Abstract
Introduction
Globally, sexually transmitted infections (STI) affect >300 million people annually, and are a major cause of sexual and reproductive health complications in women. In this commentary, we describe how STIs interact with the immune and non‐immune cells, both within and below the cervicovaginal mucosal barrier, to cause inflammation, which in turn has been associated with increased HIV acquisition risk.
Discussion
STIs have a major impact on the female genital mucosa, which is an important biological and physical barrier that forms the first line of defence against invading microorganisms such as HIV. Pattern recognition of STI pathogens, by receptors expressed either on the cell surface or inside the cell, typically triggers inflammation at the mucosal barrier. The types of mucosal responses vary by STI, and can be asymptomatic or culminate in the formation of discharge, ulcers and/or warts. While the aim of this response is to clear the invading microbes, in many cases these responses are either evaded or cause pathology that impairs barrier integrity and increases HIV access to target cells in the sub‐mucosa. In addition, innate responses to STIs can result in an increased number of immune cells, including those that are the primary targets of HIV, and may contribute to the association between STIs and increased susceptibility to HIV acquisition. Many of these cells are mediators of adaptive immunity, including tissue‐resident cells that may also display innate‐like functions. Bacterial vaginosis (BV) is another common cause of inflammation, and evidence for multiple interactions between BV, STIs and HIV suggest that susceptibility to these conditions should be considered in concert.
Conclusions
STIs and other microbes can induce inflammation in the genital tract, perturbing the normal robust function of the mucosal barrier against HIV. While the impact of STIs on the mucosal immune system and HIV acquisition is often under‐appreciated, understanding their interactions of the infections with the immune responses play an important role in improving treatment and reducing the risk of HIV acquisition. The frequent sub‐clinical inflammation associated with STIs underscores the need for better STI diagnostics to reverse the immunological consequences of infection.
Keywords: immunology, inflammation, sexually transmitted infections, women, HIV, bacterial vaginosis, mucosal immune responses, adaptive immune responses
1. Introduction
There are over 50 types of viruses, bacteria and parasites that can be sexually transmitted, eight of which are most widely recognized as sexually transmitted infections (STIs). These include syphilis, gonorrhoea, chlamydia, trichomoniasis, hepatitis B, herpes simplex virus (HSV), human papillomavirus (HPV) and HIV 1. Most often, STIs are either asymptomatic or mildly symptomatic, and therefore remain undiagnosed and under‐recognized by patients and clinicians 2. Long‐term infection by STIs can cause severe reproductive health complications in women, including still birth, preterm delivery, increased risk of HIV acquisition, infertility and cancer, among others 3, 4, 5. In most high‐income countries, policies ensure the availability of diagnostic tests, rapid delivery of results and contact tracing for those infected; however, these are not typically available in low‐ and middle‐income countries where STIs are common and managed primarily on the basis of signs and symptoms. While syndromic diagnosis is reasonably sensitive and specific for ulcerative infections, other STIs are often missed due to poor sensitivity and may remain untreated for long periods of time 6.
Another challenge is drug resistance; infections such as Neisseria gonorrhoeae are increasingly resistant to standard therapies including macrolides, tetracyclines and cephalosporins 7. This increase in antibiotic resistance, combined with high prevalence, low rates of treatment, and their association with HIV transmission and reproductive compilations, all underscore the need to better understand the mucosal immune responses to STI‐causing organisms.
The purpose of this commentary was to describe how STIs interact with the vaginal mucosal barrier, and the commensal microbes that line its luminal surface, to cause inflammation. While this commentary focuses on STIs in women, some similar mechanisms have been suggested for male genital immunology 8, 9, 10. Many of the pathological effects of STIs correspond to biological mechanisms that may favour HIV acquisition in women.
2. Discussion
2.1. Types of mucosal immune responses to STIs
There are several ways to classify STIs, the most obvious being by the type of causative organism, that is, bacterial, viral or parasitic. A second important way is by clinical presentation; although STIs are frequently asymptomatic, they can also cause (a) ulcers in genital, anal, oral and perianal tissues (e.g. Treponema pallidum, HSV), (b) urethral and vaginal discharge (e.g. Chlamydia trachomatis, N. gonorrhoeae and Mycoplasma genitalium), or (c) genital warts (e.g. HPV) 11.
Yet another way to classify STIs is by the different mechanisms through which they cause infections and evade immunity. STIs result in a large inflammatory response that can lead to pathology throughout the genital tract, including pelvic inflammatory disease, ectopic pregnancy and infertility, and degradation of the epithelium. As part of this inflammatory response, an influx of immune cells including neutrophils has been associated with discharge and lesions in the genital tract, resulting in further damage to the epithelial barrier 12. We and others have shown that this epithelial damage may be due to increased protease expression, which functions to degrade epithelial integrity 13, 14.
Although the mechanisms differ, the ability of all STI‐causing pathogens to induce an inflammatory response, damage the epithelial barrier, and impair natural innate defences is believed to increase the risk of HIV acquisition, by providing the virus better access to HIV target cells in the sub‐mucosa and beyond. Inflammation may simultaneously increase the number of and location of these cells relative to the lumen or induce phenotypic changes that increase their cellular susceptibility to virus infection 15. The inflammatory responses induced by STIs is intended to (and in some cases may) play an important role in protecting the host, but in many other cases this response favours the pathogen. This could be due to evasion of the effector mechanisms that are aimed at pathogen clearance (see Table 1), but also by causing collateral damage to host tissues 16, 17, 18. For example, in C. trachomatis infection, neutrophils are among the first immune cells to be recruited to the site of infection. Delayed apoptosis is a strategy used by C. trachomatis to avoid a complete immune response whereby it reduces the neutrophil sensitivity towards the stimuli from apoptosis, hence contributing towards pathogen persistence 19.
Table 1.
Immune evasion strategies employed by common STIs
| Strategy | Definition | Examples | References |
|---|---|---|---|
| Internalization | Epithelial cell entry, avoiding extracellular mechanisms of immune surveillance such as antibody responses | Chlamydia trachomatis Neisseria gonorrhoeae Mycoplasma genitalium | 26, 90, 91 |
| Deregulation of cellular process | Inhibition of important cellular processes in order to dampen the immune response e.g. DNA methylation, maturation of DCs, activation of immunoinhibitory pathways |
HPV, HSV2, C. trachomatis Treponema pallidum |
92, 93, 94, 95 |
| Resistance to antimicrobial peptides | Expression of genes which are highly resistant to antimicrobial peptides | Haemophilus ducreyi | 96, 97, 98, 99 |
| Interference with the processes of the complement system | Acquisition of CD59 from different host cells, which inhibits binding of C9 with C5b‐C8 that is critical for pore formation. In addition, this pathogen can stimulate iron induced cysteine protease activity. | Trichomonas vaginalis | 100, 101 |
| Structure alteration | Pathogen‐induced changes to their extracellular structure to avoid detection by the innate immune system. | M. genitalium | 37 |
| Inhibition of Th1 CD4 and CTL responses | Pathogens upregulate specific responses which results to the suppression other immune responses that would result to their clearance. For example, upregulation of Th17 response that results to the downregulation of Th1 response. | M. genitalium, Chlamydia trachomatis T. vaginalis, HSV2, HPV, Treponema pallidum, N. gonorrhoeae | 95, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112 |
| Inhibition of other types of T cell responses (Th2, 17, 22, Treg) | The pathogen downregulates the immune response in specific cells like macrophages, dendritic cells and monocytes. | T. vaginalis, HPV, N. gonorrhoeae, T. pallidum |
2.2. STIs and genital inflammation
Genital inflammation, defined by elevated cytokines, has been a strong predictor of HIV acquisition risk and decreased TFV gel efficacy 20, 21. Elevated levels of inflammatory cytokines have been highly correlated to increased protease activity, which may decrease the integrity of the epithelial barrier 13, 14. South African women with laboratory‐confirmed STI infections had increased the levels of inflammatory cytokines in the genital tract, including IL‐1α, IL‐4, fractalkine, TNF‐β, macrophage‐derived chemokine, IL‐1β and interferon‐γ 20, 22. STIs have been associated with increased genital inflammation signatures specifically among those with C. trachomatis infections 23, 24, 25, 26. Many studies have established that mucosal cytokine production occurs after STI acquisition, forming a central feature of the ensuing immune response. Therefore, consideration of the broader immune pathways that drive these cytokine responses could provide important insight into how STIs change the mucosal milieu 27, 28.
2.3. Intracellular and extracellular recognition of STIs by pattern recognition receptors
Mucosal epithelial cells are the first barrier against infection, forming an early line of defence against pathogen invasion. Epithelial cells are equipped with receptors that are crucial for pathogen detection, and these cells function to initiate and modulate the inflammatory cascade aimed at inducing pathogen clearance 29, 30. Inflammation leads to a series of reactions which induce adaptive immunity, including effector mechanisms that can clear infection. However, tight regulation of inflammation is required in order to avoid self‐damage 30, 31. In the case of STIs, a combination of immune evasion, potent induction of inflammation and poor natural immunity represents scenarios in which HIV entry may be increased (Figure 1).
Figure 1.
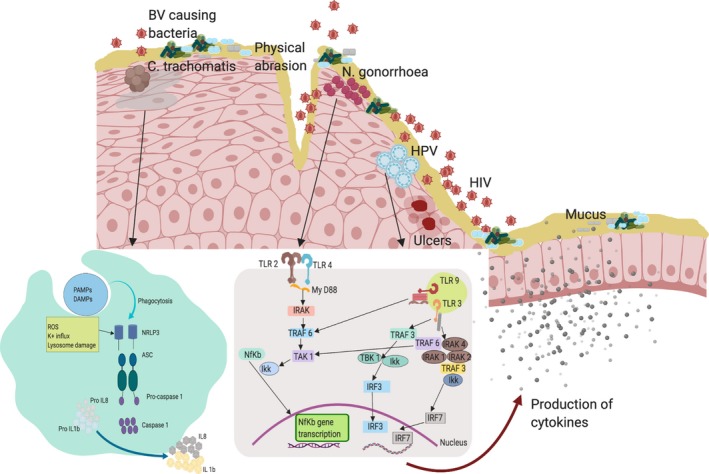
Mucosal innate immune responses to STIs in the female genital tract that could potentiate HIV transmission risk. Depicted are several of the modes through which STIs might increase the risk of HIV acquisition. Infection with STIs results to physical abrasion, ulcer formation and increase of pro‐inflammatory cytokines resulting in inflammation. Inflammation increases the availability of HIV target cells in the sub‐mucosa. During N. gonorrhoeae infection, TLR2 and 4 detect lipooligosaccharide and induce a NF‐KB driven immune response resulting to production of cytokines. Infection with C. trachomatis results in death of some cells which in turn produce elementary bodies. C. trachomatis infection is detected by inflammasomes resulting to production of IL‐1b and IL‐8 through the NLR3 pathway. TLR9 detects the CpG island in Genomic material of the HPV virus inducing an immune response through the MYD88 pathway. TLR3 detects the viral nucleic acid to induce an immune response through the IRF and IR7 pathways.
Toll‐like receptors (TLRs) play an important role in detecting pathogens including STIs, and initiating appropriate innate and adaptive immune responses. TLRs bind to their cognate ligands, resulting in a signalling cascade that culminates in the expression of pro‐inflammatory cytokines. TLRs can be classified as both intracellular (TLR3,7,8,9,11,12 and 13) and extracellular (TLR1,2,4,5,6 and 10), on the basis of their expression and where ligand recognition typically occurs 32, 33. TLRs recognize pathogen‐associated molecular patterns (PAMPs), including bacterial DNA, viral nucleic acid and viral proteins, with the eventual goal of inducing specific T‐cell and antibody responses. For example, TLR9 detects the unmethylated CpG sequences in bacterial DNA molecules. Many TLRs signal via MyD88, an important intracellular protein adaptor molecule. MyD88 is responsible for induction of the IL‐1 family, a group of 11 mainly inflammatory cytokines that regulate innate immune cell function. IL‐1R‐associated kinase is recruited via MyD88 activation, further activating the NF‐kB pathway culminating in transcription of pro‐inflammatory cytokine genes 34, 35.
Several bacterial STIs induce innate inflammatory responses by interacting with extracellular TLRs. A recent study that utilized a 3D model of endocervical cells showed that M. genitalium was recognized by TLR2, 4 and 6, a pattern of TLR usage that initiates the NF‐KB pathway and is unique to this bacterium 36, 37. In microorganisms such as Neisseria, protein elements are detected both intracellularly and extracellularly, both of which can induce an NF‐KB driven inflammatory response. TLR 2 and 4 detect LPS, outer membrane vesicles, porins and other proteins, while additional pattern recognition molecules called NOD 1 and 2 detect additional STI biochemical structures such as gamma glutamyl diaminopimelic acid and muramyl dipeptide, which also results in induction of NF‐KB‐driven inflammation 38, 39.
Intracellular TLRs mainly detect viral infections. In contrast to many extracellular TLRs, which tend to recognize protein structures, intracellular expression of TLR3, TLR7, TLR8 and TLR9 mediates viral nucleic acid sensing. In a recent study that evaluated TLR gene expression by qPCR in endocervical cells of women, increased levels of TLR and IFN‐α2 were observed among those who had cleared HPV‐16 infection, suggesting that TLR responses may be associated with viral clearance. Moreover, HPV‐16 may interfere with these responses, thus enhancing their persistence 40. In this study, TLR9 expression was upregulated during high‐risk HPV infection and was higher in HPV‐positive compared to HPV‐negative individuals, confirming that TLR9 plays an important role in the detection of CpG islands in the DNA motifs during HPV infection in vivo 41.
STIs similarly induce immune responses through inflammasomes (multi‐protein intracellular structures located in the cytosol). The inflammasome is activated by the signalling of PAMPs, DAMPs (damage associated molecular proteins), changes in the ion concentrations of cytosol and by extracellular adenosine triphosphate (ATP). Once activated, this molecular complex leads to expression of pro‐inflammatory cytokines and can also initiate an inflammatory form of cell death called pyroptosis 42, 43, 44. Activation of the inflammasome often occurs through NOD‐like receptors (NLRs, especially NLR3), which interacts with apoptosis‐associated speck‐like protein containing a CARD (ASC). This protein is located in the nucleus of macrophages and monocytes and is responsible for activating caspase‐1, which in turn cleaves and activates IL‐1β and IL‐8 44. C. trachomatis, co‐cultured with epithelial cells, were found to activate inflammasomes resulting in IL‐1β and IL‐8 production and activation of pyroptosis. An inactivated form of C. trachomatis was tested in the same model and was still found to lead to priming of the inflammasome, but without the resulting inflammatory response, implying that pathogen replication may be critical for cytokine induction 45. This inflammatory pathway also applies to other STIs; for example, the LPS of N. gonorrhoeae has been shown to harbour hexa‐acylated lipid A, which can activate the NRLP3 inflammasome 46. H. ducreyi elicits IL‐1β responses that are dependent on activation of caspase‐1, ‐5 and NLRP3 in both M1 and 2 macrophages 47. In viral STIs such as HPV, cytosolic viral DNA is detected by AIM2 inflammasome and IFI16, an intracellular DNA sensor, resulting in the production of IL‐1β and IFN‐β respectively. Blocking of AIM2 resulted in increased production of IFN‐β thus it has the ability to block the production of IFN‐β an important mediator of antiviral response 48.
2.4. Co‐infection with STIs, bacterial vaginosis and HIV
In addition to the mucosal barrier, the composition of the vaginal microbiome can play an important role in providing immune defence at the genital mucosa. In particular, women with certain Lactobacillus‐dominant communities are able to produce lactic acid and maintain a low mucosal pH, which inhibits the growth of pathogenic bacteria including STIs. In the absence of Lactobacillus spp., with the exception of Lactobacillus iners, a more diverse microbiome population is typical, which is often associated with bacterial vaginosis (BV). BV, defined either by Nugent scoring or using molecular methods 49, has been associated with an increased risk of both STI and HIV acquisition 50, 51, 52, 53, 54, 55, 56, 57. Both STIs and BV are associated with increased levels of inflammatory cytokines like IFN‐α2, IL‐1α, IL‐1β, TNF‐α, IFN‐γ and IL‐8 51, 58. Epithelial cells of the genital mucosa produce glycogen, an energy source that allows Lactobacillus spp. to flourish 59, 60, which has been suggested provide protection against Chlamydia infection 61.
Synergism between BV and STIs is in part through the production of metabolites by the BV causing bacteria, which are utilized by STIs as growth factors. An example is seen between BV and C. trachomatis infections. Bacterial species that produce tryptophan have been associated with the increased risk of C. trachomatis infection among women whereas Indoleamine‐2,3‐dehydrogenase 1 (IDO1) producing species have been associated with decreased risk. IDOL1 inhibits the availability of tryptophan. This shows that BV may play an important role in both first time and recurrent C. trachomatis infections 62, 63. Ziklo et al. found that chlamydia infection was associated with reduced IFN‐γ response. IL‐17 was also reduced among infected individuals, and this cytokine is important in boosting host defence and maintaining mucosal barrier. Therefore, the increased levels of kynurenine, the byproduct of tryptophan breakdown, is associated with increased risk of HIV acquisition 63, 64, 65. Studies have suggested that ethnicity and not metabolic mechanisms may also underlie the association between chlamydia and HIV 28, 66, 67. Increased risk of Trichomonas vaginalis acquisition has been associated with BV in both HIV‐positive and ‐negative women 68.
Durable and effective treatment of BV has been a major challenge for the field. Oral or topical metronidazole is effective in the short term, yet recurrence occurs among more that 50% of women within three to twelve months 50, 69, 70. However, periodic presumptive treatment has proven to be an effective method in reducing STI incidence 71, 72. This strengthens the case for a causal relationship between BV and STIs, and also suggests that reducing BV may help to reduce STI incidence.
STI co‐infection in HIV‐positive women, particularly by N. gonorrhoeae or HSV‐2, increases inflammatory responses and mucosal HIV shedding 22, 73, 74, 75. In addition to mucosal inflammatory response, STIs such as N. gonorrhoeae have been found to increase plasma viral load and reduce CD4 T‐cell counts, indicating that both STI and HIV act synergistically resulting in detrimental effects to the host. While studies have suggested that STI treatment could reduce HIV shedding and transmission 73, this may be a moot point in the era of effective antiretroviral therapy, which, if taken correctly, reduces HIV transmission almost completely 76.
2.5. Role of adaptive immune response in STIs
Mechanisms of immunity to STIs are poorly understood, forming an obvious barrier to vaccine development. Epidemiological evidence for immunity to Chlamydia has been shown in the context to treatment 77. While the mechanism for the immunity is unclear, C. trachomatis infection has been associated with the formation of follicles 78; the presence of IFN‐γ+ CD4+ T cells in these follicles has been thought to provide an immune response in the case of a secondary infection 79. In some STI infections, re‐infection occurs long after the primary infection 80, 81, 82, as the adaptive immune response following primary infection plays a major role in immune surveillance and forms the first line of immune response in secondary infection.
Memory T‐cells were initially divided into central and effector memory T‐cells, which preferentially home to non‐lymphoid and secondary lymphoid organs respectively. Since that time, it is clear that there is an additional population of tissue‐resident memory lymphocytes that either do not re‐circulate, or re‐circulate very slowly, and provide rapid responses to re‐infection 83, 84. The role of these cells in the STI response is only beginning to be explored, with some data emerging for HSV‐2. In HSV‐2, a persistent infection occurs at the dermal epidermal joint (DEJ) of the mucosal lining with CD8+ T cells being the most predominant immune cells at this site. An assessment of CD8+ T‐cells at the DEJ in biopsies of HSV‐2 infected individuals revealed a high proportion of CD8 TCRαβ T‐cells. A comparison of the prevalence of CD8β or CD8α subsets at the DEJ showed that there was a higher population of CD8α mRNA, which were specifically CD8αα homodimers, an indication that they are responsible for containing HSV‐2 infection. The CD8α T‐cells formed clusters around epithelial cells that were HSV‐2 specific 85.
Additional cells including mucosal associated invariant T (MAIT) cells, invariant natural killer T (iNKT) cells, γδ T‐cells, innate lymphoid cells and IELs form part of the connection between the innate and adaptive response, and play a major role in guarding the integrity of the tissue and generation of local immune responses. Some studies support the presence of these cells in the vagina 86, 87, 88, 89, however, their responses to STIs have not been extensively explored.
3. Conclusions
In summary, STIs induce inflammatory responses through interactions with the epithelial barrier and immune cells at the site of infection. There are several molecular pathways involved in the inflammatory response to a diverse range of STIs, all of which likely function to cause pathology by weakening the mucosal barrier. At the same time, STIs use a variety of immune evasion strategies to dampen the immune response and enhance their persistence. STIs and BV likely both increase the risk of HIV acquisition by damaging the mucosal barrier and increasing pro‐inflammatory cytokines, increasing the availability of HIV target cells. The impact of STIs on mucosal immune responses and HIV acquisition is often under‐appreciated, but improved control of these infections through better diagnosis, treatment and prevention could make an important contribution to reducing HIV risk and improving reproductive health outcomes.
Competing interests
The authors declare no conflicts of interest.
Authors’ contributions
RM wrote the first draft of the paper. LRM, CB, SSAK and QAK provided critical review of the paper.
Acknowledgements
Funding
LRM was funded by CIHR New Investigator Award. Some of the research mentioned in the review was conducted as part of the DST‐NRF Centre of Excellence in HIV Prevention, which is supported by the Department of Science and Technology and the National Research Foundation (UID: 96354).
Mwatelah, R. , McKinnon, L. R. , Baxter, C. , Karim, Q. A. , Abdool Karim, S. S. Mechanisms of sexually transmitted infection‐induced inflammation in women: implications for HIV risk. J Int AIDS Soc. 2019; 22(S6):e25346
References
- 1. WHO . Sexually transmitted infections. Geneva, Switzerland: World Health Organisation; 2019. [cited 2019 Jun 1]. Available from: https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis) [Google Scholar]
- 2. Tsevat DG, Wiesenfeld HC, Parks C, Peipert JF. Sexually transmitted diseases and infertility. Am J Obstet Gynecol. 2017;216(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bansal A, Singh MP, Rai B. Human papillomavirus‐associated cancers: a growing global problem. Int J Appl Basic Med Res. 2016;6(2):84–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buder S, Schofer H, Meyer T, Bremer V, Kohl PK, Skaletz‐Rorowski A, et al. Bacterial sexually transmitted infections. J Dtsch Dermatol Ges. 2019;17(3):287–315. [DOI] [PubMed] [Google Scholar]
- 5. Paavonen J. Chlamydia trachomatis and cancer. Sex Transm Infect. 2001;77(3):154–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Masson L, Barnabas S, Deese J, Lennard K, Dabee S, Gamieldien H, et al. Inflammatory cytokine biomarkers of asymptomatic sexually transmitted infections and vaginal dysbiosis: a multicentre validation study. Sex Transm Infect. 2019;95(1):5–12. [DOI] [PubMed] [Google Scholar]
- 7. WHO . WHO guidelines for the treatment of Neisseria gonorrhoeae. Geneva, Switzerland: World Health Organisation; 2016. [cited 2019 Jun 1]. Available from: https://www.who.int/reproductivehealth/publications/rtis/gonorrhoea-treatment-guidelines/en/ [PubMed] [Google Scholar]
- 8. Prodger JL, Kaul R. The biology of how circumcision reduces HIV susceptibility: broader implications for the prevention field. AIDS Res Ther. 2017;14(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anderson D, Politch JA, Pudney J. HIV infection and immune defense of the penis. Am J Reprod Immunol. 2011;65(3):220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Esra RT, Olivier AJ, Passmore JA, Jaspan HB, Harryparsad R, Gray CM. Does HIV exploit the inflammatory milieu of the male genital tract for successful infection? Front Immunol. 2016;7:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wagenlehner FM, Brockmeyer NH, Discher T, Friese K, Wichelhaus TA. The presentation, diagnosis, and treatment of sexually transmitted infections. Dtsch Arztebl Int. 2016;113(1–02):11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fichorova RN, Trifonova RT, Gilbert RO, Costello CE, Hayes GR, Lucas JJ, et al. Trichomonas vaginalis lipophosphoglycan triggers a selective upregulation of cytokines by human female reproductive tract epithelial cells. Infect Immun. 2006;74(10):5773–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arnold KB, Burgener A, Birse K, Romas L, Dunphy LJ, Shahabi K, et al. Increased levels of inflammatory cytokines in the female reproductive tract are associated with altered expression of proteases, mucosal barrier proteins, and an influx of HIV‐susceptible target cells. Mucosal Immunol. 2016;9(1):194–205. [DOI] [PubMed] [Google Scholar]
- 14. Borgdorff H, Gautam R, Armstrong SD, Xia D, Ndayisaba GF, van Teijlingen NH, et al. Cervicovaginal microbiome dysbiosis is associated with proteome changes related to alterations of the cervicovaginal mucosal barrier. Mucosal Immunol. 2016;9(3):621–33. [DOI] [PubMed] [Google Scholar]
- 15. McKinnon LR, Kaul R. Quality and quantity: mucosal CD4+ T cells and HIV susceptibility. Curr Opin HIV AIDS. 2012;7(2):195–202. [DOI] [PubMed] [Google Scholar]
- 16. Rusconi B, Greub G. Chlamydiales and the innate immune response: friend or foe? FEMS Immunol Med Microbiol. 2011;61(3):231–44. [DOI] [PubMed] [Google Scholar]
- 17. Chew T, Taylor KE, Mossman KL. Innate and adaptive immune responses to herpes simplex virus. Viruses. 2009;1(3):979–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nunes RAL, Morale MG, Silva GAF, Villa LL, Termini L. Innate immunity and HPV: friends or foes. Clinics (Sao Paulo). 2018;73 Suppl 1:e549s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vasilevsky S, Greub G, Nardelli‐Haefliger D, Baud D. Genital Chlamydia trachomatis: understanding the roles of innate and adaptive immunity in vaccine research. Clin Microbiol Rev. 2014;27(2):346–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Masson L, Passmore JA, Liebenberg LJ, Werner L, Baxter C, Arnold KB, et al. Genital inflammation and the risk of HIV acquisition in women. Clin Infect Dis. 2015;61(2):260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McKinnon LR, Liebenberg LJ, Yende‐Zuma N, Archary D, Ngcapu S, Sivro A, et al. Genital inflammation undermines the effectiveness of tenofovir gel in preventing HIV acquisition in women. Nat Med. 2018;24(4):491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mlisana K, Naicker N, Werner L, Roberts L, van Loggerenberg F, Baxter C, et al. Symptomatic vaginal discharge is a poor predictor of sexually transmitted infections and genital tract inflammation in high‐risk women in South Africa. J Infect Dis. 2012;206(1):6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Passmore JA, Jaspan HB, Masson L. Genital inflammation, immune activation and risk of sexual HIV acquisition. Curr Opin HIV AIDS. 2016;11(2):156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jha R, Srivastava P, Salhan S, Finckh A, Gabay C, Mittal A, et al. Spontaneous secretion of interleukin‐17 and ‐22 by human cervical cells in Chlamydia trachomatis infection. Microbes Infect. 2011;13(2):167–78. [DOI] [PubMed] [Google Scholar]
- 25. O'Connell CM, Ferone ME. Chlamydia trachomatis genital infections. Microb Cell. 2016;3(9):390–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Buckner LR, Lewis ME, Greene SJ, Foster TP, Quayle AJ. Chlamydia trachomatis infection results in a modest pro‐inflammatory cytokine response and a decrease in T cell chemokine secretion in human polarized endocervical epithelial cells. Cytokine. 2013;63(2):151–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thurman AR, Doncel GF. Innate immunity and inflammatory response to Trichomonas vaginalis and bacterial vaginosis: relationship to HIV acquisition. Am J Reprod Immunol. 2011;65(2):89–98. [DOI] [PubMed] [Google Scholar]
- 28. Fichorova RN, Chen PL, Morrison CS, Doncel GF, Mendonca K, Kwok C, et al. The contribution of cervicovaginal infections to the immunomodulatory effects of hormonal contraception. MBio. 2015;6(5):e00221–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McClure R, Massari P. TLR‐dependent human mucosal epithelial cell responses to microbial pathogens. Front Immunol. 2014;5:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. [DOI] [PubMed] [Google Scholar]
- 31. Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and inflammation‐associated diseases in organs. Oncotarget. 2018;9(6):7204–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kawasaki T, Kawai T. Toll‐like receptor signaling pathways. Front Immunol. 2014;5:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kawai T, Akira S. The role of pattern‐recognition receptors in innate immunity: update on Toll‐like receptors. Nat Immunol. 2010;11(5):373–84. [DOI] [PubMed] [Google Scholar]
- 34. Sims JE, Smith DE. The IL‐1 family: regulators of immunity. Nat Rev Immunol. 2010;10(2):89–102. [DOI] [PubMed] [Google Scholar]
- 35. Fichorova RN, Cronin AO, Lien E, Anderson DJ, Ingalls RR. Response to Neisseria gonorrhoeae by cervicovaginal epithelial cells occurs in the absence of toll‐like receptor 4‐mediated signaling. J Immunol. 2002;168(5):2424–32. [DOI] [PubMed] [Google Scholar]
- 36. McGowin CL, Ma L, Martin DH, Pyles RB. Mycoplasma genitalium‐encoded MG309 activates NF‐kappaB via Toll‐like receptors 2 and 6 to elicit proinflammatory cytokine secretion from human genital epithelial cells. Infect Immun. 2009;77(3):1175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dehon PM, McGowin CL. The immunopathogenesis of mycoplasma genitalium infections in women: a narrative review. Sex Transm Dis. 2017;44(7):428–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chan JM, Dillard JP. Attention seeker: production, modification, and release of inflammatory peptidoglycan fragments in neisseria species. J Bacteriol. 2017;199(20):e00354–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stevens JS, Criss AK. Pathogenesis of Neisseria gonorrhoeae in the female reproductive tract: neutrophilic host response, sustained infection, and clinical sequelae. Curr Opin Hematol. 2018;25(1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Daud II, Scott ME, Ma Y, Shiboski S, Farhat S, Moscicki AB. Association between toll‐like receptor expression and human papillomavirus type 16 persistence. Int J Cancer. 2011;128(4):879–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cannella F, Pierangeli A, Scagnolari C, Cacciotti G, Tranquilli G, Stentella P, et al. TLR9 is expressed in human papillomavirus‐positive cervical cells and is overexpressed in persistent infections. Immunobiology. 2015;220(3):363–8. [DOI] [PubMed] [Google Scholar]
- 42. Sharma D, Kanneganti TD. The cell biology of inflammasomes: mechanisms of inflammasome activation and regulation. J Cell Biol. 2016;213(6):617–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lupfer C, Anand PK. Integrating inflammasome signaling in sexually transmitted infections. Trends Immunol. 2016;37(10):703–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Verma V, Dhanda RS, Moller NF, Yadav M. Inflammasomes and their role in innate immunity of sexually transmitted infections. Front Immunol. 2016;7:540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Webster SJ, Brode S, Ellis L, Fitzmaurice TJ, Elder MJ, Gekara NO, et al. Detection of a microbial metabolite by STING regulates inflammasome activation in response to Chlamydia trachomatis infection. PLoS Pathog. 2017;13(6):e1006383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhou X, Gao X, Broglie PM, Kebaier C, Anderson JE, Thom N, et al. Hexa‐acylated lipid A is required for host inflammatory response to Neisseria gonorrhoeae in experimental gonorrhea. Infect Immun. 2014;82(1):184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li W, Katz BP, Bauer ME, Spinola SM. Haemophilus ducreyi infection induces activation of the NLRP3 inflammasome in nonpolarized but not in polarized human macrophages. Infect Immun. 2013;81(8):2997–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reinholz M, Kawakami Y, Salzer S, Kreuter A, Dombrowski Y, Koglin S, et al. HPV16 activates the AIM2 inflammasome in keratinocytes. Arch Dermatol Res. 2013;305(8):723–32. [DOI] [PubMed] [Google Scholar]
- 49. McKinnon LR, Achilles SL, Bradshaw CS, Burgener A, Crucitti T, Fredricks DN, et al. The evolving facets of bacterial vaginosis: implications for HIV transmission. AIDS Res Hum Retroviruses. 2019;35(3):219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Francis SC, Looker C, Vandepitte J, Bukenya J, Mayanja Y, Nakubulwa S, et al. Bacterial vaginosis among women at high risk for HIV in Uganda: high rate of recurrent diagnosis despite treatment. Sex Transm Infect. 2016;92(2):142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Masson L, Arnold KB, Little F, Mlisana K, Lewis DA, Mkhize N, et al. Inflammatory cytokine biomarkers to identify women with asymptomatic sexually transmitted infections and bacterial vaginosis who are at high risk of HIV infection. Sex Transm Infect. 2016;92(3):186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta‐analysis of published studies. AIDS. 2008;22(12):1493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. van de Wijgert JH, Morrison CS, Cornelisse PG, Munjoma M, Moncada J, Awio P, et al. Bacterial vaginosis and vaginal yeast, but not vaginal cleansing, increase HIV‐1 acquisition in African women. J Acquir Immune Defic Syndr. 2008;48(2):203–10. [DOI] [PubMed] [Google Scholar]
- 54. Masese L, Baeten JM, Richardson BA, Bukusi E, John‐Stewart G, Graham SM, et al. Changes in the contribution of genital tract infections to HIV acquisition among Kenyan high‐risk women from 1993 to 2012. AIDS. 2015;29(9):1077–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lennard K, Dabee S, Barnabas SL, Havyarimana E, Blakney A, Jaumdally SZ, et al. Microbial composition predicts genital tract inflammation and persistent bacterial vaginosis in South African adolescent females. Infect Immun. 2018;86(1):e00410–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gosmann C, Anahtar MN, Handley SA, Farcasanu M, Abu‐Ali G, Bowman BA, et al. Lactobacillus‐deficient cervicovaginal bacterial communities are associated with increased HIV acquisition in young south african women. Immunity. 2017;46(1):29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McClelland RS, Lingappa JR, Srinivasan S, Kinuthia J, John‐Stewart GC, Jaoko W, et al. Evaluation of the association between the concentrations of key vaginal bacteria and the increased risk of HIV acquisition in African women from five cohorts: a nested case‐control study. Lancet Infect Dis. 2018;18(5):554–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Anahtar MN, Byrne EH, Doherty KE, Bowman BA, Yamamoto HS, Soumillon M, et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity. 2015;42(5):965–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Anderson DJ, Marathe J, Pudney J. The structure of the human vaginal stratum corneum and its role in immune defense. Am J Reprod Immunol. 2014;71(6):618–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mirmonsef P, Hotton AL, Gilbert D, Burgad D, Landay A, Weber KM, et al. Free glycogen in vaginal fluids is associated with Lactobacillus colonization and low vaginal pH. PLoS One. 2014;9(7):e102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gong Z, Luna Y, Yu P, Fan H. Lactobacilli inactivate Chlamydia trachomatis through lactic acid but not H2O2. PLoS One. 2014;9(9):e107758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ziklo N, Huston WM, Taing K, Katouli M, Timms P. In vitro rescue of genital strains of Chlamydia trachomatis from interferon‐gamma and tryptophan depletion with indole‐positive, but not indole‐negative Prevotella spp. BMC Microbiol. 2016;16(1):286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ziklo N, Vidgen ME, Taing K, Huston WM, Timms P. Dysbiosis of the vaginal microbiota and higher vaginal kynurenine/tryptophan ratio reveals an association with Chlamydia trachomatis genital infections. Front Cell Infect Microbiol. 2018;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, et al. Tryptophan catabolism by indoleamine 2,3‐dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2(32):32ra6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Goodbourn S, Didcock L, Randall RE. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J Gen Virol. 2000;81 Pt 10:2341–64. [DOI] [PubMed] [Google Scholar]
- 66. Fettweis JM, Brooks JP, Serrano MG, Sheth NU, Girerd PH, Edwards DJ, et al. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology. 2014;160 Pt 10:2272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. van der Veer C, Bruisten SM, van der Helm JJ, de Vries HJ, van Houdt R. The cervicovaginal microbiota in women notified for Chlamydia trachomatis infection: a case‐control study at the sexually transmitted infection outpatient clinic in amsterdam, The Netherlands. Clin Infect Dis. 2017;64(1):24–31. [DOI] [PubMed] [Google Scholar]
- 68. Balkus JE, Richardson BA, Rabe LK, Taha TE, Mgodi N, Kasaro MP, et al. Bacterial vaginosis and the risk of Trichomonas vaginalis acquisition among HIV‐1‐negative women. Sex Transm Dis. 2014;41(2):123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bradshaw CS, Morton AN, Hocking J, Garland SM, Morris MB, Moss LM, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. 2006;193(11):1478–86. [DOI] [PubMed] [Google Scholar]
- 70. Myer L, Kuhn L, Denny L, Wright TC Jr. Recurrence of symptomatic bacterial vaginosis 12 months after oral metronidazole therapy in HIV‐positive and ‐negative women. J Infect Dis. 2006;194(12):1797–9. [DOI] [PubMed] [Google Scholar]
- 71. Steen R, Dallabetta G. Sexually transmitted infection control with sex workers: regular screening and presumptive treatment augment efforts to reduce risk and vulnerability. Reprod Health Matters. 2003;11(22):74–90. [DOI] [PubMed] [Google Scholar]
- 72. Balkus JE, Manhart LE, Lee J, Anzala O, Kimani J, Schwebke J, et al. Periodic presumptive treatment for vaginal infections may reduce the incidence of sexually transmitted bacterial infections. J Infect Dis. 2016;213(12):1932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jarvis GA, Chang TL. Modulation of HIV transmission by Neisseria gonorrhoeae: molecular and immunological aspects. Curr HIV Res. 2012;10(3):211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Xu SX, Leontyev D, Kaul R, Gray‐Owen SD. Neisseria gonorrhoeae co‐infection exacerbates vaginal HIV shedding without affecting systemic viral loads in human CD34+ engrafted mice. PLoS One. 2018;13(1):e0191672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Phipps W, Saracino M, Magaret A, Selke S, Remington M, Huang ML, et al. Persistent genital herpes simplex virus‐2 shedding years following the first clinical episode. J Infect Dis. 2011;203(2):180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sivro A, McKinnon LR. Mucosal HIV Shedding During ART. J Infect Dis. 2017;216(12):1484–6. [DOI] [PubMed] [Google Scholar]
- 77. Geisler WM, Lensing SY, Press CG, Hook EW III. Spontaneous resolution of genital Chlamydia trachomatis infection in women and protection from reinfection. J Infect Dis. 2013;207(12):1850–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Darville T, Hiltke TJ. Pathogenesis of genital tract disease due to Chlamydia trachomatis . J Infect Dis. 2010;201(Suppl 2):S114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Johnson RM, Brunham RC. Tissue‐resident T cells as the central paradigm of chlamydia immunity. Infect Immun. 2016;84(4):868–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tsadik M, Berhane Y, Worku A, Terefe W. Perceived risk of reinfection among individuals treated for sexually transmitted infections in Northern Ethiopia: implication for use in clinical practice. Pan Afr Med J. 2017;27:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Das A, Pathni AK, Narayanan P, George B, Morineau G, Saidel T, et al. High rates of reinfection and incidence of bacterial sexually transmitted infections in a cohort of female sex workers from two Indian cities: need for different STI control strategies? Sex Transm Infect. 2013;89(1):5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mehta SD, Erbelding EJ, Zenilman JM, Rompalo AM. Gonorrhoea reinfection in heterosexual STD clinic attendees: longitudinal analysis of risks for first reinfection. Sex Transm Infect. 2003;79(2):124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mueller SN, Mackay LK. Tissue‐resident memory T cells: local specialists in immune defence. Nat Rev Immunol. 2016;16(2):79–89. [DOI] [PubMed] [Google Scholar]
- 84. Schenkel JM, Masopust D. Tissue‐resident memory T cells. Immunity. 2014;41(6):886–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zhu J, Peng T, Johnston C, Phasouk K, Kask AS, Klock A, et al. Immune surveillance by CD8alphaalpha+ skin‐resident T cells in human herpes virus infection. Nature. 2013;497(7450):494–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lee SK, Kim CJ, Kim DJ, Kang JH. Immune cells in the female reproductive tract. Immune Netw. 2015;15(1):16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Alcaide ML, Strbo N, Romero L, Jones DL, Rodriguez VJ, Arheart K, et al. Bacterial vaginosis is associated with loss of gamma delta T cells in the female reproductive tract in Women in the Miami Women Interagency HIV Study (WIHS): a cross sectional study. PLoS One. 2016;11(4):e0153045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gibbs A, Leeansyah E, Introini A, Paquin‐Proulx D, Hasselrot K, Andersson E, et al. MAIT cells reside in the female genital mucosa and are biased towards IL‐17 and IL‐22 production in response to bacterial stimulation. Mucosal Immunol. 2017;10(1):35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Juno JA, Lajoie J, Stalker AT, Oyugi J, Kimani M, Kimani J, et al. Enrichment of LAG‐3, but not PD‐1, on double negative T cells at the female genital tract. Am J Reprod Immunol. 2014;72(6):534–40. [DOI] [PubMed] [Google Scholar]
- 90. Valverde‐Villegas JM, de Medeiros RM, de Andrade KP, Jacovas VC, Dos Santos BR, Simon D, et al. Novel genetic associations and gene‐gene interactions of chemokine receptor and chemokine genetic polymorphisms in HIV/AIDS. AIDS. 2017;31(9):1235–43. [DOI] [PubMed] [Google Scholar]
- 91. McGowin CL, Radtke AL, Abraham K, Martin DH, Herbst‐Kralovetz M. Mycoplasma genitalium infection activates cellular host defense and inflammation pathways in a 3‐dimensional human endocervical epithelial cell model. J Infect Dis. 2013;207(12):1857–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Westrich JA, Warren CJ, Pyeon D. Evasion of host immune defenses by human papillomavirus. Virus Res. 2017;231:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Stefanidou M, Ramos I, Mas Casullo V, Trepanier JB, Rosenbaum S, Fernandez‐Sesma A, et al. Herpes simplex virus 2 (HSV‐2) prevents dendritic cell maturation, induces apoptosis, and triggers release of proinflammatory cytokines: potential links to HSV‐HIV synergy. J Virol. 2013;87(3):1443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fankhauser SC, Starnbach MN. PD‐L1 limits the mucosal CD8+ T cell response to Chlamydia trachomatis . J Immunol. 2014;192(3):1079–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sasagawa T, Takagi H, Makinoda S. Immune responses against human papillomavirus (HPV) infection and evasion of host defense in cervical cancer. J Infect Chemother. 2012;18(6):807–15. [DOI] [PubMed] [Google Scholar]
- 96. Bahar AA, Ren D. Antimicrobial peptides. Pharmaceuticals (Basel). 2013;6(12):1543–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mahlapuu M, Hakansson J, Ringstad L, Bjorn C. Antimicrobial peptides: an emerging category of therapeutic agents. Front Cell Infect Microbiol. 2016;6:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Trombley MP, Post DM, Rinker SD, Reinders LM, Fortney KR, Zwickl BW, et al. Phosphoethanolamine transferase LptA in Haemophilus ducreyi modifies lipid A and contributes to human defensin resistance in vitro . PLoS One. 2015;10(4):e0124373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Rinker SD, Trombley MP, Gu X, Fortney KR, Bauer ME. Deletion of mtrC in Haemophilus ducreyi increases sensitivity to human antimicrobial peptides and activates the CpxRA regulon. Infect Immun. 2011;79(6):2324–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ibanez‐Escribano A, Nogal‐Ruiz JJ, Perez‐Serrano J, Gomez‐Barrio A, Escario JA, Alderete JF. Sequestration of host‐CD59 as potential immune evasion strategy of Trichomonas vaginalis . Acta Trop. 2015;149:1–7. [DOI] [PubMed] [Google Scholar]
- 101. Nemati M, Malla N, Yadav M, Khorramdelazad H, Jafarzadeh A. Humoral and T cell‐mediated immune response against trichomoniasis. Parasite Immunol. 2018;40(3): doi: 10.1111/pim.12510. [DOI] [PubMed] [Google Scholar]
- 102. Li K, Wang C, Lu H, Gu X, Guan Z, Zhou P. Regulatory T cells in peripheral blood and cerebrospinal fluid of syphilis patients with and without neurological involvement. PLoS Negl Trop Dis. 2013;7(11):e2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Busca A, Kumar A. Innate immune responses in hepatitis B virus (HBV) infection. Virol J. 2014;11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Tan A, Koh S, Bertoletti A. Immune response in hepatitis B virus infection. Cold Spring Harb Perspect Med. 2015;5(8):a021428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Morrison RP, Caldwell HD. Immunity to murine chlamydial genital infection. Infect Immun. 2002;70(6):2741–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bakshi RK, Gupta K, Jordan SJ, Chi X, Lensing SY, Press CG, et al. An adaptive Chlamydia trachomatis‐specific IFN‐gamma‐producing CD4(+) T cell response is associated with protection against chlamydia reinfection in women. Front Immunol. 2018;9:1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Paintlia MK, Kaur S, Gupta I, Ganguly NK, Mahajan RC, Malla N. Specific IgA response, T‐cell subtype and cytokine profile in experimental intravaginal trichomoniasis. Parasitol Res. 2002;88(4):338–43. [DOI] [PubMed] [Google Scholar]
- 108. Liu Y, Russell MW. Diversion of the immune response to Neisseria gonorrhoeae from Th17 to Th1/Th2 by treatment with anti‐transforming growth factor beta antibody generates immunological memory and protective immunity. MBio. 2011;2(3):e00095–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Liu Y, Liu W, Russell MW. Suppression of host adaptive immune responses by Neisseria gonorrhoeae: role of interleukin 10 and type 1 regulatory T cells. Mucosal Immunol. 2014;7(1):165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Nishimura H, Yajima T, Kagimoto Y, Ohata M, Watase T, Kishihara K, et al. Intraepithelial gammadelta T cells may bridge a gap between innate immunity and acquired immunity to herpes simplex virus type 2. J Virol. 2004;78(9):4927–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Amador‐Molina A, Hernandez‐Valencia JF, Lamoyi E, Contreras‐Paredes A, Lizano M. Role of innate immunity against human papillomavirus (HPV) infections and effect of adjuvants in promoting specific immune response. Viruses. 2013;5(11):2624–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Salazar JC, Hazlett KR, Radolf JD. The immune response to infection with Treponema pallidum, the stealth pathogen. Microbes Infect. 2002;4(11):1133–40. [DOI] [PubMed] [Google Scholar]


