Abstract
The development and use of nanomaterials are increasing significantly. Among nanomaterials, carbon nanotubes are of particular interest due to its distinctive physicochemical properties. This material composed of sheets of graphite has very high thermal conductivity, metallic-type electrical conductivity, stiffness, toughness and unique ability to bond to itself in an extended network with extraordinary strength. Its application in the industry is continuously growing, which could lead to the accumulation in the environment and a consequent impact on both humans and ecosystems. Considering that environmental systems are dynamic, it is difficult to predict the risks associated with the release of nanomaterials to the environment. Bioindicators are useful tools as primary signals of environmental risk, and their responses reveal the organism and ecosystem health.
In the present study, we evaluated the impact of multi-walled carbon nanotubes with different dimensions and agglomeration pattern on zebrafish embryo and larvae; mainly, studies were focused on physiological and behavioral responses. In embryos, measurements were hatching rate, morphology changes, and viability. In larvae, locomotor activity, heart rate, innate inflammatory response, general and tissue-specific morphology were measured. MWCNT-S (short, wide and mostly dispersed) caused depression of the locomotor activity of larvae, indicating an alteration of the central nervous system, and depression of neutrophil migration activity. MWCNT-L (long, thin and agglomerated) caused malformations during larval development, a decrease of neutrophil migration and alteration of cardiac rhythm. Results obtained for both carbon nanotubes were different, highlighting the importance of dimensions of the same nanomaterial, and also the kind of agglomeration and shape adopted, for the toxic effects on organisms.
Keywords: Toxicology, Carbon nanotubes, Zebrafish, Ecotoxicology, Developmental toxicity, Immunotoxicity
1. Introduction
Nanomaterials (NMs) are technological products of increasing interest due to unique characteristics. Carbon nanotubes (CNTs) are nanomaterials composed of sheets of graphite; according to the amounts of sheets, they are called single-walled carbon nanotubes or multi-walled carbon nanotubes (MWCNTs). These structures exhibit exceptional stiffness, strength, toughness, exceptionally high thermal conductivity due to the high-frequency carbon-carbon bond vibrations and metallic-type electrical conductivity (He et al., 2013). Notably, it is the only element which bonds to itself in a network with the strength of the carbon-carbon bond. Because of the multiple potential physicochemical properties of CNTs, their applications in the industry is continuously increasing and nowadays encompass electronics, cosmetics, cleaning materials, coatings, food packaging (Souza-Junior et al., 2012). Besides, CNTs ability to be filled with different compounds positioned them among the most promising nanomaterials for biomedical applications. Examples include the use of CNTs as biosensors, in imaging techniques, tissue engineering, targeted therapies, as carriers for drugs and gene delivery and oncological therapies (Simon et al., 2019). The increase in the production and use of CNTs could lead to their accumulation in the layers of water, the source of human and animal consumption, making imperative the assessment of possible adverse implications for both environmental and human health.
Given that the physicochemical properties of compounds vary when they are produced at the nanoscale, and that the environmental systems are dynamic and stochastic, it is not easy to predict the hazards associated with the release of NMs and their products to the environment (Kahru and Dubourguier, 2010). Therefore, it is necessary to study the behavior of new technologies in ecosystems. In this sense, fish's responses can be used for studying the health status of an organism and ecosystem (Rangasamy et al., 2018). Zebrafish is a widely used model system in nanoecotoxicology due to its small size, high fertilization rate and rapid external development of transparent embryo (Hill et al., 2005).
Despite the fact that MWCNTs tend to agglomerate when they are introduced in an aqueous medium, the biological consequences of agglomeration have not been well investigated due to its complexity. Recently studies in vitro showed that the effect of a MWCNT depends on the state of agglomeration (Song et al., 2016). This work aimed to explore the biological response in vivo of zebrafish embryos and larvae, bioindicators of ecotoxicity in aquatic systems, against the exposure to MWCNTs of different dimensions and agglomeration pattern.
2. Materials and methods
2.1. Animal husbandry and ethics statement
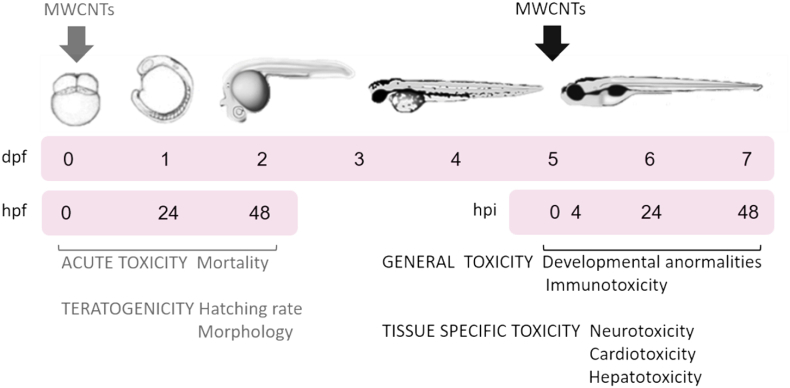
Zebrafish (Danio rerio) were kept as previously described (Prieto et al., 2012). In this study, “embryos” refer to zebrafish up to 3 days post-fertilization (dpf), while “larvae” refer to hatched animals over 3 dpf; the experimental design is shown in Fig. 1. Zebrafish procedures performed were approved by the corresponding Committees of the National University of Quilmes (CE-UNQ 2/2014, CICUAL-UNQ 013-15, 014-15, D001-17 and D002-17).
Fig. 1.
Experimental design: timeline. Zebrafish embryos of 4 h post-fertilization (hpf) (0 dpf) were incubated with MWCNTs at 0.005, 0.05, 0.5, 5 and 50 ppm final concentrations. Hatching, mortality and morphological changes were studied up to 2 dpf. On the other hand, zebrafish larvae of 5 dpf were incubated with MWCNTs at 0.005, 0.05, 0.5, 5 and 50 ppm final concentrations. Morphological changes, neurotoxicity, cardiotoxicity, hepatotoxicity and immunotoxicity were studied up to 7 dpf.
2.2. Preparation of carbon nanotubes suspensions and characterization
Multi-walled carbon nanotubes (MWCNTs) of different length and diameter were purchased from Sigma: MWCNT-S (hereafter referred to short, wide and mostly dispersed) have 110–170 nm diameter and 5–9 μm length (CAS no. 659258. Purity ≥95% based on trace metals analysis) while MWCNT-L (hereafter referred to long, thin and agglomerated) have 6–13 nm diameter and 2.5–20 μm length (CAS no. 698849. Purity ≥98% based on trace metals analysis). As our study aimed to analyse the consequence of the use of these commercial CNTs without modifications, as consumers would use it, we consider CNTs and the traces of their synthesis as a whole unit.
Pristine (non-functionalized) MWCNTs were weighed and added to distilled water in a 500 ppm final concentration. MWCNTs were homogenized by ultrasonication with a microtip (Mastersize 2000, Malvern Instruments Ltd) in an ice bath, through a program of two pulses of 10 s at 2 W and two pulses of 10 s at 10 W (adapted from (Cerrillo et al., 2015)). This program was done twice, and then zebrafish were incubated with the resulting dispersions.
With the aim of characterizing the MWCNTs, two microscopic techniques were performed. MWCNTs were analyzed by scanning electron microscopy (SEM) (HMS 6360 Lv, Jeol). For this purpose, MWCNTs suspensions were frozen (−80 °C overnight) and lyophilized in a Freezone 4.5 LABCONCO lyophilizer (LABCONCO, USA) at −50 °C maintaining the lyophilisation process pressure within the range of 33 × 10−3–65 × 10−3 mbar for 24 h (Igartúa et al., 2018b). Then, samples were adhered to the carrier (stub) with bifaz carbon tape and then metalized with gold 99.99% (JFC 1100, Jeol), thickness of 400 Å, to improve imaging. Also, MWCNTs were analyzed by transmission electron microscopy (TEM) (EM 109T, Zeiss). To this goal, samples sonicated were dropped onto the grids and dried directly in the membrane.
2.3. Zebrafish embryos: exposure to MWCNTs
Zebrafish embryos of 2 h post-fertilization (hpf) were transferred individually to a 96-well plate containing E3 medium. At 4 hpf, zebrafish were exposed to MWCNT-S, MWCNT-L diluted in E3 medium, or to E3 only (controls) for 2 days. Zebrafish were exposed to 0.005, 0.05, 0.5, 5 and 50 ppm MWCNT-S or MWCNT-L. Embryos were reared at 28 °C ± 0.5 on a 14/10 h light/dark cycle. For all the assays, the same embryos were used. Sixteen technical replicates (embryos with the same treatment within the same plate) and three biological replicates (embryos with the same treatment but performed at a different day and mating) were done (n = 48).
2.4. Zebrafish embryos: hatching, morphology and mortality
Embryonic development was monitored from 4 to 48 hpf using a stereomicroscope Nikon SMZ800. Hatching was measured at different points from 36 to 48 hpf and determined as a percentage of hatched embryos respect to the total of viable embryos at 48 hpf. Mortality was observed though endpoints -absence of heartbeats or coagulation- at 7, 24 and 48 hpf, and expressed as a percentage of dead embryos respect to total embryos at 48 hpf. Besides, teratogenicity of MWCNTs was studied through the analysis of sublethal parameters comprising abnormality of bent spine, tail, yolk, head, eyes or heart, reduction of pigmentation and delay of hatching. Embryos were designated as normal if none of the endpoints was observed, as malformed if one or more endpoints were observed, or as deceased if coagulation or no heartbeat was detected (Igartúa et al., 2018a; Martinez et al., 2018).
2.5. Zebrafish larvae: exposure to MWCNTs
Zebrafish embryos of 1 dpf were transferred to a 96-well plate containing E3 medium; three eggs per well. At 5 dpf, zebrafish were exposed to MWCNT-S, MWCNT-L diluted in E3 medium, or to E3 only (controls) for 2 days. The administration was done at 5 dpf, when organs are fully developed, allowing the study of the effects on formed and functional organs -biocompatibility and toxicity- instead of teratogenicity as at embryo stages (de Esch et al., 2012). Zebrafish were exposed to 0.005, 0.05, 0.5, 5 and 50 ppm MWCNT-S or MWCNT-L. Larvae were reared at 28 °C ± 0.5 on a 14/10 h light/dark cycle. For cardiac activity assay, sixteen technical replicates and three biological replicates (n = 48) were done. For the rest of the assays, eight technical replicates and three biological replicates (n = 24) were done.
2.6. Zebrafish larvae: neuro and cardiac activity
The locomotor activity was studied through the spontaneous movements of larvae of 5 and 7 dpf, at 4 and 48 h post-incubation (hpi) respectively, in a multichannel ADC system (WMicrotracker, Designplus SRL) with infrared beams that are interrupted by larvae swimming activity (Igartúa et al., 2015; Feas et al., 2017; Martinez et al., 2018). The cardiac rhythm was analyzed through the heart beats of larvae of 7 dpf. Animals were immobilized in slides with 2% sodium carboxymethylcellulose (CAS no. 21902, Sigma), placed under a zoom stereomicroscope (SMZ800, Nikon) and recorded in parasagittal orientation (Feas et al., 2017; Igartúa et al., 2018b).
2.7. Zebrafish larvae: morphology
Animals used for heart rate assays were also involved in morphological abnormalities tests. Larvae were photographed in parasagittal orientation at 60× magnification. The photomicrographs were observed for several morphological alterations as bent spine, jaw malformation, opaque head region, small head, opaque liver, opaque yolk salc, yolk not depleted, uninflated swim bladder, edema and tail malformation. Zebrafish were scored based on the degree of morphological anomalies [0 = no visible toxic effects; 1 = minor, one to two morphological anomalies; 2 = moderate, three to four effects; 3 = severe, more than four minor toxic effects; and 4 = dead] (adapted from (Fako and Furgeson, 2009)). The mean toxicity score for each treatment was determined by a score of individual larvae.
A separate group of larvae of 7 dpf were fixed in 4% paraformaldehyde, bleached in 10% hydrogen peroxide, 5% formamide and 0.5× SSC buffer, and then incubated in alcian blue solution (70% ethanol, 0.37% hydrochloric acid and 0.1% alcian blue; CAS no. 5268, Sigma) (Martinez et al., 2018). Larvae were photographed in parasagittal orientation at 60× magnification. Craniofacial cartilages measurements were determined using the ImageJ software (National Institutes of Health, USA) (de Peralta et al., 2016).
2.8. Zebrafish larvae: histopathology
Anesthetized animals of 7 dpf were fixed in 4% paraformaldehyde, embedded in agar 1.5% and sucrose 10% and placed into plastic molds. The blocks obtained were cryoprotected in a 30% sucrose solution and frozen. Parasagittal sections were cut in 10 μm in a Leica CM 1850 cryostat (Leica Microsystems, Nussloch, Germany). Histological sections of 10 larvae per treatment and control were performed (Prieto et al. 2012, 2014). Slides were stained with hematoxylin and eosin following standard procedures, and then mounted in Canadian balsam (no. 1302.05, Biopack) for analysis and storage. Images of samples stained were taken with a light microscope (BX41, Olympus) at 400× magnification.
2.9. Zebrafish larvae: neutrophil migration activity
Larvae of 7 dpf were anesthetized with 0.2 mg/ml tricaine and the tail was transected near its tip. Then larvae were placed in fresh E3 medium until analysis at different time points after trauma, in which larvae were fixed overnight in 4% paraformaldehyde. Larvae were incubated with benzidine dihydrochloride (cat. B-3383, Sigma) to dye myeloperoxidase in neutrophils based on the method of Kaplow (Lieschke et al., 2001; Cordero-Maldonado et al., 2013). Controls done included the stain of larvae incubated only with E3 medium, stain of larvae before trauma and stain of larvae immediately after trauma. Larvae were photographed in ventral orientation at 60× magnification. Dark staining peroxidize-positive cell was quantified with the image-J software.
2.10. Statistical analysis
Statistical analysis was performed by ONE-WAY ANOVA or TWO-WAY ANOVA tests followed by Dunnett's multiple comparison post-test using the GraphPad Prism statistical program. Data was considered statistically significant if P < 0.05; it was presented as a mean ± standard error of the mean (SEM).
3. Results and discussion
The purpose of this study was to underline the in vivo effects of the nanomaterial MWCNTs with different dimensions –length and diameter- and agglomeration pattern on zebrafish. Studies were conducted using 4 hpf embryos and 5 dpf larvae that were incubated with MWCNT-S or MWCNT-L for 48 h. Results presented here might predict possible both acute and chronic toxicity.
3.1. Characterizations of MWCNTs
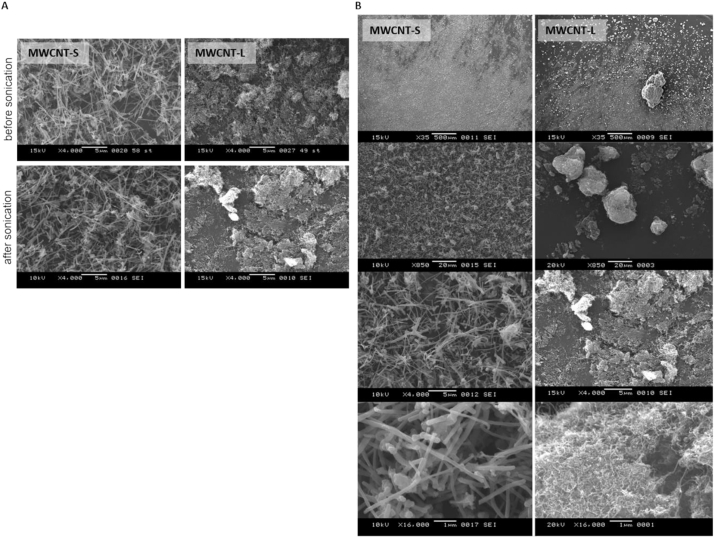
The MWCNTs were analyzed through two kinds of microscopy, SEM for information on the surface and TEM for analysis of the inner structure of the sample. In SEM analysis, MWCNT-S were mostly dispersed in the medium, while MWCNT-L formed bundles (Fig. 2). The fact that MWCNT-L exhibited bundles is common sense since nanotubes are usually hydrophobic and these nanotubes are very long, so Van der Waals attraction forces among tubes are significant (Gao et al., 2003).
Fig. 2.
SEM micrographs of MWCNTs. MWCNT-S and MWCNT-L at 50 ppm concentration were dispersed in distilled water by sonication and then dried by lyophilization. Representative micrographs of MWCNTs before (A) and after (A and B) sonication of different magnifications are shown.
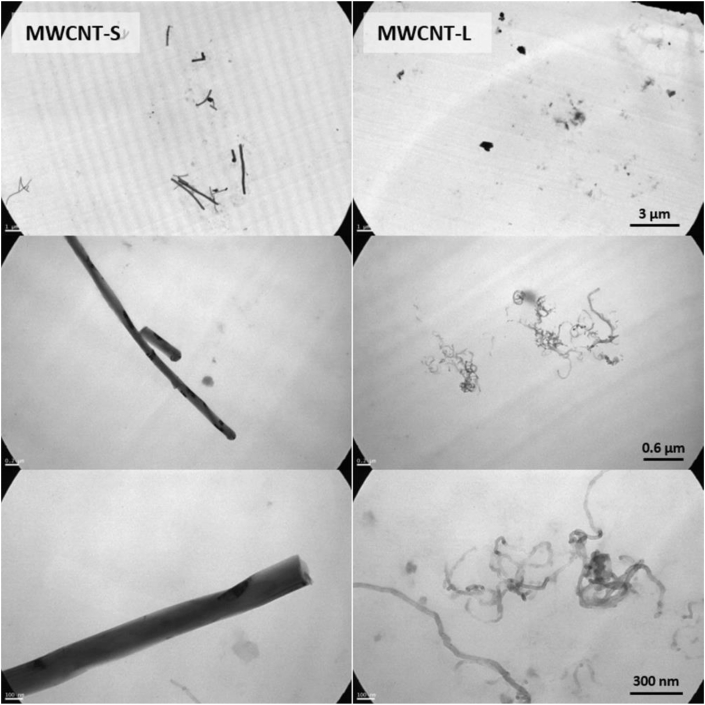
In TEM pictures, in which magnified images were obtained, dimensions were measured. For MWCNT-S, tubes length ranged between 0.6 and 3.7 um and diameter between 100 and 200 nm. For MWCNT-L, diameter ranged between 5 and 10 nm (Fig. 3). We were unable to precisely calculate the lengths due to the bundles. The data collected after sonication coincide with manufacturer information, showing that the procedure did not affect the MWCNTs significantly.
Fig. 3.
TEM micrographs of MWCNTs. MWCNT-S and MWCNT-L at 50 ppm concentration were dispersed in distilled water by sonication and then placed in TEM grids. Representative micrographs of different magnifications are shown.
Differences in size and agglomeration pattern may cause a diverse response in an organism. In fact, recent literature reported that longer MWCNTs were more cytotoxic than shorter, and it was suggested that this effect was due to a role in oxidative stress (Long et al., 2017). As well, it was reported that a MWCNT produced the generation of oxidative stress in fishes (Cimbaluk et al., 2018). For this reason, our in vivo work will help to deepen the understanding of the implications of the differences in dimensions and agglomeration pattern for the same nanomaterial.
3.2. Hatching, mortality and morphology of zebrafish embryos
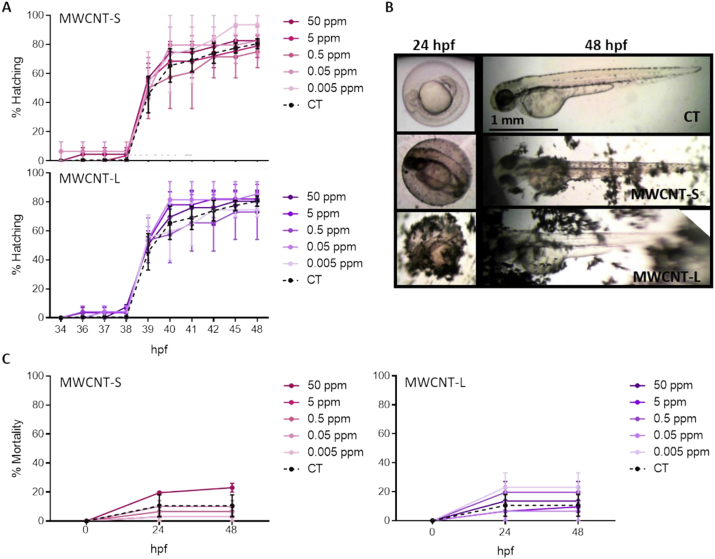
We studied the potential teratogenicity of MWCNT-S and MWCNT-L. Hatching rate was studied from 4 to 48 hpf; other toxicological endpoints were analyzed at 24 and 48 hpf. Normal hatching was observed for both MWCNTs when compared to controls (Fig. 4A), as well as the normal morphology of embryos (Fig. 4B). This is in agreement with previous works that reported a decreased hatching rate and developmental abnormalities only with concentrations of MWCNTs higher than 60 ppm (Cheng et al., 2007; Asharani et al., 2008; Liu et al., 2014). Embryos are protected by a chorion, which has pores necessary for oxygen and nutrient transport from the outer environment and the elimination of wastes. We observed agglomerates of MWCNTs adhered to the outer layer of the chorion of the embryos, indicating they were too large to enter the chorion and reach the embryos (Fig. 4B); the accumulation was greater with higher concentrations of nanotubes (data not shown). Also, these results are in accordance with earlier works (Asharani et al., 2008; Pelka et al., 2017), which analyzed embryos of 24 hpf and reported size of the pores of 0.23 μm. Since MWCNT-S and MWCNT-L have lengths greater than 2.5 μm, and they have a considerable tendency to agglomerate, it is not possible to cross through the chorion. We observed that deposition of MWCNTs on the surface of the chorion did not exert a mechanic inhibition on embryo hatching either. When mortality was studied, no reduction of survival was observed (Fig. 4C). Morphology was not altered in embryos exposed to MWCNT-S or MWCNT-L at 24 hpf nor 48 hpf (data not shown). Even though the nanotubes were not able to cross the chorion, their accumulation around might prevent the regular passage of oxygen and nutrient. Nonetheless, our results showed a different situation since embryos developed with normality. Results so far indicate that exposition to MWCNTs did not alter embryos development in concentrations up to 50 ppm.
Fig. 4.
Hatching and mortality of embryos exposed to MWCNTs. Zebrafish embryos of 4 hpf were incubated with the 0.005, 0.05, 0.5, 5 and 50 ppm of MWCNT-S or MWCNT-L. (A) Hatching was observed until 48 hpf. Results are expressed as the percentage of hatched embryos respect to total embryos. (B) Representative photographs of hatched and non-hatched zebrafish exposed to 50 ppm of MWCNTs are shown. (C) Cumulative mortality curves were constructed from 0 to 48 hpf. Results are expressed as the percentage of dead embryos respect to total embryos. Values are shown as mean ± SEM. Values are shown as mean ± SEM. Significant differences respect to control were analyzed by TWO-WAY ANOVA test followed by Dunnett's multiple comparisons post-test.
3.3. Neuro and cardiac activity of zebrafish larvae
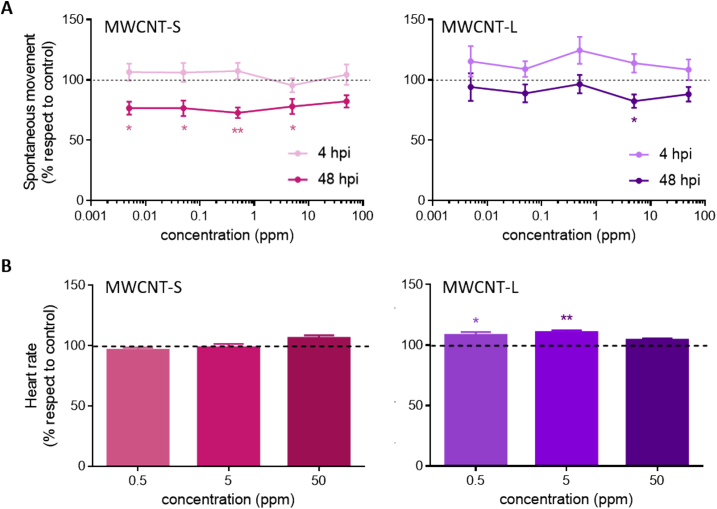
In order to determine if MWCNTs were capable of causing neurotoxicity, the spontaneous movement of zebrafish was studied. Larvae exposed to MWCNT-S showed depression of locomotor activity compared to controls at all concentrations studied except for 50 ppm, this last could be due to bigger agglomerates unable to enter into the zebrafish because of the size. On the other hand, larvae incubated with MWCNT-L showed altered movement at 5 ppm but not at lower concentrations. At 50 ppm, results were similar to those observed for MWCNT-S (Fig. 5A). The fact that spontaneous movement was reduced in larvae exposed to MWCNTs, but animals were alive, means there is affection at the neuronal level or morphology anomalies that do not allow the animal to swim properly.
Fig. 5.
Neuro and cardiac activity of larvae exposed to MWCNTs. Zebrafish larvae of 5 dpf were incubated with 0.005, 0.05, 0.5, 5 and 50 ppm of MWCNT-S and MWCNT-L for 48 h (hpi). (A) Swimming activity was measured. Results are expressed as the percentage of spontaneous movement respect to control. (B) Cardiac rhythm was quantified. Results are expressed as the percentage of heart rate respect to control. Values are shown as mean ± SEM. Significant differences respect to control were analyzed by ONE-WAY ANOVA test followed by Dunnett's multiple comparisons post-test (*p < 0.05, **p < 0.01).
The effect of MWCNTs on the heart activity of zebrafish larvae was analyzed by heart rate measurement and blood circulation observation through the ventral aorta posterior cardinal vein channel. Larvae exhibited similar results to the control group at all concentrations studied for MWCNT-S (Fig. 5B). When zebrafish exposed to MWCNT-L were analyzed, an increased heart rate was observed in those exposed to 0.5 and 5 ppm but not in those exposed to 50 ppm, the same profile observed in locomotor activity. To our knowledge, this is the first work that studied the heart rate of larvae older than 5 dpf exposed to MWCNTs, which is critical to analyze the effect of the nanomaterials over a formed and fully functional heart. So far, it was reported that MWCNTs did not cause an alteration of the cardiac rhythm in earlier life stages of zebrafish exposed to them (Liu et al., 2014; Wang et al., 2017). However, we have now shown an effect once the organ is entirely functional.
3.4. Morphology of zebrafish larvae
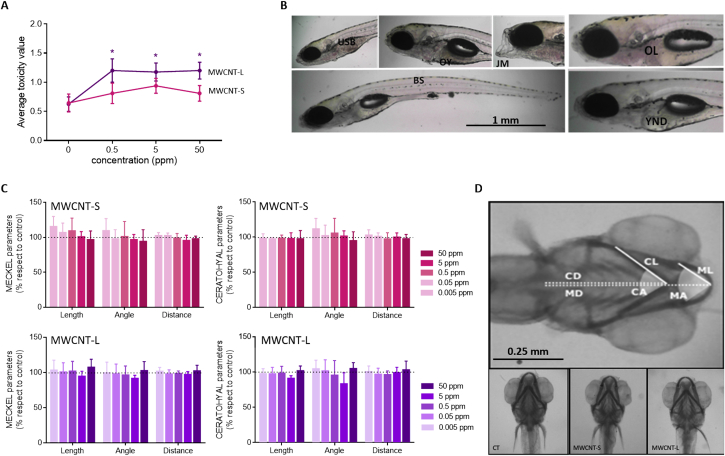
Larvae treated with MWCNT-S did not exhibit morphological alterations compared to controls, while animals incubated with MWCNT-L showed developmental abnormalities at all concentrations studied (Fig. 6A,B). Results for animals exposed to MWCNT-S discard the possibility that the decrease in the locomotor activity observed before was due to morphology anomalies, instead it must have a neurological origin. Further consequences of exposition to MWCNTs were studied concerning cartilage development. Craniofacial cartilages analyzed here were Meckel's and Ceratohyal, and the parameters included were angle, length, and distance to the fins (Fig. 6D). None of the parameters exhibited differences compared to controls (Fig. 6C). These results reinforce that the effect of MWCNT-S on the reduction of movement observed was due to neurotoxicity.
Fig. 6.
Morphology abnormalities of larvae exposed to MWCNTs. Zebrafish larvae of 5 dpf were incubated with 0.5, 5 and 50 ppm of MWCNT-S or MWCNT-L for 48 h; (A) larvae were scored based on the severity of their morphological defects. Scores range for morphological anomalies defects are: 0 for no visible toxic effects, 1 for minor degree (one to two effects), 2 for moderate (three to four effects) and 3 for severe (more than four effects). Sublethal defects studied were bent spine (BS), jaw malformation (JM), opaque head region (OH), opaque liver (OL), opaque yolk salc (OY), uninflated swim bladder (USB), edema (E), small head (SH), tail malformation (TM), yolk not depleted (YND). (B) Representative pictures of endpoints included in the scoring evaluation are shown. (C) Craniofacial parameters were analyzed. Meckel's cartilage length (ML), angle (MA), distance to fins (MD) and Ceratohyal cartilage length (CL), angle (CA), distance to fins (CD) were quantified and expressed as percentage respect to control larvae. (D) Ventral view of representative pharyngeal skeletons of MWCNT-incubated and control larvae are shown. Values are shown as mean ± SEM. Statistical analysis was performed by ONE-WAY ANOVA test followed by Dunnett's multiple comparisons post-test (*p < 0.05).
3.5. Tissue sections
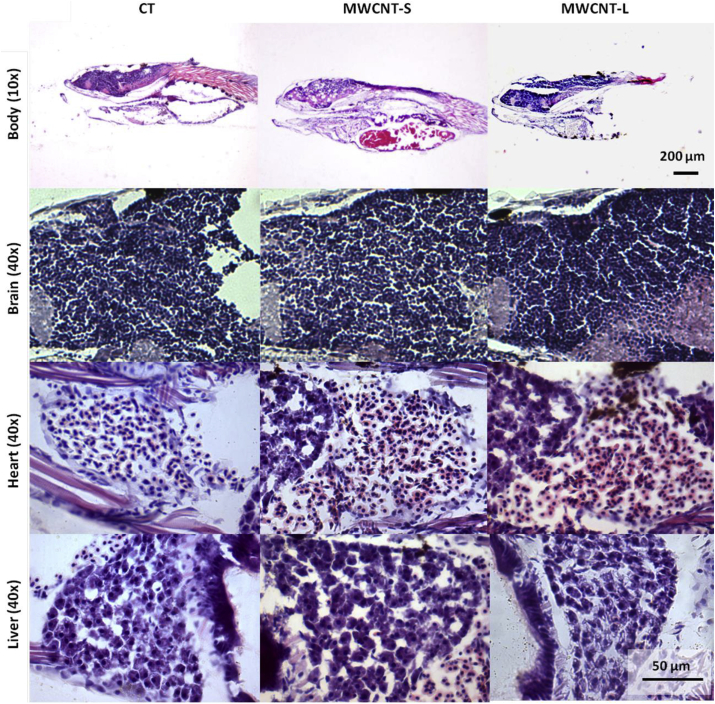
Histopathology of brain, heart and liver of larvae was performed to analyze cells size and morphology of larvae exposed to MWCNTs. For this purpose, parasagittal orientation slides were stained with hematoxylin-eosin. No differences were observed between zebrafish larvae exposed to MWCNTs and controls in any tissue (Fig. 7). Results so far reveal that short exposition to MWCNTs affects the activity of the cells of brain and heart but not their morphology.
Fig. 7.
Histopathology of larvae exposed to MWCNTs. Zebrafish larvae were incubated with 0.005, 0.05, 0.5, 5, and 50 ppm of MWCNT-S and MWCNT-L for 48 h; brain, heart and liver phenotype (cells size and shape) were analyzed. Representative photomicrographs of hematoxylin and eosin staining of the whole body and tissue sections are shown.
3.6. Migration activity of neutrophils in zebrafish larvae
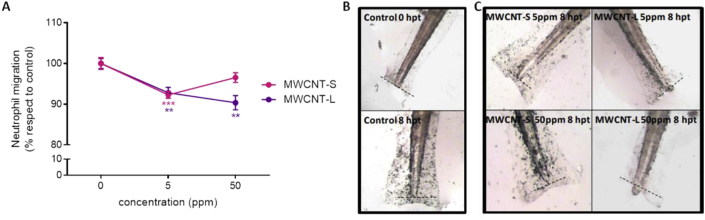
In zebrafish, innate and adaptive immune responses mature at different time points; while the innate system is detected at 1 dpf, the adaptive system appears several weeks after the fertilization of the egg (Novoa and Figueras, 2012). Inflammation is triggered when innate immune cells detect an injury; neutrophils are the first leukocytes to be recruited to the wound, accumulating in large numbers a few hours after damage and then returning to pre-damage density levels when the tissue cells begin to proliferate, ending the inflammatory phase and beginning regeneration (Ferreira, 2018). The effect of MWCNTs on the innate inflammatory response was studied through the amputation of the caudal fin of zebrafish larvae of 5dpf and the following chemotactic activity of neutrophils towards the injury. Larvae were transected at the tip and the migration of neutrophils after traumatization was followed by myeloperoxidase staining in them. For this assay, only 5 and 50 ppm of MWCNTs were studied since results so far did not show effects in larvae incubated with lower concentrations.
Before and immediately after the trauma, there was no neutrophil presence at the trauma site in any of the conditions assessed (data not shown). At 8 h post-trauma, larvae control exhibited a population of stained cells accumulated in the ventral vein region and more marked at the site of acute inflammation. Larvae exposed to MWCNT-S exhibited a decrease of the neutrophil migration to the site of inflammation at 5 ppm when compared to controls, but not at 50 ppm, which could be due to agglomerates unable to enter into the zebrafish because of the size. Larvae exposed to MWCNT-L showed affection at both concentrations (Fig. 8). As seen in the assays reported above, the lack of effect in 50 ppm MWCNT-S could be due to agglomerates unable to go into the zebrafish. To our knowledge, this is the first work that studied the migration activity of neutrophils in zebrafish larvae exposed to carbon nanotubes.
Fig. 8.
Migration activity of neutrophils in zebrafish larvae exposed to MWCNTs. Zebrafish larvae were incubated with 5 and 50 ppm of MWCNT-S and MWCNT-L for 48 h, then transection of the tail tip was performed. Immediately and 8 h post-transection, myeloperoxidase histochemical staining of larvae was done. (A) Peroxidize-positive cell were quantified. Results are expressed as the percentage of neutrophil migration respect to control. Values are shown as mean ± SEM. Significant differences respect to control were analyzed by ONE-WAY ANOVA test followed by Dunnett's multiple comparisons post-test (**p < 0.01, ***p < 0.001). Representative photographs of controls (B) and MWCNT-incubated larvae are shown (C). Dash line (--) indicates transection site.
In summary, MWCNT-S were mostly dispersed in the medium, while MWCNT-L formed bundles. Noteworthy, the medium in which MWCNTs are immersed affects their dimension since intra- and inter-nanotubes interaction change, modifying the tendency to form agglomerates. In the present work, the studies were carried out in aqueous media, the most common environment where the MWCNT could be found. MWCNTs did not reduce the survival of embryos, and neither altered embryos development at concentrations up to 50 ppm. When functions at neuro and cardiac levels were studied in zebrafish larvae, results varied for both MWCNTs. While exposition to MWCNT-S caused a depression of the locomotor activity of larvae, exposition to MWCNT-L only had effect at 5 ppm. The alteration observed could lead to a modified behavior, putting animal survival at risk, or could cause a long-term detrimental effect. On the other hand, heart rate of larvae was not affected by MWCNT-S whereas the cardiac rhythm was increased by MWCNT-L at 5 ppm concentrations. Then, the morphology of the growing larvae exposed to MWCNTs was assessed. Larvae treated with MWCNT-S did not exhibit morphological alterations, while those exposed to MWCNT-L showed enhanced developmental abnormalities. Further consequences of exposition to MWCNTs were studied with regard to craniofacial development. The craniofacial phenotype was analyzed, and it was not distorted for larvae exposed to MWCNTs. In addition, cell damage was analyzed in brain, heart, and liver but no alterations were observed in larvae exposed to MWCNTs in any tissue. This means that acute exposition to MWCNTs affects the activity of the cells mentioned but not their morphology. Finally, the innate inflammatory response was evaluated. A depression of the neutrophil migration activity was observed in larvae treated with both MWCNTs, although the effect was different in each case depending on the concentration.
4. Conclusions
The biological response of zebrafish exposed to carbon nanotubes with different size and agglomeration pattern was evaluated. Exposition of MWCNTs to embryos did not cause lethality or teratogenicity. Larvae exposed to short and dispersed MWCNTs in the aquatic environment developed neurotoxicity and immunotoxicity, whereas those exposed to long and agglomerated MWCNTs presented developmental malformations, cardiotoxicity, and immunotoxicity, showing that the adverse effects of MWCNTs depend on both dimension and agglomeration pattern. Since recent evidence showed that longer MWCNTs were more cytotoxic due to a role in oxidative stress, it will be interesting as a future perspective to study the presence of reactive oxygen species in zebrafish exposed to MWCNTs used here, to get a better understanding of the toxicity mechanisms of these nanomaterials.
Declarations
Author contribution statement
Martinez CS: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Igartúa DE, Czarnowski I, Feas DA: Performed the experiments.
Alonso S.delV: Contributed reagents, materials, analysis tools or data.
Prieto MJ: Analyzed and interpreted the data.
Funding statement
This work was supported by the Universidad Nacional de Quilmes (UNQ) (PUNQ 1388/15 and 1076/15) and the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) (PICT-CABBIO 0511/14).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Carolina Soledad Martinez, María Jimena Prieto and Silvia del Valle Alonso are members of CONICET, Argentina. Daniela Edith Igartúa acknowledge doctoral fellowships from CONICET, Argentina. Ian Czarnowski acknowledge fellowships from CIN, Argentina. We thank Laboratory of Chronobiology of the National University of Quilmes, for their technical support with the light microscope and the cryostat.
References
- Asharani P.V. Impact of multi-walled carbon nanotubes on aquatic species. J. Nanosci. Nanotechnol. 2008;8(7):3603–3609. doi: 10.1166/jnn.2008.432. [DOI] [PubMed] [Google Scholar]
- Cerrillo C. Ecotoxicity of multiwalled carbon nanotubes: standardization of the dispersion methods and concentration measurements. Environ. Toxicol. Chem. 2015;34(8):1854–1862. doi: 10.1002/etc.2999. [DOI] [PubMed] [Google Scholar]
- Cimbaluk G.V. Evaluation of multiwalled carbon nanotubes toxicity in two fish species. Ecotoxicol. Environ. Saf. 2018;150:215–223. doi: 10.1016/j.ecoenv.2017.12.034. [DOI] [PubMed] [Google Scholar]
- Cordero-Maldonado M.L. Optimization and pharmacological validation of a leukocyte migration assay in zebrafish larvae for the rapid in vivo bioactivity analysis of anti-inflammatory secondary metabolites. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0075404. e75404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J. Effect of carbon nanotubes on developing zebrafish (Danio rerio) embryos. Environ. Toxicol. Chem. 2007;26(4):708–716. doi: 10.1897/06-272r.1. [DOI] [PubMed] [Google Scholar]
- de Esch C. Zebrafish as potential model for developmental neurotoxicity testing: a mini review. Neurotoxicol. Teratol. 2012;34(6):545–553. doi: 10.1016/j.ntt.2012.08.006. [DOI] [PubMed] [Google Scholar]
- de Peralta M.S. Cnbp ameliorates Treacher Collins Syndrome craniofacial anomalies through a pathway that involves redox-responsive genes. Cell Death Dis. 2016;7(10):e2397. doi: 10.1038/cddis.2016.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fako V.E., Furgeson D.Y. Zebrafish as a correlative and predictive model for assessing biomaterial nanotoxicity. Adv. Drug Deliv. Rev. 2009;61(6):478–486. doi: 10.1016/j.addr.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Feas D.A. Nutraceutical emulsion containing valproic acid (NE-VPA): a drug delivery system for reversion of seizures in zebrafish larvae epilepsy model. J. Pharm. Investig. 2017:1–9. [Google Scholar]
- Ferreira M.G. 2018. Analysis of Neutrophil Recruitment during Tissue Regeneration in Zebrafish Models of Human Disease. [Google Scholar]
- Gao H. Spontaneous insertion of DNA oligonucleotides into carbon nanotubes. Nano Lett. 2003;3(4):471–473. [Google Scholar]
- He H. Carbon nanotubes: applications in pharmacy and medicine. BioMed Res. Int. 2013;2013:578290. doi: 10.1155/2013/578290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A.J. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005;86(1):6–19. doi: 10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- Igartúa D.E. Folic acid magnetic nanotheranostics for delivering doxorubicin: toxicological and biocompatibility studies on Zebrafish embryo and larvae. Toxicol. Appl. Pharmacol. 2018 doi: 10.1016/j.taap.2018.09.009. [DOI] [PubMed] [Google Scholar]
- Igartúa D.E. Development of nutraceutical emulsions as risperidone delivery systems: characterization and toxicological studies. J. Pharm. Sci. 2015;104(12):4142–4152. doi: 10.1002/jps.24636. [DOI] [PubMed] [Google Scholar]
- Igartúa D.E. PAMAM dendrimers as a carbamazepine delivery system for neurodegenerative diseases: a biophysical and nanotoxicological characterization. Int. J. Pharm. 2018;544(1):191–202. doi: 10.1016/j.ijpharm.2018.04.032. [DOI] [PubMed] [Google Scholar]
- Kahru A., Dubourguier H.C. From ecotoxicology to nanoecotoxicology. Toxicology. 2010;269(2-3):105–119. doi: 10.1016/j.tox.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Lieschke G.J. Morphologic and functional characterization of granulocytes and macrophages in embryonic and adult zebrafish. Blood. 2001;98(10):3087–3096. doi: 10.1182/blood.v98.10.3087. [DOI] [PubMed] [Google Scholar]
- Liu X.T. Toxicity of multi-walled carbon nanotubes, graphene oxide, and reduced graphene oxide to zebrafish embryos. Biomed. Environ. Sci. 2014;27(9):676–683. doi: 10.3967/bes2014.103. [DOI] [PubMed] [Google Scholar]
- Long J. The adverse vascular effects of multi-walled carbon nanotubes (MWCNTs) to human vein endothelial cells (HUVECs) in vitro: role of length of MWCNTs. J. Nanobiotechnol. 2017;15(1):80. doi: 10.1186/s12951-017-0318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez C. In vivo study of teratogenic and anticonvulsant effects of antiepileptics drugs in zebrafish embryo and larvae. Neurotoxicol. Teratol. 2018;66:17–24. doi: 10.1016/j.ntt.2018.01.008. [DOI] [PubMed] [Google Scholar]
- Novoa B., Figueras A. Current Topics in Innate Immunity II. Springer; 2012. Zebrafish: model for the study of inflammation and the innate immune response to infectious diseases; pp. 253–275. [DOI] [PubMed] [Google Scholar]
- Pelka K.E. Size does matter–Determination of the critical molecular size for the uptake of chemicals across the chorion of zebrafish (Danio rerio) embryos. Aquat. Toxicol. 2017;185:1–10. doi: 10.1016/j.aquatox.2016.12.015. [DOI] [PubMed] [Google Scholar]
- Prieto M.J. Optimization and in vivo toxicity evaluation of G4. 5 PAMAM dendrimer-risperidone complexes. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0090393. e90393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto M.J. Effect of risperidone and fluoxetine on the movement and neurochemical changes of zebrafish. Open J. Med. Chem. 2012;2(4):129–138. [Google Scholar]
- Rangasamy B. Developmental toxicity and biological responses of zebrafish (Danio rerio) exposed to anti-inflammatory drug ketoprofen. Chemosphere. 2018 doi: 10.1016/j.chemosphere.2018.09.013. [DOI] [PubMed] [Google Scholar]
- Simon J. Overview of carbon nanotubes for biomedical applications. Materials. 2019;12(4):624. doi: 10.3390/ma12040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z.-M. Biological effects of agglomerated multi-walled carbon nanotubes. Colloids Surfaces B Biointerfaces. 2016;142:65–73. doi: 10.1016/j.colsurfb.2016.02.032. [DOI] [PubMed] [Google Scholar]
- Souza-Junior E.J. Selective enamel etching: effect on marginal adaptation of self-etch LED-cured bond systems in aged Class I composite restorations. Oper. Dent. 2012;37(2):195–204. doi: 10.2341/11-184L. [DOI] [PubMed] [Google Scholar]
- Wang S. The presence of MWCNTs reduces developmental toxicity of PFOS in early life stage of zebrafish. Environ. Pollut. 2017;222:201–209. doi: 10.1016/j.envpol.2016.12.055. [DOI] [PubMed] [Google Scholar]