Abstract
Immunosuppressive therapies decrease the incidence of acute kidney rejection after kidney transplantation, but also increase the risk of infections and sepsis. This study aimed to identify the risk factors associated with complications and/or graft failure in kidney transplant patients with sepsis. A total of 14,658 kidney transplant patients with sepsis, identified in the National Inpatient Sample (NIS) database (data from 2005–2014), were included in the study and classified into three groups: patients without complications or graft failure/dialysis (Group 1), patients with complications only (Group 2), and patients with complications and graft failure/dialysis (Group 3). Multinomial logistic regression analyses were conducted to evaluate factors associated with kidney transplant recipients. Multivariate analysis showed that, compared to Group 1, patients from Group 2 or Group 3 were more likely to be Black and to have cytomegalovirus infection, coagulopathy, and glomerulonephritis (p ≤ 0.041). Also, Group 2 was more likely to have herpes simplex virus infection, and Group 3 was more likely to have hepatitis C infection and peripheral vascular disorders, compared to Group 1 (p ≤ 0.002). In addition, patients in Group 3 were more likely to be Black and to have hepatitis C infection, peripheral vascular disorders, coagulopathy, and hypertension compared to Group 2 (p ≤ 0.039). Age and female gender were associated with lower odds of complications after kidney transplantation regardless of graft rejection/dialysis (p ≤ 0.049). Hyperlipidemia and diabetes decreased the chance of complications and graft failure/dialysis after kidney transplant (p < 0.001). In conclusion, the study highlights that black race, male gender, and specific comorbidities can increase the risk of complications and graft failure in kidney transplant patients with sepsis.
Keywords: Renal transplantation, sepsis, dialysis, hyperlipidemia, diabetes mellitus, hepatitis C
INTRODUCTION
Potent immunosuppressive therapies used in patients undergoing kidney transplantation have lowered the incidence of acute kidney rejection by 10–15% in most centers. Unfortunately, immunosuppression inevitably increases the risk of post-transplant opportunistic infections and sepsis [1-4]. Infection is the most common non-cardiac cause of death after solid organ transplant, and sepsis is a major hurdle to disease-free survival after renal transplantation [1,2,5,6]. About 70% of renal transplant recipients have at least one episode of infection within 3 years of receiving the transplant [2]. Urinary tract infections are the most common post-transplant infections [7,8].
The occurrence and severity of infection after transplantation are determined by a balance between exposure, status of immunosuppression and nature of protection, as determined by chemoprophylaxis and vaccination status [2]. Viral and bacterial infections influence transplant outcomes. Bacterial infections are about twice as frequent as viral infections in kidney transplant recipients; in the period between 1996 and 2000, about 13% of kidney transplant patients in the United States (U.S.) required hospitalization in the first 3 months post-transplant because of bacterial infection, compared with 6% of patients with viral infections [9].
Sepsis is one of the most common causes of hospital admission for solid organ transplant recipients and is believed to suppress immune function; it is also a risk factor for cytomegalovirus (CMV) reactivation [10]. One study found that, after 6 months, sepsis occurred in 62% of transplant patients, and the primary source of infection was the urinary tract (38%). The most common pathogen was Escherichia coli (30.3%) [11]. Although the exact cause is unclear, sepsis may result from a number of factors, including urinary tract infections, pneumonia, kidney infections, abdominal infections, acute rejection, ureteral stent placement, Charlson Comorbidity Index (CCI) ≥3, and receipt of an organ from a deceased donor [10-12]. Sepsis may increase the risk for graft rejection and graft loss by mediating pro-inflammatory cytokines and upregulating histocompatibility antigens or adhesion proteins [10,12]. Several comorbidities have been found to be risk factors for complications and/or graft failure after renal transplantation, such as chronic hypertension, diabetes, obesity, glomerulonephritis and hyperlipidemia [13-17].
To our knowledge, no study has evaluated risk factors for complications and graft failure in kidney transplant patients with sepsis in a large patient population. Thus, we designed a study using a large national database to identify risk factors associated with complications and/or graft failure (as indicated by the need for dialysis) in kidney transplant patients with sepsis.
MATERIALS AND METHODS
Data sources
The National Inpatient Sample (NIS) is the largest all-payer inpatient database in the U.S. The NIS is maintained by the Agency for Healthcare Research and Quality as part of the Healthcare Cost and Utilization Project (HCUP) [18]. The NIS database contains inpatient information including sources of admission, demographics, diagnoses, procedures performed specifically for admission, comorbidity, disease severity evaluation, and health-care costs. This study used NIS data from 2005 to 2014; data before 2012 included all discharges from a 20% stratified sample of all U.S. hospitals. Data starting from 2012 contained a sample of discharges from all HCUP-participating hospitals in 44 states. The database approximates 20% of all discharges from all U.S. hospitals. To account for the new design of the database in 2012, sampling weights were created for trend analysis for the years before 2012. This study obtained the certificate number HCUP-28H23JUT1 and conforms to the data use agreement for the NIS from the HCUP Project.
Study population
The International Classification of Diseases, 9th revision, Clinical Modification code (ICD-9 CM) was used to identify all kidney transplant recipients (ICD-9-CM code V420, diagnosis [DX]2-DXn) admitted for treatment of sepsis (ICD-9-CM code 0380-0389, DX1). This group of patients formed the study population. Patients who had chronic renal dialysis or transplanted organ removal after more than a year were excluded.
Patients were divided into three groups according to the status of post-operative complications and dialysis: Group 1 was the reference group and included patients with no kidney transplant complications and without dialysis; Group 2 included patients with kidney transplant complications and without dialysis; and Group 3 patients had kidney transplant complications and dialysis.
Study variables
The following variables were assessed: demographics (age, gender, and race), the presence of viral infection, comorbidities, kidney transplant complications, and graft failure. In the HCUP-NIS, the first listed diagnostic code is the patient’s primary diagnosis (DX1), representing the primary reason why each patient was admitted to the hospital.
To identify viral infection prior to sepsis, the following diagnostic codes for each viral infection were used: cytomegalovirus [CMV] (ICD-9-CM code 0785); hepatitis C virus [HCV] (ICD-9-CM code 07044, 07054, V0262), hepatitis B virus [HBV] (ICD-9-CM code 07030-07033, V0261); and herpes simplex virus [HSV] (ICD-9-CM code 0544-0549).
To avoid confounding factors related to the study interest and outcomes, we adjusted our data for the following prognostic factors in kidney transplant patients: chronic hypertension, chronic diabetes, obesity, glomerulonephritis, and hyperlipidemia. In the NIS database, “chronic” is defined as having a disease for at least one year. To see if vascular causes played a role in kidney transplant complication and graft failure/dialysis, we also analyzed peripheral vascular disease and coagulopathies as risk factors. Although no ICD-9 codes exist for the comorbidities of glomerulonephritis and hyperlipidemia, we used the following codes to identify the diseases: glomerulonephritis (ICD-9-CM codes 5804-5812, 5821-5829) and hyperlipidemia (ICD-9-CM codes 2720 and 2724).
Outcomes
The primary outcome was defined as complications of the kidney transplant and/or graft failure/dialysis. We identified complications in patients with transplanted kidneys using the diagnostic code (ICD-9-CM code 99681), lymphocele (DX = 457.8), or artery stenosis (DX = 433.1). To differentiate patients with severe complications from those with less severe complications, acute graft loss was defined when patients had both the diagnostic code of complication in a transplanted kidney and the procedure code of hemodialysis catheter placement or hemodialysis (ICD-9-CM code 3895,3995) or peritoneal dialysis (ICD-9-CM code 5498). The NIS has no diagnostic code for “graft failure.” Thus, we assumed that when a kidney transplant patient required dialysis, this indicated a decline in graft function and, hence, graft failure.
Statistical analysis
Categorical variables were presented as counts and weighted percentages. Frequency distributions between categorical variables were assessed using the Chi-square test. Continuous variables were presented as mean with standard error (SE) and analyzed by ANOVA test. Multinomial logistic regression analyses were conducted to evaluate factors associated with kidney transplant recipients with sepsis, with or without complications or graft failure. The variables that were significantly associated with kidney transplant recipients with sepsis in the univariate multinomial logistic regression analyses were simultaneously included in multivariate multinomial logistic regression models. The mean, SE, proportions, all tests, and regression models were applied with discharge weights to account for the HCUP-NIS sampling method. Two-sided p < 0.05 was considered statistically significant. Statistical analyses were performed using the statistical software package SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
A total of 16,550 kidney transplant recipients with sepsis were identified in the HCUP-NIS database for the period between 2005 and 2014 year. After excluding missing data for age, gender and race, 14,658 patients were included in the final analysis (Figure 1). The mean age was 56.1 years; 53.1% of patients were male; and 55.7% were Caucasian.
FIGURE 1.
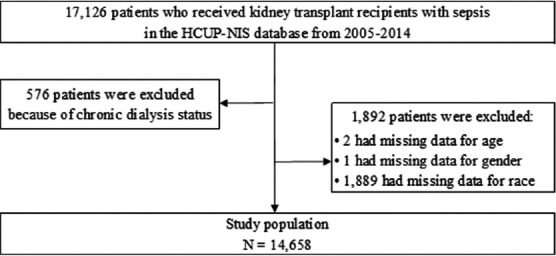
Patients’ selection flow.
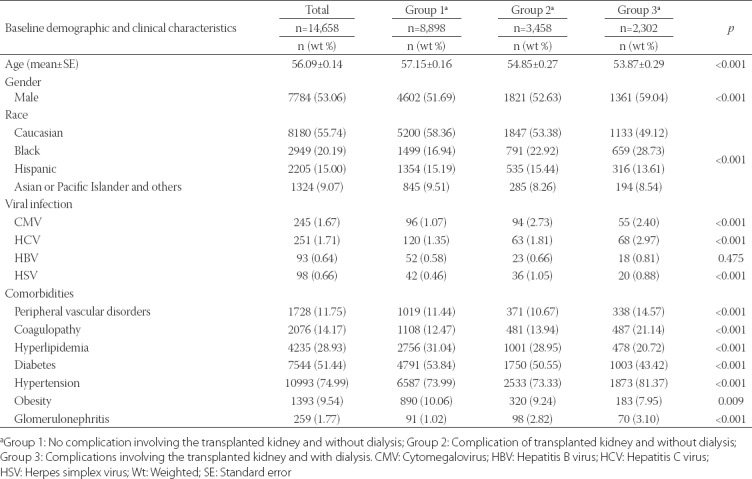
A comparison of the demographics and clinical features of each subgroup is shown in Table 1. Significant differences were observed in age (p < 0.001), gender (p < 0.001), race (p < 0.001), CMV infection (p < 0.001), HCV infection (p < 0.001), HSV infection (p < 0.001), peripheral vascular disorders (p < 0.001), coagulopathy (p < 0.001), hyperlipidemia (p < 0.001), diabetes (p < 0.001), hypertension (p < 0.001), obesity (p = 0.009), and glomerulonephritis (p < 0.001) among the three groups. Patients in Group 2 had a higher percentage of CMV infection (2.73%) and HSV infection (1.05%) than did patients in the other two groups. Patients in Group 1 had a higher percentage of hyperlipidemia (31.04%), diabetes (53.84%), and obesity (10.06%) than did patients in the other groups. Patients in Group 3 had a higher percentage of HCV infection (2.97%), peripheral vascular disorders (14.57%), coagulopathy (21.14%), hypertension (81.37%), and glomerulonephritis (3.10%) than did patients in the other groups.
TABLE 1.
Baseline demographic and clinical characteristics of kidney transplant patients with sepsis
Univariate and multivariate analysis
Group 1 vs. Group 2
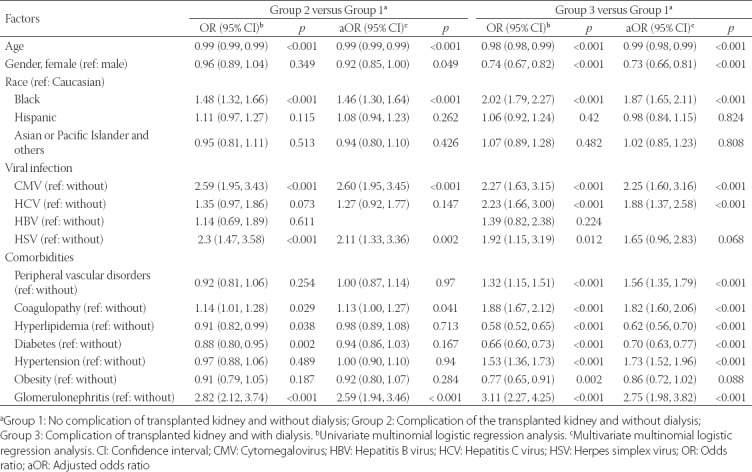
Univariate analyses indicated that the following variables were significantly more common risk factors for post-transplant complications among Group 2 patients than among Group 1 patients: age (p < 0.001), Black race (p < 0.001), CMV (p < 0.001) or HSV infection (p < 0.001), coagulopathy (p = 0.029), hyperlipidemia (p = 0.038), diabetes (p = 0.002), and glomerulonephritis [p < 0.001] (Table 2). Multivariate analyses found that Black race (p < 0.001), CMV infection (p < 0.001), HSV infection (p = 0.002), coagulopathy (p = 0.041), and glomerulonephritis (p < 0.001) were independent risk factors for post-transplant complications in Group 2 patients compared with Group 1 patients. In addition, age (p < 0.001) and female gender (p = 0.049) were protective factors in Group 2 patients compared with Group 1 patients (Table 2).
TABLE 2.
Univariate and multivariate multinomial logistic regression analyses of factors associated with different groups among kidney transplant recipients with sepsis (reference: Group 1)
Group 1 vs. Group 3
Univariate analysis found that Group 3 patients, compared with Group 1 patients, were significantly associated with Black race (p < 0.001), CMV infection (p < 0.001), HCV infection (p < 0.001), HSV infection (p = 0.012), peripheral vascular disorders (p < 0.001), coagulopathy (p < 0.001), hypertension (p < 0.001), and glomerulonephritis (p < 0.001) (Table 2). In contrast, age (p < 0.001), female gender (p < 0.001), hyperlipidemia (p < 0.001), diabetes (p < 0.001), and obesity (p = 0.002) were significantly more associated with Group 1 compared with Group 3 patients (Table 2). Results of multivariate analysis showed that 7 variables remained significantly more associated with Group 3 than Group 1 patients, as follows: Black race (p < 0.001), CMV infection (p < 0.001), HCV infection (p < 0.001), peripheral vascular disorders (p < 0.001), coagulopathy (p < 0.001), hypertension (p < 0.001), and glomerulonephritis (p < 0.001). Similarly, age (p < 0.001), female gender (p < 0.001), hyperlipidemia (p < 0.001), and diabetes (p < 0.001) remained less associated with Group 3 than with Group 1 in multivariate analysis (Table 2).
Group 2 vs. Group 3
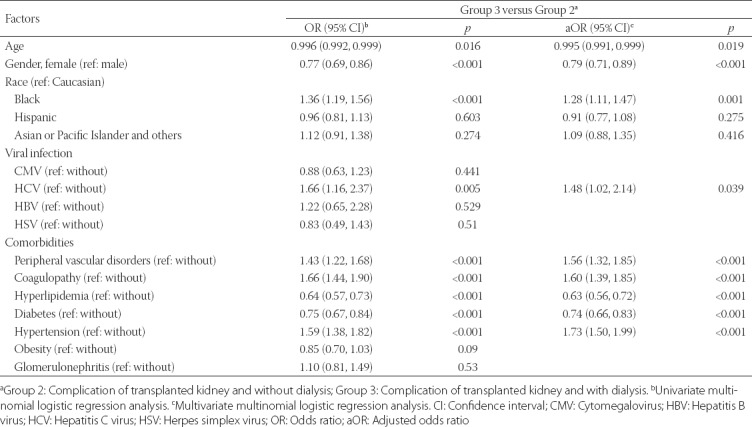
The comparison between Group 3 and Group 2 patients is shown in Table 3. The following variables were found to be significantly associated with Group 3 compared with Group 2: age (p = 0.016), female gender (p < 0.001), Black race (p < 0.001), HCV infection (p = 0.005), having peripheral vascular disorders (p < 0.001), coagulopathy (p < 0.001), hyperlipidemia (p < 0.001), diabetes (p < 0.001), and hypertension (p < 0.001) (Table 3). Multivariate analysis identified 5 variables as independent risk factors for post-transplant complications with dialysis/graft failure, including Black race (p = 0.001), HCV infection (p = 0.039), peripheral vascular disorders (p < 0.001), coagulopathy (p < 0.001), and hypertension (p < 0.001). Age (p = 0.019), female gender (p < 0.001), hyperlipidemia (p < 0.001), and diabetes (p < 0.001) were protective factors in patients who had kidney transplant complications and dialysis/graft failure.
TABLE 3.
Univariate and multivariate multinomial logistic regression analyses of factors associated with different groups among kidney transplant recipients with sepsis (reference: Group 2)
DISCUSSION
In this study, we found that Black race, male gender, and medical comorbidities were associated with an increased risk of complications and graft failure in kidney transplant recipients who developed sepsis. Medical comorbidities associated with an increased risk of complications without graft failure in post-transplant patients with sepsis were CMV infection, HSV infection, coagulopathy, and glomerulonephritis. Comorbidities associated with an increased risk for complications plus graft failure in post-transplant patients with sepsis were CMV infection, HCV infection, coagulopathy, glomerulonephritis, peripheral vascular disorders, and hypertension. In contrast, hyperlipidemia and diabetes were associated with a decreased chance of developing complications with graft failure/dialysis after kidney transplant. However, we did not find any association between diabetes or hyperlipidemia and post-transplant complications in the absence of graft failure/dialysis. When we compared kidney transplant patients who developed complications and had graft loss with patients who only developed complications, those who had graft loss were more likely to be Black and to have HCV infection, peripheral vascular disorders, coagulopathy, and hypertension. Also, female gender and higher age were associated with less risk of post-transplant complications, regardless of whether the person had or did not have graft rejection/dialysis.
Our results are consistent with prior findings that link the risk of graft rejection or failure to Black race, male gender, age, hypertension, and glomerulonephritis [16,19-22]. A retrospective, cross-sectional study that used HCUP-NIS 1995–2005 discharge data found that independent predictors of complications following renal transplant included older age, male gender, and CCI ≥ 1 [22]. Our findings are consistent with the results of previous studies that highlight racial disparities in renal transplant outcomes. For example, in their longitudinal, cohort study of 4,918 renal transplant recipients (33% were non-Hispanic Black recipients), Taber et al. [22] found that non-Hispanic Blacks were significantly more likely to experience graft loss; others have noted that Blacks have worse outcomes in sepsis, likely due to a multitude of factors [23,24]. How these factors intersect in renal transplant patients with sepsis is not understood and warrants further investigation. With respect to gender, de Carvalho et al. [12] found that male gender was associated with increased hospital mortality in renal transplant recipients with sepsis; however, they did not evaluate renal transplant complications or graft failure in their study.
Similar to our study, others have shown that certain medical comorbidities influence graft and patient survival. Wu et al. [25] used the CCI to evaluate 715 patients who underwent kidney transplantation and found that high comorbidity scores were associated with a trend toward increased risk of graft loss during the post-operative period. Similarly, in a retrospective study of 198 kidney transplant patients (age ≥18 years), Levine et al. [26] found that CCI scores, diabetes and peripheral vascular disease predicted increased risk for complications. Our results showed that glomerulonephritis was an independent risk factor for complications and graft failure following renal transplant, which is consistent with the results of several previous studies. Akl et al. [27] performed a single-site retrospective study (n = 2,000) and evaluated the influence of glomerulonephritis on graft and patient survival. The authors found that aging of the graft and mammalian target of rapamycin (mTor) immunosuppression were risk factors for glomerulonephritis [27]. The complex interplay between infection, sepsis, the systemic inflammatory response, and various types of glomerulonephritis may be amplified in the setting of renal transplantation, but these data in the transplant population are scarce. Peripheral vascular disorders and hypertension have been associated with complications and graft failure in kidney transplant patients [19,28,29], which is also in agreement with our results.
In contrast to our findings, several studies have reported that diabetes and hyperlipidemia are independent risk factors associated with graft loss in renal transplant recipients [20,30-37]. Differences between earlier studies and our study regarding diabetes and hyperlipidemia may reflect differences in patient populations and definitions of diabetes and hyperlipidemia used in the studies or limitations of the NIS database, since it was not possible to differentiate between patients who developed these conditions before and after transplantation. However, some studies have suggested a beneficial role of statins in renal disease [38-41], and perhaps this contributes to improved outcomes for renal transplant recipients in the setting of sepsis. De Rango et al. [42] reported that statin use might reduce the all-cause mortality in adult patients with chronic kidney disease and vascular access for chronic dialysis by 50% at 5 years post-transplant, and Sanada et al. [43] found that statin treatment prolonged hemodialysis vascular access survival. Additionally, Zahr et al. [44] treated humanized sickle cell mice with atorvastatin and found that this significantly reduced albuminuria and improved urine concentrating ability and glomerular filtration rate.
Other investigators have explored the use of metformin in renal and infectious diseases [42,44,45]. Rafieian-Kopaie [46] reported that metformin has a protective effect against renal tubular injury by tempering oxidative stress on the renal tubules. In an animal study from China, Zhai et al. [47] found that metformin may ameliorate glomerular podocyte damage in rats with type 2 diabetes, and Kim et al. [48] demonstrated that metformin decreased high-fat-diet-induced renal injury by regulating the expression of adipokines and the renal AMP-activated protein kinase/acetyl-CoA carboxylase pathway in mice. Moreover, prior studies have provided some support for the value of metformin use in sepsis. For example, Kim et al. [49] demonstrated that metformin can suppress lipopolysaccharide-induced inflammatory responses in animal models. Nonetheless, it remains unclear whether statin or metformin use can diminish the development of post-transplant complications or graft failure in renal transplant recipients with sepsis.
It is not surprising that our study found that CMV and HCV infections were associated with complications as well as graft failure in kidney transplant recipients with sepsis. Viral infections have previously been found to be associated with kidney transplant complications and allograft rejection [2,50], and the role of CMV disease or CMV infection with allograft nephropathy and increased graft loss is well-established [24,51-53]. However, it is also thought that CMV exerts immunomodulatory effects which, in turn, increase the risk for bacteremia and invasive fungal disease in transplant recipients [51]. The findings of our study suggest that CMV infection, through its immunomodulatory effects, amplifies the severity of complications and graft failure in transplant recipients with sepsis.
We acknowledge the limitations of this study. Typical of this type of database analysis, the data may have been confounded by coding errors or reporting bias. In addition, the NIS database includes each hospitalization as a separate entry without a unique patient identifier. Hence, it was not possible to identify readmissions. The cross-sectional study design limited any inferences about causality; however, the comorbidity measurement system of NIS implied the studied factors were chronic conditions, thereby indicating a temporal relationship between the exposures (coinfection and comorbidities) and outcome (graft complications and graft loss). However, although we deliberately looked at chronic diagnoses to identify chronic risk exposures, the length of chronic exposures was not clear.
CONCLUSION
In summary, we found that Black race, male gender, and comorbidities (CMV infection, HSV infection, hepatitis C infection, coagulopathies, glomerulonephritis, peripheral vascular disease, and hypertension) can increase the risk of complications and graft failure in kidney transplant patients with sepsis. However, unlike earlier reports, our study did not find an association of diabetes or hyperlipidemia with graft failure and dialysis. The study findings indicate the need to evaluate kidney transplant patients according to race, gender, and comorbidities to reduce the risk of complications and graft failure after kidney transplantation.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- 1.Schachtner T, Stein M, Reinke P. Sepsis after renal transplantation:Clinical, immunological, and microbiological risk factors. Transpl Infect Dis. 2017;19(3) doi: 10.1111/tid.12695. https://doi.org/10.1111/tid.12695. [DOI] [PubMed] [Google Scholar]
- 2.Jha V. Post-transplant infections:An ounce of prevention. Indian J Nephrol. 2010;20:171–8. doi: 10.4103/0971-4065.73431. https://doi.org/10.4103/0971-4065.73431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis:A novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13:260–8. doi: 10.1016/S1473-3099(13)70001-X. https://doi.org/10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heininger A, Haeberle H, Fischer I, Beck R, Riessen R, Rohde F, et al. Cytomegalovirus reactivation and associated outcome of critically ill patients with severe sepsis. Crit Care. 2011;15:R77. doi: 10.1186/cc10069. https://doi.org/10.1186/cc10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4:378–83. doi: 10.1111/j.1600-6143.2004.00332.x. https://doi.org/10.1111/j.1600-6143.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 6.Adams PL. Long-term patient survival:Strategies to improve overall health. Am J Kidney Dis. 2006;47:S65–85. doi: 10.1053/j.ajkd.2005.12.043. https://doi.org/10.1053/j.ajkd.2005.12.043. [DOI] [PubMed] [Google Scholar]
- 7.Thölking G, Schuette-Nuetgen K, Vogl T, Dobrindt U, Kahl BC, Brand M, et al. Male kidney allograft recipients at risk for urinary tract infection? PLoS One. 2017;12(11):e0188262. doi: 10.1371/journal.pone.0188262. https://doi.org/10.1371/journal.pone.0188262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giessing M. Urinary tract infection in renal transplantation. Arab J Urol. 2012;10:162–8. doi: 10.1016/j.aju.2012.01.005. https://doi.org/10.1016/j.aju.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dharnidharka VR, Agodoa LY, Abbott KC. Risk factors for hospitalization for bacterial or viral infection in renal transplant recipients-an analysis of USRDS data. Am J Transplant. 2007;7:653–61. doi: 10.1111/j.1600-6143.2006.01674.x. https://doi.org/10.1111/j.1600-6143.2006.01674.x. [DOI] [PubMed] [Google Scholar]
- 10.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression:From cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862–74. doi: 10.1038/nri3552. https://doi.org/10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva M, Jr, Marra AR, Pereira CA, Medina-Pestana JO, Camargo LF. Bloodstream infection after kidney transplantation:Epidemiology, microbiology, associated risk factors, and outcome. Transplantation. 2010;90:581–7. doi: 10.1097/TP.0b013e3181e8a680. https://doi.org/10.1097/TP.0b013e3181e8a680. [DOI] [PubMed] [Google Scholar]
- 12.de Carvalho MA, Freitas FG, Silva Junior HT, Bafi AT, Machado FR, Pestana JO, et al. Mortality predictors in renal transplant recipients with severe sepsis and septic shock. PLoS One. 2014;9:e111610. doi: 10.1371/journal.pone.0111610. https://doi.org/10.1371/journal.pone.0111610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballesteros F, Allard J, Durand C, Cardinal H, Lalonde L, Fortin MC, et al. Kidney transplant recipients'perspectives on cardiovascular disease and related risk factors after transplantation:A qualitative study. Transplant Direct. 2017;3:e162. doi: 10.1097/TXD.0000000000000679. https://doi.org/10.1097/TXD.0000000000000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodrigo E, Santos L, Piñera C, Millán JC, Quintela ME, Toyos C, et al. Prediction at first year of incident new-onset diabetes after kidney transplantation by risk prediction models. Diabetes Care. 2012;35:471–3. doi: 10.2337/dc11-2071. https://doi.org/10.2337/dc11-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meier-Kriesche HU, Arndorfer JA, Kaplan B. The impact of body mass index on renal transplant outcomes:A significant independent risk factor for graft failure and patient death. Transplantation. 2002;73:70–4. doi: 10.1097/00007890-200201150-00013. https://doi.org/10.1097/00007890-200201150-00013. [DOI] [PubMed] [Google Scholar]
- 16.Briganti EM, Russ GR, McNeil JJ, Atkins RC, Chadban SJ. Risk of renal allograft loss from recurrent glomerulonephritis. N Engl J Med. 2002;347:103–9. doi: 10.1056/NEJMoa013036. https://doi.org/10.1056/NEJMoa013036. [DOI] [PubMed] [Google Scholar]
- 17.Carvalho MF, Soares V. Hyperlipidemia as a risk factor of renal allograft function impairment. Clin Transplant. 2001;15:48–52. doi: 10.1034/j.1399-0012.2001.150108.x. https://doi.org/10.1034/j.1399-0012.2001.150108.x. [DOI] [PubMed] [Google Scholar]
- 18.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–6. doi: 10.7326/0003-4819-158-4-201302190-00009. https://doi.org/10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 19.Legendre C, Canaud G, Martinez F. Factors influencing long-term outcome after kidney transplantation. Transpl Int. 2014;27:19–27. doi: 10.1111/tri.12217. https://doi.org/10.1111/tri.12217. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal A, Prasad GV. Post-transplant dyslipidemia:Mechanisms, diagnosis and management. World J Transplant. 2016;6:125–34. doi: 10.5500/wjt.v6.i1.125. https://doi.org/10.5500/wjt.v6.i1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butler JA, Peveler RC, Roderick P, Smith PW, Horne R, Mason JC, et al. Modifiable risk factors for non-adherence to immune suppressants in renal transplant recipients:A cross-sectional study. Nephrol Dial Transplant. 2004;19:3144–9. doi: 10.1093/ndt/gfh505. https://doi.org/10.1093/ndt/gfh505. [DOI] [PubMed] [Google Scholar]
- 22.Taber DJ, Gebregziabher M, Payne EH, Srinivas T, Baliga PK, Egede LE, et al. Overall graft loss versus death-censored graft loss:Unmasking the magnitude of racial disparities in outcomes among US kidney transplant recipients. Transplantation. 2017;101:402–10. doi: 10.1097/TP.0000000000001119. https://doi.org/10.1097/TP.0000000000001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valley TS, Cooke CR. The epidemiology of sepsis:Questioning our understanding of the role of race. Crit Care. 2015;19:347. doi: 10.1186/s13054-015-1074-7. https://doi.org/10.1186/s13054-015-1074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayr FB, Yende S, Linde-Zwirble WT, Peck-Palmer OM, Barnato AE, Weissfeld LA, et al. Infection rate and acute organ dysfunction risk as explanations for racial differences in severe sepsis. JAMA. 2010;303:2495–503. doi: 10.1001/jama.2010.851. https://doi.org/10.1001/jama.2010.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu C, Evans I, Joseph R, Shapiro R, Tan H, Basu A, et al. Comorbid conditions in kidney transplantation:Association with graft and patient survival. J Am Soc Nephrol. 2005;16:3437–44. doi: 10.1681/ASN.2005040439. https://doi.org/10.1681/ASN.2005040439. [DOI] [PubMed] [Google Scholar]
- 26.Levine MA, Schuler T, Gourishankar S. Complications in the 90-day postoperative period following kidney transplant and the relationship of the Charlson comorbidity index. Can Urol Assoc J. 2017;11:388–93. doi: 10.5489/cuaj.4378. https://doi.org/10.5489/cuaj.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akl AI, Adel H, Rahim MA, Wafa EW, Shokeir AA. Outcome of glomerulonephritis in live-donor renal transplant recipients:A single-centre experience. Arab J Urol. 2015;13:295–305. doi: 10.1016/j.aju.2015.09.003. https://doi.org/10.1016/j.aju.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman AL, Cheung K, Roman SA, Sosa JA. Early clinical and economic outcomes of patients undergoing living donor nephrectomy in the United States. Arch Surg. 2010;145:356–62. doi: 10.1001/archsurg.2010.17. https://doi.org/10.1001/archsurg.2010.17. [DOI] [PubMed] [Google Scholar]
- 29.Boratyńska M, Wakulenko A, Klinger M, Szyber P. Chronic allograft dysfunction in kidney transplant recipients:Long-term single-center study. Transplant Proc. 2014;46:2673–7. doi: 10.1016/j.transproceed.2014.09.058. https://doi.org/10.1016/j.transproceed.2014.09.058. [DOI] [PubMed] [Google Scholar]
- 30.Assis DN, Schilsky ML. Testing and management of thrombocytopenia and coagulopathy in the pre- and post-liver transplant patient. Minerva Gastroenterol Dietol. 2010;56:331–43. [PubMed] [Google Scholar]
- 31.Ghisdal L, Van Laecke S, Abramowicz MJ, Vanholder R, Abramowicz D. New-onset diabetes after renal transplantation:Risk assessment and management. Diabetes Care. 2012;35:181–8. doi: 10.2337/dc11-1230. https://doi.org/10.2337/dc11-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guitard J, Rostaing L, Kamar N. New-onset diabetes and nephropathy after renal transplantation. Contrib Nephrol. 2011;170:247–55. doi: 10.1159/000325778. https://doi.org/10.1159/000325778. [DOI] [PubMed] [Google Scholar]
- 33.Memon SS, Tandon N, Mahajan S, Bansal VK, Krishna A, Subbiah A, et al. The prevalence of new onset diabetes mellitus after renal transplantation in patients with immediate post-transplant hyperglycemia in a tertiary care centre. Indian J Endocrinol Metab. 2017;21:871–5. doi: 10.4103/ijem.IJEM_309_17. https://doi.org/10.4103/ijem.IJEM_309_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alagbe SC, Voster A, Ramesar R, Swanepoel CR. New-onset diabetes after transplant:Incidence, risk factors and outcome. S Afr Med J. 2017;107:791–6. doi: 10.7196/SAMJ.2017.v107i9.12258. https://doi.org/10.7196/SAMJ.2017.v107i9.12258. [DOI] [PubMed] [Google Scholar]
- 35.Cooper L, Oz N, Fishman G, Shohat T, Rahamimov R, Mor E, et al. New onset diabetes after kidney transplantation is associated with increased mortality-a retrospective cohort study. Diabetes Metab Res Rev. 2017;33(8) doi: 10.1002/dmrr.2920. https://doi.org/10.1002/dmrr.2920. [DOI] [PubMed] [Google Scholar]
- 36.Liu FC, Lin HT, Lin JR, Yu HP. Impact of immunosuppressant therapy on new-onset diabetes in liver transplant recipients. Ther Clin Risk Manag. 2017;13:1043–51. doi: 10.2147/TCRM.S142348. https://doi.org/10.2147/TCRM.S142348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santos AH, Jr, Chen C, Casey MJ, Womer KL, Wen X. New-onset diabetes after kidney transplantation:Can the risk be modified by choosing immunosuppression regimen based on pre transplant viral serology? Nephrol Dial Transplant. 2018;33:177–84. doi: 10.1093/ndt/gfx281. https://doi.org/10.1093/ndt/gfx281. [DOI] [PubMed] [Google Scholar]
- 38.Charlton M. Obesity, hyperlipidemia, and metabolic syndrome. Liver Transpl. 2009;15(Suppl 2):S83–9. doi: 10.1002/lt.21914. https://doi.org/10.1002/lt.21914. [DOI] [PubMed] [Google Scholar]
- 39.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3:178–85. doi: 10.1034/j.1600-6143.2003.00010.x. https://doi.org/10.1046/j.1600-6143.2003.00211.x. [DOI] [PubMed] [Google Scholar]
- 40.Halloran PF, Melk A, Barth C. Rethinking chronic allograft nephropathy:The concept of accelerated senescence. J Am Soc Nephrol. 1999;10:167–81. doi: 10.1681/ASN.V101167. [DOI] [PubMed] [Google Scholar]
- 41.Bosmans JL, Holvoet P, Dauwe SE, Ysebaert DK, Chapelle T, Jürgens A, et al. Oxidative modification of low-density lipoproteins and the outcome of renal allografts at 1 1/2 years. Kidney Int. 2001;59:2346–56. doi: 10.1046/j.1523-1755.2001.00752.x. https://doi.org/10.1046/j.1523-1755.2001.00752.x. [DOI] [PubMed] [Google Scholar]
- 42.De Rango P, Parente B, Farchioni L, Cieri E, Fiorucci B, Pelliccia S, et al. Effect of statins on survival in patients undergoing dialysis access for end-stage renal disease. Semin Vasc Surg. 2016;29:198–205. doi: 10.1053/j.semvascsurg.2017.03.001. https://doi.org/10.1053/j.semvascsurg.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Sanada S, Miyasaka Y, Kanno A, Sato K, Sato M, Sugai H, et al. Efficacy of statin on vascular access patency in diabetic hemodialysis patients. J Vasc Access. 2017;18:295–300. doi: 10.5301/jva.5000739. https://doi.org/10.5301/jva.5000739. [DOI] [PubMed] [Google Scholar]
- 44.Zahr RS, Chappa P, Yin H, Brown LA, Ataga KI, Archer DR, et al. Renal protection by atorvastatin in a murine model of sickle cell nephropathy. Br J Haematol. 2018;181:111–21. doi: 10.1111/bjh.15157. https://doi.org/10.1111/bjh.15157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin SY, Lin CL, Hsu WH, Lin CC, Chang CT, Kao CH, et al. Association of statin use and the risk of end-stage renal disease:A nationwide Asian population-based case-control study. Eur J Intern Med. 2016;31:68–72. doi: 10.1016/j.ejim.2016.02.012. https://doi.org/10.1016/j.ejim.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 46.Rafieian-Kopaie M. Metformin and renal injury protection. J Renal Inj Prev. 2013;2:91–2. doi: 10.12861/jrip.2013.29. https://doi.org/10.12861/jrip.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhai L, Gu J, Yang D, Wang W, Ye S. Metformin ameliorates podocyte damage by restoring renal tissue podocalyxin expression in Type 2 diabetic rats. J Diabetes Res. 2015;6:1–8. doi: 10.1155/2015/231825. https://doi.org/10.1155/2015/231825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim D, Lee JE, Jung YJ, Lee AS, Lee S, Park SK, et al. Metformin decreases high-fat diet-induced renal injury by regulating the expression of adipokines and the renal AMP-activated protein kinase/acetyl-coA carboxylase pathway in mice. Int J Mol Med. 2013;32:1293–302. doi: 10.3892/ijmm.2013.1508. https://doi.org/10.3892/ijmm.2013.1508. [DOI] [PubMed] [Google Scholar]
- 49.Kim J, Kwak HJ, Cha JY, Jeong YS, Rhee SD, Kim KR, et al. Metformin suppresses lipopolysaccharide (LPS)-induced inflammatory response in murine macrophages via activating transcription factor-3 (ATF-3) induction. J Biol Chem. 2014;289(33):23246–55. doi: 10.1074/jbc.M114.577908. https://doi.org/10.1074/jbc.M114.577908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cukuranovic J, Ugrenovic S, Jovanovic I, Visnjic M, Stefanovic V. Viral infection in renal transplant recipients. Scientific World Journal. 2012;2012:820621. doi: 10.1100/2012/820621. https://doi.org/10.1100/2012/820621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Humar A, Gillingham KJ, Payne WD, Dunn DL, Sutherland DE, Matas AJ, et al. Association between cytomegalovirus disease and chronic rejection in kidney transplant recipients. Transplantation. 1999;68:1879–83. doi: 10.1097/00007890-199912270-00011. https://doi.org/10.1097/00007890-199912270-00011. [DOI] [PubMed] [Google Scholar]
- 52.Toupance O, Bouedjoro-Camus MC, Carquin J, Novella JL, Lavaud S, Wynckel A, et al. Cytomegalovirus-related disease and risk of acute rejection in renal transplant recipients:A cohort study with case-control analyses. Transpl Int. 2000;13:413–9. doi: 10.1007/s001470050723. https://doi.org/10.1111/j.1432-2277.2000.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 53.Tong CY, Bakran A, Peiris JS, Muir P, Herrington CS. The association of viral infection and chronic allograft nephropathy with graft dysfunction after renal transplantation. Transplantation. 2002;74:576–8. doi: 10.1097/00007890-200208270-00026. https://doi.org/10.1097/00007890-200208270-00026. [DOI] [PubMed] [Google Scholar]


