Abstract
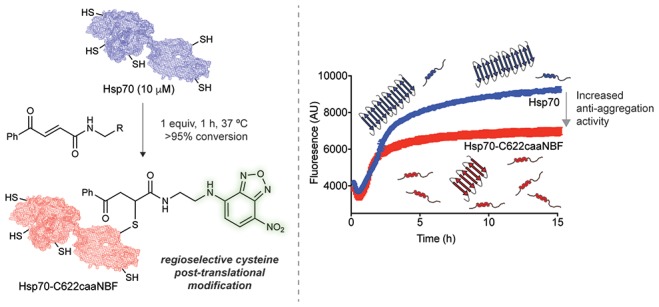
Protein behavior is closely regulated by a plethora of post-translational modifications (PTMs). It is therefore desirable to develop approaches to design rational PTMs to modulate specific protein functions. Here, we report one such method, and we illustrate its successful implementation by potentiating the anti-aggregation activity of a molecular chaperone. Molecular chaperones are a multifaceted class of proteins essential to protein homeostasis, and one of their major functions is to combat protein misfolding and aggregation, a phenomenon linked to a number of human disorders. In this work, we conjugated a small-molecule inhibitor of the aggregation of α-synuclein, a process associated with Parkinson’s disease (PD), to a specific cysteine residue on human Hsp70, a molecular chaperone with five free cysteines. We show that this regioselective conjugation augments in vitro the anti-aggregation activity of Hsp70 in a synergistic manner. This Hsp70 variant also displays in vivo an enhanced suppression of α-synuclein aggregation and its associated toxicity in a Caenorhabditis elegans model of PD.
Short abstract
A rationally designed post-translational modification, introduced by a regioselective cysteine conjugation strategy, enhances the anti-amyloid activity of a multifunctional molecular chaperone.
Introduction
In order to exert quality control over the synthesis, folding, trafficking, and turnover of proteins under normal and stress conditions, cells employ a complex network of proteins known as the protein homeostasis system.1−3 Molecular chaperones are key players in this system,1,2,4 and one of their many important roles is to control protein aggregation, particularly when proteins assemble into highly ordered aggregates, called amyloid fibrils.3,5 The formation of amyloid deposits is a characteristic event in a family of debilitating conditions, generally termed protein misfolding disorders, which includes Alzheimer’s and Parkinson’s diseases as well as type-II diabetes.1−3,5,6 The formation of these amyloid deposits involves the aggregation of specific proteins and peptides into progressively more organized assemblies, which eventually mature into amyloid fibrils.3,6 Transient and highly cytotoxic low molecular weight species, known as oligomers, are also produced during this process.7,8 The crucial physiological role of molecular chaperones in deterring amyloid formation involves the inhibition of specific microscopic steps in the aggregation reaction of certain amyloidogenic proteins.2,9 Because of their selectivity for both the steps of inhibition and the identity of the aggregating proteins, molecular chaperones have been considered as possible tools for intervention against misfolding diseases,1,2,9−13 although it has been very challenging so far to identify effective ways to exploit them for this purpose.
While general upregulation of molecular chaperones has been proven to be able to inhibit aggregation in a number of cellular and animal models of misfolding diseases,14−16 such unspecific manipulation of their activity could potentially have unintended consequences on the large number of pathways in which they are involved.17 Strategies to specifically enhance molecular chaperones toward inhibiting aggregation so far include genetic engineering approaches to selectively alter their activity toward a particular target, as illustrated in recent studies on the molecular chaperones Hsp104 and Hsp70.18,19 Molecular chaperones have also been harnessed previously, without exploiting directly their ability to affect protein aggregation, to nonspecifically increase the steric bulk of a small-molecule inhibitor of aggregation.20
In this work, we investigated the possibility of incorporating rationally designed post-translational modifications (PTMs) into molecular chaperones to specifically enhance their anti-aggregation activity. Inspired by the observation that in order to regulate the activity of molecular chaperones the cell routinely employs a host of PTMs to alter their functions, cellular locations and binding to cochaperones,21 we developed a rational method of introducing a non-natural PTM into human Hsp70 to enhance its anti-aggregation activity. To accomplish this goal, we envisioned merging the world of small-molecule inhibitors of aggregation with the world of molecular chaperones through the use of site-selective conjugation. While the rational PTM was found to compromise some of the other functions of Hsp70 related to the structural stability of the lid subdomain, it was also found to enhance the anti-aggregation in a synergistic manner in vitro and maintained its enhancement when tested in vivo in a Caenorhabditis elegans model of Parkinson’s disease.
Results and Discussion
Regioselective Cysteine Modification of Hsp70
Among molecular chaperones, the 70 kDa heat shock protein (Hsp70) family members have shown a particularly strong ability to inhibit protein aggregation.16,22 In particular, the stress inducible isoform of Hsp70 has been found to coaggregate with amyloid deposits in the brain, and upregulation of Hsp70 has also been shown to ameliorate misfolding-related toxicity in cellular and animal models.14−16,23 The structure of Hsp70 is highly conserved throughout all domains of life and consists of an N-terminal nucleotide binding domain (NBD) with ATPase activity, a C-terminal substrate binding domain (SBD), and a linker region connecting the two.24 The activity of Hsp70 is governed by a complex allosteric mechanism whereby the conformation of one domain greatly alters the activity of the other.25
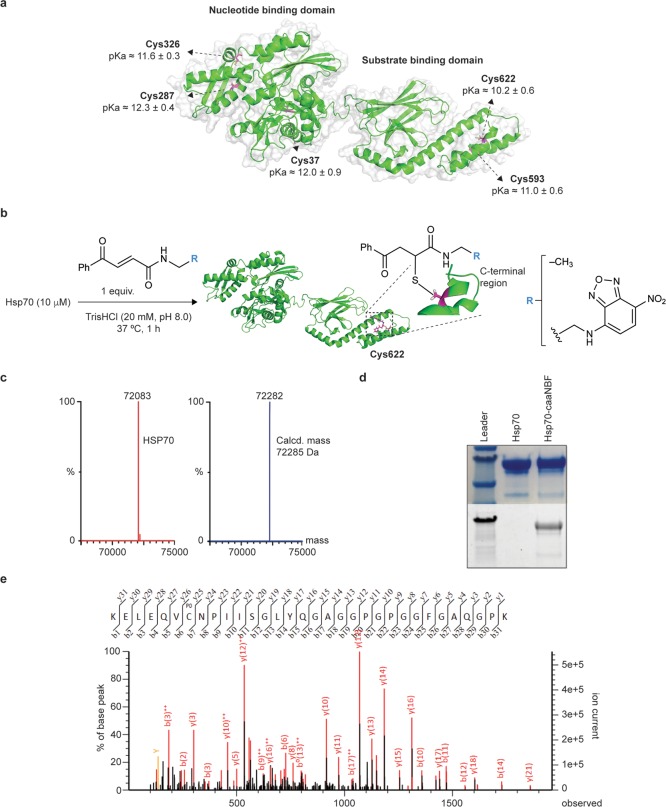
Knowing that Hsp70 has a complex mechanism of action, we sought to develop a minimally invasive conjugation strategy that could both avoid perturbing its overall function and be localized to the SBD, so that the new chemical entity would be near where this molecular chaperone naturally binds protein aggregates. The structure of the SBD of Hsp70 contains a β-sandwich subdomain, where the substrate binding pocket is located, and an α-helical lid subdomain comprising five α-helices. The entire α-helical lid has been shown to be a highly dynamic and flexible region of the protein, enabling Hsp70 to accommodate a wide range of substrates.26 While human Hsp70 contains in total five free cysteines, two of these are located in α-helices within this subdomain. We hypothesized that the positions of these residues on the exposed and dynamic α-helical lid made them likely to be the most reactive ones in the protein, potentially making it possible to create a scheme to selectively conjugate to the SBD by developing a regioselective cysteine labeling method. On the basis of the rationale that the most acidic and solvent accessible cysteines in Hsp70 should be the most reactive,27,28 we performed a theoretical calculation of the pKa values of the different cysteine residues. We used the PROPKA algorithm (PARSE force-field) on 650 Hsp70 structures derived from a molecular dynamics (MD) simulation starting from a homology model of the protein obtained through SWISS-MODEL.29,30 The results indicated that the two cysteines within the substrate binding domain of Hsp70 have the lowest pKa values, with Cys622 predicted to be the most acidic, consistent with our expectations about the cysteine reactivity profiles within Hsp70 (Figure 1a and Supporting Figure 1). This position is also quite far away from the crucial EEVD motif on the disordered C-terminus of Hsp70, which is responsible for cochaperone interaction, which in principle should minimize the risk that a modification at this position would inhibit any associations. As a small molecule to conjugate to Hsp70, we chose nitrobenzofurazan (NBF, Figure 1b) as some of its derivatives have been shown to inhibit amyloid formation and because of its simple structure and its ease of attachment to potential conjugation reagents.31
Figure 1.
Regioselective modification of Cys622 in Hsp70. (a) pKa values calculated for all cysteine side chains of Hsp70. These values were averaged over 650 structures resulting from a 650 ns MD simulation. (b) Reaction scheme for the labeling of Cys622 of Hsp70 with the linker alone (caa) and the NBF derivative (caaNBF). (c) LC-MS spectra of unlabeled Hsp70 (red) and Hsp70 modified with one equivalent of the linker (blue). (d) Fluorescence imaging of the SDS-PAGE gel of Hsp70 before and after conjugation to caaNBF. (e) Peptide mapping by LC-MS/MS preceded by trypsin digestion confirming Cys622 as the major site of labeling.
For the conjugation strategy, we adopted a recently developed system for cysteine-selective conjugation using carbonylacrylic reagents that has many advantages over traditional methods.32 Conjugates created with these reagents have been found to have fully stable bonds, even after extended exposure to glutathione and human plasma, a feat difficult to achieve with other strategies. Most importantly for our purposes, these reagents have been found to label cysteines rapidly under biocompatible conditions (aqueous media, pH 8, 37 °C) and using a single molar equivalent of the reagent to each cysteine residue and facilitated a facile fully homogeneous conjugation of an antibody.32 The latter improvement prompted us to apply this strategy to label a single cysteine within the SBD by employing mild reaction conditions and using only one equivalent of the labeling reagent (Figure 1b and Supporting Figures 2 and 3). (E)-N-Ethyl-4-oxo-4-phenylbut-2-enamide (caa) was used as a model for the conjugation methodology, and a labeling reagent was then synthesized with an NBF group preceded by a linker – (E)-N-(2-((7-nitrobenzo[c][1,2,5]oxadiazol-4-yl)amino)ethyl)-4-oxo-4-phenylbut-2-enamide (caaNBF) using an established method.32 Analysis by LC-MS confirmed that under these conditions a singularly labeled population of Hsp70 with the caa reagent was obtained (Figure 1c and Supporting Figures 4 and 5), and conjugation to caaNBF was confirmed via fluorescent imaging of the SDS-PAGE of Hsp70 post reaction (Figure 1d). The location of the conjugation site with the caa reagent was identified by peptide mapping using mass spectrometry (LC-MS/MS) preceded by trypsin digestion, which showed that Cys622 was indeed the predominant site of conjugation (Figure 1e and Supporting Figure 6). This very high selectivity of labeling for a single free cysteine residue out of five in the protein is very difficult to achieve and was enabled by the efficient conjugation strategy.33
Effects of the Conjugation on the Endogenous Activity of Hsp70
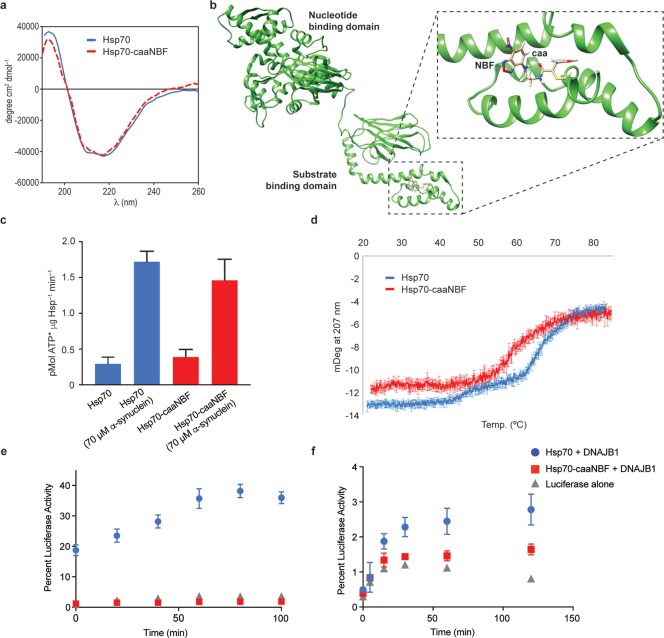
The effects of the NBF modification on the general activity Hsp70 were first assessed. The overall structural integrity of the Hsp70 conjugate (termed Hsp-caaNBF) was investigated by using circular dichroism (CD) spectroscopy, which showed a profile similar to that of the nonconjugated form (Figure 2a). In parallel, 0.5 μs MD simulations were performed on the two possible stereoisomers generated upon the addition of Cys622 to caaNBF (Figure 2b and Figure S5). According to these calculations, the addition of caaNBF makes the C-terminal region of the protein more flexible and distorts the α-helix structure in the proximity of the chemical modification. On the other hand, the NBF moiety is exposed to the solvent in both conjugates, favoring its interaction with a biological target. We then tested the ATPase activity of Hsp-caaNBF. ATPase activity is important for the overall function of Hsp70 because the on and off rates of substrate binding are highly altered depending on whether Hsp70 is bound to ATP or ADP.
Figure 2.
The endogenous in vitro activity of the Hsp70 conjugate Hsp70-caaNBF is similar to that of the unmodified Hsp70. (a) Comparison of the CD spectra of Hsp70 (blue) and Hsp70-caaNBF (red). (b) A representative frame derived from 0.5 μs MD simulations performed on a conjugate with R configuration at the new stereogenic center generated upon addition of Cys622 to caaNBF. (c) Comparison of the colorimetric ATPase assay of Hsp70 (blue) and Hsp70-caaNBF (red) for the intrinsic and stimulated hydrolysis rates in the absence and presence of monomeric α-synuclein. (d) CD melting curves of both Hsp70-caaNBF (red) and Hsp70 (blue). (e) Heat-denatured luciferase refolding assay comparing the recovery of luminescence by Hsp70 (blue), Hsp70-caaNBF (red), and luciferase alone (gray). (f) Chemically denatured luciferase refolding assay comparing the same treatments.
Since it has been shown that the oxidation of cysteines in the NBD of Hsp70 can inhibit ATPase activity, we investigated if our larger chemical modification could lead to inhibition through a conformational rearrangement, despite being limited to the SBD.34 To assess ATPase activity, we used a previously established colorimetric assay that had been implemented for high-throughput screening of Hsp70 ATPase inhibitors.35 Both the intrinsic ATPase activity and the activity stimulated by substrate (70 μM monomeric α-synuclein) were found to be largely unaltered by the conjugation to caaNBF and consistent with previous measures of Hsp70 ATPase rates (Figure 2c). To ensure that the increase in ATPase activity was not the result of contamination from the α-synuclein stock, ATP was incubated in the same manner with α-synuclein alone, which showed the same level of activity as the background hydrolysis (Figure S6).
As an important physiological role of Hsp70 is the refolding of denatured proteins, a function that Hsp70 performs by associating with a number of cochaperones to assist in substrate turnover.2 To investigate whether or not the addition of caaNBF to the SBD might perturb this more complex activity of Hsp70, we employed the well-established assay of luciferase refolding in the presence of the cochaperone DNAJB1.36 Before performing the heat denaturation assay, the thermal stability of Hsp70-caaNBF was assessed by CD (Figure 2d). Compared to unmodified Hsp70, the Hsp70-caaNBF displays a partially affected stability. This loss of structural integrity at higher temperatures was then found to have a debilitating effect on the ability of Hsp70-caaNBF to cooperate with DNAJB1 and inhibit the misfolding of luciferase at 42 °C (Figure 2e). To assess if Hsp70-caaNBF retained the ability to functionally associate with DNAJB1 under less harsh conditions, the refolding of chemically denatured luciferase was also assessed (Figure 2f). This measurement revealed that at lower temperatures the modified chaperone still retains approximately 60% of the refolding ability compared to native Hsp70. The lower activity observed in this assay could possibly arise from subtle structural variations at the C-terminus of Hsp70 where DNAJB1 associates and is relatively close to the caaNBF modification.
Enhancement of the Anti-Aggregation Activity of the Hsp70 Conjugate
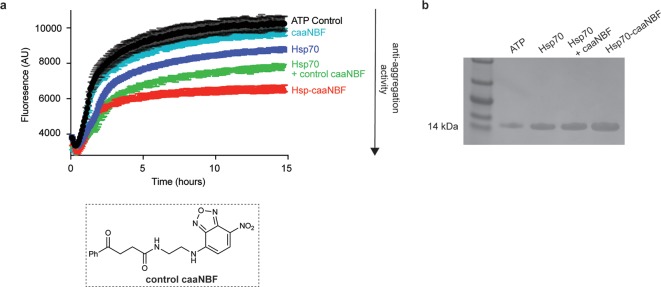
In order to test the potential of using the regioselective cysteine conjugation strategy, we considered the 140-residue intrinsically disordered protein α-synuclein, the major component of Lewy bodies, which are aberrant deposits characteristically seen in the brain tissue of patients suffering from Parkinson’s disease (PD).6 The ability of Hsp70 to combat α-synuclein aggregation in vitro has been characterized in detail, and it has been determined that Hsp70 accomplishes this task through its preferential association with aggregated species of α-synuclein.16,37,38 To test how the anti-aggregation activity of Hsp70 is altered upon conjugation to caaNBF, we ran aggregation experiments by introducing preformed α-synuclein fibrils (5%) in order to probe specifically the elongation of fibrils and overcome the problem that the spontaneous nucleation of α-synuclein is an extremely slow process.39
To accurately assess if conjugation to caaNBF imparts a synergistic benefit compared to the two components separately, an inert version of caaNBF was synthesized lacking the α/β unsaturated system of the carbonylacrylic moiety (see the Supporting Information for synthetic details), rendering it incapable of conjugating to cysteine (Figure 3a). The aggregation reactions revealed that caaNBF alone had only a very modest inhibitory activity, and Hsp70 alone was only capable of reducing the aggregation by ∼15% (Figure 3a). An equimolar mixture of the two separately displayed an enhanced inhibition compared to the two alone, but the most potent agent was indeed the conjugate form (Hsp-caaNBF), confirming that the conjugation does indeed impart a synergistic benefit (Figure 3a). To be sure that the decreased ThT signal by Hsp70-caaNBF indeed represents an overall decrease of the aggregation of α-synuclein, rather than a conformational change of the fibrils, the fraction of monomer left at the end of extended reactions was quantified. After 48 h, samples were ultracentrifuged to pellet all aggregated protein, and the monomer in solution was quantified via SDS-PAGE (Figure 3b). The intensities of the bands corroborate that the ThT signal is indeed representative of a decrease of the actual aggregation of α-synuclein.
Figure 3.
The Hsp70 conjugate Hsp70-caaNBF is more potent than its parent Hsp70 in inhibiting in vitro α-synuclein aggregation. (a) Comparison of seeded α-synuclein aggregation assays in the presence of ATP alone as control (black), caaNBF (cyan), Hsp70 (purple), an equimolar mixture of control caaNBF and Hsp70 (green), and the NBF-modified chaperone Hsp70-caaNBF (red). (b) SDS-PAGE of the supernatant of the chaperone reactions after ultracentrifugation.
Enhancement of the Hsp70 Conjugate Anti-Aggregation Activity in a C. elegans Model of Parkinson’s Disease
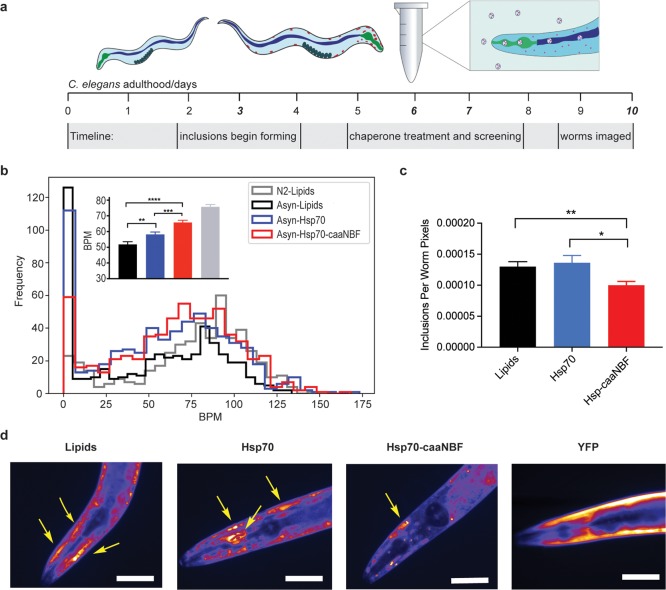
In light of the clear evidence for the enhancement of anti-aggregation activity in vitro, we assessed the properties of our modified Hsp70 in vivo using an established C. elegans model of PD.40 In this model, overexpression of α-synuclein tagged with yellow fluorescent protein (YFP) in the muscle cells of the worms leads to the formation of well-defined inclusions that can be observed in the animals from day three and onward after hatching.40,41 The gradual development of these α-synuclein inclusions is strongly correlated to an accelerated age-dependent paralysis of the animals compared to the wild-type phenotype. This model has been used successfully to screen for potential therapeutic compounds for Parkinson’s disease as well as for distinguishing genes playing roles in neurodegeneration.40,41
To test the activity of Hsp70-caaNBF in this model system, we administered the modified molecular chaperone by transducing the protein into adult worms through a recently developed method adapted from a technique for the lipid vesicle mediated transduction of mammalian cells, where the worms ingest the loaded vesicles that can then unload their cargo into the worms through their gut tissue.42,43 Using this protocol, we encapsulated, separately, both Hsp70-caaNBF and unmodified Hsp70 in lipid vesicles and incubated them with the worms for 5 h on day 6 of adulthood, when there are a significant number of inclusions; a control group of worms was also incubated with empty vesicles. The animals were then screened the following day for measures of paralysis (Figure 4a). The fitness of C. elegans can be generally be quantified by observing the number and amplitude of body bends per minute (BPMs).41 In order to improve reproducibility, we used an automated platform for the screening of the worms that utilizes a wide field of view camera to capture movies of the swimming worms which are subsequently analyzed by custom image analysis software.41,43 Each treatment was carried out in triplicate with about 200 worms per replicate for a total of approximately 600 worms. The results showed that worms incubated with Hsp70-caaNBF had significantly higher average BPM values than did the worm populations treated with Hsp70 or with the empty vesicles (65.76 BPM versus 58.17 and 51.84 respectively), rising to a level much closer to that observed for wild-type worms (designated N2) on the same day (Figure 4b). In particular, the data showed a much-reduced proportion of worms at very low BPM values (Figure 4a and Supporting Figure 8a–d). The unspecific activity of the modified chaperone was also investigated by treating wild-type worms in the same manner as the α-synuclein worms. In this case, only a very marginal increase in motility was observed, 5% increase over lipid-treated wild-type controls versus the 26% increase in the α-synuclein model worms (Supporting Figure 9). After the phenotypic analysis in liquid media, worms were plated on solid media for 4 days and then imaged using fluorescence microscopy to quantify the number of YFP tagged inclusions in each treatment population. We found that the Hsp70-caaNBF-treated worms contained a significantly decreased amount of inclusion bodies compared to either the Hsp70 or lipid-treated populations (Figure 4c,d), thus confirming that the potentiated anti-aggregation activity is also present in vivo.
Figure 4.
The Hsp70 conjugate Hsp70-caaNBF is more potent than its parent Hsp70 in inhibiting α-synuclein aggregation in a C. elegans model of Parkinson’s disease. (a) Timeline of the Hsp70 treatment for adult worms. (b) Average BPM values for the three replicates of each treatment; error bars indicate the standard error of the mean (SEM). Hsp70-caaNBF-treated worms (red) showed a significantly increased BPM compared to both Hsp70- (p-value = 0.000248) and lipid- (p-value = 6.46 × 10–9) treated populations. Worms treated with Hsp70 also displayed significantly higher BPM values than lipid-treated worms (p-value = 0.00576); statistical tests were one-sided Mann–Whitney U tests. (c) Measurements of the inclusions per worm pixel of each treatment population, Hsp70-caaNBF worms (n = 33) showed a significantly decreased inclusion burden compared to both Hsp70- (n = 34, p-value = 0.0343) and lipid- (n = 34, p-value = 0.00654) treated populations; p-values are calculated from one-sided Mann–Whitney U tests, error bars display the SEM. (d) Representative false color images of single worms from each population, and a worm expressing cytosolic YFP also imaged on day 10. Scale bars are 80 μm, and arrows point to major inclusions.
Conclusions
We have demonstrated that a rational PTM can specfically augment one of the molecular activities of a multifunctional protein. Crucial to obtain this result has been the use of a remarkably successful regioselective cysteine conjugation reaction, validating the use of carbonylacrylic reagents for specific cysteine conjugation in multicysteine containing proteins. The development of non-natural site-selective PTMs has become an area of intensive research with many potential applications in basic and applied research and biotechnology.44 Given its multiplicity of functions and mechanisms of regulation, we used Hsp70 as a model system to see if the conjugation strategy that we have presented here can be used to manipulate a specific activity of a protein. While our rational PTM is not as well tolerated as the evolutionary-optimized PTMs that the cell employs, it does indeed enhance the intended function in a synergistic manner. Considering that the preservation of endogenous functions must be of paramount importance for a desired construct, the location of conjugation and properties of the chemical modification could be improved to minimize off-target effects. We anticipate that the design and implementation of rational PTMs to augment endogenous protein functions could find wide applicability in biotechnology.
Acknowledgments
We would like to acknowledge Sam Casford for assistance with all experiments involving C. elegans and Dr. Michael Deery for conducting the LC-MS/MS analysis. We are grateful to the Centre for Misfolding Diseases for support of this research and for invaluable discussions with many of its members. We thank Alzheimer’s Society UK (Senior Research Fellowship to F.A.A., Grant No. 317, AS-SF-16-003), Xunta de Galicia (postdoctoral Fellowship to M.J.M., ED481B 2014/086-0), Ministerio de Ciencia, Innovación y Universidades (Project RTI2018-099592-B-C21 to F.C.), FCT Portugal (FCT Investigator to G.J.L.B., iFCT IF/00624/2015), CNPq Brazil (postdoctoral Fellowship 200456/2015-6 to J.B.B.), Royal Society (NIF\R1\180120 to B.B.) and FAPESP (BEPE 2017/13168-8 to B.B.) for funding. G.J.L.B. is a Royal Society URF (URF\R\180019).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.9b00467.
Supporting figures, detailed methods, and additional characterization (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Balch W. E.; Morimoto R. I.; Dillin A.; Kelly J. W. Adapting proteostasis for disease intervention. Science 2008, 319 (5865), 916–919. 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- Kim Y. E.; Hipp M. S.; Bracher A.; Hayer-Hartl M.; Ulrich Hartl F. Molecular chaperone functions in protein folding and proteostasis. Annu. Rev. Biochem. 2013, 82, 323–355. 10.1146/annurev-biochem-060208-092442. [DOI] [PubMed] [Google Scholar]
- Knowles T. P. J.; Vendruscolo M.; Dobson C. M. The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell Biol. 2014, 15, 496. 10.1038/nrm3826. [DOI] [PubMed] [Google Scholar]
- Bukau B.; Weissman J.; Horwich A. Molecular chaperones and protein quality control. Cell 2006, 125 (3), 443–451. 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Eisenberg D.; Jucker M. The amyloid state of proteins in human diseases. Cell 2012, 148 (6), 1188–1203. 10.1016/j.cell.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiti F.; Dobson C. M. Protein misfolding, amyloid formation, and human disease: A summary of progress over the last decade. Annu. Rev. Biochem. 2017, 86, 27–68. 10.1146/annurev-biochem-061516-045115. [DOI] [PubMed] [Google Scholar]
- Cohen S. I. A.; et al. A molecular chaperone breaks the catalytic cycle that generates toxic Aβ oligomers. Nat. Struct. Mol. Biol. 2015, 22 (3), 207–213. 10.1038/nsmb.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C.; Selkoe D. J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid β-peptide. Nat. Rev. Mol. Cell Biol. 2007, 8 (2), 101–112. 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Arosio P.; Michaels T. C. T.; Linse S.; Mansson C.; Emanuelsson C.; Presto J.; Johansson J.; Vendruscolo M.; Dobson C. M.; Knowles T. P. J. Kinetic analysis reveals the diversity of microscopic mechanisms through which molecular chaperones suppress amyloid formation. Nat. Commun. 2016, 7, 10948. 10.1038/ncomms10948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia S. K.; Kalia L. V.; McLean P. J. Molecular chaperones as rational drug targets for Parkinson’s disease therapeutics. CNS Neurol. Disord.: Drug Targets 2010, 9 (6), 741–753. 10.2174/187152710793237386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannini B.; et al. Molecular mechanisms used by chaperones to reduce the toxicity of aberrant protein oligomers. Proc. Natl. Acad. Sci. U. S. A. 2012, 109 (31), 12479–12484. 10.1073/pnas.1117799109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C. G.; Chang L.; Gestwicki J. E. Heat shock protein 70 (Hsp70) as an emerging drug target. J. Med. Chem. 2010, 53 (12), 4585–4602. 10.1021/jm100054f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchowski P. J.; Wacker J. L. Modulation of neurodegeneration by molecular chaperones. Nat. Rev. Neurosci. 2005, 6 (1), 11–22. 10.1038/nrn1587. [DOI] [PubMed] [Google Scholar]
- Cummings C. J.; et al. Over-expression of inducible HSP70 chaperone suppresses neuropathology and improves motor function in SCA1 mice. Hum. Mol. Genet. 2001, 10 (14), 1511–1518. 10.1093/hmg/10.14.1511. [DOI] [PubMed] [Google Scholar]
- Auluck P. K.; Chan H. Y. E.; Trojanowski J. Q.; Lee V. M. Y.; Bonini N. M. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson’s disease. Science 2002, 295 (5556), 865–868. 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- Klucken J.; Shin Y.; Masliah E.; Hyman B. T.; McLean P. J. Hsp70 reduces α-synuclein aggregation and toxicity. J. Biol. Chem. 2004, 279 (24), 25497–25502. 10.1074/jbc.M400255200. [DOI] [PubMed] [Google Scholar]
- Sherman M. Y.; Gabai V. L. Hsp70 in cancer: back to the future. Oncogene 2015, 34 (32), 4153–4161. 10.1038/onc.2014.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackrel M. E.; et al. Potentiated Hsp104 variants antagonize diverse proteotoxic misfolding events. Cell 2014, 156 (1–2), 170–182. 10.1016/j.cell.2013.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprile F. A.; Sormanni P.; Vendruscolo M. A Rational Design Strategy for the Selective Activity Enhancement of a Molecular Chaperone toward a Target Substrate. Biochemistry 2015, 54 (32), 5103–5112. 10.1021/acs.biochem.5b00459. [DOI] [PubMed] [Google Scholar]
- Gestwicki J. E.; Crabtree G. R.; Graef I. A. Harnessing chaperones to generate small-molecule inhibitors of amyloid beta aggregation. Science 2004, 306 (5697), 865–869. 10.1126/science.1101262. [DOI] [PubMed] [Google Scholar]
- Mayer M. P.; Bukau B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cell. Mol. Life Sci. 2005, 62 (6), 670–684. 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipp M. S.; Park S. H.; Hartl U. U. Proteostasis impairment in protein-misfolding and -aggregation diseases. Trends Cell Biol. 2014, 24 (9), 506–514. 10.1016/j.tcb.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Cantuti-Castelvetri I.; Klucken J.; Ingelsson M.; Ramasamy K.; McLean P. J.; Frosch M. P.; Hyman B. T.; Standaert D. G. Alpha-synuclein and chaperones in dementia with Lewy bodies. J. Neuropathol. Exp. Neurol. 2005, 64 (12), 1058–1066. 10.1097/01.jnen.0000190063.90440.69. [DOI] [PubMed] [Google Scholar]
- Bertelsen E. B.; Chang L.; Gestwicki J. E.; Zuiderweg E. R. P. Solution conformation of wild-type E. coli Hsp70 (DnaK) chaperone complexed with ADP and substrate. Proc. Natl. Acad. Sci. U. S. A. 2009, 106 (21), 8471–8476. 10.1073/pnas.0903503106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuravleva A.; Clerico E. M.; Gierasch L. M. An interdomain energetic tug-of-war creates the allosterically active state in Hsp70 molecular chaperones. Cell 2012, 151 (6), 1296–1307. 10.1016/j.cell.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlecht R.; Erbse A. H.; Bukau B.; Mayer M. P. Mechanics of Hsp70 chaperones enables differential interaction with client proteins. Nat. Struct. Mol. Biol. 2011, 18 (3), 345–351. 10.1038/nsmb.2006. [DOI] [PubMed] [Google Scholar]
- Bertoldo J. B.; et al. A Water-Bridged Cysteine-Cysteine Redox Regulation Mechanism in Bacterial Protein Tyrosine Phosphatases. Chem. 2017, 3 (4), 665–677. 10.1016/j.chempr.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoldo J. B.; Terenzi H.; Hüttelmaier S.; Bernardes G. J. L. Posttranslational chemical mutagenesis: to reveal the role of non-catalytic cysteine residues in pathogenic bacterial phosphatases. Biochemistry 2018, 57 (43), 6144–6152. 10.1021/acs.biochem.8b00639. [DOI] [PubMed] [Google Scholar]
- Dolinsky T. J.; Nielsen J. E.; McCammon J. A.; Baker N. A. PDB2PQR: An automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res. 2004, 32, W665–W667. 10.1093/nar/gkh381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasini M.; et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, W252–W258. 10.1093/nar/gku340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed M. A.; Wood T. K.; Banfield S. C.; Barden C. J.. (Treventis Corporation), WO2014031873, 2013.
- Bernardim B.; Cal P. M.S.D.; Matos M. J.; Oliveira B. L.; Martinez-Saez N.; Albuquerque I. S.; Perkins E.; Corzana F.; Burtoloso A. C.B.; Jimenez-Oses G.; Bernardes G. J. L. Stoichiometric and irreversible cysteine-selective protein modification using carbonylacrylic reagents. Nat. Commun. 2016, 7, 13128. 10.1038/ncomms13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalker J. M.; Bernardes G. J. L.; Lin Y. A.; Davis B. G. Chemical modification of proteins at cysteine: Opportunities in chemistry and biology. Chem. - Asian J. 2009, 4 (5), 630–640. 10.1002/asia.200800427. [DOI] [PubMed] [Google Scholar]
- Miyata Y.; Rauch J. N.; Jinwal U. K.; Thompson A. D.; Srinivasan S.; Dickey C. A.; Gestwicki J. E. Cysteine reactivity distinguishes redox sensing by the heat-inducible and constitutive forms of heat shock protein 70. Chem. Biol. 2012, 19 (11), 1391–1399. 10.1016/j.chembiol.2012.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L.; Bertelsen E. B.; Wisén S.; Larsen E. M.; Zuiderweg E. R.; Gestwicki J. E. High-throughput screen for small molecules that modulate the ATPase activity of the molecular chaperone DnaK. Anal. Biochem. 2008, 372 (2), 167–176. 10.1016/j.ab.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Bonomo J.; Welsh J. P.; Manthiram K.; Swartz J. R. Comparing the functional properties of the Hsp70 chaperones, DnaK and BiP. Biophys. Chem. 2010, 149 (1–2), 58–66. 10.1016/j.bpc.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprile F. A.; et al. Inhibition of α-Synuclein Fibril Elongation by Hsp70 Is Governed by a Kinetic Binding Competition between α-Synuclein Species. Biochemistry 2017, 56 (9), 1177–1180. 10.1021/acs.biochem.6b01178. [DOI] [PubMed] [Google Scholar]
- Dedmon M. M.; Christodoulou J.; Wilson M. R.; Dobson C. M. Heat shock protein 70 inhibits alpha-synuclein fibril formation via preferential binding to prefibrillar species. J. Biol. Chem. 2005, 280 (15), 14733–14740. 10.1074/jbc.M413024200. [DOI] [PubMed] [Google Scholar]
- Galvagnion C.; Buell A. K.; Meisl G.; Michaels T. C.; Vendruscolo M.; Knowles T. P. J.; Dobson C. M. Lipid vesicles trigger α-synuclein aggregation by stimulating primary nucleation. Nat. Chem. Biol. 2015, 11 (3), 229–234. 10.1038/nchembio.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ham T. J.; Thijssen K. L.; Breitling R.; Hofstra R. M.; Plasterk R. H.; Nollen E. A. C. elegans model identifies genetic modifiers of α-synuclein inclusion formation during aging. PLoS Genet. 2008, 4 (3), e1000027 10.1371/journal.pgen.1000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perni M.; et al. A natural product inhibits the initiation of α-synuclein aggregation and suppresses its toxicity. Proc. Natl. Acad. Sci. U. S. A. 2017, 114 (6), E1009–E1017. 10.1073/pnas.1610586114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perni M.; Aprile F. A.; Casford S.; Mannini B.; Sormanni P.; Dobson C. M.; Vendruscolo M. Delivery of Native Proteins into C. elegans Using a Transduction Protocol Based on Lipid Vesicles. Sci. Rep. 2017, 7, 15045. 10.1038/s41598-017-13755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprile F. A.; Sormanni P.; Perni M.; Arosio P.; Linse S.; Knowles T. P. J.; Dobson C. M.; Vendruscolo M. Selective targeting of primary and secondary nucleation pathways in Aβ42 aggregation using a rational antibody scanning method. Sci. Adv. 2017, 3 (6), e1700488 10.1126/sciadv.1700488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall N.; da Cruz F. P.; Boutureira O.; Bernardes G. J. L. Site-selective protein-modification chemistry for basic biology and drug development. Nat. Chem. 2016, 8 (2), 103–113. 10.1038/nchem.2393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.