Abstract
Septic patients suffer a ‘cytokine storm’ from proinflammatory cytokines, chemokines and other inflammatory mediators, resulting in acute kidney injury (AKI) and death. The purpose of the present study was to determine the expression patterns of microRNA-210 (miR-210), miR-494, and miR-205 in middle-aged and old patients with sepsis-induced AKI and to evaluate their association with patient prognosis. Serum blood urea nitrogen (BUN), creatinine (Cr) and cystatin C levels were determined in peripheral venous blood collected from 110 patients with sepsis-induced AKI and 110 healthy controls. The expression profile of 30 miRNAs was analyzed by TaqMan low-density array (TLDA) in plasma samples from patients and controls. Association of miRNAs with prognosis and survival of patients was analyzed by Spearman’s rank correlation coefficient, Cox multivariate analysis, and ROC curve analysis. TILDA analysis showed 11 upregulated and 11 downregulated miRNAs in patients with sepsis-induced AKI. MiR-210 and miR-494 were the most upregulated and miR-205 was the most downregulated miRNA. High expression of miR-210 and miR-494 was positively correlated with BUN, Cr and cystatin C levels of patients, while low expression of miR-205 was negatively correlated. MiR-210 and miR-494 expression was significantly decreased and miR-205 expression was increased in survivors with sepsis-induced AKI (28-day survival, n = 68) vs. non-survivors (n = 42). BUN, Cr, and miR-205 were independent risk factors for prognosis in sepsis-induced AKI. Our study showed the predictive value of miR-210, miR-494, and miR-205 in prognosis and survival of patients with sepsis-induced AKI. MiR-205 is an independent risk factor for sepsis-induced AKI and its decreased expression is associated with shorter patient survival.
Keywords: microRNA, sepsis, acute kidney injury, prognosis, predictive value
INTRODUCTION
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection [1]. As the most frequent cause of mortality in intensive care units (ICUs), sepsis accounts for approximately 250,000 deaths per year in the United States [2]. The incidence of sepsis is increasing in elderly people. In countries with advanced health-care and modern ICUs, 60% of patients who develop sepsis and 75% who succumb to the disease are older than 65 years [3]. Furthermore, sepsis is a known cause of acute kidney injury (AKI), which increases patient morbidity and affects multiple organ functions [4]. Sepsis-induced AKI is especially common among hospitalized and ICU patients, with an incidence of 11–67% and a mortality of 13–36% [5-7]. Thus, there is a need for identifying new biomarkers of sepsis that can help in the therapeutic decision making, diagnosis, monitoring of the response to therapy and risk stratification [8]. The biochemical indices blood urea nitrogen (BUN), creatinine (Cr) and cystatin C are three key indicators of kidney function, and have a predictive value in AKI [9,10]. Moreover, recent studies have reported the potential role of microRNAs (miRNAs) as biomarkers in the diagnosis, prevention and prognosis of sepsis-induced AKI [11,12].
MiRNAs are a class of noncoding RNAs that regulate gene expression in multicellular and some unicellular eukaryotes [13]. Certain miRNAs have been recently suggested as non-invasive biomarkers of organ injury [14]. A study comparing the expression of miRNAs between patients with sepsis, systemic inflammatory response syndrome (SIRS), and healthy controls identified a miRNA signature that can discriminate sepsis from SIRS [15]. Also, miRNAs have been correlated with disease stage as well as short- and long-term prognosis of patients with sepsis [16]. In post-transplant AKI, miR-182-5p was identified as a molecular regulator and was strongly correlated with changes in gene expression during kidney injury [17]. In critically ill patients with AKI, circulating levels of miR-210 were an independent predictor of survival [18]. In tumor necrosis factor (TNF)-α-treated mouse C2C12 myotubes, the expression of miR-494 was upregulated by TNF-α-mediated inflammation, worsening the insulin resistance [19]. Moreover, in renal tubular cell line HK-2 cultured under hypoxia-reoxygenation-induced oxidative stress or endoplasmic reticulum (ER) stress, miR-205 had a protective role against both stress conditions and was suggested as a novel therapeutic target in AKI and chronic kidney disease associated with oxidative or ER stress in tubules [20].
In this study, we analyzed the expression patterns of miR-210, miR-494, and miR-205 in middle-aged and old patients with sepsis-induced AKI and evaluated the association of these miRNAs with BUN, Cr, cystatin C levels, and patient prognosis.
MATERIALS AND METHODS
Patients
Middle-aged and old adults with sepsis-induced AKI diagnosed between January 2014 and December 2016 at the Emergency Department of Sir Run Run Shaw Hospital, Medicine School of Zhejiang University were included as the case group. Healthy individuals who underwent physical examination during the same period were included as the control group. The age, gender, body mass (BM), the Sequential Organ Failure Assessment (SOFA) score, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, mean arterial pressure (MAP), and heart rate of patients and controls are provided in Table 1. The patients were evaluated by two associate senior doctors in the ICU. The diagnostic criteria for sepsis were according to the American College of Chest Physicians/Society of Critical Care Medicine (ACCP/SCCM) [21] and the criteria for AKI were based on the Acute Kidney Injury Network (AKIN) classification [22]. The patients were included if they 1) were over 50 years old; 2) conformed to the diagnostic criteria of ACCP/SCCM; and 3) conformed to the diagnostic criteria of the AKIN. The patients were excluded in case they 1) were diagnosed with sepsis; 2) had concurrent viral myocarditis; 3) had concurrent severe hepatitis or cirrhosis; 4) did not have complete clinical record; or 5) their adherence was poor. Finally, from a total of 125 patients, 110 patients (57 males, 53 females) were selected as the case group. The control group included 110 healthy individuals (58 males, 52 females). This study was approved by the Ethics Committee of Sir Run Run Shaw Hospital, Medicine School of Zhejiang University, Hangzhou, China. Informed consent was obtained from all participants.
TABLE 1.
Baseline characteristics of case (patients with sepsis-induced AKI) and control (healthy individuals) group
Sample collection
Peripheral venous blood (5 ml) was collected from all participants under fasting conditions on the morning of admission to hospital and stored in vacuum blood collection tubes. The collected blood was centrifuged at 3000 r/m for 10 minutes, and the serum was isolated and stored at -80°C until further analysis. An automatic biochemical analyzer (AU400, Olympus, Tokyo, Japan) was used to determine the serum levels of BUN (mmol/L) and Cr (µmol/L). The serum levels of cystatin C (mg/L) were evaluated using a double-antibody sandwich ELISA (DAS-ELISA) kit (BlueGene Biotech, Shanghai, China). Optical density (OD) was measured at 450 nm on a Model 550 microplate reader (Bio-Rad, Hercules, CA, USA).
TLDA
A total of 30 µl plasma was aspirated from each blood sample in case and control groups and mixed. TLDA chips (V2.0, Applied Biosystems, Foster City, CA, USA) were used to screen differentially expressed 30 miRNAs in the mixed plasma samples, and the analysis was performed by the Beijing Orbital Co., Ltd. (Beijing, China).
Statistical analysis
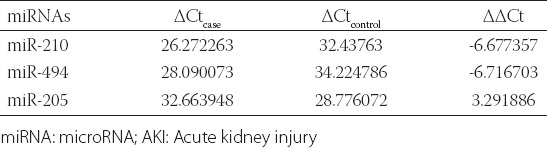
Data analysis was performed with IBM SPSS Statistics for Windows, Version 21.0. (IBM Corp., Armonk, NY) and GraphPad Prism version 7.00 for Windows (GraphPad Software, La Jolla California USA). Measurement data were presented as mean ± standard deviation (SD) and compared between two groups by Student’s t-test. Enumeration data were presented as ratio or percentage and compared between groups by Chi-square test. TLDA data were analyzed by SDS RQ Study software (Applied Biosystems, Life Technologies, USA) with the threshold cycle (Ct) value of 0.2. The ratio of miRNA expression in the plasma samples from case and control groups was calculated by the 2-ΔΔCt method as follows: ΔΔCt = (Ctsample - Ctreference)case group - (Ctsample - Ctreference)control group, with cel-miR-39 used as the reference. Receiver operating characteristic (ROC) curves were plotted to analyze the predictive value of miR-210, miR-494, and miR-205 expression in prognosis of patients with sepsis-induced AKI at 28 days. Spearman’s rank correlation analysis was also performed to evaluate the correlation of miRNAs with serum BUN, Cr, and cystatin C levels. Multivariate analysis with Cox proportional hazards model (back: LR) was used to determine independent risk factors for prognosis of patients with sepsis-induced AKI. Differences were considered to be significant at p < 0.05.
RESULTS
Baseline characteristics of case and control group
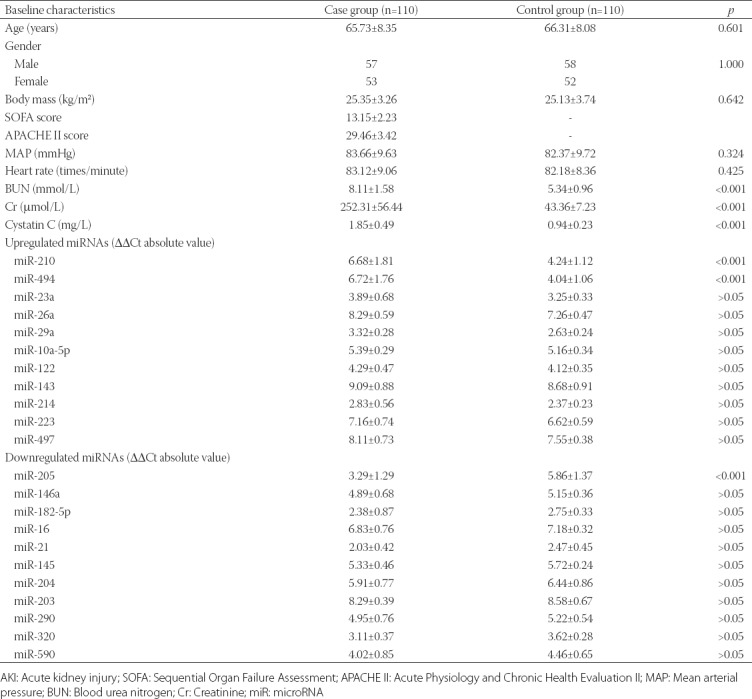
The clinical data of cases (110 middle-aged and old patients with sepsis-induced AKI) and controls (110 healthy individuals) are presented in Table 1. No significant differences were observed in the age, gender, BM, SOFA score, APACHE II score, MAP and heart rate between case and control groups (p > 0.05). Serum levels of BUN, Cr and cystatin C, and the expression of miR-201 and miR-494 in plasma were significantly higher in patients with sepsis-induced AKI compared to control group (p < 0.01); whereas, miR-205 expression in plasma was significantly lower in patient vs. control group [p < 0.01] (Table 1).
Differentially expressed miRNAs screened by TLDA
According to the criterion of Ct value < 35 and absolute Ct value > 2, eleven miRNAs (miR-210, miR-494, miR-23a, miR-26a, miR-29a, miR-10a-5p, miR-122, miR-143, miR-214, miR-223, and miR-497) were upregulated in patients with sepsis-induced AKI, and the upregulated expression of miR-210 and miR-494 was higher compared to the other miRNAs. Eleven miRNAs (miR-205, miR-146a, miR-182-5p, miR-16, miR-21, miR-145, miR-203, miR-204, miR-290, miR-320, and miR-590) were downregulated in patients with sepsis-induced AKI. Among these, miR-205 was the most downregulated miRNA. Eight miRNAs (miR-146a, miR-17, miR-100, miR-126, miR-137, miR-148, miR-306, and miR-415) were not differentially expressed between patient and control group (Table 2).
TABLE 2.
Significantly differentially expressed miRNAs in patients with sepsis-induced AKI
Correlation analysis of miR-210, miR-494, and miR-205 with biochemical indices
We performed a correlation analysis of miR-210, miR-494, and miR-205 plasma expression levels with BUN, Cr and cystatin C serum levels in case group. A positive correlation was identified between miR-210 and miR-494 expression and BUN, Cr and cystatin C levels; whereas, a negative correlation was found between miR-205 expression and BUN, Cr and cystatin C levels in patients with sepsis-induced AKI [p < 0.05] (Figure 1).
FIGURE 1.
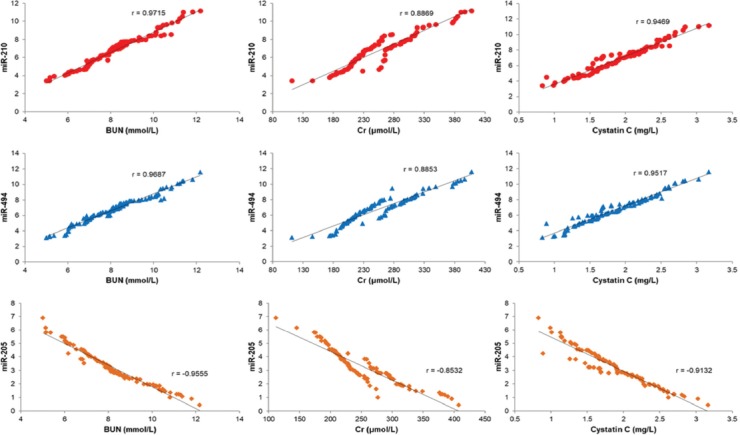
Correlation analysis of miR-210, miR-494, and miR-205 with biochemical indices of kidney injury (BUN, Cr, and cystatin C). A positive correlation was identified between miR-210 and miR-494 plasma expression and BUN, Cr and cystatin C serum levels; whereas, a negative correlation was found between miR-205 plasma expression and BUN, Cr and cystatin C serum levels in patients with sepsis-induced AKI (p < 0.05). BUN: Blood urea nitrogen; Cr: Creatinine; miR: microRNA; AKI: Acute kidney injury.
Association of miR-210, miR-494, and miR-205 and biochemical indices with survival of sepsis-induced AKI patients
Patients with sepsis-induced AKI were classified into survival (n = 68) or non-survival group (n = 42), according to 28-day survival. Plasma expression levels of miR-210, miR-494, and miR-205 were compared between the two groups. The expression levels of miR-210 (5.83 ± 1.32) and miR-494 (5.91 ± 1.37) were significantly lower in survival compared to non-survival group [8.05 ± 1.66 for miR-210 and 8.03 ± 1.54 for miR-494] (p < 0.05), while miR-205 expression was higher in survival (3.89 ± 1.14) vs. non-survival group [2.33 ± 0.87] (p < 0.05). The serum levels of BUN, Cr and cystatin C were decreased in survival compared to non-survival group [p < 0.05] (Figure 2).
FIGURE 2.
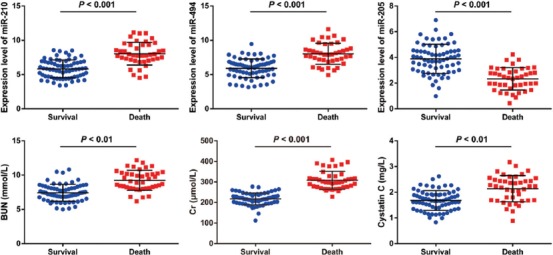
Association of miR-210, miR-494 and miR-205 expression and BUN, Cr and cystatin C levels with the survival of patients with sepsis-induced AKI. The plasma expression levels of miR-210 and miR-494 were significantly lower in survival compared to non-survival group (p < 0.05), while miR-205 plasma expression was higher in survival vs. non-survival group (p < 0.05). The serum levels of BUN, Cr, and cystatin C were decreased in survival compared to non-survival group (p < 0.05). AKI: Acute kidney injury; BUN: Blood urea nitrogen; Cr: Creatinine; miR: microRNA.
Predictive value of miR-210, miR-494, and miR-205 in prognosis of patients with sepsis-induced AKI
ROC curves were plotted to evaluate the predictive value of miR-210, miR-494, and miR-205 in prognosis of patients with sepsis-induced AKI (Figure 3). The cut-off point of miR-210, miR-494 and miR-205 was 6.995, 7.005 and 3.245 and the area under the curve (AUC) was 0.852, 0.847 and 0.860, respectively. The sensitivity of miR-210, miR-494 and miR-205 was 81.0%, 80.9% and 78.6% and the specificity was 80.9%, 72.1% and 90.5%, respectively (95% CI was 0.777–0.928 for miR-210, 0.772–0.922 for miR-494, 0.792–0.927 for miR-205). These results indicated a good predictive value of miR-210, miR-494, and miR-205 in sepsis-induced AKI.
FIGURE 3.
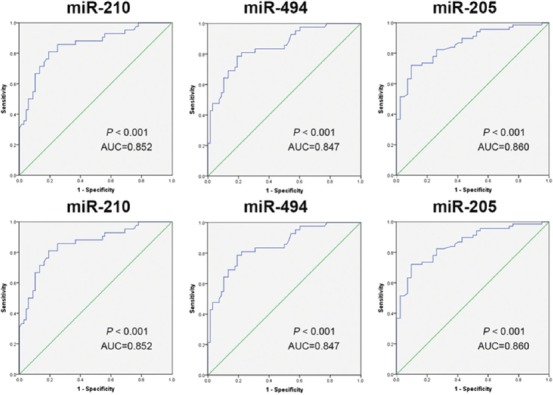
ROC curve analysis of miR-210, miR-494, miR-205 to assess their predictive value in prognosis of patients with sepsis-induced AKI. Our results indicated a good predictive value of miR-210, miR-494, and miR-205 in sepsis-induced AKI. ROC: Receiver operating characteristic; AKI: Acute kidney injury; miR: microRNA.
Univariate survival analysis
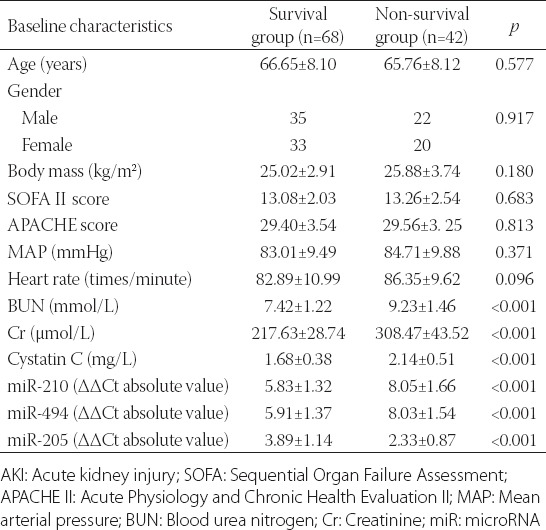
The survival rate of 110 patients with sepsis-induced AKI at 28 day was 38.18% (42 cases), which was correlated with the plasma expression levels of miR-210, miR-494 and miR-205 and serum levels of BUN, Cr and cystatin C (p < 0.01). On the other hand, the age, gender, BM, SOFA score, APACHE II score, MAP and heart rate were not significantly correlated with the survival rate of patients [p > 0.05] (Table 3).
TABLE 3.
Univariate survival analysis of factors associated with the survival of patients with sepsis-induced AKI
Multivariate survival analysis
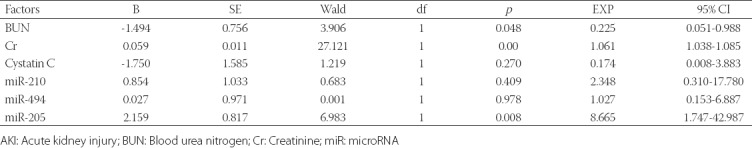
BUN, Cr, cystatin C, miR-210, miR-494, and miR-205 were included in the Cox multivariate survival analysis (Table 4). The results showed that BUN, Cr and miR-205 were risk factors for the prognosis of patients with sepsis-induced AKI, and higher BUN and Cr levels and lower miR-205 expression were associated with shorter survival of patients with sepsis-induced AKI (p < 0.05).
TABLE 4.
COX multivariate survival analysis of risk factors for the prognosis of patients with sepsis-induced AKI
DISCUSSION
Sepsis is characterized by end-organ dysfunction distant from the primary site of infection, and is a common cause of acute disease and death in patients with nosocomial and community-acquired infections [23]. AKI is associated with a prolonged length of stay in ICUs, and hence requires considerable healthcare resources [4]. MiRNAs are suggested as important diagnostic and prognostic indicators in sepsis and AKI [24,25]. In this study, we showed the predictive value of miR-210, miR-494 and miR-205 in the prognosis and 28-day survival of patients with sepsis-induced AKI.
Initially, we performed TLDA analysis to screen differentially expressed miRNAs related to sepsis-induced AKI. We identified eleven upregulated and eleven downregulated miRNAs in plasma samples of patients with sepsis-induced AKI, among which miR-210 and miR-494 were the most upregulated and miR-205 was the most downregulated miRNA. A previous study showed that miR-205-5b is upregulated in different tissues in a mouse model of lipopolysaccharide (LPS)-induced sepsis, which suppressed high-mobility group box 1 protein (HMGB1) expression at the post-transcriptional level. The authors suggested that this upregulation of miR-205-5b might be targeted in novel treatment strategies for late sepsis [26]. MiR-210 has been shown to exert its effects in the innate immune signaling [27]. Urinary miR-210 levels were able to differentiate renal transplant patients with acute rejection from patients without rejection and could be used as an indicator of long-term kidney function [28]. In a rat model of hepatic ischemia/reperfusion (I/R) injury, miR-494 showed a protective effect against the I/R injury by downregulating its downstream target gene PTEN and activating the PI3K/AKT signaling pathway. This suggests that miR-494 may be used as a biomarker in I/R injury [29]. However, to the best of our knowledge, no reports on miR-494 role in sepsis or AKI are available in the literature.
We further evaluated serum BUN, Cr, and cystatin C and showed their increased levels in patients with sepsis-induced AKI compared to controls. According to our Spearman’s rank correlation analysis, miR-210 and miR-494 were positively correlated and miR-205 was negatively correlated with the levels of BUN, Cr and cystatin C in patients with sepsis-induced AKI. BUN and Cr are important indicators of kidney function in AKI [30]. Serum cystatin C is suggested as a sensitive predictive parameter for reduced glomerular filtration rate, and may be used for the laboratory diagnosis of chronic renal failure [31,32]. Moreover, urinary cystatin C is a diagnostic indicator of AKI and sepsis, and predicts mortality in ICU patients [5]. Notably, our correlation analysis showed that the plasma expression levels of miR-210 and miR-494 were significantly lower while those of miR-205 were higher in survivors with sepsis-induced AKI vs. non-survivors. In addition, the serum levels of BUN, Cr and cystatin C were decreased in the survivors.
Our ROC curve, univariate and multivariate analysis confirmed the predictive value of miR-210, miR-494 and miR-205 in the prognosis and survival of patients with sepsis-induced AKI. We showed that BUN, Cr and miR-205 were the risk factors for prognosis of sepsis-induced AKI. As sensitive biomarkers of AKI, BUN and Cr have the potential to transform the care of patients suffering from renal disease [33,34]. A previous study indicated that specific miRNAs might regulate the progression of sepsis-induced AKI by targeting pathways related to oxidative stress and mitochondria [35]. Huo et al. showed a good predictive value of miR-29a and miR-10a-5p in evaluating the 28-day mortality of patients with sepsis-induced AKI [11]. Similarly, Lorenzen et al. indicated that miR-210 is an independent predictor of 28-day survival in AKI and showed the upregulation of this miRNA in the plasma of patients with AKI compared to healthy controls and disease controls (critically ill patients with acute myocardial infarction) [18]. In the study of Muratsu-Ikeda et al., decreased miR-205 expression in renal tubular cell line HK-2 was associated with increased cell susceptibility to oxidative and ER stress, which are critical risk factors for tubular damage in both AKI and chronic kidney disease [20].
CONCLUSION
In conclusion, our study suggest that miR-210, miR-494 and miR-205 have a predictive value in the prognosis and survival of patients with sepsis-induced AKI. MiR-205 appears to be an independent risk factor for sepsis-induced AKI and its decreased expression is associated with shorter patient survival.
ACKNOWLEDGMENTS
This study was supported by National Natural Youth Science Fund Project (81801890).
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–10. doi: 10.1001/jama.2016.0287. https://doi.org/10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression:from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862–74. doi: 10.1038/nri3552. https://doi.org/10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis:a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13(3):260–8. doi: 10.1016/S1473-3099(13)70001-X. https://doi.org/10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zarjou A, Agarwal A. Sepsis and acute kidney injury. J Am Soc Nephrol. 2011;22(6):999–1006. doi: 10.1681/ASN.2010050484. https://doi.org/10.1681/ASN.2010050484. [DOI] [PubMed] [Google Scholar]
- 5.Nejat M, Pickering JW, Walker RJ, Westhuyzen J, Shaw GM, Frampton CM, et al. Urinary cystatin C is diagnostic of acute kidney injury and sepsis, and predicts mortality in the intensive care unit. Crit Care. 2010;14(3):R85. doi: 10.1186/cc9014. https://doi.org/10.1186/cc9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagshaw SM, George C, Bellomo R. ANZICS Database Management Committee. Early acute kidney injury and sepsis:a multicentre evaluation. Crit Care. 2008;12(2):R47. doi: 10.1186/cc6863. https://doi.org/10.1186/cc6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, et al. A unified theory of sepsis-induced acute kidney injury:inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41(1):3–11. doi: 10.1097/SHK.0000000000000052. https://doi.org/10.1097/SHK.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reinhart K, Bauer M, Riedemann NC, Hartog CS. New approaches to sepsis:molecular diagnostics and biomarkers. Clin Microbiol Rev. 2012;25(4):609–34. doi: 10.1128/CMR.00016-12. https://doi.org/10.1128/CMR.00016-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell M, Granath F, Mårtensson J, Löfberg E, Ekbom A, Martling CR, et al. Cystatin C is correlated with mortality in patients with and without acute kidney injury. Nephrol Dial Transplant. 2009;24(10):3096–102. doi: 10.1093/ndt/gfp196. https://doi.org/10.1093/ndt/gfp196. [DOI] [PubMed] [Google Scholar]
- 10.Uchino S, Bellomo R, Goldsmith D. The meaning of the blood urea nitrogen/creatinine ratio in acute kidney injury. Clin Kidney J. 2012;5(2):187–91. doi: 10.1093/ckj/sfs013. https://doi.org/10.1093/ckj/sfs013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huo R, Dai M, Fan Y, Zhou JZ, Li L, Zu J. Predictive value of miRNA-29a and miRNA-10a-5p for 28-day mortality in patients with sepsis-induced acute kidney injury. [Article in Chinese] Nan Fang Yi Ke Da Xue Xue Bao. 2017;37(5):646–51. doi: 10.3969/j.issn.1673-4254.2017.05.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leelahavanichkul A, Somparn P, Panich T, Chancharoenthana W, Wongphom J, Pisitkun T, et al. Serum miRNA-122 in acute liver injury induced by kidney injury and sepsis in CD-1 mouse models. Hepatol Res. 2015;45(13):1341–52. doi: 10.1111/hepr.12501. https://doi.org/10.1111/hepr.12501. [DOI] [PubMed] [Google Scholar]
- 13.Benz F, Roderburg C, Vargas Cardenas D, Vucur M, Gautheron J, Koch A, et al. U6 is unsuitable for normalization of serum miRNA levels in patients with sepsis or liver fibrosis. Exp Mol Med. 2013;45:e42. doi: 10.1038/emm.2013.81. https://doi.org/10.1038/emm.2013.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou X, Qu Z, Zhu C, Lin Z, Huo Y, Wang X, et al. Identification of urinary microRNA biomarkers for detection of gentamicin-induced acute kidney injury in rats. Regul Toxicol Pharmacol. 2016;78:78–84. doi: 10.1016/j.yrtph.2016.04.001. https://doi.org/10.1016/j.yrtph.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Ma Y, Vilanova D, Atalar K, Delfour O, Edgeworth J, Ostermann M, et al. Genome-wide sequencing of cellular microRNAs identifies a combinatorial expression signature diagnostic of sepsis. PLoS One. 2013;8(10):e75918. doi: 10.1371/journal.pone.0075918. https://doi.org/10.1371/journal.pone.0075918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benz F, Roy S, Trautwein C, Roderburg C, Luedde T. Circulating MicroRNAs as biomarkers for sepsis. Int J Mol Sci. 2016;17(1):E78. doi: 10.3390/ijms17010078. https://doi.org/10.3390/ijms17010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilflingseder J, Sunzenauer J, Toronyi E, Heinzel A, Kainz A, Mayer B, et al. Molecular pathogenesis of post-transplant acute kidney injury:assessment of whole-genome mRNA and miRNA profiles. PLoS One. 2014;9(8):e104164. doi: 10.1371/journal.pone.0104164. https://doi.org/10.1371/journal.pone.0104164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorenzen JM, Kielstein JT, Hafer C, Gupta SK, Kümpers P, Faulhaber-Walter R, et al. Circulating miR-210 predicts survival in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol. 2011;6(7):1540–6. doi: 10.2215/CJN.00430111. https://doi.org/10.2215/CJN.00430111. [DOI] [PubMed] [Google Scholar]
- 19.Lee H, Jee Y, Hong K, Hwang GS, Chun KH. MicroRNA-494, upregulated by tumor necrosis factor-alpha, desensitizes insulin effect in C2C12 muscle cells. PLoS One. 2013;8(12):e83471. doi: 10.1371/journal.pone.0083471. https://doi.org/10.1371/journal.pone.0083471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muratsu-Ikeda S, Nangaku M, Ikeda Y, Tanaka T, Wada T, Inagi R. Downregulation of miR-205 modulates cell susceptibility to oxidative and endoplasmic reticulum stresses in renal tubular cells. PLoS One. 2012;7(7):e41462. doi: 10.1371/journal.pone.0041462. https://doi.org/10.1371/journal.pone.0041462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–55. doi: 10.1378/chest.101.6.1644. https://doi.org/10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 22.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network:report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. https://doi.org/10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis:a systematic review and meta-analysis. Lancet Infect Dis. 2013;13(5):426–35. doi: 10.1016/S1473-3099(12)70323-7. https://doi.org/10.1016/S1473-3099(12)70323-7. [DOI] [PubMed] [Google Scholar]
- 24.Khalid U, Bowen T, Fraser DJ, Jenkins RH. Acute kidney injury:a paradigm for miRNA regulation of the cell cycle. Biochem Soc Trans. 2014;42(4):1219–23. doi: 10.1042/BST20140093. https://doi.org/10.1042/BST20140093. [DOI] [PubMed] [Google Scholar]
- 25.Han Y, Dai QC, Shen HL, Zhang XW. Diagnostic value of elevated serum miRNA-143 levels in sepsis. J Int Med Res. 2016;44(4):875–81. doi: 10.1177/0300060516645003. https://doi.org/10.1177/0300060516645003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou W, Wang J, Li Z, Li J, Sang M. MicroRNA-2055b inhibits HMGB1 expression in LPS-induced sepsis. Int J Mol Med. 2016;38(1):312–8. doi: 10.3892/ijmm.2016.2613. https://doi.org/10.3892/ijmm.2016.2613. [DOI] [PubMed] [Google Scholar]
- 27.Zou L, Feng Y, Xu G, Jian W, Chao W. Splenic RNA and microRNA mimics promote complement factor B production and alternative pathway activation via innate immune signaling. J Immunol. 2016;196(6):2788–98. doi: 10.4049/jimmunol.1502106. https://doi.org/10.4049/jimmunol.1502106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lorenzen JM, Volkmann I, Fiedler J, Schmidt M, Scheffner I, Haller H, et al. Urinary miR-210 as a mediator of acute T-cell mediated rejection in renal allograft recipients. Am J Transplant. 2011;11(10):2221–7. doi: 10.1111/j.1600-6143.2011.03679.x. https://doi.org/10.1111/j.1600-6143.2011.03679.x. [DOI] [PubMed] [Google Scholar]
- 29.Su S, Luo Liu X, Liu J, Peng F, Fang C, et al. miR-494 up-regulates the PI3K/Akt pathway via targetting PTEN and attenuates hepatic ischemia/reperfusion injury in a rat model. Biosci Rep. 2017;37(5) doi: 10.1042/BSR20170798. BSR20170798. https://doi.org/10.1042/BSR20170798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takaya Y, Yoshihara F, Yokoyama H, Kanzaki H, Kitakaze M, Goto Y, et al. Risk stratification of acute kidney injury using the blood urea nitrogen/creatinine ratio in patients with acute decompensated heart failure. Circ J. 2015;79(7):1520–5. doi: 10.1253/circj.CJ-14-1360. https://doi.org/10.1253/circj.CJ-14-1360. [DOI] [PubMed] [Google Scholar]
- 31.Gökkuşu CA, Ozden TA, Gül H, Yildiz A. Relationship between plasma Cystatin C and creatinine in chronic renal diseases and Tx-transplant patients. Clin Biochem. 2004;37(2):94–7. doi: 10.1016/j.clinbiochem.2003.10.009. https://doi.org/10.1016/j.clinbiochem.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Shlipak MG, Coca SG, Wang Z, Devarajan P, Koyner JL, Patel UD, et al. Presurgical serum cystatin C and risk of acute kidney injury after cardiac surgery. Am J Kidney Dis. 2011;58(3):366–73. doi: 10.1053/j.ajkd.2011.03.015. https://doi.org/10.1053/j.ajkd.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waikar SS, Bonventre JV. Biomarkers for the diagnosis of acute kidney injury. Nephron Clin Pract. 2008;109(4):c192–7. doi: 10.1159/000142928. https://doi.org/10.1159/000142928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider J, Khemani R, Grushkin C, Bart R. Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit Care Med. 2010;38(3):933–9. doi: 10.1097/CCM.0b013e3181cd12e1. https://doi.org/10.1097/CCM.0b013e3181cd12e1. [DOI] [PubMed] [Google Scholar]
- 35.Ge QM, Huang CM, Zhu XY, Bian F, Pan SM. Differentially expressed miRNAs in sepsis-induced acute kidney injury target oxidative stress and mitochondrial dysfunction pathways. PLoS One. 2017;12(3):e0173292. doi: 10.1371/journal.pone.0173292. https://doi.org/10.1371/journal.pone.0173292. [DOI] [PMC free article] [PubMed] [Google Scholar]


