Abstract
Background
The experimental tobacco marketplace (ETM) provides a method to estimate, prior to implementation, the effects of new products or policies on purchasing across various products in a complex tobacco marketplace. We used the ETM to examine the relationship between nicotine strength and substitutability of alternative products for cigarettes to contribute to the literature on regulation of e-liquid nicotine strength.
Methods
The present study contained four sampling and four ETM purchasing sessions. During sampling sessions, participants were provided 1 of 4 e-liquid strengths (randomised) to sample for 2 days followed by an ETM purchasing session. The nicotine strength sampled in the 2 days prior to an ETM session was the same strength available for purchase in the next ETM. Each participant sampled and could purchase 0 mg/mL, 6 mg/mL, 12 mg/mL and 24 mg/mL e-liquid, among other products, during the study.
Results
Cigarette demand was unaltered across e-liquid strength. E-liquid was the only product to substitute for cigarettes across more than one e-liquid strength. Substitutability increased as a function of e-liquid strength, with the 24 mg/mL displaying the greatest substitutability of all products.
Conclusions
The present study found that e-liquid substitutability increased with nicotine strength, at least up to 24 mg/mL e-liquid. However, the effects of e-liquid nicotine strength on cigarette purchasing were marginal and total nicotine purchased increased as e-liquid nicotine strength increased.
INTRODUCTION
The nicotine strength of e-cigarette liquid (e-liquid) available for consumers varies by country. For example, retail sale of nicotine-containing e-cigarettes is currently prohibited in Australia and Canada. In the UK, only e-cigarette products with 20 mg/mL or less are available for retail sale with stronger products requiring medicinal licencing prior to supply. The US Food and Drug Administration has the authority to regulate e-liquid nicotine strength but does not currently impose restrictions. Evidence on the impact of nicotine strength on substitutability of e-cigarettes for cigarettes is needed to inform decision making to provide the greatest public health benefits. Nicotine is the primary constituent of tobacco that drives self-administration and consumption.1,2 Thus, the e-liquid nicotine strengths available for e-cigarettes may be critical in determining the extent to which e-cigarettes are subjectively valued and used, either instead of or alongside cigarettes.
The type and quantity of products in a marketplace alters a commodity’s demand elasticity (ie, sensitivity to price) as well as switching among those products.3–5 Thus, achieving the tobacco control goals of reducing purchasing of a particular product (eg, cigarettes) and/or shifting preference to another product (eg, e-cigarettes) may each be enhanced or diminished via the economic process of substitution (for review, see ref 3). Substitution, a form of product switching, occurs as an increase in purchasing of a price-constant product as a result of an increase in the price of an alternative commodity, which is defined by the slope (ie, cross-price elasticity) of the price-constant purchasing function.3,6–9, but see 10 For example, in human laboratory studies, as the price of cigarettes increases and purchasing decreases, purchasing of nicotine gum increases even though its price remains constant.11 However, the complexity of the tobacco marketplace requires a procedure that examines substitutability interactions among a broad range of products.
The experimental tobacco marketplace (ETM)12–14 is an online store that displays photos, prices and information for each of several available products. Using the ETM, Quisenberry et al14 had participants make real purchases among alternate nicotine products (eg, e-cigarettes, snus and cigarillos) across four cigarette price conditions while the prices for all alternate products remained fixed. Participants were more likely to switch to e-cigarettes (Blu brand disposables) than other non-combustible tobacco products as the price of cigarettes increased. Two additional ETM studies12,15 found that alternate product substitutability is dependent on product availability and indicated that while e-cigarettes generally demonstrate the greatest substitutability among the alternate products tested, consumer characteristics, such as gender, also modulate substitutability.
The maximum nicotine strength allowed in e-cigarettes is currently a debated topic. Given that nicotine strength may be vital to e-cigarette substitutability, governmental limits and regulations may be informed by experimental data. Indeed, understanding the relationship between nicotine strength and e-cigarette substitutability may help to maximise the substitutability of modified risk products and decrease tobacco-related harm. Thus, the present study investigated how e-liquid strength (0, 6, 12 and 24 mg/mL) interacts with the price of cigarettes to influence cigarette demand and the substitutability of e-liquid, as well as other alternate products, including snus, dip, nicotine gum and nicotine lozenges. To combat participants’ potential lack of exposure to e-cigarettes, participants were given and taught how to use and refill an e-cigarette device. To ensure that all participants had similar e-liquid experiences and exposure throughout the study and to guarantee experience with all nicotine strengths so that the relation between e-liquid nicotine strength and e-liquid substitutability could be examined in each participant, two types of sessions were incorporated: e-liquid nicotine strength sampling sessions (2-day period) and ETM purchasing sessions.
METHODS
Participants
Participants (n=25) were recruited from Roanoke, Virginia, and surrounding areas to participate at the Addiction Recovery Research Center. Eligible participants were (A) between the ages of 18 years and 65 years; (B) current cigarette smokers (≥10 cigarettes/day); and (C) willing to try e-cigarettes. Regular use of other tobacco products was not exclusionary if these products were not meant to help with smoking cessation (eg, nicotine patch, gum, lozenge and spray). Individuals who planned to quit smoking or were currently using prescription medication that might affect smoking or nicotine metabolism were excluded. Additionally, participants were excluded if they were pregnant, had immediate plans to move away from the area or had an unmanaged medical or psychiatric condition.
Participants were 52% female, 84% Caucasian and 12% African American, had a mean age of 42.72 (SD = 11.14) and had a mean of 12.56 (SD = 1.73) years of education. Of the 25 participants, 7 (28%) had used e-cigarettes in the past month, using an average of 6 days during the prior 30-day period, with all participants reporting e-cigarette use 15 or fewer times during those 30 days. Participants smoked an average of 18.72 (SD = 7.34) cigarettes per day over the last 30 days (reported via the Timeline Follow-back (TLFB) assessment16), had a mean score of 6.72 on the Fagerstrom Test of Nicotine Dependence17; and had a mean score of 3.84 on the Questionnaire on Smoking Urges-Brief.18
Procedures
After an initial screening questionnaire determined eligibility, participants completed nine sessions, including four e-liquid sampling sessions, four ETM purchase sessions and one follow-up session. Immediately following consent, participants were given an e-cigarette (eGo ONE CT, $54.90, Shenzhen Joyetech, Shenzhen, China), taught how to properly use it (eg, handling, charging and vaping) and refill it with e-liquid. Afterwards, a flavour preference assessment was conducted, in which participants used the e-cigarette to sample each of the three different e-liquid flavours at 0 mg/mL nicotine strength (VaporHQ, Oregon; ‘Blueberry Harvest’, ‘American Red’ tobacco, ‘32 Degrees’ menthol). After this process, participants selected their preferred e-liquid flavour to be used throughout the study.
Sampling sessions were typically held at the beginning of the week, always 2 days prior to an ETM purchase session. During sampling sessions, participants were given 2 mL of their preferred flavour of e-liquid, with the order of nicotine strength randomised across the four sampling sessions. Participants were also instructed to return any unused e-liquid from the sampling period. The average amount of e-liquid returned across the four sampling sessions was 0.30 mL (SD = 0.40; range = 0–1.20 mL).
Account balance
Similar to previous studies using the ETM,14,15 participants were given an account balance during ETM purchasing sessions to more appropriately approximate real-world conditions related to income constraints. After reporting nicotine product use over the past 30 days using TLFB, an individualised account balance was calculated by multiplying the local market price of each individual nicotine product by the average, reported use of each product (via TLFB). The local market prices of each product were as follows: $0.25 per cigarette, $0.50 per mL of e-liquid, $0.20 per dip and snus pouches, $0.80 per piece of nicotine gum and $0.60 per nicotine lozenge. Calculating the account balance using this method produces ETM purchasing that is representative of participants’ tobacco purchases prior to the experiment.19
ETM sessions
During the ETM sessions, participants were seated in a behavioural booth with a computer monitor and a basic four-function calculator. After completion of the questionnaires on Qualtrics, participants purchased products on OpenCart, a website individualised for each participant’s preferred cigarette and e-liquid flavour. Six products were available to purchase: typical brand of cigarette, preferred e-liquid flavour, winter-chill flavour Camel snus, wintergreen flavour Grizzly dip, white ice mint flavour Nicorette 4 mg nicotine gum and mint flavour Nicorette 4 mg nicotine lozenges. More information about the products, including nicotine content, can be found in the supplementary appendix.
The four e-liquid nicotine strengths (0 mg/mL, 6 mg/mL, 12 mg/mL and 24 mg/mL) were randomised across the four ETM sessions, with 2 mL of each strength provided at the prior sample session. During each session, participants made purchases across five ascending cigarette prices ($0.12, $0.25, $0.50, $1.00 and $2.00 per cigarette) while the price of all other available products remained constant. Participants purchased enough products to use over the next 5 days, and their account balance started over prior to each cigarette price condition. At the end of the session, the participants received all products purchased and any remaining account balance from one randomly chosen cigarette price condition.
Statistical analysis
Microsoft Excel and GraphPad Prism V7 were used for all data analyses. All statistical tests were considered significant at the a<0.05 level. Where applicable, Tukey’s multiple comparisons test was the post hoc used to further interpret significant main effects and interactions.
Consistent with prior ETM studies,14,15 purchasing data were converted from raw units purchased to mg of nicotine purchased (see supplementary materials for additional explanation, quantity data and analysis). This conversion ensured that purchasing of all products was depicted and analysed on the same scale, as the units in which the different products were sold, especially e-liquid, were product specific and prohibited direct comparisons between the different ETM products. Nicotine was the relevant reinforcer for all products in the ETM and constituted our independent variable across the four e-liquid strengths. Additional analyses can be found in the supplementary materials.
Cigarette demand in the ETM
We applied Koffarnus et al’s20 exponentiated demand model to individual participant’s cigarette demand at each e-liquid strength:
| (1) |
where Q is cigarette purchasing (mg nicotine), P is the price of the cigarette, Q0 represents demand intensity (cigarette purchasing level at zero cost), k corresponds to the range of the function in logarithmic units and a represents demand elasticity.21 The parameter k was fitted as a constant common across all prices and e-liquid strengths (k = 1.44). Model-derived values of and Q0 served as dependent measures of demand elasticity and intensity, respectively; the values of these parameter estimates at each of the four e-liquid strengths were compared using a repeated measures analysis of variance (RMANOVA).
Substitution for cigarettes
As mentioned above, substitutability was defined by the slope of the fixed-product purchasing functions3 (for review, see refs 6–8). Hursh and Roma’s5 cross-price elasticity equation, and when exponentiated, was then fit to individual participant and group mean alternate product purchasing at each e-liquid strength; however, fitting this model rarely resulted in convergence. Therefore, our approach was similar to Quisenberry et al14 in that linear regressions were fitted to individual participant’s alternate product purchasing data across e-liquid strength. Specifically, for each participant, linear regressions were applied to fixed-price, alternate product purchasing data as a function of log-transformed cigarette unit prices to estimate substitutability, represented here by the slopes of fixed-price, alternate product purchasing functions. In this analysis, because the slope of the regression line is a proxy for substitutability, product substitution was demonstrated if a product function’s slope was statistically greater than 0, with higher slopes indicating greater substitutability. All statistical tests were two tailed. As an additional measure of alternate product purchasing, the y-intercepts of the alternate product purchasing functions were also subject to analysis, which provide a measure of e-liquid purchasing in the presence of free access to cigarettes.22 To test how e-liquid strength influenced alternate product substitutability and purchasing, RMANOVAs were applied to individual participant’s slope and y-intercept estimates across e-liquid strength.
Total mg of nicotine purchased
The final analysis examined the influence of cigarette price and e-liquid nicotine strength over the total mg of nicotine purchased, when summed across all products purchased. Once total mg of nicotine purchased across all products was calculated for each individual participant, across each cigarette price and each e-liquid strength, a two-way RMANOVA, with both price and strength as within-subject factors, was used to characterise changes in total mg of nicotine purchased.
RESULTS
Cigarette demand
Figure 1A displays mean, obtained cigarette demand across the four e-liquid strengths, while the solid lines drawn through the data represent predictions from Eq. 1. Table 1 contains mean, Eq. 1-derived estimates of Q0 and and median goodness-of-fit measures. In general, Eq. 1 provided excellent fits to individual subject cigarette demand, accounting for over 90% of the variance across e-liquid strengths. Demand for cigarettes decreased as unit price increased. Although visual inspection of figure 1A indicates slight variation in Q0 from the denicotinised (0 mg/mL) to nicotinised e-liquid strengths (6 mg/mL, 12 mg/mL and 24 mg/mL), the RMANOVA applied to individual estimates of Q0 revealed no significant effect of e-liquid strength (F(3, 72) =0.74, p = 0.53), nor did the RMANOVA applied to estimates across e-liquid strength (F(3, 72) = 1.50, p = 0.23).
Figure 1.
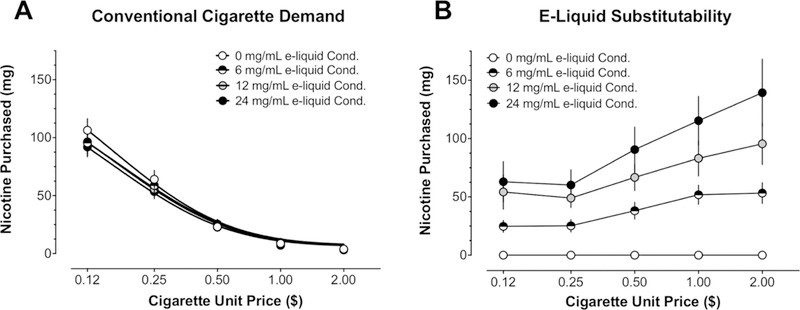
(A) Mean, conventional cigarette demand (mg of nicotine) as a function of the five conventional cigarette unit prices, which are shown in log space on the x-axis. The four different classes of symbols represent the mean, obtained conventional cigarette demand at each of the four different e-liquid nicotine strengths. The four lines drawn through the symbols represent the mean, Eq. 1-predicted cigarette demand across the four e-liquid strengths (see also table 1). (B) Mean, obtained e-liquid substitutability at each of the four e-liquid strengths. Note the increase in e-liquid substitutability (ie, slope and y-int) as a function of nicotine strength. See text for additional details. Error bars represent ±1 SEM.
Table 1.
Mean estimates of demand intensity or initial consumption (Q0) and elasticity (α) and median goodness-of-fit (R2) derived from application of Eq. 1 to individual participant’s cigarette demand functions at each of the four e-liquid strengths.
| 0 mg/mL | 6 mg/mL | 12 mg/mL | 24 mg/mL | |
|---|---|---|---|---|
| Q0 | 221.99 | 186.63 | 196.37 | 196.49 |
| se | 24.01 | 27.21 | 26.46 | 23.07 |
| α | 0.015 | 0.016 | 0.012 | 0.011 |
| se | 0.004 | 0.003 | 0.002 | 0.002 |
| R2 | 0.95 | 0.95 | 0.90 | 0.94 |
Table 2 lists the results of the linear regressions applied to individual alternate product purchasing at each e-liquid strength to determine changes in substitutability. Overall, only two of the five alternate products (e-liquid and dip) generated mean slope estimates significantly greater than 0 (ie, substitute for cigarettes) at a minimum of one e-liquid strength. Table 2 also reveals that the three nicotinic e-liquid strengths showed relatively high levels of purchasing and were each significant substitutes for cigarettes. The e-liquid purchasing functions for all participants for 0 mg/mL were perfect lines at y = 0 (see supplementary materials; table S2, figure S2B; analysis with raw mL purchased). By contrast, mean dip slope estimates were significantly greater than 0 only at the 0 mg/mL strength and only 3 of the 25 individual participant dip slopes were significantly greater than 0.
Table 2.
Mean statistics from linear regressions performed to determine substitution profiles
| slope (substitutability) | y-Intercept (initial intensity) | slope 95% CI | Model R2 | slope F test (p values) | |
|---|---|---|---|---|---|
| 0 mg/mL | |||||
| e-Liquid | 0 | 0 | 0 | 0 | 0 |
| Snus | 6.13 | 9.30 | −0.33 to 12.6 | 0.75 | 9.13 (0.06) |
| Dip* | 8.46† | 7.58† | 4.66 to 12.3 | 0.94 | 50 (<0.01)* |
| Nicotine gum | 1.63 | 2.96 | −2.06 to 5.33 | 0.40 | 1.97 (0.25) |
| Nicotine lozenge | 1.09 | 1.29 | −1.94 to 4.13 | 0.31 | 1.32 (0.33) |
| 6 mg/mL | |||||
| e-Liquid* | 27.44† | 26.3† | 11.4 to 33.1 | 0.92 | 32.5 (0.01)* |
| Snus | −0.15 | 2.34 | −5.17 to 4.86 | 0.01 | 0.01 (0.93) |
| Dip | −0.48 | 0.45 | −3.41 to 2.45 | 0.08 | 0.27 (0.64) |
| Nicotine gum | −0.80 | 2.22 | −1.57 to −0.02 | 0.78 | 5.32 (0.10) |
| Nicotine lozenge | −0.26 | 1.26 | −1.26 to 0.74 | 0.19 | 0.69 (0.46) |
| 12 mg/mL | |||||
| e-Liquid* | 39.56† | 50.1† | 12.3 to 67.6 | 0.91 | 31.1 (0.01)* |
| Snus | 1.78 | 3.19 | −3.32 to 6.88 | 0.29 | 1.24 (0.35) |
| Dip | 0.36 | 0.24 | −0.06 to 0.78 | 0.71 | 7.47 (0.07) |
| Nicotine gum | 1.58 | 3.62 | −0.37 to 3.53 | 0.69 | 6.69 (0.08) |
| Nicotine lozenge | 0.26 | 0.50 | −2.27 to 2.79 | 0.03 | 0.10 (0.76) |
| 24 mg/mL | |||||
| e-Liquid* | 68.05† | 58.1† | 34.5 to 108.2 | 0.90 | 26.3 (0.01)* |
| Snus | 2.10 | 3.29 | −3.81 to 8.01 | 0.30 | 1.28 (0.34) |
| Dip | −0.05 | −0.00 | −0.15 to 0.04 | 0.51 | 3.14 (0.17) |
| Nicotine gum | −0.38 | 0.49 | −1.31 to 0.54 | 0.37 | 1.74 (0.28) |
| Nicotine lozenge | −0.44 | 0.57 | −2.17 to 1.29 | 0.18 | 0.66 (0.48) |
Purchasing is expressed as mg of nicotine purchased for each product.
Indicates that a particular alternate product was a significant substitute for cigarettes at a particular e-liquid strength.
Indicates that the mean slope at a given strength was significantly >0.
The RMANOVA applied to individual e-liquid slope estimates revealed a significant effect of e-liquid strength on e-liquid substitutability (ie, slope) (F(3, 72) = 8.48, p<0.001), with mean slope estimates increasing as a function of strength. Concurrently, the number of participants with e-liquid slopes significantly greater than 0 increased as a function of strength, with 8, 11 and 16 participants showing positive slopes at the 6 mg/mL, 12 mg/mL and 24 mg/mL strengths, respectively. Tukey’s post hoc tests confirmed that the e-liquid slope estimates across all three nicotinic strengths were greater than at the denicotinised strength (all ps<0.031). In addition, while the 12 mg/ mL slope estimate was nominally greater than that of the 6 mg/mL strength and, similarly, the 24 mg/mL slope was nominally greater than the slopes of both the 6 mg/mL and 12 mg/mL strengths, the only significant difference among e-liquid slopes across the four strengths was between the 6 mg/mL and 24 mg/mL strengths, with the 24 mg/mL e-liquid demonstrating greater substitutability than the 6 mg/mL e-liquid (p = 0.03).
The one-way RMANOVA applied to individual e-liquid y-intercept estimates also revealed a significant effect of e-liquid strength (F(3, 72) = 18.70, p<0.001), with mean e-liquid y-intercept estimates also increasing as a function of e-liquid strength. Tukey’s post hoc tests confirmed that the mean y-intercept estimates for each of the three nicotinic e-liquid functions (6 mg/mL,12 mg/mL and 24 mg/mL) was greater than the mean y-intercept estimate obtained for purchasing of the denicotinised e-liquid strength (all ps<0.001). In addition, the y-intercept estimates at the 12 mg/mL and 24 mg/mL strengths were significantly greater than the y-intercept estimates at the 6 mg/mL strength (p<0.01), but the difference between y-intercept estimates at the 24 mg/mL and 12 mg/mL strength was not significant (p = 0.49). Similar to e-liquid slopes, however, purchasing at the 24 mg/mL e-liquid strength demonstrated the highest nominal y-intercepts.
Figure 1B summarises the reports in table 2 and the most important results of the study. Specifically, nicotinised e-liquid was the only alternate product available that occasioned a significant amount of purchasing at each cigarette price and across more than one e-liquid condition. Third, individual subject and mean estimates of both e-liquid slopes and y-intercepts demonstrated large increases as a function of e-liquid strength.
Total mg of nicotine purchased
Figure 2 illustrates how total mg of nicotine purchased (summed across all products) changed as a function of cigarette price and e-liquid nicotine strength. Visual analysis of figure 2 indicates that a greater amount of total nicotine was purchased as e-liquid nicotine strength increased. In addition, total mg of nicotine purchased was a negatively decelerating function of cigarette price. The two-way RMANOVA applied to total mg of nicotine purchased revealed a significant e-liquid nicotine strength × cigarette price interaction (F(12, 288)=3.12, p = 0.0004). Tukey’s multiple comparisons test across e-liquid strength revealed that total nicotine purchased was significantly greater when the available e-liquid was 12 mg/mL and 24 mg/mL compared with when the available e-liquid was 0 mg/mL (ps<0.0001) and was significantly greater when the available e-liquid was 24 mg/mL compared with 6 mg/mL (p=0.004).
Figure 2.
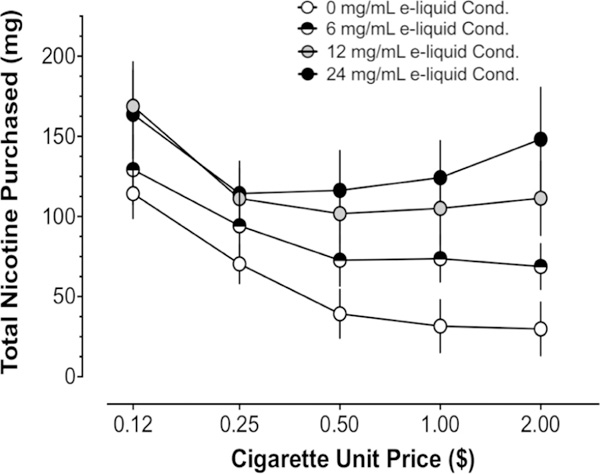
Total mg of nicotine purchased, when summed across all products, as a function of cigarette price and e-liquid nicotine strength. On average, the total amount of nicotine purchased increased as a function of the e-liquid nicotine strength available and decreased as a function of cigarette price. See text for statistical details. Error bars represent ±1 SEM.
DISCUSSION
The present study replicated and extended recent work investigating real purchasing of several nicotine/tobacco products available concurrently in the ETM12–14 by determining if and how cigarette demand and alternate product substitutability were affected by the strength of e-liquid available in the ETM. Typical of product demand profiles, cigarette purchasing decreased as unit price increased. Interestingly, cigarette demand was unaffected by the e-liquid nicotine strength available, which may suggest that all participants were relatively inexperienced e-cigarette users. Furthermore, the lack of such an effect may also indicate that while the e-liquid sampling exposed all participants to each e-liquid nicotine strength, equating experience during the study, the sampling period may have been insufficient to affect cigarette demand but only e-liquid substitutability. Indeed, similar studies have demonstrated the sensitivity of cigarette demand to the availability of e-cigarette products but only in users with more e-cigarette experience than the participants in the present study, for example, dual users.23,24 When the total amount of nicotine purchased was summed across all products, there was a clear positive relation between e-liquid nicotine strength and total mg of nicotine purchased.
Evaluation of the substitutability of alternate products revealed that dip functioned as a substitute for cigarettes only when the e-liquid strength was 0 mg/mL, while e-liquid had the highest substitutability of all products tested. Indeed, e-liquid functioned as a strong substitute for cigarettes across all three nicotine strengths (see also supplementary materials for quantity data illustrating that 0 mg/mL also functioned as a significant substitute). There was very limited purchasing of any other alternate products across all cigarette prices. Thus, few participants purchased anything other than cigarettes and e-liquid in the ETM throughout the experiment.
Perhaps the most significant result was the orderly relationship between e-liquid nicotine strength and e-liquid substitutability. That is, e-liquid substitutability (ie, slopes and y-intercepts) increased nominally as a function of e-liquid strength. The 24 mg/mL e-liquid was the strongest substitute for cigarettes because 24 mg/mL slope and y-intercept estimates were significantly higher than those at the 6 mg/mL, and there were no significant differences between the 12 mg/mL and 6 mg/mL strengths. Additionally, the 24 mg/mL e-liquid functioned as a significant substitute for the greatest number of participants (see supplementary materials). When the total amount of nicotine purchased was summed across all products, however, there was a clear positive relation between e-liquid nicotine strength and total mg of nicotine purchased. Thus, while the 24 mg/mL e-liquid demonstrated the highest substitutability and resulted in the lowest quantity purchased (ie, mL of e-liquid) among the three active e-liquid strengths, this strength also resulted in more mg of total nicotine purchased. To the extent that e-liquid consumption has any negative health effects, higher e-liquid strengths tend to minimise the number of puffs required to obtain satisfactory nicotine consumption relative to lower nicotine strengths (comperared with refs25,26). Thus, higher strength e-liquid may expose users to less harm than lower strength e-liquids.
While previous ETM studies examining alternate nicotine product substitutability have typically observed a greater variety of product selection,13,14 the present study observed almost exclusive e-liquid purchasing. This result alongside the graded substitutability as a function of nicotine strength were likely due to at least two major factors that differed between the current study and previous ETM studies. First, in the present study, participants were explicitly exposed to e-liquid, were taught how to properly use and refill the device and were given a device to use throughout the experiment. Such exposure required participants to at least try e-liquid, whereas previous studies examining substitutability of alternate products may have underestimated e-liquid (or other product) substitutability due to lack of participant experience or exposure.14 Second, to ensure that each participant had sampled and been exposed to the device and the different e-liquid nicotine strengths, the present study added a sampling session for each e-liquid strength 2 days prior to each ETM session. This additional exposure and use of different e-liquid strengths increased general exposure to e-liquids and allowed participants to find the strength they preferred. Future studies may benefit from investigating the effects of pre-exposure and product sampling on alternate product substitutability.
Some limitations of this study must be acknowledged. First, a moderating effect of e-cigarette use prior to the study was unable to be explored because of the relatively small, unequal sample sizes of naive e-cigarette users and those with e-cigarette exposure prior to the study. Second, while e-liquid clearly functioned as a significant substitute for cigarettes, including an additional cigarette price condition that was sufficiently high to completely suppress cigarette purchasing may have resulted in other products substituting for cigarettes and/or more robust e-liquid substitution. Finally, the present study was conducted over a relatively short period (7 days per e-liquid strength), and studies of longer durations may yield different demand and substitution profiles.
CONCLUSION
The results of the present study suggest that e-liquid substitutability increases as a function of nicotine strength when purchasing was analysed as mg of nicotine, with 24 mg/mL having the greatest substitutability. In contrast, when purchasing was analysed as product quantity, mL of e-liquid purchased was an inverted U-shaped function of e-liquid nicotine strength, with the 24 mg/mL resulting in the fewest mL purchased of any active nicotine strength e-liquid (see supplementary materials). However, e-liquid nicotine strength had only a marginal effect on cigarette demand. Indeed, total mg of nicotine purchased strength-dependently increased. Further research is required to examine if extended exposure to higher nicotine strength e-liquid results in a greater decline of cigarette use, rather than maintaining dual use. To the extent that e-liquid consumption has any negative health effects, higher e-liquid strengths tend to minimise the number of puffs required to obtain satisfactory nicotine consumption relative to lower nicotine strengths (c.f., 25,26).
Supplementary Material
What this paper adds.
-
►
The nicotine and tobacco marketplace has seen sizeable growth in the number of nicotine/tobacco products available for purchase.
-
►
There is a lack of experimental research on the influence of e-cigarette nicotine strength on substitutability of e-cigarettes for cigarettes compared with other non-cigarette alternatives (snus, dip, nicotine gum and nicotine lozenges).
-
►
E-cigarette e-liquid was the only product to substitute for cigarettes across more than one nicotine strength.
-
►
Substitutability increased as a function of e-liquid nicotine strength, with the 24 mg/mL displaying the greatest substitutability of all products.
Acknowledgments
Funding This work was supported by the Virginia Tech Carilion Research Institute and by NIH Grant No. P01 cA200512, 201 5. BWH was supported by K23 DA041616.
Footnotes
Competing interests WKB is a principal in HealthSim, LLC, and NotifiUs, LLC.
Patient consent Obtained.
Ethics approval The Virginia Tech Institutional Review Board approved all procedures and protocols.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Readers are encouraged to email wkbickel@vtc.vt.edu to obtain more data for this study.
REFERENCES
- 1.Henningfield JE, Goldberg SR. Control of behavior by intravenous nicotine injections in human subjects. Pharmacol Biochem Behav 1983;19:1021–6. [DOI] [PubMed] [Google Scholar]
- 2.Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology 2006;31:1203–11. [DOI] [PubMed] [Google Scholar]
- 3.Bickel WK, DeGrandpre RJ, Higgins ST. The behavioral economics of concurrent drug reinforcers: a review and reanalysis of drug self-administration research. Psychopharmacology 1995;118:250–9. [DOI] [PubMed] [Google Scholar]
- 4.Bickel WK, Johnson MW, Koffarnus MN, et al. The behavioral economics of substance use disorders: reinforcement pathologies and their repair. Annu Rev Clin Psychol 2014;10:641–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hursh SR, Roma PG. Behavioral economics and empirical public policy. J Exp Anal Behav 2013;99:98–124. [DOI] [PubMed] [Google Scholar]
- 6.Bickel WK, Vuchinich RE. Reframing Health Behavior Change With Behavioral Economics. London: Psychology Press, 2000. [Google Scholar]
- 7.Hursh SR. Economic concepts for the analysis of behavior. J Exp Anal Behav 1980;34:219–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hursh SR. Behavioral economics. J Exp Anal Behav 1984;42:435–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson MW, Bickel WK. The behavioral economics of cigarette smoking: The concurrent presence of a substitute and an independent reinforcer. Behav Pharmacol 2003;14:137–44. [DOI] [PubMed] [Google Scholar]
- 10.Green L, Freed DE. The substitutability of reinforcers. J Exp Anal Behav 1993;60:141–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shahan TA, Odum AL, Bickel WK. Nicotine gum as a substitute for cigarettes: a behavioral economic analysis. Behav Pharmacol 2000;11:71–9. [DOI] [PubMed] [Google Scholar]
- 12.Heckman B, Cummings KM, Hirsch A, et al. A Novel Method for Evaluating the Acceptability of Substitutes for Cigarettes: The Experimental Tobacco Marketplace. Tob Regul Sci 2017;3:266–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quisenberry AJ, Koffarnus MN, Epstein LH, et al. The Experimental Tobacco Marketplace II: Substitutability and sex effects in dual electronic cigarette and conventional cigarette users. Drug Alcohol Depend 2017;178:551–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quisenberry AJ, Koffarnus MN, Hatz LE, et al. The Experimental Tobacco Marketplace I: Substitutability as a Function of the Price of Conventional Cigarettes. Nicotine Tob Res 2016;18:1642–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quisenberry A, Koffarnus MN, Bianco A, et al. The experimental tobacco marketplace II: Substitutability in dual e-cigarette and cigarette users. Drug Alcohol Depend 2017;171:e171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sobell LC, Sobell MB. Timeline Follow-Back In: Litten RZ, Allen JP, eds. Measuring Alcohol Consumption. New York, USA: Humana Press, 1992. [Google Scholar]
- 17.Fagerström KO, Kunze M, Schoberberger R, et al. Nicotine dependence versus smoking prevalence: comparisons among countries and categories of smokers. Tob Control 1996;5:52–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res 2001;3:7–16. [DOI] [PubMed] [Google Scholar]
- 19.Koffarnus MN, Wilson AG, Bickel WK. Effects of experimental income on demand for potentially real cigarettes. Nicotine Tob Res 2015;17:292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koffarnus MN, Franck CT, Stein JS, et al. A modified exponential behavioral economic demand model to better describe consumption data. Exp Clin Psychopharmacol 2015;23:504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev 2008;115:186–98. [DOI] [PubMed] [Google Scholar]
- 22.Stein JS, Koffarnus MN, Stepanov I, et al. Cigarette and e-liquid demand and substitution in e-cigarette-naïve smokers. Exp. Clin. Psychopharmacol. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson MW, Johnson PS, Rass O, et al. Behavioral economic substitutability of e-cigarettes, tobacco cigarettes, and nicotine gum. J Psychopharmacol 2017;31:851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rass O, Pacek LR, Johnson PS, et al. Characterizing use patterns and perceptions of relative harm in dual users of electronic and tobacco cigarettes. Exp Clin Psychopharmacol 2015;23:494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeGrandpre RJ, Bickel WK, Hughes JR, et al. Behavioral economics of drug self-administration. Psychopharmacology 1992;108:1–10. [DOI] [PubMed] [Google Scholar]
- 26.Kosmider L, Kimber CF, Kurek J, et al. Compensatory Puffing With Lower Nicotine Concentration E-liquids Increases Carbonyl Exposure in E-cigarette Aerosols. Nicotine Tob Res 2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


