Abstract
The social impairments of autism spectrum disorder (ASD) have a major impact on quality of life, yet there are no medications that effectively treat these core social behavior deficits. Preclinical research suggests that arginine vasopressin (AVP), a neuropeptide involved in promoting mammalian social behaviors, may be a possible treatment for ASD. Using a double-blind, randomized, placebo-controlled, parallel study design, we tested the efficacy and tolerability of a 4-week intranasal AVP daily treatment in 30 children with ASD. AVP-treated participants aged 6 to 9.5 years received the maximum daily target dose of 24 International Units (IU); participants aged 9.6 to 12.9 years received the maximum daily target dose of 32 IU. Intranasal AVP treatment compared to placebo enhanced social abilities as assessed by change from baseline in this phase 2 trial’s primary outcome measure, the Social Responsiveness Scale, 2nd Edition total score (SRS-2 T score; F1,20 = 9.853; P = 0.0052; ηp2 = 33.0%; Cohen’s d = 1.40). AVP treatment also diminished anxiety symptoms and some repetitive behaviors. Most of these findings were more pronounced when we accounted for pretreatment AVP concentrations in blood. AVP was well tolerated with minimal side effects. No AVP-treated participants dropped out of the trial, and there were no differences in the rate of adverse events reported between treatment conditions. Last, no changes from baseline were observed in vital signs, electrocardiogram tracings, height and body weight, or clinical chemistry measurements after 4 weeks of AVP treatment. These preliminary findings suggest that AVP has potential for treating social impairments in children with ASD.
INTRODUCTION
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by social impairments (e.g., diminished eye gaze, abnormal face and emotion processing, and impaired social judgment) and the presence of restricted, repetitive behaviors (1). Although several medications are approved for the treatment of ASD (i.e., the antipsychotic drugs risperidone and aripiprazole), they have negative side effects (e.g., lethargy and weight gain), target only associated behaviors (e.g., irritability), and are ineffective in ameliorating ASD’s core social features. Research that identifies the underlying biology of social impairments and tests medications that directly target these pathways is therefore urgently needed (2).
An accumulating body of research points to the arginine vasopressin (AVP) signaling pathway as a promising ASD target. It has been known for several decades that AVP plays a critical role in promoting mammalian social behavior (3) and that dysregulation of the AVP signaling pathway produces social deficits in rodents (4, 5). Researchers have begun to translate these preclinical findings to evaluate the effects of intranasal AVP administration on social and cognitive abilities in adult humans. These studies have shown that single doses of intranasal AVP enhance a variety of social abilities, including memory for emotional faces (6), identification of social words (7), and cooperative behavior (8) in healthy individuals. Single doses of intranasal AVP have also been shown to enhance speech and word formation in patients with poststroke aphasia (9) and to improve short- and long-term memory in patients with central diabetes insipidus (10), suggesting that this compound can boost cognition. Although the precise central mechanisms by which intranasally administered AVP and other neuropeptides achieve behavioral effects remain to be determined (11), intranasal AVP administration results in elevated cerebrospinal fluid (CSF) concentrations of AVP, suggesting that intranasally administered AVP achieves access to the central nervous system (12).
Despite scientific evidence documenting the prosocial and cognitive-enhancing effects of AVP administration, the use of intranasal AVP for treating individuals with ASD has not been tested. Several lines of evidence further underscore the necessity of such research. First, our group recently reported that children with ASD have lower CSF AVP concentrations compared to control children and that patients with ASD with the lowest CSF AVP concentrations have the most severe symptoms (13, 14). Second, animal models demonstrate that AVP’s prosocial effects are largely mediated through AVP1A receptors (AVPR1A) (15–17), which, in primates, are widely distributed throughout the brain (in regions such as the anterior cingulate cortex, amygdala, bed nucleus of the stria terminalis, and insular cortex) (18). This suggests that AVP administration can target neural pathways known to regulate social behavior. Last, AVP’s pharmacological effects are especially evident in male animals (15, 19), and given ASD’s male-biased prevalence (20), the AVP signaling pathway may be particularly relevant to understanding the risk for, and treatment of, ASD.
Here, we used a double-blind, randomized, placebo-controlled, parallel design to test the efficacy and tolerability of 4-week intranasal administration of AVP to children with ASD aged 6 to 12 years. Our central hypothesis was that AVP compared to placebo treatment would improve social abilities in children with ASD and that pretreatment measures of neuropeptide biology (e.g., pretreatment AVP concentrations in blood) would predict treatment response.
The primary outcome measure in this pilot trial was change from baseline in social ability as determined by parent ratings on the Social Responsiveness Scale, 2nd Edition (SRS-2) total score, expressed as a gender-normed T score. Secondary outcome measures included change from baseline in social communication and social cognition abilities as assessed by clinician evaluation and child performance on laboratory-based tests, respectively. We also tested whether AVP treatment ameliorated other core or associated symptoms of ASD using parent rating scales, and whether AVP was safe and well tolerated in individuals with ASD, given that limited safety and tolerability data are available for repeated intranasal administration of AVP in humans, especially in children.
RESULTS
Participant characteristics, blinding procedures, and dosing compliance
A Consolidated Standards of Reporting Trials (CONSORT) flow diagram is presented in Fig. 1. An a priori power analysis determined that N = 30 was a sufficient sample size for our planned analyses. A total of N = 30 participants (25 male, 5 female) completed the 4-week intranasal AVP treatment trial. Participant demographic and phenotypic characteristics are presented in Table 1 and table S1. Participants’ stable concomitant medications, which did not differ between treatment conditions, are presented in table S2.
Fig. 1. The CONSORT flow diagram for the phase 2 clinical trial.
The CONSORT flow diagram details the progress, from screening through to study completion, of participants in the double-blind randomized placebo-controlled trial testing a 4-week intranasal vasopressin treatment versus placebo in children with ASD. A total of 149 prospective participants were screened. Thirty-eight of the 68 individuals enrolled in the study were not randomized owing to either not meeting eligibility criteria or declining to participate. All 30 participants who were randomized to a treatment condition or placebo completed the study, independent of whether they were in the vasopressin or placebo group.
Table 1. Characteristics of participants in the study.
Fisher’s exact test was used to test whether the distribution of individuals randomized to the treatment conditions differed by sex and by ethnicity; no significant effects were found. For age, IQ, pretreatment SRS-2 T score, pretreatment CGI-S score, pretreatment blood AVP concentration (in picograms per milliliter), and pretreatment blood AVPR1A:OXTR gene expression, differences between treatment conditions were tested with a simple one-way general linear model; no significant effects were discerned. The values are reported as means ± SE. F, female; M, male; IQ, Intelligence Quotient; SRS-2, Social Responsiveness Scale, 2nd Edition; CGI-S, Clinical Global Impression-Severity; AVP, arginine vasopressin AVPR1A:OXTR, relative gene expression as the ΔΔCt of AVPR1A and OXTR gene expression.
| Treatment | n | Sex | Ethnicity | Age (years) | Full-scale IQ score |
SRS-2 T score | CGI-S score | Blood AVP concentration |
Blood AVPR1A: OXTR |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | Caucasian | Other | ||||||||
| AVP | 17 | 3 | 14 | 8 | 9 | 9.14 ± 0.57 | 77.65 ± 5.03 | 78.12 ± 1.66 | 4.82 ± 0.15 | 1.32 ± 0.25 | 2.25 ± 0.31 |
| Placebo | 13 | 2 | 11 | 11 | 2 | 9.86 ± 0.65 | 91.85 ± 5.76 | 83.00 ± 1.90 | 4.77 ± 0.17 | 1.28 ± 0.29 | 2.21 ± 0.35 |
Because the clinical trial’s primary, and many of its secondary, outcome measures relied on parent report measures, we took multiple precautions to keep the study blinded as described in Materials and Methods. We also empirically evaluated whether parents were able to ascertain the treatment condition to which their child had been randomized before breaking the study’s blind. Parents were not able to do so accurately (χ2 likelihood ratio, 0.002; P = 0.9607; table S3), suggesting that parent ratings were not influenced by inadvertent knowledge of their child’s treatment condition. Dosing compliance was also monitored in several ways as described in Materials and Methods. We also empirically evaluated compliance by weighing participants’ spray bottles after completion of the 4-week trial. Bottle weights did not differ between treatment conditions (AVP, 60.2 ± 1.24 g versus placebo, 59.1 ± 1.30 g; F1,121 = 0.3487; P = 0.5611; table S4), suggesting that our clinical trial findings were not driven by treatment condition–related variations in dosing compliance.
Primary and secondary outcome measures after intranasal AVP treatment
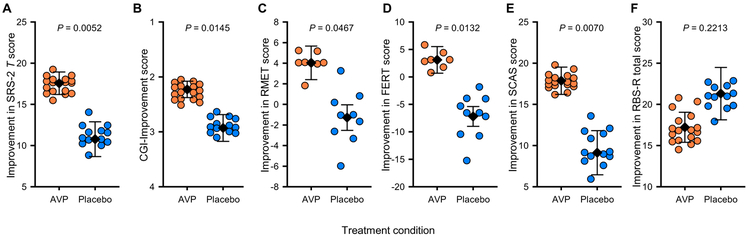
Individuals with ASD treated with intranasal AVP for 4 weeks showed greater improvement in their social abilities as assessed by the primary outcome measure, the SRS-2 T score, compared to those individuals receiving placebo (F1,20 = 9.853; P = 0.0052; ηp2 = 33.0%; equivalent Cohen’s d = 1.40; Fig. 2A, Table 2, and table S5). This effect was also dependent on pretreatment AVP concentrations in blood (F1,20 = 50.49; P < 0.0001; ηp2 = 71.6%; Table 2 and table S5). Higher pretreatment blood AVP concentrations predicted a greater treatment response in AVP-treated participants (F1,20 = 60.13; P < 0.0001; Table 2 and table S5). The AVPR1A-to-OXTR (oxytocin receptor) gene expression ratio did not show this selective response and was thus retained in this and all other relevant analyses as a blocking (control) factor only. The same pattern of results was observed if different study blocking (control) factors were included or excluded, confirming the robustness of the model. In addition, the core result of the treatment condition–by–blood AVP concentration interaction held if we did not weight the model according to the reliability of pretreatment parent-reported SRS-2 scores (F1,20 = 7.508; P = 0.0126; table S6). Last, AVP’s treatment effect was even more pronounced when we examined the Social Communication and Interaction (SCI) subscale of the SRS-2 (Table 2 and table S7) (21).
Fig. 2. Improvement scores for participants receiving 4-week intranasal arginine vasopressin (AVP) treatment versus placebo.
Participants’ improvement scores for the clinical trial’s key primary and secondary outcome measures are provided for each treatment condition. (A) Primary outcome measure: the Social Responsiveness Scale, 2nd Edition (SRS-2) T score. (B to F) Secondary outcome measures: Clinical Global Impression (CGI)-Improvement score (B), Reading the Mind in the Eyes Test (RMET) score (C), Facial Emotion Recognition Test (FERT) score (D), Spence Children’s Anxiety Scale (SCAS) score (E), and Repetitive Behaviors Scale-Revised (RBS-R) total score (F). General linear model F tests were used to evaluate whether participants treated with AVP (orange circles) versus placebo (blue circles) differed as a main effect of treatment (i.e., at the mean pretreatment blood AVP concentration). Data are presented as least-squares means (LSM)±SEM, with individual data points plotted as residuals from their LSM adjacent to the respective error bar. Thus, all data are plotted corrected for other variables in the analysis. Data depicted in (A) and (C) to (F) are presented as absolute difference scores (between posttreatment and baseline pretreatment) such that positive numbers on the y axis indicate improvement. Data depicted in (B) are presented as clinician improvement ratings where 1 = very much improved since the initiation of treatment, 2 = much improved, 3 = minimally improved, and 4 = no change since the initiation of treatment. AVP-treated participants differed significantly from placebo-treated participants in their improvement scores on nearly all measures except for the RBS-R, in which there was no overall group difference. n = 30 for the SRS, CGI, and SCAS; n = 29 for the RBS-R; n = 17 for the FERT; and n = 16 for the RMET.
Table 2. Change from baseline in the behavioral outcome measures for the 4-week arginine vasopressin (AVP) treatment trial.
Data were analyzed using the same model throughout (which controlled for ethnicity, IQ, pretreatment blood differential receptor gene expression, and baseline behavioral severity for each measure). All analyses tested for a main effect of treatment condition at the mean pretreatment blood AVP concentration. This LSM response takes into account any predictive effect of pretreatment blood AVP concentration on treatment response and gives an overall measure of efficacy in the population of participants as a whole. The ability of pretreatment blood AVP concentration to predict treatment response differentially in the drug-treated group is tested by the treatment condition–by–pretreatment blood AVP concentration interaction. Clinical Global Impression-Improvement (CGI-I) is a single score taken after a 4-week treatment. Otherwise, all other scores are given as a change from baseline, normalized for the direction of the scale, so that symptomatic improvement is reported as a positive change. For all measures, significant overall effects of treatment condition are in the predicted direction (i.e., AVP-treated participants improve more than placebo-treated participants). When a treatment condition–by–pretreatment blood AVP concentration interaction is significant, post hoc tests are reported for AVP-treated and placebo-treated participants: “Greater” indicates that symptomatic improvement increased with higher pretreatment blood concentrations of AVP; “Lesser” indicates that less improvement was observed at higher pretreatment blood concentrations of AVP; “ns” indicates that the scale was not significantly affected by pretreatment blood AVP concentrations. Subscales of an instrument are only tested if the overall score is significant at P < 0.05 and are tested at appropriate Bonferroni-corrected critical α to minimize the risk of false discovery. (Social Behavior Scale, 2nd Edition (SRS-2), critical α < 0.025; Repetitive Behaviors Scale-Revised (RBS-R), critical α < 0.0083). Any post hoc tests of treatment condition–by–pretreatment blood AVP concentration interactions are further Bonferroni-corrected to a critical α half that of the original critical α for the interaction. Effect sizes are given as ηp2. SCI, Social Communication and Interaction; RRB, Restricted Interests and Repetitive Behavior; CGI-S, Clinical Global Impression-Severity; FERT, Facial Emotion Recognition Test; RMET, Reading the Mind in the Eyes Test; NEPSY, Developmental NEuroPSYchological Assessment.
| Measure | Treatment condition main effect |
Improvement in score LSM ± SE |
*Significant after correction for multiple comparisons |
Treatment condition– by–pretreatment blood AVP concentration interaction |
*Significant after correction for multiple comparisons |
Improvement in score becomes greater or lesser with higher pretreatment blood AVP concentrations |
||
|---|---|---|---|---|---|---|---|---|
| AVP | Placebo | AVP | Placebo | |||||
| Primary outcome measure | ||||||||
| SRS-2 T score | F1,20 = 9.853; P = 0.0052; ηp2 = 33.0% | 17.6 ± 1.37 | 10.8 ± 2.11 | * | F1,20 = 50.49; P < 0.0001; ηp2 = 71.6% | * | Greater | Lesser |
| SCI | F1,20 = 12.74; P = 0.0019; ηp2 = 38.9% | 17.5 ± 1.37 | 9.75 ± 2.11 | * | F1,20 = 58.38; P < 0.0001; ηp2 = 74.5% | * | Greater | Lesser |
| RRB | F1,20 = 1.045; P = 0.3188; ηp2 = 5.0% | 15.6 ± 1.73 | 12.7 ± 2.70 | ns | F1,20 = 7.601; P = 0.0122; ηp2 = 27.5% | * | Greater | ns |
| Secondary outcome measures (clinician-evaluated) | ||||||||
| CGI-S, social and communication | F1,20 = 0.549; P = 0.4674; ηp2 = 2.7% | 0.873 ± 0.126 | 0.712 ± 0.202 | ns | F1,20 = 1.519; P = 0.2320; ηp2 = 7.1% | ns | ||
| CGI-I, social and communication | F1,21 = 7.098; P = 0.0145; ηp2 = 25.3% | 2.23 ± 0.149 | 2.93 ± 0.244 | * | F1,21 = 9.520; P = 0.0056; ηp2 = 31.2% | * | Greater | ns |
| Secondary outcome measures (parent-rated) | ||||||||
| Spence Children’s Anxiety Scale | F1,20 = 9.014; P = 0.0070; ηp2 = 31.1% | 17.9 ± 1.66 | 9.14 ± 2.68 | * | F1,20 = 19.48; P = 0.0003; ηp2 = 49.3% | * | Greater | Lesser |
| RBS-R—total | F1,19 = 1.600; P = 0.2213; ηp2 = 7.8% | 17.2 ± 1.83 | 21.3 ± 3.18 | ns | F1,19 = 18.57; P = 0.0004; ηp2 = 49.4% | * | Greater | ns |
| Stereotypic | F1,19 = 0.001; P = 0.9783; ηp2 = 0.0% | 2.24 ± 0.313 | 2.23 ± 0.486 | ns | F1,19 = 4.932; P = 0.0381; ηp2 = 20.6% | ns | ||
| Self-injurious | F1,19 = 0.552; P = 0.4666; ηp2 = 2.8% | 0.594 ± 0.467 | 1.18 ± 0.828 | ns | F1,19 = 27.54; P < 0.0001; ηp2 = 59.2% | * | Greater | Lesser |
| Compulsive | F1,19 = 2.028; P = 0.1707; ηp2 = 9.6% | 3.35 ± 0.511 | 4.59 ± 0.819 | ns | F1,19 = 22.49 ; P = 0.0001; ηp2 = 54.2% | * | Greater | ns |
| Ritualistic | F1,19 = 3.133; P = 0.0920; ηp2 = 14.2% | 3.08 ± 0.504 | 4.63 ± 0.818 | ns | F1,19 = 3.643; P = 0.0708; ηp2 = 16.1% | ns | ||
| Sameness | F1,19 = 0.043; P = 0.8375; ηp2 = 0.2% | 5.61 ± 0.661 | 5.85 ± 1.03 | ns | F1,19 = 3.071; P = 0.0950; ηp2 = 13.9% | ns | ||
| Restricted | F1,19 = 0.623; P = 0.4392; ηp2 = 3.2% | 2.58 ± 0.54 | 3.4 ± 1.01 | ns | F1,19 = 4.261; P = 0.0522; ηp2 = 18.3% | ns | ||
| Secondary outcome measures (child performance) | ||||||||
| FERT | F1,7 = 10.85; P = 0.0132; ηp2 = 60.8% | 3.10 ± 2.42 | −7.19 ± 1.81 | * | F1,7 = 0.971; P = 0.3573; ηp2 = 12.2% | ns | ||
| RMET | F1,6 = 6.2341; P = 0.0467; ηp2 = 51.0% | 4.04 ± 1.63 | −1.28 ± 1.24 | * | F1,6 = 0.068; P = 0.8031; ηp2 = 1.1% | ns | ||
| NEPSY—Theory of Mind | F1,12 = 0.647; P =0.4367; ηp2= 5.1% | 2.1 ± 1.41 | 0.677 ± 1.20 | ns | F1,12 = 1.162; P =0.3022; ηp2= 8.8% | ns | ||
| NEPSY—Affect Recognition | F1,12 = 0.946; P = 0.3500; ηp2 = 7.3% | 0.094 ± 2.20 | −2.58 ± 1.90 | ns | F1,12 = 0.251; P = 0.6254; ηp2 = 2.0% | ns | ||
Blinded parent ratings of treatment-related improvements in social behavior (i.e., SRS-2 scores) were significantly correlated with blinded clinician evaluations, i.e., Clinical Global Impression–Improvement (CGI-I) scores in the drug-treated group (r = 0.8753; P < 0.0001; table S8. Parent ratings were corroborated by both clinician evaluations and child performance on laboratory tests. Specifically, AVP-treated individuals showed greater clinician-evaluated improvement in social communication abilities as assessed by the CGI-I scale after 4-week intranasal AVP administration compared to placebo-treated individuals (F1,21, = 7.098; P = 0.0145; ηp2 = 25.3%; equivalent Cohen’s d = 1.16; Fig. 2B, Table 2, and table S9). As with the SRS-2, this finding was more pronounced in AVP-treated participants who had higher pretreatment AVP concentrations in blood (F1,21 = 9.52; P = 0.0056; Table 2 and table S9). Similarly, child participants treated with intranasal AVP versus placebo showed enhanced theory of mind abilities, that is, the ability to interpret the mental/emotional states of others, as assessed by the Reading the Mind in the Eyes Test (RMET) (F1,6 = 6.2341; P = 0.047; ηp2 = 51.0%; equivalent Cohen’s d = 2.04; Fig. 2C, Table 2, and table S10 Children with ASD treated with intranasal AVP versus placebo also showed increased facial emotion recognition abilities as assessed by the Facial Emotion Recognition Test (FERT) (F1,7 = 10.85; P = 0.0132; ηp2 = 60.8%; equivalent Cohen’s d = 2.49; Fig. 2D, Table 2, and table S11). The effects of AVP treatment on child performance–related assessments were independent of pretreatment blood AVP concentrations.
AVP treatment effects on other core and associated symptom measures
AVP treatment reduced anxiety symptoms compared to placebo as assessed by the Spence Children’s Anxiety Scale (SCAS) (F1,20 = 9.014; P = 0.0070; ηp2 = 31.1%; equivalent Cohen’s d = 1.34; Fig. 2E, Table 2, and table S12). As observed for social abilities, this effect, too, was magnified in AVP-treated participants with the highest pretreatment AVP concentrations in blood (F1,20 = 19.48; P = 0.0003; Table 2 and table S12). Although AVP compared to placebo as a main effect of treatment did not significantly reduce repetitive behaviors as assessed by the Repetitive Behaviors Scale–Revised (RBS-R; ηp2 = 7.8%; equivalent Cohen’s d = 0.58; Fig. 2F, Table 2, and table S13), it did diminish repetitive behaviors overall in AVP-treated participants with the highest pretreatment AVP concentrations in blood (F1,19 = 18.57; P = 0.0004; Table 2 and table S13). A similar result was also observed for the Restricted Interests and Repetitive Behavior (RRB) subscale of the SRS-2 (Table 2 and table S14) (21).
AVP treatment effects on safety and tolerability measures
AVP was overall well tolerated with minimal adverse effects. No participants dropped out during this study. There were no significant differences in adverse event rates reported in the AVP-treated compared to placebo-treated groups as assessed by parent ratings on the Dosage Record Treatment Emergent Symptom Scale (DOTES) and Overt Aggression Scale (OAS) (Table 3). No significant changes from baseline in vital signs, 12-lead electrocardiogram, clinical chemistry, or physiological measures were discerned after 4 weeks of intranasal AVP treatment (tables S15 and S16).
Table 3. Reported adverse events during the 4-week arginine vasopressin (AVP) treatment trial assessed by the DOTES and OAS scales.
Adverse events are reported as counts and percentages. Fisher’s exact test was used to test for differences in adverse events between the AVP and placebo treatment conditions. No significant effects were discerned. DOTES, Dosage Record Treatment Emergent Symptom Scale; OAS, Overt Aggression Scale; HEENT, head, ears, eyes, nose, and throat.
| Adverse event | AVP (n = 17) | Placebo (n = 13) |
|---|---|---|
| General | ||
| Fever | 2 (12%) | 1 (8%) |
| Cough | 1 (6%) | 0 (0%) |
| Body ache | 1 (6%) | 0 (0%) |
| Neurological/psychiatric | ||
| Excitement/agitation | 4 (24%) | 1 (8%) |
| Insomnia | 4 (24%) | 1 (8%) |
| Increased motor activity | 4 (24%) | 0 (0%) |
| Depressive affect | 2 (12%) | 1 (8%) |
| Headache | 2 (12%) | 0 (0%) |
| Drowsiness | 1 (6%) | 3 (23%) |
| Decreased motor activity | 1 (6%) | 2 (15%) |
| Aggression | 1 (6%) | 1 (8%) |
| Akathisia | 1 (6%) | 0 (0%) |
| Head banging | 1 (6%) | 0 (0%) |
| Dizziness | 0 (0%) | 1 (8%) |
| Lethargy/tiredness | 0 (0%) | 1 (8%) |
| HEENT | ||
| Nasal congestion | 3 (18%) | 4 (31%) |
| Dry mouth | 1 (6%) | 3 (23%) |
| Blurred vision | 1 (6%) | 1 (8%) |
| Ear infection | 0 (0%) | 1 (8%) |
| Runny nose | 0 (0%) | 1 (8%) |
| Sore throat | 0 (0%) | 1 (8%) |
| Cold sore | 0 (0%) | 1 (8%) |
| Gastrointestinal | ||
| Decreased appetite | 4 (24%) | 5 (38%) |
| Nausea/vomiting | 2 (12%) | 2 (15%) |
| Constipation | 1 (6%) | 0 (0%) |
| Diarrhea | 0 (0%) | 1 (8%) |
| Renal | ||
| Increased urination | 1 (6%) | 1 (8%) |
| Bed-wetting | 1 (6%) | 0 (0%) |
| Dermatological | ||
| Skin rash | 1 (6%) | 1 (8%) |
| Bug bite | 1 (6%) | 0 (0%) |
| Skin burn | 0 (0%) | 1 (8%) |
DISCUSSION
Using a double-blind, randomized, placebo-controlled, parallel clinical trial design, we found that the 4-week intranasal AVP treatment enhanced social abilities in children with ASD as assessed by the trial’s primary outcome measure, the SRS-2 T score. The robustness of this parent-reported social improvement score was corroborated by convergent evidence from clinician evaluation of the social communication abilities of trial participants and by performance of trial participants on laboratory tests of social cognition. These preliminary findings suggest that intranasally administered AVP may be a promising medication for treatment of core social impairments in children with ASD.
We also sought to investigate whether pretreatment neuropeptide concentrations in blood could predict AVP treatment response. We found that participants with the highest pretreatment AVP concentrations in blood benefitted the most from intranasal AVP treatment. This finding may seem counterintuitive, particularly in light of our recent studies showing that low AVP concentrations in CSF could be used to differentiate ASD cases from non-ASD control individuals (13, 14). One might therefore expect that it would be those children with the lowest endogenous AVP concentrations that stood to benefit the most from intranasal AVP treatment. However, being mindful of safety in this pediatric population, our pilot study used a conservative dose escalation regimen in which children were treated with fairly low doses of AVP throughout much of the trial. Assuming that blood AVP concentrations are related, in some manner, to brain AVP activity—a notion about which there is debate (14, 22–25)—it is possible that participants with lower endogenous AVP concentrations at the trial’s outset were “underdosed” in terms of drug amount or duration of treatment and, therefore, would not benefit as fully from AVP administration as those with higher endogenous AVP concentrations. This interpretation is consistent with our finding that AVP treatment enhanced simple social perceptual abilities independent of pretreatment AVP concentrations in blood, whereas it was only those AVP-treated individuals with higher pretreatment blood AVP concentrations who showed gains in complex social behaviors and a reduction in repetitive behaviors.
There exists another explanation for this finding, however. Similar to insulin-resistant patients with high “compensatory” insulin concentrations, patients with ASD with decreased AVP sensitivity may present with high AVP concentrations, and it is these individuals who may respond most robustly to AVP administration. Our blood AVP concentration finding points to the need for larger clinical trials to evaluate endogenous AVP concentrations before treatment in relation to maximum tolerated AVP dose and outcome measures more directly related to brain function, e.g., electroencephalography. Larger trials will help to elucidate the biological importance of blood AVP concentrations and to determine whether pretreatment blood AVP concentrations may be a useful measure for establishing AVP dosing guidelines.
The actions of AVP are mediated by four G protein–coupled receptors: AVPR1A, AVPR1B (also known as AVPR3), AVPR2, and the OXTR. These four receptors are distributed in a tissue-specific manner throughout the brain and body (26). In the brain, in addition to prosocial functioning, the three AVPRs and OXTR have been implicated in regulating stereotyped behaviors, anxiogenesis/anxiolysis, and territorial/parental aggression and mate guarding, although their roles in these phenomena are complex and vary markedly by species, context, brain region, and receptor type (27, 28). AVPRs are also known for their roles in the periphery in vasoconstriction, thrombosis, plasma volume and osmolality control, and release of the anterior pituitary hormone corticotrophin (26). AVP concentrations increase in both CSF and blood after intranasal administration of AVP (12), suggesting that a variety of neuropeptide receptor–mediated behavioral and physiological processes might be affected (in both desirable and undesirable ways) by intranasal AVP treatment. In the present study, we investigated this possibility. We found that intranasal AVP treatment reduced repetitive behaviors (particularly self-injurious and compulsive ones) and anxiety symptoms in children with ASD. We also determined intranasal AVP treatment to be safe, well tolerated, and with minimal side effects in our pilot study population. However, participants in our study were required to have normal cardiac function, vital signs, and clinical chemistry values, and be free of uncontrolled seizure disorders as well as serious liver, renal, and cardiac illnesses. Participants were also excluded if they habitually consumed high fluid volumes. The selected age range of treated participants (the majority of whom were male) was prepubertal (i.e., 6 to 12 years of age), out of consideration that testosterone can enhance AVP’s aggression-promoting effects, at least in male rodents (29). As AVP treatment trials expand to include higher dosing regimens and postpubertal individuals, “off-target” AVP effects will continue to require careful monitoring.
There are other research and clinical efforts to modulate the AVP signaling pathway for ASD treatment that warrant comment. First, there is evidence that nonpharmacological interventions may facilitate endogenous AVP release, for example, electroacupuncture stimulation increases brain AVP concentrations in rats (30). Transcutaneous electrical acupoint stimulation (TEAS) therapy improves social functioning and anxiety symptoms in children with ASD, particularly in those with the largest posttreatment increase in blood AVP concentrations (31). The authors of this prior report theorized that increased AVP signaling may be the mechanism by which the prosocial and anxiolytic benefits of TEAS treatment were achieved. Second, a synthetic analog of AVP, desmopressin, has been widely used for over 40 years to treat nocturnal enuresis (nighttime bed-wetting) (32). Nocturnal enuresis is common in individuals with ASD (33), but to our knowledge, there are no reports that desmopressin enhances social functioning in ASD (or in any other clinical population). This may be because desmopressin is typically administered at bedtime (so prosocial effects would be less evident) and orally (oral desmopressin does not cross the blood-brain barrier) (34). The most likely explanation, however, is that desmopressin acts selectively on AVPR2, rather than on AVPR1A (35).
Last, small-molecule AVPR1A antagonists are currently being developed and tested by Hoffmann–La Roche to treat ASD. The molecules are identified through high-throughput screening (36). A previous study tested the effects of a single-dose intravenous administration of RG7713 in a small sample of high-functioning adults with ASD (37). This study reported nonsignificant drug-related improvements on overall composite tests, with a few post hoc subtest findings (which were not corrected for multiple comparisons), showing that RG7713 increased biological motion orienting preference and reduced the ability to detect lust on an affective speech recognition task. Hoffmann–La Roche also recently reported findings from their Vasopressin ANtagonist to Improve sociaL communication in Autism (VANILLA) phase 2 trial, which used the AVPR1A antagonist, balovaptan (RG7314/RO5285119), to treat social impairments in high-functioning adults with ASD as assessed by caregiver ratings and clinician evaluation (38). Although the primary outcome measure (the SRS-2) was negative, a dose-dependent improvement on a secondary outcome measure, the Vineland-II Adaptive Behavior Scale, was observed compared to placebo. This caregiver-reported outcome measure was not corroborated by clinician evaluation on the CGI-I (which was negative) or participant performance on social cognition tests (which were not administered).
The presumed rationale for treating patients with ASD with an AVPR1A antagonist is based, at least in part, on studies showing that AVP administration increases aggression in healthy male rodents (39, 40) and enhances threat perception in healthy adult male volunteers (41). In addition, AVP administration has been shown to increase anxiety in rats, with AVPR1A blockade leading to a reduction in anxiety (42). However, many of these previous studies were conducted using “neurotypical” animal and human subjects, with presumably intact AVP neural circuitry. In contrast, our rationale for treating patients with ASD with AVP was based on long-standing evidence of increased AVP release during social bond formation in rodents (15, 17, 19) and from studies of naturally low-social male rhesus monkeys and of children with ASD (13, 14, 43). This latter work has demonstrated that low CSF AVP concentration is a robust marker of impaired social functioning in both human and nonhuman primates. It is therefore presently difficult to reconcile these seemingly opposite pharmacological approaches to ASD treatment. However, ASD is clinically heterogeneous, and it is possible that each AVP-related treatment strategy—the vasopressin agonist we used here and the AVPR1A antagonist used in the VANILLA trial (38)—may prove efficacious in distinct, biologically well-characterized ASD subgroups.
There are several limitations to the present study. First, our sample size was small and therefore potentially vulnerable to sampling bias in a disease population that is heterogeneous in nature. Second, our sample was male-biased (83%) and not statistically powered to detect sex differences or sex-by-group interactions in our analyses. Third, our study participants were not medication free, but their concomitant medications were stable during the intervention. These medications were not known to interact with intranasal AVP and did not differ by treatment condition. It is therefore unlikely that the observed social improvements in AVP-treated individuals with ASD were driven by concomitant medications. Fourth, our primary outcome measure (and many of our secondary outcome measures) relied on parent reporting to ascertain AVP treatment–related changes. Although we used gold-standard reporting instruments, these measures were nevertheless subjective in nature. Here, we mitigated this subjectivity, at least for the primary outcome measure, by accounting for measurable influences in parent SRS-2 score reporting reliability, and by determining that blinded clinician assessment and child performance on laboratory tests corroborated parent ratings. It would be valuable in future studies to also include a measure of treatment-related change in clinical significance, such as the brief observation of social communication change (44), a sensitive test designed specifically to assess changes in core ASD symptoms, particularly in the context of treatment trials.
In conclusion, the present pilot study determined that 4-week intranasal AVP treatment compared to placebo enhanced social communication abilities, diminished anxiety symptoms, and reduced repetitive behaviors in children with ASD. On nearly all behavioral measures, participants with the highest pretreatment blood AVP concentrations benefitted the most from AVP treatment, suggesting that pretreatment blood AVP concentrations may be useful for setting dosing guidelines for this medication. Last, intranasal AVP treatment was well tolerated with minimal side effects in this pediatric study population. These preliminary findings suggest that intranasal AVP treatment has potential to enhance social abilities in an ASD patient population characterized by currently intractable social impairments.
MATERIALS AND METHODS
Study design and regulatory approval
This phase 2 clinical trial was conducted in the Autism and Developmental Disorders Clinic (ADDC) in the Division of Child and Adolescent Psychiatry at Stanford University. Recruitment began in December 2013 and ended in May 2017. Before initiating this trial, an Investigational New Drug application (#118327) was filed with the U.S. Food and Drug Administration (FDA), and this study was approved by the Institutional Review Board of Stanford University. This trial was also registered on ClinicalTrials.gov (#NCT01962870). Parents or legal guardians of the study’s participants provided written consent before initiation of experimental procedures. If the child was deemed intellectually capable of understanding the study, written assent was also obtained. Last, this study was overseen by an independent Data Safety Monitoring Board composed of clinicians with expertise in clinical trials, ASD, or pediatric medical care. The study was a double-blind, randomized, placebo-controlled, parallel design that tested the efficacy and tolerability of 4-week intranasal daily AVP treatment in 30 children with ASD. All participants, their parents, investigators, and research staff involved in completing trial end-points were blind to treatment assignment. The active compound and the placebo were identical with regard to smell, color, and consistency.
Participant recruitment and eligibility criteria
Children with a history of an ASD diagnosis were recruited to participate in this study. Participants were recruited through (i) the Autism and Developmental Disorders Research Registry at Stanford University, (ii) flyers posted in the ADDC or in the surrounding community (e.g., pediatrician offices), (iii) advertisements posted online (e.g., Interactive Autism Network), or (iv) special events (e.g., Autism Speaks Walk). Participants were screened by phone for initial study eligibility and then underwent a medical assessment and a comprehensive psychiatric evaluation. The psychiatric evaluation included a clinical interview with a child psychiatrist (A.Y.H., L.K.F., or K.E.H.) to confirm the child’s previous ASD diagnosis based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) (45). This diagnosis was further confirmed by the Autism Diagnostic Interview–Revised (ADI-R) (46) and the Autism Diagnostic Observation Schedule, 2nd Edition (ADOS-2) (47). The ADI-R and ADOS-2 were administered by assessors trained and closely supervised by a research-reliable child psychologist (J.M.P.), following standardized ADI-R and ADOS-2 research reliability protocols.
In addition to meeting diagnostic criteria for ASD, other study inclusion criteria included the following: (i) medically healthy outpatients between 6 and 12.9 years of age; (ii) intelligence quotient (IQ) ≥ 50 as determined by the Stanford Binet 5th Edition (48); (iii) CGI-Severity (CGI-S) scale rating of ≥4 (49); (iv) care provider who can reliably bring participant to clinic visits, provides trustworthy ratings, and interacts with the participant on a regular basis; (v) stable concomitant medications for at least 4 weeks; (vi) no planned changes in psychosocial interventions during the trial; and (vii) willingness and ability to provide blood samples and undergo electrocardiograms.
Study exclusion criteria included the following: (i) previous or current use of AVP; (ii) abnormal chemistry result; (iii) electrocardiogram abnormality as determined by the study pediatric cardiologist (K.S.M.); (iv) DSM-IV-TR diagnosis of schizophrenia, schizoaffective disorder, or psychotic disorder; (v) regular nasal obstruction or nosebleeds; (vi) active medical problems: uncontrolled seizures and physical illness (e.g., serious liver, renal, or cardiac pathology); (vii) sensitivity to preservatives (e.g., chlorobutanol); (viii) evidence of a genetic mutation known to cause ASD (e.g., fragile X syndrome); (ix) hearing or vision impairments; (x) habitually drinks large volumes of water; (xi) pregnancy, breastfeeding, or childbirth within the last 6 months; or (xii) sexually active females not using a reliable method of contraception. In addition, any female aged 11 years or older, or who had started menstruating, was required to have a (negative) urine pregnancy test.
Pharmacological intervention
Commercially available injectable sterile AVP was used in this study. It was initially purchased from JHP Pharmaceuticals (Rochester, MI), which was subsequently acquired by Par Sterile Products (Chestnut Ridge, NY) in 2014. The placebo solution was prepared by Koshland Pharm (San Francisco, CA) and consisted of ingredients used in the active solution except for the AVP compound. A pharmacist transferred 25 ml of AVP (20 International Units (IU)/ml) or placebo solutions into standard sterile amber glass bottles with metered (0.1 ml per puff) nasal spray applicators to ensure that the AVP and placebo applicators were visually indistinguishable to the research team. These applicators were coded and given to the Stanford Health Care’s Investigational Drug Service for refrigerated storage (2°C to 8°C) and subsequent dispensing. After the first AVP dose (see below), the dose-escalation regimen at home for all participants involved administration of 4 IU twice daily (or BID) of AVP during week 1 and 8 IU BID of AVP during week 2. Participants aged 6 to 9.5 years then received 12 IU BID of AVP during weeks 3 and 4, whereas participants aged 9.6 to 12.9 years received 16 IU BID of AVP during weeks 3 and 4. A range of possible AVP doses was identified by review of the published literature; the final study doses were then determined in close consultation with the FDA.
Overview of study visits and procedures
After the screening phase, baseline (pretreatment) measures were obtained from participants continuing to meet inclusion and exclusion criteria. These measures included assessments of participants’ behavioral symptoms and abilities, safety/tolerability monitoring, and blood sample collection for later neuropeptide quantification. One to four weeks later, participants were randomly assigned to a treatment condition (i.e., AVP or placebo), stratified by age (6 to 9.5 years versus 9.6 to 12.9 years) and sex (male versus female). Randomization was performed by an unblinded investigator (K.J.P.) using a machine-generated treatment schedule, which allocated each participant to an intervention by using the method of randomly permuted blocks of two or four (which were then concatenated together to form the schedule). This practice allowed the clinical research team, parents/legal guardians, and child participants to remain blinded throughout the trial’s duration.
Parents were trained in the clinic by research staff to administer the nasal spray to their child. The first dose was administered in the ADDC under the direct supervision of the research team (with the first dose being 12 IU for participants aged 6 to 9.5 years and 16 IU for participants aged 9.6 to 12.9 years). Vital signs were monitored before and 20 min after initial single-dose nasal spray administration to monitor for acute, unanticipated reactions to AVP. Participants’ parents then received two 25-ml nasal spray bottles containing either AVP or placebo for 4 weeks of at-home dispensing (as described above), for which they were responsible for their child’s continued twice daily dosing. Compliance was monitored by weekly phone calls to participants’ parents, by review of parent-reported daily dosing logs, and by verification that spray bottle weights upon completion of the trial did not differ between AVP-treated and placebo-treated groups. Parents were instructed to keep the bottles refrigerated with only brief room temperature excursions (i.e., for dosing). Participants underwent weekly safety/tolerability assessments in the clinic to monitor for adverse events. On completion of the 4-week treatment period, behavioral data, safety/tolerability data, and blood samples were again collected. After completion of the trial (but before breaking the blind), parents were asked to ascertain the treatment condition to which their child had been randomized. After breaking the blind, those participants randomized to the placebo group were invited to participate in an optional open-label 4-week AVP treatment phase to ensure that all children had the opportunity to receive AVP treatment.
Laboratory social cognition tests
In addition to parent-rated questionnaires and clinical evaluation of behavioral symptoms (detailed below), children were assessed at baseline and following 4-week treatment on laboratory tests of social functioning designed to assess the ability to perceive others’ intentions or emotions. These tests included the 28-item child RMET, which consists of images of eyes depicting emotional states and from which the participant makes a forced choice from four mental state terms per image (50); the Developmental NEuroPSYchological (NEPSY) Assessment, 2nd Edition, Social Perception Domain subtests of Affect Recognition (which assesses the ability to recognize affect from photographs of children’s faces) and Theory of Mind (which assesses the ability to understand complex mental functions through a series of visual and verbally presented stories and questions) (51); and the FERT, which was derived from the NimStim Set of Facial Expressions (52). Here, the FERT required participants to identify the correct facial emotion displayed by each face stimulus. In the FERT, participants were required to select an emotion from a list of seven possible emotions (angry, calm, disgusted, happy, sad, scared, and surprised) and were administered a total of 42 face stimuli per test session.
Safety and tolerability monitoring
Throughout the trial, safety and tolerability were assessed weekly using the following measurements: vital signs, 12-lead electrocardiogram, clinical chemistry laboratories, and height and body weight. Side effects were evaluated with the DOTES (53) and modified to assess potential specific side effects that might be related to AVP such as hyponatremia/water intoxication. Last, on the basis of animal studies showing that AVP can induce aggressive behavior under some circumstances (39, 40), we used the OAS to monitor for observable aggressive or violent behavior in this trial (54).
Blood sample collection and processing procedures
Blood was drawn from the child’s antecubital region by a pediatric phlebotomist, using standard protocols at the Lucile Packard Children’s Hospital outpatient laboratory. A portion of the sample was collected per standard laboratory protocols and sent for clinical chemistry analysis. To evaluate AVP concentrations, blood was also collected into chilled EDTA-treated vacutainer tubes and immediately placed on wet ice. Samples were promptly centrifuged (1600g at 4°C for 15 min), and the plasma fraction was aliquoted into polypropylene tubes and flash-frozen on dry ice. Blood was also collected into PAXgene RNA tubes (Qiagen, CA) and processed per manufacturer instruction to evaluate neuropeptide receptor gene expression (i.e., OXTR and AVPR1A). All samples were stored at −80°C until quantification.
Quantification of blood AVP concentrations
AVP concentrations were quantified using a commercially available enzyme immunoassay kit (Enzo Life Sciences Inc., Farmingdale, NY). This kit is highly specific and selectively recognizes AVP and not related peptides (i.e., cross-reactivity with OXT is <0.001%). A research team member blinded to treatment condition performed sample preparation and AVP quantification following established procedures (14, 22, 23). Briefly, blood samples (1000 μl per participant) were extracted per manufacturer’s instructions and evaporated using compressed nitrogen. Each evaporated sample was reconstituted in 250 μl of assay buffer before AVP quantification to provide sufficient sample volume to run each participant’s sample in duplicate wells (100 μl per well). This practice ensured that the plated samples contained high enough AVP quantities to be read above the limit of detection (2.84 pg/ml). Samples were assayed with a tunable microplate reader (Molecular Devices, CA) for the 96-well format per manufacturer’s instructions.
Quantification of blood OXTR and AVPR1A gene expression
A research team member blinded to treatment condition performed sample preparation and quantitative polymerase chain reaction (qPCR). Total RNA was isolated and purified using a PAXgene blood RNA kit from blood stabilized in PAXgene RNA tubes (Qiagen, CA). RNA integrity was assessed with the Agilent 2100 Bioanalyzer (Agilent Technologies, CA) and consistently found to have RNA integrity number values greater than 9.5. The first-strand complementary DNA (cDNA) synthesis reaction was carried out with a QuantiTect reverse transcription kit (Qiagen, CA), with a starting RNA quantity of 1 μg in a 20-μl final volume. The primer sequence information for OXTR and AVPR1A genes was obtained from published studies and was designed as follows: OXTR, 5′-CTGAACATCCCGAGGAACTG-3′ (forward) and 5′-CTCTGAGCCACTGCAAATGA-3′ (reverse) (55); and AVPR1A, 5′-CTTTTGTGATCGTGACGGCTTA-3′ (forward) and 5′-TGATGGTAGGGTTTTCCGATTC-3′ (reverse) (56). Two housekeeping genes, hypoxanthine phosphoribosyltransferase 1 [HPRT1; 5′-GGACAGGACTGAACGTCTTGC-3′ (forward) and 5′-ATAGCCCCCCTTGAGCACAC-3′ (reverse) (56)] and ubiquitin C [UBC; 5′-GCTGCTCATAAGACTCGGCC-3′ (forward) and 5′-GTCACCCAAGTCCCGTCCTA-3′ (reverse) (56)], were selected for normalization using geNorm. qPCR was performed on the StepOnePlus Real-Time PCR System (Life Technologies, CA) with SYBR Green (Thermo Fisher Scientific, MA). cDNA was PCR-amplified in triplicate, and Ct values from each sample were obtained using StepOnePlus software. The relative expression of each gene was calculated on the basis of the ΔΔCt value, where the results were normalized to the average Ct value of HPRT1 and UBC (57).
Trial outcome measures
The primary outcome measure was the change from baseline in social ability as assessed by parent ratings on the SRS-2 T score. The SRS measures the severity of social deficits as they occur in natural environments, and its variance has been well studied in the general population and in individuals with ASD (58–60). The SRS is also a sensitive measure (i.e., it strongly correlates with DSM criterion scores) (61) and has been used as a primary measure of response to intervention in previous clinical trials (61–64). Here, we also examined two DSM-compatible SRS subscales, the SCI and RRB (21).
The trial’s secondary behavioral outcome measures included social cognition improvement assessed by child performance on laboratory tests (as detailed above) and symptom improvement in other core or associated nonsocial symptom domains. CGI-S and CGI-I scales were also obtained and were completed by a clinician with extensive experience in the assessment and treatment of children with developmental disabilities (49). The CGI-S uses a seven-point scale, ranging from not at all ill (1) to extremely ill (7). The CGI-I uses a seven-point scale ranging from marked improvement (1) to no change (4) to very much worse (7). In this study, the ratings were specifically focused on social communication skills.
Treatment effect generalizability was evaluated using (i) the RBS-R, which measures a comprehensive list of repetitive and stereotyped behaviors (65), and (ii) the SCAS, which assesses the severity of trait anxiety symptoms broadly in line with DSM dimensions of anxiety disorder (66). Anxiety was selected as an outcome measure due to its frequent comorbidity with ASD (67) and the existence of literature linking AVP to anxiety (42). Last, safety and tolerability were evaluated using (i) the DOTES (53) and (ii) change from baseline in electrocardiogram, clinical chemistry laboratory tests, vital signs (i.e., blood pressure, heart rate, temperature), and height and body weight.
A priori power analysis
No computational method exists for estimating a priori power for the complex factorial design and analysis required in a study of this kind. Instead, we used “Mead’s rule” (also known as the “Mead’s resource equation”) (68). This method has several advantages over traditional power calculations for simple tests (such as t tests). In particular, it does not require an estimate of effect size, but instead relies on the curves of decreasing return in estimating the variance components in a general linear or mixed model as sample size increases. Accordingly, we were well powered at N = 30 participants, given our planned experimental and control variables and the planned analyses.
Statistical analyses
Data were managed using REDCap (69) and analyzed using least-squares general linear models (LS-GLMs) in JMP Pro 13 and SAS 9.4 for Windows (SAS Institute Inc.). Efficacy analyses were guided by our prestudy aims, which included (i) testing for treatment main effects on the primary and secondary outcome measures and (ii) testing whether pretreatment blood–based biological measures predicted who benefited from treatment. To minimize the risk of false discovery, we first identified a robust model using our primary outcome measure (i.e., change in the SRS-2 T score) and then applied that model to all other outcomes. P < 0.05 was considered significant, and two-tailed tests were used throughout. Post hoc tests were Bonferroni-corrected to maintain a family level of P < 0.05 (as detailed below).
The initial model included sex, ethnicity, body weight, IQ, and blood collection time as blocking (control) factors. Pretreatment SRS-2 T score was included as a blocking (control) factor to account for the range of possible improvement and thus reduce possible floor or ceiling effects. Last, we included treatment condition (i.e., AVP or placebo) to test our main hypothesis and pretreatment blood AVP concentration and pretreatment blood neuropeptide receptor gene expression (expressed as an AVPR1A-to-OXTR ratio to account for within-individual differences in expression) as measures of endogenous AVP function that might predict treatment efficacy. As we administered two different AVP doses according to age, dose was nested within treatment condition to explicitly test for an overall effect of AVP treatment and to control for any dose-related effects. We also tested for treatment condition–by–biological measure and dose–by–biological measure interactions, as pretreatment biological measures should generally only predict treatment outcome in the drug-treated individuals. Last, because the SRS is a parent-reported measure, we collected SRS scores at two pretreatment time points to identify the reliability of an individual participant’s scores. This enabled us to use weighted LS-GLM (WLS-GLM) analyses whenever parent-reported measures were assessed. WLS ideally uses the inverse of the variance of a mean estimate as the weight (70), which we could obtain directly from our two pretreatment SRS scores.
Initial analyses showed that sex, blood collection time, and body weight introduced collinearities, were nonsignificant, and did not improve the R2 of the model, and so were removed following best practices for linear models (model simplification is important to avoid overspecification and the associated risk for false discovery, especially in small sample sizes) (71). In particular, sex was collinear with IQ and so could be safely removed from the model while still being controlled for in the analysis. Similarly, nonsignificant interactions were removed to avoid confounds of marginality for the main effects and to distinguish blocking (control) factors. Treatment condition and dose interaction with AVPR1A-to-OXTR gene expression ratio were nonsignificant and were removed following best practices (71). The AVPR1A-to-OXTR gene expression ratio main effect improved R2 and was retained as a blocking (control) factor. Thus, the final model contained ethnicity, IQ, pretreatment SRS-2 T score, and AVPR1A-to-OXTR gene expression ratio as blocking (control) variables. We tested dose nested within treatment condition and pretreatment blood AVP concentration as main effects and the interaction of pretreatment blood AVP concentration with dose nested within treatment condition. Thus, the model contained the biologically and experimentally essential variables, regardless of significance. We further tested the robustness of this model by confirming that key results held when different blocking (control) factors were included or excluded and if WLS-GLM was not used. Effect sizes were calculated as ηp2 (partial eta-squared), as appropriate for complex linear models. Equivalent Cohen’s d is provided for main effects where justifiable.
Once this model was identified for our primary outcome measure, the same model was applied to all secondary outcome measures with the exception that the baseline, pretreatment behavioral measure was replaced to match the outcome variable. For child performance measures, LS-GLM, not WLS-GLM, was used (and as we would predict, WLS-GLM did not improve these models, confirming that the WLS-GLM approach was specific to parent-rated measures by capturing variance in parent ratings). For CGI-I, in which there is no baseline pretreatment measure, the model did not include a baseline control. Effect sizes for these secondary outcome measures were likewise calculated as ηp2.
To minimize the risk of false discovery from multiplicity (71), we first tested the total score for each instrument and then only tested subscales if the total score was significant. Subscales were Bonferroni-corrected for multiple comparisons. The assumptions of WLS-GLM (linearity, homogeneity of variance, and normality of error) were confirmed post hoc, and suitable transformations were applied as needed (71). Post hoc tests were performed as planned contrasts and further Bonferroni-corrected for multiple comparisons. As these models consistently showed an interaction between treatment condition and pretreatment blood AVP concentration (i.e., pretreatment blood AVP concentration predicted the magnitude of the behavioral response to drug), we tested our first aim by testing for an overall effect of treatment condition at the mean pretreatment blood AVP concentration.
Fisher’s exact test was used to test for differences between treatment conditions for participant characteristics and concomitant medications. χ2 likelihood ratio was used to test whether parents were able to accurately ascertain the treatment condition to which their child had been randomized. Pearson’s correlation coefficient was used to test the relationship between change from baseline in parent-reported SRS-2 T score and clinician-evaluated CGI-I score in AVP-treated participants. One-way LS-GLMs were used to test the change from baseline for adverse events and safety measures between the AVP and placebo treatment conditions after the 4-week treatment and to test the difference in posttreatment bottle weights between treatment conditions. Suitable transformations were applied as needed.
SUPPLEMENTARY MATERIAL
Table S1. Raw data and SAS code for the data and analyses shown in Table 1.
Table S2. Participants’ stable concomitant medications during the 4-week AVP treatment trial.
Table S3. Raw data and SAS code testing whether parents could ascertain treatment condition.
Table S4. Raw data and SAS code testing whether bottle weights differed between treatment conditions.
Table S5. Raw data and SAS code for Fig. 2A and associated analyses.
Table S6. Raw data and SAS code testing treatment effects for the SRS-2 using an unweighted analysis.
Table S7. Raw data and SAS code testing treatment effects for the SCI subscale of the SRS-2.
Table S8. Raw data and SAS code correlating parent SRS-2 ratings with clinician CGI evaluations.
Table S9. Raw data and SAS code for Fig. 2B and associated analyses.
Table S10. Raw data and SAS code for Fig. 2C and associated analyses.
Table S11. Raw data and SAS code for Fig. 2D and associated analyses.
Table S12. Raw data and SAS code for Fig. 2E and associated analyses.
Table S13. Raw data and SAS code for Fig. 2F and associated analyses.
Table S14. Raw data and SAS code testing treatment effects for the RRB subscale of the SRS-2.
Table S15. Change from baseline in the safety assessments for the 4-week AVP treatment trial.
Table S16. Raw data and SAS code for the data and analyses shown in table S15.
Acknowledgments:
We are grateful to members of the Parker and Hardan laboratories, the pharmacists at the Stanford Health Care Investigational Drug Services and Koshland Pharm, the study participants and their families for their participation, and Par Sterile Products for providing (free of cost) a portion of the drug used in this study. The REDCap platform services are made possible by Stanford School of Medicine Research Office. The REDCap platform services at Stanford are subsidized by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1 TR001085.
Funding: This research was supported by the NIH (R21MH100387 to K.J.P. and A.Y.H., R21HD083629 to K.J.P., R01HD091972 to K.J.P. and A.Y.H., K08MH111750 to L.K.F., and T32MH019908 to D.S.K.), an Autism Speaks Meixner Postdoctoral Fellowship in Translational Research (#7895 to D.S.C.), a Bass Society Pediatric Fellowship (D.S.C.), the Mosbacher Family Fund for Autism Research (K.J.P.), the Teresa and Charles Michael Endowed Fund for Autism Research and Education (A.Y.H.), the Child Health Research Institute (K.J.P., A.Y.H., and O.O.), the Yani Calmidis Memorial Fund for Autism Research (K.J.P.), and Stanford’s Department of Psychiatry (K.J.P., L.K.F, and A.Y.H.).
Footnotes
Competing interests: D.S.C. received financial compensation from Janssen Pharmaceuticals Inc., Syneos Health, Saniona, Vista Point Capital, Katana Pharmaceuticals Inc., Anatomics, Giner Inc., and Trigemina Inc.; D.S.K. was a paid consultant for GSW Creative Corporation and Infinite Skin Inc.; L.K.F. was a paid consultant for Autism Speaks; and A.Y.H. served as either a paid consultant or an advisory board member for Hoffmann–LaRoche, Hoffman Technologies, CheckOrphan, and Realiteer and received research funding from BioElectron Technology Corp and Hoffmann–LaRoche. J.P.G. was a paid consultant for Genentech and Hoffmann–LaRoche (on animal welfare issues unrelated to autism or this trial). The other authors declare no competing interests. The Board of Trustees of the Leland Stanford Junior University has filed a provisional patent application related to this study, #US 62/696,234, entitled “Intranasal Vasopressin Treatment for Social Deficits in Children with Autism”.
Data and materials availability: All data associated with this study are presented in the paper or the Supplementary Materials. A portion of the commercially available study drug was supplied free of cost by Par Sterile Products under a material transfer agreement.
REFERENCES AND NOTES
- 1.APA, Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, ed. 5, 2013). [Google Scholar]
- 2.Webb S, Drugmakers dance with autism. Nat. Biotechnol. 28, 772–774 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Albers HE, The regulation of social recognition, social communication and aggression: Vasopressin in the social behavior neural network. Horm. Behav. 61, 283–292 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Bielsky IF, Hu S-B, Szegda KL, Westphal H, Young LJ, Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology 29, 483–493 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Paul MJ, Peters NV, Holder MK, Kim AM, Whylings J, Terranova JI, de Vries GJ, Atypical social development in vasopressin-deficient brattleboro rats. eNeuro 3, ENEURO.0150-15.2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guastella AJ, Kenyon AR, Alvares GA, Carson DS, Hickie IB, Intranasal arginine vasopressin enhances the encoding of happy and angry faces in humans. Biol. Psychiatry 67, 1220–1222 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Guastella AJ, Kenyon AR, Unkelbach C, Alvares GA, Hickie IB, Arginine vasopressin selectively enhances recognition of sexual cues in male humans. Psychoneuroendocrinology 36, 294–297 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Brunnlieb C, Nave G, Camerer CF, Schosser S, Vogt B, Münte TF, Heldmann M, Vasopressin increases human risky cooperative behavior. Proc. Natl. Acad. Sci. U.S.A. 113, 2051–2056 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsikunov SG, Belokoskova SG, Psychophysiological analysis of the influence of vasopressin on speech in patients with post-stroke aphasias. Span. J. Psychol. 10, 178–188 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Laczi F, Valkusz Z, Lászlo FA, Wagner Á, Járdánházy T, Szász A, Szilárd J, Telegdy G, Effects of lysine-vasopressin and 1-deamino-8-D-arginine-vasopressin on memory in healthy individuals and diabetes insipidus patients. Psychoneuroendocrinology 7, 185–193 (1982). [DOI] [PubMed] [Google Scholar]
- 11.Quintana DS, Guastella AJ, Westlye LT, Andreassen OA, The promise and pitfalls of intranasally administering psychopharmacological agents for the treatment of psychiatric disorders. Mol. Psychiatry 21, 29–38 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL, Sniffing neuropeptides: A transnasal approach to the human brain. Nat. Neurosci. 5, 514–516 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Oztan O, Garner JP, Partap S, Sherr EH, Hardan AY, Farmer C, Thurm A, Swedo SE, Parker KJ, Cerebrospinal fluid vasopressin and symptom severity in children with autism. Ann. Neurol. 84, 611–615 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker KJ, Garner JP, Oztan O, Tarara ER, Li J, Sclafani V, Del Rosso LA, Chun K, Berquist SW, Chez MG, Partap S, Hardan AY, Sherr EH, Capitanio JP, Arginine vasopressin in cerebrospinal fluid is a marker of sociality in nonhuman primates. Sci. Transl. Med. 10, eaam9100 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parker KJ, Lee TM, Central vasopressin administration regulates the onset of facultative paternal behavior in Microtus pennsylvanicus (meadow voles). Horm. Behav. 39, 285–294 (2001). [DOI] [PubMed] [Google Scholar]
- 16.Young LJ, Nilsen R, Waymire KG, MacGregor GR, Insel TR, Increased affiliative response to vasopressin in mice expressing the V1a receptor from a monogamous vole. Nature 400, 766–768 (1999). [DOI] [PubMed] [Google Scholar]
- 17.Donaldson ZR, Spiegel L, Young LJ, Central vasopressin V1a receptor activation is independently necessary for both partner preference formation and expression in socially monogamous male prairie voles. Behav. Neurosci. 124, 159–163 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman SM, Inoue K, Smith AL, Goodman MM, Young LJ, The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta). Psychoneuroendocrinology 45, 128–141 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR, A role for central vasopressin in pair bonding in monogamous prairie voles. Nature 365, 545–548 (1993). [DOI] [PubMed] [Google Scholar]
- 20.Christensen DL, Baio J, Van Naarden Braun K, Bilder D, Charles J, Constantino JN, Daniels J, Durkin MS, Fitzgerald RT, Kurzius-Spencer M, Lee L-C, Pettygrove S, Robinson C, Schulz E, Wells C, Wingate MS, Zahorodny W, Yeargin-Allsopp M, Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR Surveill. Summ. 65, 1–23 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frazier TW, Ratliff KR, Gruber C, Zhang Y, Law PA, Constantino JN, Confirmatory factor analytic structure and measurement invariance of quantitative autistic traits measured by the social responsiveness scale-2. Autism 18, 31–44 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Carson DS, Garner JP, Hyde SA, Libove RA, Berquist SW, Hornbeak KB, Jackson LP, Sumiyoshi RD, Howerton CL, Hannah SL, Partap S, Phillips JM, Hardan AY, Parker KJ, Arginine vasopressin is a blood-based biomarker of social functioning in children with autism. PLOS ONE 10, e0132224 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carson DS, Howerton CL, Garner JP, Hyde SA, Clark CL, Hardan AY, Penn AA, Parker KJ, Plasma vasopressin concentrations positively predict cerebrospinal fluid vasopressin concentrations in human neonates. Peptides 61C, 12–16 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Wotjak CT, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M, Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: New insights into the secretory capacities of peptidergic neurons. Neuroscience 85, 1209–1222 (1998). [DOI] [PubMed] [Google Scholar]
- 25.Kagerbauer SM, Martin J, Schuster T, Blobner M, Kochs EF, Landgraf R, Plasma oxytocin and vasopressin do not predict neuropeptide concentrations in human cerebrospinal fluid. J. Neuroendocrinol. 25, 668–673 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Thibonnier M, Coles P, Thibonnier A, Shoham M, The basic and clinical pharmacology of nonpeptide vasopressin receptor antagonists. Annu. Rev. Pharmacol. Toxicol. 41, 175–202 (2001). [DOI] [PubMed] [Google Scholar]
- 27.Johnson ZV, Young LJ, Oxytocin and vasopressin neural networks: Implications for social behavioral diversity and translational neuroscience. Neurosci. Biobehav. Rev. 76, 87–98 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baribeau DA, Anagnostou E, Oxytocin and vasopressin: Linking pituitary neuropeptides and their receptors to social neurocircuits. Front. Neurosci. 9, 335 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delville Y, Mansour KM, Ferris CF, Testosterone facilitates aggression by modulating vasopressin receptors in the hypothalamus. Physiol. Behav. 60, 25–29 (1996). [DOI] [PubMed] [Google Scholar]
- 30.Zhang H-F, Li H-X, Dai Y-C, Xu X-J, Han S-P, Zhang R, Han J-S, Electro-acupuncture improves the social interaction behavior of rats. Physiol. Behav. 151, 485–493 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Zhang R, Jia M-X, Zhang J-S, Xu X-J, Shou X-J, Zhang X-T, Li L, Li N, Han S-P, Han J-S, Transcutaneous electrical acupoint stimulation in children with autism and its impact on plasma levels of arginine-vasopressin and oxytocin: A prospective single-blinded controlled study. Res. Dev. Disabil. 33, 1136–1146 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Vande Walle J, Stockner M, Raes A, Norgaard JP, Desmopressin 30 years in clinical use: A safety review. Curr. Drug Saf. 2, 232–238 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Niemczyk J, Wagner C, von Gontard A, Incontinence in autism spectrum disorder: A systematic review. Eur. Child Adolesc. Psychiatry 27, 1523–1537 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Sørensen PS, Vilhardt H, Gjerris F, Warberg J, Impermeability of the blood-cerebrospinal fluid barrier to 1-deamino-8-D-arginine-vasopressin (DDAVP) in patients with acquired, communicating hydrocephalus. Eur. J. Clin. Invest. 14, 435–439 (1984). [DOI] [PubMed] [Google Scholar]
- 35.Robben JH, Knoers NVAM, Deen PMT, Regulation of the vasopressin V2 receptor by vasopressin in polarized renal collecting duct cells. Mol. Biol. Cell 15, 5693–5699 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ratni H, Rogers-Evans M, Bissantz C, Grundschober C, Moreau JL, Schuler F, Fischer H, Alvarez Sanchez R, Schnider P, Discovery of highly selective brain-penetrant vasopressin 1a antagonists for the potential treatment of autism via a chemogenomic and scaffold hopping approach. J. Med. Chem. 58, 2275–2289 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Umbricht D, del Valle Rubido M, Hollander E, McCracken JT, Shic F, Scahill L, Noeldeke J, Boak L, Khwaja O, Squassante L, Grundschober C, Kletzl H, Fontoura P, A single dose, randomized, controlled proof-of-mechanism study of a novel vasopressin 1a receptor antagonist (RG7713) in high-functioning adults with autism spectrum disorder. Neuropsychopharmacology 42, 1924 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bolognani F, del Valle Rubido M, Squassante L, Wandel C, Derks M, Murtagh L, Sevigny J, Khwaja O, Umbricht D, Fontoura P, A phase 2 clinical trial of a vasopressin V1a receptor antagonist shows improved adaptive behaviors in men with autism spectrum disorder. Sci. Transl. Med. 11, eaat7838 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Gobrogge KL, Liu Y, Young LJ, Wang Z, Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proc. Natl. Acad. Sci. U.S.A. 106, 19144–19149 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferris CF, Melloni RH Jr., Koppel G, Perry KW, Fuller RW, Delville Y, Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J. Neurosci. 17, 4331–4340 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson R, Gupta S, Miller K, Mills S, Orr S, The effects of vasopressin on human facial responses related to social communication. Psychoneuroendocrinology 29, 35–48 (2004). [DOI] [PubMed] [Google Scholar]
- 42.Neumann ID, Landgraf R, Balance of brain oxytocin and vasopressin: Implications for anxiety, depression, and social behaviors. Trends Neurosci. 35, 649–659 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Sclafani V, Del Rosso LA, Seil SK, Calonder LA, Madrid JE, Bone KJ, Sherr EH, Garner JP, Capitanio JP, Parker KJ, Early predictors of impaired social functioning in male rhesus macaques (Macaca mulatta). PLOS ONE 11, e0165401 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grzadzinski R, Carr T, Colombi C, McGuire K, Dufek S, Pickles A, Lord C, Measuring changes in social communication behaviors: Preliminary development of the brief observation of social communication change (BOSCC). J. Autism Dev. Disord. 46, 2464–2479 (2016). [DOI] [PubMed] [Google Scholar]
- 45.APA, Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, ed. 4, 2000). [Google Scholar]
- 46.Lord C, Rutter M, Le Couteur A, Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 24, 659–685 (1994). [DOI] [PubMed] [Google Scholar]
- 47.Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop SL, Luyster RJ, Guthrie W, Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) Manual (part 1): Modules 1–4 (Western Psychological Services, 2012). [Google Scholar]
- 48.Roid GH, Stanford Binet's Intelligence Scales, Fifth Edition, Technical Manual (Riverside Publishing, 2003). [Google Scholar]
- 49.Guy W, ECDEU Assessment Manual for Psychopharmacology (U.S. Department of Health, Education, and Welfare, 1976). [Google Scholar]
- 50.Baron-Cohen S, Wheelwright S, Spong A, Scahill V, Lawson J, Are intuitive physics and intuitive psychology independent? A test with children with Asperger syndrome. J. Dev. Learn Disord. 5, 47–78 (2001). [Google Scholar]
- 51.Korkman M, Kirk U, Kemp S, NEPSY-II Clinical and Interpretive Manual (Harcourt Assessment Inc., 2007). [Google Scholar]
- 52.Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C, The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res. 168, 242–249 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garvey CA, Gross D, Freeman L, Assessing psychotropic medication side effects among children. A reliability study. J. Child Adolesc. Psychiatr. Ment. Health Nurs. 4, 127–131 (1991). [DOI] [PubMed] [Google Scholar]
- 54.Yudofsky SC, Silver JM, Jackson W, Endicott J, Williams D, The overt aggression scale for the objective rating of verbal and physical aggression. Am. J. Psychiatry 143, 35–39 (1986). [DOI] [PubMed] [Google Scholar]
- 55.Gregory SG, Connelly JJ, Towers AJ, Johnson J, Biscocho D, Markunas CA, Lintas C, Abramson RK, Wright HH, Ellis P, Langford CF, Worley G, Delong GR, Murphy SK, Cuccaro ML, Persico A, Pericak-Vance MA, Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med. 7, 62 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang S-S, Kamphuis W, Huitinga I, Zhou J-N, Swaab DF, Gene expression analysis in the human hypothalamus in depression by laser microdissection and real-time PCR: The presence of multiple receptor imbalances. Mol. Psychiatry 13, 786–799 (2008). [DOI] [PubMed] [Google Scholar]
- 57.Lossie AC, Lo C-L, Baumgarner KM, Cramer MJ, Garner JP, Justice MJ, ENU mutagenesis reveals that Notchless homolog 1 (Drosophila) affects Cdkn1a and several members of the Wnt pathway during murine pre-implantation development. BMC Genet. 13, 106 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Constantino JN, Todd RD, Intergenerational transmission of subthreshold autistic traits in the general population. Biol. Psychiatry 57, 655–660 (2005). [DOI] [PubMed] [Google Scholar]
- 59.Constantino JN, Todd RD, Autistic traits in the general population: A twin study. Arch. Gen. Psychiatry 60, 524–530 (2003). [DOI] [PubMed] [Google Scholar]
- 60.Constantino JN, The quantitative nature of autistic social impairment. Pediatr. Res. 69, 55R-62R (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, Metzger LM, Shoushtari CS, Splinter R, Reich W, Validation of a brief quantitative measure of autistic traits: Comparison of the social responsiveness scale with the autism diagnostic interview-revised. J. Autism Dev. Disord. 33, 427–433 (2003). [DOI] [PubMed] [Google Scholar]
- 62.Yatawara CJ, Einfeld SL, Hickie IB, Davenport TA, Guastella AJ, The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: A randomized clinical crossover trial. Mol. Psychiatry 21, 1225–1231 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aman MG, Findling RL, Hardan AY, Hendren RL, Melmed RD, Kehinde-Nelson O, Hsu H-A, Trugman JM, Palmer RH, Graham SM, Gage AT, Perhach JL, Katz E, Safety and efficacy of memantine in children with autism: Randomized, placebo-controlled study and open-label extension. J. Child Adolesc. Psychopharmacol. 27, 403–412 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parker KJ, Oztan O, Libove RA, Sumiyoshi RD, Jackson LP, Karhson DS, Summers JE, Hinman KE, Motonaga KS, Phillips JM, Carson DS, Garner JP, Hardan AY, Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism. Proc. Natl. Acad. Sci. U.S.A. 114, 8119–8124 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lam KSL, Aman MG, The repetitive behavior scale-revised: Independent validation in individuals with autism spectrum disorders. J. Autism Dev. Disord. 37, 855–866 (2007). [DOI] [PubMed] [Google Scholar]
- 66.Wigham S, McConachie H, Systematic review of the properties of tools used to measure outcomes in anxiety intervention studies for children with autism spectrum disorders. PLOS ONE 9, e85268 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.White SW, Oswald D, Ollendick T, Scahill L, Anxiety in children and adolescents with autism spectrum disorders. Clin. Psychol. Rev. 29, 216–229 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mead R, The Design of Experiments: Statistical Principles for Practical Applications (Cambridge Univ. Press, 1988). [Google Scholar]
- 69.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG, Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 42, 377–381 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neter J, Kutner MH, Nachtscheim C, Wasserman W, Applied Linear Statistical Models (McGraw-Hill, ed. 4, 1996). [Google Scholar]
- 71.Grafen A, Hails R, Modern Statistics for the Life Sciences (Oxford Univ. Press, 2002), pp. XV, 351. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Raw data and SAS code for the data and analyses shown in Table 1.
Table S2. Participants’ stable concomitant medications during the 4-week AVP treatment trial.
Table S3. Raw data and SAS code testing whether parents could ascertain treatment condition.
Table S4. Raw data and SAS code testing whether bottle weights differed between treatment conditions.
Table S5. Raw data and SAS code for Fig. 2A and associated analyses.
Table S6. Raw data and SAS code testing treatment effects for the SRS-2 using an unweighted analysis.
Table S7. Raw data and SAS code testing treatment effects for the SCI subscale of the SRS-2.
Table S8. Raw data and SAS code correlating parent SRS-2 ratings with clinician CGI evaluations.
Table S9. Raw data and SAS code for Fig. 2B and associated analyses.
Table S10. Raw data and SAS code for Fig. 2C and associated analyses.
Table S11. Raw data and SAS code for Fig. 2D and associated analyses.
Table S12. Raw data and SAS code for Fig. 2E and associated analyses.
Table S13. Raw data and SAS code for Fig. 2F and associated analyses.
Table S14. Raw data and SAS code testing treatment effects for the RRB subscale of the SRS-2.
Table S15. Change from baseline in the safety assessments for the 4-week AVP treatment trial.
Table S16. Raw data and SAS code for the data and analyses shown in table S15.