Abstract
Mesenchymal stem cells are undifferentiated cells able to acquire different phenotypes under specific stimuli. In vitro manipulation of these cells is focused on understanding stem cell behavior, proliferation and pluripotency. Latest advances in the field of stem cells concern epigenetics and its role in maintaining self-renewal and differentiation capabilities. Chemical and physical stimuli can modulate cell commitment, acting on gene expression of Oct-4, Sox-2 and Nanog, the main stemness markers, and tissue-lineage specific genes. This activation or repression is related to the activity of chromatin-remodeling factors and epigenetic regulators, new targets of many cell therapies. The aim of this review is to afford a view of the current state of in vitro and in vivo stem cell applications, highlighting the strategies used to influence stem cell commitment for current and future cell therapies. Identifying the molecular mechanisms controlling stem cell fate could open up novel strategies for tissue repairing processes and other clinical applications.
Keywords: Stem cells, Epigenetics, Self-renewal, In vitro differentiation, Physical stimuli, Stem cell fate, Clinical practice, Cell transplantation
Core tip: The latest advances in the field of stem cells concern epigenetics and its role in self-renewal and differentiation capability. Activation or silencing of genes controlling stemness and tissue-lineage specification are related to chromatin-remodeling factors and epigenetic regulators. In this review, we focused on the principal epigenetic markers that regulate stem cell pluripotency, in vitro manipulation and the current state-of-the-art in vivo applications of human mesenchymal stem cells.
INTRODUCTION
Stem cells are known for their self-renewal and their capability to differentiate into various lineages, participating in tissue regeneration after damage[1]. Since human embryonic stem cells (ESCs) are isolated from the inner cell mass of the blastocyst[2] their application in vitro and in vivo is burdened by ethical issues, causing researchers to turn their interests toward other sources[3,4]. Mesenchymal stem cells, defined by other authors as mesenchymal stromal cells[5], have shown a high proliferative potential in vitro, being identified as the elements that maintain the bone marrow microenvironment, improve hematopoiesis and give rise to various cell lineages[6,7]. The most common source for human mesenchymal stem cells (hMSCs) is the bone marrow, usually obtained from the iliac crest of adult patients. Bone marrow-derived stem cells (BM-MSCs) can be separated from the tissue by centrifugation in a density gradient media and, once placed in culture, they can be easily induced to differentiate towards different phenotypes[8]. MSCs are found in many other adult tissues, including the dental pulp[9], adipose tissue (ASCs)[10], umbilical cord blood[11] and Wharton’s jelly of umbilical cord12]. Despite some differences in terms of growth kinetics and pluripotency, donor age- and -gender-related features[13,14], MSCs can differentiate under a variety of external cues, acting to replace damaged cells and maintain tissue homeostasis[15]. In order to reduce manipulation of the stromal fraction, minimize enzymatic digestion and ensure maximum yield in culture, the interest of researchers has turned to the optimization of MSC isolation protocols[16,17]. In particular, new devices have been developed for adipose tissue, based only upon mechanical forces, thus allowing a micro-fragmented tissue fraction in one-step that is enriched in hMSCs and pericytes[18,19]. Stem cells represent an important model to study the molecular pathways involved in disease onset and progression and to develop drug delivery system and differentiation processes, in view of a successful application in tissue engineering and clinical practice[20,21]. In this review, we summarize the influence of specific chemical and physical agents able to affect stem cell behavior and fate, pointing out the current development of hMSCs applications in vivo.
EPIGENETIC REGULATION OF SELF-RENEWAL AND PLURIPOTENCY
Stem cell differentiation is an essential complex process involved in the maintenance of tissues homeostasis, being in turn orchestrated by a wide range of signaling pathways[22]. In vitro differentiation involves different molecular mechanisms influencing the expression of the main markers of stemness: Octamer-binding transcription factor 4 (Oct-4), sex determining region Y-box 2 (Sox-2) and Homeobox protein Nanog[23,24]. These transcription factors are essential for maintaining stem cell pluripotency and are also involved in adult somatic cell reprogramming[25,26].
Epigenetics refers to the range of heritable changes in the structure of chromatin able to affect gene expression and represents the molecular reaction to all the environmental changes[27]. These chromatin modifications are orchestrated by different kind of enzymes, such as DNA methyltransferases (DNMTs), or enzymes controlling post-translational histone modification, as Histone deacetylase (HDACs) and histone acetyltransferases[28]. Epigenetic mechanisms are involved in the progression from the undifferentiated to differentiated state, through silencing of self-renewal genes and activation of differentiation markers. The onset of these specific gene expression patterns is stimulated by developmental and environmental stimuli, causing changes in the chromatin structure, thus allowing a specific transcriptional program, with a mechanism not fully clarified yet[29-31]. Therefore, epigenetics has a central role not only during embryogenesis but also in maintaining tissue homeostasis and controlling the regenerative potential through adulthood[32]. Wang et al[33] demonstrated that HDAC6 takes part in dental MSC differentiation and osteoblast maturation by maintaining dental and periodontal tissue homeostasis. Interactions between the HDAC Sirtuin 6 (Sirt6) and Ten-eleven translocation (Tet) enzyme are directly involved in the regulation of Oct-4, Sox-2 and Nanog genes, finely tuning pluripotency and differentiation balance in ESCs[34]. Santaniello et al[35] (2018) demonstrated that a combination of melatonin and vitamin D activates HDAC1 and the (NAD)-dependent deacetylases Sirtuins 1 and 2 in ASCs. The final effect was an inhibition of adipogenic differentiation, even when cells were cultured in a medium able to prime adipogenic differentiation[35].
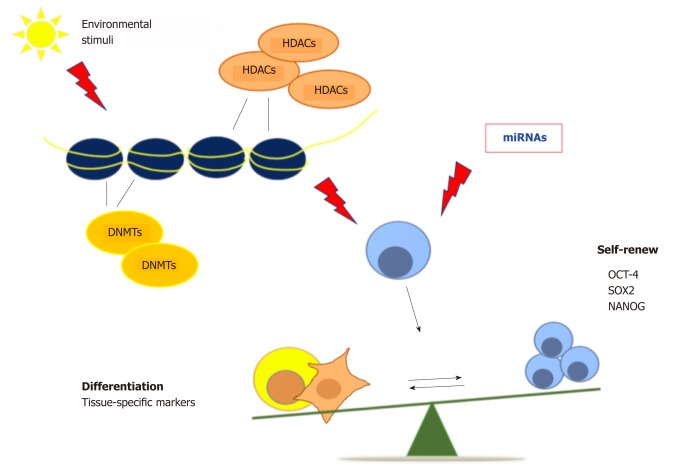
Exposure of human amniotic fluid stem cells to DNMT inhibitors induces cardiomyogenic differentiation via chromatin remodeling, upregulation of cardiac-related genes and repression of HDAC1 expression[36]. In addition, a combination of DNMT and HDAC inhibitors counteracts cancer stem cell growth, reducing the tumor mass in mouse mammary tumor models, thus increasing mice survival, and unfolding novel epigenetic-based therapies for drug-resistant breast cancer[37]. DNA methylation plays a key role in maintaining the undifferentiated state in stem cells by silencing the differentiation genes, and it is also implicated in somatic cell reprogramming[38,39]. All of these classes of enzymes promote changes in chromatin structure, exerting a crucial role in regulating the balance between pluripotency and differentiation[40]. On the whole, continuous efforts to unravel epigenetic regulation holds promise for continuous innovation in strategies aimed at controlling stem cell pluripotency and tissue homeostasis. MicroRNAs (miRNAs), small non-coding RNAs, have been discovered as regulators of different signaling pathways, stem cell pluripotency and somatic cell reprogramming[41]. The modulation of cell differentiation by miRNAs could be used to treat various kind of diseases, including myocardial infarction, neurodegenerative and muscle diseases[42]. Moreover, epigenetic mechanisms could unravel many deregulated cellular dynamics, as those involved in cancer, aging and age-related diseases[43] (Figure 1).
Figure 1.
Epigenetic regulation of stem cell fate. Chromatin remodeling affects cell behavior and regulates the balance between pluripotency and differentiation. HDACs: Histone deacetylases; Oct-4: Octamer-binding transcription factor 4; Sox-2: Sex determining region Y-box 2; NANOG: Nanog homeobox.
IN VITRO MODULATION OF STEM CELL BEHAVIOR
In the last years, several molecules capable of orchestrating the multilineage repertoire of stem cells have been largely used to generate specific conditioned media[44,45]. Within this context, some authors found that medium conditioned by factors such as activin A, bone morphogenetic protein 4 (BMP4), vascular endothelial growth factor (VEGF) or Dickkopf-related protein 1, can optimize cardiac development in mouse and human stem cell lines[46,47]. BMP4 itself, in combination with inhibitors of the Activin/Nodal signaling pathways, induces differentiation of ESCs into trophoblastic cells, which show similar trophectoderm profile and are able to secrete placental hormones[48]. Concerning the use of chemistry to push stem cells to specific phenotypes, molecules that can affect the epigenetic code to activate a molecular differentiation program have largely been used. Ventura et al[49,50] described for the first time how a hyaluronan mixed ester of butyric and retinoic acids (HBR) increases the transcription of cardiogenic genes, acting through the epigenetic regulation of a cardiogenesis program in vitro. HBR was also able to promote cardiac regeneration in infarcted rat hearts, decreasing the number of apoptotic cardiomyocytes without the need for stem cell transplantation[49-52]. More recently, a mixture of HBR and melatonin was successfully employed to induce an osteogenic phenotype in dental pulp stem cells, suggesting the use of this cocktail for future in vivo orthopedic and dental applications[53].
MODULATION OF STEM CELL COMMITMENT BY PHYSICAL STIMULI
Electromagnetic fields can interact with cells, tissues and biological systems in general[54,55] and are able to influence phenotypic features, gene expression patterns and differentiation in MSCs, acting in a dose and time-dependent manner[56,57]. It has been shown that 7 d of MSC growth on an electroconductive polymeric substrate was sufficient to promote Nestin and β-3 Tubulin upregulation and the appearance of neural-like morphological extensions[58]. MSCs can be employed to improve cartilage regeneration[59]. Synthetic scaffolds and biopolymers are incorporated in stem cell cultures to induce their growth, mimicking the stem cell niche[60]. Biomaterials provide a physical environment that can control cell function. The interaction between stem cells and these surfaces modulates multiple processes such as cell migration, proliferation and differentiation, as well as extracellular matrix deposition, providing dynamic signaling able to regulate cell behavior[61,62]. Non-invasive electrical stimulation therapy exerts an important role in controlling calcium channels, thus regulating the intracellular calcium concentration during chondrogenic and osteogenic stem cell differentiation, and opening novel approaches to improve tissue repair in vivo[63,64]. Extracorporeal shock wave therapy (ESWT) is largely used to treat orthopedic diseases, including tendinopathies or bone disorders, as well as wound healing stimulation in radiation-damaged skin[65,66]. ESWT stimulates angiogenesis, neovascularization, and recruitment of MSCs, inducing their proliferation and differentiation. These processes have been shown to involve ATP release and increased extracellular signal–regulated kinases Erk1/2 and p38 MAPK activation, which is responsible for the proliferative and reparative effects[67]. Human and rat ASCs exposed to repetitive ESWT retained all cell surface markers and exhibited increased multipotency into osteogenic and adipogenic lineages[68].
Radio electric fields asymmetrically conveyed by a medical device, referred to radioelectric asymmetric conveyer (REAC), are able to induce the transcription of GATA-4, Nkx-2.5, VEGF, hepatocyte growth factor (HGF), Von Willebrand factor (vWF), neurogenin-1, and myoD, genes orchestrating different tissue lineages, both in mouse embryonic and human adult stem cells[69,70]. Moreover, REAC exposure counteracted MSC senescence by downregulating the expression of p16INK4, ARF, p53, and p21, involved in cell cycle regulation, reducing the number of senescence associated-beta-galactosidase positive cells, while also preserving TERT expression and telomere length[71-74]. Radio electric conveyed fields allowed for the direct reprogramming of human skin fibroblasts toward cardiac and neurogenic lineages and synergistically enhanced the cardiogenic commitment in induced pluripotent stem cells (iPSCs) cultured in cardiogenic medium[47,75]. In addition, radio electric conveyed fields were sufficient to induce the neurogenic phenotype in PC12 cells, a model for dopaminergic neuron studies[76]. Finally, concerning cell reprogramming, several authors have shown that mechanical stimuli such as equiaxial stretching have an important role in reprogramming somatic cells into iPSCs, with the formation of a great number of iPSC colonies without using common viral mediated gene transduction[77]. These findings showed the prominence of physical stimuli in opening up new strategies for cell manipulation and regenerative medicine[78,79].
BIOACTIVE MOLECULES IN ORCHESTRATING CELL DIFFERENTIATION
The use of nutraceuticals has recently been largely employed in regenerative medicine.
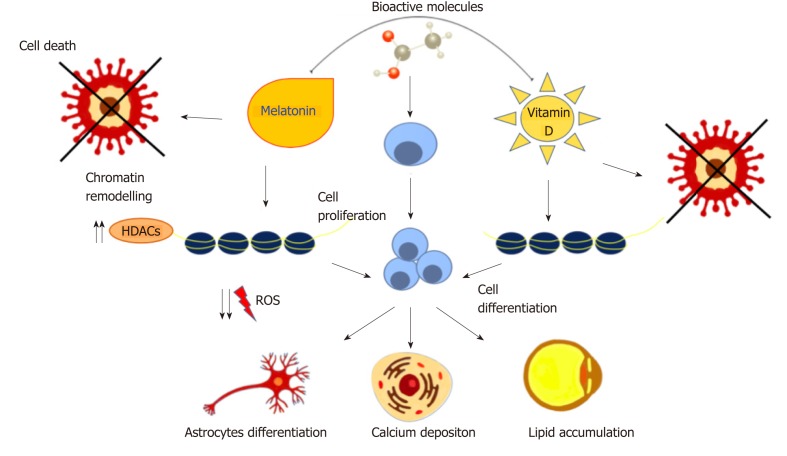
A wide range of natural molecules and compounds has been described as capable to orchestrate stem cell commitment. Known as nutraceuticals or functional foods, these molecules are largely used for their therapeutic or preventive effects[80,81]. Melatonin, the hormone secreted by the pineal gland, regulates many physiological functions such as circadian rhythm, hemostasis and the immune system. An alteration in its secretion is related to the onset of pathological manifestations[82,83]. In vitro studies with MSCs demonstrated that melatonin exerts anti-oxidant and anti-apoptotic effects, regulating the expression of pro- and anti-apoptotic proteins, ameliorating the outcome of stem cell transplantation[84,85]. Mendivil-Perez et al[86] demonstrated that melatonin in transplanted mice was able to induce proliferation and differentiation of neural stem cells into oligodendrocytes and astrocytes, reducing oxidative stress produced by mitochondrial activity. Oxidative stress has a crucial role in osteogenesis inhibition and in aging-related osteoporosis[87]. MSCs exposed to melatonin exhibit increased calcium stores and osteogenic differentiation. These events include the recruitment of AMP-activated protein kinase (AMPK), Runt-related transcription factor 2 and Forkhead box O3, with the latter usually being downregulated under stress conditions[88]. AMPK activation is also involved in the regulation of adipogenesis. It regulates the expression of peroxisome proliferator-activated receptor γ (PPARγ), the main adipogenic orchestrator gene and a molecular target of natural compounds used in obesity management[89,90].In combination with other molecules, including vitamin D, melatonin has a synergistic effect on inhibiting adipogenesis[91]. The active form of Vitamin D is calcitriol, which is naturally synthesized following sun exposure or taken as dietary supplements. It controls calcium metabolism, apoptosis, and stimulates macrophages and immune responses[92,93]. When ASCs are cultured in the presence of melatonin and vitamin D in adipogenic-conditioned medium, adipogenic differentiation is blocked. This inhibitory effect is through the downregulation of specific genes controlling adipogenesis, protein contents, and fat depots[91]. Moreover, the synergistic effect of these two molecules epigenetically modulates ASC commitment towards osteogenic differentiation through the activation of HDAC1 and SIRT1, even in the presence of adipogenic conditions[35]. Natural compounds can therefore be considered potent differentiating agents able to drive cell proliferation and apoptosis resistance by epigenetic regulations and post-transcriptional modifications[94,95]. At the same time, they can act as anti-proliferative agents against many tumor cells, including hepatocarcinoma cells, without affecting the cell cycle or viability of non-cancer cells, thus representing novel specific tools for cancer prevention[96,97] (Figure 2).
Figure 2.
Natural molecules and stem cell fate. Bioactive molecules induce cell proliferation and differentiation, reducing ROS production and apoptosis, through chromatin remodeling and epigenetic modifications. ROS: Reactive oxygen species; HDACs: Histone deacetylases.
FROM BENCH TO BEDSIDE
MSCs have largely attracted the attention of clinicians in regenerative medicine for their easy expansion and differentiation potential, avoiding the ethical issues related to the use of ESCs[98,99]. Stem cells are currently applied in gene therapy and treatment of serious pathologies, sometimes representing the only alternative to conventional treatments, to slow down the progression of the disease and improve life qualities of the patients[100,101]. Moreover, when transplanted in both autologous and allogenic fashion, MSCs can migrate into the damaged tissue to control inflammation and immune responses[102]. The use of stem cells represents the most frequently applied cell therapy in hematological diseases[103], although with the risk of rejection and potential failure[104]. Starting with allogenic bone marrow transplantation in 1957[105], stem cell therapy nowadays represent the main actor in many different clinical trials for several diseases, such as neurological diseases like amyotrophic lateral sclerosis (commonly known as ALS)[106].
BONE MARROW HEMATOPOIETIC STEM CELLS IN CLINICAL PRACTICE
MSCs are multipotent cells that are able to differentiate into different lineages, and they can also be easily expanded for clinical practice[107]. Bone marrow is a mesen-chymal specialized connective tissue composed of progenitor cells that can undergo adipogenic, osteogenic, chondrogenic and myogenic differentiation[108]. Thus, bone represents a microenvironment in which hematopoietic stem cells (HSCs) can maintain their undifferentiated state and participate in hematopoiesis when exposed to different stimuli[109]. Hematopoiesis is a complex process, during which HSCs undergo asymmetric division to become progenitor blood and bone marrow cells, as erythrocytes, lymphocytes and monocytes[110]. HSC self-renewal potential is regulated by different signaling pathways. Among them, physiological Notch signaling is required for bone formation, regulates the HSC microenvironment and cell fate decisions, and is also associated with tumorigenic potential and leukemia when dysregulated[111]. Moreover, the crosstalk between Notch and Wnt signaling is crucial for tissue development and turnover[112]. Wnt/β-catenin signal is essential for HSC growth and homeostasis in vitro and in vivo, and its inhibition causes cell growth arrest with a related decline in self-renewal potential of stem cells. On the other hand, activation of Wnt patterning increases Notch expression and supports the self-renewal potential of progenitor cells from different tissues, suppressing differentiation[113,114]. Alterations in signaling pathways and normal microenvironment play a crucial role in the development of hematopoietic diseases, such as chronic and acute myeloid leukemia[115]. HSCs are employed as therapeutic tools in stem cell transplantations[116] due to their immunomodulatory properties, secretion of growth factors and regeneration of injured tissues, especially in patients refractory to conventional chemotherapy[117]. Autologous transplantations are used in leukemia, lymphomas, multiple myeloma and other hematological malignancies[118]. There are several retrospective studies in which patients were monitored after 10-12 years from the transplant to evaluate survival and transplant-related mortality[119-121]. HSC transplantation was shown to be effective in counteracting the progression of the disease, notably at the early stages of disease[122].
MSC TRANSPLANTATION FOR AMYOTROPHIC LATERAL SCLEROSIS
ALS is the most frequent neurodegenerative dysfunction of the midlife[123]. ALS is characterized by progressive degeneration of spinal cord motor neurons, muscle paralysis and death in 3-5 years due to respiratory failure. Degeneration involves toxicity and inflammatory processes associated with proliferation of resident cellular populations[124]. Genetic and epigenetic risk factors are certainly the main causes related to progression of the disease. Superoxide dismutase 1 (SOD1), which encodes Cu/Zn superoxide dismutase 1, was the first gene whose alteration was associated with ALS. Its mutation is related to protein misfolding and loss-of-function, and it is found in many familiar forms[125,126]. Misfolded proteins have a central role in neurodegenerative disease, since in their abnormally aggregated forms, cellular proteins are prevented from exerting their essential roles in RNA binding/ metabolism and cellular homeostasis[127]. MicroRNAs (miRNAs) are able to regulate gene expression and promote or repress mRNA stabilization through post-transcriptional modification and by binding specific targets[128]. MiRNAs are involved in different physiological mechanisms, such as cell growth and apoptotic processes, while orchestrating pluripotency and differentiation in stem cells[129]. Altered miRNA expression in the skeletal muscle is related to neurological symptoms and disease progression. Some in vivo and in vitro studies have described how MiR-206 is enrolled upon muscle denervation in the attempt to regenerate neuromuscular synapses, highlighting the role of this miRNA in different stages of ALS progression[130,131]. Actually, there are no curative therapies for ALS. While drugs that suppress oxidative stress can be used to try to maintain motor neuron function[132] to slightly increase patient survival, novel compounds are now being tested[133]. An alternative to conventional therapy may be autologous MSC transplantation. Stem cells, thanks to their immunomodulatory properties, secrete neurotrophic factors and other anti-inflammatory cytokines, thus supporting motor neuron survival and functionality[134,135]. Notwithstanding, bone marrow is the most common source for MSCs, Wharton jelly, umbilical cord blood and in particular ASCs, represent a valid alternative in ALS therapy[136], due to their efficient isolation and high toleration by the patients.
In several clinical studies, patients received intravenous injection of MSCs while being monitored at regular time intervals. In all trials, autologous cell therapy proved to be a safe procedure. The recipient tissues did not exhibit any structural changes, tumor formation or toxicity related to transplantation, while it was shown to be effective in counteracting disease progression, improving the quality of patient’s life[137-139].
CONCLUSION
Epigenetic regulators were identified as new promising therapeutic targets in patients with hematological, breast cancer and other malignancies, as well as in neurode-generative diseases[140,141]. The rescuing potential of stem cells is under control of different kinds of signals, including the environment, which epigenetically regulate their differentiation processes[142]. Understanding the molecular pathways involved in stem cell fate is critical to develop novel tools for both the prevention and treatment of a variety of diseases, with great impact in regenerative medicine, bioengineering and clinical transplantation.
Footnotes
Conflict-of-interest statement: The authors declare no conflicts of interest.
Manuscript source: Invited manuscript
Peer-review started: February 20, 2019
First decision: April 15, 2019
Article in press: June 12, 2019
Specialty type: Cell and tissue engineering
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bonartsev AP, Najafzadeh N S-Editor: Ji FF L-Editor: Filipodia E-Editor: Xing YX
Contributor Information
Sara Cruciani, Department of Biomedical Sciences, University of Sassari, Sassari 07100, Italy; Laboratory of Molecular Biology and Stem Cell Engineering, National Institute of Biostructures and Biosystems – Eldor Lab, Innovation Accelerator, Consiglio Nazionale delle Ricerche, Bologna 40129, Italy.
Sara Santaniello, Department of Biomedical Sciences, University of Sassari, Sassari 07100, Italy; Laboratory of Molecular Biology and Stem Cell Engineering, National Institute of Biostructures and Biosystems – Eldor Lab, Innovation Accelerator, Consiglio Nazionale delle Ricerche, Bologna 40129, Italy.
Andrea Montella, Department of Biomedical Sciences, University of Sassari, Sassari 07100, Italy; Operative Unit of Clinical Genetics and Developmental Biology, Sassari 07100, Italy.
Carlo Ventura, Laboratory of Molecular Biology and Stem Cell Engineering, National Institute of Biostructures and Biosystems – Eldor Lab, Innovation Accelerator, Consiglio Nazionale delle Ricerche, Bologna 40129, Italy.
Margherita Maioli, Department of Biomedical Sciences, University of Sassari, Sassari 07100, Italy; Laboratory of Molecular Biology and Stem Cell Engineering, National Institute of Biostructures and Biosystems – Eldor Lab, Innovation Accelerator, Consiglio Nazionale delle Ricerche, Bologna 40129, Italy; Istituto di Ricerca Genetica e Biomedica, Consiglio Nazionale delle Ricerche, Cagliari 09042, Italy; Center for Developmental Biology and Reprogramming-CEDEBIOR, Department of Biomedical Sciences, University of Sassari, Sassari 07100, Italy. mmaioli@uniss.it.
References
- 1.Biehl JK, Russell B. Introduction to stem cell therapy. J Cardiovasc Nurs. 2009;24:98–103; quiz 104-5. doi: 10.1097/JCN.0b013e318197a6a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fischbach GD, Fischbach RL. Stem cells: science, policy, and ethics. J Clin Invest. 2004;114:1364–1370. doi: 10.1172/JCI23549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Wert G, Mummery C. Human embryonic stem cells: research, ethics and policy. Hum Reprod. 2003;18:672–682. doi: 10.1093/humrep/deg143. [DOI] [PubMed] [Google Scholar]
- 4.Wood A. Ethics and embryonic stem cell research. Stem Cell Rev. 2005;1:317–324. doi: 10.1385/SCR:1:4:317. [DOI] [PubMed] [Google Scholar]
- 5.Mizukami A, Swiech K. Mesenchymal Stromal Cells: From Discovery to Manufacturing and Commercialization. Stem Cells Int. 2018;2018:4083921. doi: 10.1155/2018/4083921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding DC, Shyu WC, Lin SZ. Mesenchymal stem cells. Cell Transplant. 2011;20:5–14. doi: 10.3727/096368910X. [DOI] [PubMed] [Google Scholar]
- 8.Baghaei K, Hashemi SM, Tokhanbigli S, Asadi Rad A, Assadzadeh-Aghdaei H, Sharifian A, Zali MR. Isolation, differentiation, and characterization of mesenchymal stem cells from human bone marrow. Gastroenterol Hepatol Bed Bench. 2017;10:208–213. [PMC free article] [PubMed] [Google Scholar]
- 9.Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 10.Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45:115–120. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- 12.Ranjbaran H, Abediankenari S, Mohammadi M, Jafari N, Khalilian A, Rahmani Z, Momeninezhad Amiri M, Ebrahimi P. Wharton's Jelly Derived-Mesenchymal Stem Cells: Isolation and Characterization. Acta Med Iran. 2018;56:28–33. [PubMed] [Google Scholar]
- 13.Siegel G, Kluba T, Hermanutz-Klein U, Bieback K, Northoff H, Schäfer R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013;11:146. doi: 10.1186/1741-7015-11-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balzano F, Bellu E, Basoli V, Dei Giudici S, Santaniello S, Cruciani S, Facchin F, Oggiano A, Capobianco G, Dessole F, Ventura C, Dessole S, Maioli M. Lessons from human umbilical cord: gender differences in stem cells from Wharton's jelly. Eur J Obstet Gynecol Reprod Biol. 2019;234:143–148. doi: 10.1016/j.ejogrb.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 15.Chen Q, Shou P, Zheng C, Jiang M, Cao G, Yang Q, Cao J, Xie N, Velletri T, Zhang X, Xu C, Zhang L, Yang H, Hou J, Wang Y, Shi Y. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ. 2016;23:1128–1139. doi: 10.1038/cdd.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aronowitz JA, Lockhart RA, Hakakian CS. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. Springerplus. 2015;4:713. doi: 10.1186/s40064-015-1509-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaput B, Bertheuil N, Escubes M, Grolleau JL, Garrido I, Laloze J, Espagnolle N, Casteilla L, Sensebé L, Varin A. Mechanically Isolated Stromal Vascular Fraction Provides a Valid and Useful Collagenase-Free Alternative Technique: A Comparative Study. Plast Reconstr Surg. 2016;138:807–819. doi: 10.1097/PRS.0000000000002494. [DOI] [PubMed] [Google Scholar]
- 18.Bianchi F, Maioli M, Leonardi E, Olivi E, Pasquinelli G, Valente S, Mendez AJ, Ricordi C, Raffaini M, Tremolada C, Ventura C. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant. 2013;22:2063–2077. doi: 10.3727/096368912X657855. [DOI] [PubMed] [Google Scholar]
- 19.Coccè V, Brini A, Giannì AB, Sordi V, Berenzi A, Alessandri G, Tremolada C, Versari S, Bosetto A, Pessina A. A Nonenzymatic and Automated Closed-Cycle Process for the Isolation of Mesenchymal Stromal Cells in Drug Delivery Applications. Stem Cells Int. 2018;2018:4098140. doi: 10.1155/2018/4098140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003;5:32–45. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nöth U, Rackwitz L, Steinert AF, Tuan RS. Cell delivery therapeutics for musculoskeletal regeneration. Adv Drug Deliv Rev. 2010;62:765–783. doi: 10.1016/j.addr.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Biteau B, Hochmuth CE, Jasper H. Maintaining tissue homeostasis: dynamic control of somatic stem cell activity. Cell Stem Cell. 2011;9:402–411. doi: 10.1016/j.stem.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, Oron E, Nelson B, Razis S, Ivanova N. Distinct lineage specification roles for NANOG, OCT4, and SOX2 in human embryonic stem cells. Cell Stem Cell. 2012;10:440–454. doi: 10.1016/j.stem.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Gagliardi A, Mullin NP, Ying Tan Z, Colby D, Kousa AI, Halbritter F, Weiss JT, Felker A, Bezstarosti K, Favaro R, Demmers J, Nicolis SK, Tomlinson SR, Poot RA, Chambers I. A direct physical interaction between Nanog and Sox2 regulates embryonic stem cell self-renewal. EMBO J. 2013;32:2231–2247. doi: 10.1038/emboj.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 26.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 27.Laird PW. Principles and challenges of genomewide DNA methylation analysis. Nat Rev Genet. 2010;11:191–203. doi: 10.1038/nrg2732. [DOI] [PubMed] [Google Scholar]
- 28.Boland MJ, Nazor KL, Loring JF. Epigenetic regulation of pluripotency and differentiation. Circ Res. 2014;115:311–324. doi: 10.1161/CIRCRESAHA.115.301517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheung P, Allis CD, Sassone-Corsi P. Signaling to chromatin through histone modifications. Cell. 2000;103:263–271. doi: 10.1016/s0092-8674(00)00118-5. [DOI] [PubMed] [Google Scholar]
- 30.Fischle W, Wang Y, Allis CD. Histone and chromatin cross-talk. Curr Opin Cell Biol. 2003;15:172–183. doi: 10.1016/s0955-0674(03)00013-9. [DOI] [PubMed] [Google Scholar]
- 31.Wu H, Sun YE. Epigenetic regulation of stem cell differentiation. Pediatr Res. 2006;59:21R–25R. doi: 10.1203/01.pdr.0000203565.76028.2a. [DOI] [PubMed] [Google Scholar]
- 32.Rinaldi L, Benitah SA. Epigenetic regulation of adult stem cell function. FEBS J. 2015;282:1589–1604. doi: 10.1111/febs.12946. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Shi ZY, Feng J, Cao JK. HDAC6 regulates dental mesenchymal stem cells and osteoclast differentiation. BMC Oral Health. 2018;18:190. doi: 10.1186/s12903-018-0624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Etchegaray JP, Chavez L, Huang Y, Ross KN, Choi J, Martinez-Pastor B, Walsh RM, Sommer CA, Lienhard M, Gladden A, Kugel S, Silberman DM, Ramaswamy S, Mostoslavsky G, Hochedlinger K, Goren A, Rao A, Mostoslavsky R. The histone deacetylase SIRT6 controls embryonic stem cell fate via TET-mediated production of 5-hydroxymethylcytosine. Nat Cell Biol. 2015;17:545–557. doi: 10.1038/ncb3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santaniello S, Cruciani S, Basoli V, Balzano F, Bellu E, Garroni G, Ginesu GC, Cossu ML, Facchin F, Delitala AP, Ventura C, Maioli M. Melatonin and Vitamin D Orchestrate Adipose Derived Stem Cell Fate by Modulating Epigenetic Regulatory Genes. Int J Med Sci. 2018;15:1631–1639. doi: 10.7150/ijms.27669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gasiūnienė M, Zubova A, Utkus A, Navakauskienė R. Epigenetic and metabolic alterations in human amniotic fluid stem cells induced to cardiomyogenic differentiation by DNA methyltransferases and p53 inhibitors. J Cell Biochem. 2018 doi: 10.1002/jcb.28092. [DOI] [PubMed] [Google Scholar]
- 37.Pathania R, Ramachandran S, Mariappan G, Thakur P, Shi H, Choi JH, Manicassamy S, Kolhe R, Prasad PD, Sharma S, Lokeshwar BL, Ganapathy V, Thangaraju M. Combined Inhibition of DNMT and HDAC Blocks the Tumorigenicity of Cancer Stem-like Cells and Attenuates Mammary Tumor Growth. Cancer Res. 2016;76:3224–3235. doi: 10.1158/0008-5472.CAN-15-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khavari DA, Sen GL, Rinn JL. DNA methylation and epigenetic control of cellular differentiation. Cell Cycle. 2010;9:3880–3883. doi: 10.4161/cc.9.19.13385. [DOI] [PubMed] [Google Scholar]
- 39.Cheng Y, Xie N, Jin P, Wang T. DNA methylation and hydroxymethylation in stem cells. Cell Biochem Funct. 2015;33:161–173. doi: 10.1002/cbf.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keenen B, de la Serna IL. Chromatin remodeling in embryonic stem cells: regulating the balance between pluripotency and differentiation. J Cell Physiol. 2009;219:1–7. doi: 10.1002/jcp.21654. [DOI] [PubMed] [Google Scholar]
- 41.Gangaraju VK, Lin H. MicroRNAs: key regulators of stem cells. Nat Rev Mol Cell Biol. 2009;10:116–125. doi: 10.1038/nrm2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li N, Long B, Han W, Yuan S, Wang K. microRNAs: important regulators of stem cells. Stem Cell Res Ther. 2017;8:110. doi: 10.1186/s13287-017-0551-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beerman I, Rossi DJ. Epigenetic Control of Stem Cell Potential during Homeostasis, Aging, and Disease. Cell Stem Cell. 2015;16:613–625. doi: 10.1016/j.stem.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodrigues M, Griffith LG, Wells A. Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cell Res Ther. 2010;1:32. doi: 10.1186/scrt32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hwang KC, Kim JY, Chang W, Kim DS, Lim S, Kang SM, Song BW, Ha HY, Huh YJ, Choi IG, Hwang DY, Song H, Jang Y, Chung N, Kim SH, Kim DW. Chemicals that modulate stem cell differentiation. Proc Natl Acad Sci U S A. 2008;105:7467–7471. doi: 10.1073/pnas.0802825105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 47.Basoli V, Santaniello S, Rinaldi S, Fontani V, Pigliaru G, Wieser M, Strajeriu A, Castagna A, Redl H, Ventura C, Grillari R, Maioli M. Physical stimulation by REAC and BMP4/WNT-1 inhibitor synergistically enhance cardiogenic commitment in iPSCs. PLoS One. 2019;14:e0211188. doi: 10.1371/journal.pone.0211188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J, Anguera MC. In Vitro Differentiation of Human Pluripotent Stem Cells into Trophoblastic Cells. J Vis Exp. 2017 doi: 10.3791/55268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ventura C, Maioli M, Asara Y, Santoni D, Scarlata I, Cantoni S, Perbellini A. Butyric and retinoic mixed ester of hyaluronan. A novel differentiating glycoconjugate affording a high throughput of cardiogenesis in embryonic stem cells. J Biol Chem. 2004;279:23574–23579. doi: 10.1074/jbc.M401869200. [DOI] [PubMed] [Google Scholar]
- 50.Ventura C, Cantoni S, Bianchi F, Lionetti V, Cavallini C, Scarlata I, Foroni L, Maioli M, Bonsi L, Alviano F, Fossati V, Bagnara GP, Pasquinelli G, Recchia FA, Perbellini A. Hyaluronan mixed esters of butyric and retinoic Acid drive cardiac and endothelial fate in term placenta human mesenchymal stem cells and enhance cardiac repair in infarcted rat hearts. J Biol Chem. 2007;282:14243–14252. doi: 10.1074/jbc.M609350200. [DOI] [PubMed] [Google Scholar]
- 51.Lionetti V, Cantoni S, Cavallini C, Bianchi F, Valente S, Frascari I, Olivi E, Aquaro GD, Bonavita F, Scarlata I, Maioli M, Vaccari V, Tassinari R, Bartoli A, Recchia FA, Pasquinelli G, Ventura C. Hyaluronan mixed esters of butyric and retinoic acid affording myocardial survival and repair without stem cell transplantation. J Biol Chem. 2010;285:9949–9961. doi: 10.1074/jbc.M109.087254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maioli M, Santaniello S, Montella A, Bandiera P, Cantoni S, Cavallini C, Bianchi F, Lionetti V, Rizzolio F, Marchesi I, Bagella L, Ventura C. Hyaluronan esters drive Smad gene expression and signaling enhancing cardiogenesis in mouse embryonic and human mesenchymal stem cells. PLoS One. 2010;5:e15151. doi: 10.1371/journal.pone.0015151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maioli M, Basoli V, Santaniello S, Cruciani S, Delitala AP, Pinna R, Milia E, Grillari-Voglauer R, Fontani V, Rinaldi S, Muggironi R, Pigliaru G, Ventura C. Osteogenesis from Dental Pulp Derived Stem Cells: A Novel Conditioned Medium Including Melatonin within a Mixture of Hyaluronic, Butyric, and Retinoic Acids. Stem Cells Int. 2016;2016:2056416. doi: 10.1155/2016/2056416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santini MT, Rainaldi G, Indovina PL. Cellular effects of extremely low frequency (ELF) electromagnetic fields. Int J Radiat Biol. 2009;85:294–313. doi: 10.1080/09553000902781097. [DOI] [PubMed] [Google Scholar]
- 55.Collodel G, Fioravanti A, Pascarelli NA, Lamboglia A, Fontani V, Maioli M, Santaniello S, Pigliaru G, Castagna A, Moretti E, Iacoponi F, Rinaldi S, Ventura C. Effects of regenerative radioelectric asymmetric conveyer treatment on human normal and osteoarthritic chondrocytes exposed to IL-1β. A biochemical and morphological study. Clin Interv Aging. 2013;8:309–316. doi: 10.2147/CIA.S42229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maziarz A, Kocan B, Bester M, Budzik S, Cholewa M, Ochiya T, Banas A. How electromagnetic fields can influence adult stem cells: positive and negative impacts. Stem Cell Res Ther. 2016;7:54. doi: 10.1186/s13287-016-0312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ventura C, Maioli M, Asara Y, Santoni D, Mesirca P, Remondini D, Bersani F. Turning on stem cell cardiogenesis with extremely low frequency magnetic fields. FASEB J. 2005;19:155–157. doi: 10.1096/fj.04-2695fje. [DOI] [PubMed] [Google Scholar]
- 58.Thrivikraman G, Madras G, Basu B. Intermittent electrical stimuli for guidance of human mesenchymal stem cell lineage commitment towards neural-like cells on electroconductive substrates. Biomaterials. 2014;35:6219–6235. doi: 10.1016/j.biomaterials.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 59.Vinatier C, Mrugala D, Jorgensen C, Guicheux J, Noël D. Cartilage engineering: a crucial combination of cells, biomaterials and biofactors. Trends Biotechnol. 2009;27:307–314. doi: 10.1016/j.tibtech.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 60.Singh A, Elisseeff J. Biomaterials for stem cell differentiation. J Mater Chem. 2010;20:8832–8847. [Google Scholar]
- 61.Ghasemi-Mobarakeh L, Prabhakaran MP, Tian L, Shamirzaei-Jeshvaghani E, Dehghani L, Ramakrishna S. Structural properties of scaffolds: Crucial parameters towards stem cells differentiation. World J Stem Cells. 2015;7:728–744. doi: 10.4252/wjsc.v7.i4.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakayama KH, Hou L, Huang NF. Role of extracellular matrix signaling cues in modulating cell fate commitment for cardiovascular tissue engineering. Adv Healthc Mater. 2014;3:628–641. doi: 10.1002/adhm.201300620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun S, Liu Y, Lipsky S, Cho M. Physical manipulation of calcium oscillations facilitates osteodifferentiation of human mesenchymal stem cells. FASEB J. 2007;21:1472–1480. doi: 10.1096/fj.06-7153com. [DOI] [PubMed] [Google Scholar]
- 64.Fodor J, Matta C, Oláh T, Juhász T, Takács R, Tóth A, Dienes B, Csernoch L, Zákány R. Store-operated calcium entry and calcium influx via voltage-operated calcium channels regulate intracellular calcium oscillations in chondrogenic cells. Cell Calcium. 2013;54:1–16. doi: 10.1016/j.ceca.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 65.Haupt G. Use of extracorporeal shock waves in the treatment of pseudarthrosis, tendinopathy and other orthopedic diseases. J Urol. 1997;158:4–11. doi: 10.1097/00005392-199707000-00003. [DOI] [PubMed] [Google Scholar]
- 66.Mittermayr R, Antonic V, Hartinger J, Kaufmann H, Redl H, Téot L, Stojadinovic A, Schaden W. Extracorporeal shock wave therapy (ESWT) for wound healing: technology, mechanisms, and clinical efficacy. Wound Repair Regen. 2012;20:456–465. doi: 10.1111/j.1524-475X.2012.00796.x. [DOI] [PubMed] [Google Scholar]
- 67.Weihs AM, Fuchs C, Teuschl AH, Hartinger J, Slezak P, Mittermayr R, Redl H, Junger WG, Sitte HH, Rünzler D. Shock wave treatment enhances cell proliferation and improves wound healing by ATP release-coupled extracellular signal-regulated kinase (ERK) activation. J Biol Chem. 2014;289:27090–27104. doi: 10.1074/jbc.M114.580936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schuh CM, Heher P, Weihs AM, Banerjee A, Fuchs C, Gabriel C, Wolbank S, Mittermayr R, Redl H, Rünzler D, Teuschl AH. In vitro extracorporeal shock wave treatment enhances stemness and preserves multipotency of rat and human adipose-derived stem cells. Cytotherapy. 2014;16:1666–1678. doi: 10.1016/j.jcyt.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 69.Maioli M, Rinaldi S, Santaniello S, Castagna A, Pigliaru G, Gualini S, Fontani V, Ventura C. Radiofrequency energy loop primes cardiac, neuronal, and skeletal muscle differentiation in mouse embryonic stem cells: a new tool for improving tissue regeneration. Cell Transplant. 2012;21:1225–1233. doi: 10.3727/096368911X600966. [DOI] [PubMed] [Google Scholar]
- 70.Maioli M, Rinaldi S, Santaniello S, Castagna A, Pigliaru G, Delitala A, Bianchi F, Tremolada C, Fontani V, Ventura C. Radioelectric asymmetric conveyed fields and human adipose-derived stem cells obtained with a nonenzymatic method and device: a novel approach to multipotency. Cell Transplant. 2014;23:1489–1500. doi: 10.3727/096368913X672037. [DOI] [PubMed] [Google Scholar]
- 71.Maioli M, Rinaldi S, Santaniello S, Castagna A, Pigliaru G, Delitala A, Lotti Margotti M, Bagella L, Fontani V, Ventura C. Anti-senescence efficacy of radio-electric asymmetric conveyer technology. Age (Dordr) 2014;36:9–20. doi: 10.1007/s11357-013-9537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maioli M, Rinaldi S, Pigliaru G, Santaniello S, Basoli V, Castagna A, Fontani V, Ventura C. REAC technology and hyaluron synthase 2, an interesting network to slow down stem cell senescence. Sci Rep. 2016;6:28682. doi: 10.1038/srep28682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rinaldi S, Maioli M, Santaniello S, Castagna A, Pigliaru G, Gualini S, Margotti ML, Carta A, Fontani V, Ventura C. Regenerative treatment using a radioelectric asymmetric conveyor as a novel tool in antiaging medicine: an in vitro beta-galactosidase study. Clin Interv Aging. 2012;7:191–194. doi: 10.2147/CIA.S33312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rinaldi S, Maioli M, Pigliaru G, Castagna A, Santaniello S, Basoli V, Fontani V, Ventura C. Stem cell senescence. Effects of REAC technology on telomerase-independent and telomerase-dependent pathways. Sci Rep. 2014;4:6373. doi: 10.1038/srep06373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maioli M, Rinaldi S, Santaniello S, Castagna A, Pigliaru G, Gualini S, Cavallini C, Fontani V, Ventura C. Radio electric conveyed fields directly reprogram human dermal skin fibroblasts toward cardiac, neuronal, and skeletal muscle-like lineages. Cell Transplant. 2013;22:1227–1235. doi: 10.3727/096368912X657297. [DOI] [PubMed] [Google Scholar]
- 76.Maioli M, Rinaldi S, Migheli R, Pigliaru G, Rocchitta G, Santaniello S, Basoli V, Castagna A, Fontani V, Ventura C, Serra PA. Neurological morphofunctional differentiation induced by REAC technology in PC12. A neuro protective model for Parkinson's disease. Sci Rep. 2015;5:10439. doi: 10.1038/srep10439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim YM, Kang YG, Park SH, Han MK, Kim JH, Shin JW, Shin JW. Effects of mechanical stimulation on the reprogramming of somatic cells into human-induced pluripotent stem cells. Stem Cell Res Ther. 2017;8:139. doi: 10.1186/s13287-017-0594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baek S, Choi H, Park H, Cho B, Kim S, Kim J. Effects of a hypomagnetic field on DNA methylation during the differentiation of embryonic stem cells. Sci Rep. 2019;9:1333. doi: 10.1038/s41598-018-37372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baek S, Quan X, Kim S, Lengner C, Park JK, Kim J. Electromagnetic fields mediate efficient cell reprogramming into a pluripotent state. ACS Nano. 2014;8:10125–10138. doi: 10.1021/nn502923s. [DOI] [PubMed] [Google Scholar]
- 80.Aronson JK. Defining 'nutraceuticals': neither nutritious nor pharmaceutical. Br J Clin Pharmacol. 2017;83:8–19. doi: 10.1111/bcp.12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cruciani S, Santaniello S, Garroni G, Fadda A, Balzano F, Bellu E, Sarais G, Fais G, Mulas M, Maioli M. <i>Myrtus</i> Polyphenols, from Antioxidants to Anti-Inflammatory Molecules: Exploring a Network Involving Cytochromes P450 and Vitamin D. Molecules. 2019;24:pii: E1515. doi: 10.3390/molecules24081515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Claustrat B, Leston J. Melatonin: Physiological effects in humans. Neurochirurgie. 2015;61:77–84. doi: 10.1016/j.neuchi.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 83.Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev. 2005;9:11–24. doi: 10.1016/j.smrv.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 84.Zhang S, Chen S, Li Y, Liu Y. Melatonin as a promising agent of regulating stem cell biology and its application in disease therapy. Pharmacol Res. 2017;117:252–260. doi: 10.1016/j.phrs.2016.12.035. [DOI] [PubMed] [Google Scholar]
- 85.Lee JH, Yoon YM, Han YS, Jung SK, Lee SH. Melatonin protects mesenchymal stem cells from autophagy-mediated death under ischaemic ER-stress conditions by increasing prion protein expression. Cell Prolif. 2019;52:e12545. doi: 10.1111/cpr.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mendivil-Perez M, Soto-Mercado V, Guerra-Librero A, Fernandez-Gil BI, Florido J, Shen YQ, Tejada MA, Capilla-Gonzalez V, Rusanova I, Garcia-Verdugo JM, Acuña-Castroviejo D, López LC, Velez-Pardo C, Jimenez-Del-Rio M, Ferrer JM, Escames G. Melatonin enhances neural stem cell differentiation and engraftment by increasing mitochondrial function. J Pineal Res. 2017:63. doi: 10.1111/jpi.12415. [DOI] [PubMed] [Google Scholar]
- 87.Domazetovic V, Marcucci G, Iantomasi T, Brandi ML, Vincenzini MT. Oxidative stress in bone remodeling: role of antioxidants. Clin Cases Miner Bone Metab. 2017;14:209–216. doi: 10.11138/ccmbm/2017.14.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee S, Le NH, Kang D. Melatonin alleviates oxidative stress-inhibited osteogenesis of human bone marrow-derived mesenchymal stem cells through AMPK activation. Int J Med Sci. 2018;15:1083–1091. doi: 10.7150/ijms.26314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Feng S, Reuss L, Wang Y. Potential of Natural Products in the Inhibition of Adipogenesis through Regulation of PPARγ Expression and/or Its Transcriptional Activity. Molecules. 2016;21:pii: E1278. doi: 10.3390/molecules21101278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vingtdeux V, Chandakkar P, Zhao H, Davies P, Marambaud P. Small-molecule activators of AMP-activated protein kinase (AMPK), RSVA314 and RSVA405, inhibit adipogenesis. Mol Med. 2011;17:1022–1030. doi: 10.2119/molmed.2011.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Basoli V, Santaniello S, Cruciani S, Ginesu GC, Cossu ML, Delitala AP, Serra PA, Ventura C, Maioli M. Melatonin and Vitamin D Interfere with the Adipogenic Fate of Adipose-Derived Stem Cells. Int J Mol Sci. 2017;18:pii: E981. doi: 10.3390/ijms18050981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21:319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Szymczak I, Pawliczak R. The Active Metabolite of Vitamin D3 as a Potential Immunomodulator. Scand J Immunol. 2016;83:83–91. doi: 10.1111/sji.12403. [DOI] [PubMed] [Google Scholar]
- 94.Morceau F, Chateauvieux S, Orsini M, Trécul A, Dicato M, Diederich M. Natural compounds and pharmaceuticals reprogram leukemia cell differentiation pathways. Biotechnol Adv. 2015;33:785–797. doi: 10.1016/j.biotechadv.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 95.Nobili S, Lippi D, Witort E, Donnini M, Bausi L, Mini E, Capaccioli S. Natural compounds for cancer treatment and prevention. Pharmacol Res. 2009;59:365–378. doi: 10.1016/j.phrs.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 96.Maioli M, Basoli V, Carta P, Fabbri D, Dettori MA, Cruciani S, Serra PA, Delogu G. Synthesis of magnolol and honokiol derivatives and their effect against hepatocarcinoma cells. PLoS One. 2018;13:e0192178. doi: 10.1371/journal.pone.0192178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ferhi S, Santaniello S, Zerizer S, Cruciani S, Fadda A, Sanna D, Dore A, Maioli M, D'hallewin G. Total Phenols from Grape Leaves Counteract Cell Proliferation and Modulate Apoptosis-Related Gene Expression in MCF-7 and HepG2 Human Cancer Cell Lines. Molecules. 2019;24:pii: E612. doi: 10.3390/molecules24030612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Squillaro T, Peluso G, Galderisi U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant. 2016;25:829–848. doi: 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]
- 99.Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient's bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211:27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- 100.Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441:1094–1096. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- 101.Cohen JA. Mesenchymal stem cell transplantation in multiple sclerosis. J Neurol Sci. 2013;333:43–49. doi: 10.1016/j.jns.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 103.Esiashvili N, Pulsipher MA. Hematopoietic stem cell transplantation. Pediatric Oncology. 2018:301–311. [Google Scholar]
- 104.Robin M, Porcher R, De Castro Araujo R, de Latour RP, Devergie A, Rocha V, Larghero J, Adès L, Ribaud P, Mary JY, Socié G. Risk factors for late infections after allogeneic hematopoietic stem cell transplantation from a matched related donor. Biol Blood Marrow Transplant. 2007;13:1304–1312. doi: 10.1016/j.bbmt.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 105.Henig I, Zuckerman T. Hematopoietic stem cell transplantation-50 years of evolution and future perspectives. Rambam Maimonides Med J. 2014;5:e0028. doi: 10.5041/RMMJ.10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim SU, de Vellis J. Stem cell-based cell therapy in neurological diseases: a review. J Neurosci Res. 2009;87:2183–2200. doi: 10.1002/jnr.22054. [DOI] [PubMed] [Google Scholar]
- 107.Kim N, Cho SG. Clinical applications of mesenchymal stem cells. Korean J Intern Med. 2013;28:387–402. doi: 10.3904/kjim.2013.28.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ogawa M, Larue AC, Watson PM, Watson DK. Hematopoietic stem cell origin of connective tissues. Exp Hematol. 2010;38:540–547. doi: 10.1016/j.exphem.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 109.Kunisaki Y. [The hematopoietic stem cell niche] Rinsho Ketsueki. 2015;56:1888–1893. doi: 10.11406/rinketsu.56.1888. [DOI] [PubMed] [Google Scholar]
- 110.Hoggatt J, Kfoury Y, Scadden DT. Hematopoietic Stem Cell Niche in Health and Disease. Annu Rev Pathol. 2016;11:555–581. doi: 10.1146/annurev-pathol-012615-044414. [DOI] [PubMed] [Google Scholar]
- 111.Guruharsha KG, Kankel MW, Artavanis-Tsakonas S. The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat Rev Genet. 2012;13:654–666. doi: 10.1038/nrg3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Collu GM, Hidalgo-Sastre A, Brennan K. Wnt-Notch signalling crosstalk in development and disease. Cell Mol Life Sci. 2014;71:3553–3567. doi: 10.1007/s00018-014-1644-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 114.Liu J, Sato C, Cerletti M, Wagers A. Notch signaling in the regulation of stem cell self-renewal and differentiation. Curr Top Dev Biol. 2010;92:367–409. doi: 10.1016/S0070-2153(10)92012-7. [DOI] [PubMed] [Google Scholar]
- 115.García-García A, de Castillejo CL, Méndez-Ferrer S. BMSCs and hematopoiesis. Immunol Lett. 2015;168:129–135. doi: 10.1016/j.imlet.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 116.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 117.Stiff PJ, Unger JM, Cook JR, Constine LS, Couban S, Stewart DA, Shea TC, Porcu P, Winter JN, Kahl BS, Miller TP, Tubbs RR, Marcellus D, Friedberg JW, Barton KP, Mills GM, LeBlanc M, Rimsza LM, Forman SJ, Fisher RI. Autologous transplantation as consolidation for aggressive non-Hodgkin's lymphoma. N Engl J Med. 2013;369:1681–1690. doi: 10.1056/NEJMoa1301077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ali N, Adil SN, Shaikh MU. Autologous Hematopoietic Stem Cell Transplantation-10 Years of Data From a Developing Country. Stem Cells Transl Med. 2015;4:873–877. doi: 10.5966/sctm.2015-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Farge D, Labopin M, Tyndall A, Fassas A, Mancardi GL, Van Laar J, Ouyang J, Kozak T, Moore J, Kötter I, Chesnel V, Marmont A, Gratwohl A, Saccardi R. Autologous hematopoietic stem cell transplantation for autoimmune diseases: an observational study on 12 years' experience from the European Group for Blood and Marrow Transplantation Working Party on Autoimmune Diseases. Haematologica. 2010;95:284–292. doi: 10.3324/haematol.2009.013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gratwohl A, Baldomero H, Aljurf M, Pasquini MC, Bouzas LF, Yoshimi A, Szer J, Lipton J, Schwendener A, Gratwohl M, Frauendorfer K, Niederwieser D, Horowitz M, Kodera Y Worldwide Network of Blood and Marrow Transplantation. Hematopoietic stem cell transplantation: a global perspective. JAMA. 2010;303:1617–1624. doi: 10.1001/jama.2010.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Annaloro C, Onida F, Lambertenghi Deliliers G. Autologous hematopoietic stem cell transplantation in autoimmune diseases. Expert Rev Hematol. 2009;2:699–715. doi: 10.1586/ehm.09.60. [DOI] [PubMed] [Google Scholar]
- 122.Klingemann HG, Storb R, Fefer A, Deeg HJ, Appelbaum FR, Buckner CD, Cheever MA, Greenberg PD, Stewart PS, Sullivan KM. Bone marrow transplantation in patients aged 45 years and older. Blood. 1986;67:770–776. [PubMed] [Google Scholar]
- 123.Bourke SC, Gibson GJ. Non-invasive ventilation in ALS: current practice and future role. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5:67–71. doi: 10.1080/14660820410020330. [DOI] [PubMed] [Google Scholar]
- 124.Morgan S, Orrell RW. Pathogenesis of amyotrophic lateral sclerosis. Br Med Bull. 2016;119:87–98. doi: 10.1093/bmb/ldw026. [DOI] [PubMed] [Google Scholar]
- 125.Brown RH., Jr Amyotrophic lateral sclerosis: recent insights from genetics and transgenic mice. Cell. 1995;80:687–692. doi: 10.1016/0092-8674(95)90346-1. [DOI] [PubMed] [Google Scholar]
- 126.Grad LI, Cashman NR. Prion-like activity of Cu/Zn superoxide dismutase: implications for amyotrophic lateral sclerosis. Prion. 2014;8:33–41. doi: 10.4161/pri.27602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sibilla C, Bertolotti A. Prion Properties of SOD1 in Amyotrophic Lateral Sclerosis and Potential Therapy. Cold Spring Harb Perspect Biol. 2017;9:pii: a024141. doi: 10.1101/cshperspect.a024141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Catalanotto C, Cogoni C, Zardo G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int J Mol Sci. 2016;17:pii: E1712. doi: 10.3390/ijms17101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Balzano F, Cruciani S, Basoli V, Santaniello S, Facchin F, Ventura C, Maioli M. MiR200 and miR302: Two Big Families Influencing Stem Cell Behavior. Molecules. 2018;23:pii: E282. doi: 10.3390/molecules23020282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Di Pietro L, Lattanzi W, Bernardini C. Skeletal Muscle MicroRNAs as Key Players in the Pathogenesis of Amyotrophic Lateral Sclerosis. Int J Mol Sci. 2018;19:pii: E1534. doi: 10.3390/ijms19051534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Williams AH, Valdez G, Moresi V, Qi X, McAnally J, Elliott JL, Bassel-Duby R, Sanes JR, Olson EN. MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice. Science. 2009;326:1549–1554. doi: 10.1126/science.1181046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yacila G, Sari Y. Potential therapeutic drugs and methods for the treatment of amyotrophic lateral sclerosis. Curr Med Chem. 2014;21:3583–3593. doi: 10.2174/0929867321666140601162710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Martinez A, Palomo Ruiz MD, Perez DI, Gil C. Drugs in clinical development for the treatment of amyotrophic lateral sclerosis. Expert Opin Investig Drugs. 2017;26:403–414. doi: 10.1080/13543784.2017.1302426. [DOI] [PubMed] [Google Scholar]
- 134.Meamar R, Nasr-Esfahani MH, Mousavi SA, Basiri K. Stem cell therapy in amyotrophic lateral sclerosis. J Clin Neurosci. 2013;20:1659–1663. doi: 10.1016/j.jocn.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 135.Gowing G, Svendsen CN. Stem cell transplantation for motor neuron disease: current approaches and future perspectives. Neurotherapeutics. 2011;8:591–606. doi: 10.1007/s13311-011-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mazzini L, Ferrari D, Andjus PR, Buzanska L, Cantello R, De Marchi F, Gelati M, Giniatullin R, Glover JC, Grilli M, Kozlova EN, Maioli M, Mitrečić D, Pivoriunas A, Sanchez-Pernaute R, Sarnowska A, Vescovi AL BIONECA COST ACTION WG Neurology. Advances in stem cell therapy for amyotrophic lateral sclerosis. Expert Opin Biol Ther. 2018;18:865–881. doi: 10.1080/14712598.2018.1503248. [DOI] [PubMed] [Google Scholar]
- 137.Rushkevich YN, Kosmacheva SM, Zabrodets GV, Ignatenko SI, Goncharova NV, Severin IN, Likhachev SA, Potapnev MP. The Use of Autologous Mesenchymal Stem Cells for Cell Therapy of Patients with Amyotrophic Lateral Sclerosis in Belarus. Bull Exp Biol Med. 2015;159:576–581. doi: 10.1007/s10517-015-3017-3. [DOI] [PubMed] [Google Scholar]
- 138.Mazzini L, Gelati M, Profico DC, Sgaravizzi G, Projetti Pensi M, Muzi G, Ricciolini C, Rota Nodari L, Carletti S, Giorgi C, Spera C, Domenico F, Bersano E, Petruzzelli F, Cisari C, Maglione A, Sarnelli MF, Stecco A, Querin G, Masiero S, Cantello R, Ferrari D, Zalfa C, Binda E, Visioli A, Trombetta D, Novelli A, Torres B, Bernardini L, Carriero A, Prandi P, Servo S, Cerino A, Cima V, Gaiani A, Nasuelli N, Massara M, Glass J, Sorarù G, Boulis NM, Vescovi AL. Human neural stem cell transplantation in ALS: initial results from a phase I trial. J Transl Med. 2015;13:17. doi: 10.1186/s12967-014-0371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mazzini L, Vercelli A, Mareschi K, Ferrero I, Testa L, Fagioli F. Mesenchymal stem cells for ALS patients. Amyotroph Lateral Scler. 2009;10:123–124. doi: 10.1080/17482960802572707. [DOI] [PubMed] [Google Scholar]
- 140.Gallipoli P, Giotopoulos G, Huntly BJ. Epigenetic regulators as promising therapeutic targets in acute myeloid leukemia. Ther Adv Hematol. 2015;6:103–119. doi: 10.1177/2040620715577614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chuang DM, Leng Y, Marinova Z, Kim HJ, Chiu CT. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci. 2009;32:591–601. doi: 10.1016/j.tins.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fagnocchi L, Mazzoleni S, Zippo A. Integration of Signaling Pathways with the Epigenetic Machinery in the Maintenance of Stem Cells. Stem Cells Int. 2016;2016:8652748. doi: 10.1155/2016/8652748. [DOI] [PMC free article] [PubMed] [Google Scholar]