., Jajour N.N., Mauad T., Sorkness R.L., Teague W.G. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2014;43:343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 9.FDA approves Nucala to treat severe asthma, n.d, FDA News Release. U.S. Food and Drug Administration.
- 10.Menzella F., Galeone C., Lusuardi M., Simonazzi A., Castagnett C., Ruggiero P., Facciolongo N. Near-fatal asthma responsive to mepolizumab after failure of omalizumab and bronchial thermoplasty. TCRM. 2017;13:1489–1493. doi: 10.2147/TCRM.S149775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsubouchi H., Tsuchida S., Yanagi S., Shigekusa T., Miura M., Sakaguchi K., Matsumoto N., Nakazato M. Successful treatment with mepolizumab in a case of allergic bronchopulmonary aspergillosis complicated with nontuberculous mycobacterial infection. Respir. Med. Case Rep. 2019;28:100875. doi: 10.1016/j.rmcr.2019.100875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haldar P., Brightling C.E., Singapuri A., Hargadon B., Gupta S., Monteiro W., Bradding P., Green R.H., Wardlaw A.J., Ortega H., Pavord I.D. Outcomes after cessation of mepolizumab therapy in severe eosinophilic asthma: a 12-month follow-up analysis. J. Allergy Clin. Immunol. 2014;133:921–923. doi: 10.1016/j.jaci.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 13.Prazma C.M., Wenzel S., Barnes N., Douglass J.A., Hartley B.F., Ortega H. Characterisation of an OCS-dependent severe asthma population treated with mepolizumab. Thorax. 2014;69:1141–1142. doi: 10.1136/thoraxjnl-2014-205581. [DOI] [PubMed] [Google Scholar]
- 14.Smith S.G., Chen R., Kjarsgaard M., Huang C., Oliveria J.-P., O'Byrne P.M., Gauvreau G.M., Boulet L.-P., Lemiere C., Martin J., Nair P., Sehmi R. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J. Allergy Clin. Immunol. 2016;137:75–86. doi: 10.1016/j.jaci.2015.05.037. e8. [DOI] [PubMed] [Google Scholar]
- 15.Sehmi R., Smith S.G., Kjarsgaard M., Radford K., Boulet L.-P., Lemiere C., Prazma C.M., Ortega H., Martin J.G., Nair P. Role of local eosinophilopoietic processes in the development of airway eosinophilia in prednisone-dependent severe asthma. Clin. Exp. Allergy. 2016;46:793–802. doi: 10.1111/cea.12695. [DOI] [PubMed] [Google Scholar]