Abstract
Daclizumab, a humanized monoclonal antibody, binds CD25 and blocks formation of the IL-2 receptor on T cells. A study of daclizumab as acute graft-versus-host disease (GVHD) prophylaxis after unrelated bone marrow transplantation was conducted before the importance of CD25+FOXP3+ regulatory T cells (Tregs) was recognized. Tregs can abrogate the onset of GVHD. The relation between Tregs and a graft-versus-malignancy effect is not fully understood. An international, multicenter, double-blind clinical trial randomized 210 adult or pediatric patients to receive 5 weekly doses of daclizumab at 0.3 mg/kg (n = 69) or 1.2 mg/kg (n = 76) or placebo (n = 65) after unrelated marrow transplantation for treatment of hematologic malignancies or severe aplastic anemia. The risk of acute GVHD did not differ among the groups (P = .68). Long-term follow-up of clinical outcomes and correlative analysis of peripheral blood T cell phenotype suggested that the patients treated with daclizumab had an increased risk of chronic GVHD (hazard ratio [HR], 1.49; 95% confidence interval [CI], 1.0 to 2.3; P = .08) and a decreased risk of relapse (HR, 0.57; 95% CI, 0.3 to 1.0; P = .05), but similar survival (HR, 0.89; 95% CI, 0.6 to 1.3; P = .53). T cells from a subset of patients (n = 107) were analyzed by flow cytometry. Compared with placebo, treatment with daclizumab decreased the proportion of Tregs among CD4 T cells at days 11–35 and increased the proportion of central memory cells among CD4 T cells at 1 year. Prophylactic administration of daclizumab does not prevent acute GVHD, but may increase the risk of chronic GVHD and decrease the risk of relapse. By delaying Treg reconstitution and promoting immunologic memory, anti-CD25 therapy may augment alloreactivity and antitumor immunity.
Keywords: Antitumor immunity, Daclizumab, Regulatory T cells
INTRODUCTION
Daclizumab is a humanized monoclonal antibody that targets the p55 subunit of the IL-2 receptor α (IL-2Rα, or CD25) on human lymphocytes. On binding of CD25, daclizumab (like the similar monoclonal antibody basiliximab) blocks IL-2 binding to the high-affinity IL-2 receptor and impairs IL-2–mediated activation of T lymphocytes. Several investigators have reported on the activity of daclizumab in adults and children as primary [1] or secondary [2–9] therapy for acute graft-versus-host disease (aGVHD). Favorable safety and pharmacokinetic data prompted the design of a multicenter, randomized, double-blind clinical trial adding daclizumab to cyclosporine/methotrexate prophylaxis in unrelated donor bone marrow transplantation.
At the time that this trial was designed, it was thought that daclizumab would block T cell activation and prevent aGVHD. Since that time, however, the central role of regulatory T cells (Tregs) as negative regulators of immune responses has been established [10–15]. Tregs, characterized by CD25 cell surface expression and the nuclear transcription factor FOXP3, depend on IL-2 [16,17], and blockade of CD25 could inhibit their expansion and function, thereby augmenting the immune response. Tregs play a key role in preventing and abrogating the severity of GVHD in both murine and human models [18,19]. The study failed to meet the primary endpoint of decreasing the risk of aGVHD, and the results were reported in abstract form [20].
We recently hypothesized that IL-2 blockade with daclizumab after immune ablation and transplantation of hematopoietic cells hindered repopulation by Tregs while allowing the expansion of memory T cells, preventing the expected clinical response. Treg biology was unknown at the time of initial study design; however, banked samples allowed us to address this question. In the present study, we obtained long-term follow-up clinical data from this trial and analyzed the corresponding stored patient samples for Treg markers.
MATERIALS AND METHODS
Study Design and Objectives
Protocol NO14348 was a randomized, multicenter, double-blind, placebo-controlled study designed to evaluate the efficacy of 5 weekly doses of 0.3 mg/kg or 1.2 mg/kg of daclizumab when administered with cyclosporine and methotrexate for the prevention of aGVHD in recipients of a first unrelated allogeneic bone marrow transplant. The study’s primary endpoint was the 100-day cumulative incidence of aGVHD necessitating steroid therapy. Secondary endpoints included the incidence of chronic GVHD (cGVHD), disease relapse, and survival.
Blood samples were obtained throughout the first 101 days and at 1 year post-transplantation to evaluate daclizumab binding to CD25, the number of free CD25 binding sites, and the overall expression of CD25 on T cells. The long-term follow-up protocol was designed to evaluate Treg and memory T cell phenotype by flow cytometry and to evaluate overall survival and the incidence of cGVHD. The trial protocol and updated analysis were approved by the relevant Institutional Review Boards.
Eligibility and Transplantation Therapy
Adult and pediatric patients undergoing bone marrow transplant for any malignancy or severe aplastic anemia with total body irradiation at a dose of 1200 to 1440 cGy as part of the conditioning regimen were eligible for this study. HLA matching was determined by serologic typing for HLA-A and HLA-B and by allele typing by PCR for HLA-DRB1. A single mismatch within the 6 A, B, or DRB1 loci was permitted. Exclusion criteria were patient age over 55 years or under 1 year, previous autologous or allogeneic marrow transplantation, cardiac disease or arrhythmia, active or suspected pulmonary infection, serum glutamic oxalocetic transaminase more than twice the normal level or direct bilirubin >2 mg/dL, serum creatinine more than twice the normal level, involved field radiation to the chest within the previous 6 months or at any time if the total exposure exceeded 1500 cGy, and human immunodeficiency virus infection.
GVHD prophylaxis consisted of i.v. methotrexate 15 mg/m2 on day +1 and 10 mg/m2 on days +3, +6, and +11. Administration of cyclosporine 1.5 mg/kg i.v. every 12 hours began on day −1 and continued until recovery from gastrointestinal toxicity of the conditioning regimen. Thereafter, cyclosporine was administered orally at 6.25 mg/kg every 12 hours until day +50. Dosage reduction was allowed: methotrexate for impaired liver function as evaluated by elevated bilirubin and both cyclosporine and methotrexate for impaired renal function based on serum creatinine. Tapering of cyclosporine doses at 5% per week began on day +51 for patients who did not require systemic corticosteroids and had no GVHD. Patients developing grade II, III, or IV aGVHD were eligible for prednisone therapy. Concomitant enrollment onto GVHD therapy trials was allowed. Patients who received any study drug were evaluated for the study endpoints. The use of hematopoietic growth factor was at the discretion of the principal investigator. Fluconazole prophylaxis was to continue from conditioning until day +75.
Study Treatment
Randomization was stratified by donor HLA (matched versus mismatched), patient age (<20 versus ≥20 years), and center (Fred Hutchinson Cancer Research Center (FHCRC) versus United States non-FHCRC versus non–United States). Therapy consisted of 5 weekly i.v. doses of placebo (arm A), 0.3 mg/kg daclizumab (arm B), or 1.2 mg/kg daclizumab (arm C) to a maximum of 100 mg beginning on the day before transplantation. Assessments were performed no less than weekly following transplantation up to day +55 and then regularly until day +100, with cGVHD assessment at days +180 and +365. Weekly clinical assessments for aGVHD onset were recorded until day +100. Staging of aGVHD was based on the Glucksberg scale [21]. cGVHD was classified as limited (localized skin or hepatic dysfunction not accompanied by liver histology changes or other organ involvement) or extensive [22]. Adverse events were defined as any adverse change from the patient’s baseline (pretreatment) condition, including intercurrent illness, occurring during the course of the study.
Data Collection and Sample Analysis
Data from all 210 originally randomized patients were available for a summary of baseline characteristics and reported trial primary outcome measure (aGVHD requiring steroid therapy). Additional outcome data (grade II-IV aGVHD, grade III-IV aGVHD, cGVHD, relapse, death) were available from the original trial data for only 209 patients (data missing for 1 patient on the placebo arm). From these original 209 patients, additional long-term clinical data (beyond the data available at study closure through full duration of surviving patient follow-up) were secured from 95% of the patients at risk for events (obtained for 99 of the 104 patients alive at trial closure). Investigators at the various sites were contacted to update study records with the following long-term data: duration of follow-up, maximal grade of aGVHD, maximal grade of cGVHD, attainment of complete remission from primary disease, primary disease relapse, date of death, cause of death, and date of last contact.
Blood samples were collected at 5 distinct time windows from patients treated at FHCRC: before conditioning, on therapy (days +11 to +35), off therapy (days +36 to +80), washed (days +81 to +101), and long-term follow-up (day +365 ± 30). These samples were incubated with fluorescent-tagged antibodies or with primary antibodies and secondary fluorescent-tagged antibodies and analyzed to determine the fraction of T cells expressing CD25, to quantify free CD25 daclizumab-binding sites, and to determine the effectiveness of daclizumab binding to CD25+ cells. Cell aliquots were cryopreserved for later studies.
Permission from FHCRC and Moffitt Internal Review Board approval were obtained to analyze cryopreserved samples so that Treg and T cell memory phenotype could be evaluated. It was hypothesized that differences in T cell repopulation due to daclizumab would be most evident in comparisons between placebo (arm A) and daclizumab 1.2 mg/kg (arm C). Cells were stained with L/D yellow, and surfaces were stained with CD3 PB, CD4 Alexa Fluor 700, CD15 PerCp-Cy5.5, CD25 PE-Cy7, and CD127-Alexa Fluor 647 (BD Biosciences, San Jose, CA). The cells were then fixed and permeabilized using the Foxp3 buffer set (BD Biosciences), and then stained with Foxp3-PE. In another panel, cells were surface-stained with L/D yellow, CD3 PB, CD4 Alexa Fluor 700, CD15 Percp-Cy5.5, CD8 APC-H7, CD127 Alexa Fluor 647, CD45RA FITC, and CCR7 PE-Cy7. Cells were analyzed by flow cytometry (LSRII; BD Biosciences). Results from each post-transplantation time window (on therapy [days +11 to +35], washed [days +81 to +101], and long-term follow-up [day +365 ± 30) were compared between arms A and C. Neither absolute lymphocyte count nor T cell count data were available, and thus the absolute number of Treg or central memory subsets could not be derived.
Statistical Analyses
All study endpoints were analyzed according to the principle of intent-to-treat analysis. The study was designed to detect a decrease in the incidence of aGVHD (grade II-IV, requiring treatment with corticosteroids to day +100) from 80% to 55% with 80% power and 5% type 1 error. Baseline characteristics were summarized using descriptive statistics, including mean, median, standard deviation, and range for continuous measures and counts and frequencies for categorical measures. The cumulative incidence rates of aGVHD, grade III-IV aGVHD, cGVHD, primary disease relapse, and nonrelapse mortality were estimated using standard methods for competing-risks analysis and compared using Wald tests in a Cox regression model [23]. Overall survival was analyzed using the Kaplan-Meier method and compared using the log-rank test. Flow cytometry data for the daclizumab arms were compared with the placebo arm at each time window using the 2-sample t-test.
RESULTS
Patient and Transplantation Characteristics
A total of 210 patients were enrolled between April 13, 1993, and July 19, 1995. After stratification by age, degree of HLA matching, and transplantation center, patients were randomized to placebo (arm A; n = 65), daclizumab 0.3 mg/kg (arm B; n = 69), or daclizumab 1.2 mg/kg (arm C; n = 76) treatment added to cyclosporine/methotrexate GVHD prophylaxis. Patient and transplantation characteristics for the full original trial patient population (n = 210) are presented in Table 1. Most patients received the 5 intended doses (n = 62 in arm A, 63 in arm B, and 68 in arm C) and completed the 100-day follow-up (n = 49, 52, and 61, respectively).
Table 1.
Patient Characteristics
| Characteristic | Randomized Patients (n = 210)* | ||
|---|---|---|---|
| Arm A (Placebo; n = 65) | Arm B (0.3 mg/kg Daclizumab; n = 69) | Arm C (1.2 mg/kg Daclizumab; n = 76) | |
| Study site, n (%) | |||
| FHCRC | 35 (54) | 34 (49) | 39 (51) |
| United States, non-FHCRC | 16(24) | 19(28) | 19(25) |
| Non-US centers | 14(22) | 16(23) | 18(24) |
| Patient sex, n (%) | |||
| Female | 19(29) | 23 (33) | 40 (53) |
| Male | 46(71) | 46 (67) | 36 (47) |
| Donor sex, n (%) | |||
| Female | 28 (43) | 26 (38) | 30 (39) |
| Male | 37 (57) | 43 (62) | 46 (61) |
| HLA matching, | |||
| n(%) | |||
| Matched | 45 (69) | 51 (74) | 53 (70) |
| Mismatched | 20(31) | 18(26) | 23 (30) |
| Diagnosis, n (%) | |||
| ALL | 11 (17) | 11 (16) | 9(12) |
| AML | 6 (9) | 11 (16) | 13(17) |
| CML | 35 (54) | 37 (54) | 47 (62) |
| MDS | 9 (14) | 5(7) | 2 (3) |
| Other | 4 (6) | 5(7) | 5 (7) |
| Disease risk†,‡, n(%) | |||
| High | 31 (48) | 30 (43) | 33 (43)‡ |
| Low | 34 (52) | 39 (57) | 41 (54)‡ |
| Age | |||
| Median (range), yr | 35 (3–54) | 31 (1–52) | 30(1–54) |
| <20 yr, n (%) | 13(20) | 13(19) | 17(22) |
| ≥20 yr, n (%) | 52 (80) | 56(81) | 59 (78) |
| Cytomegalovirus serology, donor/patient, n (%) | |||
| +/− | 16(25) | 23 (34) | 19(25) |
| −/+ | 18(28) | 18(26) | 26 (34) |
| +/− | 11 (17) | 7(10) | 11 (14) |
| −/− | 20(31) | 20 (29) | 20 (26) |
| Doses of study treatment, n (%) | |||
| 5 | 62 (95) | 63 (91) | 68 (89) |
| <5 | 3 (5) | 6(9) | 8(11) |
FHCRC indicates Fred Hutchinson Cancer Research Center; ALL, acute lymphoblastic leukemia; AML, acute myelogenous leukemia; CML, chronic myelogenous leukemia; MDS, myelodysplastic syndrome.
Baseline characteristics are inclusive of all randomized patients (n = 210).
Disease risk defined as follows: high risk, acute leukemia in relapse or greater than second remission, transfusion-dependent aplastic anemia, CML beyond first chronic phase, and MDS with refractory anemia with excess blasts or refractory anemia with excess blasts in transformation; low risk, acute leukemia in first or second remission, non–transfusion-dependent aplastic anemia, CML in first chronic phase, and MDS with refractory anemia.
Risk category data not available for 2 patients.
aGVHD
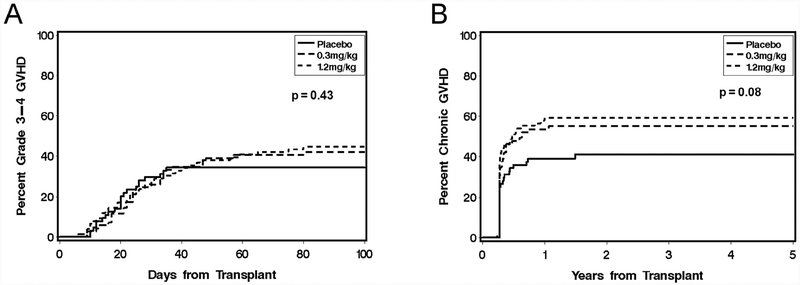
The primary endpoint of the trial was not achieved. Analysis of data on study closure (n = 210) demonstrated that the addition of daclizumab at doses of 0.3 mg/kg and 1.2 mg/kg to a standard immunosuppressive regimen of cyclosporine/methotrexate did not decrease the 100-day incidence of aGVHD necessitating high-dose steroid therapy: 66% for arm A, 72% for arm B, and 75% for arm C (P > .05). All subsequent analyses reported here are based on the complete data available in both the original and long-term follow-up datasets (n = 209). The cumulative incidence of any grade IIIIV aGVHD was similar in the 3 arms (38% for arm A, 42% for arm B, and 47% for arm C; P > .05) (Figure 1). With one exception, subgroup analyses of patients stratified on the basis of sex, age (<20 years versus ≥20 years), HLA match (matched versus mismatched), geographical study site (FHCRC versus United States non-FHCRC versus non-United States), and relapse risk category (low versus high) showed no statistically significant differences in the incidence or severity of aGVHD. The single exception was a greater incidence of aGVHD in arm C compared with arm A (88% versus 46%) in patients age <20 years (P = .02).
Figure 1.
Cumulative incidence of grade III-IV acute GVHD (A) and chronic GVHD (B) according to treatment with placebo (arm A), daclizumab 0.3 mg/kg (arm B), or daclizumab 1.2 mg/kg (arm C).
Secondary Endpoints
The Kaplan-Meier curve for overall survival and the cumulative incidence of nonrelapse mortality and relapse are presented in Figure 2. Analysis of overall mortality showed no statistical differences between either arm B (hazard ratio [HR], 1.02; 95% confidence interval [CI], 0.67 to 1.55; P = .93) or arm C (HR, 0.78; 95% CI, 0.51 to 1.20; P = .26) compared with arm A. Similarly, analysis of nonrelapse mortality showed no statistically significant differences between either arm B (HR, 1.23; 95% CI, 0.74 to 2.05; P = .43) or arm C (HR, 0.81; 95% CI, 0.48 to 1.39; P = .45) compared with arm A. For post hoc exploratory analyses, the 2 daclizumab dosage arms (B and C) were combined, because the results showed no statistically significant differences between these 2 arms (Table 2). Daclizumab therapy did not alter overall mortality (HR, 0.89; 95% CI, 0.6 to 1.3; P = .53) compared with placebo, but trends suggested that daclizumab decreased the risk of relapse (HR, 0.57; 95% CI, 0.3 to 1.0; P = .05) but increased the risk of cGVHD (HR, 1.49; 95% CI, 1.0 to 2.3; P = .08) compared with placebo.
Figure 2.
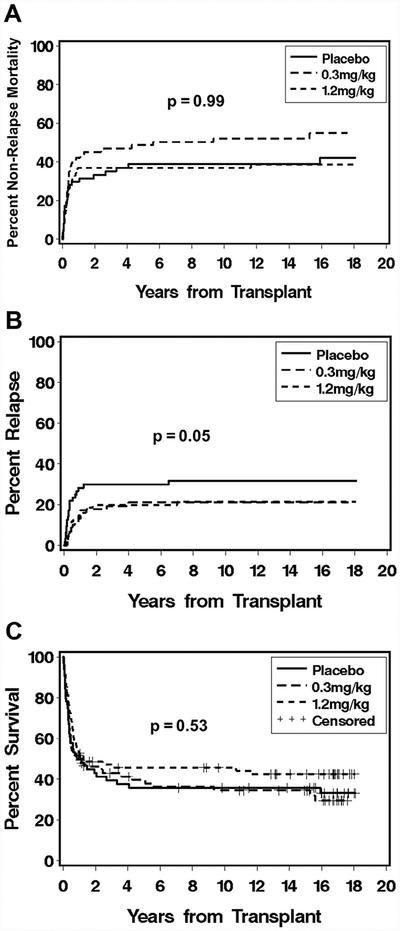
Cumulative incidence curves for nonrelapse mortality (A) and relapse (B), and Kaplan-Meier curve for overall survival (C), according to treatment with placebo, daclizumab 0.3 mg/kg, or daclizumab 1.2 mg/kg.
Table 2.
Clinical Outcomes: Daclizumab Therapy Compared with Placebo*
| Outcome | Therapy | HR (95% CI) | P versus Placebo |
|---|---|---|---|
| Overall mortality | Placebo | 1.0 | |
| Daclizumab | 0.89 (0.61–1.29) | .53 | |
| Relapse | Placebo | 1.0 | |
| Daclizumab | 0.57 (0.32–1.00) | .05 | |
| Nonrelapse mortality | Placebo | 1.0 | |
| Daclizumab | 1.00 (0.63–1.59) | .99 | |
| Grade II-IV GVHD | Placebo | 1.0 | |
| Daclizumab | 1.22 (0.75–1.98) | .42 | |
| Chronic GVHD | Placebo | 1.0 | |
| Daclizumab | 1.49(1.00–2.31) | .08 |
GVHD indicates graft-versus-host disease; HR, hazard ratio; CI, confidence interval.
A total of 209 patients contributed data to these outcome analyses.
To explore whether this relapse effect was limited to particular malignancies, we performed subset analysis. In the cohort of patients with chronic myelogenous leukemia (CML), relapse was decreased with daclizumab therapy compared with placebo (HR, 0.31; 95% CI, 0.1–0.8; P = .01). This was not seen in the combined group of patients with acute myelogenous leukemia/acute lymphoblastic leukemia/myelodysplastic syndrome (HR, 1.03; 95% CI, 0.5–2.4; P = .94).
Characteristics of patients with samples drawn and those without samples were well matched, with no differences in sex, HLA matching, diagnoses, disease risk, age, or treatment arm. Analyses of the median absolute total lymphocyte counts and median absolute CD3+, CD4+, CD8+, CD16+/CD56+, and CD20+ lymphocyte subset counts showed no statistically significant differences among the arms at any time point. The analyses on days +13 and +27 demonstrated significant differences in the numbers of circulating CD25+ T cells when stained by an antibody that did not cross-block with daclizumab (arm A, 27 ± 2; arm B, 20 ± 1 [P = .000007]; arm C, 16 ± 1 [P = .000002]; data not shown), but differences in CD25+ T cells were not apparent on days +55 and +83. These data demonstrate that daclizumab was effective in delaying the repopulation of CD25+ T cells.
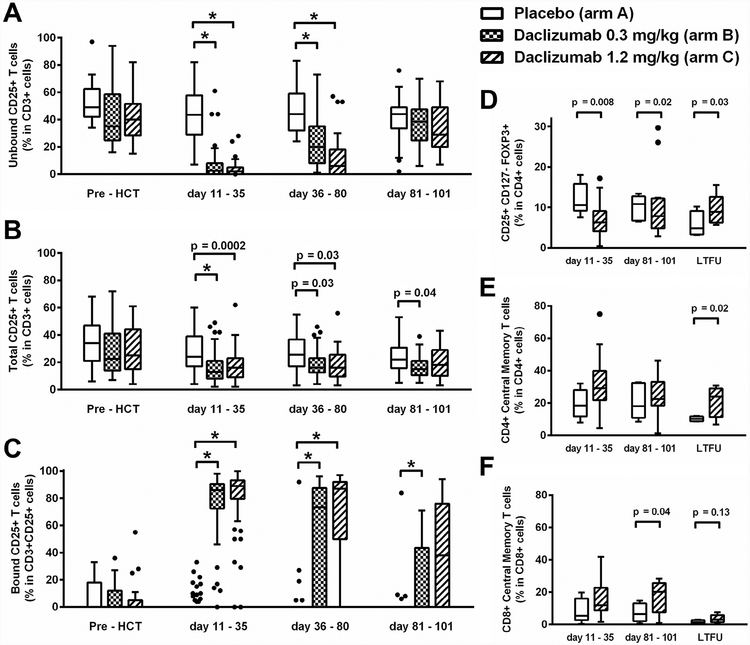
In studies of T cell subsets, daclizumab administration reduced the mean percentage of CD4 T cells expressing CD25 at days +11 to +35 (arm A, 28%; arm B, 16%; arm C, 18%) and at days +36 to +80 (26%, 19%, and 19%, respectively) but not at days +81 to +101 (Figure 3A). Daclizumab administration decreased the numbers of cells with free CD25-binding sites at days +11 to +35 (arm A, 45%; arm B, 7%; arm C, 3%) and at days +36 to +80 (49%, 25%, and 11%, respectively) but not at days +81 to +101 (Figure 3B). Daclizumab was bound to CD25 in vivo at days +11 to +35 (arm A, 3%; arm B, 74%; arm C, 82%) and persisted at days +81 to +101 (4.3%, 17%, and 39%, respectively) (Figure 3C).
Figure 3.
Flow cytometry analysis of lymphocyte subsets according to study arm. (A) Administration of daclizumab decreased the percentage of T cells expressing CD25 as measured by 7G7, a non–daclizumab-competing antibody against CD25, at days +11 to +35 and days +36 to +80, but not at days +81 to +101. (B) Daclizumab administration decreased the percentage of T cells with free CD25-binding sites at days +11 to +35 and days +36 to +80. (C) Daclizumab was effectively bound to CD25+ T cells in vivo at days +11 to +35, days +36 to +90, and days +81 to +101. (D) The percentage of Tregs in the CD4 T cell population (CD3+CD4+CD127lowCD25+FOXP3+) was decreased at days +11 to +35 and days +81 to +101. (E) The percentage of CD4 cells with a central memory phenotype (CD3+CD4+CCR7+CD45RA-) was increased at 1 year (LTFU). (F) The percentage of CD8 cells with a central memory phenotype (CD3+CD8+CCR7+CD45RA-) was increased at days +81 to +101. *P < .0001, 2-sample t-test. In all graphs, the box extends the interquartile range (IQR) from the 25th to 75th percentiles, the whiskers extend to the nearest data point 1.5 times the IQR beyond the 25th and 75th percentiles, and the dots represent data >1.5 times the IQR above the 75th quartile or below the 25th quartile. In some cases (C), the box and whiskers are all at 0 and not represented on the graph.
Available samples from arm A (n = 18) and arm C (n = 40) were tested for Treg (CD4+CD127−CD25+FOXP3+) (Figure 3D) and central memory (CD4+CD45RA−CCR7+) phenotype (Figure 3E). Compared with placebo, daclizumab 1.2 mg/kg administration decreased the percentage of Tregs in CD4 cells at days +11 to +35 (12% versus 7%) but not at days +81 to +101 or at 1 year. Daclizumab increased the percentage of central memory cells in CD4 cells at 1 year (10% versus 21%).
DISCUSSION
The results of this controlled trial of daclizumab do not support the efficacy of CD25 blockade for GVHD prophylaxis. Although the trial was concluded many years ago, long-term follow-up and recent analysis of stored blood samples revealed novel biological insights. Blockade of CD25 led to a sustained decrease in Tregs during the critical period of aGVHD onset and promoted CD4+ memory. This explains the suggested clinical effects of this therapy when used after allogeneic hematopoietic cell transplantation, including increased risk of cGVHD and decreased risk of relapse. By delaying Treg reconstitution and promoting immunologic memory, anti-CD25 therapy may augment alloreactivity and antitumor immunity. Using strict criteria for the identification of Tregs, CD4+CD127−CD25+FoxP3+, we show that the percentage of Tregs in the CD4 population was reduced after daclizumab administration. It was previously hypothesized that CD25 blockade would decrease T cell activation by preventing IL-2 signaling. It is now recognized that CD25+ cells are highly enriched for FOXP3+ Tregs, which are necessary to prevent untoward autoimmunity. In allogeneic hematopoietic cell transplantation, Tregs are critical in decreasing the onset of GVHD in both human and animal models.
These data are important in the context of other clinical trials reporting Treg analysis after hematopoietic cell transplantation. Both in vivo and ex vivo manipulations are under development to increase Tregs relative to T effectors to reduce the incidence of GVHD [24,25]. Matsuoka et al. demonstrated that low-dose IL-2 improves thymic export, increases proliferation, and enhances the resistance to apoptosis in Tregs but not conventional CD4 T cells [26]. Our findings show that when IL-2 signaling is blocked, a reciprocal mechanism acts to either eliminate or inhibit the proliferation of circulating Tregs rather than simply down-regulate T cell CD25 expression. This is in contrast to findings by others suggesting that CD25-specific antibody administration merely down-regulates CD25 expression without depleting Tregs or altering the FoxP3 transcription factor [27].
In principle, CD25 blockade or depletion could reduce CD8+ effectors and CD4+ helper cells to a degree that impairs immunity or increases the risk of relapse [28]. In the present study, rates of infection and lymphocyte reconstitution were similar among the groups. In line with our predetermined hypothesis, we examined central memory subsets after transplantation and found that daclizumab was associated with an increase in the percentages of cells with a central memory phenotype among the CD4 and CD8 cells in patients at 1 year after transplantation. Animal studies have established that alloreactive memory T cells are responsible for persistence of cGVHD, although with the small numbers of patients presented here, a direct role cannot be established [29]. Homeostatic proliferation refers to the brisk in vivo expansion of transferred T cells made possible by lymphodepleting chemoradiation therapy. The increased proportion of CD4 central memory cells may be due to a competitive advantage gained by the absence of Tregs during this early expansion phase [30]. Careful examination of Treg reconstitution early after allogeneic transplantation and its impact on the later development of T cell subsets merits further investigation.
Interpretation of the present study must take into account the following factors. First, a regimen of cyclosporine and methotrexate was used for GVHD prophylaxis. Newer GVHD prophylaxis regimens may promote the regeneration or survival of Tregs post-transplantation [25,31], further supporting the idea that reconstitution of the Treg compartment immediately after transplantation prevents alloreactivity. Second, all patients received bone marrow grafts. Mobilized blood cells accelerate engraftment but also increase the incidence of aGVHD and cGVHD [32–35]. Third, matching for 6/6 HLA-A, HLA-B, and HLA-DR antigens leads to higher rates of GVHD compared with 8/8 allele typing that includes HLA-C, which is more commonly used today [36]. Fourth, all patients received a total body irradiation–based myeloablative regimen. The degree of lymphodepletion and the effects on Treg repopulation may vary with reduced-intensity or nonmyeloablative regimens. Finally, we were unable to evaluate the absolute Treg numbers, and there may be immunologic differences between the absolute numbers and percentages of Treg in the peripheral blood. However, multiple studies have shown that Tregs expressed as a percentage of the total number of T cells and Tregs expressed as an absolute number per microliter track together, and both correlate with clinical outcomes after adoptive cellular therapy [37,38].
Although daclizumab did not affect the incidence or severity of aGVHD or the risk of mortality, there was a suggestion of an increased risk of cGVHD (P = .08) and a decreased risk of relapse (P = .05) compared with placebo, consistent with other observations that Treg reconstitution early after transplantation is linked to the incidence of cGVHD and relapse [39,40]. The decrease in relapse was most notable in patients with CML (P = .01). A direct effect of daclizumab on CML leukemia stem cells, which may express CD25, cannot be ruled out; however, CD25 expression acts as a negative regulator of CML cell growth, suggesting that daclizumab blockade of CD25 is unlikely to decrease the survival of CML cells directly [41]. Despite recent changes in the makeup of transplantation recipients with CML and the advent of reduced-intensity conditioning regimens, CML is a disease in which the graft-versus-leukemia effect is well described. Our finding supports the concept that even a transient reduction in Tregs can influence long-term disease control.
Early data suggested that CD25-blocking antibodies might be therapeutic against aGVHD, although it was subsequently shown that daclizumab was detrimental, increasing the likelihood of death when added as an adjuvant to the initial steroid therapy of aGVHD [1]. Results of that study and others decreased interest in the targeting of CD25 as a therapy against GVHD [42]. The data presented here highlight the potential utility of using CD25 blockade to promote antitumor immunity, an effect that may be exploited after either autologous or allogeneic hematopoietic cell transplantation [43]. Further efforts should be made to reduce Treg numbers to enhance antitumor immunity after donor lymphocyte infusion in allogeneic transplant recipients and after tumor vaccine administration in autologous transplant recipients. Although the i.v. formulation of daclizumab (Zenapax) is no longer marketed, a subcutaneous formulation of daclizumab (Zinbryta) was recently approved for the treatment of multiple sclerosis, or basiliximab, which targets the same CD25 subunit, could be administered to patients. Alternatively, a magnetic column and bead-based depletion of the cell product could be used [44–46]. Antibody blockade of CD25 immediately after transplantation did not decrease the risk of aGVHD; however, the suggestions for decreased risk of relapse and increased risk of cGVHD indicate that further exploration of this strategy should be considered to promote antitumor immunity.
ACKNOWLEDGMENTS
The authors thank Rasa Hamilton for editing the manuscript.
Footnotes
Conflict of interest statement: S.L. owns stock in Protein Design Labs, Inc. which is the holding company for the antibody humanization patents, and owns stock in Biogen-Idec, which is developing daclizumab for the therapy of multiple sclerosis. All of the other authors have no conflicts of interest to report.
REFERENCES
- 1.Lee SJ, Zahrieh D, Agura E, et al. Effect of up-front daclizumab when combined with steroids for the treatment of acute graft-versus-host disease: results of a randomized trial. Blood 2004;104:1559–1564. [DOI] [PubMed] [Google Scholar]
- 2.Przepiorka D, Kernan NA, Ippoliti C, et al. Daclizumab, a humanized anti-interleukin-2 receptor alpha chain antibody, for treatment of acute graft-versus-host disease. Blood 2000;95:83–89. [PubMed] [Google Scholar]
- 3.Willenbacher W, Basara N, Blau IW, et al. Treatment of steroid-refractory acute and chronic graft-versus-host disease with daclizumab. Br J Haematol 2001;112:820–823. [DOI] [PubMed] [Google Scholar]
- 4.Srinivasan R, Chakrabarti S, Walsh T, et al. Improved survival in steroid-refractory acute graft versus host disease after non-myeloablative allogeneic transplantation using a daclizumab-based strategy with comprehensive infection prophylaxis. Br J Haematol 2004;124:777–786. [DOI] [PubMed] [Google Scholar]
- 5.Wolff D, Roessler V, Steiner B, et al. Treatment of steroid-resistant acute graft-versus-host disease with daclizumab and etanercept. Bone Marrow Transplant 2005;35:1003–1010. [DOI] [PubMed] [Google Scholar]
- 6.Bordigoni P, Dimicoli S, Clement L, et al. Daclizumab, an efficient treatment for steroid-refractory acute graft-versus-host disease. Br J Haematol 2006;135:382–385. [DOI] [PubMed] [Google Scholar]
- 7.Perales MA, Ishill N, Lomazow WA, et al. Long-term follow-up of patients treated with daclizumab for steroid-refractory acute graft-vs-host disease. Bone Marrow Transplant 2007;40:481–486. [DOI] [PubMed] [Google Scholar]
- 8.Schechter T, Afzal S, Finkelstein Y, et al. Daclizumab therapy for children with corticosteroid-resistant acute graft-vs.-host disease. Pediatr Transplant 2009;13:332–337. [DOI] [PubMed] [Google Scholar]
- 9.Miano M, Cuzzubbo D, Terranova P, et al. Daclizumab as useful treatment in refractory acute GVHD: a paediatric experience. Bone Marrow Transplant 2009;43:423–427. [DOI] [PubMed] [Google Scholar]
- 10.Albert MH, Anasetti C, Yu XZ. T regulatory cells as an immunotherapy for transplantation. Expert Opin Biol Ther 2006;6:315–324. [DOI] [PubMed] [Google Scholar]
- 11.Allan SE, Broady R, Gregori S, et al. CD4+ T-regulatory cells: toward therapy for human diseases. Immunol Rev 2008;223:391–421. [DOI] [PubMed] [Google Scholar]
- 12.Godfrey WR, Ge YG, Spoden DJ, et al. In vitro-expanded human CD4(+)CD25(+) T-regulatory cells can markedly inhibit allogeneic dendritic cell-stimulated MLR cultures. Blood 2004;104:453–461. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann P, Ermann J, Edinger M, et al. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med 2002;196:389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen VH, Zeiser R, Negrin RS. Role of naturally arising regulatory T cells in hematopoietic cell transplantation. Biol Blood Marrow Transplant 2006;12:995–1009. [DOI] [PubMed] [Google Scholar]
- 15.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity 2009;30:646–655. [DOI] [PubMed] [Google Scholar]
- 16.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004;10:942–949. [DOI] [PubMed] [Google Scholar]
- 17.Rudensky AY. Regulatory T cells and Foxp3. Immunol Rev 2011;241:260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edinger M, Hoffmann P, Ermann J, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med 2003;9:1144–1150. [DOI] [PubMed] [Google Scholar]
- 19.Koreth J, Matsuoka K, Kim HT, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med 2011;365:2055–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anasetti C, Lin A, Nademanee A, et al. A phase II/III randomized, double-blind, placebo-controlled multicentre trial of humanized anti-Tac for prevention of acute graft-versus-host disease in recipients of marrow transplants from unrelated donors. Blood 1995;86(Suppl 1):621a. [Google Scholar]
- 21.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HLA-matched sibling donors. Transplantation 1974;18:295–304. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan KM. Acute and chronic graft-versus-host disease in man. Int J Cell Cloning 1986;4 Suppl 1:42–93. [DOI] [PubMed] [Google Scholar]
- 23.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. New York: Wiley; 1980. [Google Scholar]
- 24.Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood 2011;117:1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pidala J, Kim J, Jim H, et al. A randomized phase II study to evaluate tacrolimus in combination with sirolimus or methotrexate after allogeneic hematopoietic cell transplantation. Haematologica 2012; 97:1882–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuoka K, Koreth J, Kim HT, et al. Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med 2013;5:179ra43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohm AP, McMahon JS, Podojil JR, et al. Cutting edge: anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J Immunol 2006;176:3301–3305. [DOI] [PubMed] [Google Scholar]
- 28.Müller AM, Linderman JA, Florek M, et al. Allogeneic T cells impair engraftment and hematopoiesis after stem cell transplantation. Proc Natl Acad Sci USA 2010;107:14721–14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Joe G, Hexner E, et al. Alloreactive memory T cells are responsible for the persistence of graft-versus-host disease. J Immunol 2005;174:3051–3058. [DOI] [PubMed] [Google Scholar]
- 30.Shen S, Ding Y, Tadokoro CE, et al. Control of homeostatic proliferation by regulatory T cells. J Clin Invest 2005;115:3517–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanakry CG, Ganguly S, Zahurak M, et al. Aldehyde dehydrogenase expression drives human regulatory T cell resistance to posttransplantation cyclophosphamide. Sci Transl Med 2013;5:211ra157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bensinger WI, Martin PJ, Storer B, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med 2001;344:175–181. [DOI] [PubMed] [Google Scholar]
- 33.Couban S, Simpson DR, Barnett MJ, et al. A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood 2002;100:1525–1531. [DOI] [PubMed] [Google Scholar]
- 34.Eapen M, Logan BR, Confer DL, et al. Peripheral blood grafts from unrelated donors are associated with increased acute and chronic graft-versus-host disease without improved survival. Biol Blood Marrow Transplant 2007;13:1461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367:1487–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flomenberg N, Baxter-Lowe LA, Confer D, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood 2004;104: 1923–1930. [DOI] [PubMed] [Google Scholar]
- 37.Wolf AM, Wolf D, Steurer M, et al. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res 2003;9:606–612. [PubMed] [Google Scholar]
- 38.Yao X, Ahmadzadeh M, Lu YC, et al. Levels of peripheral CD4(+)FoxP3(+) regulatory T cells are negatively associated with clinical response to adoptive immunotherapy of human cancer. Blood 2012;119:5688–5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol 1999;163:5211–5218. [PubMed] [Google Scholar]
- 40.Onizuka S, Tawara I, Shimizu J, et al. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res 1999;59:3128–3133. [PubMed] [Google Scholar]
- 41.Sadovnik I, Hoelbl-Kovacic A, Herrmann H, et al. Identification of CD25 as STAT5-dependent growth regulator of leukemic stem cells in Ph+ CML. Clin Cancer Res 2016;22:2051–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin PJ, Pei J, Gooley T, et al. Evaluation of a CD25-specific immunotoxin for prevention of graft-versus-host disease after unrelated marrow transplantation. Biol Blood Marrow Transplant 2004;10:552–560. [DOI] [PubMed] [Google Scholar]
- 43.Jing W, Yan X, Hallett WH, et al. Depletion of CD25+ T cells from hematopoietic stem cell grafts increases posttransplantation vaccine-induced immunity to neuroblastoma. Blood 2011;117:6952–6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nikiforow S, Kim H, Kao GS, et al. CD25+ regulatory T cell-depleted donor lymphocyte infusion for relapse after allogeneic transplantation: a PHASE 1 study. Biol Blood Marrow Transplant 2013;19:S135–S136. [Google Scholar]
- 45.Maury S, Lemoine FM, Hicheri Y, et al. CD4+CD25+ regulatory T cell depletion improves the graft-versus-tumor effect of donor lymphocytes after allogeneic hematopoietic stem cell transplantation. Sci Transl Med 2010;2:41ra52. [DOI] [PubMed] [Google Scholar]
- 46.Gold R, Giovannoni G, Selmaj K, et al. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): a randomised, double-blind, placebo-controlled trial. Lancet 2013;381:2167–2175. [DOI] [PubMed] [Google Scholar]