Abstract
Treatment of Mycobacterium abscessus pulmonary infection requires long-term administration of multiple antibiotics. Little is known, however, about the impact of each antibiotic on treatment outcomes. A retrospective analysis was conducted to evaluate the efficacy and adverse effects of antibiotics administered in 244 cases of M. abscessus pulmonary disease. Only 110 (45.1%) patients met the criteria for treatment success. The efficacy of treating M. abscessus pulmonary disease continues to be unsatisfactory especially for infections involving M. abscessus subsp. abscessus. Treatment with drug combinations that included amikacin [adjusted odds ratio (AOR), 3.275; 95% confidence interval (CI), 1.221–8.788], imipenem (AOR, 2.078; 95% CI, 1.151–3.753), linezolid (AOR, 2.231; 95% CI, 1.078–4.616), or tigecycline (AOR, 2.040; 95% CI, 1.079–3.857) was successful. Adverse side effects affected the majority of patients (192/244, 78.7%). Severe effects that resulted in treatment modification included: gastrointestinal distress (29/60, 48.3%) mostly caused by tigecycline, ototoxicity (14/60, 23.3%) caused by amikacin; and myelosuppression (6/60, 10%) caused mainly by linezolid. In conclusion, the success rate of treatment of M. abscessus pulmonary disease is still unsatisfactory. The administration of amikacin, imipenem, linezolid, and tigecycline correlated with increased treatment success. Adverse side effects are common due to long-term, combination antibiotic therapy. Ototoxicity, gastrointestinal distress, and myelosuppression are the most severe.
Keywords: Mycobacterium abscessus, pulmonary disease, drug, efficacy, adverse effect
Introduction
The incidence of pulmonary infections caused by non-tuberculous mycobacteria (NTM) has increased dramatically worldwide in recent years (Hoefsloot et al., 2013; Lin et al., 2018; Lee et al., 2019). Among them, Mycobacterium abscessus (M. abscessus) infections are the most difficult to manage (Nessar et al., 2012; Griffith, 2019). M. abscessus infections, which are even refractory to combined, long-term antibiotic therapy, often result in mortality.
Mycobacterium abscessus treatment is challenging, albeit effective treatment options are evolving. In 2007, the American Thoracic Society (ATS)/Infectious Disease Society of America (IDSA) introduced a clarithromycin-based multidrug therapy with amikacin plus cefoxitin or imipenem administered parenterally (Griffith et al., 2007). In 2017, the British Thoracic Society guidelines recommended a revision in antibiotic therapy that consisted of intravenous amikacin, tigecycline, and imipenem with a macrolide, e.g., clarithromycin, for the initial treatment phase (Haworth et al., 2017). This was followed by a continuation phase composed of nebulized amikacin and a macrolide in combination with additional oral antibiotics. It was further recommended that selection of a specific agent should consider the antibiotic susceptibility of the isolate and the antibiotic tolerance of the patient.
Patients with pulmonary disease due to M. abscessus infection require long-term treatment with multiple antibiotics. Little is known about the impact of each antibiotic on treatment outcomes. Recently, the NTM International Network released a consensus statement defining the treatment outcomes of NTM pulmonary disease, allowing for a better evaluation of the efficacy of each antibiotic used in clinical studies (van Ingen et al., 2018). Using these criteria, Kwak et al. (2019) conducted an excellent meta-analysis of 14 studies with detailed individual patient data. Patients treated with drug combinations that included azithromycin, amikacin, or imipenem exhibited better outcomes, emphasizing the import of different therapeutic approaches. However, two important antibiotics specifically recommended in the 2017 British Thoracic Society guidelines, i.e., linezolid and tigecycline, were not used or were administered in very few cases. Moreover, despite identifying the antibiotics most effective, the adverse effects of these antibiotics were not considered.
We previously reported a series of studies demonstrating the antibiotic susceptibility of clinical M. abscessus isolates and the treatment outcomes of patients diagnosed with M. abscessus pulmonary disease (Li B. et al., 2017, 2018; Guo et al., 2018; Ye et al., 2019). A number of cases accumulated during the course of these studies dealt with the long-term treatment with antibiotics, including linezolid and tigecycline; the adverse effects of antibiotic treatment were well documented. The retrospective analysis reported herein was undertaken to evaluate the efficacy and adverse effect of a variety of antibiotics used to treat M. abscessus pulmonary disease. The results of this analysis should facilitate therapeutic choices in clinical practice.
Materials and Methods
Study Population
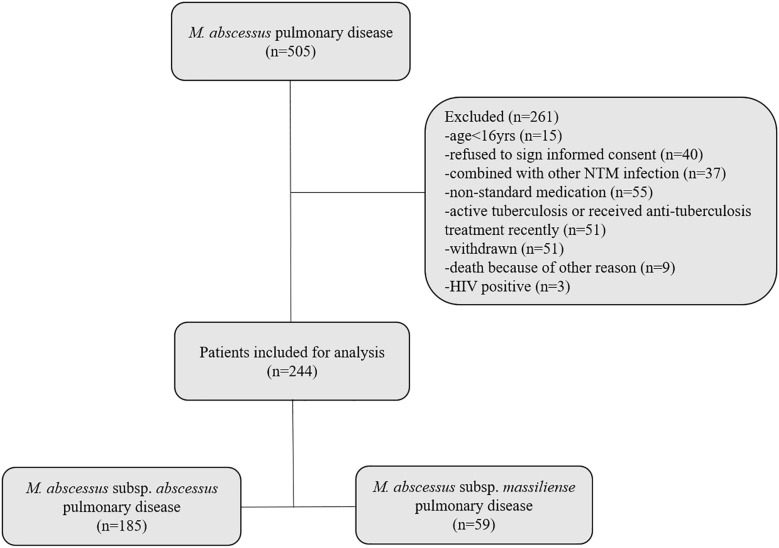
A retrospective review was conducted of the medical records of all patients entering Shanghai Pulmonary Hospital between January 2012 and December 2017 with M. abscessus lung disease. Participating patients were followed-up on a regular basis; sputum culture and chest CT examination were performed once a month and once every 3 months, respectively. The inclusion criteria were: (1) age > 16 years; (2) having undergone initial diagnosis and treatment at the Shanghai Pulmonary Hospital in accordance with the 2007 ATS/IDSA Guidelines or the 2017 British Thoracic Society Guidelines; and (3) follow-up period lasting >12 months. Exclusion criteria were: (1) age < 16 years; (2) co-infection with active tuberculosis or another NTM; (3) refusal to sign informed consent form; and (4) AIDS. Notably, patients with cystic fibrosis were never found and are essentially non-existent in Asia. A detailed, patient enrollment flow chart is shown in Figure 1. This study was approved by the Ethics Committees of Shanghai Pulmonary Hospital and Tongji University School of Medicine, ethics number K17-150. All participants signed informed consent forms before enrollment.
FIGURE 1.
Flow diagram of the study. Two hundred forty-four pulmonary disease patients, who conformed to the inclusion criteria, were enrolled. One hundred eighty-five patients were infected with M. abscessus subsp. abscessus; 59 patients were infected with M. abscessus subsp. massiliense.
Collection, Identification, and Preservation of Bacteria
All clinical M. abscessus isolates used in this study were preserved in the Clinical Microbiology Laboratory of Shanghai Pulmonary Hospital. Shanghai Pulmonary Hospital is one of the designated treatment centers for tuberculosis and NTM in China, attracting NTM cases nationwide. M. abscessus isolates were obtained from sputum and bronchoalveolar lavage fluid. The detailed process of M. abscessus identification was described previously by us using rpoB, erm(41), and PRA-hsp65 genes to identify and differentiate abscessus, massiliense, and bolletii subspecies (Guo et al., 2018). M. abscessus subsp. bolletii is extremely rare and, therefore, was excluded. Identified isolates, stored at −80°C, were recovered for microbiology and molecular biology studies.
Genotype Analysis
Genomic information of rpoB, erm(41), and PRA-hsp65 genes for 182 isolates was obtained by whole genome sequencing, which was available at DDBJ/ENA/GenBank under the BioProject PRJNA448987, PRJNA398137, and PRJNA488058. The genotype of the remaining isolates was determined by PCR and sequencing the rpoB, erm(41), and PRA-hsp65 genes.
Treatment Regimen
All patients were treated with antibiotics recommended by the ATS/IDSA or the British Thoracic Society guidelines (Griffith et al., 2007; Haworth et al., 2017). Clarithromycin, azithromycin, amikacin, tigecycline, linezolid, imipenem, meropenem, cefoxitin, ciprofloxacin, moxifloxacin, doxycycline, minocycline, and levofloxacin (among the most common antibiotics used to treat M. abscessus infections) were included in the analysis. These antibiotics were selected based upon: drug susceptibility, adverse side effects, medical history, economic considerations, and the ease with which the regimens could be modified during the course of treatment.
Treatment Efficacy and Adverse Drug Effects
Treatment outcomes were defined in accordance with the NTM International Network consensus statement (van Ingen et al., 2018); a microbiological cure was considered successful treatment. Since all patients enrolled in the current study were simultaneously or sequentially treated with more than one drug, analysis of the direct response to a single drug was impossible. Rather, the efficacy of individual drugs was assessed based upon a comparison of the frequency of drug usage in successfully versus unsuccessfully treated patients (Kwak et al., 2019). Evaluation of chest images and symptoms was determined by the treating physician. Adverse drug effects and the drugs responsible were identified by referring to the medical records and confirmed by the diminution or elimination of symptoms following drug cessation.
Statistical Analysis
Statistical analysis was conducted using SPSS version 20 (IBM Corporation, Chicago, IL, United States). Group comparisons for continuous data were performed using Mann–Whitney U-test. Group comparisons of proportions were made using Pearson’s chi-squared test or Fisher’s exact test. Multivariable logistic regression was used to confirm the association of specific drug use with treatment success; symptomatic and radiographic improvement; and adjusting for age, sex, BMI, and radiographic features. Statistical significance was set at a two-sided p-value of <0.05.
Results
Patient Characteristics
Two hundred and forty-four patients who conformed to the recruitment criteria were enrolled. Among them, 75.8% of the patients were infected with M. abscessus subsp. abscessus; 24.2% were infected with M. abscessus subsp. massiliense (Table 1). Patients experiencing M. abscessus pulmonary disease were 73.0% female and had relatively low body mass indices. Most of the patients had comorbidities consisting of prior TB/NTM infection or bronchiectasis. The main symptoms were cough and sputum production. The proportion of pulmonary disease patients infected with M. abscessus subsp. abscessus exhibited more fibrocavitary and less nodular bronchiectasis in chest images relative to patients infected with M. abscessus subsp. massiliense.
TABLE 1.
Baseline patient characteristicsa.
| Total (n = 244) | M. abscessus subsp. abscessus pulmonary disease (n = 185) | M. abscessus subsp. massiliense pulmonary disease (n = 59) | P-value | |
| Median age (years) | 56.0(49.0,65.8)b | 56(49.0,66.0)b | 54.0(48.0,63.0)b | 0.207 |
| Sex, male | 66(27.0) | 53(28.6) | 13(22.0) | 0.319 |
| Body mass index (kg/m2) | 19.6(18.6,20.5)b | 19.7(18.6,20.5)b | 19.4(18.6,20.6)b | 0.536 |
| Respiratory comorbidities | ||||
| Prior TB/NTMc | 127(52.0) | 92(49.7) | 35(59.3) | 0.199 |
| Bronchiectasis | 208(85.2) | 154(83.2) | 54(91.5) | 0.118 |
| COPDc | 16(6.6) | 13(7.0) | 3(5.1) | 0.768 |
| Cor pulmonale | 12(4.9) | 10(5.4) | 2(3.4) | 0.736 |
| Asthma | 15(6.1) | 12(6.5) | 3(5.1) | 1.000 |
| Main respiratory symptoms | ||||
| Cough | 201(82.4) | 153(87.4) | 48(81.4) | 0.246 |
| Sputum | 206(84.4) | 158(85.4) | 48(81.4) | 0.455 |
| Hemoptysis | 59(24.2) | 47(25.4) | 12(20.3) | 0.429 |
| Shortness of breath | 75(30.7) | 54(29.2) | 21(35.6) | 0.353 |
| Chest pain | 48(19.7) | 38(20.5) | 10(16.9) | 0.546 |
| Radiographic features | <0.001 | |||
| Fibrocavitary | 61(25.0) | 57(30.8) | 4(6.8) | |
| Nodular bronchiectatic | 171(70.1) | 116(62.7) | 55(93.2) | |
| Indeterminate | 12(4.9) | 12(6.5) | 0(0) | |
aData are the medians (interquartile range) or numbers (percentage). bRange. cTB, tuberculosis; NTM, non-tuberculous mycobacterium; COPD, chronic obstructive pulmonary disease.
Treatment Outcomes and Modalities
Only 45.1% of total patients (110/244) met the criteria for treatment success (Table 2). Significantly greater success was observed among patients infected with M. abscessus subsp. massiliense [81.4% (48/59)] compared to those infected with subsp. abscessus [33.5% (62/185)]. Clarithromycin used in drug regiments to treat patients infected with M. abscessus subsp. abscessus was more commonly associated with treatment failure than treatment success (85.4 vs. 71.0%, respectively). Treatments that included a different macrolide (azithromycin), on the other hand, achieved significantly greater success (37.1%) than failure (19.5%). These differences were not found upon analysis of the entire study population or patients infected with M. abscessus subsp. massiliense. Treatment with drug combinations that included amikacin, imipenem, linezolid, or tigecycline also exhibited far greater success than failure in treating the entire patient population, as well as treating those patients infected with M. abscessus subsp. abscessus. Drug combinations that included these same four antibiotics did not exert the same beneficial effects on patients infected with M. abscessus subsp. massiliense.
TABLE 2.
Comparison of treatment modalities: success versus failurea.
| Antibiotic | M. abscessus pulmonary disease | M. abscessus subsp. abscessus pulmonary disease | M. abscessus subsp. massiliense pulmonary disease | |||||||||
| Total (n = 244) | Success (n = 110) | Failure (n = 134) | P-value | Total (n = 185) | Success (n = 62) | Failure (n = 123) | P-value | Total (n = 59) | Success (n = 48) | Failure (n = 11) | P-value | |
| Clarithromycin | 199 | 86(78.2) | 113(84.3) | 0.218 | 149 | 44(71.0) | 105(85.4) | 0.020 | 50 | 42(87.5) | 8(72.7) | 0.347 |
| Azithromycin | 61 | 32(29.1) | 29(21.6) | 0.181 | 47 | 23(37.1) | 24(19.5) | 0.010 | 14 | 9(18.8) | 5(45.5) | 0.110 |
| Amikacin | 218 | 104(94.5) | 114(85.1) | 0.017 | 166 | 60(96.8) | 106(86.2) | 0.025 | 52 | 44(91.7) | 8(72.7) | 0.112 |
| Imipenem | 67 | 39(35.5) | 28(20.9) | 0.011 | 47 | 22(35.5) | 25(20.3) | 0.025 | 20 | 17(35.4) | 3(27.3) | 0.734 |
| Meropenem | 13 | 7(6.4) | 6(4.5) | 0.514 | 10 | 5(8.1) | 5(4.1) | 0.256 | 3 | 2(4.2) | 1(9.1) | 0.468 |
| Cefoxitin | 144 | 65(59.1) | 79(59.0) | 0.983 | 110 | 38(61.3) | 72(58.5) | 0.719 | 34 | 27(56.2) | 7(63.6) | 0.745 |
| Linezolidb | 38 | 24(21.8) | 14(10.4) | 0.015 | 27 | 15(24.2) | 12(9.8) | 0.009 | 11 | 9(18.8) | 2(18.2) | 1.000 |
| Tigecycline | 53 | 32(29.1) | 21(15.7) | 0.011 | 39 | 19(30.6) | 20(16.3) | 0.024 | 14 | 13(27.1) | 1(9.1) | 0.269 |
| Doxycycline | 30 | 10(9.1) | 20(14.9) | 0.167 | 23 | 6(9.7) | 17(13.8) | 0.420 | 7 | 4(8.3) | 3(27.3) | 0.112 |
| Minocycline | 22 | 10(9.1) | 12(9.0) | 0.971 | 15 | 4(6.5) | 11(8.9) | 0.558 | 7 | 6(12.5) | 1(9.1) | 1.000 |
| Moxifloxacinb | 53 | 28(25.5) | 25(18.7) | 0.200 | 34 | 13(21.0) | 21(17.1) | 0.519 | 19 | 15(31.2) | 4(36.4) | 0.734 |
| Levofloxacinb | 26 | 8(7.3) | 18(13.4) | 0.121 | 20 | 4(6.5) | 16(13.0) | 0.175 | 6 | 4(8.3) | 2(18.2) | 0.310 |
| Ciprofloxacin | 17 | 8(7.3) | 9(6.7) | 0.865 | 13 | 4(6.5) | 9(7.3) | 1.000 | 4 | 4(8.3) | 0(0) | 1.000 |
| Number of patients administered: | – | – | – | 0.810 | – | – | – | 0.148 | – | – | – | 0.367 |
| One parenteral drug | 15 | 6(5.5) | 9(6.7) | – | 9 | 1(1.6) | 8(6.5) | – | 6 | 5(10.4) | 1(9.1) | – |
| Two parenteral drugs | 161 | 70(63.6) | 91(67.9) | – | 124 | 38(61.3) | 86(69.9) | – | 37 | 32(66.7) | 5(45.5) | – |
| Three parenteral drugs | 62 | 31(28.2) | 31(23.1) | – | 47 | 21(33.9) | 26(21.1) | – | 15 | 10(20.8) | 5(45.5) | – |
| More than three parenteral drugs | 6 | 3(2.7) | 3(2.2) | – | 5 | 2(3.2) | 3(2.4) | – | 1 | 1(2.1) | 0(0) | – |
| Months of treatment | 25.6 (18.8, 37.8) | 20.7 | 30.0 | <0.001 | 27.7 (20.7, 40.8) | 23.4 | 30.0 | 0.001 | 20.2 (15.9, 29.8) | 18.0 | 28.0 | 0.179 |
| (16.2, 31.0) | (22.0, 43.3) | (18.1, 34.6) | (22.0, 44.0) | (15.9, 26.8) | (16.0, 43.0) | |||||||
| Surgical resection | 10 | 2 | 8 | 0.192 | 7 | 1 | 6 | 0.427 | 3 | 1 | 2 | 0.086 |
aData are the number (percentage; the number of patients who succeeded or failed treatment with the indicated drug divided by the total number of patients who succeeded or failed treatment) or median (interquartile range) of cases. Each antibiotic listed was included regardless of whether it was discontinued during the course of treatment. bAdministered orally and/or intravenously.
The duration of treatment was significantly shorter for the total population of patient who were successfully treated versus patients who failed treatment. Similarly, the treatment duration was substantially shorter for M. abscessus subsp. abscessus infected patients who were successfully treated. Successfully treated patients infected with M. abscessus subsp. massiliense exhibited the same trend, but failed to achieve statistical significance. Efficacy of treatment modalities with respect to symptomatic and raidiographic improvement has also been made and similar outcome profiles are obtained (Supplementary Tables 1, 2).
Effects of Individual Drugs on Treatment Outcomes
Multiple logistic regression analysis (adjusted for age, gender, BMI, and radiographic findings) indicated that azithromycin was clinically superior to clarithromycin in treating patients infected with M. abscessus subsp. abscessus (Table 3). The superiority of azithromycin was not observed in treating the total patient population or patients infected with M. abscessus subsp. massiliense. Amikacin, imipenem, linezolid, and tigecycline were also associated with success in treating the entire patient population, as well as those patients infected with M. abscessus subsp. abscessus. Notably, amikacin was the only drug showing clinical efficacy in treating M. abscessus subsp. massiliense infected patients in our study. The association of each drug with symptomatic and radiographic improvements was also subjected to multivariable logistic regression analysis (Supplementary Tables 3, 4).
TABLE 3.
Treatment success with individual antibiotics.
| Antibiotic | Total (n = 244) | M. abscessus subsp. abscessus pulmonary disease (n = 185) | M. abscessus subsp. massiliense pulmonary disease (n = 59) | ||||||
| Adjusted ORa | 95% CIa,b | P-value | Adjusted OR | 95% CI | P-value | Adjusted OR | 95% CI | P-value | |
| Clarithromycin | 0.588 | 0.290–1.194 | 0.142 | 0.425 | 0.191–0.945 | 0.036 | 1.460 | 0.214–9.962 | 0.699 |
| Azithromycin | 1.558 | 0.844–2.877 | 0.156 | 2.339 | 1.141–4.794 | 0.020 | 0.295 | 0.061–1.418 | 0.128 |
| Amikacin | 3.275 | 1.221–8.788 | 0.018 | 5.911 | 1.247–28.012 | 0.025 | 15.023 | 1.294–174.400 | 0.030 |
| Imipenem | 2.078 | 1.151–3.753 | 0.015 | 2.050 | 1.018–4.126 | 0.044 | 1.357 | 0.280–6.575 | 0.705 |
| Meropenem | 1.218 | 0.390–3.806 | 0.735 | 1.787 | 0.486–6.574 | 0.382 | 0.341 | 0.026–4.487 | 0.413 |
| Cefoxitin | 1.121 | 0.659–1.908 | 0.672 | 1.253 | 0.656–2.394 | 0.495 | 0.610 | 0.133–2.795 | 0.524 |
| Linezolidc | 2.231 | 1.078–4.616 | 0.031 | 2.875 | 1.221–6.772 | 0.016 | 1.286 | 0.189–8.746 | 0.797 |
| Tigecycline | 2.040 | 1.079–3.857 | 0.028 | 1.971 | 0.931–4.173 | 0.076 | 2.614 | 0.291–23.514 | 0.391 |
| Doxycycline | 0.599 | 0.260–1.380 | 0.229 | 0.628 | 0.222–1.772 | 0.379 | 0.408 | 0.053–3.147 | 0.390 |
| Minocycline | 0.992 | 0.399–2.467 | 0.986 | 0.691 | 0.206–2.315 | 0.549 | 1.312 | 0.116–14.876 | 0.827 |
| Moxifloxacinc | 0.695 | 0.372–1.300 | 0.255 | 0.866 | 0.393–1.908 | 0.720 | 1.495 | 0.303–7.388 | 0.622 |
| Levofloxacinc | 0.474 | 0.193–1.162 | 0.103 | 0.453 | 0.142–1.445 | 0.181 | 0.242 | 0.032–1.857 | 0.172 |
| Ciprofloxacin | 1.026 | 0.372–2.831 | 0.960 | 1.155 | 0.330–4.039 | 0.822 | 0 | 0 | 0 |
aOR, odds ratio; CI, confidence interval. bAdjusted for age, sex, body mass index, and radiographic findings. cAdministered orally and/or intravenously.
Adverse Effects of Antibiotics
One hundred and ninety-two of the 244 patients enrolled in the study experienced 319 adverse events caused by therapeutic intervention (Table 4). The most frequent adverse events were gastrointestinal complaints that included nausea, vomiting, diarrhea, and abdominal pain. Hematologic toxicity and nephrotoxicity were the next most frequent events documented. Most of these were mild, tolerable, and did not result in disability or death. Serious adverse reactions, however, occurred in 60 (24.6%) patients resulting in a discontinuation or modification of the treatment regimen. Notably, severe myelosuppression was mainly a consequence of linezolid treatment. Gastrointestinal side effects were most often due to tigecycline; amikacin caused most cases of serious ototoxicity and nephrotoxicity. Fortunately, all severe side effects disappeared or were remarkably alleviated after changes in the treatment regimen.
TABLE 4.
Adverse events∗.
| Antibiotic-specific adverse events leading to treatment modification (n = 60) | ||||||||
| Total patients (n = 192) | Total frequency of adverse events (n = 319) | Clarithromycin (n = 4) (199 patients) | Azithromycin (n = 3) (61 patients) | Amikacin (n = 26) (218 patients) | Imipenem (n = 3) (67 patients) | Linezolid (n = 9) (38 patients) | Tigecycline (n = 15) (53 patients) | |
| Gastrointestinal distress | 79(41.1) | 143(44.8) | 4 | 3 | 4 | 0 | 4 | 14 |
| Diarrhea | 15(7.8) | 22(6.9) | 0 | 0 | 0 | 0 | 0 | 0 |
| Abdominal pain | 13(6.8) | 25(7.8) | 1 | 1 | 0 | 0 | 1 | 0 |
| Nausea | 35(18.2) | 66(20.7) | 1 | 2 | 4 | 0 | 2 | 10 |
| Vomiting | 16(8.3) | 30(9.4) | 2 | 0 | 0 | 0 | 1 | 4 |
| Dizziness | 7(2.9) | 15(4.7) | 0 | 0 | 0 | 0 | 0 | 0 |
| Ototoxicity | 11(5.7) | 15(4.7) | 0 | 0 | 14 | 0 | 0 | 0 |
| Nephrotoxicity | 20(10.4) | 34(10.7) | 0 | 0 | 5 | 0 | 0 | 0 |
| Hepatotoxicity | 9(4.7) | 15(4.7) | 0 | 0 | 0 | 0 | 0 | 1 |
| Hematologic toxicity | 11(5.7) | 26(8.2) | 0 | 0 | 0 | 1 | 5 | 0 |
| Leukopenia | 5(2.6) | 11(3.4) | 0 | 0 | 0 | 1 | 2 | 0 |
| Thrombocytopenia | 2(1.0) | 5(1.6) | 0 | 0 | 0 | 0 | 2 | 0 |
| Anemia | 4(2.1) | 10(3.13) | 0 | 0 | 0 | 0 | 1 | 0 |
| Insomnia | 3(1.6) | 6(1.9) | 0 | 0 | 0 | 0 | 0 | 0 |
| Fever | 3(1.6) | 5(1.6) | 0 | 0 | 0 | 0 | 0 | 0 |
| Headache | 14(7.3) | 22(6.9) | 0 | 0 | 0 | 0 | 0 | 0 |
| Myoclonus | 3(1.6) | 4(1.3) | 0 | 0 | 0 | 0 | 0 | 0 |
| Agitation | 3(1.6) | 3(0.9) | 0 | 0 | 0 | 1 | 0 | 0 |
| Taste alteration | 10(5.2) | 11(3.4) | 0 | 0 | 0 | 0 | 0 | 0 |
| Allergic reactions | 19(9.9) | 20(6.3) | 0 | 0 | 3 | 1 | 0 | 0 |
∗The patients affected by the adverse event listed are enumerated. Notably, a single patient is often affected by more than one event. Those antibiotics, which most frequently cause events that necessitates treatment modification, are listed.
Discussion
The study reported here evaluated the efficacy and adverse effects of different antibiotics used in combination to treat patients with pulmonary disease caused by M. abscessus. A variety of antibiotics recommended by the British Thoracic Society guidelines were analyzed including linezolid and tigecycline, two important drugs recently used more frequently. While the overall rate of treatment success remained very low, the incorporation of amikacin, imipenem, linezolid, and/or tigecycline into treatment regimens was associated with increased success. The overall safety of macrolide-based regimens was moderately satisfactory since no fatalities or disabilities resulted from treatment. However, the total incidence of adverse effects was high. Indeed, there were cases in which patients were unable to tolerate one or more potentially effective drugs, i.e., azithromycin, amikacin, imipenem, linezolid, and tigecycline, during the course of treatment.
Two recent meta-analyses reported disappointing treatment outcomes for M. abscessus pulmonary disease. The therapeutic efficiency rates were 54 and 45.6% for all patients, and 35 and 33.0% for patients diagnosed with pulmonary, M. abscessus subsp. abscessus infections (Pasipanodya et al., 2017; Kwak et al., 2019). Similar rates of treatment success are reported here, i.e., 45.1% for all cases of M. abscessus pulmonary disease and 33.5% for cases involving M. abscessus subsp. abscessus. As such, the therapeutic efficacy of M. abscessus pulmonary disease continues to be unsatisfactory, and is even worse for M. abscessus subsp. abscessus infections.
Amikacin exhibits a high level of antibacterial activity and a low rate of resistance in vitro; its successful use to treat pulmonary, M. abscessus infections has been reported (Olivier et al., 2014; Lee H. et al., 2017). Indeed, amikacin administered parenterally is regarded as one of the most active antibiotics available to treat M. abscessus pulmonary disease (Griffith et al., 2007). Consistent with this perception, amikacin administered in our study was strongly associated with the alleviation of symptoms and treatment success suggesting that amikacin remains an ideal, first choice for treating M. abscessus infections. Clinicians should be aware, however, that amikacin is ototoxic. As such, blood concentration of amikacin should be monitored continually to ensure safety.
The anti-M. abscessus activity of imipenem in vitro is variable; bacterial resistance was over 60% in some studies (Chua et al., 2015; Lee M.C. et al., 2017; Li B. et al., 2017). Imipenem was efficacious, however, in treating pulmonary M. abscessus disease in our study. Similar results were reported by Kwak et al. (2019). The elevated antimicrobial activity expressed by imipenem intracellularly provides one plausible explanation for the apparent difference in activity exhibited in vitro versus in vivo (Rominski et al., 2017). In this regard, the high in vivo killing activity of imipenem in an embryonic zebrafish test system was reported (Lefebvre et al., 2016). Moreover, it is likely that the combination of imipenem with other antibiotics has a synergistic or additive effect, which contributes to the treatment success associated with imipenem (Miyasaka et al., 2007; Le Run et al., 2019). Notably, imipenem caused the fewest severe, adverse side effects among the four dominant drugs (i.e., amikacin, imipenem, linezolid, and tigecycline) identified in this study suggesting that it should be included as a treatment option provided in vitro sensitivity testing demonstrates the susceptibility of the clinical M. abscessus isolate. Furthermore, a newly developed beta-lactamase inhibitor, relebactam, has been shown to significantly improve the anti-M. abscessus activity of imipenem in vitro and no additional consideration needed to be addressed when imipenem and relebactam are used together (Zhanel et al., 2018; Kaushik et al., 2019a).
Accumulated evidence suggests that linezolid possesses elevated anti-M. abscessus activity. Recently, we reported the high activity expressed by linezolid in vitro against clinical M. abscessus isolates collected from patients with lung diseases (Ye et al., 2019). A study conducted using a Drosophila melanogaster-infection model demonstrated the anti-M. abscessus activity of linezolid in vivo (Oh et al., 2014); the successful use of linezolid in treating clinical M. abscessus infections was also reported (Inoue et al., 2018). These results are supported by data presented here. Better outcomes occurred when linezolid was a component of multi-drug therapy used to treat M. abscessus pulmonary disease. Linezolid has the advantage that it can be administered orally. It penetrates well into both extracellular fluid and cells, making linezolid one of the more important options for treating M. abscessus infections (Honeybourne et al., 2003). Linezolid-induced myelosuppression, however, was the most severe event leading to treatment intervention in our study. Considering its high price and limited availability in some areas, linezolid may be a more appropriate secondary treatment choice, especially when antibiotic sensitivity testing demonstrates alternatives.
Tigecycline exhibits the potentially strongest antibacterial activity of any antibiotic against M. abscessus in vitro. One study conducted in Japan showed it exerts 100% bacteriostasis against M. abscessus at very low concentrations (MIC ≤ 0.5 μg/ml), which is far superior to the antibacterial effect of clarithromycin (62%) and linezolid (77%) at the CLSI recommended breakpoint (Hatakeyama et al., 2017). Similar results were found in both France (90%, MIC ≤ 1 μg/ml) and China (94.3%, MIC ≤ 2 μg/ml) (Mougari et al., 2016; Li G. et al., 2017). Moreover, the combination of tigecycline with clarithromycin in vitro produces synergistic antibacterial effects against M. abscessus (Zhang et al., 2017). Tigecycline also showed excellent therapeutic effects against M. abscessus infection in a clinical study. Wallace et al. (2014) reported that daily treatment of M. abscessus disease with 50–100 mg tigecycline for 1 month resulted in a clinical remission rate that exceeded 60%. Tigecycline also proved superior in treating M. abscessus infections in the study reported here, supporting the British Thoracic Society guidelines that list tigecycline as a first-line solution for treating M. abscessus infections (Haworth et al., 2017). It is pertinent to note that tigecycline-treated patients often suffered from severe nausea and vomiting. Notably, two newly developed tetracycline analogs, omadacycline and eravacycline, have been reported to show therapeutic potential in treatment of M. abscessus infection (Kaushik et al., 2019b; Shoen et al., 2019), with similar in vitro activity to tigecycline, but better tolerated.
The study described herein has several limitations. First, it is a retrospective analysis of data obtained at a single center, which could limit the generalization and accuracy of the results. Second, only a relatively small number of M. abscessus subsp. massiliense infected cases were included, consequently, their characteristics may not be well representative. Third, due to the simultaneous administration of multiple antibiotics, conclusions regarding the adverse effects of individual drugs may be inaccurate. Four, this study excluded subjects who failed to complete their follow-up visits. Conceivably, this failure occurs as a consequence of adverse drug side effects resulting in an underestimation of the adverse events that could otherwise lead to treatment modification. Finally, antibiotics are selected strictly according to guidelines or sputum culture results in our study, rather than at random, resulting in the occurrence of prescription bias. However, it is inevitable.
Conclusion
The success rate of M. abscessus pulmonary disease treatment is still unsatisfactory, albeit the use of amikacin, imipenem, linezolid, and tigecycline is associated with increased treatment success. Adverse effects are common due to the long-term combination anti-M. abscessus therapy. Ototoxicity caused by amikacin, gastrointestinal side effects caused primarily by tigecycline, and myelosuppression caused by linezolid were the most severe adverse effects observed.
Data Availability
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
All authors contributed to the preparation of the final manuscript. JC, LZ, YM, BL, and HC conceived and designed the study. MY, QG, YZ, LX, BL, and ZZ collected the clinical data and performed the clinical evaluations. BL and JC performed the statistical analyses. JC, LZ, and YM wrote the manuscript, which was reviewed, edited, and approved by all authors.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the patients who agreed to participate in this study. Dr. Stephen H. Gregory (Providence, RI, United States) helped write and edit this manuscript. This manuscript has been released as a preprint at BioRxiv (Chen et al., 2019).
Footnotes
Funding. This work was supported by grants obtained from the National Natural Science Foundation of China (Nos. 81672063 and 81800003); the Natural Science Foundation of Shanghai Municipal Science and Technology Commission (No. 18ZR1431600); the Medical Guide Program of Shanghai Science and Technology Committee (Nos. 18411970600 and 19411969600); the New Frontier Technology Joint Project of Municipal Hospital, Shanghai Shenkang Hospital Development Center (No. SHDC12017113); and the Project of Top Clinical Medicine Centers and Key Disciplines Construction in Shanghai (No. 2017ZZ02012).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.01977/full#supplementary-material
References
- Chen J., Zhao L., Mao Y., Ye M., Guo Q., Zhang Y., et al. (2019). Clinical efficacy and adverse effects of antibiotics used to treat Mycobacterium abscessus pulmonary disease. BioRxiv [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua K. Y., Bustamante A., Jelfs P., Chen S. C., Sintchenko V. (2015). Antibiotic susceptibility of diverse Mycobacterium abscessus complex strains in new South Wales, Australia. Pathology 47 678–682. 10.1097/PAT.0000000000000327 [DOI] [PubMed] [Google Scholar]
- Griffith D. E. (2019). Mycobacterium abscessus and antibiotic resistance: same as it ever was. Clin. Infect. Dis. 10.1093/cid/ciz071 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Griffith D. E., Aksamit T., Brown-Elliott B. A., Catanzaro A., Daley C., Gordin F., et al. (2007). An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 175 367–416. 10.1164/rccm.200604-571ST [DOI] [PubMed] [Google Scholar]
- Guo Q., Chu H., Ye M., Zhang Z., Li B., Yang S., et al. (2018). The Clarithromycin susceptibility genotype affects the treatment outcome of patients with Mycobacterium abscessus lung disease. Antimicrob. Agents Chemother. 62:e2360-17. 10.1128/AAC.02360-2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama S., Ohama Y., Okazaki M., Nukui Y., Moriya K. (2017). Antimicrobial susceptibility testing of rapidly growing mycobacteria isolated in Japan. BMC Infect. Dis. 17:197. 10.1186/s12879-017-2298-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth C. S., Banks J., Capstick T., Fisher A. J., Gorsuch T., Laurenson I. F., et al. (2017). British thoracic society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 72(Suppl. 2), ii1–ii64. 10.1136/thoraxjnl-2017-210927 [DOI] [PubMed] [Google Scholar]
- Hoefsloot W., van Ingen J., Andrejak C., Angeby K., Bauriaud R., Bemer P., et al. (2013). The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur. Respir. J. 42 1604–1613. 10.1183/09031936.00149212 [DOI] [PubMed] [Google Scholar]
- Honeybourne D., Tobin C., Jevons G., Andrews J., Wise R. (2003). Intrapulmonary penetration of linezolid. J. Antimicrob. Chemother. 51 1431–1434. 10.1093/jac/dkg262 [DOI] [PubMed] [Google Scholar]
- Inoue T., Tsunoda A., Nishimoto E., Nishida K., Komatsubara Y., Onoe R., et al. (2018). Successful use of linezolid for refractory Mycobacterium abcessus infection: a case report. Respir. Med. Case Rep. 23 43–45. 10.1016/j.rmcr.2017.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik A., Ammerman N. C., Lee J., Martins O., Kreiswirth B. N., Lamichhane G., et al. (2019a). In vitro activity of the new β-Lactamase inhibitors relebactam and vaborbactam in combination with β-Lactams against Mycobacterium abscessus complex clinical isolates. Antimicrob. Agents Chemother. 63:e2623-18. 10.1128/AAC.02623-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik A., Ammerman N. C., Martins O., Parrish N. M., Nuermberger E. L. (2019b). In vitro activity of new tetracycline analogs omadacycline and eravacycline against drug-resistant clinical isolates of Mycobacterium abscessus. Antimicrob. Agents Chemother. 63:e470-19. 10.1128/AAC.00470-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak N., Dalcolmo M. P., Daley C. L., Eather G., Gayoso R., Hasegawa N., et al. (2019). Mycobacterium abscessus pulmonary disease: individual patient data meta-analysis. Eur. Respir. J. 54:1801991. 10.1183/13993003.01991-2018 [DOI] [PubMed] [Google Scholar]
- Le Run E., Arthur M., Mainardi J. L. (2019). In vitro and intracellular activity of imipenem combined with tedizolid, rifabutin, and avibactam against Mycobacterium abscessus. Antimicrob. Agents Chemother. 63:e1915-18. 10.1128/AAC.01915-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Myung W., Koh W. J., Moon S. M., Jhun B. W. (2019). Epidemiology of nontuberculous mycobacterial infection, South Korea, -2016. Emerg. Infect. Dis. 25 569–572. 10.3201/eid2503.181597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Sohn Y. M., Ko J. Y., Lee S. Y., Jhun B. W., Park H. Y., et al. (2017). Once-daily dosing of amikacin for treatment of Mycobacterium abscessus lung disease. Int. J. Tuberc. Lung Dis. 21 818–824. 10.5588/ijtld.16.0791 [DOI] [PubMed] [Google Scholar]
- Lee M. C., Sun P. L., Wu T. L., Wang L. H., Yang C. H., Chung W. H., et al. (2017). Antimicrobial resistance in Mycobacterium abscessus complex isolated from patients with skin and soft tissue infections at a tertiary teaching hospital in Taiwan. J. Antimicrob. Chemother. 72 2782–2786. 10.1093/jac/dkx212 [DOI] [PubMed] [Google Scholar]
- Lefebvre A. L., Dubee V., Cortes M., Dorchene D., Arthur M., Mainardi J. L. (2016). Bactericidal and intracellular activity of beta-lactams against Mycobacterium abscessus. J. Antimicrob. Chemother. 71 1556–1563. 10.1093/jac/dkw022 [DOI] [PubMed] [Google Scholar]
- Li B., Yang S., Chu H., Zhang Z., Liu W., Luo L., et al. (2017). Relationship between antibiotic susceptibility and genotype in Mycobacterium abscessus clinical isolates. Front. Microbiol. 8:1739 10.3389/fmicb.2017.01739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Pang H., Guo Q., Huang M., Tan Y., Li C., et al. (2017). Antimicrobial susceptibility and MIC distribution of 41 drugs against clinical isolates from China and reference strains of nontuberculous mycobacteria. Int. J. Antimicrob. Agents 49 364–374. 10.1016/j.ijantimicag.2016.10.024 [DOI] [PubMed] [Google Scholar]
- Li B., Ye M., Guo Q., Zhang Z., Yang S., Ma W., et al. (2018). Determination of MIC distribution and mechanisms of decreased susceptibility to bedaquiline among clinical isolates of Mycobacterium abscessus. Antimicrob. Agents Chemother 62:e175-18. 10.1128/aac.00175-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., Russell C., Soll B., Chow D., Bamrah S., Brostrom R., et al. (2018). increasing prevalence of nontuberculous mycobacteria in respiratory specimens from US-affiliated pacific island jurisdictions(1). Emerg. Infect. Dis. 24 485–491. 10.3201/eid2403.171301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaka T., Kunishima H., Komatsu M., Tamai K., Mitsutake K., Kanemitsu K., et al. (2007). In vitro efficacy of imipenem in combination with six antimicrobial agents against Mycobacterium abscessus. Int. J. Antimicrob. Agents 30 255–258. 10.1016/j.ijantimicag.2007.05.003 [DOI] [PubMed] [Google Scholar]
- Mougari F., Amarsy R., Veziris N., Bastian S., Brossier F., Bercot B., et al. (2016). Standardized interpretation of antibiotic susceptibility testing and resistance genotyping for Mycobacterium abscessus with regard to subspecies and erm41 sequevar. J. Antimicrob. Chemother. 71 2208–2212. 10.1093/jac/dkw130 [DOI] [PubMed] [Google Scholar]
- Nessar R., Cambau E., Reyrat J. M., Murray A., Gicquel B. (2012). Mycobacterium abscessus: a new antibiotic nightmare. J. Antimicrob. Chemother. 67 810–818. 10.1093/jac/dkr578 [DOI] [PubMed] [Google Scholar]
- Oh C. T., Moon C., Park O. K., Kwon S. H., Jang J. (2014). Novel drug combination for Mycobacterium abscessus disease therapy identified in a drosophila infection model. J. Antimicrob. Chemother. 69 1599–1607. 10.1093/jac/dku024 [DOI] [PubMed] [Google Scholar]
- Olivier K. N., Shaw P. A., Glaser T. S., Bhattacharyya D., Fleshner M., Brewer C. C., et al. (2014). Inhaled amikacin for treatment of refractory pulmonary nontuberculous mycobacterial disease. Ann. Am. Thorac. Soc. 11 30–35. 10.1513/AnnalsATS.201307-231OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasipanodya J. G., Ogbonna D., Ferro B. E., Magombedze G., Srivastava S., Deshpande D., et al. (2017). Systematic review and meta-analyses of the effect of chemotherapy on pulmonary Mycobacterium abscessus outcomes and disease recurrence. Antimicrob. Agents Chemother. 61:e1206-17. 10.1128/AAC.01206-1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rominski A., Schulthess B., Muller D. M., Keller P. M., Sander P. (2017). Effect of beta-lactamase production and beta-lactam instability on MIC testing results for Mycobacterium abscessus. J. Antimicrob. Chemother. 72 3070–3078. 10.1093/jac/dkx284 [DOI] [PubMed] [Google Scholar]
- Shoen C., Benaroch D., Sklaney M., Cynamon M. (2019). In vitro activities of omadacycline against rapidly growing mycobacteria. Antimicrob. Agents Chemother. 63:e2522-18. 10.1128/AAC.02522-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ingen J., Aksamit T., Andrejak C., Bottger E. C., Cambau E., Daley C. L., et al. (2018). Treatment outcome definitions in nontuberculous mycobacterial pulmonary disease: an NTM-NET consensus statement. Eur. Respir. J. 51:1800170. 10.1183/13993003.00170-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Dukart G., Brown-Elliott B. A., Griffith D. E., Scerpella E. G., Marshall B. (2014). Clinical experience in 52 patients with tigecycline-containing regimens for salvage treatment of Mycobacterium abscessus and Mycobacterium chelonae infections. J. Antimicrob. Chemother. 69 1945–1953. 10.1093/jac/dku062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M., Xu L., Zou Y., Li B., Guo Q., Zhang Y., et al. (2019). Molecular analysis of linezolid-resistant clinical isolates of Mycobacterium abscessus. Antimicrob. Agents Chemother. 63:e1842-18. 10.1128/AAC.01842-1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhanel G. G., Lawrence C. K., Adam H., Schweizer F., Zelenitsky S., Zhanel M., et al. (2018). Imipenem–relebactam and meropenem–vaborbactam: two novel carbapenem-b-lactamase inhibitor combinations. Drugs 78 65–98. 10.1007/s40265-017-0851-859 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Lu J., Liu M., Wang Y., Zhao Y., Pang Y. (2017). In vitro activity of clarithromycin in combination with other antimicrobial agents against Mycobacterium abscessus and Mycobacterium massiliense. Int. J. Antimicrob. Agents 49 383–386. 10.1016/j.ijantimicag.2016.12.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.