Abstract
A systemic feature of eukaryotic cells is the spatial organization of functional components through compartmentalization. Developing protocells with compartmentalized synthetic organelles is, therefore, a critical milestone toward emulating one of the core characteristics of cellular life. Here we demonstrate the bottom-up, multistep, noncovalent, assembly of rudimentary subcompartmentalized protocells through the spontaneous encapsulation of semipermeable, polymersome proto-organelles inside cell-sized coacervates. The coacervate microdroplets are membranized using tailor-made terpolymers, to complete the hierarchical self-assembly of protocells, a system that mimics both the condensed cytosol and the structure of a cell membrane. In this way, the spatial organization of enzymes can be finely tuned, leading to an enhancement of functionality. Moreover, incompatible components can be sequestered in the same microenvironments without detrimental effect. The robust stability of the subcompartmentalized coacervate protocells in biocompatible milieu, such as in PBS or cell culture media, makes it a versatile platform to be extended toward studies in vitro, and perhaps, in vivo.
Short abstract
Herein, hierarchical protocells are engineered by multistep, noncovalent assembly generating subcompartmentalized, membranized, coacervates—a functional model of eukaryotic spatial organization.
Introduction
Compartmentalization is key for the emergence of eukaryotic life, facilitating stepwise enhancements in complexity toward increasingly functional forms of hierarchically structured matter.1,2 With growing interest in the development of synthetic cell-like architectures (protocells), various forms of micro- and nanostructures that mimic essential cellular properties and processes have been presented.3−5 To date, protocell research has largely focused on the development of discrete chemical platforms such as membrane-free protocells (coacervates,6−11 hydrogel particles,12,13 or aqueous two-phase systems14,15) and membrane-bound protocells (liposomes,16 proteinosomes,8 colloidosomes,5,17 polymeric nanoparticles,18 or polymersomes19,20). These first-generation protocells have been implemented to advance fundamental understanding into the physicochemical hallmarks of living systems.21 With an emphasis on the engineering of hybrid systems, next-generation protocells seek to take this a step further, increasing structural and functional complexity by implementing multicompartmentalization.22−28 Exemplifying this, bottom-up engineering of cell-mimetic liposomes (loaded with enzymes)29 have been subcompartmentalized within polymeric particles, displaying temperature-dependent localization.30 Top-down approaches have also been adopted, bridging the divide between natural and synthetic systems by incorporating biological organelles within protocells31 and, conversely, amalgamating synthetic organelles with cells, imparting new functionality.32−36 The hierarchical assembly of subcompartmentalized systems is a key milestone in the development of protocells—a process that showcases the multistep, noncovalent assembly of complex materials that resemble lifelike systems.37 Here, we present a multicompartmentalized, coacervate-based protocell capable of spontaneously assimilating new functional elements, in a process akin to protocellular endosymbiosis. This hybrid system integrates distinct attributes of eukaryotic cells: where crowdedness, hierarchical structure, spatial organization of enzymes, and compatibility with cellular media are realized combined to establish a truly groundbreaking synthetic cell platform. In living systems, the semipermeable nature of both internal organelles and outer cell membrane allows the exchange of chemical information on the intracellular and extracellular level, owing to the ubiquitous lipid bilayer. Seeking to emulate this in the next generation of protocells, this hierarchical platform provides an important advance toward mimicking the systemic complexity observed in eukaryotic cells.
This route toward the hierarchical assembly of such a subcompartmentalized protocell harnesses the potential of coacervates to spontaneously enrich their function via sequestration of polymersomal organelles. In this case, polymersomes comprising poly(ethylene glycol)-b-poly(caprolactone-gradient-trimethylene carbonate) (PEG–PCLgTMC) block copolymers are implemented, which possess a semipermeable membrane and can be readily loaded with enzymatic cargo. For example, PEG–PCLgTMC polymersomes, loaded with an antioxidant enzyme, have been utilized as synthetic organelles after incorporation into living cells.32 Here we demonstrate the spontaneous recruitment of such synthetic (proto-) organelles by coacervate microdroplets, shuttling various functional cargoes inside the protocell. The ability of coacervates to sequester functional subcomponents is an important aspect of their protocellular behavior;15,31 however, the lack of structural stability imbued by a membrane undermines this advantageous property. Recently, we presented a strategy for the membranization of complex coacervate microdroplets using a bespoke terpolymer (based upon PEG–PCLgTMC) that interacts electrostatically with amylose-based coacervates.38 Membranized coacervates have prolonged stability under aqueous buffer conditions, as well as a semipermeable exterior that allows transmembrane diffusion of small molecule substrates.39 Chemical homology between the external membrane and inner proto-organelles (embedded in the cytosol-mimetic coacervate) makes this an exciting system for the exploration of more complex cell-mimetic behaviors (as depicted in Scheme 1). This hierarchical protocell affords us control over the spatial organization of enzymes and their reactions, facilitating the study of two main functional consequences of compartmentalization: the creation of favorable microenvironments and the ability to segregate incompatible components. Having observed the sequestration of functional (enzyme-loaded) polymersomal proto-organelles into coacervates, we then studied the effect of a spatially organized cascade reaction using the glucose oxidase (GOx)/horseradish peroxidase (HRP) cascade. Thereafter, we established the ability to segregate incompatible digestive enzymes (in this case proteinase K) into proto-lysosomes, where susceptible macromolecular species are protected from degradation. Lastly, the stability of this subcompartmentalized protocellular system was demonstrated in a coculture experiment, attempting to bridge the conceptual interface between living and nonliving architectures. The conceptual relationship between biological and protocellular architectures was showcased in a synthetic coculture experiment, constituting a significant advancement at the chemical biology interface. Overall, this hierarchical system represents a significant step forward for the field of multicompartmentalized synthetic cells, where it not only incorporates both cytosol-mimetic and membrane-bound features but also exists as a fully open and addressable system—with aqueous stability and semipermeability facilitating chemical communication with the outside environment.
Scheme 1. Formation of a Hierarchical Protocell.
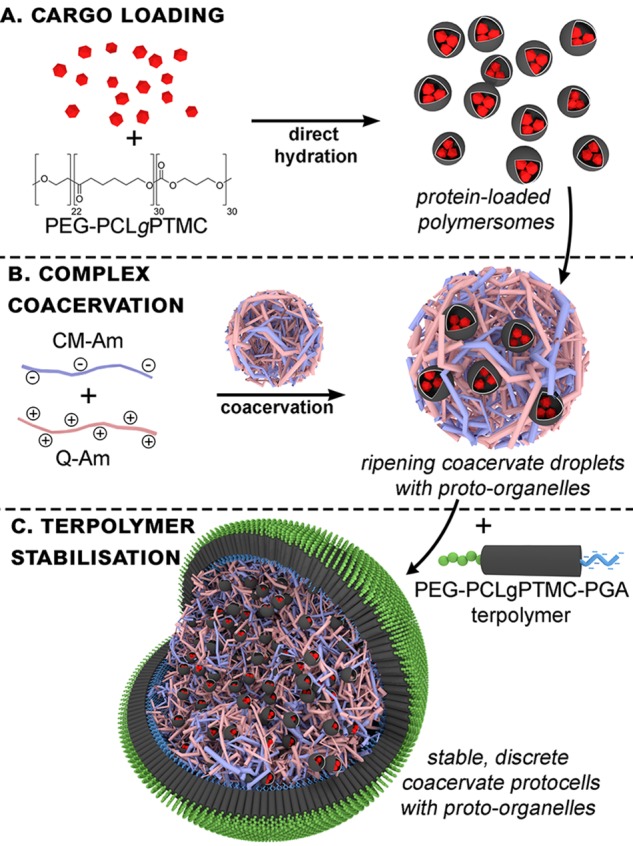
Through the spontaneous sequestration of polymersomal proto-organelles by a coacervate microdroplet (A); subsequent membranization with a synthetic terpolymer (B) provides stability to the overall construct, which was evaluated to demonstrate the advantageous properties of a spatially organized, subcompartmentalized system (C) that mimics the advanced properties of a eukaryotic cell.
Results and Discussion
In general, the sequestration of cargo by coacervates is dependent on physicochemical complementarity (based on electrostatic, hydrophobic, and steric factors), whereby bulky, uncharged hydrophilic or unfavorably charged components are excluded.40 For example, we have recently observed that electrostatically driven sequestration occurs, where negatively charged proteins preferentially partition within coacervates through interaction with positively charged amylose, which is present in stoichiometric excess in the coacervate phase.38 This was enhanced by increasing the negative surface charge of proteins through succinylation, increasing their encapsulation efficiency to ∼90% in the case of bovine serum albumin (BSA). The capacity for electrostatically driven sequestration of components into coacervates provides a pathway for the uptake of polymersomal proto-organelles. The formation of functional proto-organelles was achieved by encapsulating proteins inside PEG–PCLgTMC polymersomes via the direct hydration method (Scheme 1A), where the encapsulating proteins in PBS are added quickly to a solution of block copolymer in low molecular weight PEG.32 Nonencapsulated proteins were removed using extensive dialysis. Polymersome purification was validated using a combination of size exclusion chromatography and UV (SEC-UV), which showed no trace of unencapsulated proteins (Figure S3A). These functional nanostructures were determined to be approximately 160 nm in diameter, using cryotransmission electron microscopy (Figure S3B). The sequestration efficiency (i.e., the amount of material sequestered by the coacervate under the conditions used) of polymersomes loaded with neutral fluorescent protein (sfGFP) was low (2.9 ± 0.2%, Figure S4A), presumably due to the hydrophilic nature of surface PEG chains despite the slight negative zeta-potential of −7 ± 2 mV (Figure S5A). In order to drive integration of polymersomes, without altering the chemical nature of the compartment directly, it was possible to modulate charge characteristics through the encapsulation of charged cargo. Following the general principles of Coulombic potential, it was expected that the incorporation of negatively charged (succinylated) proteins in the polymersome core would result in an increase in the potential at the surface (radius ca. 80 nm, Figure S6).
Indeed, the zeta-potential of polymersomes loaded with succinylated cargo (in this case FITC-BSA) became more negative (−22 ± 4 mV, Figure S5A) and resulted in a marked increase of the sequestration efficiency up to 8.9 ± 0.4% (Figure S4B). In order to confirm that the structure of cargo-loaded polymersome is as expected (unequivocally showing that proteins were loaded in the core rather than at the corona), a combination of asymmetric flow field-flow fractionation and multiangle light scattering (AF4-MALS) was used. Light scattering analysis of polymersomes with encapsulated succinylated proteins clearly showed a reduction in the radius of gyration (Rg) values when compared with empty polymersomes, indicating the presence of proteins within the inner cavity (Figure S5B). In this way, succinylated cargo can enhance sequestration of polymersomal proto-organelles by coacervates, driving functional assimilation in such a hierarchical system.
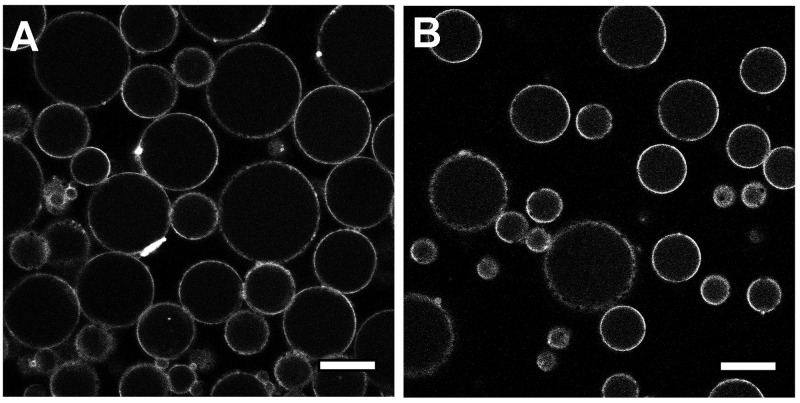
To recap, the formation of subcompartmentalized protocells was achieved using negatively charged polymersomes (loaded with succinylated protein cargo) that were spontaneously sequestered by the coacervates (Scheme 1B), followed by addition of membranizing terpolymer once the freely coalescing coacervate microdroplets were cell-sized (ca. 20 μm, Scheme 1C). The structural integrity imparted to the coacervate by the terpolymer membrane provides a stable protocell interface that maintains its discrete structure even after gentle centrifugation (Figure S7). Without membranization, coacervate microdroplets rapidly coalesced to form an unstructured condensed phase (Figure S8). We demonstrated the capacity of this system to control spatial organization of macromolecular cargo (located within proto-organelles) by using three separate populations of polymersomes, containing different fluorescent proteins, sequestered into coacervate microdroplets (Figure 1). The uptake of polymersomes by coacervates did not undermine their structural integrity, as confocal images showed distinct, punctuated structures, indicative of intact nanoparticles that have retained their cargo within discrete subcompartments. Importantly, we observed a homogeneous distribution of these proto-organelles without undesirable aggregation, modeling the organization of organelles within the cell (Figure S9).
Figure 1.
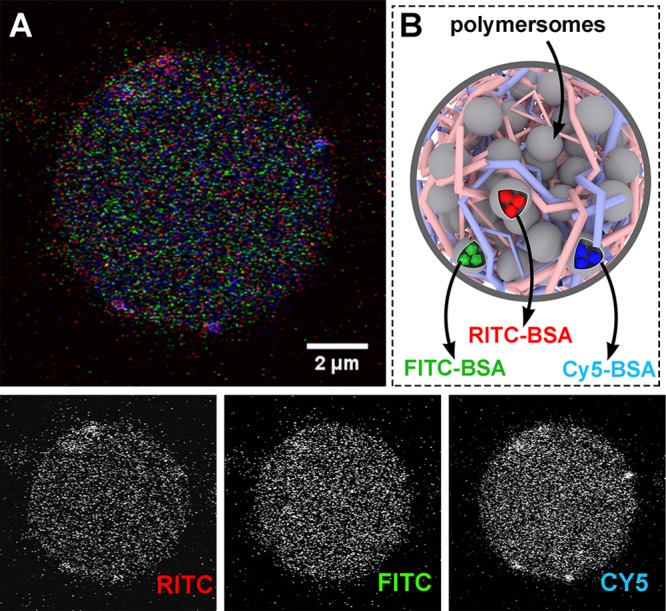
Hierarchical protocell sequestering three distinct subpopulations of polymersomal proto-organelles loaded with fluorescently labeled proteins. (A) Confocal micrograph of multicompartmentalized protocell (containing FITC-, RITC-, and Cy5-labeled succinylated bovine serum albumin (BSA) in separate vesicles) encapsulated within membranized coacervate protocells—depicted in (B), which is not drawn to scale.
One of the hallmarks of compartmentalization is the propensity of condensed functional components to enhance internal processing of molecular signals in communication cascades or metabolic cycles. To realize this functional benefit of spatial organization using the subcompartmentalized copolymer/coacervate protocell model, the well-studied enzymatic cascade of glucose oxidase (GOx) and horseradish peroxidase (HRP) was chosen.41−46 As GOx consumes glucose, it produces gluconolactone and hydrogen peroxide, the latter of which is thereafter used by HRP in the oxidation of pro-fluorescent amplex red (10-acetyl-3,7-dihydroxyphenoxazine) to resorufin (Figure 2A). All of the proteins were succinylated, and, in each experimental scenario, the encapsulated and coencapsulated concentrations of proteins were matched so that results were directly comparable (Table S3). The zeta-potentials of different polymersomes, containing either succinylated GOx, succinylated HRP, or both succinylated GOx and HRP, were comparable (Table S2), leading to similar degrees of sequestration into the coacervate. Two main conclusions can be drawn from the results of these enzymatic assays (Figure 2B, Figure S10). First, the kinetic advantages of coencapsulation (Figure 2B, black trace) over separate enzyme encapsulation (red trace) were clearly observed. In the case of separately encapsulated GOx and HRP, the intermediate substrate (H2O2) produced by GOx faces diffusometric barriers before it can be converted by HRP.32 Conversely, in the coencapsulated system, H2O2 can diffuse directly to HRP before escaping the proto-organelle, thereby increasing the overall turnover rate. Second, the cascade still functions despite physical separation of the catalytic components, which highlights the ability of such proto-organelles to communicate in a cytosol-like environment. This is a direct consequence of the semipermeable nature of both the protocell terpolymer membrane and the membrane of the polymersomes (that are chemically homologous). Regarding functional activation, due to colocalization or substrate channeling, this is an active subject of ongoing research debate and one that requires further, dedicated study.47−50 However, these studies are often performed in open systems that do not take into account the effect of compartmentalization and how this affects substrate, intermediate, and product diffusion.51 Here, our system provides a platform for the further investigation of these phenomena, by spatially confining (different) enzymes within polymersomal proto-organelles, encapsulated in the condensed cytosol-like coacervate core of the protocells.
Figure 2.
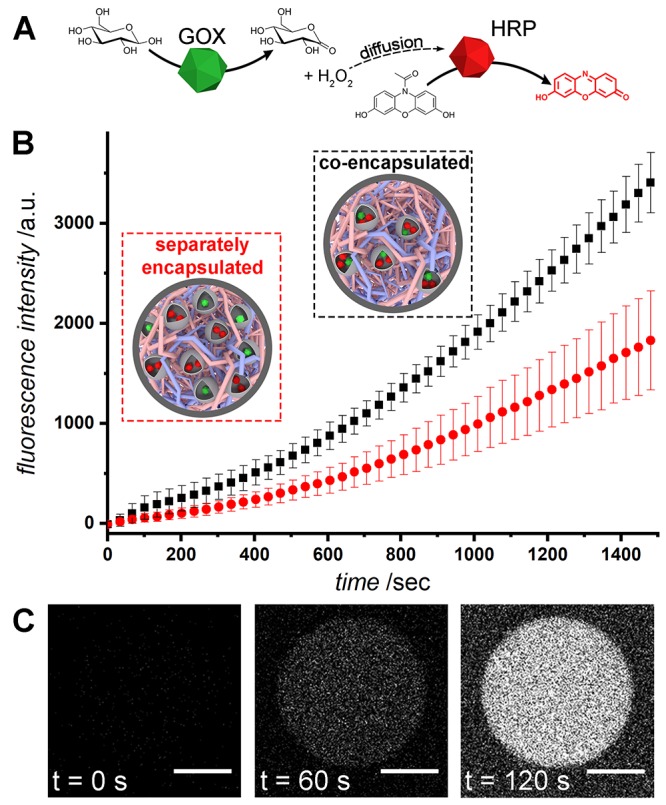
Coencapsulation of enzymes in the multicompartment protocell leads to an enhancement in the overall rate of reaction. (A) The enzyme cascade utilized. Glucose is oxidized by glucose oxidase to form gluconolactone and H2O2, which then diffuses to horseradish peroxidase, catalyzing the formation of fluorescent resorufin from amplex red. (B) Overall enzymatic rates of reaction as determined by bulk resorufin fluorescence in a plate reader. Coencapsulation of enzymes in polymersomes results in a rate increase compared to separately encapsulated enzymes. (C) Confocal images showing resorufin production over time inside a protocell containing coencapsulated GOx/HRP polymersomes (scale bars = 5 μm).
Another consequence of spatial organization in the cell is to separate and stabilize discrete processes so that, for example, digestive enzymes are confined to the lysosomal system and are not permitted to interfere with the complex chemistry occurring in the rest of the cell.52 This is facilitated by the size of digestive enzymes, which are unable to permeate the lipid bilayer, a key characteristic mirrored by the copolymeric membranes surrounding the coacervate and the polymersomal proto-organelles. In order to study this, the accessibility of proteinase K (ProtK), a powerful proteolytic enzyme, was restricted through confinement in polymersomal proto-lysosomes that prevented digestion of a pro-fluorescent substrate (BSA-FITC). Through labeling BSA with a large excesses of fluorescein (FITC), which self-quenched on the surface, a pro-fluorescent protease substrate was prepared that released highly fluorescent polypeptide fragments upon proteolytic degradation by ProtK (Figure 3A). The protocellular membrane was able to shield internally sequestered FITC-BSA from degradation by external ProtK enzymes.
Figure 3.
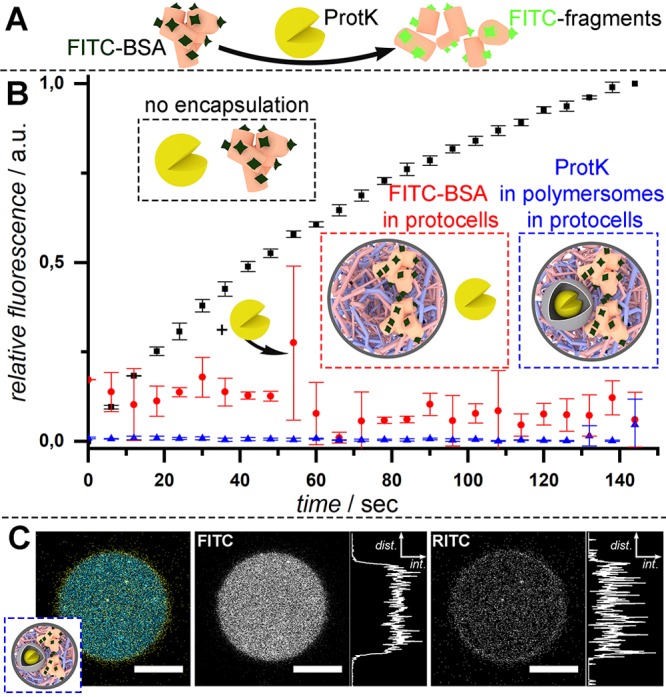
Spatial organization and isolation of macromolecules leads to control over their degradation. (A) Overlabeling of BSA with FITC leads to self-quenching, until ProtK digestion. (B) Bulk fluorescence emission spectroscopy of self-assembled systems. When free in solution, this model reaction progresses rapidly (black curve); however, BSA-FITC can be protected from degradation via both encapsulation in coacervate protocells (red curve) and subcompartmentalization of ProtK in polymersomes (blue curve). Please note: at 50 s, the protease K solution was added to the FITC-BSA protocell experiment (red), and this accounts for the high error observed. (C) Confocal micrograph demonstrating coencapsulation of both FITC-BSA substrate (false colored blue) with RITC-labeled ProtK polymersomes (false colored yellow) within coacervate protocells (scale bars = 20 μm).
Furthermore, through subcompartmentalization in polymersomal proto-lysosomes, ProtK could be integrated within the protocell with no detectable degradation of the coacervate-sequestered FITC-BSA (Figure 3B). This coexistence of substrate and proto-lysosome within the protocell was confirmed using confocal microscopy (Figure 3C), which shows superposition of homogeneous fluorescence from the substrate and, highly punctuated, fluorescence from the ProtK-loaded polymersomes. Such control over the spatial organization and localization of incompatible components behind semipermeable membranes is akin to the protection of proteins from the digestive environment within the lysosome, paving the way for incorporation of highly functional, yet potentially incompatible microenvironments within the next generation of protocells.
The physical interface between proto- and living cells is an exciting frontier, where we can explore the boundaries of biomimetic functionality in synthetic systems. However, the limited stability of existing protocellular constructs, reflected by the use of nonbiological medium during their fabrication, has meant this interface between living systems and synthetic protocells is largely unexplored. Typically, complex coacervates are prone to disassembly under high salt concentrations due to their electrostatic nature.53,54 In contrast, our subcompartmentalized coacervate protocells display stability in high salt, physiologically relevant milieu such as vascular cell basal medium (VCBM) (Figure 4). Even after 24 h of incubation, protocells retained structural integrity, highlighting the robust nature of this platform for the future exploration of protocellular interactions with living cells (Figure S11). With the capacity to generate protocellular architectures that now mimic core features of the cell, such as spatial organization and subcompartmentalization, which are stable in physiological conditions, the opportunities to take this forward in complexity are tantalizing.
Figure 4.
Confocal microscopy image of coacervate protocells stained with Nile red (A) formed in VCBM and (B) after 24 h of dialysis (scale bars = 20 μm).
Conclusion
In conclusion, we have demonstrated the ability to generate subcompartmentalized protocells, through multistep, noncovalent assembly, whereby proto-organelles are spontaneously captured by coacervate microdroplets prior to membranization—inspired by the structure of eukaryotic cells. Such hierarchical protocells are able to control the spatial organization of macromolecular cargo, localized within polymersomal organelles. In this way, spontaneous uptake of loaded polymersomes can imbue function into coacervate-based protocells. Using this novel system, we showcase both activation of cascade processes and segregation of incompatible enzymes (mimicking localization of proteases to lysosomal organelles). Finally, we highlight the stability and versatility of this subcompartmentalized protocell in physiologically relevant media, proof-of-principle for the potential of this platform at the chemical biology interface.
Acknowledgments
The Dutch Ministry of Education, Culture and Science (Gravitation Program 024.001.035) and the ERC Advanced Grant (Artisym 694120) are acknowledged for funding. We thank the Ser Cymru II programme for support of D.S.W.; this project received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie Grant Agreement No. 663830. D. F. M. Vervoort and W. J. Altenburg are thanked for providing sfGFP.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.9b00345.
Materials and methods, supplementary figures (PDF)
Author Contributions
# A.F.M. and N.A.Y. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Gabaldón T.; Pittis A. A. Origin and Evolution of Metabolic Sub-Cellular Compartmentalization in Eukaryotes. Biochimie 2015, 119, 262–268. 10.1016/j.biochi.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York-Duran M. J.; Godoy-Gallardo M.; Labay C.; Urquhart A. J.; Andresen T. L.; Hosta-Rigau L. Recent Advances in Compartmentalized Synthetic Architectures as Drug Carriers, Cell Mimics and Artificial Organelles. Colloids Surf., B 2017, 152, 199–213. 10.1016/j.colsurfb.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Nijemeisland M.; Abdelmohsen L. K. E. A.; Huck W. T. S.; Wilson D. A.; van Hest J. C. M. A Compartmentalized Out-of-Equilibrium Enzymatic Reaction Network for Sustained Autonomous Movement. ACS Cent. Sci. 2016, 2, 843–849. 10.1021/acscentsci.6b00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansy S. S.; Schrum J. P.; Krishnamurthy M.; Tobé S.; Treco D. A.; Szostak J. W. Template-Directed Synthesis of a Genetic Polymer in a Model Protocell. Nature 2008, 454, 122–125. 10.1038/nature07018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.; Harbron R. L.; Weaver J. V. M.; Binks B. P.; Mann S. Electrostatically Gated Membrane Permeability in Inorganic Protocells. Nat. Chem. 2013, 5, 529–536. 10.1038/nchem.1644. [DOI] [PubMed] [Google Scholar]
- Poudyal R. R.; Guth-Metzler R. M.; Veenis A. J.; Frankel E. A.; Keating C. D.; Bevilacqua P. C. Template-Directed RNA Polymerization and Enhanced Ribozyme Catalysis inside Membraneless Compartments Formed by Coacervates. Nat. Commun. 2019, 10, 1–13. 10.1038/s41467-019-08353-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douliez J. P.; Martin N.; Gaillard C.; Beneyton T.; Baret J. C.; Mann S.; Beven L. Catanionic Coacervate Droplets as a Surfactant-Based Membrane-Free Protocell Model. Angew. Chem., Int. Ed. 2017, 56, 13689–13693. 10.1002/anie.201707139. [DOI] [PubMed] [Google Scholar]
- Huang X.; Li M.; Green D. C.; Williams D. S.; Patil A. J.; Mann S. Interfacial Assembly of Protein–polymer Nano-Conjugates into Stimulus-Responsive Biomimetic Protocells. Nat. Commun. 2013, 4, 2239. 10.1038/ncomms3239. [DOI] [PubMed] [Google Scholar]
- Nakashima K. K.; Baaij J. F.; Spruijt E. Reversible Generation of Coacervate Droplets in an Enzymatic Network. Soft Matter 2018, 14, 361–367. 10.1039/C7SM01897E. [DOI] [PubMed] [Google Scholar]
- Fanalista F.; Deshpande S.; Lau A.; Pawlik G.; Dekker C. FtsZ-Induced Shape Transformation of Coacervates. Adv. Biosyst. 2018, 2, 1800136. 10.1002/adbi.201800136. [DOI] [Google Scholar]
- Sokolova E.; Spruijt E.; Hansen M. M. K.; Dubuc E.; Groen J.; Chokkalingam V.; Piruska A.; Heus H. A.; Huck W. T. S. Enhanced Transcription Rates in Membrane-Free Protocells Formed by Coacervation of Cell Lysate. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 11692–11697. 10.1073/pnas.1222321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douliez J. P.; Martin N.; Beneyton T.; Eloi J. C.; Chapel J. P.; Navailles L.; Baret J. C.; Mann S.; Béven L. Preparation of Swellable Hydrogel-Containing Colloidosomes from Aqueous Two-Phase Pickering Emulsion Droplets. Angew. Chem., Int. Ed. 2018, 57, 7780–7784. 10.1002/anie.201802929. [DOI] [PubMed] [Google Scholar]
- Mytnyk S.; Olive A. G. L.; Versluis F.; Poolman J. M.; Mendes E.; Eelkema R.; van Esch J. H. Compartmentalizing Supramolecular Hydrogels Using Aqueous Multi-Phase Systems. Angew. Chem., Int. Ed. 2017, 56, 14923–14927. 10.1002/anie.201706272. [DOI] [PubMed] [Google Scholar]
- Strulson C. A.; Molden R. C.; Keating C. D.; Bevilacqua P. C. RNA Catalysis through Compartmentalization. Nat. Chem. 2012, 4, 941–946. 10.1038/nchem.1466. [DOI] [PubMed] [Google Scholar]
- Poudyal R. R.; Pir Cakmak F.; Keating C. D.; Bevilacqua P. C. Physical Principles and Extant Biology Reveal Roles for RNA-Containing Membraneless Compartments in Origins of Life Chemistry. Biochemistry 2018, 57, 2509–2519. 10.1021/acs.biochem.8b00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L.; Yelleswarapu M.; Vibhute M. A.; Huck W. T. S.; Deng N.-N.; Zhao H. Macromolecularly Crowded Protocells from Reversibly Shrinking Monodisperse Liposomes. J. Am. Chem. Soc. 2018, 140, 7399–7402. 10.1021/jacs.8b03123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.; De Queiros Silveira G.; Ma X.; Xie Y.; Wu Y. A.; Barry E.; Rajh T.; Fry H. C.; Laible P. D.; Rozhkova E. A. Light-Gated Synthetic Protocells for Plasmon-Enhanced Chemiosmotic Gradient Generation and ATP Synthesis. Angew. Chem., Int. Ed. 2019, 58, 4896–4900. 10.1002/anie.201813963. [DOI] [PubMed] [Google Scholar]
- Price A. D.; Zelikin A. N.; Wark K. L.; Caruso F. A Biomolecular “Ship-in-a-Bottle”: Continuous RNA Synthesis within Hollow Polymer Hydrogel Assemblies. Adv. Mater. 2010, 22, 720–723. 10.1002/adma.200903411. [DOI] [PubMed] [Google Scholar]
- Discher D. E.; Eisenberg A. Polymer Vesicles. Science 2002, 297, 967–973. 10.1126/science.1074972. [DOI] [PubMed] [Google Scholar]
- Mason A. F.; Thordarson P. Polymersomes as Protocellular Constructs. J. Polym. Sci., Part A: Polym. Chem. 2017, 55, 3817–3825. 10.1002/pola.28780. [DOI] [Google Scholar]
- Mansy S. S.; Szostak J. W. Reconstructing the Emergence of Cellular Life through the Synthesis of Model Protocells. Cold Spring Harbor Symp. Quant. Biol. 2009, 74, 47–54. 10.1101/sqb.2009.74.014. [DOI] [PubMed] [Google Scholar]
- Marguet M.; Bonduelle C.; Lecommandoux S. Multicompartmentalized Polymeric Systems: Towards Biomimetic Cellular Structure and Function. Chem. Soc. Rev. 2013, 42, 512–529. 10.1039/C2CS35312A. [DOI] [PubMed] [Google Scholar]
- Trantidou T.; Friddin M.; Elani Y.; Brooks N. J.; Law R. V.; Seddon J. M.; Ces O. Engineering Compartmentalized Biomimetic Micro- and Nanocontainers. ACS Nano 2017, 11, 6549–6565. 10.1021/acsnano.7b03245. [DOI] [PubMed] [Google Scholar]
- Bayoumi M.; Bayley H.; Maglia G.; Sapra K. T. Multi-Compartment Encapsulation of Communicating Droplets and Droplet Networks in Hydrogel as a Model for Artificial Cells. Sci. Rep. 2017, 7, 45167. 10.1038/srep45167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbo P.; Patil A. J.; Li M.; Harniman R.; Briscoe W. H.; Mann S. Programmed Assembly of Synthetic Protocells into Thermoresponsive Prototissues. Nat. Mater. 2018, 17, 1145–1153. 10.1038/s41563-018-0183-5. [DOI] [PubMed] [Google Scholar]
- Peyret A.; Ibarboure E.; Pippa N.; Lecommandoux S. Liposomes in Polymersomes: Multicompartment System with Temperature-Triggered Release. Langmuir 2017, 33, 7079–7085. 10.1021/acs.langmuir.7b00655. [DOI] [PubMed] [Google Scholar]
- Jo S.; Wurm F. R.; Landfester K. Biomimetic Cascade Network between Interactive Multicompartments Organized by Enzyme-Loaded Silica Nanoreactors. ACS Appl. Mater. Interfaces 2018, 10, 34230–34237. 10.1021/acsami.8b11198. [DOI] [PubMed] [Google Scholar]
- Lu A. X.; Oh H.; Terrell J. L.; Bentley W. E.; Raghavan S. R. A New Design for an Artificial Cell: Polymer Microcapsules with Addressable Inner Compartments That Can Harbor Biomolecules, Colloids or Microbial Species. Chem. Sci. 2017, 8, 6893–6903. 10.1039/C7SC01335C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R. J. R. W.; Marguet M.; Marais S.; Fraaije M. W.; van Hest J. C. M.; Lecommandoux S. Cascade Reactions in Multicompartmentalized Polymersomes. Angew. Chem., Int. Ed. 2014, 53, 146–150. 10.1002/anie.201308141. [DOI] [PubMed] [Google Scholar]
- Caruso F.; Chandrawati R.; Städler B.; Postma A.; Chung S. F.; Hosta-Rigau L. Capsosomes with “Free-Floating” Liposomal Subcompartments. Adv. Mater. 2011, 23, 4082–4087. 10.1002/adma.201100908. [DOI] [PubMed] [Google Scholar]
- Pavan Kumar B. V. V. S.; Fothergill J.; Bretherton J.; Tian L.; Patil A. J.; Davis S. A.; Mann S. Chloroplast-Containing Coacervate Micro-Droplets as a Step towards Photosynthetically Active Membrane-Free Protocells. Chem. Commun. 2018, 54, 3594–3597. 10.1039/C8CC01129J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oppen L. M. P. E.; Abdelmohsen L. K. E. A.; van Emst-de Vries S. E.; Welzen P. L. W.; Wilson D. A.; Smeitink J. A. M.; Koopman W. J. H.; Brock R.; Willems P. H. G. M.; Williams D. S.; van Hest J. C. M. Biodegradable Synthetic Organelles Demonstrate ROS Shielding in Human-Complex-I-Deficient Fibroblasts. ACS Cent. Sci. 2018, 4, 917–928. 10.1021/acscentsci.8b00336. [DOI] [Google Scholar]
- Thingholm B.; Schattling P.; Zhang Y.; Städler B. Subcompartmentalized Nanoreactors as Artificial Organelle with Intracellular Activity. Small 2016, 12, 1806–1814. 10.1002/smll.201502109. [DOI] [PubMed] [Google Scholar]
- Godoy-Gallardo M.; Labay C.; Trikalitis V. D.; Kempen P. J.; Larsen J. B.; Andresen T. L.; Hosta-Rigau L. Multicompartment Artificial Organelles Conducting Enzymatic Cascade Reactions inside Cells. ACS Appl. Mater. Interfaces 2017, 9, 15907–15921. 10.1021/acsami.6b16275. [DOI] [PubMed] [Google Scholar]
- Zhao R.; Liu X.; Yang X.; Jin B.; Shao C.; Kang W.; Tang R. Nanomaterial-Based Organelles Protect Normal Cells against Chemotherapy-Induced Cytotoxicity. Adv. Mater. 2018, 30, 1801304. 10.1002/adma.201801304. [DOI] [PubMed] [Google Scholar]
- Lee K. Y.; Park S.-J.; Lee K. A.; Kim S.-H.; Kim H.; Meroz Y.; Mahadevan L.; Jung K.-H.; Ahn T. K.; Parker K. K.; et al. Photosynthetic Artificial Organelles Sustain and Control ATP-Dependent Reactions in a Protocellular System. Nat. Biotechnol. 2018, 36, 530–535. 10.1038/nbt.4140. [DOI] [PubMed] [Google Scholar]
- Vantomme G.; Meijer E. W. The Construction of Supramolecular Systems. Science 2019, 363, 1396–1397. 10.1126/science.aav4677. [DOI] [PubMed] [Google Scholar]
- Mason A. F.; Buddingh’ B. C.; Williams D. S.; van Hest J. C. M. Hierarchical Self-Assembly of a Copolymer-Stabilized Coacervate Protocell. J. Am. Chem. Soc. 2017, 139, 17309–17312. 10.1021/jacs.7b10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdall N. A.; Buddingh’ B. C.; Altenburg W. J.; Timmermans S. B. P. E.; Vervoort D. F. M.; Abdelmohsen L. K. E. A.; Mason A. F.; van Hest J. Physicochemical Characterization of Polymer-stabilized Coacervate Protocells. ChemBioChem 2019, 10.1002/cbic.201900195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. S.; Koga S.; Hak C. R. C.; Majrekar A.; Patil A. J.; Perriman A. W.; Mann S. Polymer/Nucleotide Droplets as Bio-Inspired Functional Micro-Compartments. Soft Matter 2012, 8, 6004–6014. 10.1039/c2sm25184a. [DOI] [Google Scholar]
- Bäumler H.; Georgieva R. Coupled Enzyme Reactions in Multicompartment Microparticles. Biomacromolecules 2010, 11, 1480–1487. 10.1021/bm1001125. [DOI] [PubMed] [Google Scholar]
- Kuiper S. M.; Nallani M.; Vriezema D. M.; Cornelissen J. J. L. M.; van Hest J. C. M.; Nolte R. J. M.; Rowan A. E. Enzymes Containing Porous Polymersomes as Nano Reaction Vessels for Cascade Reactions. Org. Biomol. Chem. 2008, 6, 4315–4318. 10.1039/b811196k. [DOI] [PubMed] [Google Scholar]
- van Dongen S. F. M.; Nallani M.; Cornelissen J. J. L. M.; Nolte R. J. M.; van Hest J. C. M. A Three-Enzyme Cascade Reaction through Positional Assembly of Enzymes in a Polymersome Nanoreactor. Chem. - Eur. J. 2009, 15, 1107–1114. 10.1002/chem.200802114. [DOI] [PubMed] [Google Scholar]
- Siti W.; de Hoog H.-P. M.; Fischer O.; Shan W. Y.; Tomczak N.; Nallani M.; Liedberg B. An Intercompartmental Enzymatic Cascade Reaction in Channel-Equipped Polymersome-in-Polymersome Architectures. J. Mater. Chem. B 2014, 2, 2733–2737. 10.1039/C3TB21849J. [DOI] [PubMed] [Google Scholar]
- Elani Y.; Law R. V.; Ces O. Vesicle-Based Artificial Cells as Chemical Microreactors with Spatially Segregated Reaction Pathways. Nat. Commun. 2014, 5, 5305. 10.1038/ncomms6305. [DOI] [PubMed] [Google Scholar]
- Tan H.; Guo S.; Dinh N.-D.; Luo R.; Jin L.; Chen C.-H. Heterogeneous Multi-Compartmental Hydrogel Particles as Synthetic Cells for Incompatible Tandem Reactions. Nat. Commun. 2017, 8, 663. 10.1038/s41467-017-00757-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellana M.; Wilson M. Z.; Xu Y.; Joshi P.; Cristea I. M.; Rabinowitz J. D.; Gitai Z.; Wingreen N. S. Enzyme Clustering Accelerates Processing of Intermediates through Metabolic Channeling. Nat. Biotechnol. 2014, 32, 1011–1018. 10.1038/nbt.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner A.; Tostevin F.; Hinzpeter F.; Gerland U. Optimization of Collective Enzyme Activity via Spatial Localization. J. Chem. Phys. 2013, 139, 135101. 10.1063/1.4823504. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Tsitkov S.; Hess H. Proximity Does Not Contribute to Activity Enhancement in the Glucose Oxidase-Horseradish Peroxidase Cascade. Nat. Commun. 2016, 7, 1–9. 10.1038/ncomms13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmak A.; Carmali S.; von Lieres E.; Russell A. J.; Kondrat S. Can Enzyme Proximity Accelerate Cascade Reactions?. Sci. Rep. 2019, 9, 455. 10.1038/s41598-018-37034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinzpeter F.; Gerland U.; Tostevin F. Optimal Compartmentalization Strategies for Metabolic Microcompartments. Biophys. J. 2017, 112, 767–779. 10.1016/j.bpj.2016.11.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann Y.; Pereira-Leal J. B. Evolution of Intracellular Compartmentalization. Biochem. J. 2013, 449, 319–331. 10.1042/BJ20120957. [DOI] [PubMed] [Google Scholar]
- Blocher W. C.; Perry S. L. Complex Coacervate-Based Materials for Biomedicine. Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology 2017, 9, 76–78. [DOI] [PubMed] [Google Scholar]
- Meka V. S.; Sing M. K. G.; Pichika M. R.; Nali S. R.; Kolapalli V. R. M.; Kesharwani P. A Comprehensive Review on Polyelectrolyte Complexes. Drug Discovery Today 2017, 22, 1697–1706. 10.1016/j.drudis.2017.06.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.