Abstract
Aims
Plant extracts have long been used for the ethnomedical treatment of diabetes, microbial infections and as a source of antioxidant. This study was aimed at investigating the antidiabetic, antioxidant, and antimicrobial activities of the n-hexane and ethyl acetate extract of Tephrosia bracteolata leaves (TBL) as associated with the ethnobotanical knowledge of the local people of Nigeria.
Main methods
The phytochemical composition of the n-hexane and ethyl acetate extract of the leaves of T. bracteolata were determined following standard procedures in literature, and it's in vitro inhibitory activities against α-glucosidase enzyme. 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS.+) and 1,1-diphenyl-2-picrylhydrazyl (DPPH+) antioxidant activities were also examined. Well diffusion method was employed in evaluating the antimicrobial property of the extracts.
Key findings
The ethyl acetate extract of T. bracteolata leaves had the greatest inhibitory effect on α-glucosidase, followed by the n-hexane with IC50 43.95 μg/ml and IC50 ˃50 μg/ml respectively. The ethyl acetate also exhibited significant DPPH+ and ABTS.+ antioxidant activity with IC50 of 24.96 μg/ml and 6.48 μg/ml as compared to Ascorbic acid and Trolox (12.24 μg/ml and 5.91 μg/ml) respectively. The zones of inhibition of the ethyl acetate extract of T. bracteolata leaves ranges from 10 – 25 mm at a concentration of 6.25–200 mg/ml, and it showed a greater antibacterial activity than the n-hexane extract, having a zone of inhibition from 10 – 20 mm at concentration of 12.5–200 mg/ml when compared to the standard Gentamycin. Similarly, the ethyl acetate extract of T. bracteolata showed a better anti fungi activity at concentration range 12.5–200 mg/ml than the n-hexane extract at concentration range of 25–200 mg/ml with reference to Tioconazole. These results indicated for the first time that the ethyl acetate extract of T. bracteolata leaves extracts exerted potent inhibitory effects against α-glucosidase, actively scavenge DPPH+ and ABTS.+ free radicals and successfully inhibits the proliferation of Gram positive and Gram negative microorganism.
Significance
TBL is an important source of antidiabetic, antimicrobial and antioxidant agent.
Keywords: Agriculture, Microbiology, Plant biology, Antidiabetic, Antimicrobial, Antioxidants, Phytochemical composition, Alpha glucosidase, Tephrosia bracteolata
1. Introduction
Pathogenic micro-organisms have built great resistance against several synthetic antibiotics; as a result, much attention is being paid to isolate biologically active compounds from plant species used in primitive medicine [1]. Thus, offering a new lead towards drug discovery [2]. Extracts from plants have been used since ancient times for the traditional treatment of diabetes, microbial infections, stress related disorder, sources of antioxidant, etc., and now they are widely accepted as supplementary alternative for orthodox drugs [3]. Plants represents majorly the useful dietary supplements for improving blood glucose control and preventing long-term complications in type 2 diabetes mellitus (T2DM) [4]. Research has revealed further credence to oxidative stress as a major contributor to hyperglycemia induced tissue injury likewise early events leading to the development of T2DM [5]. Presently, there is a surge of interest in plant-based remedies capable of arresting physiological effects in order to advert or cure diabetes, therefore, in vitro inhibition α -glucosidase enzymes is currently a major research focus. In recent past, in vitro studies have been performed giving rise to potential α-glucosidase inhibitors from various natural food and plant sources [6, 7, 8, 9]. Therefore, natural α-glucosidase inhibitors of plant origin offer an interesting control for postprandial hyperglycemia.
Tephrosia bracteolata is a useful Plants of West Tropical Africa [10, 11]. It is an herbaceous annual, or shrubby, erect under bush to 1 m high, or more, of open country throughout the Region from Senegal to Nigeria, and widespread in tropical Africa eastwards to Ethiopia and southwards to Angola [12]. The plant has been used for various purposes in folklore medicine. The root is used as medicine for venereal diseases, e.g. treatment of pregnant women with syphilis, the aerial parts has been used for animal grazing [13] and, used in Ivory Coast as fish poison [14]. In the northern part of Nigeria, the leaves and roots are used by hunters and warriors for charms against treatment of injury [10]. This study is aimed at determining the antidiabetic, antioxidant and antimicrobial properties of the leaves of T. bracteolate since there is dearth of information on its activity.
2. Materials and methods
2.1. Reagents
Potassium persulfate, 1,1-diphenyl-2-picrylhydrazyl (DPPH), methanol, glucose, BSA (Bovine serum albumin), sodium phosphate buffer, ascorbic acid, ethanol, Acarbose Ascorbic acid, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid), para-nitrophenyl-alpha-D-glucopyranoside, etc, were purchased from Sigma-Aldrich chemicals, USA, Merck Limited, India and S.D. Fine Chemicals Limited India.
2.2. Materials
Gram-negative bacteria: Salmonella typhii (UCH 4801), Escherica coli (UCH 00260), Pseudomonas aeruginosa (UCH 1102) and Klebsiellae pneumonae (UCH 2894) while Bacillus subtilis (UCH 74230) and Staphylococcus aureus (UCH 2473) are Gram-positive, clinical strains from the Medical Microbiology unit, University College Hospital, Ibadan, were screened at the Department of Pharmaceutical Microbiology laboratory, University of Ibadan, Ibadan, Nigeria. Solvent of n-hexane and ethyl acetate were used as negative controls in the assays. Antimicrobial agents: Gentamycin (10 μg/ml) and Ticonazole (0.7 μg/ml) were included as standard reference drugs in the study.
2.3. Collection of plant
The plant was collected from Jalala Estate, University of Ilorin, Tanke Oke-Odo, Ilorin, Kwara State, Nigeria. The plant materials were taxonomically identified and varified at the Department of Plant Biology, University of Ilorin, Ilorin, where a voucher specimen (UIH004/1189) was deposited.
2.4. Preparation of plant extracts
Fresh leaves of T. bracteolata were harvested, weeded, washed, and air-dried under shade. The air-dried leaves were gradiently extracted in n-hexane and ethyl acetate respectively. The n-hexane and ethyl acetate crude extracts were concentrated and stored at 4 °C.
2.5. Preliminary phytochemical screening of T.bracteolata leaves extract
The n-hexane extract (TbHL) and the ethyl acetate extract (TbEAL) of the leaves of T. bracteolata were each screened for various bioactive pharmaceutical constituents such as anthraquinones, steroids, cardiac glycosides, tannins, saponins, phlobotannins, terpenoids, flavanoids, and alkaloids using standard methods [14, 15].
2.6. Antidiabetic study
The antidiabetic Studies was carried out using Alpha-Glucosidase Inhibition Assay [16]. Briefly, in a 96-well microplate, 20 μL of each extract was incubated with 50 μL of crude intestinal α-glucosidase for 5 min and 50 μL of substrate (5 mM, p-nitrophenyl-α-glucopyranoside, prepared in 100 mM phosphate buffer, pH 6.8) was added. The pale yellow colour due to the release of p-nitrophenol from α-linkage of glucopyranoside by the action of enzyme α-glucosidase was measured spectrophotometrically at 405 nm (BioTek synergy4, BioTek Instruments Inc, Winooski, VT, USA) after incubation for 10 min. An individual blank for each extract was prepared to counterbalance absorbance due to the colour of samples, where, in lieu of enzyme, 50 μL of normal saline was added. Percentage of enzyme inhibition was obtained applying the following formula:
The antidiabetic Studies was carried out using Alpha-Glucosidase Inhibition Assay [16]. Acarbose was used as the reference standard.
2.7. Free radical scavenging assay
2.7.1. DPPH free radical scavenging assay
Decolorisation of DPPH radical was determined by reported standard method [11]. In brief, 25 μL of various dilutions of the extracts (n-hexane and ethyl acetate), 100 μL of Tris HCl buffer (0.1 M, pH 7.4) and 125 μL DPPH solution (0.5 mM in methanol) were added in a 96-well micro plate and incubated in the dark for 15 min. Absorbance was recorded at 517 nm, and the percentage of DPPH scavenging by extracts was calculated applying the formula:
Several serial dilutions of respective extracts were prepared and analysed [17]. Ascorbic acid was used as standard. The percentage of DPPH scavenging was calculated as above. The IC50 of the extracts were calculated applying a suitable regression analysis.
2.7.2. ABTS·+ scavenging assay
Scavenging of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) ABTS·+ cation radical was performed with suitable modifications. Briefly, 100 ml stock solution of ABTS·+ (0.5 mM) was prepared by addition of 1 ml potassium persulfate [6.89 mM in Phosphate-buffered saline (PBS) (pH 8.0)]. Mixture was stored in dark for 16 h. 10 μL of each of the different extracts (5 μg/ml in PBS) were added to 190 μL of ABTS·+ in 96-well microplate. Percentage scavenging of ABTS·+ by test samples were calculated as follows using:
Necessary calculations were applied to obtain the IC50 [18].
2.8. Antimicrobial assay
Microorganism's cultures of six human pathogenic bacteria made up of four Gram negative and two Gram positive were used for the antibacterial assay. The Antifungal assay was tested on four fungi: Candida albicans; Aspergillus niger; Rhizopus stolon; and Penicillum notatum.
Media: Nutrient agar, Sabouraud dextrose agar, nutrient broth and tryptone soya agar were used in this study.
2.8.1. Antimicrobial activity determination
Agar diffusion-pour plate method (bacteria) [19, 20]. An overnight culture of each organism was prepared by taking two wire loop of the organism from the stock and inoculated each into the sterile nutrient broth of 5ml, each incubated for 18–24 h at 37 °C. From overnight culture, 0.1 ml of each organism was taken and put into the 9.9 ml of sterile distilled water to obtained 10−2 inoculum concentration of the organism. From the diluted organism (10−2), 0.2 ml was taken into the prepared sterile nutrient agar cooled to about 40–45 °C, then poured into sterile Petri dishes and allowed to solidify for about 1 h. Using a sterile cork-borer of 8 mm diameter, the wells were made according to the number of the test tubes for the experiment. For this work 8 wells were made. The graded concentrations of the extracts were put into the wells accordingly including the controls. The studies were done in duplicates to ascertain the results obtained. The plates were left on the bench for about 2 h to allow the extract diffuse properly into the nutrient agar i.e. pre-diffusion. The plates were incubated for 18–24 h at 37 °C.
Agar diffusion-surface plate method (fungi): A sterile sabouraud dextrose agar was prepared.
accordingly, and aseptically poured into the sterile plates in triplicates and solidified properly. 0.2 ml of the 10−2 inoculum concentration of the organism was spread on the surface of the agar using a sterile Petri-dish to cover all the surface of the agar. Eight wells were bored using a sterile cork-borer of 8 mm diameter. The graded concentrations of the extracts were put into the including the controls. All the plates were left on the bench for 2 h to allow the extract diffuse properly into the agar i.e. pre-diffusion. The plates were incubated at 25 °C for 72 h [19,20].
2.9. Statistical analysis
Results were presented as mean ± standard error of mean (SEM). Statistical analysis was carried out with Graph Pad Prism 6 software, Incorporated, USA. There were significant differences between the extracts and the positive control. it may be less because its a crude extract which might have antagonistic compounds.
3. Results and discussion
3.1. Phytochemical screening
The results of the phytochemical screening carried out on the TbHL and TbEAL extracts of T. bracteolata leaves is shown in Table 1 below. The result revealed the presence of alkaloids, steroids, tannins, flavanoids and terpenoids. The analysis revealed the presence of constituents which are known to exhibit medicinal activities (Lwande et al., 1985).
Table 1.
Qualitative phytochemical composition of the n-hexane and ethyl acetate extract of T. bracteolata.
| Secondary metabolites | Extracts |
|
|---|---|---|
| TbHL | TbEAL | |
| Alkaloids | + | + |
| Steroids | + | + |
| Cardiac glycosides | - | - |
| Tannins | - | + |
| Saponins | - | - |
| Terpenoids | - | - |
| Flavonoids | - | + |
| Anthraquinones | - | - |
3.2. Antidiabetic activity
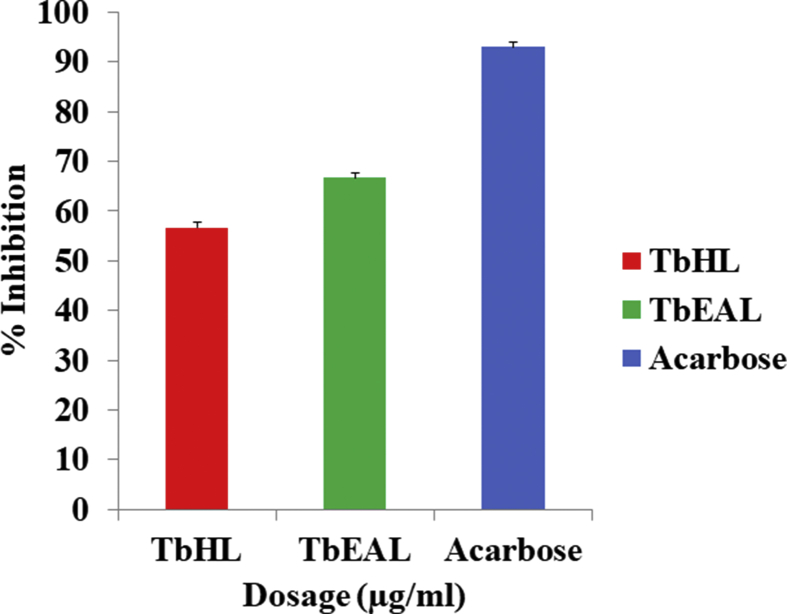
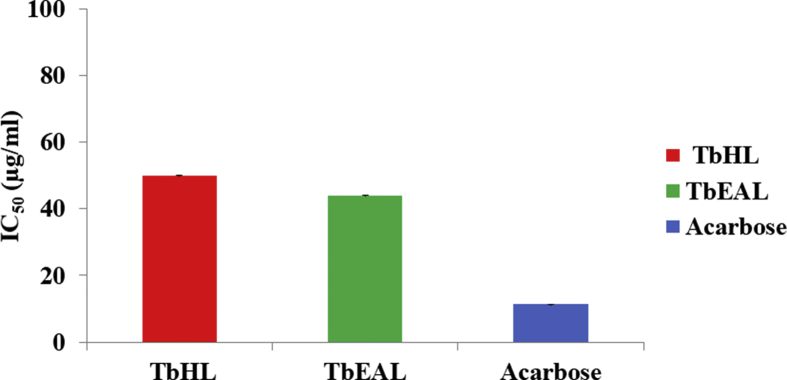
The results of the in vitro α-glucosidase enzyme inhibition assay (Fig. 1) of the TbHL and TbEAL extracts of T. bracteolata leaves revealed moderate antidiabetic activities. TbHL extract of T. bracteolata showed a lower α-glucosidase enzyme percentage inhibition activity (57%), when compared to the TbEAL (67%) extract and the standard, Acarbose (92.95%). Between these two plant extracts, TbEAL extract of T. bracteolata exhibited IC50 43.95 μg/ml as compared to Acarbose 11.31 μg/ml though not statistically significant (p < 0.05) compared to the standard of (Fig. 2). Rajaram and Suresh [21] reported the ethyl acetate stem extract of Tephrosia tinctoria to have shown significant inhibitory activity (IC50 94.33 ± 3.65 μg/ml) compared to standard (Acarbose - IC50 38.92 ± 3.52 μg/ml). The aqueous seed extract of Tephrosia purpurea was reported to have demonstrated significant in vivo antihyperglycemic activity in streptozotocin induced diabetic rats [22]. Likewise, the ethanolic extract of Tephrosia villosa leaves showed reduction in glucose level and pancreatic cell regeneration in alloxan induced diabetes in rats [23]. These results also lend credence to the fact that TbEAL extract of T. bracteolata demonstrates anti diabetic activity.
Fig. 1.
α - Glucosidase % Inhibition of TbHL and TbEAL.
Fig. 2.
IC50 Value of TbHL and TbEAL Extracts on α – Glucosidase Inhibitory Assay.
3.3. In vitro antioxidant activities
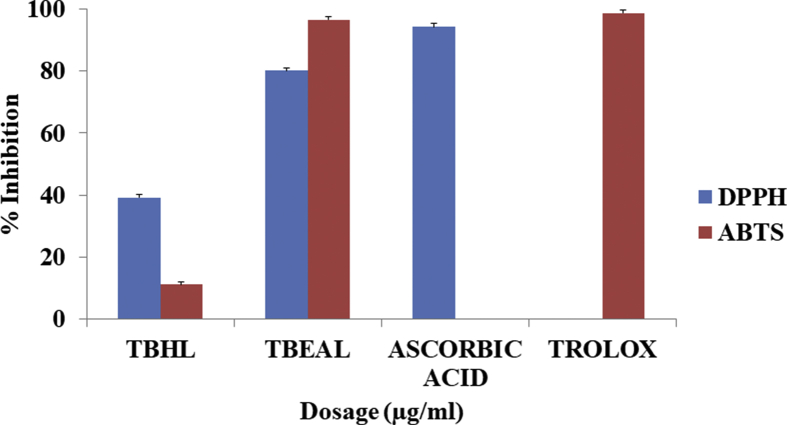
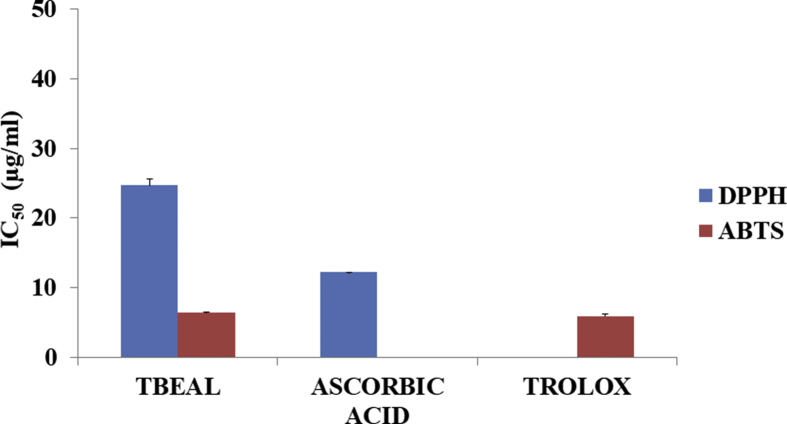
The results of the in vitro antioxidant activity of TbHL and TbEAL extracts of T. bracteolata presented in Figs. 3 and 4 showed moderate activity respectively as revealed by the DPPH and ABTS + free radical scavenging activity. The TbHL extract of T. bracteolata showed a lower percentage inhibition of DPPH antioxidant activities of (39.29 ± 0.07) when compared to that of the extract of TbEAL (80.12 ± 1.22). The percentage inhibition of TbEAL extract was not significantly different from that of the standard compound, Ascorbic acid (94.23 ± 0.00). Between these two plant extracts, the TbEAL extract of T. bracteolata exhibited significant inhibition of IC50 24.69 μg/ml as compared to the standard compound, Ascorbic acid 12.24 μg/ml. Similarly, the TbHL extract of T. bracteolata showed a lower percentage inhibition of ABTS + antioxidant activities of (11.14 ± 0.36) as compared to that of the TbEAL extract (96.46 ± 0.00). The percentage inhibition of TbEAL extract was not significantly different from that of the standard compound, Trolox (98.73 ± 0.30). The IC50 also of TbEAL extract 6.48 μg/ml was also not statistically different from the standard 5.91 μg/ml. From the results discussion above of the two plant extracts, TbEAL extract of T. bracteolata exhibited significant DPPH and ABTS + antioxidant activity IC50 of 24.96 μg/ml and 6.48 μg/ml as compared to Ascorbic acid and Trolox (12.24 μg/ml and 5.91 μg/ml) respectively.
Fig. 3.
% inhibition of DPPH and ABTS + assay respectively.
Fig. 4.
IC50 value of DPPH anad ABTS + Assay.
The result of the in vitro antioxidant activity of the ethyl acetate extracts of the leaves of T. bracteolata corroborates the fact that the Ethyl acetate fraction of stem of Tephrosia tinctoria exhibited significant antioxidant activity [21]. Other species of the Genus Tephrosia that have been studied that reveled their antioxidant activity includes: the ethanolic extract of Tephrosia purpurea [24, 25], ethyl acetate extracts of Tephrosia egregia [26], Tephrosia villosa [27], the ethanol ether extract of Tephrosia vogelii seeds [28].
3.4. Antimicrobial activities
3.4.1. Antibacterial activities
The result of the antibacterial activities of the n-hexane and ethyl acetate extracts at concentrations between 6.25 and 200 mg/ml is presented in Table 2. The antimicrobial activities of the extract in this study varies with the solvent type used in the extraction and the concentrations of the extract used. TbHL extract showed antibacterial activity on S. aureus, B. subtilis, and E. coli, at concentration range of 12.5–25 mg/ml and significant inhibitory effect on all six investigated bacteria at 50–200 mg/ml when compared to the standard, Gentamycin. Similarly, TbEAL extract inhibited the growth of all six bacteria at 12.5–200 mg/ml, except on E. coli, where the inhibition concentration ranges from 25 – 200 mg/ml. Furthermore, TbEAL extract, even at 6.25 mg/ml, inhibited the growth of S. aureus and B. subtilis (Gram positive). The TbEAL extract showed a greater antibacterial activity than the TbHL extract.
Table 2.
Antibacterial activities of the n-hexane and ethyl acetate extract of T. bracteolata leaves.
| Plant Extract | Concentration (mg/ml) | Zone of inhibition of Bacteria (mm) Mean ± Standard deviation |
|||||
|---|---|---|---|---|---|---|---|
| S. aureus | E. coli | B. subtilis | P. aeruginosa | K. pneumonae | S. typhi | ||
| n-Hexane | 200 | 20.0 ± 0*b | 20.0 ± 0*b | 18.0 ± 0*b | 14.0 ± 0*b | 14.0 ± 0*b | 14.0 ± 0*b |
| 100 | 18.0 ± 0*b | 18.0 ± 0*b | 16.0 ± 0*b | 12.0 ± 0*b | 12.0 ± 0*b | 12.0 ± 0*b | |
| 50 | 14.0 ± 0*b | 14.0 ± 0*b | 14.0 ± 0*b | 10.0 ± 0*b | 10.0 ± 0*b | 10.0 ± 0*b | |
| 25 | 12.0 ± 0*b | 12.0 ± 0*b | 12.0 ± 0*b | - | - | - | |
| 12.5 | 10.0 ± 0*b | 10.0 ± 0*b | 10.0 ± 0*b | - | - | - | |
| 6.25 | - | - | - | - | - | - | |
| Ethyl Acetate | 200 | 25.0 ± 1.4*b | 24.0 ± 0*b | 25.0 ± 1.3*b | 20.0 ± 0*b | 19.0 ± 1.5*b | 19.0 ± 1.4*b |
| 100 | 22.0 ± 2.8*b | 19.0 ± 1.4*b | 20.0 ± 0*b | 18.0 ± 0*b | 16.0 ± 2.8*b | 16.0 ± 2.8*b | |
| 50 | 19.0 ± 1.4*b | 17.0 ± 1.3*b | 16.0 ± 0*b | 14.0 ± 0*b | 13.0 ± 1.3*b | 13.0 ± 1.4*b | |
| 25 | 16.0 ± 2.8*b | 13.0 ± 1.4*b | 14.0 ± 0*b | 12.0 ± 0*b | 11.0 ± 1.2*b | 11.0 ± 1.5*b | |
| 12.5 | 13.0 ± 1.5*b | - | 12.0 ± 0*b | 10.0 ± 0*b | 5.0±0b | 5.0±0b | |
| 6.25 | 10.0 ± 0*b | - | 10.0 ± 0*b | - | - | - | |
| Control | n-hexane | - | - | - | - | - | - |
| Ethyl Acetate | - | - | - | - | - | - | |
| Gentamycin (10 mg/ml) | 40.0 ± 0 | 39.0 ± 1.4 | 38.0 ± 0 | 39.0 ± 1.39 | 39.0 ± 1.41 | 40.0 ± 0 | |
Key: - = no inhibition; * = values significantly different when compared to negative control using t test; alphabetic superscript other than that of Gentamycin means significantly different (P < 0.05) when compared to positive control.
Several species from Genus Tephrosia have been studied for their antibacterial activity. Kumar [29] investigated the antimicrobial effects of the roots of Tephosia purpurea against acne-inducing bacteria Propionibacterium acnes and Staphylococcus epidermidis. The results from the disc diffusion method they carried out showed that T. purpurea strongly inhibited the growth of Propionibacterium acnes. Ganapaty also reported that Pumilanol, an antiprotozoal isoflavanol from Tephrosia pumila exhibited significant antiprotozoal activity against T. b. rhodensiense, T. cruzi and L. donovani with IC50 of 3.7, 3.35 and 17.2 mg/ml respectively [30]. Li investigated the bacteriostatic activity of extracts in different solvents from Tephrosia vogelii Hook F. seeds and found out that the extracts successfully inhibited the activity of E. coli, S. aureus and S. paratyphi B. hence proved the antibacterial efficacy of the plant to be significant at high doses [28].
3.4.2. Antifungal activities
The antifungal activities of the TbHL extracts and TbEAL extracts of T. bracteolata leaves at concentrations between 6.25 and 200 mg/ml is presented in Table 3. Four clinical strains of fungi were used; Candida albicans, Aspergillus niger, Rhizopus stolon, and Penicillum notatum. The TbHL extract exhibited low antifungal activities on C. albicans even at low concentration of 25 mg/ml and on all four fungi strains: C. albicans, A. niger, R. stolon, and P. notatum at concentrations between 50 and 200 mg/ml (Table 3). The TbEAL extract exhibited higher antifungal activities on the tested organisms as shown by the values of the zone of inhibition with reference to the standard drug, Ticonazole. It inhibited the growth of C. albicans, A. niger, R. stolon, and P. notatum between the concentration of 25 and 200 mg/ml, and even at low concentration of 12.5 mg/ml it also inhibited the growth of C. albicans and P. notatum. The TbEAL extract showed a fairly reasonably zone of inhibition on all four tested organisms than the TbHL extract.
Table 3.
Anti fungi activities of n-hexane and ethyl acetate extract of T. bracteolata leaves.
| Extract | Concentration (mg/ml) | Zone of inhibition of fungi Mean ± S. Dev. (mm) |
|||
|---|---|---|---|---|---|
| C. albicans | A. niger | R. stolon | P. notatum | ||
| n-Hexane | 200 | 17.0 ± 1.31*b | 15.0 ± 1.38* b | 15.0 ± 1.21* b | 17.0 ± 1.11* b |
| 100 | 14.0 ± 0* b | 13.0 ± 1.50* b | 12.0 ± 0* b | 13.0 ± 1.39* b | |
| 50 | 12.0 ± 0* b | 10.0 ± 0* b | 10.0 ± 0* b | 10.0 ± 0* b | |
| 25 | 10.0 ± 0* b | - | - | - | |
| 12.5 | - | - | - | - | |
| 6.25 | - | - | - | - | |
| Ethyl Acetate | 200 | 19.0 ± 1.37* b | 17.0 ± 1.43* b | 17.0 ± 1.35* b | 20.0 ± 0* b |
| 100 | 17.0 ± 1.4* b | 14.0 ± 0* b | 14.0 ± 0* b | 17.0 ± 1.4* b | |
| 50 | 14.0 ± 0* b | 12.0 ± 0* b | 12.0 ± 0* b | 14.0 ± 0* b | |
| 25 | 12.0 ± 0* b | 10.0 ± 0* b | 10.0 ± 0* b | 12.0 ± 0* b | |
| 12.5 | 10.0 ± 0* b | - | - | 10.0 ± 0* b | |
| 6.25 | - | - | - | - | |
| Control | n-hexane | - | - | - | - |
| Ethyl Acetate | - | - | - | - | |
| Ticonazole (70%) | 28.0 ± 0.00a | 28.0 ± 0 a | 28.0 ± 0 a | 28.0 ± 0 a | |
Key: - = no inhibition; * = values significantly different when compared to negative control using t test; alphabetic superscript other than that of Ticonazole means significantly different (P < 0.05) when compared to positive control.
Touqeer, in their literature survey reported that there are few work relating to the antifungal activity of species from Genus Tephrosia, reported that only three (3) species are known to possess antifungal activity [31], namely Tephrosia purpurea which showed significant activity against A. niger and C. albicans [32], Tephrosia hildebrandtii was shown to have antifungal activity against C. cucumerinum. The activity was found to be related to a chemical compound isolated from its roots [33], and Tephrosia tinctoria which also showed activity against A. niger, C. albicans. The methanolic extract was found to be more active against the aforementioned organisms [30]. It is very interesting to note that both the n-hexane and the ethyl acetate extract of T. bracteolata successfully inhibited the growth of all four tested organisms with the ethyl acetate extract showing more activity, as much as 70% inhibition when compared to the standard, Ticonazole.
Thus, following the trend of the antimicrobial activity of T. bracteolata, it shows that the plant is a good source of antimicrobial agent. The zone of inhibition of the test bacteria and fungi on both extracts were concentration dependent, that is, activity was higher as concentrations of the extracts increases.
4. Conclusion
The results of the in vitro α-glucosidase enzyme inhibition assay and the DPPH and ABTS.+ antioxidant activity of the extracts of Tephrosia bracteolata leaves revealed that the leaves exhibited moderate α-glucosidase enzymatic inhibition activities and an appreciable antioxidant activity respectively. The antimicrobial activity exhibited by the n-hexane (TbHL) and ethyl acetate (TbEAL) supports the use of the leaves of T. bracteolata in traditional medicine by hunters and warriors for the treatment of injury. Extracts of T. bracteolata leaves have potential to yield active antimicrobial compounds. These observed biological activities may also be the reason why this plant is used as animal feeds. Further studies to investigate the bioactive compounds responsible for the observed biological effects are suggested.
Declarations
Author contribution statement
Godshelp Osas Egharevba: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Omotayo O. Dosumu: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Stephen O. Oguntoye: Conceived and designed the experiments; Analyzed and interpreted the data.
Ngaitad S. Njinga, Samuel Olatunde Dahunsi: Analyzed and interpreted the data; Wrote the paper.
A. Abdulmumeen Hamid: Analyzed and interpreted the data.
Ajay Anand, Zehra Amtul: Performed the experiments; Analyzed and interpreted the data.
Ukkujuri Priyanka: Performed the experiments.
Funding statement
This work was supported by the Postgraduate Fellowship awarded to Egharevba Gods'help by The World Academy of Science (TWAS), Trieste, Italy in collaboration with Council of Scientific and Industrial Research (CSIR), India, tenable at the CSIR-Indian Institute of Chemical Technology, MCP Division, Hyderabad, India.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The Departments of Industrial Chemistry, Chemistry and Plant Biology of the University of Ilorin, Nigeria are highly appreciated for providing the facilities for this research. The support of my beloved wife Mrs. Egharevba P.B and my family is priceless. Authors also appreciate the technical assistance of Mr. Olowe Kehinde of the Department of Chemistry and Mr. Bolu of the Department of Plant Biology, University of Ilorin for collection of plant and lastly to Dr. O.T. Olaniyi for his immense contribution.
References
- 1.Egharevba G.O., Dosunu O.O., Oguntoye S.O., Njinga N.S., Abdulmumeen H.A., Adebayo M.B.A. Phytochemical screening, antimicrobial and antioxidant activities of the crude extract of Senecio abyssinicus flower. J. Pharmaceut. Res. Develop. Pract. 2018;2(1):16–24. [Google Scholar]
- 2.Samuelson G. fourth ed. a Swedish Pharmaceutical Press; 1999. Drugs of Natural Origin: a Textbook of Pharmacognosy. [Google Scholar]
- 3.Egharevba God'shelp O., Dosumu Omotayo O., Njinga N.S., Oguntoye Steven O., Abdulmumeen H. Amao, Abimbola Oluyori P., Tiwari Ashok K., Anand Ajay, Zehra Amtul, Priyanka U. International Conference on Science and Sustainable Development. ICSSD 2019. 2019. phytochemical constituents, antioxidant and antimicrobial activities of extracts of Tephrosia bracteolata leaves. [Google Scholar]
- 4.Gallagher A.M., Flatt P.R., Duffy G., Abdel-Wahab Y.H. The effects of traditional antidiabetic plants on in vitro glucose diffusion. Nutr. Res. 2003;23(3):413–424. [Google Scholar]
- 5.Nowotny K., Jung T., Höhn A., Weber D., Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules. 2015;5(1):194–222. doi: 10.3390/biom5010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anis E., Anis I., Ahmed S., Mustafa G., Malik A., Afza N., Hai S.M., Shahzad-ul-hussan S., Choudhary M.I. ALPHA.-Glucosidase inhibitory constituents from cuscuta reflexa. Chem. Pharm. Bull. 2002;50(1):112–114. doi: 10.1248/cpb.50.112. [DOI] [PubMed] [Google Scholar]
- 7.Emmanouil Apostolidis M.S., Kwon Y.I. Potential of cranberry-based herbal synergies for diabetes and hypertension management. Asia Pac. J. Clin. Nutr. 2006;15:433–441. [PubMed] [Google Scholar]
- 8.Pullela S.V., Tiwari A.K., Vanka U.S., Vummenthula A., Tatipaka H.B., Dasari K.R., Khan I.A., Janaswamy M.R. HPLC assisted chemobiological standardization of α-glucosidase-I enzyme inhibitory constituents from Piper longum Linn-An Indian medicinal plant. J. Ethnopharmacol. 2006;108(3):445–449. doi: 10.1016/j.jep.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Gad M.Z., El-Sawalhi M.M., Ismail M.F., El-Tanbouly N.D. Biochemical study of the anti-diabetic action of the Egyptian plants Fenugreek and Balanites. Mol. Cell. Biochem. 2006;281(1-2):173–183. doi: 10.1007/s11010-006-0996-4. [DOI] [PubMed] [Google Scholar]
- 10.Dalziel J.M. 1937. The Useful Plants of West Tropical Africa. [Google Scholar]
- 11.Hutchinson J., Dalziel J.M. second ed. vol. 1. 1954. (Flora of West Tropical Africa). [Google Scholar]
- 12.Evans S.V., Fellows L.E., Bell E.A. Distribution and systematic significance of basic non-protein amino acids and amines in the Tephrosieae. Biochem. Syst. Ecol. 1985;13(3):271–302. [Google Scholar]
- 13.Burkill H.M. vols. 1–3. Royal Botanic Gardens; Kew: 1995. (The Useful Plants of West Tropical Africa). [Google Scholar]
- 14.Newman D.J., Cragg G.M., Snader K.M. Natural products as sources of new drugs over the period 1981-2002. J. Nat. Prod. 2003;66(7):1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 15.Harborne A.J. Springer Science & Business Media; 1998. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis. [Google Scholar]
- 16.Tiwari A.K., Swapna M., Ayesha S.B., Zehra A., Agawane S.B., Madhusudana K. Identification of proglycemic and antihyperglycemic activity in antioxidant rich fraction of some common food grains. Int. Food Res. J. 2011;18(3):915–923. [Google Scholar]
- 17.Tiwari A.K., Manasa K., Kumar D.A., Zehra A. Raw horse gram seeds possess more in vitro antihyperglycaemic activities and antioxidant properties than their sprouts. Nutrafoods. 2013;12(2):47–54. [Google Scholar]
- 18.Walker R.B., Everette J.D. Comparative reaction rates of various antioxidants with ABTS radical cation. J. Agric. Food Chem. 2009;57(4):1156–1161. doi: 10.1021/jf8026765. [DOI] [PubMed] [Google Scholar]
- 19.Pandey G., Madhuri S. Pharmacological activities of Ocimum sanctum (tulsi): a review. Int. J. Pharm. Sci. Rev. Res. 2010;5(1):61–66. [Google Scholar]
- 20.Afolayan A.J., Meyer J.J. The antimicrobial activity of 3, 5, 7-trihydroxyflavone isolated from the shoots of Helichrysum aureonitens. J. Ethnopharmacol. 1997;57(3):177–181. doi: 10.1016/s0378-8741(97)00065-2. [DOI] [PubMed] [Google Scholar]
- 21.Rajaram K., Suresh K.P. In-vitro antioxidant and antidiabetic activity of Tephrosia tinctoria Pers. An endemic medicinal plant of South India. J. Pharm. Res. 2011;3:891–893. [Google Scholar]
- 22.Pavana P., Sethupathy S., Santha K., Manoharan S. Effects of Tephrosia purpurea aqueous seed extract on blood glucose and antioxidant enzyme activities in streptozotocin induced diabetic rats. Afr. J. Tradit., Complementary Altern. Med. 2009;6(1) doi: 10.4314/ajtcam.v6i1.57077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmad S., Balakrishnan B.R., Akhtar R., Pimprikar R. Antidiabetic activity of leaves of Tephrosia villosa Pers. in alloxan induced diabetic rats. J. Pharm. Res. 2009;2(3) [Google Scholar]
- 24.Choudhary G.P. In vitro antioxidant studies of the ethanolic extract of Tephrosia purpurea L. Ancient Sci. Life. 2007;(1):26. [PMC free article] [PubMed] [Google Scholar]
- 25.Gunjegaonkar S.M., Saraswathi C.D., Hrishikeshavan H.J., Harish M.S., Nargund L.V. Hepatoprotective and antioxidant activity of Tephrosia purpurea whole plant aqueous extract. Pharmacologyonline. 2010;2:568–574. [Google Scholar]
- 26.Arriaga A.M., Lima J.Q., de Oliveira M.C., Lemos T.L., Fonseca A.M., Malcher G.T., Santiago G.M., Mafezoli J., Braz-Filho R. Antioxidant and larvicidal activities of Tephrosia egregia Sandw against Aedes aegypti. Nat. Prod. Commun. 2009;4(4):529–530. [PubMed] [Google Scholar]
- 27.Kim E.M., Jeong H.R., Min T.J. Purification, Structure Determination and Biological Activities of 20 (29)-lupen-3-one from Daedaleopsis tricolor (Bull. ex Fr.) Bond. et Sing. Bull. Korean Chem. Soc. 2001;22(1):59–62. [Google Scholar]
- 28.Li X.H., Huang X.L., Yu X., Zhu X.Y., Huang X.H. Study on scavenging free radial activity of extracts from Tephrosia vogelii Hook F. Seeds. Food Res. Dev. 2010;1:5–9. [Google Scholar]
- 29.Kumar G.S., Jayaveera K.N., Kumar C.K., Sanjay U.P., Swamy B.M., Kumar D.V. Antimicrobial effects of Indian medicinal plants against acne-inducing bacteria. Trop. J. Pharm. Res. 2007;6(2):717–723. [Google Scholar]
- 30.Ganapaty S., Pannakal S.T., Srilakshmi G.V., Lakshmi P., Waterman P.G., Brun R. Pumilanol, an antiprotozoal isoflavanol from Tephrosia pumila. Phytochem. Lett. 2008;1(4):175–178. [Google Scholar]
- 31.Touqeer S., Saeed M.A., Ajaib M. A review on the phytochemistry and pharmacology of genus Tephrosia. Phytopharmacology. 2013;4(3):598–637. [Google Scholar]
- 32.Gupta M., Mazumder U., Gomathi P., Selvan V.T. Antimicrobial activity of methanol extracts of Plumeria acuminata Ait. leaves and Tephrosia purpurea (Linn.) Pers. roots. Nat. Radiance. 2008;7(2):102–105. [Google Scholar]
- 33.Lwande W., Hassanali A., Njoroge P.W., Bentley M.D., Delle Monache F., Jondiko J.I. A new 6a-hydroxypterocarpan with insect antifeedant and antifungal properties from the roots of Tephrosia hildebrandtii Vatke. Int. J. Trop. Insect Sci. 1985;6(04):537–541. [Google Scholar]