Abstract
Mitochondria sense and respond to many stressors and can support either cell survival or death through energy production and signaling pathways. Mitochondrial responses depend on fusion-fission dynamics that dilute and segregate damaged mitochondria. Mitochondrial motility and inter-organellar interactions, including with the endoplasmic reticulum, also function in cellular adaptation to stress. In this Review, we discuss how stressors influence these components, and how they contribute to the complex adaptive and pathological responses that lead to disease.
Cells maintain a dynamic balance of key intracellular parameters, which is constantly perturbed by internal and external “stressors”. The “stress response” is the process elicited by stressors to restore homeostasis. A critical aspect of all molecular and physiological stress response systems is their requirement for energy, in part provided by mitochondria 1. Mitochondria are unique organelles with their own genome, which sustain life via energy transformation and perform several biochemical functions implicated in intracellular signaling and dynamics. Mitochondrial stress responses are central to cell fate,,health and disease at the tissue and organismal level (Figure 1)2, 3. Mitochondrial dynamics are also critical to stress responses2. Understanding the types of mitochondrial stressors, their interplay with mitochondrial dynamics and the mechanisms that orchestrate how cells or organisms respond to them is critical to understanding the transition between health and disease. In the following sections we discuss the mechanisms underlying these facets of mitochondrial biology and their integration with other contributing factors in adaptation and maladaptation of cells, tissues and organisms.
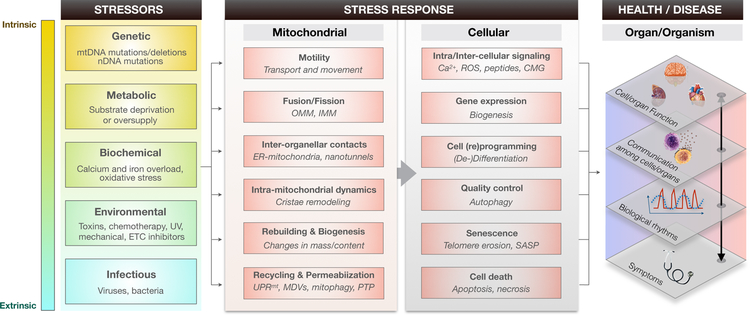
Figure 1. Framework outlining elements of the overall mitochondrial stress response and the link to disease, with a focus on mitochondrial dynamics.
Stressors affecting mitochondria vary in nature and origin (left). Intrinsic stressors are those that arise from the molecular and biological components of the organism itself, such as DNA mutations and the product of chemical reactions. Stressors induce specific stress responses involving multiple facets of mitochondrial dynamics and key cellular processes (center). Components of the mitochondrial and cellular stress responses are not mutually exclusive, and also interact and influence each other (not depicted in the figure). Collectively, stress responses affect health and disease trajectories in a multi-level way by influencing inter-related domains of organ and organism function (right). Physiology and pathology can therefore manifest at each level, clinically at the level of symptoms and disorders (e.g., fatigue, ataxia, ophtalmoplegia), and sub-clinically in the disruption of biological rhythms (e.g., circadian oscillations, mitochondrial membrane potential oscillations), of cell-cell communication (e.g., production of mitochondria-derived metabolites, pro-inflammatory molecules and cytokines), or of organ systems (e.g., brain and cognitive function, cardiac contractility, hormone biosynthesis). Levels of function are interconnected. Abbreviations: mtDNA, mitochondrial DNA; nDNA, nuclear DNA; OMM, outer mitochondrial membrane; IMM, inner mitochondrial membrane; ER, endoplasmic reticulum; UPRmt, mitochondrial unfolded protein response; MDVs, mitochondria-derived vesicles; PTP, permeability transition pore; ROS, reactive oxygen species; CMG, circulating mitochondrial genome (also ccf-mtDNA); SASP, senescence-associated secretory profile; ETC, electron transport chain.
Mitochondrial stressors and cellular stress responses
Stressors can be chemical or physical, acute or chronic (Figure 1, left). Many stressors target non-mitochondrial cell constituents, but pathways often converge on the mitochondrion, reflecting its key role in energy production and signaling required for surviving and adapting to stressors1. Mitochondria are integral to programmed cell death required for removal of cells fatally damaged by stressors that exceed the cell’s adaptive capacity 2, 3. Other stressors directly target and interfere with mitochondrial functions, including oxidative phosphorylation, intermediate metabolism, cell death, calcium signaling or cell dynamics2.
Among stressors that directly target mitochondria are genetic alterations in mitochondrial and nuclear DNA genes (mtDNA and nDNA genes respectively) that encode >1,000 mitochondrial proteins, including the mtDNA maintenance machinery3. Accumulation of mtDNA mutations or mtDNA depletion interferes with oxidative metabolism and disrupts electron transport chain (ETC) function, which impairs mitochondrial ATP production, one-and two-carbon metabolism, and the transmembrane potential (ΔΨm) and proton motive force that drive multiple mitochondrial functions3. nDNA mutations may directly alter other mitochondrial functions, including their dynamics4.
Deprivation of mitochondrial fuel substrates or oversupply of nutrients, including glucose and fatty acids, are also mitochondrial stressors4. Disturbances of intracellular iron5 and calcium (Ca2+) homeostasis, such as prolonged stimulation by Ca2+-linked agonists, insufficient cytoplasmic Ca2+ clearance or decreased mitochondrial Ca2+ gatekeeping are also stressors6, 7. Various stressors induce increased reactive oxygen species (ROS) production by the respiratory chain, with mitochondria being a prominent source of ROS and subject to ROS-mediated injury8. Physiological ETC activity generates ROS that may support signaling mechanisms. However, ETC dysfunction leads to increased ROS production that is commonly pathogenic3, 8. Although mitochondrial ROS is an ETC stress response, mitochondria can also be the target of ROS produced by other organelles or of extracellular origin (e.g. generated by ultraviolet (UV) light), in which ROS is a mitochondrial stressor that can elicit changes in mtDNA (e.g. mutations or deletions), mitochondrial lipids and proteins8.
Numerous cell permeable toxins also target mitochondria. Rotenone and Antimycin A inhibit complex I and III, respectively, to enhance ROS, whereas the proton ionophores FCCP and DNP depolarize mitochondria to uncouple ETC from ATP production9. Alcohol is metabolized in the mitochondria to give rise to toxic products10. Staurosporine targets apoptosis-related proteins located at or translocated to mitochondria, including BAX and BAK11. Pro-apoptotic chemicals are commonly used research reagents, whereas inhibitors of anti-apoptotic BCL-2 family proteins have been developed for anti-tumor therapy11. Infectious agents such as bacteria and viruses also commonly target mitochondrial function and structure, and elicit immune responses involving mitochondria.
Mitochondrial dynamics
Mitochondrial function and response to stimuli is defined by their complex structure and dynamics. Mitochondria contain outer and inner mitochondrial membranes (OMM and IMM), which border the intermembrane space (IMS) and the matrix. The IMM associates with the OMM at contact points and forms extensive inward folding to form cristae. Each of these compartments has discrete functions in oxidative metabolism, biosynthetic pathways and signaling12. Mitochondrial dynamics involve reshaping, rebuilding and recycling events that support mitochondrial stability, abundance, distribution and quality and allow compensatory changes when cells are challenged (Figure 2AB).
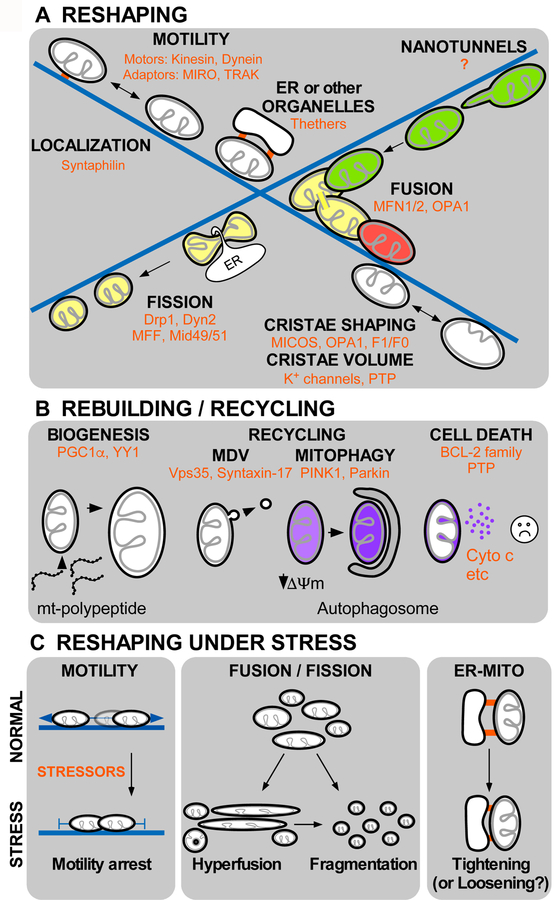
Figure 2. Components of mitochondrial dynamics and their response to stress:
A. Reshaping, localization and motility of mitochondria (depicted by black OMM and gray IMM) along the microtubules (blue) supported by molecular motors (Kinesin and Dynein) and adaptors (MIRO and TRAK) facilitates the inter-organelle communication and physical tethering with the ER or other organelles. Mitochondrial fusion (green and red organelles merge to result yellow post-fusion content) occurs in association to microtubules and mediated by GTPase proteins located at the OMM (MFN1/2) and IMM (OPA1). Fission of mitochondria is also supported by association with the ER, and triggered by DRP1 and Dynamin2 GTPases. Recently described dynamic processes are mitochondrial nanotunnel formation that also depends on interaction with microtubules, intra-mitochondrial dynamics directed by MICOS (mitochondrial contact site and cristae organizing system), OPA1 and F1/F0 (ATP synthase) and matrix volume changes, depending on IMM K+ channels and the Permeability Transition Pore (PTP). B. Rebuilding and recycling processes, mitochondrial biogenesis involves expression of organelle-targeted proteins upon activation of transcriptional factors PGC1-α and YY1, and phospholipids biosynthesis. Recycling of mitochondria can be mediated by mitochondria derived vesicles (MDVs) regulated by Vps35, Syntaxin-17, and mitophagy, driven by PINK1 and Parkin. Mitochondria host cell death signaling pathways that control cytochrome c release to decide on cell survival or removal C. Mitochondrial reshaping under stress. Diverse stressors (red) trigger adaptive responses in mitochondrial reshaping processes. Stressors commonly cause mitochondrial motility arrest, hyperelongation and donut formation or total fragmentation. Under stress, ER-mitochondria contacts usually become tighter but loosening has also been documented.
Reshaping mechanisms do not affect total mitochondrial mass and commonly represent reversible changes at the individual organelle level. By contrast, rebuilding and recycling alter mitochondrial mass and include unidirectional processes such as de novo synthesis of mitochondrial building blocks by mitochondrial biogenesis13, and recycling via mitochondrial derived vesicles (MDVs) or mitophagy, in which mitochondria are selectively targeted to autophagosomes for degradation14. Large-scale OMM or IMM permeabilization triggers cells to die, allowing replacement by surviving cells carrying healthy organelles. Whereas recycling has been covered by recent reviews2, emerging evidence links stress to reshaping, and is the main subject of this Review. Reshaping, rebuilding and recycling mechanisms are complexly interrelated, for example with respect to metabolic adaptation to changes in substrate availability after birth15.
Mitochondrial reshaping mechanisms and responses to stressors
Mitochondrial Motility
Mitochondrial trafficking and localization throughout the cytoplasm depends on interactions with the cytoskeleton and molecular motors16. MIRO1/2 are OMM-localized small GTPase-like proteins17 that anchor mitochondria to either kinesin or dynein (Figure 2A), motors for anterograde or retrograde displacement along microtubules, respectively, via TRAK1/2 adaptors16. Myosin motors can also facilitate mitochondrial positioning18 and short-distance movement along actin filaments19. Stable mitochondrial localization in axons is supported by syntaphilin, an OMM protein directly linking mitochondria to microtubules20.
Asymmetry and compartmentalization of cellular behavior require mitochondrial transport to different cellular regions. Motility is central to partitioning mitochondria for cell division21, 22. Mitochondrial transport along axons and dendrites and accumulation in the regions of high energy demand are required to maintain neural activity23, 24. Movements of energy-producing organelles may redistribute the spatial pattern of ATP production and Ca2+ buffering25. Mitochondrial movements also support fusion-fission26 and organelle recycling27. Impairment of axonal mitochondrial transport is linked to neurological phenotypes in mouse models targeting MIRO23, 28, 29 or MFN230, 31 (Table1).
Table 1: Mouse models for mitochondrial reshaping proteins.
A summary of defects observed for reshaping proteins at the level of mitochondrial dynamics and bioenergetics, targeted organs, systemic consequences and response upon stressor exposure. MEF=Mouse Embryonic Fibroblasts; SM= Skeletal muscle, CM=Cardiomyocytes, ETC= Electron transport chain activity.
| Genetic stressor | Mitochondrial dynamics & bioenergetics defects | Affected organs | Pathology & Symptoms | Interaction with other stressors | Ref. |
|---|---|---|---|---|---|
| Mfn1−/− | Fragmentation, ↓fusion & ΔΨm | Not known | Development delay, Lethal E12.5 | Not known | 62 |
| Mfn2−/− | Fragmentation, ↓ fusion &ΔΨm | Placenta | Lethal E11.5 | Not known | 62 |
| Cerebellum, conditional66 | Fragmentation, altered distribution & cristae, ↓ COX & SOD & nucleoid | Cerebellum | Locomotion defects | Not known | 66 |
| Heart, inducible70 | ↑ Area, ↓ [Ca2+]mt uptake & SR-mito tethering | Heart | Not known | Isoproterenol: Ca2+ and ETC, dysregulation | 70 |
| T105M+/+78 T105M+/− neuroectoderm79 | Altered distribution, ↓abundance in peripheral nerves | Hindlimbs, SM | CMT2A-like | Not known | 78,79 |
| Mfn2 R94W +/+ Mfn2 R94W +/− | Fragmentation, ↓ATP | Brain, pan-neuronal | Lethal @ P1 CMT2A-like | Not known | 68 |
| Mfn2 R94Q, neuro | ↑ number in motoneurons | Neurons | CMT2A-like | Not known | 67 |
| Mfn1/2 SM, conditional | ↑ Area, cristae defects, ↓mtDNA,↑deletions,↓COX | SM | Low body weight, SM atrophy | Exercise: ↑ lactate | 75 |
| Mfn1/2 Heart, conditional71–73 Heart, inducible adult71, 74 | Fragmentation, cristae defects72, 73, ↓mtDNA, ↓biogenesis, ↓COX72 Fragmentation, ↓OCR71, 74 ↓ER-mito tethering74 | Heart | Lethal E9.571, E1573. ↓cardiac function (P7) & death <3wks, heart failure 72, ↓ cardiac development73 Dilated cardiomyopathy71 | Ischemia/Reperfusion: Protection 74 | 71–74 |
| Mfn1/2 &Drp1 KO, Inducible | Clustering, ↓OXPHOS & impaired mitophagy | Heart | Cardiac hypertrophy Heart failure | Not known | 177 |
| Opa1−/− | Not known | Not known | Developmental delay, lethal E13.5 | Not known | 63 |
| Opa1−/+ Q285STOP | Fragmentation63, 81, ↓ OCR & Complex IV81. Heart: cristae defects, ↓ mtDNA, OCR, ATP & ETC82 | Retina, brain, spleen, liver, heart | ADOA-like63, Dendro-pathy80, Late-onset cardiomyopathy82 | ER-stress-induced apoptosis: resistance81 Ischemia/Reperfusion: ↓ viability82 | 63, 80–82 |
| Opa1−/+ (c.1065 + 5G→A) | Cristae defects in optic nerve | Optic Nerve | (+/+): Lethal <E12 (+/−): ADOA –like, ↓retinal ganglion cells | Not known | 69 |
| Opa1delTTAG−/+83, 178 | Fragmentation, ↑ cristae area83, ↓Ca2+ uptake in CM 178 | Optic nerve, SNS, SM | (+/+): Lethal E10.5 ADOA-like, deafness, locomotion defects 83 | Ischemia/Reperfusion: ↑ infarct area178 |
83, 178 |
| Opa1−/−, SM, conditional76 and inducible76, 77 | ↓ mass, mosaic topology, cristae, ↓ ETC & supercomplex76, ↓ mtDNA, nucleoid # & OCR | SM, adipose tissue, liver, epithelium | Lethal P9, hypoglycemia, SM atrophy76, weakness, atrophy, inflammation, early aging76, myopathy77 | Aging: ↓ Opa1 Diet-induced obesity: Normal glucose level (28607005) | 76, 77 |
| Opa1−/−, β−Cells179 | Fragmentation, altered cristae & Complex IV | Pancreas | Hyperglycemia Glucose intolerance | Not known | 179 |
| Drp1−/− | Aggregation, hyperelongation | Placenta, brain, heart, vessels | Lethal E11.5–12.5, Brain hypoplasia | Bax-and Ca2+-linked apoptosis inducers: resistance | 64, 65 |
| Miro1−/− | ↓retrograde23 & anterograde28 transport. | Brainstem | Lethal P0, brainstem motor-neuron loss, short neurite. | Not known | 23, 28 |
| Miro2−/− | Normal shape & transport | No animal phenotype | Not known | 28 | |
| Miro1−/−neuronal | Lack of mitochondria in spinal cord axons | Brainstem, lumbar spinal cord | Upper motoneuron disease, Death P35 | Not known | 23 |
| Miro1/2−/− | Short and rounded, perinuclear gathering. | Placenta | Lethal E10.5, lack of vascularization | Not known | 29 |
Mitochondrial motility is controlled by cytoplasmic [Ca2+] ([Ca2+]c)25. Physiological [Ca2+]c transients suppress mitochondrial movements through MIRO1/2 EF-hand Ca2+ sensing domains32 and may involve other Ca2+ sensors23. The dynamic interplay between Ca2+ release, mitochondrial motility and mitochondrial Ca2+ uptake forms the basis for a homeostatic mechanism in mitochondrial distribution and calcium signaling25, 33. ROS also suppress mitochondrial motility in Ca2+-dependent and independent manners34, 35. Furthermore, extracellular glucose elevation leads to mitochondrial motility inhibition by activating O-GlcNAc transferase to target TRAK36.
Whereas Ca2+ signaling transients, ROS and glucose fluctuations are physiological regulators of mitochondrial motility, larger and more prolonged changes in the same factors can pathologically alter movement dynamics (Figure 2C) 7, 8. Ca2+ and ROS mutually strengthen each other and can generate cycles impairing motility35, 37. In skeletal myoblasts, H2O2 inhibits mitochondrial motility and prompts fragmentation38. In neurons and other cell types, ROS induces Ca2+ transients and activates mitogen-activated protein kinases (MAPKs) (JNK, p38) to cause mitochondrial motility inhibition35, 37. Starvation also activates p38 MAPK phosphorylation of ubiquitin ligase Gp78, interfering with mitochondrial motility and disrupting ER–mitochondrial contacts39. In injured axon zones, mitochondrial density increases to support axon regeneration by local energy production40, highlighting an adaptive response to acute injury. Mitochondrial density might increase because mitochondria are retained by stabilized syntaphilin41. Yet, in cortical neurons after mild, reversible mitochondrial stress induced by Antimycin A, mitochondria carrying syntaphilin are recycled by retrograde trafficking and fusion with late endosomes and lysosomes42. In cancer cells, hypoxia and ROS target alternatively spliced syntaphilin, enhancing mitochondrial trafficking associated with tumor cell migration and invasion43.
Mitochondrial transport in axons is suppressed by deletion or expression of disease mutants of the fusion protein mitofusin 2 (MFN2) that interacts with MIRO30. The mitochondrial motility machinery is also targeted by degradative pathways upon stress. Dissipation of ΔΨm leads to PINK1 stabilization, inducing Parkin to mark MIRO for proteasome degradation and halting mitochondrial movement, possibly to prevent spreading of dysfunctional organelles along neurons44. In cortical neurons, mitochondrial damage triggers PINK1/Parkin to induce MIRO1 ubiquitination on Lys27, arresting mitochondria for degradation45. Oxidative stress activates the OMM-associated PGAM5-KEAP1-Nrf2 pathway leading to MIRO2 proteasomal degradation, causing mitochondrial retrograde trafficking and perinuclear localisation46. Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription47. Oxidative stress and starvation are also sensed by Myo19, an actin-linked motor that retains mitochondria in areas of low ATP/ADP ratio48. Upon mechanical injury to neurons, axon regeneration depends on ARMCX1 expression localized to mitochondria, which enhances mitochondrial transport49. Mitochondrial stress responses involve the mitochondrial motility machinery, allowing mitochondrial redistribution to areas where bioenergetic needs are increased, or by recycling damaged organelles25.
Mitochondrial fusion and fission dynamics
Mitochondrial movements along cellular tracks facilitate encounters between two distant organelles, permitting fusion 26, 32 involving successive mixing of compartments: i) OMM merging mediated by the mitofusin 1 (MFN1) and MFN2 GTPases, ii) IMS mixing, iii) IMM fusion mediated by OPA1 GTPase, and iv) matrix complementation50, 51. The fusion product can remain an individual organelle or undergo division upon cytoplasmic DRP1 GTPase recruitment to the OMM by MFF52 mediated by MID49/5153. Transient actin polymerization at the OMM constriction site54 and cytoplasmic Dynamin 2 facilitate fission completion55. Mitochondria fission can also be facilitated by motors of opposing directions26 (Figure 2A).
Fusion-mediated mitochondrial component sharing supports multiple elements of mitochondrial biology: mtDNA integrity56, mitochondrial respiration57, ΔΨm equilibration58, apoptosis51 and signaling events such as [Ca2+]c oscillations59. Fission can facilitate motility and is required for segregation of damaged mitochondria for mitophagy58, mtDNA replication60 and mitochondrial redistribution during cell division61. Thus, mitochondrial fusion/fission dynamics is central to organelle quality control and a variety of cellular functions. To test the physiological relevance of fusion-fission proteins, and the pathophysiology associated with their perturbation, genetic models have been created for MFNs, OPA1 and DRP1 in mouse (Table 1). Whole body knockouts for each interfere with early development and are embryonic lethal62–65. Organ-specific knockouts are either lethal or cause severe dysfunction of the affected organ:, as observed in the nervous system23, 66–69, heart70–74 and skeletal muscle75–77. Mice expressing human disease-causing mutations of MFN2 or OPA1 display the symptoms of Charcot-Marie-Tooth type 2A disease (CMT2A) or Autosomal Dominant Optical Atrophy (ADOA), respectively63, 67, 78–82,83 (Table 1).
Mitochondrial fusion-fission balance is regulated transcriptionally and post-transcriptionally. MFN2 expression is enhanced by PGC-1α (peroxisome proliferator gamma coactivator 1 alpha)84. MFN1/2 and OPA1 levels and activity are affected by phosphorylation85, redox modifications86, acetylation87 and ubiquitination88, 89. OPA1 is proteolytically processed by the AAA proteases OMA1 and YME1L, which are regulated by ΔΨm90 and OXPHOS activity91, respectively. OPA1 function is regulated by cardiolipin in the IMM to prompt fusion92. DRP1 recruitment to the OMM and fission are controlled by Ca2+-and cAMP-stimulated phosphoregulatory events93.
Through a combination of the mechanisms described above, stressors can cause a hyper-elongated mitochondrial network by stimulation of fusion and/or inhibition of fission; or fragmented mitochondria by stimulation of fission and/or inhibition of fusion. Hyper-elongation and fragmentation can occur sequentially within minutes to hours35 (Figure 2C). A given stressor can induce distinct mitochondrial fusion-fission phenotypes in different cells and tissues, and different stressors can induce opposing phenotypes in the same cell type. Although incompletely understood, specific mitochondrial responses to a given stressor are likely determined by a combination of interacting factors, including fusion-fission factor basal expression, biochemical and metabolic cell state, chronic stressors, and other unknown factors. Thus, a complex relationship exists between stressors and dynamic fusion-fission phenotypes.
In terms of mechanisms affecting fusion, several stressors converge on OMA1 and YMEL1 proteases, which control OPA1 fusogenic activity94. Increased OXPHOS activity in cells grown on ketogenic carbon sources promotes YME1L-mediated OPA1 processing, increasing fusion91. Mitochondrial poisons causing oxidative stress and ATP depletion suppress YME1L activity via degradation94. Mouse embryonic fibroblasts (MEFs) and cell line exposure to UV-irradiation, serum deprivation or protein synthesis inhibitors leads to mitochondrial hyperfusion, dependent on MFN1 and OPA1 and an IMM scaffold protein, SLP295. SLP2 restricts OMA1-mediated OPA1 processing to support hyperfusion96. Downstream of hyperfusion and engagement of the mitochondrial E3 ubiquitin ligase/SUMOylase MULAN/MAPL, NFkB is activated, likely as an adaptive mechanism to promote anti-apoptotic protein expression97. Pathological cardiac stress leads to mitochondrial fusion inhibition through OPA1 hyperacetylation, normally prevented by the mitochondrial matrix deacetylase, SIRT387. Conversely, pressure overload challenge in the heart activates TNFα receptor type 2, OPA1 expression and fusion dependent on STAT3 and NFkB activation98.
As to fission mechanisms and regulation, starvation induces hyperfused mitochondria by inhibiting fission, protecting the organelle from autophagic degradation93, 99. High glucose causes mitochondrial fission to mediate apoptosis in pancreatic beta-cells100. Similarly, high glucose exposure of cardiac cells leads to elevated ROS and mitochondrial fission101, whereas acute intracellular ROS elevation leads to DRP1-mediated mitochondrial fission102. ETC inhibition by rotenone or antimycin A recruits DRP1, promoting fission to support autophagic removal of damaged organelles via AMPK activation mediated by MFF phosphorylation103. AMPK likely senses an AMP/ATP ratio increase to phosphorylate MFF but other factors may function in this process, as glucose starvation-related increase in AMP/ATP ratio is associated with inhibited fission. An oxidative stress-response pathway, Keap1-NRF2 is a key regulator of DRP1 levels, leading to hyperelongated mitochondria and cell survival104. Mitochondrial depolarization combined with sustained [Ca2+]c elevation activates the cytoplasmic phosphatase calcineurin that dephosphorylates DRP1 Ser637 to stimulate mitochondrial fission105. In skeletal muscle, metabolic oversupply during sustained contractile inactivity also causes DRP1 Ser616 phosphorylation associated with mitochondrial fragmentation106. Conversely, upon high fat diet, calcineurin inhibition prevents DRP1 Ser637 dephosphorylation leading to hyperelongated mitochondria and improved metabolic performance in skeletal muscle107. Mechanical stress induced by bacterial infection also leads to mitochondrial fission108. DRP1 activation has been linked to apoptosis mediated by BCL-2 family proteins2. However, most of the above results suggest that long mitochondria provide protection against stressors. Whereas shorter mitochondria are thought to be maladaptive, this is not always the case, as mitochondrial shortening by fission supports lymphocyte migration109 and effector T cell activation110.
Fusion-fission perturbation by stressors gives rise to complex mitochondrial shapes. Hypoxia-reoxygenation and other stressors can cause donut-shaped mitochondria via autofusion between the two ends of tubular mitochondria111. In H9c2 cells, this is preceded by matrix expansion dependent on PTP or K+ channel opening and ensuing partial detachment from the microtubular track111. Donut formation is a stress response and may protect against swelling-induced structural damage111.
Nanotunnels
Even when physically separated by several microns, mitochondria can form connections through 60–200 nm wide double membrane tubules called mitochondrial nanotunnels112. Nanotunnels have been observed in cardiac113 and skeletal muscle59, and can be generated in a cell-free system by kinesin (Kif5b) in a microtubule-and ATP-dependent manner114. Protein exchange along nanotunnels115 suggests that mitochondrial nanotunnels may serve functional but possibly not genetic complementation between non-adjacent mitochondria, in tissues with restricted mitochondrial motility112. In mice carrying a ryanodine receptor (RyR) 2 mutation (A4860G) associated with human catecholaminergic polymorphic ventricular tachycardia due to aberrant Ca2+ homeostasis, mitochondria display increased nanotunnel communication116. Mitochondrial nanotunnels are frequent in skeletal muscle of mitochondrial myopathy patients carrying mtDNA deletions or mutations, suggesting that nanotunnels support mitochondrial adaptation to genetic stressors117.
Homotypic mitochondrial contacts
Mitochondria can exhibit trans-mitochondrial coordination in muscle tissues. When joined by molecularly undefined electron-dense intermitochondrial junctions (IMJs), two adjacent mitochondria can exhibit aligned cristae118. IMJs are molecularly independent from MFNs but are induced within 30 minutes by physically tethering mitochondria through inter-organellar linkers in vitro118. Increased energetic demand during muscle contraction119, and decreased energetic demand during inactivity106, increase and decrease IMJ number, respectively. MitoNEET, an OMM iron-sulphur cluster forming protein, functions in IMJs and has been linked to H2O2-induced mitochondrial fragmentation120.
Heterotypic inter-organelle communication
Mitochondria dynamically form close contacts with various intracellular organelles (<100nm gap width), which represent a small fraction of the total organellar surface and allow effective local communication without altering the global milieu (Figure 2A)121, 122. The most frequent mitochondrial companion is the endoplasmic reticulum (ER). ER-mitochondrial membrane contacts are reorganized to meet local needs123 and are supported by physical protein-based tethers124. Over 60 proteins have been implicated in tethering and many support specific functions122. MFN2 can cause diverse contact phenotypes in different paradigms, which may be determined by other tethering proteins122. These contacts function in phospholipid biosynthesis, Ca2+ transfer between ER and mitochondria, ROS signaling, mitochondrial fission, autophagy and mtDNA synthesis121, 122. Thus, ER-mitochondrial contacts represent a dynamic aspect of mitochondrial behavior impacted by stressors and relevant to other mitochondrial functions.
Contact dynamics are controlled by physical tether formation and destruction. This can be induced by physiological changes in tethering protein abundance, membrane phospholipids and [Ca2+]121. The ER may stop other organelles in their vicinity by emitting Ca2+ signals favoring contact formation25. Serum starvation or ER-specific stressors such as tunicamycin cause ER-mitochondrial contact tightening to promote cell death124, 125. Stressors converging on ROS/redox dysregulation have also been linked to changes in ER-mitochondrial contact architecture: hypoxia widens contacts in a Nogo-dependent manner126, whereas cardiac ischemia/reperfusion causes tighter contacts via PTPIP51127. Virus infections including CMV128, chronic hepatitis C or acute RNA virus129 enhance ER-mitochondrial contacts. Thus, a variety of stressors promote closer contacts that might facilitate local communication between interacting organelles (Figure 2C).
Stressors also alter the distribution of specific proteins relative to organelle contacts, influencing contact function. Palmitoylation affects calnexin distribution130 and a shift to a hypoxic/reducing environment influences ERO1α to leave ER-mitochondrial contacts131. Stressors affect Ca2+ and ROS signaling pathways at the ER-mitochondrial interface via several mechanisms. The IP3 receptor (IP3R), which mediates local Ca2+ transfer in the ER, and the RYR in the sarcoplasmic reticulum, are targets of redox regulation132, 133. Moreover, alteration of ER-mitochondrial Ca2+ communication affects other aspects of mitochondrial dynamics including fragmentation134 and autophagy135.
Several signaling pathways of BCL-2 family proteins that reside and exert pro-survival or pro-death functions in the ER and OMM have been linked to the ER-mitochondrial contacts136, 137. Sphingolipid metabolism and ceramide production at ER-mitochondrial contacts is central to Bak/Bax-mediated OMM permeabilization and ensuing cell death138. In addition to the ER, mitochondria form dynamic contacts with other organelles, including lysosomes, peroxisomes and lipid droplets, which may function during stress, such as in fatty acid shuttling from lipid droplets to mitochondria during starvation139.
Intra-mitochondrial dynamics to shape cristae and adjust matrix volume
A distinctive feature of mitochondrial ultrastructure is IMM folding into cristae12 that allows ETC component organization into supercomplexes to enhance bioenergetic efficiency140. Mitochondrial cristae display dynamic changes with different metabolic states141. Cristae shape is supported by F1F0 ATPase localization at the IMM bending regions142. Cristae junctions are secured by the mitochondrial contact site protein complex (MICOS) that helps shape cristae and organize the ETC complexes143, 144. MIC60, a MICOS component critical to IMM bending to support cristae formation145, interacts with OPA184. Oligomeric OPA1 is needed to keep cristae junctions closed146.
As a physiological adaptation to increased metabolic demands, cristae remodeling with increased density occurs in exercised skeletal muscle147. During starvation, OPA1 oligomerization is enhanced to keep cristae narrow, which is required to promote F1F0 ATPase assembly and maintain ATP-linked respiration. Mutant OPA1(Q297V) that undergoes oligomerization but is defective in fusion can support survival during starvation148. Apoptosis-promoting stressors through the BH3-only protein BID interfere with OPA1 oligomerization and trigger cristae junction opening to make cytochrome c available for release146, 149. ROS modulator 1 (ROMO1), regulates OPA1 to control cristae organization and enhance mitochondrial resistance to BID-induced cristae junction opening and cytochrome c release150.
Mitochondrial dynamics and stress responses leading to disease
The previous section discussed specific mechanisms by which stressors influence different facets of mitochondrial dynamics, promoting cellular adaptation or demise. Below we discuss three stereotypic mitochondrial stress response patterns and how they are translated into disease states. Abnormal mitochondrial dynamics are associated with morphological, genetic, and biochemical mitochondrial recalibrations that trigger cellular stress responses2, 3. These recalibrations engender the production of diffusible signals that influence the organism at multiple levels (Figure 1, right), and cause disease in some cases by inducing mtDNA instability3.
Stress responses can have both adaptive and maladaptive effects. Adaptive effects contribute to resilience, whereas maladaptive effects contribute stress pathophysiology and disease state development. As adaptation becomes exhausted and maladaptation becomes dominant, the organism transitions from physiology to pathology (Figure 3A). Based on onset and duration, we distinguish 3 types of stressors which cause different stress response patterns: i) Early onset, chronic; ii) Late onset, acute; and iii) Late onset, chronic (Figure 3B–D).
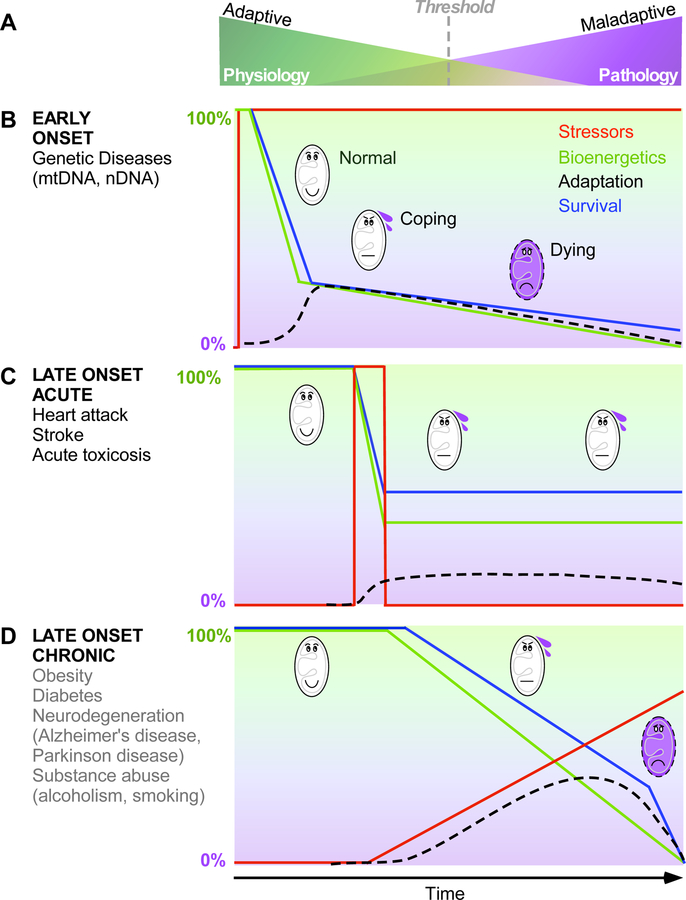
Figure 3. Influence of stressor types on mitochondrial stress responses that progress to disease.
A. Biological systems stand in balance between adaptative and maladaptative states that determine physiological and pathological outcomes. The threshold between these states can vary between individuals, and over time. B-D. Three main types of stressors can be identified based on their onset and duration. Stressors refer to a singular perturbation with a time of onset and specific duration; Bioenergetics is the capacity to use OXPHOS to transform energetic substrates and oxygen into ΔΨm to generate ATP and perform work (e.g., Ca2+ uptake), illustrated here as the transition from green to purple; Adaptation reflects the activation of secondary processes (e.g., gene expression, mitochondrial biogenesis, increased contractility) that act to compensate for bioenergetic defects. Survival indicates the ability of cells and organs to sustain viability and normal functions. B. EARLY ONSET stressors are generally chronic in nature, such as inherited mtDNA and nDNA mutations that alter key components of mitochondrial dynamics and bioenergetics. Early onset chronic stressors may cause a substantial initial loss in bioenergetic capacity (i.e., fitness) and lead to progressive decline in survival. C. LATE ONSET ACUTE stressors are punctual, arising from chemical exposure, ischemia, or other reversible event. Late onset acute stressors generally induce a rapid and substantial loss of bioenergetic capacity and survival associated with an induction of compensatory adaptive processes that may remain elevated beyond the duration of the stressor, enabling the maintenance of sub-maximal but sufficient functional capacity. D. LATE ONSET CHRONIC stressors are those that also arise punctually later in life but remain active and often progress in intensity, such as metabolic dysregulation in diabetes, neurodegenerative processes, and toxic compound exposure from substance abuse. Late onset chronic stressors generally lead to progressive decline in bioenergetics and survival, the progression of which is reduced by compensatory adaptive mechanisms.
Early onset chronic stressors
Early onset stressors are generally chronic and produce progressive disease (Figure 3B). Inherited genetic defects in genes of the fusion/fission machineries are stressors that permanently alter mitochondrial dynamics or motility throughout life 3, 83. The consequences can be devastating but often the initial defect can be compensated for by increased activity of the quality control provided by mitochondrial dynamics. When the defect impairs a fraction of normal dynamics, these defects can be compensated for, but may be aggravated beyond compensation by the accumulation of subsequent stressors3. The accumulation of stressors, such as secondary mtDNA mutations, may overwhelm the system and cause disease once the biochemical threshold is reached151. In humans, the threshold between physiological adaptation and pathology may vary based on particular mutations, but is estimated to be around 60% of mtDNA mutation load151.
As in animal models (Table 1), autosomal mutations particularly in the fusion machinery (MFN2152, OPA163), but also in fission factors (DRP1153, MFF154) and a motor adaptor (TRAK1155) lead to mitochondrial disorders. The shared clinical symptoms for these neurodegenerative diseases are neurological impairments such as retinal ganglion cell degeneration and neuromuscular symptoms. OPA1, named after its genetic mutation, was shown to be the main cause of ADOA156, 157. MFN2 mutations cause approximately 20% of CMT2A cases, an inherited peripheral neuropathy characterized by abnormal mitochondrial trafficking30, 31. To date, no disease has been associated with mutations in MFN1. In part, the pathogenic mechanism involves the accumulation of mtDNA mutations and deletions that perturb OXPHOS75, 158, 159. However, in many cases, ETC dysfunction is absent, indicating that abnormal fusion activity and motility represent a sufficient stressor to affect cell-level and organ-level function153, 154. The canonical mitochondrial fusion-fission and motility dynamics proteins regulate other aspects of mitochondrial behaviors, and proteins such as MFN2 can cause disease via impairments of ER-mitochondrial communication, Ca2+ signaling or mitophagy160.
Late onset acute stressors
Late onset stressors are generally acquired, can be relatively short-lived, and do not generally affect mitochondrial quality or the ability to produce functional organelles (Figure 3C). Excess of metabolic substrates such as acute hyperglycemia and hyperlipidemia can activate PKA to promote DRP1-dependent fission4, 161. Hyperglycemia aIso increases ROS to mediate fission, and fission further augments ROS emission162. Both MFN1 and MFN2 are involved in metabolic sensing and regulation of whole-body energy homeostasis163, 164, illustrating the adaptive cellular role of mitochondrial dynamics in response to acute metabolic stressors. Ischemia-reperfusion injury, such as myocardial infarction or stroke, usually occur late in adult life. The acute drop in oxygen and metabolic substrates followed by rapid reoxygenation causes substantial remodeling of mitochondrial morphology dominated by DRP1-mediated fission165, 166, and the system rarely recovers full function. Some toxic insults cause an acute and permanent tissue injury such as doxorubicin that engages mostly ROS and mtDNA damage the cardiomyocytes167.
Late onset chronic stressors
Late onset chronic stressors occur mostly in adult life but produce lasting deleterious effects on the system (Figure 3D). Poorly controlled diabetes, which manifests as the chronic elevation of blood glucose and lipids, represents a chronic stressor that generally develops later in life168. The metabolic oversupply of diabetes increases fission with concurrent accumulation of mtDNA defects in various tissues168. Obesity is associated with reorganization of ER-mitochondrial contacts resulting in mitochondrial Ca2+ overload, compromised mitochondrial oxidative capacity and augmented oxidative stress169. Repeated environmental and chemical stressors, such as smoking and chronic alcohol abuse, are also late onset chronic stressors that influence mitochondrial dynamics and potentially alter the trajectory of primary mitochondrial diseases170. Chronic alcohol exposure leads to mitochondrial fusion inhibition in cardiac myocytes115 and in skeletal muscle by targeting MFN1 protein levels59. A number of stressors may therefore converge on different facets of mitochondrial dynamics and, when too high in duration and intensity, lead to maladaptive changes which alone or in combination with other stressors, may culminate in disease.
Most neurodegenerative diseases have been linked to primary or secondary changes in mitochondrial dynamics171, 172. In addition to the inherited genetic defects in the proteins assigned to mitochondrial dynamics (see Early Onset Chronic), mutations in other proteins including amyloid precursor protein, presenilins, and α-synuclein, common in neurodegenerative diseases, causes interference with mitochondrial dynamics’ 169.173. The dynamic structure and function of the ER-mitochondrial contacts seems to be affected in many of these cases173, 174. However, altered ER-mitochondrial contacts and other impairments of mitochondrial dynamics (i.e. fragmentation) are also documented in sporadic cases supporting the view that mitochondrial dynamics is central to the pathogenesis of neurodegeneration174, 175. ROS and Ca2+ dysregulation, often documented in neurodegenerative diseases, can interfere with various aspects of mitochondrial dynamics and can be part of cycles that drive disease progression34.
Conclusions and looking forward
Much progress has been made in dissecting the molecular mechanisms that underlie mitochondrial dynamics. Recent in vitro and in vivo work has begun to map the effects of specific disease-causing stressors on various facets of mitochondrial and cellular responses. A challenge ahead will be to understand how the resulting mitochondrial and cellular recalibrations, both acute and chronic, interact to produce symptoms. Single models with limited readouts do not appear sufficiently precise or inclusive to explain the complex phenotypic variability in symptoms that manifest in animals and individuals with abnormal mitochondrial dynamics. Given the interaction of stressors and responses at the molecular, cellular and organismal levels (see Figure 1, right), future efforts may require advances in concurrent measurement of functions across multiple levels of organization, and development of multivariate and biologically meaningful methods and concepts to integrate such multi-level data. This would contribute to understanding the processes that translate stressors into symptoms and disease.
Future work should aim to influence adaptive and maladaptive dynamic physiological responses (see Figure 3) and to restore them towards healthy states. This will require the ability to accurately map dynamic processes at the molecular and organellar levels, and to monitor changes in bioenergetics over considerable time periods. A further challenge is how best to address these questions in physiologically relevant disease models. Most studies highlighting the pathophysiological relevance of mitochondrial motility have been performed in experimentally convenient systems, such as neural axons and dendrites in vitro in which mitochondrial trafficking is prominent and easily tracked16, but may not represent in vivo behavior176. Understanding the physiological role of mitochondrial reshaping, rebuilding and recycling in specialized tissues remains vastly unexplored and an inspiring challenge for the field.
Acknowledgements
We thank David Weaver and Orian Shirihai for comments. This work was supported by FONDECYT 1150677 to V.E., the Wharton Fund, NIH-R35-GM119793 and R21-MH113011 to M.P., and, R01-DK51526, R33-ES025672 and UO1-AA021122 to G.H.
Footnotes
Authors declare that they do not have any competing interests.
References
- 1.Picard M, McEwen BS, Epel ES & Sandi C An energetic view of stress: Focus on mitochondria. Front Neuroendocrinol 49, 72–85 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Youle RJ & van der Bliek AM Mitochondrial fission, fusion, and stress. Science 337, 1062–5 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suomalainen A & Battersby BJ Mitochondrial diseases: the contribution of organelle stress responses to pathology. Nat Rev Mol Cell Biol (2017). [DOI] [PubMed] [Google Scholar]
- 4.Liesa M & Shirihai OS Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab 17, 491–506 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemasters JJ Rusty notions of cell injury. J Hepatol 40, 696–8 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Rizzuto R, De Stefani D, Raffaello A & Mammucari C Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol 13, 566–78 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Bagur R & Hajnoczky G Intracellular Ca2+ Sensing: Its Role in Calcium Homeostasis and Signaling. Mol Cell 66, 780–788 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holmstrom KM & Finkel T Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol 15, 411–21 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Nicholls DGF, S. J. . (2013). [Google Scholar]
- 10.Szabo G et al. RSA 2004: combined basic research satellite symposium - session three: alcohol and mitochondrial metabolism: at the crossroads of life and death. Alcohol Clin Exp Res 29, 1749–52 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Montero J & Letai A Why do BCL-2 inhibitors work and where should we use them in the clinic? Cell Death Differ 25, 56–64 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mannella CA Structure and dynamics of the mitochondrial inner membrane cristae. Biochim Biophys Acta 1763, 542–8 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Dominy JE & Puigserver P Mitochondrial biogenesis through activation of nuclear signaling proteins. Cold Spring Harb Perspect Biol 5 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Youle RJ & Narendra DP Mechanisms of mitophagy. Nat Rev Mol Cell Biol 12, 9–14 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong G et al. Parkin-mediated mitophagy directs perinatal cardiac metabolic maturation in mice. Science 350, aad2459 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwarz TL Mitochondrial trafficking in neurons. Cold Spring Harb Perspect Biol 5 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fransson A, Ruusala A & Aspenstrom P Atypical Rho GTPases have roles in mitochondrial homeostasis and apoptosis. J Biol Chem 278, 6495–502 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Pathak D, Sepp KJ & Hollenbeck PJ Evidence that myosin activity opposes microtubule-based axonal transport of mitochondria. J Neurosci 30, 8984–92 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quintero OA et al. Human Myo19 is a novel myosin that associates with mitochondria. Curr Biol 19, 2008–13 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y & Sheng ZH Kinesin-1-syntaphilin coupling mediates activity-dependent regulation of axonal mitochondrial transport. J Cell Biol 202, 351–64 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rohn JL et al. Myo19 ensures symmetric partitioning of mitochondria and coupling of mitochondrial segregation to cell division. Curr Biol 24, 2598–605 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung JY, Steen JA & Schwarz TL Phosphorylation-Induced Motor Shedding Is Required at Mitosis for Proper Distribution and Passive Inheritance of Mitochondria. Cell Rep 16, 2142–2155 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen TT et al. Loss of Miro1-directed mitochondrial movement results in a novel murine model for neuron disease. Proc Natl Acad Sci U S A 111, E3631–40 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaccaro V, Devine MJ, Higgs NF & Kittler JT Miro1-dependent mitochondrial positioning drives the rescaling of presynaptic Ca2+ signals during homeostatic plasticity. EMBO Rep 18, 231–240 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yi M, Weaver D & Hajnoczky G Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J Cell Biol 167, 661–72 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Weaver D, Shirihai O & Hajnóczky G Mitochondrial ‘kiss-and-run’: interplay between mitochondrial motility and fusion-fission dynamics. EMBO J 28, 3074–89 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheng ZH Mitochondrial trafficking and anchoring in neurons: New insight and implications. J Cell Biol 204, 1087–98 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Domenech G et al. Loss of Dendritic Complexity Precedes Neurodegeneration in a Mouse Model with Disrupted Mitochondrial Distribution in Mature Dendrites. Cell Rep 17, 317–327 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez-Domenech G et al. Miro proteins coordinate microtubule- and actin-dependent mitochondrial transport and distribution. EMBO J 37, 321–336 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Misko A, Jiang S, Wegorzewska I, Milbrandt J & Baloh RH Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J Neurosci 30, 4232–40 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baloh RH Mitochondrial dynamics and peripheral neuropathy. Neuroscientist 14, 12–8 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Saotome M et al. Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc Natl Acad Sci U S A 105, 20728–33 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stephen TL et al. Miro1 Regulates Activity-Driven Positioning of Mitochondria within Astrocytic Processes Apposed to Synapses to Regulate Intracellular Calcium Signaling. J Neurosci 35, 15996–6011 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang C, Bourdette D & Banker G Oxidative stress inhibits axonal transport: implications for neurodegenerative diseases. Mol Neurodegener 7, 29 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Debattisti V, Gerencser AA, Saotome M, Das S & Hajnoczky G ROS Control Mitochondrial Motility through p38 and the Motor Adaptor Miro/Trak. Cell Rep 21, 1667–1680 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pekkurnaz G, Trinidad JC, Wang X, Kong D & Schwarz TL Glucose regulates mitochondrial motility via Milton modification by O-GlcNAc transferase. Cell 158, 54–68 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao PC, Tandarich LC & Hollenbeck PJ ROS regulation of axonal mitochondrial transport is mediated by Ca2+ and JNK in Drosophila. PLoS One 12, e0178105 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iqbal S & Hood DA Oxidative stress-induced mitochondrial fragmentation and movement in skeletal muscle myoblasts. Am J Physiol Cell Physiol 306, C1176–83 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li L et al. p38 MAP kinase-dependent phosphorylation of the Gp78 E3 ubiquitin ligase controls ER-mitochondria association and mitochondria motility. Mol Biol Cell 26, 3828–40 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han SM, Baig HS & Hammarlund M Mitochondria Localize to Injured Axons to Support Regeneration. Neuron 92, 1308–1323 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou B et al. Facilitation of axon regeneration by enhancing mitochondrial transport and rescuing energy deficits. J Cell Biol 214, 103–19 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin MY et al. Releasing Syntaphilin Removes Stressed Mitochondria from Axons Independent of Mitophagy under Pathophysiological Conditions. Neuron 94, 595–610.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caino MC et al. Syntaphilin controls a mitochondrial rheostat for proliferation-motility decisions in cancer. J Clin Invest 127, 3755–3769 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X et al. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell 147, 893–906 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Birsa N et al. Lysine 27 ubiquitination of the mitochondrial transport protein Miro is dependent on serine 65 of the Parkin ubiquitin ligase. J Biol Chem 289, 14569–82 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Mealey GB et al. A PGAM5-KEAP1-Nrf2 complex is required for stress-induced mitochondrial retrograde trafficking. J Cell Sci 130, 3467–3480 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Al-Mehdi AB et al. Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription. Sci Signal 5, ra47 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Usaj M & Henn A Kinetic adaptation of human Myo19 for active mitochondrial transport to growing filopodia tips. Sci Rep 7, 11596 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cartoni R et al. The Mammalian-Specific Protein Armcx1 Regulates Mitochondrial Transport during Axon Regeneration. Neuron 92, 1294–1307 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song Z, Ghochani M, McCaffery JM, Frey TG & Chan DC Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol Biol Cell 20, 3525–32 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weaver D et al. Distribution and apoptotic function of outer membrane proteins depend on mitochondrial fusion. Mol Cell 54, 870–8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Otera H et al. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J Cell Biol 191, 1141–58 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Osellame LD et al. Cooperative and independent roles of the Drp1 adaptors Mff, MiD49 and MiD51 in mitochondrial fission. J Cell Sci 129, 2170–81 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ji WK, Hatch AL, Merrill RA, Strack S & Higgs HN Actin filaments target the oligomeric maturation of the dynamin GTPase Drp1 to mitochondrial fission sites. Elife 4, e11553 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee JE, Westrate LM, Wu H, Page C & Voeltz GK Multiple dynamin family members collaborate to drive mitochondrial division. Nature 540, 139–143 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ono T, Isobe K, Nakada K & Hayashi JI Human cells are protected from mitochondrial dysfunction by complementation of DNA products in fused mitochondria. Nat Genet 28, 272–5 (2001). [DOI] [PubMed] [Google Scholar]
- 57.Chen H, Chomyn A & Chan DC Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem 280, 26185–92 (2005). [DOI] [PubMed] [Google Scholar]
- 58.Twig G et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. Embo J 27, 433–46 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eisner V, Lenaers G & Hajnoczky G Mitochondrial fusion is frequent in skeletal muscle and supports excitation-contraction coupling. J Cell Biol 205, 179–95 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lewis SC, Uchiyama LF & Nunnari J ER-mitochondria contacts couple mtDNA synthesis with mitochondrial division in human cells. Science 353, aaf5549 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boldogh IR et al. A protein complex containing Mdm10p, Mdm12p, and Mmm1p links mitochondrial membranes and DNA to the cytoskeleton-based segregation machinery. Mol Biol Cell 14, 4618–27 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen H et al. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160, 189–200 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davies VJ et al. Opa1 deficiency in a mouse model of autosomal dominant optic atrophy impairs mitochondrial morphology, optic nerve structure and visual function. Hum Mol Genet 16, 1307–18 (2007). [DOI] [PubMed] [Google Scholar]
- 64.Ishihara N et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nature Cell Biology 11, 958 (2009). [DOI] [PubMed] [Google Scholar]
- 65.Wakabayashi J et al. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J Cell Biol 186, 805–16 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen H, McCaffery JM & Chan DC Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell 130, 548–62 (2007). [DOI] [PubMed] [Google Scholar]
- 67.Cartoni R et al. Expression of mitofusin 2(R94Q) in a transgenic mouse leads to Charcot-Marie-Tooth neuropathy type 2A. Brain 133, 1460–9 (2010). [DOI] [PubMed] [Google Scholar]
- 68.Strickland AV et al. Characterization of the mitofusin 2 R94W mutation in a knock-in mouse model. J Peripher Nerv Syst 19, 152–64 (2014). [DOI] [PubMed] [Google Scholar]
- 69.Alavi MV et al. A splice site mutation in the murine Opa1 gene features pathology of autosomal dominant optic atrophy. Brain 130, 1029–42 (2007). [DOI] [PubMed] [Google Scholar]
- 70.Chen Y et al. Mitofusin 2-containing mitochondrial-reticular microdomains direct rapid cardiomyocyte bioenergetic responses via interorganelle Ca(2+) crosstalk. Circ Res 111, 863–75 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen Y, Liu Y & Dorn GW 2nd. Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ Res 109, 1327–31 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Papanicolaou KN et al. Mitofusins 1 and 2 are essential for postnatal metabolic remodeling in heart. Circ Res 111, 1012–26 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kasahara A, Cipolat S, Chen Y, Dorn GW & Scorrano L Mitochondrial fusion directs cardiomyocyte differentiation via calcineurin and Notch signaling. Science 342, 734–7 (2013). [DOI] [PubMed] [Google Scholar]
- 74.Hall AR et al. Hearts deficient in both Mfn1 and Mfn2 are protected against acute myocardial infarction. Cell Death Dis 7, e2238 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen H et al. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 141, 280–9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tezze C et al. Age-Associated Loss of OPA1 in Muscle Impacts Muscle Mass, Metabolic Homeostasis, Systemic Inflammation, and Epithelial Senescence. Cell Metab 25, 1374–1389.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rodríguez-Nuevo A et al. Mitochondrial DNA and TLR9 drive muscle inflammation upon Opa1 deficiency. EMBO J (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Detmer SA, Vande Velde C, Cleveland DW & Chan DC Hindlimb gait defects due to motor axon loss and reduced distal muscles in a transgenic mouse model of Charcot-Marie-Tooth type 2A. Hum Mol Genet 17, 367–75 (2008). [DOI] [PubMed] [Google Scholar]
- 79.Bannerman P, Burns T, Xu J, Miers L & Pleasure D Mice Hemizygous for a Pathogenic Mitofusin-2 Allele Exhibit Hind Limb/Foot Gait Deficits and Phenotypic Perturbations in Nerve and Muscle. PLoS One 11, e0167573 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Williams PA, Morgan JE & Votruba M Opa1 deficiency in a mouse model of dominant optic atrophy leads to retinal ganglion cell dendropathy. Brain 133, 2942–51 (2010). [DOI] [PubMed] [Google Scholar]
- 81.Kushnareva Y et al. Mitochondrial dysfunction in an Opa1(Q285STOP) mouse model of dominant optic atrophy results from Opa1 haploinsufficiency. Cell Death Dis 7, e2309 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen L et al. OPA1 mutation and late-onset cardiomyopathy: mitochondrial dysfunction and mtDNA instability. J Am Heart Assoc 1, e003012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sarzi E et al. The human OPA1delTTAG mutation induces premature age-related systemic neurodegeneration in mouse. Brain 135, 3599–613 (2012). [DOI] [PubMed] [Google Scholar]
- 84.Soriano FX et al. Evidence for a mitochondrial regulatory pathway defined by peroxisome proliferator-activated receptor-gamma coactivator-1 alpha, estrogen-related receptor-alpha, and mitofusin 2. Diabetes 55, 1783–91 (2006). [DOI] [PubMed] [Google Scholar]
- 85.Chen Y & Dorn GW, 2nd. PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science 340, 471–5 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shutt T, Geoffrion M, Milne R & McBride HM The intracellular redox state is a core determinant of mitochondrial fusion. EMBO Rep 13, 909–15 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Samant SA et al. SIRT3 deacetylates and activates OPA1 to regulate mitochondrial dynamics during stress. Mol Cell Biol 34, 807–19 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nakamura N, Kimura Y, Tokuda M, Honda S & Hirose S MARCH-V is a novel mitofusin 2- and Drp1-binding protein able to change mitochondrial morphology. EMBO Rep 7, 1019–22 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Park YY et al. Loss of MARCH5 mitochondrial E3 ubiquitin ligase induces cellular senescence through dynamin-related protein 1 and mitofusin 1. J Cell Sci 123, 619–26 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Head B, Griparic L, Amiri M, Gandre-Babbe S & van der Bliek AM Inducible proteolytic inactivation of OPA1 mediated by the OMA1 protease in mammalian cells. J Cell Biol 187, 959–66 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mishra P, Carelli V, Manfredi G & Chan DC Proteolytic cleavage of Opa1 stimulates mitochondrial inner membrane fusion and couples fusion to oxidative phosphorylation. Cell Metab 19, 630–41 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ban T et al. Molecular basis of selective mitochondrial fusion by heterotypic action between OPA1 and cardiolipin. Nat Cell Biol 19, 856–863 (2017). [DOI] [PubMed] [Google Scholar]
- 93.Gomes LC, Di Benedetto G & Scorrano L During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol 13, 589–98 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rainbolt TK, Lebeau J, Puchades C & Wiseman RL Reciprocal Degradation of YME1L and OMA1 Adapts Mitochondrial Proteolytic Activity during Stress. Cell Rep 14, 2041–2049 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tondera D et al. SLP-2 is required for stress-induced mitochondrial hyperfusion. EMBO J 28, 1589–600 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wai T et al. The membrane scaffold SLP2 anchors a proteolytic hub in mitochondria containing PARL and the i-AAA protease YME1L. EMBO Rep 17, 1844–1856 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zemirli N et al. Mitochondrial hyperfusion promotes NF-κB activation via the mitochondrial E3 ligase MULAN. FEBS J 281, 3095–112 (2014). [DOI] [PubMed] [Google Scholar]
- 98.Nan J et al. TNFR2 Stimulation Promotes Mitochondrial Fusion via Stat3- and NF-kB-Dependent Activation of OPA1 Expression. Circ Res 121, 392–410 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rambold AS, Kostelecky B & Lippincott-Schwartz J Together we are stronger: fusion protects mitochondria from autophagosomal degradation. Autophagy 7, 1568–9 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Molina AJ et al. Mitochondrial networking protects beta-cells from nutrient-induced apoptosis. Diabetes 58, 2303–15 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yu T, Sheu SS, Robotham JL & Yoon Y Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res 79, 341–51 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu S, Zhou F, Zhang Z & Xing D Mitochondrial oxidative stress causes mitochondrial fragmentation via differential modulation of mitochondrial fission-fusion proteins. FEBS J 278, 941–54 (2011). [DOI] [PubMed] [Google Scholar]
- 103.Toyama EQ et al. Metabolism. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science 351, 275–281 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sabouny R et al. The Keap1-Nrf2 Stress Response Pathway Promotes Mitochondrial Hyperfusion Through Degradation of the Mitochondrial Fission Protein Drp1. Antioxid Redox Signal (2017). [DOI] [PubMed] [Google Scholar]
- 105.Cereghetti GM et al. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci U S A 105, 15803–8 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Picard M et al. Mechanical ventilation triggers abnormal mitochondrial dynamics and morphology in the diaphragm. J Appl Physiol (1985) 118, 1161–71 (2015). [DOI] [PubMed] [Google Scholar]
- 107.Pfluger PT et al. Calcineurin Links Mitochondrial Elongation with Energy Metabolism. Cell Metab 22, 838–50 (2015). [DOI] [PubMed] [Google Scholar]
- 108.Helle SCJ et al. Mechanical force induces mitochondrial fission. Elife 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Campello S et al. Orchestration of lymphocyte chemotaxis by mitochondrial dynamics. J Exp Med 203, 2879–86 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Buck MD et al. Mitochondrial Dynamics Controls T Cell Fate through Metabolic Programming. Cell 166, 63–76 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu X & Hajnoczky G Altered fusion dynamics underlie unique morphological changes in mitochondria during hypoxia-reoxygenation stress. Cell Death Differ 18, 1561–72 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vincent AE, Turnbull DM, Eisner V, Hajnoczky G & Picard M Mitochondrial Nanotunnels. Trends Cell Biol 27, 787–799 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huang X et al. Kissing and nanotunneling mediate intermitochondrial communication in the heart. Proc Natl Acad Sci U S A 110, 2846–51 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang C et al. Dynamic tubulation of mitochondria drives mitochondrial network formation. Cell Res 25, 1108–20 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Eisner V et al. Mitochondrial fusion dynamics is robust in the heart and depends on calcium oscillations and contractile activity. Proc Natl Acad Sci U S A 114, E859–E868 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lavorato M et al. Increased mitochondrial nanotunneling activity, induced by calcium imbalance, affects intermitochondrial matrix exchanges. Proc Natl Acad Sci U S A 114, E849–E858 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vincent AE et al. The Spectrum of Mitochondrial Ultrastructural Defects in Mitochondrial Myopathy. Sci Rep 6, 30610 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Picard M et al. Trans-mitochondrial coordination of cristae at regulated membrane junctions. Nat Commun 6, 6259 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Picard M et al. Acute exercise remodels mitochondrial membrane interactions in mouse skeletal muscle. J Appl Physiol (1985) 115, 1562–71 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vernay A et al. MitoNEET-dependent formation of intermitochondrial junctions. Proc Natl Acad Sci U S A 114, 8277–8282 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Prinz WA Bridging the gap: membrane contact sites in signaling, metabolism, and organelle dynamics. J Cell Biol 205, 759–69 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Csordas G, Weaver D & Hajnoczky G Endoplasmic Reticular-Mitochondrial Contactology: Structure and Signaling Functions. Trends Cell Biol (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang Z, Zhao X, Xu J, Shang W & Tong C A novel fluorescent reporter detects plastic remodeling of mitochondria-ER contact sites. J Cell Sci (2017). [DOI] [PubMed] [Google Scholar]
- 124.Csordas G et al. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol 174, 915–21 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cieri D et al. SPLICS: a split green fluorescent protein-based contact site sensor for narrow and wide heterotypic organelle juxtaposition. Cell Death Differ (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sutendra G et al. The role of Nogo and the mitochondria-endoplasmic reticulum unit in pulmonary hypertension. Sci Transl Med 3, 88ra55 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Qiao X et al. PTPIP51 regulates mouse cardiac ischemia/reperfusion through mediating the mitochondria-SR junction. Sci Rep 7, 45379 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang A et al. Quantitative proteomic analyses of human cytomegalovirus-induced restructuring of endoplasmic reticulum-mitochondrial contacts at late times of infection. Mol Cell Proteomics 10, M111 009936 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Horner SM, Wilkins C, Badil S, Iskarpatyoti J & Gale M Jr. Proteomic analysis of mitochondrial-associated ER membranes (MAM) during RNA virus infection reveals dynamic changes in protein and organelle trafficking. PLoS One 10, e0117963 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lynes EM et al. Palmitoylation is the switch that assigns calnexin to quality control or ER Ca2+ signaling. J Cell Sci 126, 3893–903 (2013). [DOI] [PubMed] [Google Scholar]
- 131.Gilady SY et al. Ero1alpha requires oxidizing and normoxic conditions to localize to the mitochondria-associated membrane (MAM). Cell Stress Chaperones 15, 619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Joseph SK Role of thiols in the structure and function of inositol trisphosphate receptors. Curr Top Membr 66, 299–322 (2010). [DOI] [PubMed] [Google Scholar]
- 133.Hidalgo C, Aracena P, Sanchez G & Donoso P Redox regulation of calcium release in skeletal and cardiac muscle. Biol Res 35, 183–93 (2002). [DOI] [PubMed] [Google Scholar]
- 134.SanMartín CD et al. RyR2-Mediated Ca2+ Release and Mitochondrial ROS Generation Partake in the Synaptic Dysfunction Caused by Amyloid β Peptide Oligomers. Front Mol Neurosci 10, 115 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Khan MT & Joseph SK Role of inositol trisphosphate receptors in autophagy in DT40 cells. J Biol Chem 285, 16912–20 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hacki J et al. Apoptotic crosstalk between the endoplasmic reticulum and mitochondria controlled by Bcl-2. Oncogene 19, 2286–95 (2000). [DOI] [PubMed] [Google Scholar]
- 137.Nutt LK et al. Bax and Bak promote apoptosis by modulating endoplasmic reticular and mitochondrial Ca2+ stores. J Biol Chem 277, 9219–25 (2002). [DOI] [PubMed] [Google Scholar]
- 138.Chipuk JE et al. Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell 148, 988–1000 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rambold AS, Cohen S & Lippincott-Schwartz J Fatty acid trafficking in starved cells: regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev Cell 32, 678–92 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lapuente-Brun E et al. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science 340, 1567–70 (2013). [DOI] [PubMed] [Google Scholar]
- 141.Hackenbrock CR Ultrastructural bases for metabolically linked mechanical activity in mitochondria. I. Reversible ultrastructural changes with change in metabolic steady state in isolated liver mitochondria. J Cell Biol 30, 269–97 (1966). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Strauss M, Hofhaus G, Schröder RR & Kühlbrandt W Dimer ribbons of ATP synthase shape the inner mitochondrial membrane. EMBO J 27, 1154–60 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Friedman JR, Mourier A, Yamada J, McCaffery JM & Nunnari J MICOS coordinates with respiratory complexes and lipids to establish mitochondrial inner membrane architecture. Elife 4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Harner M et al. The mitochondrial contact site complex, a determinant of mitochondrial architecture. EMBO J 30, 4356–70 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tarasenko D et al. The MICOS component Mic60 displays a conserved membrane-bending activity that is necessary for normal cristae morphology. J Cell Biol 216, 889–899 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Frezza C et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 126, 177–89 (2006). [DOI] [PubMed] [Google Scholar]
- 147.Nielsen J et al. Plasticity in mitochondrial cristae density allows metabolic capacity modulation in human skeletal muscle. J Physiol 595, 2839–2847 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Patten DA et al. OPA1-dependent cristae modulation is essential for cellular adaptation to metabolic demand. EMBO J 33, 2676–91 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yamaguchi R et al. Opa1-mediated cristae opening is Bax/Bak and BH3 dependent, required for apoptosis, and independent of Bak oligomerization. Mol Cell 31, 557–69 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Norton M et al. ROMO1 is an essential redox-dependent regulator of mitochondrial dynamics. Sci Signal 7, ra10 (2014). [DOI] [PubMed] [Google Scholar]
- 151.Rossignol R et al. Mitochondrial threshold effects. Biochem J 370, 751–62 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zuchner S et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet 36, 449–51 (2004). [DOI] [PubMed] [Google Scholar]
- 153.Gerber S et al. Mutations in DNM1L, as in OPA1, result indominant optic atrophy despite opposite effectson mitochondrial fusion and fission. Brain 140, 2586–2596 (2017). [DOI] [PubMed] [Google Scholar]
- 154.Koch J et al. Disturbed mitochondrial and peroxisomal dynamics due to loss of MFF causes Leigh-like encephalopathy, optic atrophy and peripheral neuropathy. J Med Genet 53, 270–8 (2016). [DOI] [PubMed] [Google Scholar]
- 155.Barel O et al. Deleterious variants in TRAK1 disrupt mitochondrial movement and cause fatal encephalopathy. Brain 140, 568–581 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Alexander C et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet 26, 211–5 (2000). [DOI] [PubMed] [Google Scholar]
- 157.Delettre C et al. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet 26, 207–10 (2000). [DOI] [PubMed] [Google Scholar]
- 158.Amati-Bonneau P et al. OPA1 mutations induce mitochondrial DNA instability and optic atrophy ‘plus’ phenotypes. Brain 131, 338–51 (2008). [DOI] [PubMed] [Google Scholar]
- 159.Hudson G et al. Mutation of OPA1 causes dominant optic atrophy with external ophthalmoplegia, ataxia, deafness and multiple mitochondrial DNA deletions: a novel disorder of mtDNA maintenance. Brain 131, 329–37 (2008). [DOI] [PubMed] [Google Scholar]
- 160.Schrepfer E & Scorrano L Mitofusins, from Mitochondria to Metabolism. Mol Cell 61, 683–694 (2016). [DOI] [PubMed] [Google Scholar]
- 161.Wikstrom JD et al. Hormone-induced mitochondrial fission is utilized by brown adipocytes as an amplification pathway for energy expenditure. EMBO J 33, 418–36 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yu T, Robotham JL & Yoon Y Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A 103, 2653–8 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Schneeberger M et al. Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell 155, 172–87 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ramirez S et al. Mitochondrial Dynamics Mediated by Mitofusin 1 Is Required for POMC Neuron Glucose-Sensing and Insulin Release Control. Cell Metab 25, 1390–1399 e6 (2017). [DOI] [PubMed] [Google Scholar]
- 165.Kumar R et al. Mitochondrial dynamics following global cerebral ischemia. Mol Cell Neurosci 76, 68–75 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tian L et al. Ischemia-induced Drp1 and Fis1-mediated mitochondrial fission and right ventricular dysfunction in pulmonary hypertension. J Mol Med (Berl) 95, 381–393 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Meyer JN et al. Mitochondria as a target of environmental toxicants. Toxicol Sci 134, 1–17 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Picard M & Turnbull DM Linking the metabolic state and mitochondrial DNA in chronic disease, health, and aging. Diabetes 62, 672–8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Arruda AP et al. Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat Med 20, 1427–35 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kirkman MA et al. Gene-environment interactions in Leber hereditary optic neuropathy. Brain 132, 2317–26 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Schon EA & Przedborski S Mitochondria: the next (neurode)generation. Neuron 70, 1033–53 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Smith EF, Shaw PJ & De Vos KJ The role of mitochondria in amyotrophic lateral sclerosis. Neurosci Lett (2017). [DOI] [PubMed] [Google Scholar]
- 173.Guardia-Laguarta C, Area-Gomez E, Schon EA & Przedborski S A new role for alpha-synuclein in Parkinson’s disease: Alteration of ER-mitochondrial communication. Mov Disord 30, 1026–33 (2015). [DOI] [PubMed] [Google Scholar]
- 174.Area-Gomez E & Schon EA On the Pathogenesis of Alzheimer’s Disease: The MAM Hypothesis. FASEB J 31, 864–867 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hedskog L et al. Modulation of the endoplasmic reticulum-mitochondria interface in Alzheimer’s disease and related models. Proc Natl Acad Sci U S A 110, 7916–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lewis TL Jr., Turi GF, Kwon SK, Losonczy A & Polleux F Progressive Decrease of Mitochondrial Motility during Maturation of Cortical Axons In Vitro and In Vivo. Curr Biol 26, 2602–2608 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Song M, Franco A, Fleischer JA, Zhang L & Dorn GW Abrogating Mitochondrial Dynamics in Mouse Hearts Accelerates Mitochondrial Senescence. Cell Metab 26, 872–883.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Le Page S et al. Increase in Cardiac Ischemia-Reperfusion Injuries in Opa1+/− Mouse Model. PLoS One 11, e0164066 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhang Z et al. The dynamin-related GTPase Opa1 is required for glucose-stimulated ATP production in pancreatic beta cells. Mol Biol Cell 22, 2235–45 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]