Abstract
The cytokine interleukin-19 (IL-19) is a member of the IL-10 family that includes IL-20, IL-22, IL-24, and IL-26. Previous studies indicated that IL-19 is produced by keratinocytes, epithelial cells, macrophages, and B-cells. Especially, the number of IL-4-producing T cells increased, whereas the number of IFN-γ-producing T cells decreased when naive T cells from healthy people were cultured in the presence of IL-19. There is an increasing body of data demonstrating that IL-19 is associated with the development of type 1 helper T cell-responses, although IL-19 was originally associated with the development of type 2 helper T cell-responses. In this review, we will attempt to discuss current knowledge about the role of IL-19 on several T cell response-mediated inflammatory diseases including inflammatory bowel disease and hypersensitivity.
Keywords: anti-inflammation, hypersensitivity, inflammatory bowel disease, interleukin-19
The maintenance of immunological homeostasis in healthy life has been appreciated for a long time. Excessive uncontrolled inflammatory responses can result in dysregulation of immunological homeostasis, followed by a variety of inflammatory diseases. While inflammatory response is recently recognized as one of the major mechanisms for many inflammatory diseases, including inflammatory bowel diseases (IBDs) and hypersensitivities [9, 21, 30], it still remains unknown how inflammatory response through several organs affects the homeostasis of the living organism. The pathogenesis of inflammatory diseases is characterized by an imbalanced activation of inflammatory and anti-inflammatory response (Fig. 1). Current therapy of inflammatory diseases has developed as a result of inhibition of an inflammatory response. In contrast, IL-10 is a well-known anti-inflammatory and immunosuppressive cytokine and has shown promise in clinical trials for the treatment of an inflammatory disease. The potentiation of an anti-inflammatory response is strong approach for the future therapy of inflammatory diseases. Inflammatory mediators including TNF-α, IL-1β, and IL-6 are released during acute inflammation. Then, anti-inflammatory mediator such as IL-10 is released behind inflammatory mediators. Interestingly, IL-19 is released behind IL-10 (Fig. 2). The cytokine IL-19 was originally found by sequence homology to IL-10 [14] and is a member of the IL-10 family, which also includes IL-20, IL-22, IL-24, IL-26, IL-28A, IL-28B, and IL-29 [4, 34]. In this review, we aimed to elucidate novel role of IL-19 on the inflammatory diseases. We especially focus on IBDs and hypersensitivities as inflammatory diseases of the intestine and the skin.
Fig. 1.
An imbalanced activation of inflammatory and anti-inflammatory response causes inflammatory diseases.
Fig. 2.
Time course of inflammatory and anti-inflammatory mediators.
IL-19 AND IBD
IBDs are characterized by dysregulated chronic inflammation and mucosal tissue damage in parts of the gastrointestinal tract [38]. Clinically, Ulcerative colitis (UC) and Crohn’s disease (CD) are two of the most common types of IBDs. IL-10 is a well-known anti-inflammatory and immunosuppressive cytokine. Consistently, IL-10 knockout (KO) mice spontaneously developed colitis, indicating that IL-10 is critical for intestinal homeostasis [24, 25]. Like IL-10 KO mice, IL-2 KO mice also spontaneously developed colitis [35]. These findings discovered new insight into the pathogenesis of IBD. Genetic factors lead to mucosal inflammation that resembles IBD. In addition to these KO mice, animal models of UC and CD are indispensable for both the identification of immune responses involved in the pathogenesis of IBD. The most widely used IBD animal models are colitis chemically induced by 2,4,6-trinitrobenzene sulfonic acid (TNBS) [32], oxazolone [6], and dextran sodium sulfate (DSS) [1]. DSS-induced colitis is a well-characterized model of colonic inflammation. The innate immune system mainly plays a major role in the DSS-induced colitis model, because T- and B-cell-deficient mice also developed severe colonic inflammation in this model [8]. CD is associated with a type 1 helper T cell (Th1) response mainly driven by IL-12 and IFN-γ [29]. TNBS-induced colitis model, which shows a clear Th1 phenotype, resembles the symptom seen in CD [22]. In contrast, UC is driven by type 2 helper T cell (Th2) cytokines, such as IL-4, IL-5, and IL-13 [37]. Oxazolone-induced colitis model, which shows a clear Th2 phenotype, resembles the symptom seen in UC [6, 20].
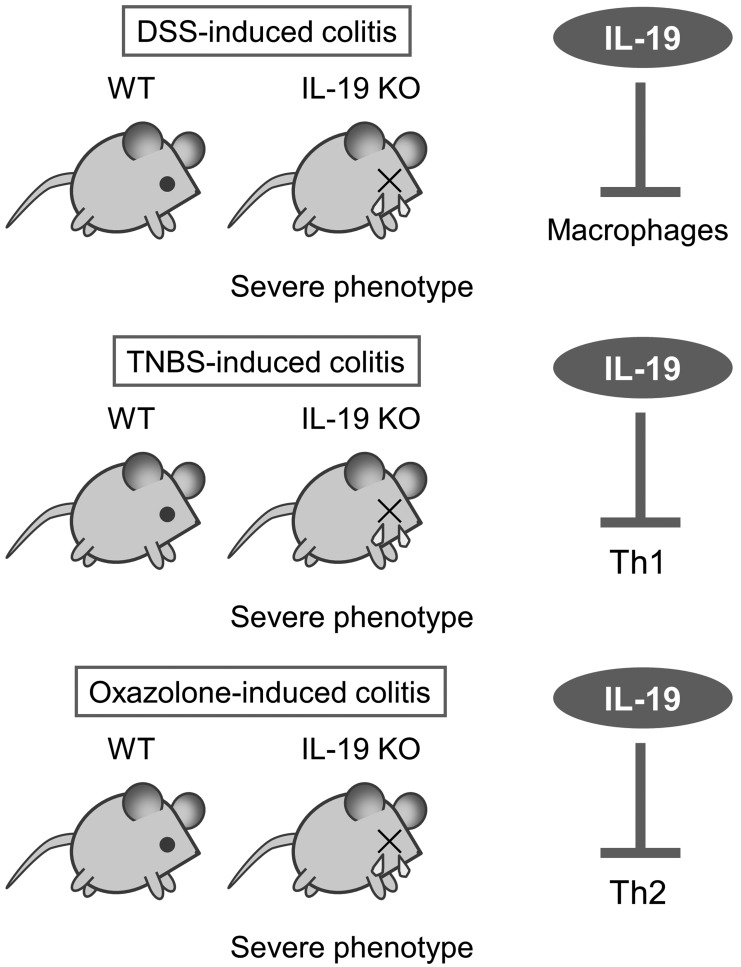
To more specifically assess the immunological role of IL-19, we generated IL-19 KO mice [2]. During these 10 years, we reported novel roles for IL-19 as a regulator of colonic inflammations using IL-19 KO mice. First, IL-19 KO mice showed severe phenotype upon DSS-induced colitis (Fig. 3 upper panel). This severe phenotype is correlated with the accumulation of macrophages and the increased production of IL-1β, IL-6, IL-12, TNF-α, IFN-γ, and CXCL1 [2]. Second, IL-19 KO mice showed severe phenotype upon TNBS-induced colitis (Fig. 3 middle panel). This severe phenotype is correlated with the increased production of IFN-γ, IL-12, IL-17, IL-22, and IL-33, and the decreased production of IL-4 [27]. Third, IL-19 KO mice showed severe phenotype upon oxazolone-induced colitis (Fig. 3 lower panel). This severe phenotype is correlated with the increased production of IL-4 and IL-9 [11]. In addition, we reported that IL-19 control the autophagy and apoptosis in cerulein-induced pancreatitis [13]. At the beg-nning of the start, we expected that IL-19 KO mice will be less susceptible to oxazolone-induced colitis because Th1 response and Th2 response should be balanced for inflammation control. However, our results are different from what we expected. We found that IL-19 can have anti-inflammatory roles in the gut, regardless of the type of colonic inflammation. In line with these previous findings, our results suggest that IL-19 plays a regulatory role in the gut by inhibiting macrophages to produce inflammatory cytokines, Th1 to produce IFN-γ, and Th2 to produce IL-4 (Fig. 3 each right panel).
Fig. 3.
Increased susceptibility of IL-19-KO mice to colitis models induced by dextran sodium sulfate (DSS), 2,4,6-trinitrobenzene sulfonic acid (TNBS), and oxazolone.
Next, we introduce clinical studies related to IL-19 of IBD patients. The first study to introduce reported that IL-19 gene polymorphisms AA (rs2243188) and AA (rs2243193) were significantly decreased in UC patients as compared with healthy controls (CC+AC; rs2243188 and GG+AG; rs2243193) [39]. The results suggest that IL-19 polymorphisms (rs2243188 and rs2243193) might have a protective role in the development of UC. A second study to introduce investigated the expression of IL-19 on cells from active CD patients. This study reported that unstimulated and Toll-like receptor (TLR) -activated monocytes produced significantly lower IL-19 in active CD patients than in healthy controls (HC) [7]. In addition, exogenous IL-19 had an anti-inflammatory effect on peripheral blood mononuclear cells from HC but not CD patients. These results suggested that IL-19 had an anti-inflammatory role in CD patients. A third study to introduce reported the expression of IL-19 on active IBD patients. IL-19 gene expression was elevated significantly in patients with both active CD and active UC versus the inactive disease and non-inflammatory control groups [10]. More precisely, IL-19 expression showed a 30-fold increase in active CD patients in contrast to a 2-fold increase in active UC patients. Furthermore, IL-19 immunoreactive cells in active CD patients were increased conspicuously in colonic mucosa compared to active UC patients and healthy donors. In the peripheral blood from active UC and CD patients, the relative percentage of circulating IL-19-producing CD4+ T cells, IL-19-producing CD8+ T cells, IL-19-producing active B cells, and IL-19-producing monocytes were all decreased compared to the relative percentage of healthy donors. This study explained that IL-19 may act on a local tissue level as anti-inflammatory effecter from the periphery into the tissue, correlating the decrease of circulating IL-19-producing cells and the increase of IL-19 in colonic mucosa. They also suggest that the increase of IL-19 in active IBD patients could be a compensatory mechanism for the anti-inflammatory response. Fourth study to introduce reported that the expression of IL-19 was significantly increased in biopsies from patients with active UC compared with quiescent UC, whereas the expression level of IL-19 was not affected by the disease status in CD patients [36]. Serum level of IL-19 was elevated in IBD patients with active disease compared with patients with quiescent disease. As a consequence, IL-19 expression was not increased in CD patients in this study, which is in contrast to a third study [10]. Further studies should be needed for resolving the discrepancy between these studies.
IL-19 AND TYPE IV HYPERSENSITIVITY
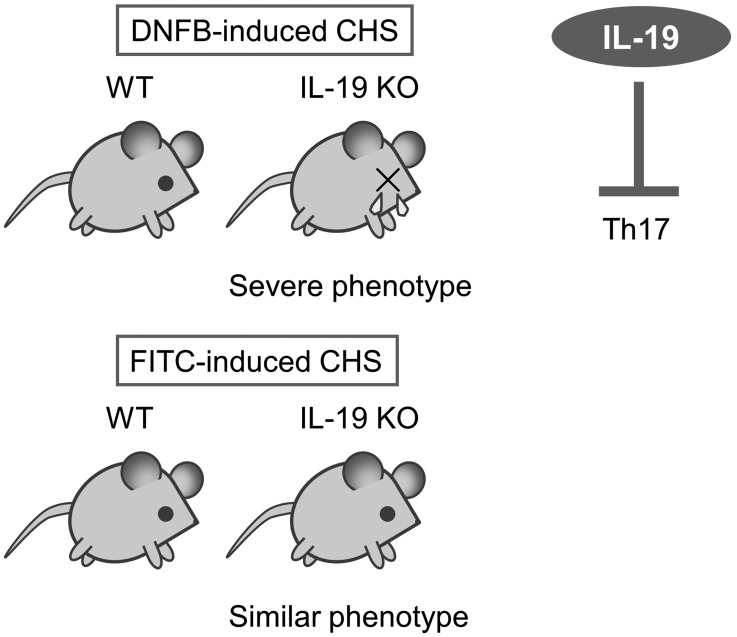
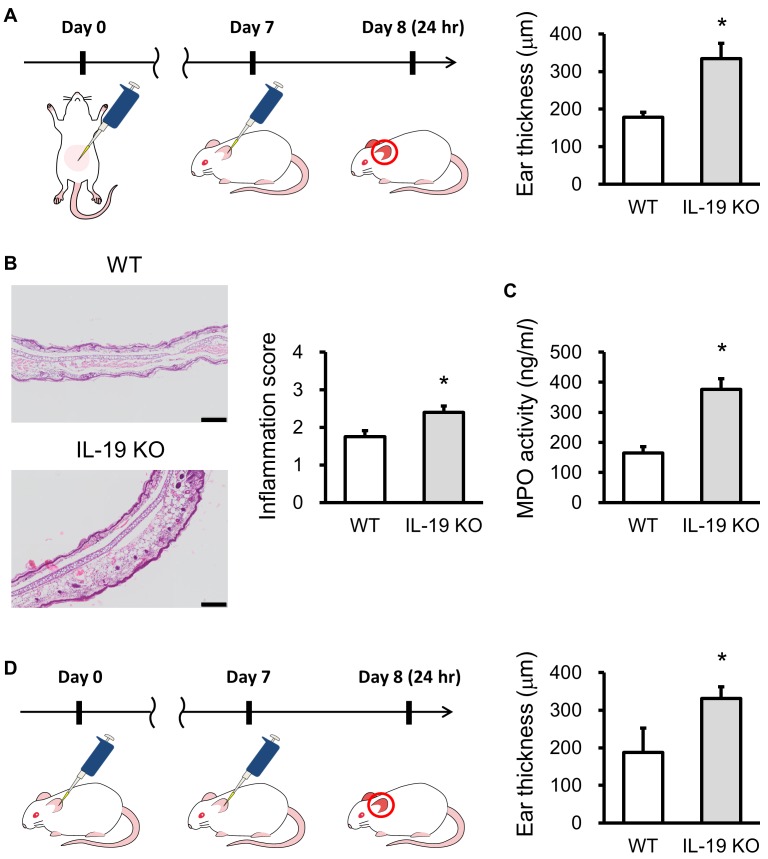
Hypersensitivity including allergy basically does not occur at the only one time exposure of hapten or allergen. When exposure to the same hapten or allergen again, the immune system results in specific reactions. This phase is known as sensitization. Sensitization may take several days or more. Hypersensitivity is the increased reactivity or increased sensitivity by the body to a hapten or allergen. There are four different types of hypersensitivity. Types I, II and III hypersensitivities all can involve antibodies. Type IV hypersensitivity is a delayed and cell-mediated reactions and is mediated by special immune cells such as a macrophage and T cell. This reaction usually takes from several hr to several days to develop an allergic response. Example disease includes allergic contact dermatitis (ACD) which is an inflammatory disease of the skin [26]. ACD is induced by frequent exposure to foreign substances such as metals, cosmetics, drugs, and plant materials [17]. In the pathogenesis of ACD, many types of immune cells, including keratinocytes, macrophages, T cells, and B cells, play important roles in the initiation and termination mechanisms of ACD [5]. Contact hypersensitivity (CHS) and delayed-type hypersensitivity (DTH) as mouse models of ACD have been used for two decades. There is only one report that IL-19 is clinically related to human dermatitis. IL-19 levels were significantly higher in the serum from patients with atopic dermatitis than in normal healthy controls [33]. Similar to human dermatitis, we have reported that IL-19 expression was increased in the ear from 1-fluoro-2,4-dinitrofluorobenzene (DNFB) -induced CHS response [12]. To elucidate the role of IL-19 in hapten-specific skin disease, we investigated CHS responses in IL-19 KO mice. IL-19 KO mice showed severe phenotype upon DNFB-induced CHS (Fig. 4 upper panels). This severe phenotype is correlated with the increased production of IL-17 and IL-6, but no change in the production of IFN-γ and IL-4. These results suggest that IL-19 can regulate the activation of IL-17-producing cells such as Th17 cells via the promotion of dermal dendritic cells and/or Langerhans cells. In contrast, ear swelling was almost the same between the IL-19 KO mice and WT mice in FITC-induced CHS (Fig. 4 lower panel). The response to a DNFB challenge is mediated by Th1 IFN-γ-inducing haptens, [18] whereas the response to a FITC challenge is mediated by a Th2 response [28]. Our results suggest that IL-19 can influence Th1-mediated CHS response through the regulation of IL-17-producing cells such as Th17 cells. In the model of CHS, many types of immune cells, including keratinocytes, dendritic cells, Langerhans cells, macrophages, T cells, and B cells, play important roles in the initiation and termination mechanisms of CHS [15]. Hapten-carrier complex is taken up by dermal dendritic cells and Langerhans cells that are the major antigen-presenting cells in CHS response. Both cells move from the skin to draining lymph nodes after activation with hapten-carrier complex and can present to naïve T cells by the hapten-carrier-MHC complex in human [16] and mouse [31]. Thereafter, effector T cells produce cytokines, such as INF-γ, IL-4, and IL-17 depend on the types of hapten. To more specifically assess the selectivity of the types of hapten, we used another hapten oxazolone. Mice were sensitized by applying 150 µl of 3% oxazolone in ethanol to the shaved abdominal skin. Seven days after sensitization, the mice were challenged by painting 20 µl of 1% oxazolone in vehicle solution (acetone: olive oil 3:1, v/v) on their ears (Fig. 5A left panel). IL-19KO mice showed increased ear swelling compared with WT mice at 24 hr after the oxazolone challenge (Fig. 5A right panel). IL-19 KO mice exhibited infiltration of a large number of inflammatory cells into the oxazolone-challenged ear epidermis. Ear tissue from IL-19KO mice revealed increased inflammation score 24 hr after the oxazolone challenge (Fig. 5B). Furthermore, ear tissue from IL-19KO mice revealed increased MPO activity (Fig. 5C), consistent with an increase in inflammation score. We also performed the repeated challenge of oxazolone on the ear (Fig. 5D left panel). IL-19KO mice also showed severe ear swelling compared with WT mice at 24 hr after the repeated challenge of oxazolone (Fig. 5D right panel). The response to an oxazolone challenge is mediated by Th1 IFN-γ-inducing haptens as well as DNFB. These results and our previous data suggest that IL-19 is a new mediator with anti-inflammatory effects on skin inflammation.
Fig. 4.
Exacerbation of 1-fluoro-2,4-dinitrofluorobenzene (DNFB)-induced contact hypersensitivity (CHS) response in IL-19 KO mice.
Fig. 5.
Exacerbation of oxazolone-induced contact hypersensitivity (CHS) response in IL-19 KO mice. (A) The protocol is shown. WT mice (n=5) and IL-19 KO mice (n=5) were sensitized with oxazolone. A CHS response was elicited by oxazolone on the ears 7 days later. Ear swelling was measured 24 hr after the challenges. (B) HE staining in the ears of sensitized WT mice (n=5) and IL-19 KO mice (n=5) 24 hr after the oxazolone challenge. Bars=100 µm. Inflammation scores were determined. (C) MPO activity in WT mice (n=5) and IL-19 KO mice (n=8). (D) The protocol of repeated challenge with oxazolone is shown. Ear swelling was measured 24 hr after the repeated challenges in WT mice (n=4) and IL-19 KO mice (n=8). *P<0.05 compared with WT mice.
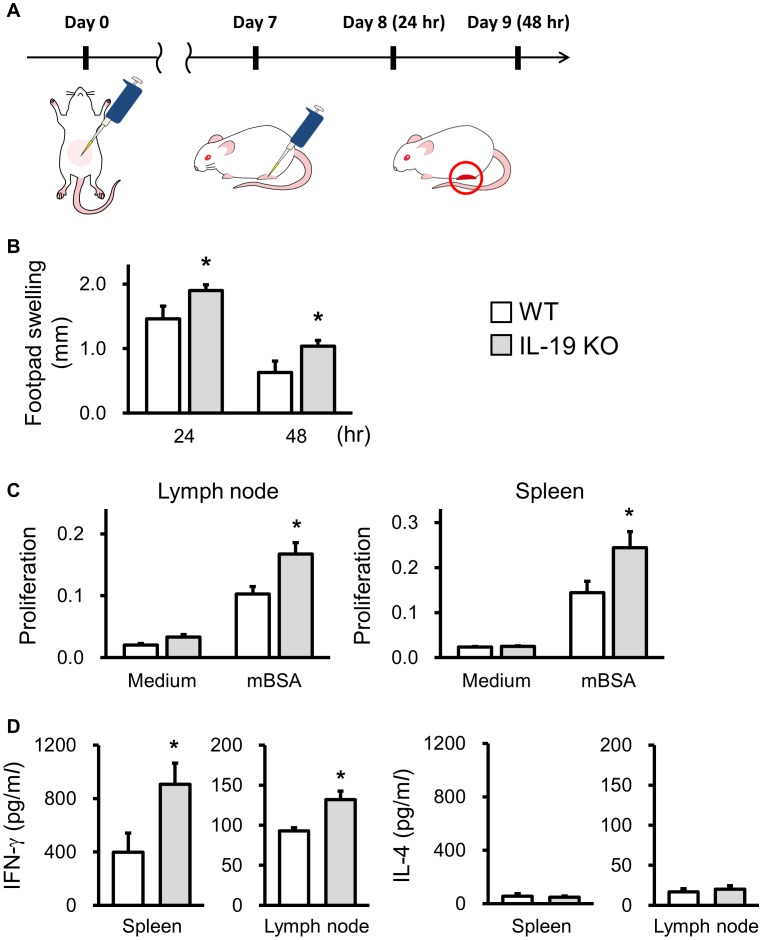
Immune responses which result in the hypersensitivity are specific for the types of antigens from different sources. We listed the difference between CHS and DTH (Table 1). The antigens, the place of the inflammation, and the type of immune responses are different. As far as we know, there is no report that IL-19 is related to a DTH response. To more specifically assess the selectivity of the type of type IV hypersensitivity, we tried to examine the role of IL-19 in methylated BSA (mBSA) -induced DTH response. Mice were immunized with 50 µl ×2 spots of 2.5 mg/ml mBSA in 50% CFA to abdominal skin by s.c. injection. Seven days later, the mice were challenged with 20 µl of 10 mg/ml mBSA in PBS on their hind footpad (Fig. 6A). IL-19KO mice showed increased footpad swelling compared with WT mice at 24 and 48 hr after the mBSA challenge (Fig. 6B). In IL-19 KO mice, cell infiltration at the inflammatory site was severer than WT mice (data not shown). The proliferation of T cells in the lymph node and spleen from IL-19 KO mice against mBSA was significantly enhanced compared with that of WT mice (Fig. 6C). Consistent with the increased proliferation of a T cell in IL-19 KO mice, IFN-γ production in the culture supernatant of T cell in the lymph node and spleen from IL-19 KO mice was significantly higher than that of WT mice (Fig. 6D left panels). In contrast, IL-4 production was below the level of detection in both WT and IL-19 KO culture supernatants (Fig. 6D right panels). These results suggest that IL-19 also plays an important role in the induction of the Th1-mediated DTH response.
Table 1. The different features in contact hypersensitivity (CHS) and delayed-type hypersensitivity (DTH).
| CHS | DTH | |
|---|---|---|
| Antigen | Low molecular weight chemicals as haptens | High molecular weight chemicals as proteins |
| Region | Skin inflammation in the epidermis | Skin inflammation in the dermis |
| Cell | CD4+ T cells-mediated macrophage activation | CD8+T cells-mediated cytotoxicity in addition to CD4+T cells-mediated macrophage activation |
Fig. 6.
Exacerbation of mBSA-induced delayed-type hypersensitivity (DTH) response in IL-19 KO mice. (A) The protocol is shown. WT mice (n=8) and IL-19 KO mice (n=22) were sensitized with mBSA. A DTH response was elicited by mBSA on the footpad 7 days later. (B) Footpad swelling was measured 24 and 48 hr after the challenges. (C, D) The cells prepared from axillary and inguinal lymph nodes, and spleen in WT mice (n=4) and IL-19KO mice (n=10) 7 days after mBSA sensitization were stimulated with mBSA (40 µg/ml) for 72 hr. (C) Proliferation was measured MTS assay. (D) The cytokine concentration was measured by ELISA. *P<0.05 compared with WT mice.
CONCLUSION
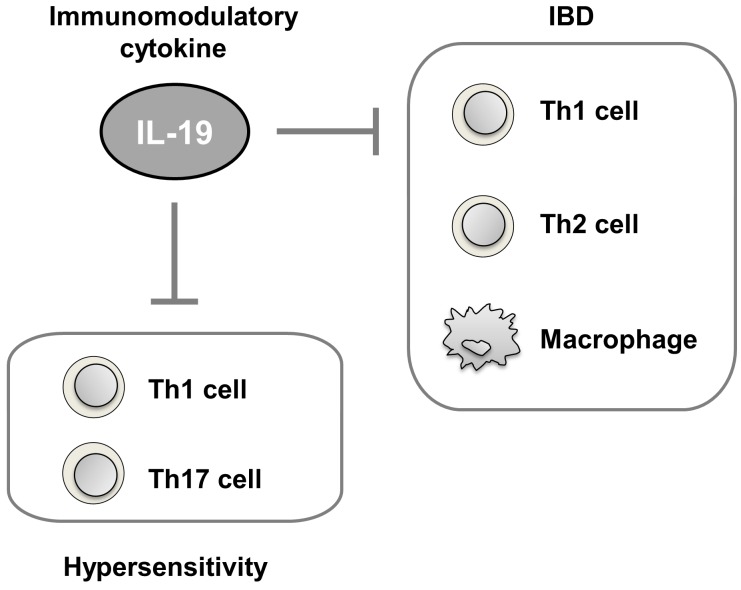
Our previous and present studies provide solid evidence for the immunopathological relevance of IL-19 as an immunomodulatory cytokine in IBD and type IV hypersensitivity against intestinal and skin inflammations (Fig. 7). To date, there are no studies related to the clinical efficacy of recombinant IL-19 and neutral antibody for IL-19 in any disease including IBD and hypersensitivity. Nonetheless, our results obtained from several animal models and basic researches data obtained from human disease indicate that IL-19 might be a strong candidate for inflammatory disorders such as IBDs and hypersensitivities. As we already reported IL-19 KO mice were born at the expected Mendelian frequency and had no overt abnormalities [2, 3]. IL-19 KO adult mice had similar weight and 1.5-year survival compared to sex-matched littermate controls, and IL-19 loss did not seem to affect a lymphoid and myeloid cellular composition in the thymus, spleen, and lymph node [3]. Although the IL-19 KO mice showed no apparent gross phenotype and abnormality, IL-19 KO mice had striking molecular phenotypes, as reported for other genes [19, 23]. Different experimental approaches could be useful to query a gene loss of function, despite its KO mouse seemingly normal appearance. Thus, targeting IL-19 may be useful for preventing inflammatory disorders, with few side effects.
Fig. 7.
Schema of the possible associations of IL-19 in the inflammatory bowel disease (IBD) and hypersensitivity.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest to declare.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (B) 25292174 (Y.T.A.).
REFERENCES
- 1.Azuma Y. T., Hagi K., Shintani N., Kuwamura M., Nakajima H., Hashimoto H., Baba A., Takeuchi T.2008. PACAP provides colonic protection against dextran sodium sulfate induced colitis. J. Cell. Physiol. 216: 111–119. doi: 10.1002/jcp.21381 [DOI] [PubMed] [Google Scholar]
- 2.Azuma Y. T., Matsuo Y., Kuwamura M., Yancopoulos G. D., Valenzuela D. M., Murphy A. J., Nakajima H., Karow M., Takeuchi T.2010. Interleukin-19 protects mice from innate-mediated colonic inflammation. Inflamm. Bowel Dis. 16: 1017–1028. doi: 10.1002/ibd.21151 [DOI] [PubMed] [Google Scholar]
- 3.Azuma Y. T., Matsuo Y., Nakajima H., Yancopoulos G. D., Valenzuela D. M., Murphy A. J., Karow M., Takeuchi T.2011. Interleukin-19 is a negative regulator of innate immunity and critical for colonic protection. J. Pharmacol. Sci. 115: 105–111. doi: 10.1254/jphs.10R02CR [DOI] [PubMed] [Google Scholar]
- 4.Azuma Y. T., Nakajima H., Takeuchi T.2011. IL-19 as a potential therapeutic in autoimmune and inflammatory diseases. Curr. Pharm. Des. 17: 3776–3780. doi: 10.2174/138161211798357845 [DOI] [PubMed] [Google Scholar]
- 5.Bian R., Tang J., Hu L., Huang X., Liu M., Cao W., Zhang H.2018. (E)‑phenethyl 3‑(3,5‑dihydroxy‑4‑isopropylphenyl) acrylate gel improves DNFB-induced allergic contact hypersensitivity via regulating the balance of Th1/Th2/Th17/Treg cell subsets. Int. Immunopharmacol. 65: 8–15. doi: 10.1016/j.intimp.2018.09.032 [DOI] [PubMed] [Google Scholar]
- 6.Boirivant M., Fuss I. J., Chu A., Strober W.1998. Oxazolone colitis: A murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J. Exp. Med. 188: 1929–1939. doi: 10.1084/jem.188.10.1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantó E., Garcia Planella E., Zamora-Atenza C., Nieto J. C., Gordillo J., Ortiz M. A., Metón I., Serrano E., Vegas E., García-Bosch O., Juárez C., Vidal S.2014. Interleukin-19 impairment in active Crohn’s disease patients. PLoS One 9: e93910. doi: 10.1371/journal.pone.0093910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dieleman L. A., Palmen M. J., Akol H., Bloemena E., Peña A. S., Meuwissen S. G., Van Rees E. P.1998. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 114: 385–391. doi: 10.1046/j.1365-2249.1998.00728.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elaidy S. M., Essawy S. S., Hussain M. A., El-Kherbetawy M. K., Hamed E. R.2018. Modulation of the IL-23/IL-17 axis by fenofibrate ameliorates the ovalbumin/lipopolysaccharide-induced airway inflammation and bronchial asthma in rats. Naunyn Schmiedebergs Arch. Pharmacol. 391: 309–321. doi: 10.1007/s00210-017-1459-z [DOI] [PubMed] [Google Scholar]
- 10.Fonseca-Camarillo G., Furuzawa-Carballeda J., Granados J., Yamamoto-Furusho J. K.2014. Expression of interleukin (IL)-19 and IL-24 in inflammatory bowel disease patients: a cross-sectional study. Clin. Exp. Immunol. 177: 64–75. doi: 10.1111/cei.12285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujimoto Y., Azuma Y. T., Matsuo Y., Kuwamura M., Kuramoto N., Miki M., Azuma N., Teramoto M., Nishiyama K., Izawa T., Nakajima H., Takeuchi T.2017. Interleukin-19 contributes as a protective factor in experimental Th2-mediated colitis. Naunyn Schmiedebergs Arch. Pharmacol. 390: 261–268. doi: 10.1007/s00210-016-1329-0 [DOI] [PubMed] [Google Scholar]
- 12.Fujimoto Y., Fujita T., Kuramoto N., Kuwamura M., Izawa T., Nishiyama K., Yoshida N., Nakajima H., Takeuchi T., Azuma Y. T.2018. The Role of Interleukin-19 in Contact Hypersensitivity. Biol. Pharm. Bull. 41: 182–189. doi: 10.1248/bpb.b17-00594 [DOI] [PubMed] [Google Scholar]
- 13.Fujimoto Y., Tsuneyama K., Kuramoto N., Hayashi S., Yoshida N., Morioka A., Teramoto M., Nakajima H., Takeuchi T., Azuma Y. T.2017. Exacerbated experimental pancreatitis in interleukin-19 knockout mice. Glob. Drugs Therap. 2: 1–5. [Google Scholar]
- 14.Gallagher G., Dickensheets H., Eskdale J., Izotova L. S., Mirochnitchenko O. V., Peat J. D., Vazquez N., Pestka S., Donnelly R. P., Kotenko S. V.2000. Cloning, expression and initial characterization of interleukin-19 (IL-19), a novel homologue of human interleukin-10 (IL-10). Genes Immun. 1: 442–450. doi: 10.1038/sj.gene.6363714 [DOI] [PubMed] [Google Scholar]
- 15.Gaudenzio N., Marichal T., Galli S. J., Reber L. L.2018. Genetic and Imaging Approaches Reveal Pro-Inflammatory and Immunoregulatory Roles of Mast Cells in Contact Hypersensitivity. Front. Immunol. 9: 1275. doi: 10.3389/fimmu.2018.01275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grabbe S., Schwarz T.1998. Immunoregulatory mechanisms involved in elicitation of allergic contact hypersensitivity. Immunol. Today 19: 37–44. doi: 10.1016/S0167-5699(97)01186-9 [DOI] [PubMed] [Google Scholar]
- 17.Hajavi J., Esmaeili S. A., Varasteh A. R., Vazini H., Atabati H., Mardani F., Momtazi-Borojeni A. A., Hashemi M., Sankian M., Sahebkar A.2019. The immunomodulatory role of probiotics in allergy therapy. J. Cell. Physiol. 234: 2386–2398. doi: 10.1002/jcp.27263 [DOI] [PubMed] [Google Scholar]
- 18.Han N. R., Moon P. D., Yoo M. S., Ryu K. J., Kim H. M., Jeong H. J.2018. Regulatory effects of chrysophanol, a bioactive compound of AST2017-01 in a mouse model of 2,4-dinitrofluorobenzene-induced atopic dermatitis. Int. Immunopharmacol. 62: 220–226. doi: 10.1016/j.intimp.2018.06.046 [DOI] [PubMed] [Google Scholar]
- 19.Hara T., Mimura K., Abe T., Shioi G., Seiki M., Sakamoto T.2011. Deletion of the Mint3/Apba3 gene in mice abrogates macrophage functions and increases resistance to lipopolysaccharide-induced septic shock. J. Biol. Chem. 286: 32542–32551. doi: 10.1074/jbc.M111.271726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heller F., Fuss I. J., Nieuwenhuis E. E., Blumberg R. S., Strober W.2002. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity 17: 629–638. doi: 10.1016/S1074-7613(02)00453-3 [DOI] [PubMed] [Google Scholar]
- 21.Kalra J., Lingaraju M. C., Mathesh K., Kumar D., Parida S., Singh T. U., Sharma A. K., Kumar D., Tandan S. K.2018. Betulinic acid alleviates dextran sulfate sodium-induced colitis and visceral pain in mice. Naunyn Schmiedebergs Arch. Pharmacol. 391: 285–297. doi: 10.1007/s00210-017-1455-3 [DOI] [PubMed] [Google Scholar]
- 22.Kitani A., Fuss I. J., Nakamura K., Schwartz O. M., Usui T., Strober W.2000. Treatment of experimental (Trinitrobenzene sulfonic acid) colitis by intranasal administration of transforming growth factor (TGF)-beta1 plasmid: TGF-beta1-mediated suppression of T helper cell type 1 response occurs by interleukin (IL)-10 induction and IL-12 receptor beta2 chain downregulation. J. Exp. Med. 192: 41–52. doi: 10.1084/jem.192.1.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleaveland B., Shi C. Y., Stefano J., Bartel D. P.2018. A Network of Noncoding Regulatory RNAs Acts in the Mammalian Brain. Cell 174: 350–362.e17. doi: 10.1016/j.cell.2018.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W.1993. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75: 263–274. doi: 10.1016/0092-8674(93)80068-P [DOI] [PubMed] [Google Scholar]
- 25.Mähler M., Leiter E. H.2002. Genetic and environmental context determines the course of colitis developing in IL-10-deficient mice. Inflamm. Bowel Dis. 8: 347–355. doi: 10.1097/00054725-200209000-00006 [DOI] [PubMed] [Google Scholar]
- 26.Maeda N., Yamada C., Takahashi A., Kuroki K., Maenaka K.2017. Therapeutic application of human leukocyte antigen-G1 improves atopic dermatitis-like skin lesions in mice. Int. Immunopharmacol. 50: 202–207. doi: 10.1016/j.intimp.2017.06.026 [DOI] [PubMed] [Google Scholar]
- 27.Matsuo Y., Azuma Y. T., Kuwamura M., Kuramoto N., Nishiyama K., Yoshida N., Ikeda Y., Fujimoto Y., Nakajima H., Takeuchi T.2015. Interleukin 19 reduces inflammation in chemically induced experimental colitis. Int. Immunopharmacol. 29: 468–475. doi: 10.1016/j.intimp.2015.10.011 [DOI] [PubMed] [Google Scholar]
- 28.Matsuoka T., Endo Y., Kurohane K., Imai Y.2018. Skin Sensitization to Fluorescein Isothiocyanate Is Enhanced by Butyl Paraben in a Mouse Model. Biol. Pharm. Bull. 41: 1853–1858. doi: 10.1248/bpb.b18-00584 [DOI] [PubMed] [Google Scholar]
- 29.Monteleone G., Monteleone I., Fina D., Vavassori P., Del Vecchio Blanco G., Caruso R., Tersigni R., Alessandroni L., Biancone L., Naccari G. C., MacDonald T. T., Pallone F.2005. Interleukin-21 enhances T-helper cell type I signaling and interferon-gamma production in Crohn’s disease. Gastroenterology 128: 687–694. doi: 10.1053/j.gastro.2004.12.042 [DOI] [PubMed] [Google Scholar]
- 30.Moulahoum H., Boumaza B. M. A., Ferrat M., Bounaama A., Djerdjouri B.2019. Arsenic trioxide ameliorates murine colon inflammation through inflammatory cell enzymatic modulation. Naunyn Schmiedebergs Arch. Pharmacol. 392: 259–270. doi: 10.1007/s00210-018-1578-1 [DOI] [PubMed] [Google Scholar]
- 31.Nakae S., Komiyama Y., Nambu A., Sudo K., Iwase M., Homma I., Sekikawa K., Asano M., Iwakura Y.2002. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 17: 375–387. doi: 10.1016/S1074-7613(02)00391-6 [DOI] [PubMed] [Google Scholar]
- 32.Neurath M. F., Fuss I., Kelsall B. L., Stüber E., Strober W.1995. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J. Exp. Med. 182: 1281–1290. doi: 10.1084/jem.182.5.1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oka T., Sugaya M., Takahashi N., Nakajima R., Otobe S., Kabasawa M., Suga H., Miyagaki T., Asano Y., Sato S.2017. Increased Interleukin-19 Expression in Cutaneous T-cell Lymphoma and Atopic Dermatitis. Acta Derm. Venereol. 97: 1172–1177. doi: 10.2340/00015555-2723 [DOI] [PubMed] [Google Scholar]
- 34.Sabat R., Wallace E., Endesfelder S., Wolk K.2007. IL-19 and IL-20: two novel cytokines with importance in inflammatory diseases. Expert Opin. Ther. Targets 11: 601–612. doi: 10.1517/14728222.11.5.601 [DOI] [PubMed] [Google Scholar]
- 35.Sadlack B., Merz H., Schorle H., Schimpl A., Feller A. C., Horak I.1993. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 75: 253–261. doi: 10.1016/0092-8674(93)80067-O [DOI] [PubMed] [Google Scholar]
- 36.Steinert A., Linas I., Kaya B., Ibrahim M., Schlitzer A., Hruz P., Radulovic K., Terracciano L., Macpherson A. J., Niess J. H.2017. The Stimulation of Macrophages with TLR Ligands Supports Increased IL-19 Expression in Inflammatory Bowel Disease Patients and in Colitis Models. J. Immunol. 199: 2570–2584. doi: 10.4049/jimmunol.1700350 [DOI] [PubMed] [Google Scholar]
- 37.Tanaka J., Saga K., Kido M., Nishiura H., Akamatsu T., Chiba T., Watanabe N.2010. Proinflammatory Th2 cytokines induce production of thymic stromal lymphopoietin in human colonic epithelial cells. Dig. Dis. Sci. 55: 1896–1904. doi: 10.1007/s10620-009-0979-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tong M., Li X., Wegener Parfrey L., Roth B., Ippoliti A., Wei B., Borneman J., McGovern D. P., Frank D. N., Li E., Horvath S., Knight R., Braun J.2013. A modular organization of the human intestinal mucosal microbiota and its association with inflammatory bowel disease. PLoS One 8: e80702. doi: 10.1371/journal.pone.0080702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto-Furusho J. K., Álvarez-León E., Fragoso J. M., Gozalishvilli A., Vallejo M., Vargas-Alarcón G.2011. Protective role of interleukin-19 gene polymorphisms in patients with ulcerative colitis. Hum. Immunol. 72: 1029–1032. doi: 10.1016/j.humimm.2011.08.013 [DOI] [PubMed] [Google Scholar]